Detecting the Endangered San Joaquin Kit Fox (Vulpes macrotis mutica) and Other Canine Species in Kern County, CA: Applying a Non-Invasive PCR-Based Method to Four Case Study Sites
Abstract
1. Introduction
- Establish an economical and reliable non-invasive PCR-based method to detect the presence of SJKFs and other canine species in urban Bakersfield and surrounding environments in Kern County in our lab at CSUB.
- Apply this method to selected locations in Kern County where SJKFs are suspected of roaming, including areas where a suitable habitat is threatened by development.
- Communicate and promote this non-invasive PCR-based method to local environmental consulting companies to support efforts to identify SJKF habitats in the Central Valley of California prior to development in a timely and cost-efficient manner.
- Contribute to SJKF protection and conservation efforts in the Central Valley of California.
2. Materials and Methods
2.1. Reference Samples
2.2. Site Selection and Description
2.3. Collection of Environmental Scat Samples
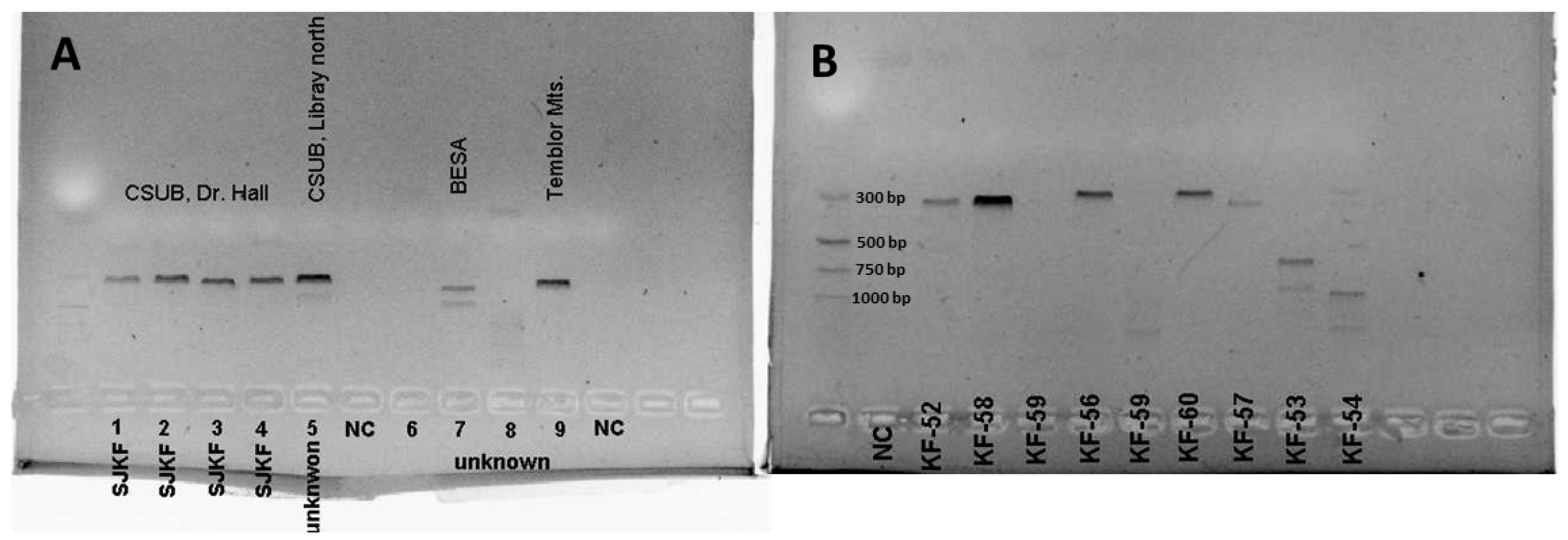
2.4. DNA Extraction, Polymerase Chain Reaction (PCR), and Agarose Gel Electrophoresis
2.5. Sequencing of PCR Products
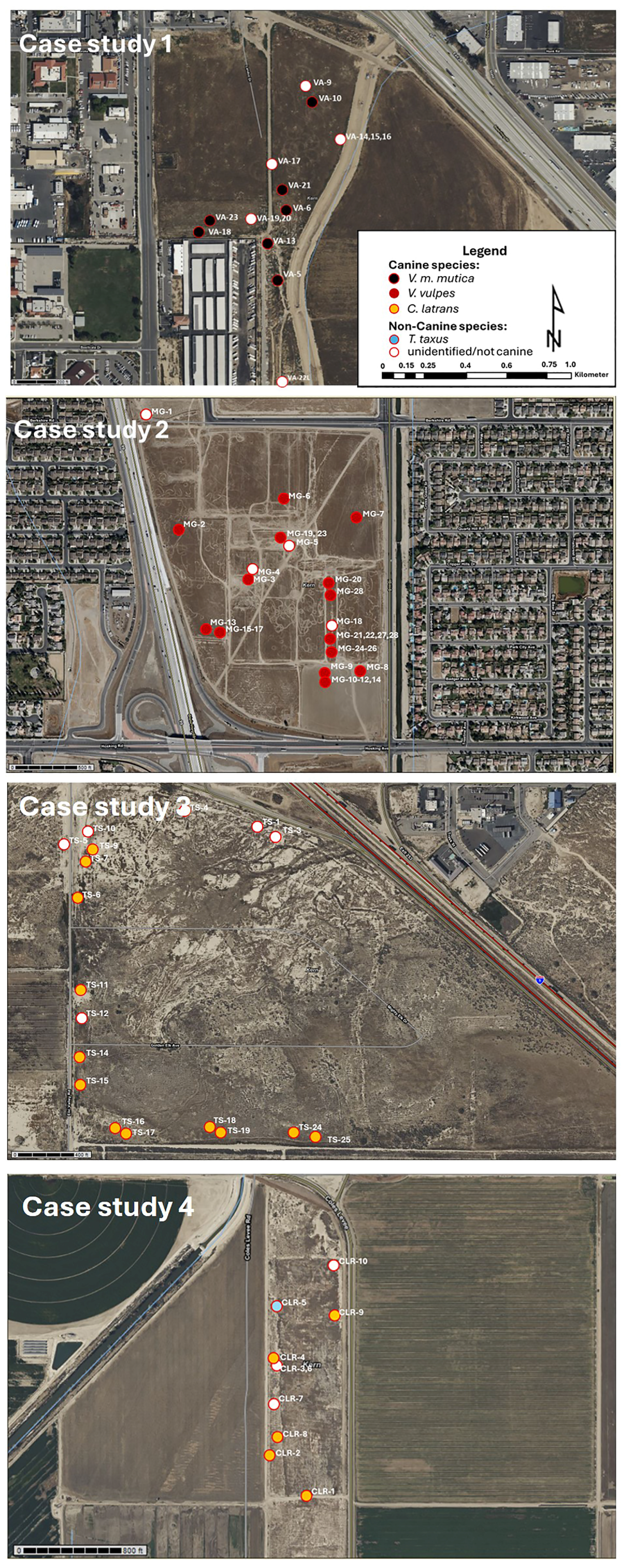
3. Results
4. Discussion
- Continuing data collection (potentially in collaboration with environmental consulting companies) in areas of Kern County where the presence of SJKFs is unknown or anecdotal (e.g., potential mitigation sites).
- Developing primer pairs that are more specific to canines, or, alternatively, allow the identification of all non-canine species that are common in Kern County, thereby reducing the number of unidentified scat samples.
- Amplifying the DNA extracts that failed to produce an amplicon using an additional primer pair [54] that amplifies a shorter fragment of the mitochondrial DNA, which might help with slightly compromised DNA from older scat samples and exclude the amplification of non-target species.
- Performing a phylogenetic analysis of SJKF sequences to investigate if differences in populations can be detected based on the mitochondrial DNA indicating potential mixing of the gene pool from different populations.
- Alerting Kern County officials and the city of Bakersfield about results from this work and future work prior to certifying developmental projects that could destroy SJKF habitat.
- Communicating this method to local environmental consultants to improve future environmental surveys for SJKF populations for EIRs in a cost and time-effective way.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- O’Farrell, T. San Joaquin Kit Fox Recovery Plan; US Fish and Wildlife Service: Washington, DC, USA, 1983; Contract No. DE-ACOB-76NV01183. [Google Scholar]
- Sacks, B.N.; Milburn, P.J. Genetic characterization of kit foxes at their northern range extent and monitoring recommendations. Wildl. Soc. Bull. 2018, 42, 684–692. [Google Scholar] [CrossRef]
- Cypher, B.L. Kit Foxes. In Urban Carnivores: Ecology, Conflict, and Conservation; Ghert, S.D., Riley, S.P.D., Cypher, B.L., Eds.; John Hopkins University Press: Baltimore, MD, USA, 2010; pp. 49–60. [Google Scholar]
- Cypher, B.L.; Spencer, K.A. Competitive Interactions between Coyotes and San Joaquin Kit Foxes. J. Mammal. 1998, 79, 204–214. [Google Scholar] [CrossRef]
- Knapp, D.K. Effects of Agricultural Development in Kern County, California, on the San Joaquin Kit Fox in 1977; Department of Fish and Game, The Resource Agency, State of California: Sacramento, CA, USA, 1978. [Google Scholar]
- Deatherage, N.A.; Cypher, B.L.; Murdoch, J.; Westall, T.L.; Kelly, E.C.; Germano, D.J. Urban landscape attributes affect occupancy patterns of the San Joaquin kit fox during an epizootic. Pac. Conserv. Biol. 2021, 27, 256–266. [Google Scholar] [CrossRef]
- Cypher, B.L.; Deatherage, N.A.; Westall, T.L.; Kelly, E.C.; Phillips, S.E. Potential habitat and carrying capacity of endangered San Joaquin kit foxes in an urban environment: Implications for conservation and recovery. Urban Ecosyst. 2023, 26, 173–183. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service. Species Status Assessment Report for the San Joaquin Kit Fox (Vulpes macrotis mutica). 2020. Available online: https://www.fws.gov/sites/default/files/documents/san_joaquin_kit_fox_SSA_5-year_review_2019-11.pdf (accessed on 7 August 2024).
- U.S. Fish and Wildlife Service (FR 1967). Native Fish and Wildlife: Endangered Species. Federal Register. 1967; Volume 32, p. 4001. Available online: https://www.fws.gov/species-publication-action/endangered-species-list-1967-5 (accessed on 4 May 2024).
- Morrell, S.H. San Joaquin Kit Fox Distribution and Abundance in 1975; Wildlife Management Branch Administration Report; California Department of Fish and Game: Sacramento, CA, USA, 1975; No. 75–3; 28p. [Google Scholar]
- U.S. Fish and Wildlife Service. Recovery Plan for Upland Species of the San Joaquin Valley, California; U.S. Fish and Wildlife Service, Region 1: Portland, OR, USA, 1998; 319p, (Executive summary). [Google Scholar]
- Cypher, B.L.; Rudd, J.L.; Westall, T.L.; Woods, L.; Stephenson, W.N.; Foley, J.E.; Richardson, D.; Clifford, D.L. Sarcoptic Mange in Endangered Kit Foxes (Vulpes macrotis mutica): Case Histories, Diagnoses, and Implications for Conservation. Epidemiology 2017, 53, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Cypher, B.L.; Westall, T.L.; Kelly, E.C.; Deatherange, N.A. Demographic and Ecological Responses of Endangered San Joaquin Kit Foxes to the Panoche Valley Solar Farm. Final Report for Con Edison Clean Energy Business. Endangered Species Recovery Program California State University Stanislaus. 2022. Available online: https://esrp.csustan.edu/publications/pdf/Cypher%20et%20al%202022_Kit%20fox%20response%20to%20Panoche%20Solar%20Farm_CSUS%20ESRP.pdf (accessed on 1 May 2023).
- Foley, P.; Foley, J.; Rudd, J.; Clifford, D.; Westall, T.L.; Cypher, B.L. Spatio-temporal and transmission dynamics of sarcoptic mange in an endangered New World kit fox. PLoS ONE 2023, 18, e0280283. [Google Scholar] [CrossRef]
- Maclean, S.A.; Dominguez, A.F.R.; Valpine, P.D.; Beissinger, S.R. A century of climate and land-use change cause species turnover without loss of beta diversity in California’s Central Valley. Glob. Change Biol. 2018, 24, 5882–5894. [Google Scholar] [CrossRef] [PubMed]
- California Natural Diversity Database (CNDDB) Special Animals List. 2024. Available online: https://nrm.dfg.ca.gov/FileHandler.ashx?DocumentID=109406&inline (accessed on 10 October 2024).
- Deatherage, N.A.; Cypher, B.L.; Westall, T.L.; Kelly, E.C. Spatial relationships between mesocarnivores and domestic species in an urban environment and implications for endangered San Joaquin kit foxes. Urban Ecosyst. 2024, 27, 321–334. [Google Scholar] [CrossRef]
- Endangered Species Recovery Program. San Joaquin Kit Fox (Vulpes macrotis mutica) (csustan.edu). 2024. Available online: https://esrp.csustan.edu/speciesprofiles/profile.php?sp=vuma (accessed on 6 May 2024).
- Mitchel, J.M. Recovery Areas for the San Joaquin Kit Fox 2007; Sacramento Fish and Wildlife Office: Sacramento, CA, USA, 2015. [Google Scholar]
- Newsome, S.D.; Ralls, K.; Van Horn Job, C.; Fogel, L.M.; Cypher, B.L. Stable isotopes evaluate exploitation of anthropogenic foods by the endangered San Joaquin Kit Fox (Vulpes macrotis mutica). J. Mammal. 2010, 91, 1313–1321. [Google Scholar] [CrossRef]
- Cypher, B.L.; Van Horn Job, C.L. Management and Conservation of San Joaquin Kit Foxes in Urban Environments. In Proceedings of the 25th Vertebrate Pest Conference, 5–8 March 2012, Monterey, CA, USA; University of California: Davis, CA, USA, 2012. No. 25. pp. 347–352. [Google Scholar]
- Sacramento Fish and Wildlife Office. U.S. Fish and Wildlife Service Standardized Recommendations for Protection of The Endangered San Joaquin Kit Fox Prior to or During Ground Disturbance. 2011. Available online: https://www.fws.gov/sites/default/files/documents/survey-protocols-for-the-san-joaquin-kit-fox.pdf (accessed on 12 August 2024).
- Schwartz, M.K.; Ralls, K.; Williams, D.F.; Cypher, B.L.; Pilgrim, K.L.; Fleischer, R.C. Gene flow among San Joaquin kit fox populations in a severely changed ecosystem. Conserv. Gen. 2005, 6, 25–37. [Google Scholar] [CrossRef]
- Sacks, B.N.; Statham, M.J.; Serieys, L.E.; Riley, S.P. Population genetics of California gray foxes clarify origins of the island fox. Genes 2022, 13, 1859. [Google Scholar] [CrossRef] [PubMed]
- Taberlet, P.; Luikart, G. Non-invasive genetic sampling and individual identification. Biol. J. Linn. Soc. 1999, 68, 41–55. [Google Scholar] [CrossRef]
- Dalén, L.; Götherström, A.; Angerbjörn, A. Identifying species from pieces of faeces. Conserv. Gen. 2004, 5, 109–111. [Google Scholar] [CrossRef]
- Cypher, B.L.; Phillips, S.E.; Kelly, P.A. Quantity and distribution of suitable habitat for endangered San Joaquin Kit fox: Conservation implications. Canid Biol. Conserv. 2013, 16, 25–31. [Google Scholar]
- Cypher, B.L.; Phillips, S.E.; Kelly, P.A. Habitat Suitability and Potential Corridors for San Joaquin Kit Fox in the San Luis Unit. California State University, Stanislaus. 2007. Available online: https://esrp-csustan-edu.carmenjimenezphillips.com/publications/reports/usbr/esrp_2007_sanluisunitkitfox_e.pdf (accessed on 16 April 2024).
- McCormick Biological, Inc. Biological Evaluation for VA Community-Based Outpatient Clinic, City of Bakersfield, Kern County, California, Dated September 2020. 61p. Available online: https://files.ceqanet.opr.ca.gov/266280-2/attachment/mmheXfFFSLxEaat2Q5-pJztDYMEOonGIB7U6mCHPzzXiwInn1Uvk7z9FsknXm-1DI1yLKLmdzfj31VU70 (accessed on 8 September 2024).
- Noel, E.; McCormick Biological. Biological Evaluation for VA Community-Based Outpatient Clinic, City of Bakersfield, Kern County, California. December 2022 (Revision-3). Prepared for SASD Development Group, LLC, San Diego, California. 2022, 66p. Available online: https://files.ceqanet.opr.ca.gov/280782-2/attachment/hRdXSvj_3cj3t_zqYume2y3X9CqfBaZNal-tjS1imv3sPaK_FzuqvqOsfSHb0S3sq-5PDbWbwX9xCeCY0 (accessed on 9 September 2024).
- Wilbert, T. Appeal of Environmental Impact Report for Site Plan Review Nol 21-0399; SCH No. 2022080337, 2 November 2023, Submitted to the Bakersfield City Council; The City of Bakersfield Planning Department: Bakersfield, CA, USA, 2023. [Google Scholar]
- Grant, B.; McCormick Biological, Inc. MBI. 2021 Biological Resources Evaluation, SR 99/Hosking Commercial Center Project. April 2022; Biological Resources Existing Conditions Letter. 5 May 2022. Biological Resources Offsite Conditions Letter. Available online: https://files.ceqanet.opr.ca.gov/276905-2/attachment/w3mVYWAZnTPZam5ofPPgZRvnGbqDAbplA6BwVQYCmLSjoBOgKVRUQyynvsSbYfGzo_uYiOkl8cK_MF3A0 (accessed on 3 April 2024).
- Cox, J. The Bakersfield Californian, Stockdale Exit Along I-5 May Get New Travel Center with 4 Drive-Thrus, 19 November 2023. Available online: https://www.aol.com/stockdale-exit-along-5-may-045900899.html (accessed on 3 September 2024).
- Green Acres Farm Biosolids Land Application Project. 2014. Findings and Statement of Overriding Considerations. Final Environmental Impact Report. October 2014. Available online: https://clkrep.lacity.org/onlinedocs/2014/14-1556_misc_a_11-5-14.pdf (accessed on 10 March 2024).
- Ceqanet. Veteran’s Affairs Community-Based Outpatient Medical Clinic; Site Plan Review No. 21-0399. 2022. Available online: https://ceqanet.opr.ca.gov/2022080337 (accessed on 10 October 2024).
- Ceqanet. Majestic Gateway Project. 2022. Available online: https://ceqanet.opr.ca.gov/2022030196/2 (accessed on 9 August 2024).
- Los Angeles Bureau of Engineering. Green Acres Biosolids Land Application Project. 2024. Available online: https://engineering.lacity.gov/about-us/divisions/environmental-management/projects/green-acres-biosolids-land-application-project (accessed on 9 October 2024).
- Central Sierra Environmental Resource Center (CSERC). What Scat Can Tell You About Your Wildlife Neighbors. 2018. Available online: https://www.cserc.org/news/what-scat-can-tell-you-about-your-wildlife-neighbors/ (accessed on 14 January 2022).
- Bryson, J. Scat Identification. A Visual Aid to Identifying Scat. Available online: https://www.thinktrees.org/wp-content/uploads/2019/03/Scat-Identification.pdf (accessed on 14 January 2022).
- De Barba, M.; Adams, J.R.; Goldberg, C.S.; Stansbury, C.R.; Arias, D.; Cisneros, R.; Waits, L.P. Molecular species identification for multiple carnivores. Conserv. Gen. Res. 2014, 6, 821–824. [Google Scholar] [CrossRef]
- Murphy, M.A.; Waits, L.P.; Kendall, K.C. Quantitative evaluation of fecal drying methods for brown bear DNA analysis. Wildl. Soc. Bull. 2000, 28, 951–957. Available online: https://www.jstor.org/stable/3783853 (accessed on 12 April 2022).
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2012, 41, D36–D42. [Google Scholar] [CrossRef]
- Ralls, K.; Smith, D.A. Latrine use by San Joaquin kit foxes (Vulpes macrotis mutica) and coyotes (Canis latrans). West. N. Am. Nat. 2004, 64, 544–547. Available online: https://www.jstor.org/stable/41717412 (accessed on 7 February 2023).
- Smith, D.A.; Ralls, K.; Cypher, B.L.; Maldonado, J.E. Assessment of scat-detection dog surveys to determine kit fox distribution. Wildl. Soc. Bull. 2005, 33, 897–904. [Google Scholar] [CrossRef]
- Murdoch, J.D. Scent Marking Behavior of the San Joaquin Kit Fox (Vulpes macrotis mutica). Master’s Thesis, University of Denver, Denver, CO, USA, 2003. [Google Scholar]
- Clark Jr, H.O.; Warrick, G.D.; Cypher, B.L.; Kelly, P.A.; Williams, D.F.; Grubbs, D.E. Competitive interactions between endangered kit foxes and nonnative red foxes. West. N. Am. Nat. 2005, 65, 153–163. Available online: https://www.jstor.org/stable/41717441 (accessed on 3 February 2023).
- City of Bakersfield. Planning Division, Ongoing Planning Programs. 2024. Available online: https://bakersfield2045.com/general-plan/ (accessed on 24 January 2024).
- Clark, H.O. Marking of novel objects by kit foxes. Calif. Fish Game 2007, 93, 103. [Google Scholar]
- Cypher, B.L.; Deatherage, N.A.; Westall, T.L.; Kelly, E.C. Intraguild competition between endangered kit foxes and a novel predator in a novel environment. Animals. 2022, 12, 2727. [Google Scholar] [CrossRef] [PubMed]
- Zemanova, M.A. Poor implementation of non-invasive sampling in wildlife genetics studies. Rethink. Ecol. 2019, 4, 119–132. [Google Scholar] [CrossRef]
- Zemanova, M.A. Noninvasive genetic assessment is an effective wildlife research tool when compared with other approaches. Genes 2021, 12, 1672. [Google Scholar] [CrossRef] [PubMed]
- Aterio, Bakersfield, CA, 2025 and 2039 Projected Population. Available online: https://www.aterio.io/insights/us-population-forecast/ca/bakersfield (accessed on 5 May 2024).
- Kern County Department of Planning and Resources. General Plan 2024, Specific & Rural Community Plans. 2024. Available online: https://kernplanning.com/planning/planning-documents/specific-plans/ (accessed on 2 October 2024).
- Perez, A.D. A Plan for Conservation of Endangered Species Kit Fox for California State University, Bakersfield. 8 June 2001. Available online: https://scholarworks.calstate.edu/concern/projects/xs55mk86x (accessed on 6 June 2023).
- Bozarth, C.A.; Alva-Campbell, Y.R.; Ralls, K.; Henry, T.R.; Smith, D.A.; Westphal, M.F.; Maldonado, J.E. An efficient noninvasive method for discriminating among faeces of sympatric North American canids. Conserv. Gen. Res. 2010, 2, 173–175. [Google Scholar] [CrossRef]
- Pfau, R.S.; Goetze, J.R.; Martin, R.E.; Matocha, K.G.; Nelson, A.D. Spatial and temporal genetic diversity of the Texas kangaroo rat, Dipodomys elator (Rodentia: Heteromyidae). J. Mammal. 2019, 100, 1169–1181. [Google Scholar] [CrossRef]
- Riddle, B.R. Molecular biogeography in the pocket mice (Perognathus and Chaetodipus) and grasshopper mice (Onychomys): The late Cenozoic development of a North American aridlands rodent guild. J. Mammal. 1995, 76, 283–301. [Google Scholar] [CrossRef]
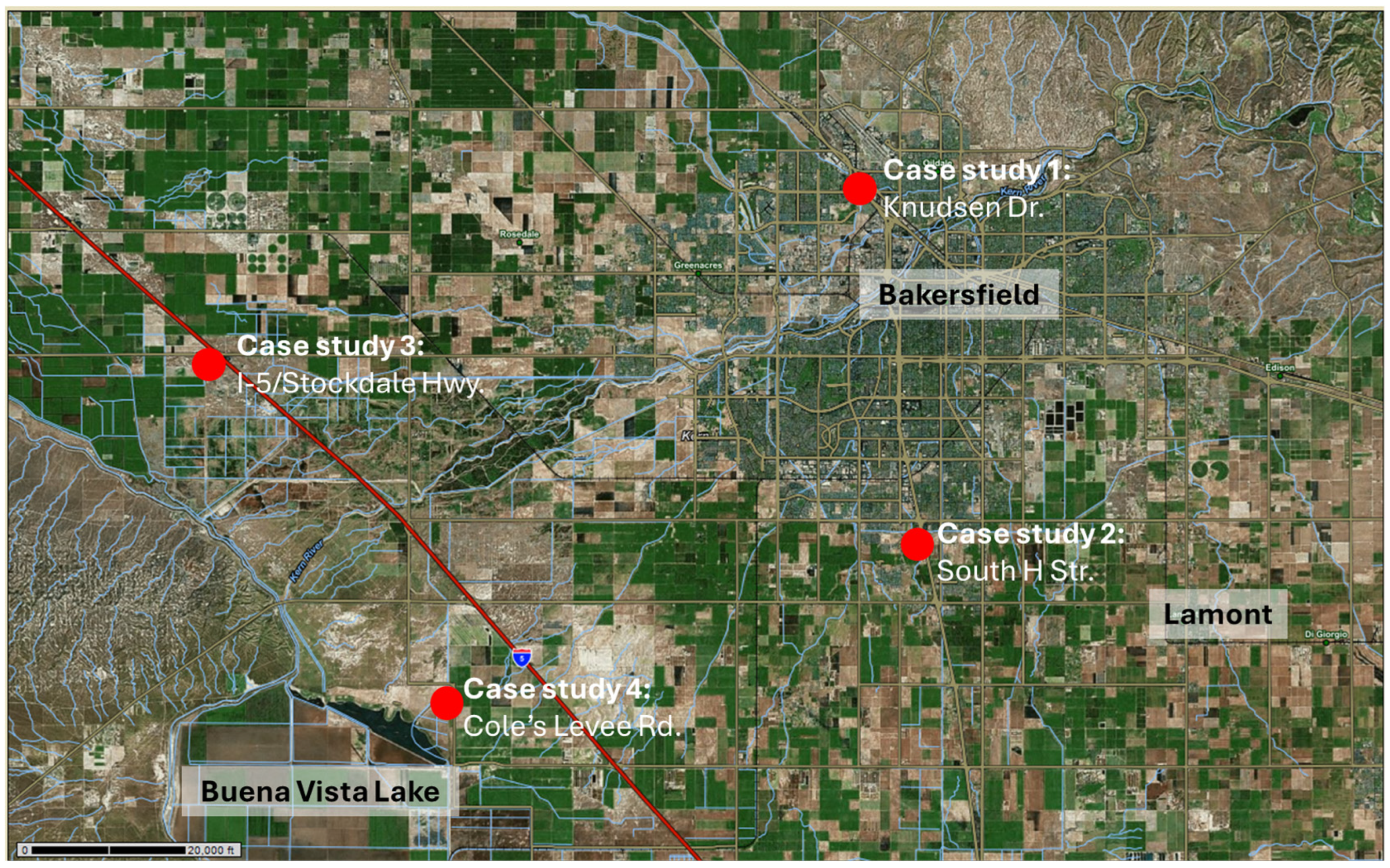
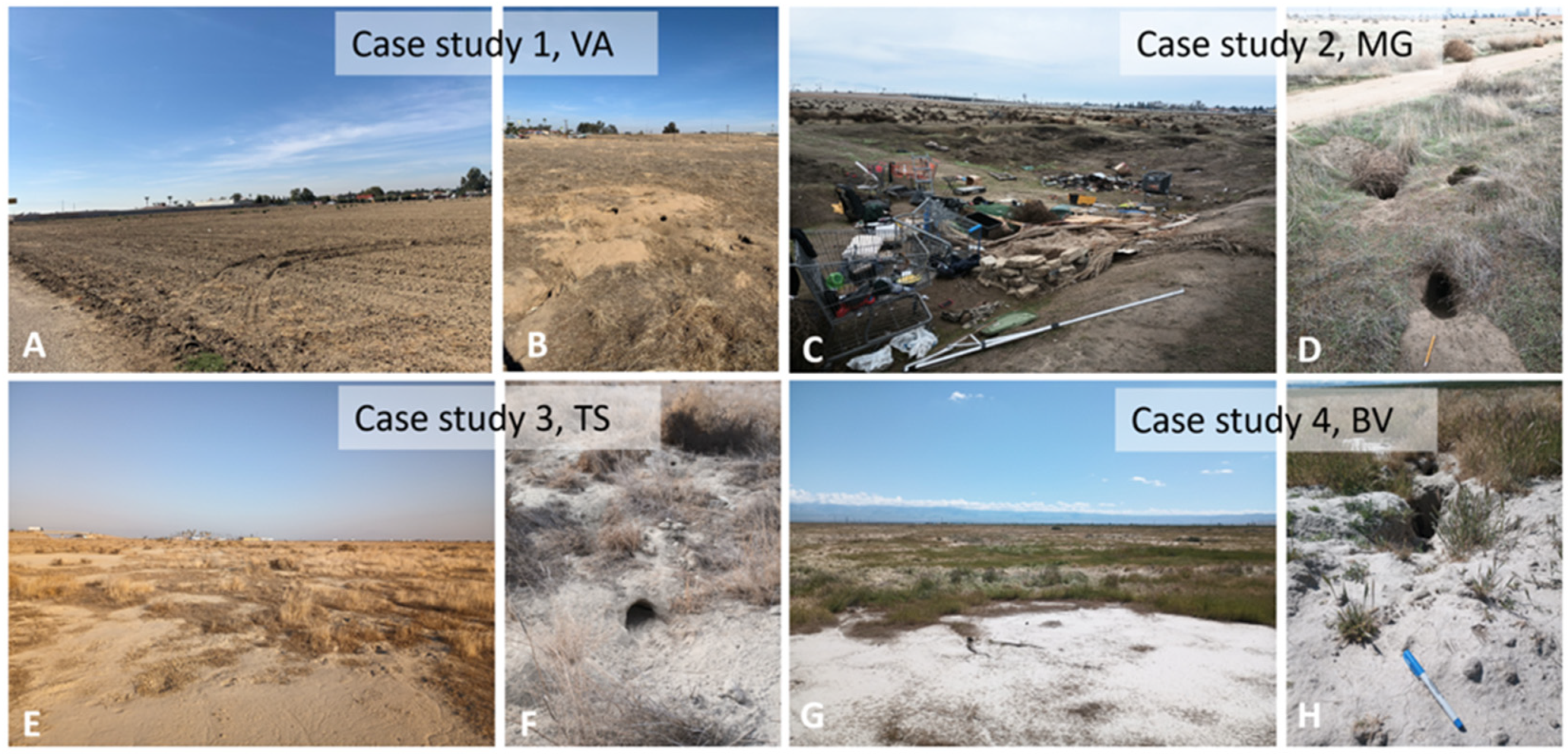
| Sampling Locations and Sites | Samples (n) | Habitat Type and Rating (Class 1) [27] | Sampling Date |
|---|---|---|---|
| Central Bakersfield: | |||
| Knudsen Dr. (VA) (case study 1) | 22 | Grassland/ruderal, 85 | 30 October 2023 |
| South Bakersfield: | |||
| South H Str. (MG) (case study 2) | 31 | Grassland/ruderal, 85 | 31 December 2023 and 15 June 2024 |
| West Bakersfield: | |||
| I5-Stockdale Hwy. (TS) (case study 3) | 18 | Scrub, 95 | 13 + 17 December 2023 |
| Southwest Bakersfield: | |||
| Cole’s Levee Rd. (BV) (case study 4) | 9 | Scrub, 95 | 1 April 2024 |
| Case Study Site | Size (sf) | Description of Site | Additional Information |
|---|---|---|---|
| Case study 1 (VA) Knudsen Dr. | ±39,648 | Grassland, mostly invasive species, disked at least once, partially trashed, presence of SJKF confirmed by previous environmental surveys | Construction of Veteran’s Affairs Community-Based Outpatient Medical Clinic [35]. |
| Case study 2 (MG) South H Street. | 187,500 + 1,012,185 | Grassland, mostly invasive species, disked at least once, partially trashed, presence of SJKF suspected by previous environmental surveys | Majestic Gateway Project, 12 commercial buildings and warehouses planned [36]. |
| Case study 3 (TS) I-5/Stockdale Hwy. | 1,141,272 | Natural valley grassland with occasional shrubs, presence of SJKF not known | Travel center with 4 fast food restaurants, 28 fuel pumps and a nine-bay, island-like diesel truck fueling station associated with truck scales and parking [33]. |
| Case study 4 (BV) Cole’s Levee Rd. | 3450 | Unused agricultural plot with semi-natural grassland vegetation, invasive species, and occasional shrubs, presence of SJKF suspected | Los Angeles Bureau of Engineering, Green Acres Biosolids Land Application Project [37]. |
| Samples | Species | ||||||
|---|---|---|---|---|---|---|---|
| Sampling Location | # of Samples | % of id. Samples | % of unid. Samples | V. m. mutica | V. vulpes | C. latrans | T. taxus |
| City of Bakersfield | |||||||
| Case study 1 (VA) Knudsen Dr. | 22 | 45.5 | 54.55 | 6 | 0 | 4 | 0 |
| South of Bakersfield | |||||||
| Case study 2 (MG) South H Str. | 31 | 80.65 | 19.35 | 0 | 25 | 0 | 0 |
| West of Bakersfield | |||||||
| Case study 3 (TS) I-5/Stockdale Hwy. | 18 | 66.67 | 33.33 | 0 | 0 | 12 | 0 |
| Buena Vista Lake Area | |||||||
| Case study 4 (BV) Cole’s Levee Rd. | 9 | 55.56 | 44.44 | 0 | 0 | 4 | 1 |
| n | Pos PCR * | Neg PCR | Multiple Amplicons | Amplicon > 500 bp ** |
|---|---|---|---|---|
| 80 | 52 | 11 | 12 | 5 |
| 100% | 65.00% | 13.75% | 15.00% | 6.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauer, A.; Alame, S.; Calvillo, J.A.; Gaytan, M.E.; Juarez, J.R.; Lopez, J.J.; Medina, K.; Owens, I.; Romero, A.; Sheppard, J. Detecting the Endangered San Joaquin Kit Fox (Vulpes macrotis mutica) and Other Canine Species in Kern County, CA: Applying a Non-Invasive PCR-Based Method to Four Case Study Sites. Conservation 2025, 5, 8. https://doi.org/10.3390/conservation5010008
Lauer A, Alame S, Calvillo JA, Gaytan ME, Juarez JR, Lopez JJ, Medina K, Owens I, Romero A, Sheppard J. Detecting the Endangered San Joaquin Kit Fox (Vulpes macrotis mutica) and Other Canine Species in Kern County, CA: Applying a Non-Invasive PCR-Based Method to Four Case Study Sites. Conservation. 2025; 5(1):8. https://doi.org/10.3390/conservation5010008
Chicago/Turabian StyleLauer, Antje, Sarah Alame, Julian A. Calvillo, Mario E. Gaytan, Jonathan R. Juarez, Jocelyne J. Lopez, Kayla Medina, Isaac Owens, Alejandro Romero, and Jarred Sheppard. 2025. "Detecting the Endangered San Joaquin Kit Fox (Vulpes macrotis mutica) and Other Canine Species in Kern County, CA: Applying a Non-Invasive PCR-Based Method to Four Case Study Sites" Conservation 5, no. 1: 8. https://doi.org/10.3390/conservation5010008
APA StyleLauer, A., Alame, S., Calvillo, J. A., Gaytan, M. E., Juarez, J. R., Lopez, J. J., Medina, K., Owens, I., Romero, A., & Sheppard, J. (2025). Detecting the Endangered San Joaquin Kit Fox (Vulpes macrotis mutica) and Other Canine Species in Kern County, CA: Applying a Non-Invasive PCR-Based Method to Four Case Study Sites. Conservation, 5(1), 8. https://doi.org/10.3390/conservation5010008






