Biodiversity Conservation and Survival Factors of Charophyte Algal Communities in Protected High-Mountain Lakes of Kaçkar Mountains National Park (Rize, Turkey)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Laboratory Studies
3. Results
3.1. Physical and Chemical Properties of Waters
3.2. Floristic Composition and Diversity of Charophyta
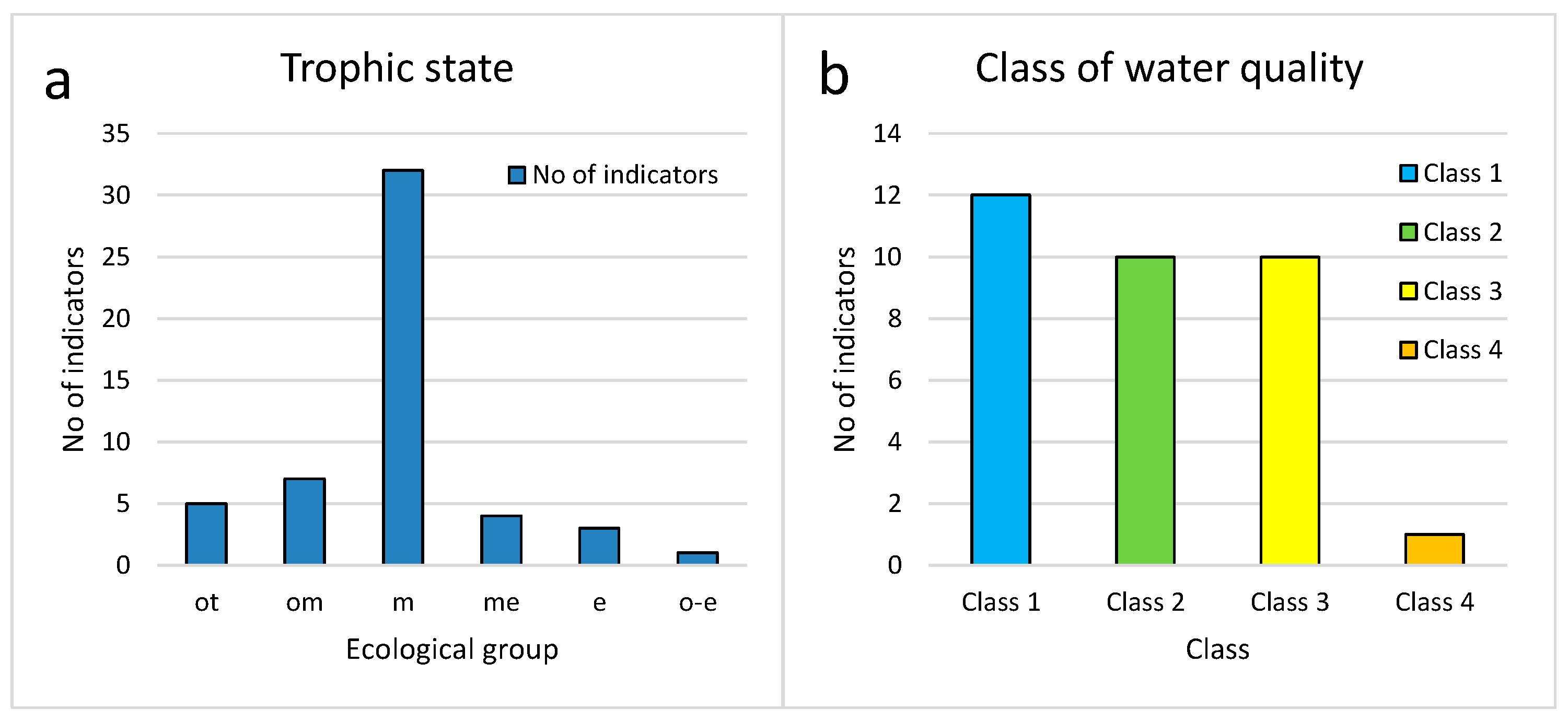
3.3. Bioindicators
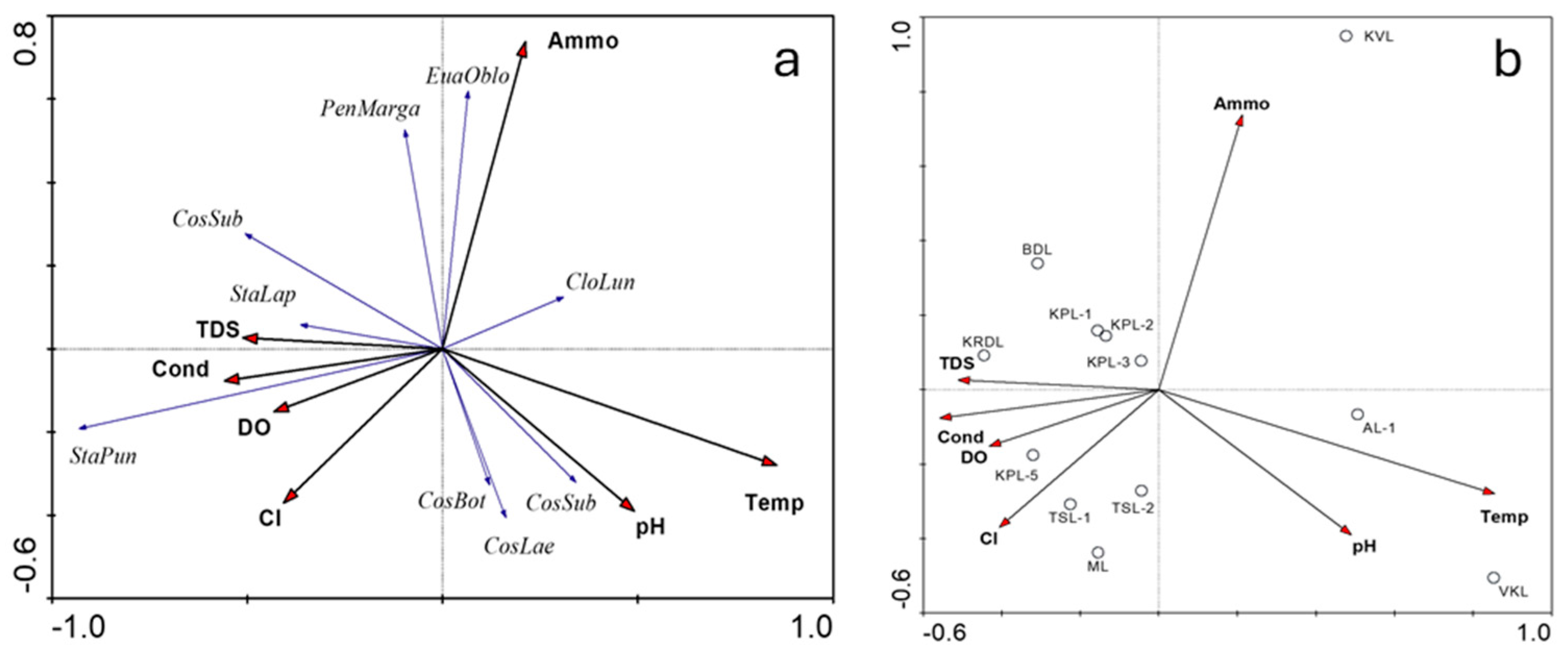
3.4. Species–Environmental Relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falasco, E.; Ector, L.; Ciaccio, E.; Hoffmann, L.; Bona, F. Alpine freshwater ecosystems in a protected area: A source of diatom diversity. Hydrobiologia 2012, 695, 233–251. [Google Scholar] [CrossRef]
- Tarabay, A.B.; Villela, R.G.; Espino, G.L. Limnological aspects of a high-mountain lake in Mexico. Hydrobiologia 1991, 224, 1–10. [Google Scholar] [CrossRef]
- Chapin, F.S.; Körner, C. Patterns, causes, changes and consequences of biodiversity in arctic and alpine ecosystems. In Arctic and Alpine Biodiversity: Patterns, Causes and Ecosystems Consequences, 2nd ed.; Chapin, F.S., III, Körner, C., Eds.; Springer: Berlin, Germany, 1995; pp. 313–320. [Google Scholar]
- Sommaruga, R. The role of solar UV radiation in the ecology of alpine lakes. J. Photochem. Photobiol. B Biol. 2001, 62, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Psenner, R. Alpine lakes: Extreme ecosystems under the pressures of global change. EAWAG News 2003, 55, 12–14. [Google Scholar]
- Catalan, J.; Camerero, L.; Felip, M.; Pla, S.; Ventura, M. High mountain lakes: Extreme habitats and witnesses of environmental changes. Limnetica 2006, 25, 551–584. [Google Scholar] [CrossRef]
- Coesel, P.F.M. A method for quantifying conservation value in lentic freshwater habitats using desmids as indicator organisms. Biodivers. Conserv. 2001, 10, 177–187. [Google Scholar] [CrossRef]
- Wehr, J.D.; Sheath, R.G.; Kociolek, J.P. Freshwater Algae of North America: Ecology and Classification, 3rd ed.; Academic Press: San Diego, USA, 2015; pp. 1–15. [Google Scholar]
- Magner, M. Desmid Communities and Environmental Conditions of New England Wetlands. Bachelor’s Thesis, Assumption Universty, Worcester, MA, USA, 2022. [Google Scholar]
- Geçen, R.; Toprak, V.; Tonbul, S. Glacial lakes on Eastern Blacksea Mountain their distribution and morphometric properties. In Proceedings of the International Geography Symposium on the 30th Anniversary of TUCAUM, Ankara, Turkey, 3–6 October 2018. [Google Scholar]
- Şahin, B.; Akar, B.; Barinova, S. Cohabitant charophyte algal flora and its ecology in high-mountain lakes of the Artabel Lakes Nature Park (Gümüşhane, Turkey). Bot. Serbica Program 2020, 44, 11–25. [Google Scholar] [CrossRef]
- Şahin, B.; Barinova, S. Assessment of Charophyta flora and ecological status in two high-mountain lakes (Rize, Turkey). Transylv. Rev. Syst. Ecol. Res. 2022, 24, 35–54. [Google Scholar] [CrossRef]
- Anonymous. Kaçkar Dağları Milli Parkı Uzun Devreli Gelişme Planı Analitik Etüt ve Sentez Raporu; Doğa ve Milli Parklar Genel Müdürlüğü: Ankara, Turkey, 2006. [Google Scholar]
- Sarı, D. A method to determine the potential for flora tourism in mountainous regions: A case study of the Kaçkar Mountains National Park, Turkey. Research 2019, 11, 27–35. [Google Scholar] [CrossRef]
- Eyüpoğlu, Y.; Dudas, F.O.; Zhu, D.; Liu, Z.; Chatterjee, N. Late cretaceous I- and A-type magmas in eastern Turkey: Magmatic response to double-sided subduction of Paleo- and Neo- Tethyan lithospheres. Lıthos 2019, 326, 39–70. [Google Scholar]
- Kurdoğlu, O. Kaçkar Dağları Milli Parkı ve Yakın Çevresinin Doğal Kaynak Yönetimi Açısından Incelenmesi. Ph.D. Thesis, Karadenizz Technical University, Ortahisar, Turkey, 2002. (In Turkish). [Google Scholar]
- Erinç, S. Klimatoloji ve Metodları; Istanbul University Faculty of Geography Publications: İstanbul, Turkey, 1969. [Google Scholar]
- Davis, P.H. Flora of Turkey and the East Aegean Islands; University Press: Edinburg, UK, 1965–1985; Volume 1–9. [Google Scholar]
- Gürpınar, T. Kuş göçü açısından Türkiye’nin önemi, Türkiye ve Balkan Ülkelerinde Yaban Hayatı. In Proceedings of the Uluslararası Sempozyumu Bildiriler Kitabı, İstanbul, Turkey, 16–20 September 1987. [Google Scholar]
- Round, F.E. An investigation of two benthic algal communities in Malharm Tarn, Yorkshire. J. Ecol. 1953, 41, 174–197. [Google Scholar] [CrossRef]
- Sládečková, A. Limnological investigation methods for the periphyton (“Aufwuchs”) community. Bot. Rev. 1962, 28, 286–350. [Google Scholar] [CrossRef]
- Schmidle, W. Beiträge zur alpinen algenflora. Osterr. Bot. Z. 1895, 45, 249–253. (In German) [Google Scholar] [CrossRef]
- West, W.; West, G.S. A Monograph of the British Desmidiaceae, I, 3rd ed.; Printed for the Ray Society: London, UK, 1904; 224p. [Google Scholar]
- West, W.; West, G.S. A Monograph of the British Desmidiaceae, II, 3rd ed.; Printed for the Ray Society: London, UK, 1905; 204p. [Google Scholar]
- West, W.; West, G.S. A Monograph of the British Desmidiaceae, III, 3rd ed.; Printed for the Ray Society: London, UK, 1908; 274p. [Google Scholar]
- West, W.; West, G.S. A Monograph of the British Desmidiaceae, IV, 3rd ed.; Printed for the Ray Society: London, UK, 1912; 194p. [Google Scholar]
- West, W.; West, G.S. A Monograph of the British Desmidiaceae, V, 3rd ed.; Printed for the Ray Society: London, UK, 1923; 300p. [Google Scholar]
- Ruzicka, J. Die Desmidiaceen Mitteleuropas, Band 1, 3rd ed.; E. Schweizerbart’sche Verlangbuchhandlung (Nägele u. Obermiller): Stuttgart, Germany, 1977; 292p. (In German) [Google Scholar]
- Lind, E.M.; Brook, A.J. Desmids of the English Lake District, 3rd ed.; Freshwater Biological Association Scientific Publication: Cumbria, UK, 1980; 123p. [Google Scholar]
- Förster, K. Conjugatophyceae Zygnematales und Desmidiales (excl. Zygnemataceae), in Das Phytoplankton des Süßwassers Systematik und Biologie (Band) 8, Teil 1, Hälfte, 3rd ed.; E. Schweizerbart’sche Verlangbuchhandlung (Nägele u. Obermiller): Stuttgart, Germany, 1982; 543p. (In German) [Google Scholar]
- Croasdale, H.; Flint, E.A. Flora of New Zealand Desmids, I, 3rd ed.; Ward, V.R., Ed.; Government Printer: Wellington, New Zealand, 1986; 133p. [Google Scholar]
- Croasdale, H.; Flint, E.A. Flora of New Zealand Desmids, II, 3rd ed.; Ward, V.R., Ed.; Goverment Printer: Wellington, New Zealand, 1988; 147p. [Google Scholar]
- Croasdale, H.; Flint, E.A.; Racine, M.M. Flora of New Zealand Desmids, III, 3rd ed.; Manaaki Whenua Press: Canterbury, New Zealand, 1994; 218p. [Google Scholar]
- Ling, H.U.; Tyler, P.A. A Limnological Survey of the Alligator Rivers Region, II, Freshwater Algae, Exclusive of Diatoms, 3rd ed.; Australian Government Publishing Service: Canberra, Australian, 1986; 173p. [Google Scholar]
- Dillard, G.E. Freshwater Algae of the Southeastern United States: Part 3, Chlorophyceae: Zygnematales: Zygnemataceae, Mesotaeniaceae and Desmidiaceae, 1, 3rd ed.; Cramer, J.: Stuttgart, Germany, 1990; 172p. [Google Scholar]
- Dillard, G.E. Freshwater Algae of the Southeastern United States: Part 4, Chlorophycea: Zygnematales: Desmidiaceae, 2, 3rd ed.; Cramer, J.: Stuttgart, Germany, 1991; 205p. [Google Scholar]
- Dillard, G.E. Freshwater Algae of the Southeastern United States: Part 5, Chlorophyceae: Zygnematales: Desmidiaceae, 3, 3rd ed.; Cramer, J.: Stuttgart, Germany, 1991; 155p. [Google Scholar]
- Dillard, G.E. Freshwater Algae of the Southeastern United States, 6, Chlorophycea: Zygnematales: Desmidiaceae, 4, 3rd ed.; Cramer, J.: Stuttgart, Germany, 1993; 166p. [Google Scholar]
- Bourrelly, P.; Couté, A. Desmidiees de Madagascar (Chlorophyta, Zygophyceae), 3rd ed.; Cramer, J.: Stuttgart, Germany, 1991; 348p. (In French) [Google Scholar]
- Lenzenweger, R. Desmidiaceenflora von Östereich, 1, 3rd ed.; Bibliotheca Phycologica, 101; Cramer, J.: Stuttgart, Germany, 1996; 162p. (In German) [Google Scholar]
- Lenzenweger, R. Desmidiaceenflora von Östereich, 2, 3rd ed.; Bibliotheca Phycologica, 102; Cramer, J.: Stuttgart, Germany, 1997; 216p. (In German) [Google Scholar]
- Lenzenweger, R. Desmidiaceenflora von Östereich, 3, 3rd ed.; Bibliotheca Phycologica, 104; Cramer, J.: Stuttgart, Germany, 1999; 218p. (In German) [Google Scholar]
- Kouwets, F.A.C. Contributions to the knowledge of the French Desmid Flora, I, New and noteworty taxa from the Central and Eastern Pyrenees. Arch. Protistenkd. 1997, 148, 33–51. [Google Scholar] [CrossRef]
- Dingley, M. Desmids of New South Wales: New species and new records. Telopea 2001, 9, 601–637. [Google Scholar] [CrossRef]
- John, D.M.; Whitton, B.A.; Brook, A.J. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae, 3rd ed.; Cambridge University Press: Cambridge, USA, 2003; 702p. [Google Scholar]
- Brook, A.J.; Williamson, D.B. A Monograph on Some British Desmids, 3rd ed.; Ray Society: London, UK, 2010; 364p. [Google Scholar]
- Štastny, J. Desmids (Conjugatophyceae, Viridiplantae) from the Czech Republic; new and rare taxa, distribution, ecology. Fottea 2010, 10, 1–74. [Google Scholar] [CrossRef]
- Coesel, P.F.M.; Meesters, K.J. Desmids of the Lowlands Mesotaeniaceae and Desmidiaceae of the European Lowlands, 3rd ed.; KNNV Publishing: Zeist, The Netherlands, 2007; 351p. [Google Scholar]
- Coesel, P.F.M.; Meesters, K.J. European Flora of the Desmid Genera Staurastrum and Staurodesmus, 3rd ed.; KNNV Publishing: Zeist, The Netherlands, 2013; 357p. [Google Scholar]
- Kim, H.S. Algal Flora of Korea, 6, 1, 3rd ed.; Republic of Korea National Institute of Biological Resources Ministry of Environment: Incheon, Republic of Korea, 2012; 94p. [Google Scholar]
- Kim, H.S. Algal Flora of Korea, 6, 6, 3rd ed.; Republic of Korea National Institute of Biological Resources Ministry of Environment: Incheon, Republic of Korea, 2015; 95p. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase, World-Wide Electronic Publication. Available online: https://www.algaebase.org (accessed on 21 October 2024).
- Kocataş, A. Ekoloji (Çevre Biyolojisi), 3rd ed.; Ege Üniversitesi Matbaası: İzmir, Turkey, 1992; 570p. (In Turkish) [Google Scholar]
- Barinova, S. Essential and practical bioindication methods and systems for the water quality assessment. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Perspectives; Publishing House of Haifa University: Haifa, Israel, 2019; 367p. (In Russian) [Google Scholar]
- McAleece, N.; Gage, J.D.G.; Lambshead, P.J.D.; Paterson, G.L.J. BioDiversity Professional Statistics Analysis Software, Jointly Developed by the Scottish Association for Marine Science and the Natural History Museum London; Publisher Informer Technologies, Inc., The Scottish Association for Marine Science: Oban, UK, 1997. [Google Scholar]
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.A.; Ly, A.; Gronau, F.Q.; Smira, M.; Epskamp, S.; et al. JASP Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power Press: Ithaca, NY, USA, 2002; 500p. [Google Scholar]
- Şahin, B. New desmid records from Kaçkar Mountains National Park (Rize/Turkey). Biol. Divers. Conserv. 2022, 15, 171–178. [Google Scholar] [CrossRef]
- Şahin, B. Türkiye’nin Tatlısu suyosunu florası için yeni kayıtlar. Bağbahçe Bilim Derg. 2023, 10, 41–44. (In Turkish) [Google Scholar]
- Sterlyagova, I.N. Desmids in mountain lakes of the subpolar Urals. Biologia 2008, 63, 915–920. [Google Scholar] [CrossRef]
- Šovran, S.; Jovanović, V.; Krizmanić, J.; Cvijan, M. Desmid flora from peat bogs in Serbia. Arch. Biol. Sci. 2013, 65, 721–732. [Google Scholar] [CrossRef]
- Barinova, S. The effect of altitude on distribution of freshwater algae in continental Israel. Curr. Top. Plant Biol. 2011, 12, 89–95. [Google Scholar]
- Coesel, P.F.M. Sieralgen en Natuurwaarden; KNNV Publishing: Utrecht, The Netherlands, 1998; 56p. (In Dutch) [Google Scholar]
- Shakhmatov, A.S.; Pavlovskiy, E.V.; Paukov, A.G. Desmids algae (Charophyta: Conjugatophyceae) of Ekaterinburg, Middle Urals, Russia. Folia Cryptogam. Est. 2018, 55, 7–17. [Google Scholar] [CrossRef]
- Coesel, P.F.M. Biogeography of desmids. Hydrobiologia 1996, 336, 41–53. [Google Scholar] [CrossRef]
- Kosteviciene, L.; Briskaite, R.; Bakunaite, J.; Jakimaviciute, I. Desmids (Chlorophyta, Desmidiales) from Lithuania. Biologia 2003, 58, 685–695. [Google Scholar]
- Stamenkovic, M.; Cvijan, M. Some new and interesting ecological observation on desmids from yhe Province of Vojvodina (Northern Serbia). Biologia 2008, 63, 917–923. [Google Scholar] [CrossRef][Green Version]
- Fuzinato, S.; Cvijan, M.; Stamenkovic, M. A Checklist of desmids (Conjugatophyceae, Chlorophyta) of Serbian. II. Genus Cosmarium. Cryptogam. Algol. 2011, 32, 77–95. [Google Scholar] [CrossRef]
- Fuzinato, S.; Cvijan, M.; Stamenkovic, M. A Checklist of desmids (Conjugatophyceae, Chlorophyta) of Serbian. III. Genus Staırastrum. Cryptogam. Algol. 2011, 4, 363–377. [Google Scholar] [CrossRef]
- Kopyrina, L.; Pshennıkova, E.; Barinova, S. Diversity and ecology of non-diatom algae in a swampy mountain lake of the Suntar-Khayat Ridge (Republic Sakha, Yakutia, Russia). Transylv. Rev. Syst. Ecol. Res. 2022, 24, 17–34. [Google Scholar] [CrossRef]
- Bjork, S. Redevelopment of lake system-a case study approach. Ambio 1988, 17, 90–98. [Google Scholar]
- Lotter, A.F.; Pienitz, R.; Schmidt, R. Diatoms as indicators of environmental changes near Arctic and Alpine treeline. In The Diatoms: Application for the Environmental and Earth Sciences, 2nd ed.; Stoermer, E.F., Smol, J.P., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 205–226. [Google Scholar]
- van Dam, H.; Meesters, K. Did desmid assemblages in Dutch moorland pools recover from acidification in the past century? Hydrobiologia 2021, 848, 5011–5031. [Google Scholar] [CrossRef]
- Muro, C.; Sandoval, J.; García, F.G.; Andrade, M.; Diaz-Nava, M. del C.; Alvarado, Y. Evaluation of environmental risks by heavy metals pollution and algae accumulations in two alpine lakes. Chem. Ecol. 2024, 41, 1–21. [Google Scholar] [CrossRef]
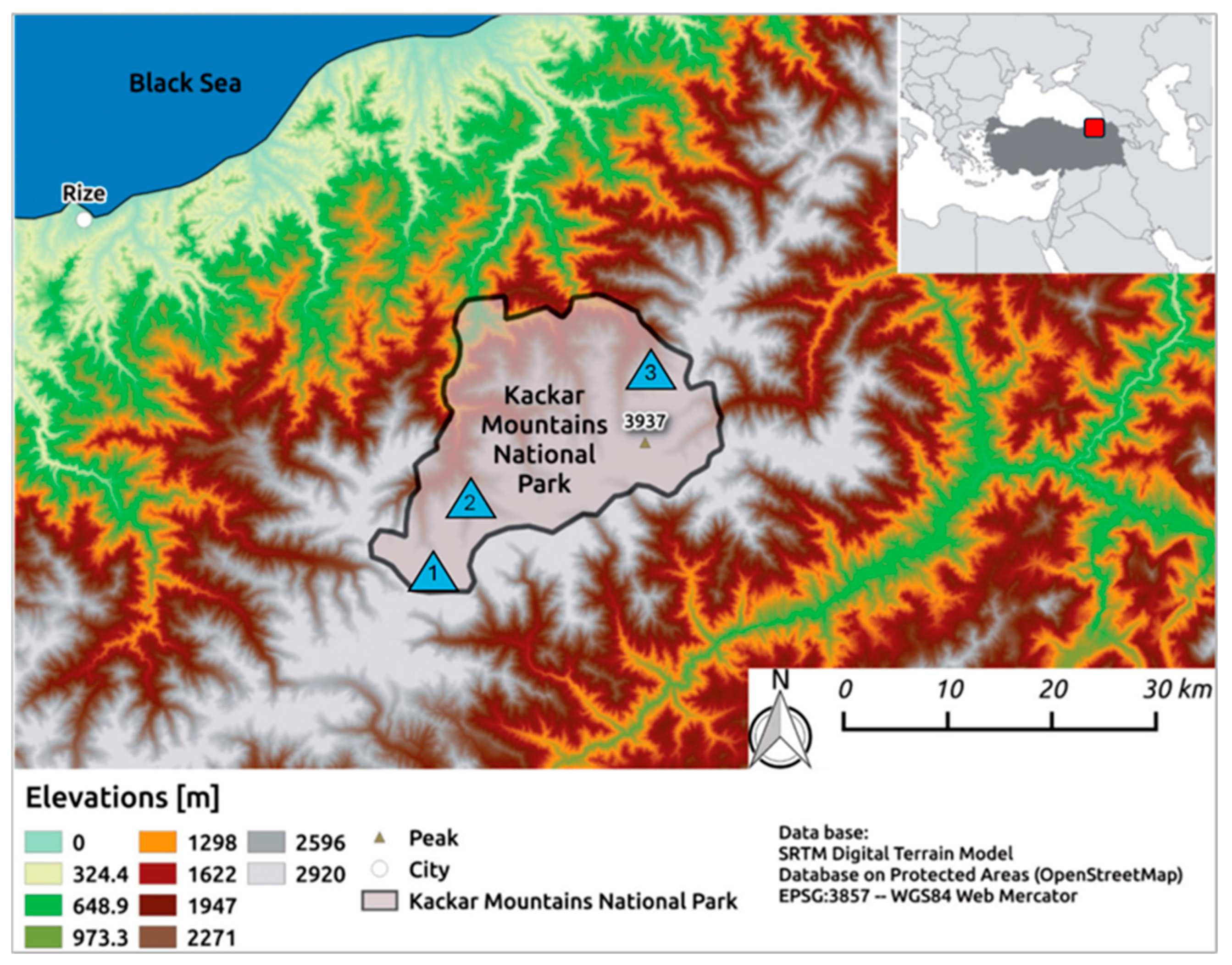

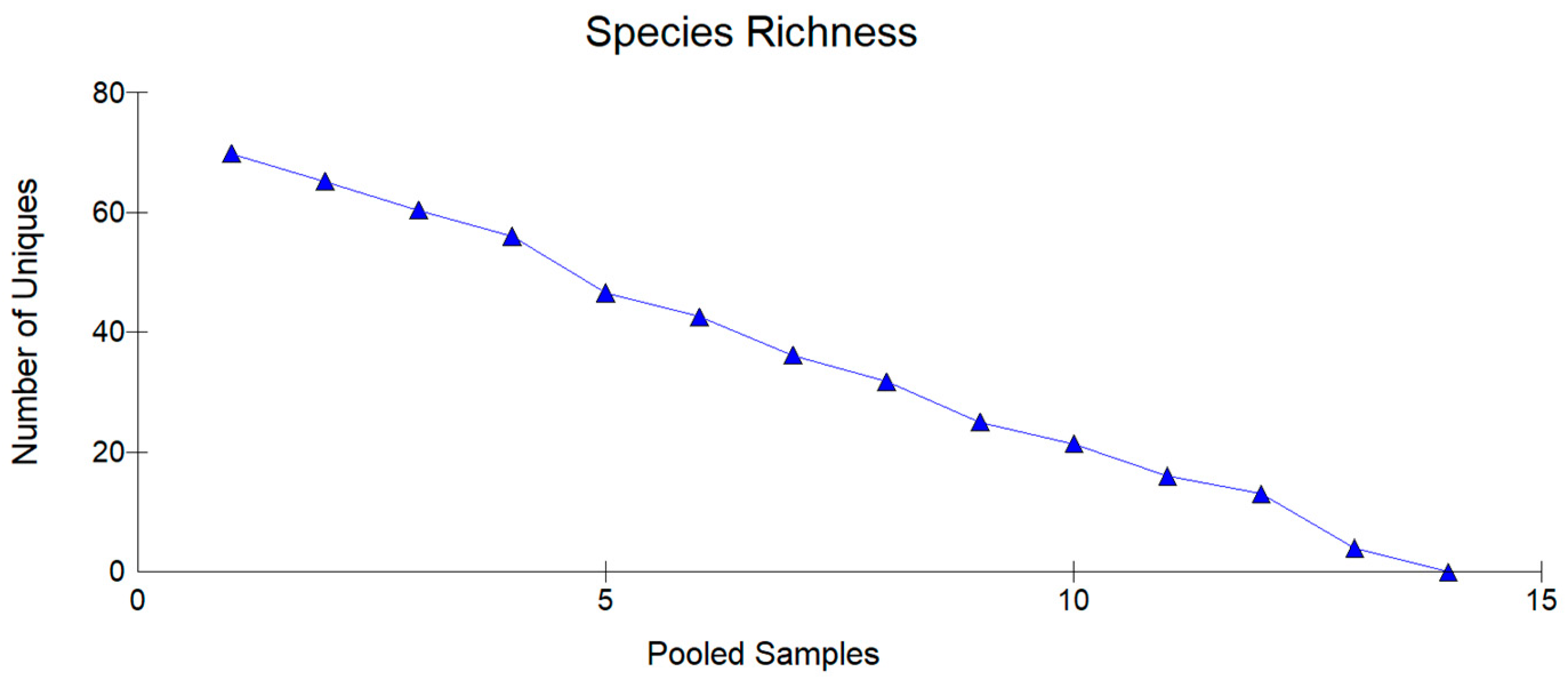
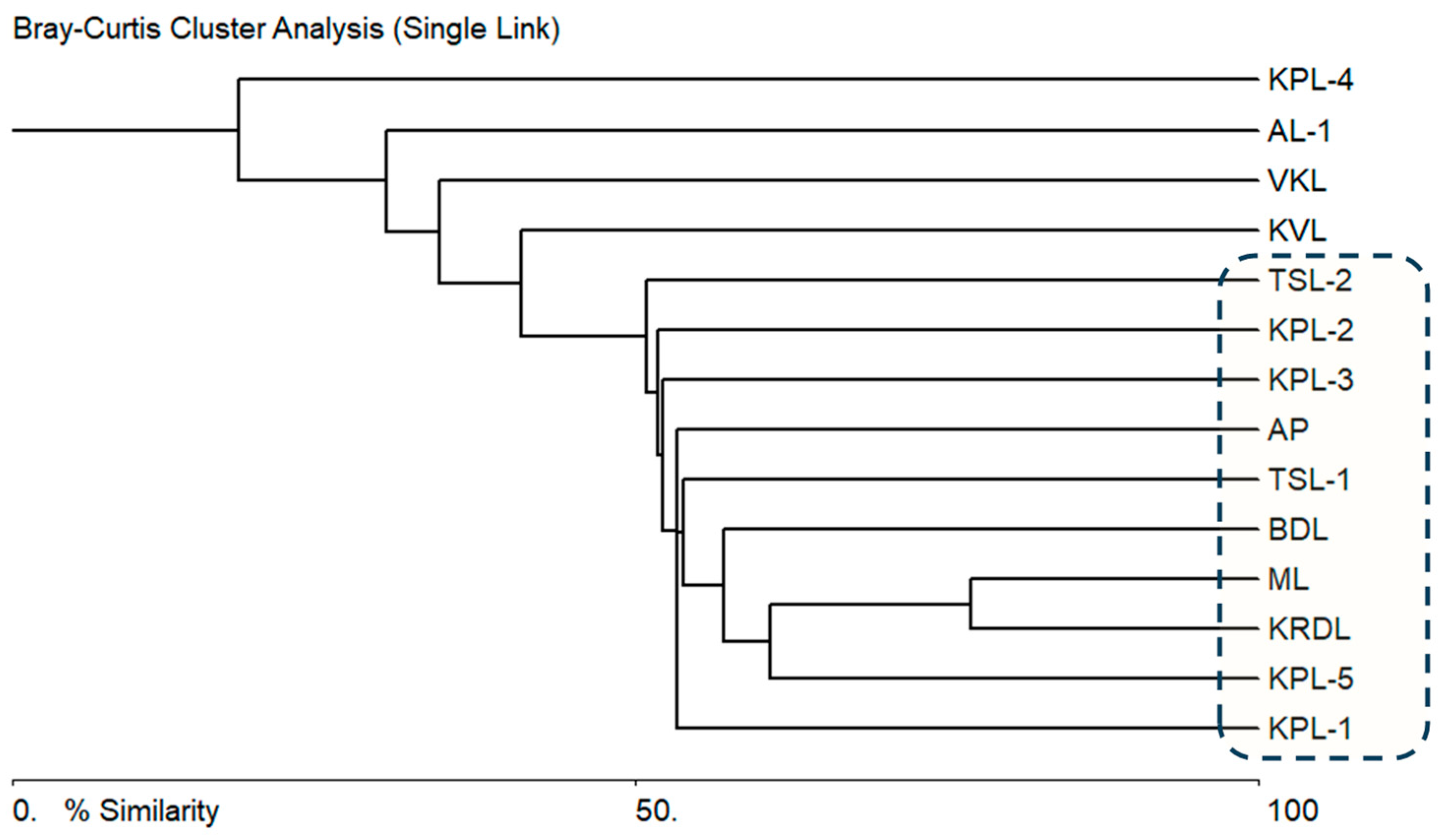

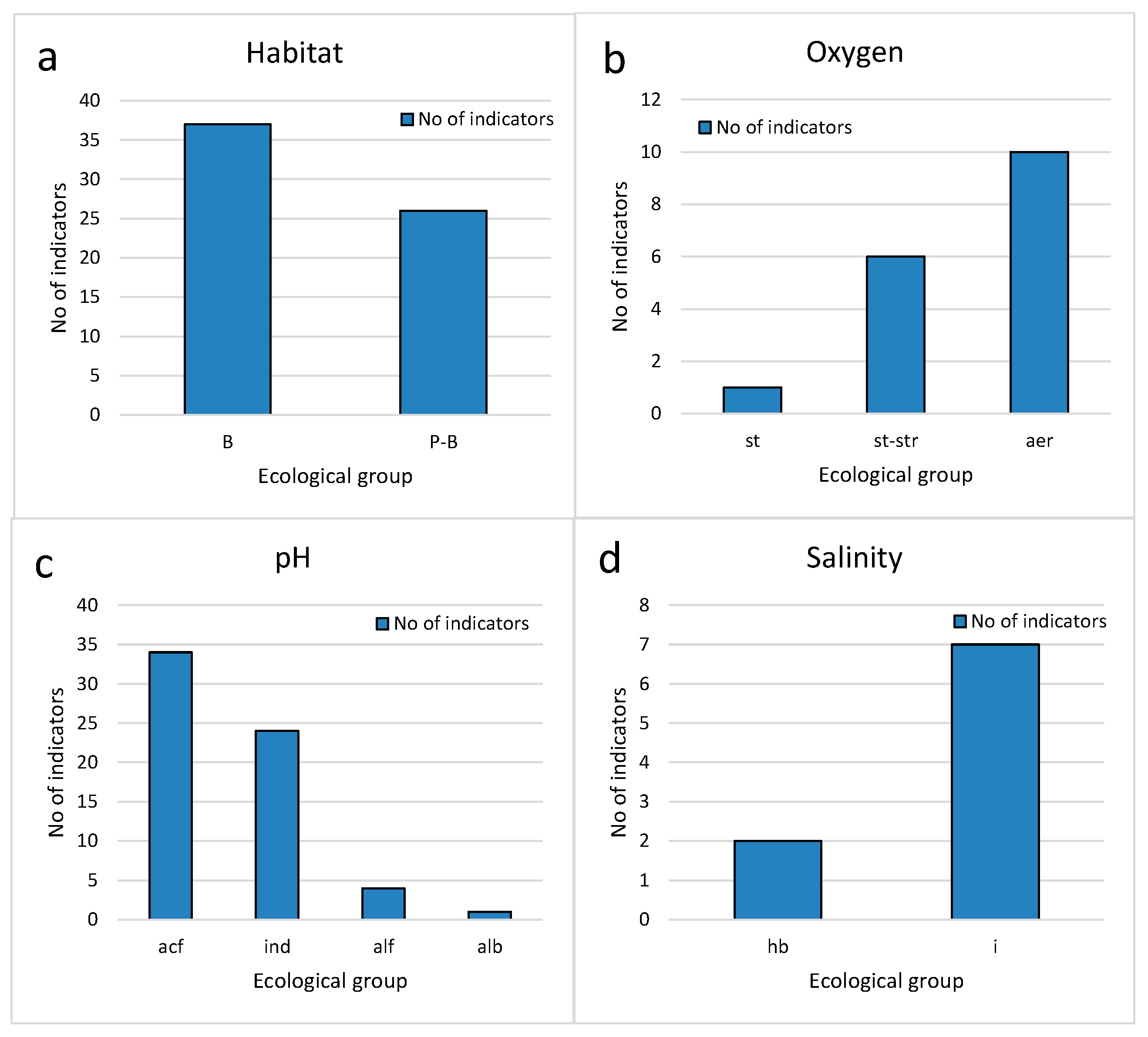
| Lake with Abbreviation | North | South | Altitude (m) | Area (m2) |
|---|---|---|---|---|
| Kapılı Lakes-1 (KPL-1) | 40°42′56″.59 N | 40°54′51″.71 E | 2980 | 70.529 |
| Kapılı Lakes-2 (KPL-2) | 40°43′08″.70 N | 40°54′55″.30 E | 2973 | 14.879 |
| Kapılı Lakes-3 (KPL-3) | 40°42′34″.73 N | 40°54′49″.02 E | 3074 | 35.738 |
| Kapılı Lakes-4 (KPL-4) | 40°42′43″.97 N | 40°54′47″.24 E | 3028 | 4.732 |
| Kapılı Lakes-5 (KPL-5) | 40°42′59″.03 N | 40°54′20″.06 E | 2926 | 7.272 |
| Vercenik Kumlu Lake (VKL) | 40°43′17″.91 N | 40°54′16″.58 E | 2864 | 3.279 |
| Karadeniz Lake (KRDL) | 40°52′39″.19 N | 41°10′ 02″.06 E | 2782 | 24.141 |
| Kavron Lake (KVL) | 40°52′24″.39 N | 41°09′45″.73 E | 2911 | 9.007 |
| Büyük Deniz Lake (BDL) | 40°52′04″.60 N | 41°09′38″.54 E | 2922 | 68.244 |
| Adsız Lake-1 (AL-1) | 40°42′39″.75 N | 40°54′57″.65 E | 3075 | 162 |
| Moçar Lake (ML) | 40°44′11″.63 N | 40°56′05″.36 E | 2958 | 22.246 |
| Tatos Sulak Lakes-1 (TSL-1) | 40°44′16″.11 N | 40°56′42″.25 E | 2976 | 46.421 |
| Tatos Sulak Lakes-2 (TSL-2) | 40°44′25″.50 N | 40°56′51″.18 E | 2940 | 17.337 |
| Artificial pond (AP) | 40°52′53″.00 N | 41°07′52″.65 E | 2272 | 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şahin, B.; Barinova, S. Biodiversity Conservation and Survival Factors of Charophyte Algal Communities in Protected High-Mountain Lakes of Kaçkar Mountains National Park (Rize, Turkey). Conservation 2025, 5, 14. https://doi.org/10.3390/conservation5010014
Şahin B, Barinova S. Biodiversity Conservation and Survival Factors of Charophyte Algal Communities in Protected High-Mountain Lakes of Kaçkar Mountains National Park (Rize, Turkey). Conservation. 2025; 5(1):14. https://doi.org/10.3390/conservation5010014
Chicago/Turabian StyleŞahin, Bülent, and Sophia Barinova. 2025. "Biodiversity Conservation and Survival Factors of Charophyte Algal Communities in Protected High-Mountain Lakes of Kaçkar Mountains National Park (Rize, Turkey)" Conservation 5, no. 1: 14. https://doi.org/10.3390/conservation5010014
APA StyleŞahin, B., & Barinova, S. (2025). Biodiversity Conservation and Survival Factors of Charophyte Algal Communities in Protected High-Mountain Lakes of Kaçkar Mountains National Park (Rize, Turkey). Conservation, 5(1), 14. https://doi.org/10.3390/conservation5010014







