A Multi-Wavelength Raman Study of Some Oligothiophenes and Polythiophene
Abstract
1. Introduction
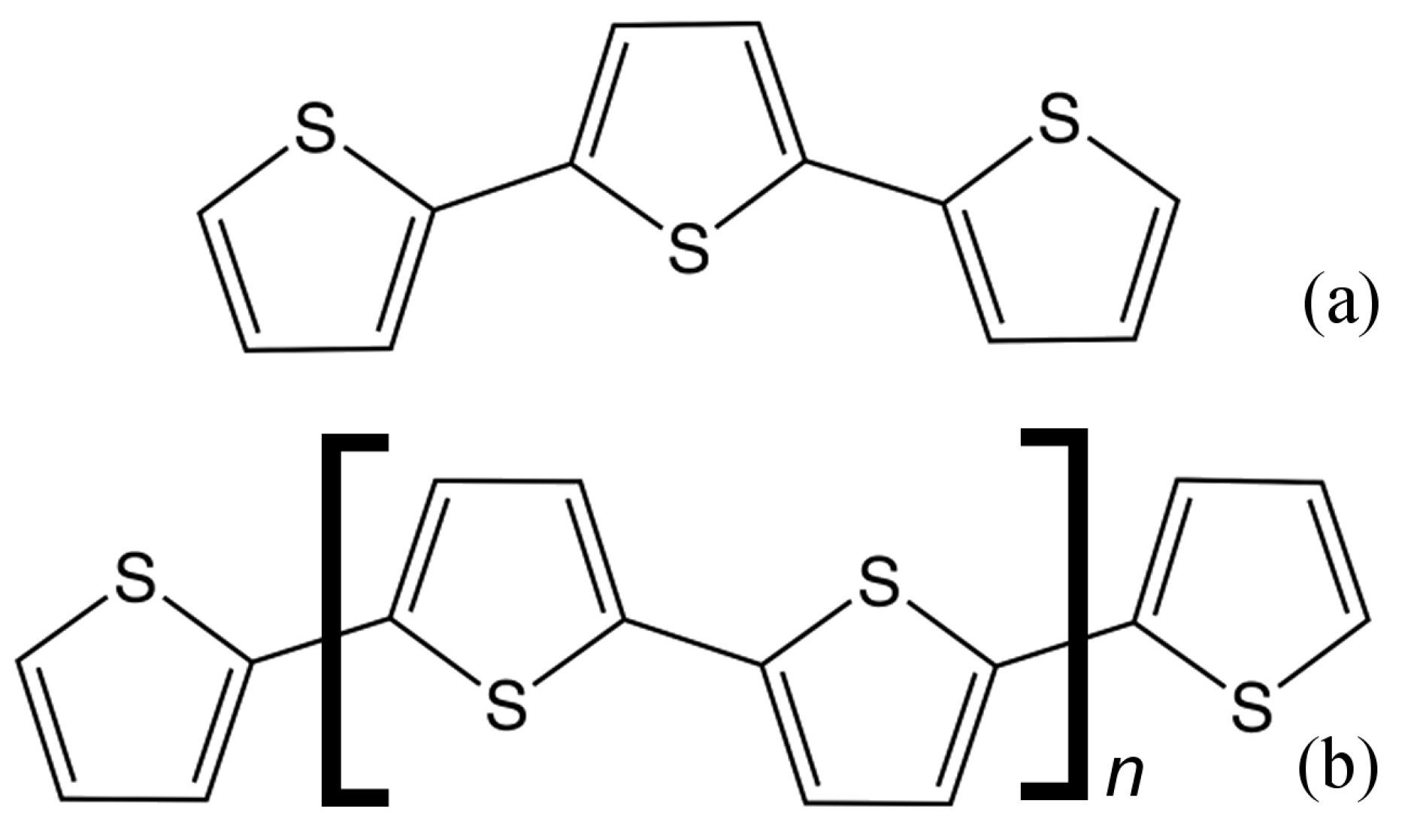
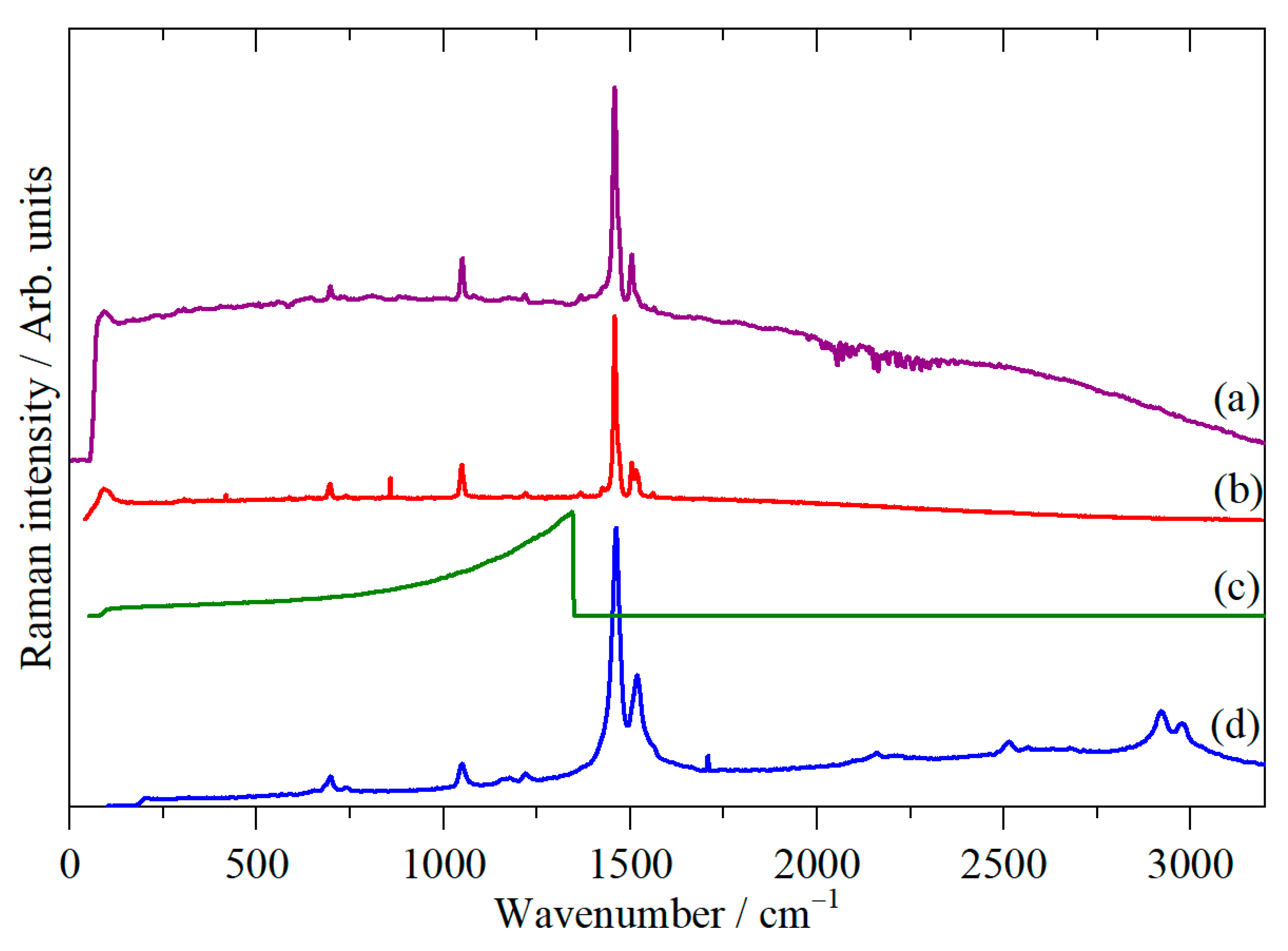
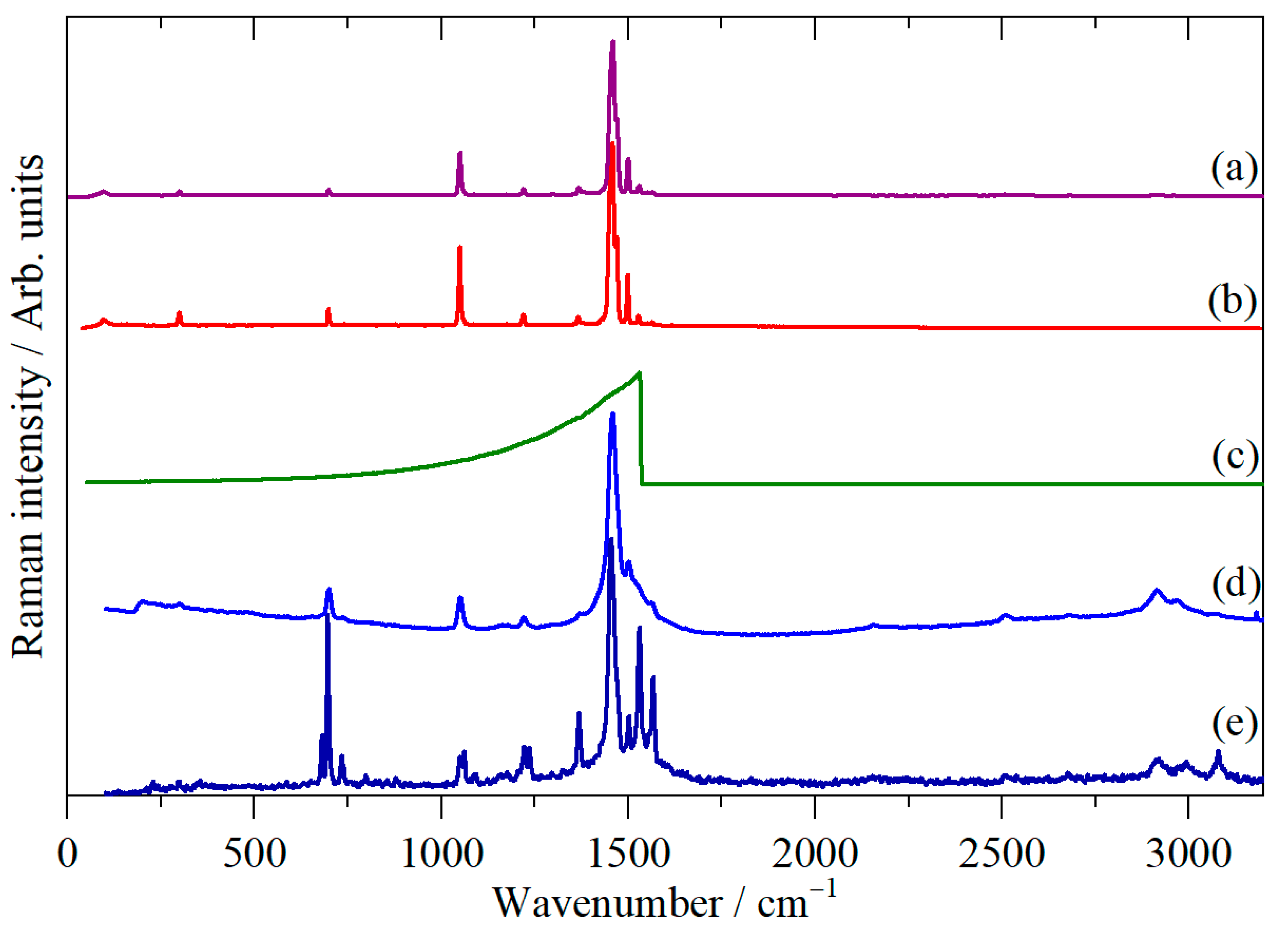
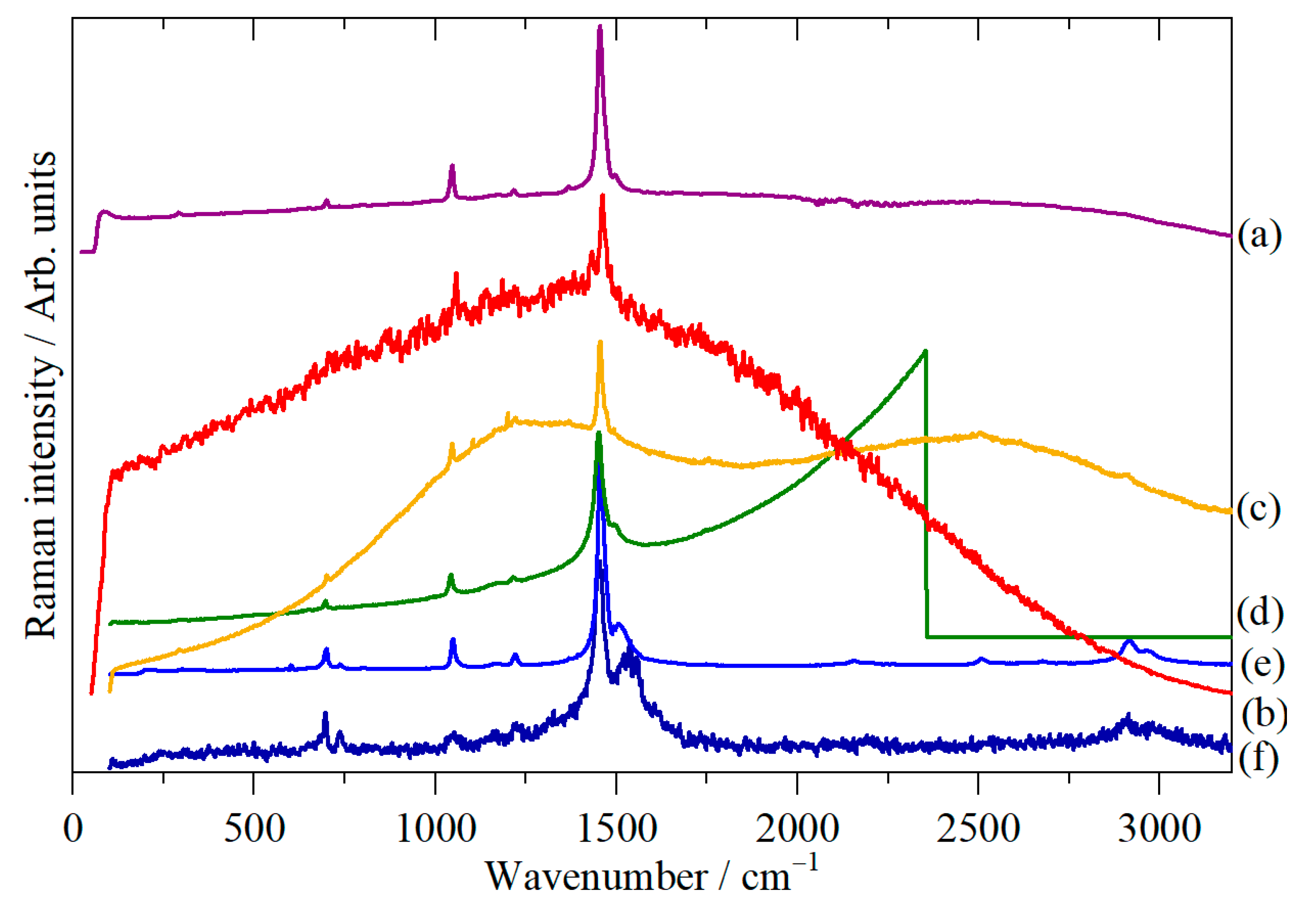
2. Materials and Methods
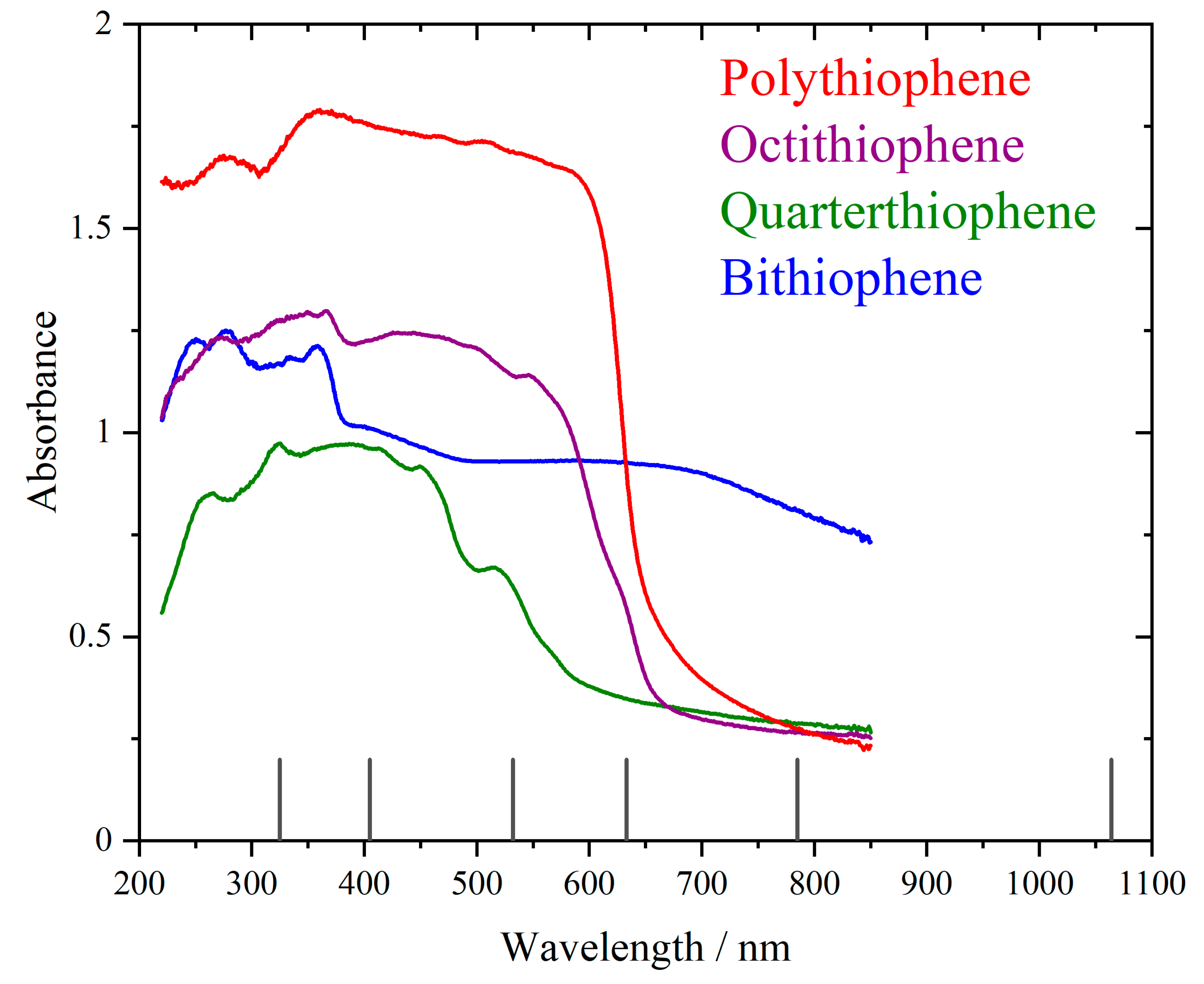
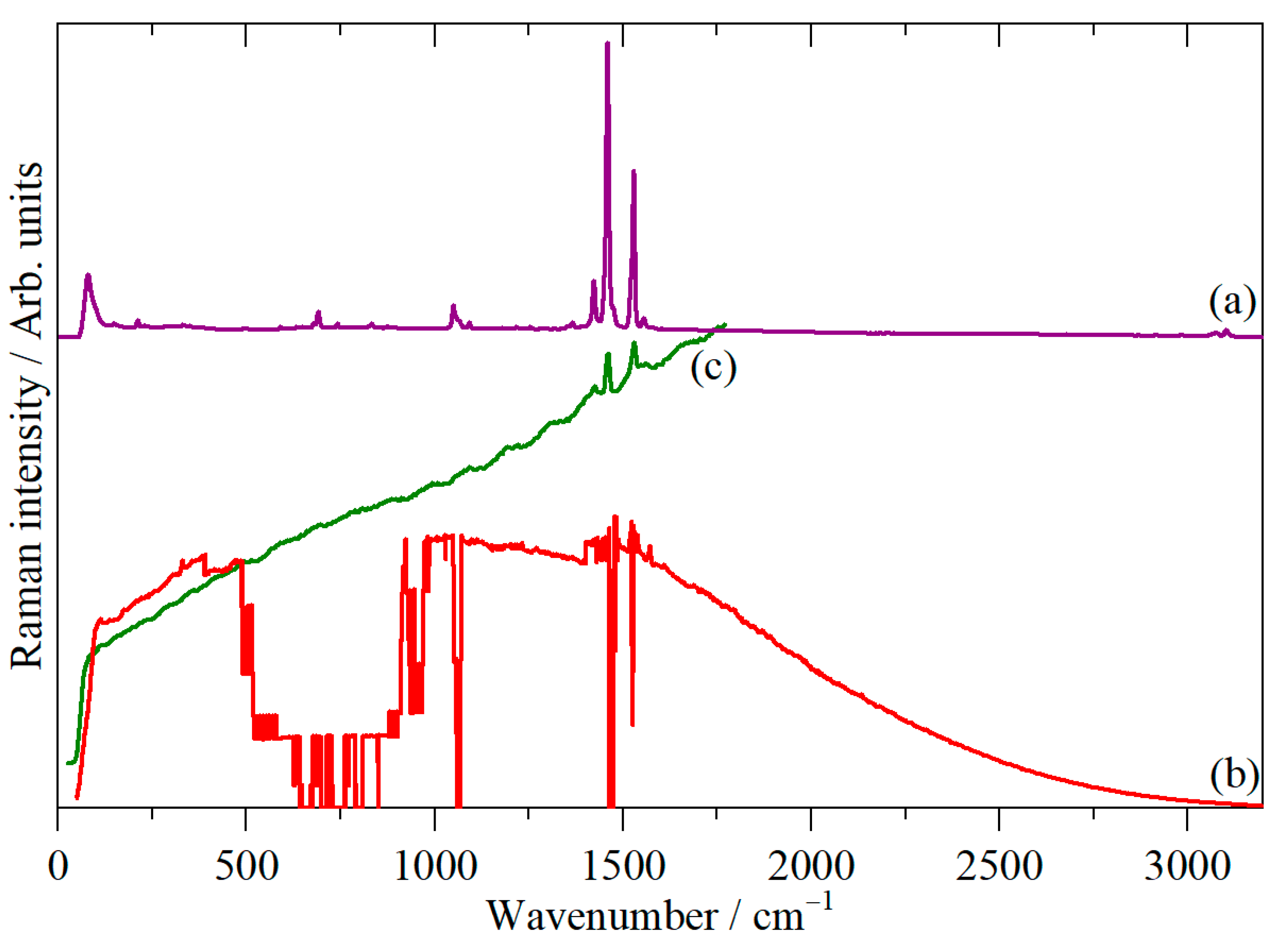
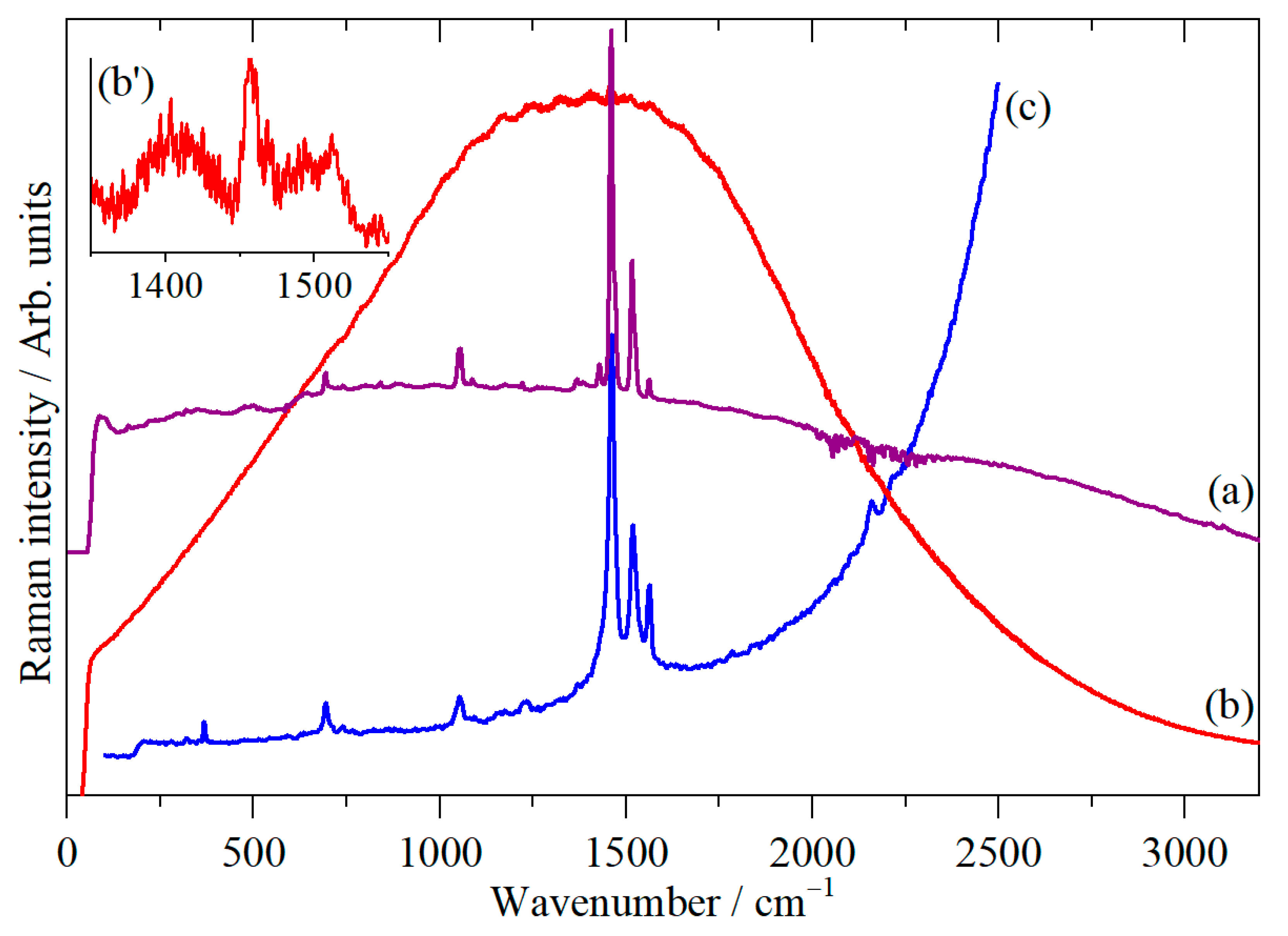
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Nobel Prize in Chemistry 2000. NobelPrize.org. Nobel Prize Outreach AB 2022. Mon. Available online: https://www.nobelprize.org/prizes/chemistry/2000/summary/ (accessed on 28 November 2022).
- Loo, Y.-L.; McCulloch, I. Progress and challenges in commercialization of organic electronics. MRS Bull. 2008, 33, 553–562. [Google Scholar] [CrossRef]
- Mazzio, K.A.; Luscombe, C.K. The future of organic photovoltaics. Chem. Soc. Rev. 2015, 44, 78–90. [Google Scholar] [CrossRef]
- Bracciale, M.P.; Kim, C.; Marrocchi, A. Organic electronics: An overview of key materials, processes, and devices. In Sustainable Strategies in Organic Electronics; Marrocchi, A., Ed.; Woodhead Publishing: San Francisco, CA, USA, 2022; pp. 3–71. [Google Scholar]
- Wang, C.; Dong, H.; Jiang, L.; Hu, W. Organic semiconductor crystals. Chem. Soc. Rev. 2018, 47, 422–500. [Google Scholar] [CrossRef] [PubMed]
- Yeha, N.; Yeh, P. Organic solar cells: Their developments and potentials. Renew. Sust. Energy Rev. 2013, 21, 421–431. [Google Scholar] [CrossRef]
- Tong, Y.; Xiao, Z.; Du, X.; Zuo, C.; Li, Y.; Lv, M.; Yuan, Y.; Yi, C.; Hao, F.; Hua, Y.; et al. Progress of the key materials for organic solar cells. Sci. China Chem. 2020, 63, 758–765. [Google Scholar] [CrossRef]
- Zhang, L.; Colella, N.S.; Cherniawski, B.P.; Mannsfeld, S.C.B.; Briseno, A.L. Oligothiophene semiconductors: Synthesis, characterization, and applications for organic devices. ACS Appl. Mater. Interfaces 2014, 6, 5327–5343. [Google Scholar] [CrossRef] [PubMed]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From fundamental perspectives to applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Chaloner, P.A.; Gunatunga, S.R.; Hitchcock, P.B. Redetermination of 2,2′-bithiophene. Acta Cryst. 1994, C50, 1941–1942. [Google Scholar] [CrossRef]
- Pelletier, M.; Brisse, F. Bithiophene at 133 K. Acta Cryst. 1994, C50, 1942–1945. [Google Scholar] [CrossRef]
- van Bolhuis, F.; Wynberg, H.; Havinga, E.E.; Meijer, E.W.; Staring, E.G.J. The X-ray structure and MNDO calculations of α-terthienyl: A model for polythiophenes. Synth. Metals 1989, 30, 381–389. [Google Scholar] [CrossRef]
- Azumi, R.; Goto, M.; Honda, K.; Matsumoto, M. Conformation and packing of odd-numbered α-oligothiophenes. Bull. Chem. Soc. Jpn. 2003, 76, 1561–1567. [Google Scholar] [CrossRef]
- Siegrist, T.; Kloc, C.; Laudise, R.A.; Katz, H.E.; Haddon, R.C. Crystal growth, structure, and electronic band structure of α-4T polymorphs. Adv. Mater. 1998, 10, 379–382. [Google Scholar] [CrossRef]
- Horowitz, G.; Bachet, B.; Yassar, A.; Lang, P.; Demanze, F.; Fave, J.-L.; Garnier, F. Growth and characterization of sexithiophene single crystals. Chem. Mater. 1995, 7, 1337–1341. [Google Scholar] [CrossRef]
- Fichou, D.; Bachet, B.; Demanze, F.; Billy, I.; Horowitz, G.; Garnier, F. Growth and structural characterization of the quasi-2D single crystal of α-octithiophene. Adv. Mater. 1996, 8, 500–504. [Google Scholar] [CrossRef]
- Mo, Z.; Lee, K.-B.; Moon, Y.B.; Kobayashi, M.; Heeger, A.J.; Wudl, F. X-ray scattering from polythiophene: Crystallinity and crystallographic structure. Macromolecules 1985, 18, 1972–1977. [Google Scholar] [CrossRef]
- Brückner, S.; Porzio, W. The structure of neutral polythiophene. An application of the Rietveld method. Makromol. Chem. 1988, 189, 961–967. [Google Scholar] [CrossRef]
- Mishra, A.; Ma, C.-Q.; Bäuerle, P. Functional oligothiophenes: Molecular design for multidimensional nanoarchitectures and their application. Chem. Rev. 2009, 109, 1141–1276. [Google Scholar] [CrossRef]
- Agosti, E.; Rivola, M.; Hernandez, V.; Del Zoppo, M.; Zerbi, G. Electronic and dynamical effects from the unusual features of the Raman spectra of oligo and polythiophenes. Synth. Metals 1999, 100, 101–112. [Google Scholar] [CrossRef]
- Esposti, A.D.; Moze, O.; Taliani, C.; Tomkinson, J.; Zamboni, R.; Zerbetto, F. The intramolecular vibrations of prototypical polythiophenes. J. Chem. Phys. 1996, 104, 9704–9718. [Google Scholar] [CrossRef]
- van Eijck, L.; Johnson, M.R.; Kearley, G.J. Intermolecular interactions in bithiophene as a model for polythiophene. J. Phys. Chem. A 2003, 107, 8980–8984. [Google Scholar] [CrossRef]
- Hermet, P.; Bantignies, J.-L.; Rahmani, A.; Sauvajol, J.-L.; Johnson, M.R. Density-of-states of crystalline 2,2′-bithiophene: Ab initio analysis and comparison with inelastic neutron scattering response. J. Phys. Condens. Matter 2004, 16, 7385–7396. [Google Scholar] [CrossRef]
- Hermet, P.; Izzard, N.; Rahmani, A.; Ghosez, P. Raman scattering in crystalline oligothiophenes: A comparison between density functional theory and bond polarizability model. J. Phys. Chem. B 2006, 110, 24869–24875. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Brambilla, L.; Del Zoppo, M.; Zerbi, G. Raman dispersion and intermolecular interactions in unsubstituted thiophene oligomers. J. Phys. Chem. B 2007, 111, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Louarn, G.; Buisson, J.P.; Lefran, S.; Fichou, D. Vibrational studies of a series of α-oligothiophenes as model systems of polythiophene. J. Phys. Chem. 1995, 99, 11399–11404. [Google Scholar] [CrossRef]
- Hermet, P.; Bantignies, J.-L.; Rahmani, A.; Sauvajol, J.-L.; Johnson, M.R. Polymorphism of crystalline α-quarterthiophene and α-sexithiophene: Ab initio analysis and comparison with inelastic neutron scattering response. J. Phys. Chem. A 2005, 109, 4202–4207. [Google Scholar] [CrossRef]
- Ranzieri, P.; Girlando, A.; Tavazzi, S.; Campione, M.; Raimondo, L.; Bilotti, I.; Brillante, A.; Della Valle, R.G.; Venuti, E. Polymorphism and phonon dynamics of α-quarterthiophene. Chem. Phys. Chem. 2009, 10, 657–663. [Google Scholar] [CrossRef]
- Degli Esposti, A.; Fanti, M.; Muccini, M.; Taliani, C.; Ruani, G. The polarized infrared and Raman spectra of α-T6 single crystal: An experimental and theoretical study. J. Chem. Phys. 2000, 112, 5957–5967. [Google Scholar] [CrossRef]
- Furukawa, Y.; Akimoto, M.; Harada, I. Vibrational key bands and electrical conductivity of polythiophene. Synth. Metals 1987, 18, 151–156. [Google Scholar] [CrossRef]
- Sauvajol, J.L.; Poussigue, G.; Benoit, C. Sample dependence of Raman spectrum on polythiophene films. Synth. Metals 1991, 41, 1237–1242. [Google Scholar] [CrossRef]
- Vardeny, Z.; Ehrenfreund, E.; Brafman, O. Photoinduced absorption and resonant Raman scattering of polythiophene. Synth. Metals 1987, 18, 183–188. [Google Scholar] [CrossRef]
- Bazzaoui, E.A.; Marsault, J.P.; Aeiyach, S.; Lacaze, P.C. Resonance Raman study of polythiophene films in the doped and undoped states. Relations between spectral data and physicochemical properties. Synth. Metals 1994, 66, 217–224. [Google Scholar] [CrossRef]
- Nejati, S.; Lau, K.K.S. Chemical vapor deposition synthesis of tunable unsubstituted polythiophene. Langmuir 2011, 27, 15223–15229. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.F.; Parker, J.L.; Jura, M. Structure and vibrational spectra of 2,5-diiodothiophene: A model for polythiophene. J. Phys. Chem. C 2017, 121, 12636–12642. [Google Scholar] [CrossRef]
- Parker, S.F.; Trevelyan, J.E.; Cavaye, H. Vibrational spectra of neutral and doped oligothiophenes and polythiophene. RSC Adv. 2023, 13, 5419–5427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, H.; Lic, J.; Zhang, J. Synthesis of polythiophene/graphite composites and their enhanced electrochemical performance for aluminum ion batteries. New. J. Chem. 2019, 43, 15014–15022. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Shariq, M.U.; Khan, M.M.A. Ultra-sensitive, highly selective and completely reversible ammonia sensor based on polythiophene/SWCNT nanocomposite. Materialia 2020, 10, 100704. [Google Scholar] [CrossRef]
- Mosca, S.; Milani, A.; Castiglioni, C.; Hernández Jolín, V.; Meseguer, C.; López Navarrete, J.T.; Zhao, C.; Sugiyasu, K.; Carmen, M.; Delgado, R. Raman fingerprints of π-electron delocalization in polythiophene-based insulated molecular wires. Macromolecules 2022, 55, 3458–3468. [Google Scholar] [CrossRef]
- El Guerraf, A.; Ben Jadi, S.; Bouabdallaoui, M.; Aouzal, Z.; Bazzaoui, M.; Aubard, J.; Lévi, G.; Bazzaoui, E.A. Investigation of the geometry and anchoring mode of conducting polythiophene films electrosynthesized on aluminium working electrodes. Mater. Today Proc. 2020, 22, 73–77. [Google Scholar] [CrossRef]
- Fichou, D.; Horowitz, G.; Xu, B.; Garnier, F. Low temperature optical absorption of polycrystalline thin films of α-quaterthiophene, α-sexithiophene and α-octithiophene, three model oligomers of polythiophene. Synth. Metals 1992, 48, 167–179. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: Boston, MA, USA, 1991; p. 17. [Google Scholar]
- Parker, S.F. INS Studies of Conducting Polymers: Oligothiophenes and Polythiophene; STFC ISIS Facility: Swindon, UK, 2017. [Google Scholar] [CrossRef]

| Thiophene | 325 nm | 405 nm | 532 nm | 632 nm | 785 nm | 1064 nm | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNR 1 | S:B 2 | SNR | S:B | SNR | S:B | SNR | S:B | SNR | S:B | SNR | S:B | |
| Bithiophene | 4 | 1.32 | 16 | 1.74 | 140 | 5.37 | 44 | 1.62 | 253 | 3.93 | ||
| Terthiophene | 3 | 0.11 | 0 3 | F 4 | 649 | 18.56 | ||||||
| Quarterthiophene | 126 | 2.21 | 2 | 0.03 | 132 | 2.17 | ||||||
| Sexithiophene | 52 | 3.24 | F | F | 288 | 7.03 | 110 | 1.33 | ||||
| Octithiophene | 71 | 4.14 | 43 | 1.87 | 0 | F | 734 | 46.62 | 1159 | 113.15 | ||
| Polythiophene | 11 | 1.62 | 124 | 6.16 | 25 | 1.13 | 28 | 0.34 | 11 | 0.22 | 414 | 2.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, S.F.; Trevelyan, J.E.; Smith, T.; Williams, K.P.J. A Multi-Wavelength Raman Study of Some Oligothiophenes and Polythiophene. Physchem 2023, 3, 210-219. https://doi.org/10.3390/physchem3020014
Parker SF, Trevelyan JE, Smith T, Williams KPJ. A Multi-Wavelength Raman Study of Some Oligothiophenes and Polythiophene. Physchem. 2023; 3(2):210-219. https://doi.org/10.3390/physchem3020014
Chicago/Turabian StyleParker, Stewart F., Jessica E. Trevelyan, Timothy Smith, and Kenneth P. J. Williams. 2023. "A Multi-Wavelength Raman Study of Some Oligothiophenes and Polythiophene" Physchem 3, no. 2: 210-219. https://doi.org/10.3390/physchem3020014
APA StyleParker, S. F., Trevelyan, J. E., Smith, T., & Williams, K. P. J. (2023). A Multi-Wavelength Raman Study of Some Oligothiophenes and Polythiophene. Physchem, 3(2), 210-219. https://doi.org/10.3390/physchem3020014






