Soundscapes: Species Richness and Community Composition of Neotropical Atlantic Forest Avifauna
Abstract
1. Introduction
2. Materials and Methods
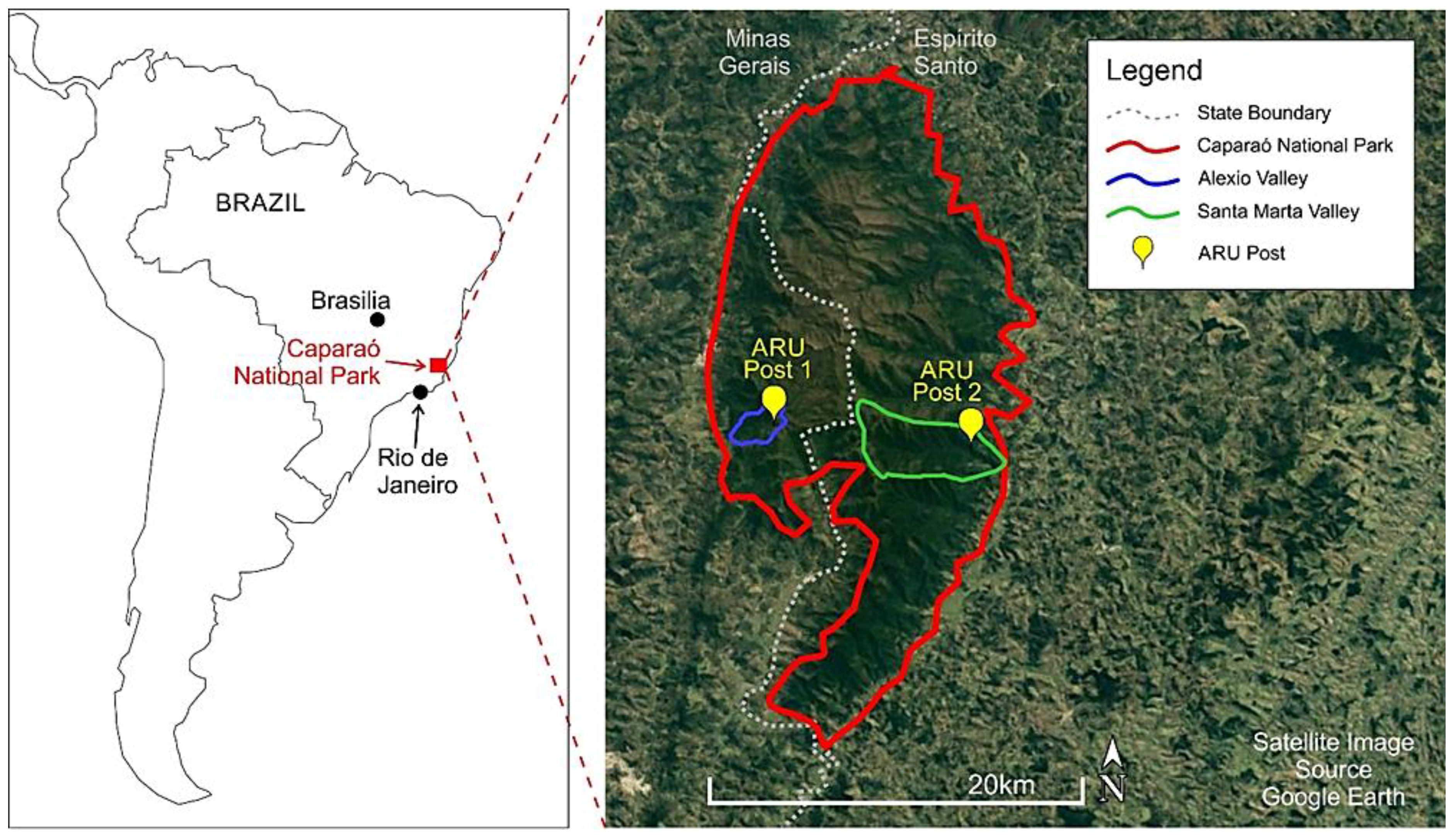
2.1. Study Site
2.2. Sampling Methods
2.3. Data Processing and Analysis
3. Results
3.1. Species Occurrence and Diversity
3.2. Avian Vocalisation Patterns and Acoustic Soundscape Dynamics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | Aleixo Valley |
| ARU | Autonomous recording units |
| ACI | Acoustic Complexity Index |
| ADI | Acoustic Diversity Index |
| AEI | Acoustic Evenness Index |
| BI | Bioacoustic Index |
| CNP | Caparao National Park |
| E | Endangered |
| H | Acoustic Entropy |
| IUCN | International Union for Conservation of Nature |
| kHz | Kilohertz |
| LC | Least Concern |
| NDSI | Normalised Difference Soundscape Index |
| NT | Near-threatened |
| PAM | Passive acoustic monitoring |
| SMV | Santa Marta Valley |
| STD | Standard deviation |
| V | Vulnerable |
Appendix A
| Species | Common Name | Location | Number of Recordings |
|---|---|---|---|
| Asthenes moreirae | Itatiaia spinetail | Aleixo, Santa Marta | 32 |
| Scytalopus speluncae | Mouse-coloured tapaculo | Aleixo, Santa Marta | 28 |
| Myiarchus ferox | Short-crested flycatcher | Aleixo, Santa Marta | 24 |
| Saltator similis | Green-winged saltator | Aleixo, Santa Marta | 24 |
| Geothlypis velata | Southern yellowthroat | Aleixo | 23 |
| Sporophila frontalis | Buffy-fronted seedeater | Aleixo, Santa Marta | 23 |
| Basileuterus culicivorus | Golden-crowned warbler | Aleixo, Santa Marta | 21 |
| Chamaeza meruloides | Cryptic antthrush | Aleixo, Santa Marta | 21 |
| Pyrrhura frontalis | Maroon-bellied parakeet | Aleixo, Santa Marta | 20 |
| Aves | Unknown 8 | Aleixo, Santa Marta | 19 |
| Aves | Unknown 9 | Aleixo, Santa Marta | 19 |
| Aves | Unknown 16 | Aleixo | 18 |
| Aves | Unknown 4 | Aleixo, Santa Marta | 18 |
| Colibri serrirostris | White-vented violetear | Aleixo | 17 |
| Stephanoxis lalandi | Green-crowned plovercrest | Aleixo, Santa Marta | 17 |
| Conirostrum speciosum speciosum | Chestnut-vented conebill | Aleixo | 16 |
| Zonotrichia capensis | Rufous-collared sparrow | Aleixo | 16 |
| Aves | Unknown 21 | Aleixo | 16 |
| Leucochloris albicollis | White-throated hummingbird | Aleixo, Santa Marta | 15 |
| Trogon surrucura | Surucua trogon | Aleixo, Santa Marta | 15 |
| Aves | Unknown 18 | Aleixo | 15 |
| Campylorhamphus falcularius | Black-billed scythebill | Santa Marta | 14 |
| Drymophila genei | Rufous-tailed antbird | Aleixo, Santa Marta | 14 |
| Sporophila caerulescens | Double-collared seedeater | Aleixo, Santa Marta | 14 |
| Turdus flavipes | Yellow-legged thrush | Aleixo, Santa Marta | 14 |
| Aves | Unknown 11 | Aleixo, Santa Marta | 14 |
| Mimus saturninus | Chalk-browed mockingbird | Aleixo, Santa Marta | 13 |
| Aves | Unknown 6 | Aleixo, Santa Marta | 13 |
| Elaenia flavogaster | Yellow-bellied elaenia | Aleixo, Santa Marta | 12 |
| Polystictus superciliaris | Grey-backed tachuri | Aleixo, Santa Marta | 12 |
| Sporophila falcirostris | Temminck’s seedeater | Aleixo | 12 |
| Aves | Unknown 1 | Aleixo, Santa Marta | 12 |
| Elaenia mesoleuca | Olivaceous elaenia | Aleixo, Santa Marta | 11 |
| Hemitriccus diops | Drab-breasted bamboo tyrant | Aleixo | 11 |
| Streptoprocne biscutata | Biscutate swift | Aleixo | 11 |
| Thraupis ornata | Golden-chevroned tanager | Santa Marta | 11 |
| Corythopis delalandi | Southern antpipit | Aleixo, Santa Marta | 10 |
| Hylophilus poicilotis | Rufous-crowned greenlet | Aleixo, Santa Marta | 10 |
| Tangara desmaresti | Brassy-breasted tanager | Aleixo, Santa Marta | 10 |
| Aves | Unknown 17 | Aleixo | 10 |
| Aves | Unknown 7 | Aleixo, Santa Marta | 10 |
| Amazona vinacea | Vinaceous-breasted Amazon | Aleixo, Santa Marta | 9 |
| Ramphastos dicolorus | Green-billed toucan | Aleixo | 9 |
| Aves | Unknown 10 | Aleixo, Santa Marta | 9 |
| Aves | Unknown 13 | Aleixo, Santa Marta | 9 |
| Chiroxiphia caudata | Blue manakin | Aleixo | 8 |
| Haplospiza unicolor | Uniform finch | Aleixo, Santa Marta | 8 |
| Procnias nudicollis | Bare-throated bellbird | Aleixo, Santa Marta | 8 |
| Stephanophorus diadematus | Diademed tanager | Aleixo | 8 |
| Tyranniscus burmeisteri | Rough-legged tyrannulet | Aleixo | 8 |
| Aves | Unknown 12 | Aleixo, Santa Marta | 8 |
| Aves | Unknown 3 | Aleixo | 8 |
| Aves | Unknown 19 | Aleixo | 7 |
| Aves | Unknown 23 | Aleixo | 7 |
| Arremon semitorquatus | Half-collared sparrow | Aleixo, Santa Marta | 6 |
| Cyclarhis gujanensis | Rufous-browed peppershrike | Aleixo, Santa Marta | 6 |
| Lepidocolaptes squamatus | Scaled woodcreeper | Aleixo, Santa Marta | 6 |
| Mackenziaena severa | Tufted antshrike | Aleixo | 6 |
| Penelope obscura | Dusky-legged guan | Aleixo | 6 |
| Pteroglossus aracari | Black-necked aracari | Aleixo | 6 |
| Syndactyla rufosuperciliata | Buff-browed foliage-gleaner | Aleixo, Santa Marta | 6 |
| Drymophila ochropyga | Ochre-rumped antbird | Aleixo | 5 |
| Formicivora serrana | Serra antwren | Aleixo | 5 |
| Phacellodomus rufifrons | Rufous-fronted thornbird | Santa Marta | 5 |
| Pheugopedius genibarbis | Moustached wren | Aleixo | 5 |
| Piculus aurulentus | Yellow-browed woodpecker | Aleixo, Santa Marta | 5 |
| Pyriglena leucoptera | White-shouldered fire-eye | Aleixo | 5 |
| Aves | Unknown 15 | Aleixo | 5 |
| Mackenziaena leachii | Large-tailed antshrike | Aleixo | 4 |
| Parabuteo leucorrhous | White-rumped hawk | Aleixo | 4 |
| Synallaxis spixi | Spix’s spinetail | Aleixo | 4 |
| Aves | Unknown 22 | Aleixo | 4 |
| Chlorophonia cyanea | Blue-naped chlorophonia | Santa Marta | 3 |
| Hemitriccus diops | drab-breasted bamboo tyrant | Santa Marta | 3 |
| Phylloscartes ventralis | Mottle-cheeked tyrannulet | Santa Marta | 3 |
| Serpophaga subcristata | White-crested tyrannulet | Aleixo | 3 |
| Aves | Unknown 2 | Aleixo | 3 |
| Aves | Unknown 20 | Aleixo | 3 |
| Aves | Unknown 5 | Aleixo | 3 |
| Crypturellus obsoletus | Brown tinamou | Aleixo | 2 |
| Ilicura militaris | Pin-tailed manakin | Aleixo | 2 |
| Patagioenas cayennensis | Pale-vented pigeon | Aleixo | 2 |
| Thamnophilus caerulescens | Variable antshrike | Aleixo | 2 |
| Turdus albicollis | White-necked thrush | Aleixo | 2 |
| Turdus rufiventris | Rufous-bellied thrush | Aleixo, Santa Marta | 2 |
| Aves | Unknown 24 | Aleixo | 2 |
| Capsiempis flaveola | Yellow tyrannulet | Aleixo | 1 |
| Cyclarhis gujanensis ochrocephala | Rufous-browed peppershrike | Aleixo | 1 |
| Leptodon cayanensis | Grey-headed kite | Aleixo | 1 |
| Myiarchus swainsoni | Swainson’s flycatcher | Aleixo | 1 |
| Picumnus cirratus | The white-barred piculet | Aleixo | 1 |
| Ramphastos dicolorus | Green-billed Toucan | Santa Marta | 1 |
| Ramphastos vitellinus | Channel-billed toucan | Aleixo | 1 |
| Sittasomus griseicapillus | Olivaceous woodcreeper | Aleixo | 1 |
| Thamnophilus ruficapillus | Rufous-capped antshrike | Aleixo | 1 |
| Aves | Unknown 25 | Aleixo | 1 |
| Aves | Unknown 26 | Aleixo | 1 |
References
- Furumo, P.R.; Mitchell Aide, T. Using soundscapes to assess biodiversity in Neotropical oil palm landscapes. Landsc. Ecol. 2019, 34, 911–923. [Google Scholar] [CrossRef]
- de Camargo, U.; Roslin, T.; Ovaskainen, O. Spatio-temporal scaling of biodiversity in acoustic tropical bird communities. Ecography 2019, 42, 1936–1947. [Google Scholar] [CrossRef]
- Shaw, T.; Hedes, R.; Sandstrom, A.; Ruete, A.; Hiron, M.; Hedblom, M.; Eggers, S.; Mikusiński, G. Hybrid bioacoustic and ecoacoustic analyses provide new links between bird assemblages and habitat quality in a winter boreal forest. Environ. Sustain. Indic. 2021, 11, 100141. [Google Scholar] [CrossRef]
- Fraixedas, S.; Lindén, A.; Piha, M.; Cabeza, M.; Gregory, R.; Lehikoinen, A. A state-of-the-art Review on Birds as Indicators of biodiversity: Advances, challenges, and Future Directions. Ecol. Indic. 2020, 118, 106728. [Google Scholar] [CrossRef]
- Gasc, A.; Pavoine, S.; Lellouch, L.; Grandcolas, P.; Sueur, J. Acoustic indices for biodiversity assessments: Analyses of bias based on simulated bird assemblages and recommendations for field surveys. Biol. Conserv. 2015, 191, 306–312. [Google Scholar] [CrossRef]
- Gasc, A.; Francomano, D.; Dunning, J.B.; Pijanowski, B.C. Future directions for soundscape ecology: The importance of ornithological contributions. Am. Ornithol. Soc. 2017, 1341, 215–228. [Google Scholar] [CrossRef]
- Hart, P.J.; Ibanez, T.; Paxton, K.; Tredinnick, G.; Sebastián-González, E.; Tanimoto-Johnson, A. Timing Is Everything: Acoustic Niche Partitioning in Two Tropical Wet Forest Bird Communities. Front. Ecol. Evol. 2021, 9, 753363. [Google Scholar] [CrossRef]
- To, A.W.Y.; Dingle, C.; Collins, S.A. Multiple constraints on urban bird communication: Both abiotic and biotic noise shape songs in cities. Behav. Ecol. 2021, 32, 1042–1053. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; Peet, M. Birds sing at a higher pitch in urban noise. Nature 2003, 424, 267. [Google Scholar] [CrossRef]
- Duarte, M.H.L.; Sousa-Lima, R.S.; Young, R.J.; Farina, A.; Vasconcelos, M.; Rodrigues, M.; Pieretti, N. The impact of noise from open-cast mining on Atlantic forest biophony. Biol. Conserv. 2015, 191, 623–631. [Google Scholar] [CrossRef]
- Alcocer, I.; Lima, H.; Sugai, L.S.M.; Llusia, D. Acoustic indices as proxies for biodiversity: A meta-analysis. Biol. Rev. 2022, 97, 2209–2236. [Google Scholar] [CrossRef]
- Fairbrass, A.J.; Rennert, P.; Williams, C.; Titheridge, H.; Jones, K.E. Biases of acoustic indices measuring biodiversity in urban areas. Ecol. Indic. 2017, 83, 169–177. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Metzger, J.P.; Martensen, A.C.; Ponzoni, F.J.; Hirota, M.M. The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009, 142, 1141–1153. [Google Scholar] [CrossRef]
- Fuzessy, L.; Pavoine, S.; Cardador, L.; Maspons, J.; Sol, D. Loss of species and functions in a deforested megadiverse tropical forest. Conserv. Biol. 2024, 38, e14250. [Google Scholar] [CrossRef]
- Forti, L.R.; Passetti, A.; Oliveira, T.; Lima, J.; Queiros, A.; Alice, M.; Szabo, J.K. Declining representation of imperiled Atlantic Forest birds in community-science datasets. Perspect. Ecol. Conserv. 2024, 22, 277–287. [Google Scholar] [CrossRef]
- Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). Plano de Manejo do Parque Nacional do Caparaó. [pdf] Brasília: ICMBio. 2018. Available online: https://www.gov.br/icmbio/pt-br/assuntos/biodiversidade/unidade-de-conservacao/unidades-de-biomas/mata-atlantica/lista-de-ucs/parna-de-caparao/arquivos/dcom_plano_de_manejo_parna_caparao.pdf (accessed on 27 May 2025).
- Aguiar, A.P.; Chiarello, A.G.; Mendes, S.L.; Matos, E.N. Os corredores Central e da Serra do Mar na Mata Atlântica brasileira. In Mata Atlântica: Biodiversidade, Ameaças e Perspectivas; Galindo-Leal, C., Câmara, I.G., Eds.; Fundação SOS Mata Atlântica, Conservação Internacional e Centro de Ciências Aplicadas à Biodiversidade: Belo Horizonte, Brasil, 2005; Chapter 11; pp. 119–132. [Google Scholar]
- Ferreira, C.D.; Baptista, M.N.d.M. Birds of Parque Nacional do Caparaó, Atlantic Forest, southeastern Brazil. Check List 2021, 17, 1557–1584. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Perbiche-neves, G. The importance of the standardizing sampling methodology to detect altitudinal gradients in mountains: A study case for the resident bird community in a hotspot (Atlantic forest) and the Middle Domain Effect. Acta Oecologica 2021, 110, 103677. [Google Scholar] [CrossRef]
- Chazdon, R.L. Towards more effective integration of tropical forest restoration and conservation. Biotropica 2019, 51, 463–472. [Google Scholar] [CrossRef]
- Jorge, F.C.; Machado, C.G.; da Cunha Nogueira, S.S.; Nogueira-Filho, S.L.G. The effectiveness of acoustic indices for forest monitoring in Atlantic rainforest fragments. Ecol. Indic. 2018, 91, 71–76. [Google Scholar] [CrossRef]
- Metcalf, O.C.; Barlow, J.; Marsden, S.; Gomes de Moura, N.; Berenguer, E.; Ferreira, J.; Lees, A.C. Optimizing tropical forest bird surveys using passive acoustic monitoring and high temporal resolution sampling. Remote Sens. Ecol. Conserv. 2021, 8, 45–56. [Google Scholar] [CrossRef]
- Darras, K.; Batáry, P.; Furnas, B.; Celis-Murillo, A.; Van Wilgenburg, S.L.; Mulyani, Y.A.; Tscharntke, T. Comparing the sampling performance of sound recorders versus point counts in bird surveys: A meta-analysis. J. Appl. Ecol. 2018, 55, 2575–2586. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Traba, J. Estimating bird density using passive acoustic monitoring: A review of methods and suggestions for further research. Ibis 2021, 163, 765–783. [Google Scholar] [CrossRef]
- Kleyn, T.; Kaizer, C.; Passos, L. Sharing sound: Avian acoustic niches in the Brazilian Atlantic Forest. Biotropica 2021, 53, 658–670. [Google Scholar] [CrossRef]
- Moreira, M.M.; Carrijo, T.T.; Alves-Araújo, A.G.; Rapini, A.; Salino, A.; Firmino, A.D.; Chagas, A.P.; Versiane, A.F.A.; Amorim, A.M.A.; da Silva, A.N.S.; et al. A list of land plants of Parque Nacional do Caparaó, Brazil, highlights the presence of sampling gaps within this protected area. Biodivers. Data J. 2020, 8, e59664. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Google Earth. Parque Nacional do Caparao, Alto Caparao, MG, 20.422186°S, 41.853447°W. [Online]. 2025. Available online: https://earth.google.com/web/ (accessed on 19 May 2025).
- K. Lisa Yang Center for Conservation Bioacoustics at the Cornell Lab of Ornithology. Raven Pro: Interactive Sound Analysis Software (Version 1.6.5) [Computer Software]; The Cornell Lab of Ornithology: Ithaca, NY, USA, 2024; Available online: https://www.ravensoundsoftware.com/ (accessed on 23 August 2024).
- Villanueva-Rivera, L.J.; Pijanowski, B.C.; Luis, M.; Villanueva-Rivera, J. Soundscape Ecology. 2016. Available online: https://cran.r-project.org/web/packages/soundecology/soundecology.pdf (accessed on 13 May 2025).
- Hou, Y.; Yu, X.; Yang, J.; Ouyang, X.; Fan, D. Acoustic Sensor-Based Soundscape Analysis and Acoustic Assessment of Bird Species Richness in Shennongjia National Park, China. Sensors 2022, 22, 4117. [Google Scholar] [CrossRef]
- Pieretti, N.; Farina, A.; Morri, D. A new methodology to infer the singing activity of an avian community: The Acoustic Complexity Index (ACI). Ecol. Indic. 2011, 11, 868–873. [Google Scholar] [CrossRef]
- Villanueva-Rivera, L.J.; Pijanowski, B.C.; Doucette, J.; Pekin, B. A primer of acoustic analysis for landscape ecologists. Landsc. Ecol. 2011, 26, 1233–1246. [Google Scholar] [CrossRef]
- Kasten, E.P.; Gage, S.H.; Fox, J.; Joo, W. The remote environmental assessment laboratory’s acoustic library: An archive for studying soundscape ecology. Ecol. Inform. 2012, 12, 50–67. [Google Scholar] [CrossRef]
- Sueur, J.; Farina, A.; Gasc, A.; Pieretti, N.; Pavoine, S. Acoustic Indices for Biodiversity Assessment and Landscape Investigation. Acta Acust. United Acust. 2014, 100, 772–781. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package (R Package Version 2.0–3). 2012. Available online: http://CRAN.R-project.org/package=vegan (accessed on 13 May 2025).
- Hutcheson, K. A test for comparing diversities based on the shannon formula. J. Theor. Biol. 1970, 29, 151–154. [Google Scholar] [CrossRef]
- Marcon, E.; Hérault, B. entropart: An R Package to Measure and Partition Diversity. J. Stat. Softw. 2015, 67, 1–26. [Google Scholar] [CrossRef]
- Grundy, V.; Kaizer, M.; Passos, L.; Schork, I.; Soundscapes composition of neotropical Atlantic Forest avifauna. Mendeley Data, V2. Available online: https://data.mendeley.com/datasets/p2vjjwnmwy/2 (accessed on 13 May 2025). [CrossRef]
- Eldridge, A.; Guyot, P.; Moscoso, P.; Johnston, A.; Eyre-Walker, Y.; Peck, M. Sounding out ecoacoustic metrics: Avian species richness is predicted by acoustic indices in temperate but not tropical habitats. Ecol. Indic. 2018, 95, 939–952. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Fontes, M.A.L. Patterns of floristic differentiation among atlantic forests in southeastern Brazil and the influence of climate. Biotropica 2000, 32, 793–810. [Google Scholar] [CrossRef]
- De Araújo, C.B.; Lima, M.R.; Albuquerque, P.; Alquezar, R.D.; Barreiros, M.; Jardim, M.; Gangenova, E.; Machado, R.B.; Phalan, B.T.; Roos, A.L.; et al. Acoustic monitoring of anurans and birds in tropical biomes. Biotropica 2024, 56, e13307. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Z.; Zhang, Z.; Xiong, Y. Investigating the drivers of temporal and spatial dynamics in urban forest bird acoustic patterns. J. Environ. Manag. 2025, 376, 124554. [Google Scholar] [CrossRef] [PubMed]
- Karp, D.S.; Frishkoff, L.O.; Echeverri, A.; Zook, J.; Juárez, P.; Chan, K.M.A. Agriculture erases climate-driven β-diversity in Neotropical bird communities. Glob. Change Biol. 2017, 24, 338–349. [Google Scholar] [CrossRef]
- Mereta, S.T.; Lemmens, P.; De Meester, L.; Goethals, P.L.M.; Boets, P. The Relative Importance of Human Disturbance, Environmental and Spatial Factors on the Community Composition of Wetland Birds. Water 2021, 13, 3448. [Google Scholar] [CrossRef]
- Buxton, R.T.; Brown, E.; Sharman, L.; Gabriele, C.M.; McKenna, M.F. Using bioacoustics to examine shifts in songbird phenology. Ecol. Evol. 2016, 6, 4697–4710. [Google Scholar] [CrossRef]
- Metcalf, O.C.; Barlow, J.; Devenish, C.; Marsden, S.; Berenguer, E.; Lees, A.C. Acoustic indices perform better when applied at ecologically meaningful time and frequency scales. Methods Ecol. Evol. 2020, 12, 421–431. [Google Scholar] [CrossRef]
- Gaspar, L.P.; Scarpelli, M.D.A.; Oliveira, E.G.; Alves, R.S.-C.; Gomes, A.M.; Wolf, R.; Ferneda, R.V.; Kamazuka, S.H.; Gussoni, C.O.A.; Ribeiro, M.C. Predicting bird diversity through acoustic indices within the Atlantic Forest biodiversity hotspot. Front. Remote Sens. 2023, 4, 1283719. [Google Scholar] [CrossRef]
- Briseño-Jaramillo, M.; Hutschenreiter, A.; Aureli, F.; Ríos-Chelén, A.A. Passive acoustic monitoring and acoustic indices reveal noise-related changes in bird vocalisations. Bioacoustics 2025, 34, 167–187. [Google Scholar] [CrossRef]
- Apol, C.A.; Valentine, E.C.; Proppe, D.S. Ambient noise decreases detectability of songbird vocalizations in passive acoustic recordings in a consistent pattern across species, frequency, and analysis method. Bioacoustics 2020, 29, 322–336. [Google Scholar] [CrossRef]
- Luther, D.; Gentry, K. Sources of background noise and their influence on vertebrate acoustic communication. Behaviour 2013, 150, 1045–1068. [Google Scholar] [CrossRef]
- Halfwerk, W.; Bot, S.; Buikx, J.; van der Velde, M.; Komdeur, J.; ten Cate, C.; Slabbekoorn, H. Low-frequency songs lose their potency in noisy urban conditions. Proc. Natl. Acad. Sci. USA 2011, 108, 14549–14554. [Google Scholar] [CrossRef]
- Derrberry, E.P.; Luther, D. What is known—And not known—About acoustic communication in an urban soundscape. Integr. Comp. Biol. 2021, 61, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Depraetere, M.; Pavoine, S.; Jiguet, F.; Gasc, A.; Duvail, S.; Sueur, J. Monitoring animal diversity using acoustic indices: Implementation in a temperate woodland. Ecol. Indic. 2012, 13, 46–54. [Google Scholar] [CrossRef]
- Menezes, T.; Podos, J. Diverse relationships between amplitude and frequency in bird vocalizations. Proc. R. Soc. B Biol. Sci. 2025, 292, 20250781. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, Z.; Bellisario, K.; Zeng, R.; Li, N.; Zhou, W.; Pijanowski, B.C. How well do acoustic indices measure biodiversity? Computational experiments to determine effect of sound unit shape, vocalization intensity, and frequency of vocalization occurrence on performance of acoustic indices. Ecol. Indic. 2019, 107, 105588. [Google Scholar] [CrossRef]
- Sebastianelli, M.; Blumstein, D.T.; Kirschel, A.N.G. Ambient noise from ocean surf drives frequency shifts in non-passerine bird song. bioRxiv 2020. [CrossRef]
- Mullet, T.C.; Farina, A.; Gage, S.H. The Acoustic Habitat Hypothesis: An Ecoacoustics Perspective on Species Habitat Selection. Biosemiotics 2017, 10, 319–336. [Google Scholar] [CrossRef]
- Kleist, N.J.; Guralnick, R.P.; Cruz, A.; Francis, C.D. Sound settlement: Noise surpasses land cover in explaining breeding habitat selection of secondary cavity-nesting birds: Noise. Ecol. Appl. 2017, 27, 260–273. [Google Scholar] [CrossRef]
- Roca, I.T.; Desrochers, L.; Giacomazzo, M.; Bertolo, A.; Bolduc, P.; Deschesnes, R.; Martin, C.A.; Rainville, V.; Rheault, G.; Proulx, R. Shifting song frequencies in response to anthropogenic noise: A meta-analysis on birds and anurans. Behav. Ecol. 2016, 27, 1269–1274. [Google Scholar] [CrossRef]
- Fossesca, M.; Henry, K.S.; Chou, T.L.; Gall, M.D. The silent assumption of the masking hypothesis: Avian auditory processing and implications for behavioral responses to anthropogenic noise. Front. Ecol. Evol. 2023, 11, 1233911. [Google Scholar] [CrossRef]
- BirdLife International. Species Factsheet: Channel-Billed Toucan Ramphastos Vitellinus. 2022. Available online: https://datazone.birdlife.org/species/factsheet/channel-billed-toucan-ramphastos-vitellinus (accessed on 31 May 2025).
- Eiterer, N.; de Oliveira, J.A.; Cox, B. Channel-billed Toucan · Ramphastos Vitellinus, Xeno-Canto, [Online]. 2017. Available online: https://xeno-canto.org/ (accessed on 28 April 2025).
- Galetti, M.; Laps, R.; Pizo, M.A. Frugivory by toucans (Ramphastidae) at two altitudes in the Atlantic Forest of Brazil. Biotropica 2000, 32, 842–850. [Google Scholar] [CrossRef]
- BirdLife International. Species Factsheet: Buffy-Fronted Seedeater Sporophila Frontalis. 2018. Available online: https://datazone.birdlife.org/species/factsheet/buffy-fronted-seedeater-sporophila-frontalis (accessed on 31 May 2025).
- Veríssimo, N.; Safford, H.D.; Behling, H. Holocene vegetation and fire history of the Serra do Caparaó, SE Brazil. Holocene 2012, 22, 1243–1250. [Google Scholar] [CrossRef]
- Entringer, H.; Silva-Soares, T.; Silva, E.P.; Srbek-Araujo, A.C. New occurrence records and notes on habitat use and antipredator behavior by Urostrophus vautieri (Squamata: Leiosauridae) in southeastern Brazil. Herpetol. Notes 2022, 15, 241–246. [Google Scholar]
- Silveira, L.F.; Olmos, F.; Long, A.J. Birds in Atlantic Forest fragments in north-east Brazil. Cotinga 2003, 20, 32–46. [Google Scholar]
- De Araújo, C.B.; Zurano, J.P.; Torres, I.M.D.; Simões, C.R.M.A.; Rosa, G.L.M.; Aguiar, A.G.; Nogueira, W.; Vilela, H.A.L.S.; Magnago, G.; Phalan, B.T.; et al. The sound of hope: Searching for critically endangered species using acoustic template matching. Bioacoustics 2023, 32, 708–723. [Google Scholar] [CrossRef]
- Teixeira, D.; Roe, P.; van Rensburg, B.J.; Linke, S.; McDonald, P.G.; Tucker, D.; Fuller, S. Effective ecological monitoring using passive acoustic sensors: Recommendations for conservation practitioners. Conserv. Sci. Pract. 2024, 6, e13132. [Google Scholar] [CrossRef]
- Campos, I.B.; Fewster, R.; Truskinger, A.; Towsey, M.; Roe, P.; Vasques Filho, D.; Lee, W.; Gaskett, A. Assessing the potential of acoustic indices for protected area monitoring in the Serra do Cipó National Park, Brazil. Ecol. Indic. 2021, 120, 106953. [Google Scholar] [CrossRef]
- Moura, N.G.; Lees, A.C.; Andretti, C.B.; Davis, B.J.W.; Solar, R.R.C.; Aleixo, A.; Barlow, J.; Ferreira, J.; Gardner, T.A. Avian biodiversity in multiple-use landscapes of the Brazilian Amazon. Biol. Conserv. 2013, 167, 339–348. [Google Scholar] [CrossRef]
- Cardozo, R.; Machado, R.B. Bird Diversity of the Dry Chaco: Impacts of Land Use Change on Communities and Soundscapes. Austral Ecol. 2025, 50, e70032. [Google Scholar] [CrossRef]
- Shaw, T.; Scherer-Lorenzen, M.; Müller, S. Forest structural heterogeneity positively affects bird richness and acoustic diversity in a temperate, central European forest. Front. Ecol. Evol. 2024, 12, 1387879. [Google Scholar] [CrossRef]
- Charity, S.; Ferreira, J.M. Wildlife Trafficking in Brazil; TRAFFIC International: Cambridge, UK, 2020. [Google Scholar]
- Helena Torres da Rocha, A.; Guedes-Santos, J.; Alexandre Santos Vieira, F.; Claudia Mendes Malhado, A.; Ladle, R.J.; Lizandro Schmitt, J.; Bragagnolo, C. What threats do Brazilian National parks face? J. Nat. Conserv. 2025, 84, 126813. [Google Scholar] [CrossRef]
- Darras, K.; Pütz, P.; Rembold, K.; Tscharntke, T. Measuring sound detection spaces for acoustic animal sampling and monitoring. Biol. Conserv. 2016, 201, 29–37. [Google Scholar] [CrossRef]
- Lin, T.; Tsao, T. Source separation in ecoacoustics: A roadmap towards versatile soundscape information retrieval. Remote Sens Ecol Conserv. 2019, 6, 236–247. [Google Scholar] [CrossRef]
- Siddagangaiah, S.; Chen, C.-F.; Hu, W.-C.; Pieretti, N. A Complexity-Entropy Based Approach for the Detection of Fish Choruses. Entropy 2019, 21, 977. [Google Scholar] [CrossRef]
- Bradfer-Lawrence, T.; Bunnefeld, N.; Gardner, N.; Willis, S.G.; Dent, D.H. Rapid assessment of avian species richness and abundance using acoustic indices. Ecol. Indic. 2020, 115, 106400. [Google Scholar] [CrossRef]
- Gibb, R.; Browning, E.; Glover-Kapfer, P.; Jones, K.E. Emerging opportunities and challenges for passive acoustics in ecological assessment and monitoring. Methods Ecol. Evol. 2018, 10, 169–185. [Google Scholar] [CrossRef]
- Pardo, J.M.; Cruz, P.; Moya, S.; Pizzio, E.; Foletto, F.; Robino, F.; Aquino, J.; Costa, S.; Barros, Y.; Cleo, F.; et al. Predicting poaching hotspots in the largest remnant of the Atlantic Forest by combining passive acoustic monitoring and occupancy models. Biol. Conserv. 2022, 272, 109600. [Google Scholar] [CrossRef]
- Müller, S.; Gossner, M.M.; Penone, C.; Jung, K.; Renner, S.C.; Farina, A.; Anhäuser, L.; Ayasse, M.; Boch, S.; Haensel, F.; et al. Land-use intensity and landscape structure drive the acoustic composition of grasslands. Agric. Ecosyst. Environ. 2022, 328, 107845. [Google Scholar] [CrossRef]
- Sethi, S.S.; Jones, N.S.; Fulcher, B.D.; Picinali, L.; Clink, D.J.; Klinck, H.; Orme, C.D.L.; Wrege, P.H.; Ewers, R.M. Characterizing soundscapes across diverse ecosystems using a universal acoustic feature set. Proc. Natl. Acad. Sci. USA 2020, 117, 17049–17055. [Google Scholar] [CrossRef] [PubMed]



| Avian Species | Occurrence | Location | Call Frequency Range (kHz) | IUCN Conservation Status |
|---|---|---|---|---|
| Itatiaia Spinetail Asthenes moreirae | 32 | AV, SMV | 1.0–20.0 | LC |
| Mouse-coloured Tapaculo Scytalopus speluncae | 28 | AV, SMV | 2.0–22.0 | LC |
| Green-winged Saltator Saltator similis | 24 | AV, SMV | 1.0–10.0 | LC |
| Short-crested Flycatcher Myiarchus ferox | 24 | AV, SMV | 4.0–6.0 | LC |
| Buffy-fronted seedeater Sporophila frontalis | 23 | AV, SMV | 1.5–10.5 | V |
| Southern Yellowthroat Geothlypis velata | 23 | AV | 3.0–11.0 | LC |
| Cryptic Ant thrush Chamaeza meruloides | 21 | AV, SMV | 1.0–2.0 | LC |
| Golden-crowned warbler Basileuterus culicivorus | 21 | AV, SMV | 3.0–9.0 | LC |
| Maroon-bellied Parakeet Pyrrhura frontalis | 20 | AV, SMV | 2.0–23.0 | LC |
| Green-crowned Plovercrest Stephanoxis lalandi | 17 | AV, SMV | 5.0–7.0 | LC |
| White-vented Violetear Colibri serrirostris | 17 | AV | 5.0–8.0 | LC |
| Chestnut-vented Conebill C. speciosum speciosum | 16 | AV | 5.0–8.0 | LC |
| Rufous-collared Sparrow Zonotrichia capensis | 16 | AV | 4.0–8.0 | LC |
| Surucua Trogon Trogon surrucura | 15 | AV, SMV | 1.0–2.5 | LC |
| White-throated Hummingbird Leucochloris albicollis | 15 | AV, SMV | 3.0–10.0 | LC |
| Black-billed Scythebill Campylorhamphus falcularius | 14 | SMV | 4.0–22.0 | LC |
| Double-collared Seedeater Sporophila caerulescens | 14 | AV, SMV | 3.0–22.0 | LC |
| Drab-breasted bamboo tyrant Hemitriccus diops | 14 | AV | 5.0–8.0 | LC |
| Rufous-tailed Antbird Drymophila genei | 14 | AV, SMV | 2.0–10.0 | LC |
| Yellow-legged Thrush Turdus flavipes | 14 | AV, SMV | 6.0–11.0 | LC |
| Chalk-browed Mockingbird Mimus saturninus | 13 | AV, SMV | 6.0–16.0 | LC |
| Temminck’s Seedeater Sporophila falcirostris | 12 | AV | 5.0–11.0 | V |
| Yellow-bellied Elaenia Elaenia flavogaster | 12 | AV, SMV | 2.0–20.0 | LC |
| Golden-chevroned Tanager Thraupis ornata | 11 | SMVV | 4.0–20.0 | LC |
| Olivaceous Elaenia Elaenia mesoleuca | 11 | AV, SMV | 5.0–22.0 | LC |
| Acoustic Indices | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACI a | NDSI b | BI c | ADI d | AEI e | H f | |||||||
| Site | AV * | SMV | AV | SMV | AV * | SMV | AV | SMV | AV * | SMV | AV * | SMV |
| Min | 9142.97 | 4492.3 | 0.53 | 0.47 | 108.19 | 22.72 | 2.1 | 2.38 | 0.4 | 0.16 | 0.85 | 0.83 |
| Max | 122.58 | 11.59 | 0.2 | 0.09 | 7.08 | 4.97 | 0.23 | 0.14 | 0.15 | 0.11 | 0.03 | 0.02 |
| Mean | 9009.9 | 4475.31 | −0.03 | 0.35 | 92.97 | 13.64 | 1.71 | 2.03 | 0.23 | 0.06 | 0.78 | 0.81 |
| STD | 9464.53 | 4508.05 | 0.83 | 0.61 | 114.67 | 26.63 | 2.34 | 2.48 | 0.65 | 0.41 | 0.89 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grundy, V.; Kaizer, M.C.; F. Passos, L.; Schork, I. Soundscapes: Species Richness and Community Composition of Neotropical Atlantic Forest Avifauna. Conservation 2025, 5, 48. https://doi.org/10.3390/conservation5030048
Grundy V, Kaizer MC, F. Passos L, Schork I. Soundscapes: Species Richness and Community Composition of Neotropical Atlantic Forest Avifauna. Conservation. 2025; 5(3):48. https://doi.org/10.3390/conservation5030048
Chicago/Turabian StyleGrundy, Vanessa, Mariane C. Kaizer, Luiza F. Passos, and Ivana Schork. 2025. "Soundscapes: Species Richness and Community Composition of Neotropical Atlantic Forest Avifauna" Conservation 5, no. 3: 48. https://doi.org/10.3390/conservation5030048
APA StyleGrundy, V., Kaizer, M. C., F. Passos, L., & Schork, I. (2025). Soundscapes: Species Richness and Community Composition of Neotropical Atlantic Forest Avifauna. Conservation, 5(3), 48. https://doi.org/10.3390/conservation5030048






