Influence of Climatic Variables on the Stem Growth Rate in Trees of a Tropical Wet Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Species Selection
2.2. Growth Measurement
2.3. Wood Density Sampling
2.4. Weather Measurement
2.5. Statistic Analysis
3. Results
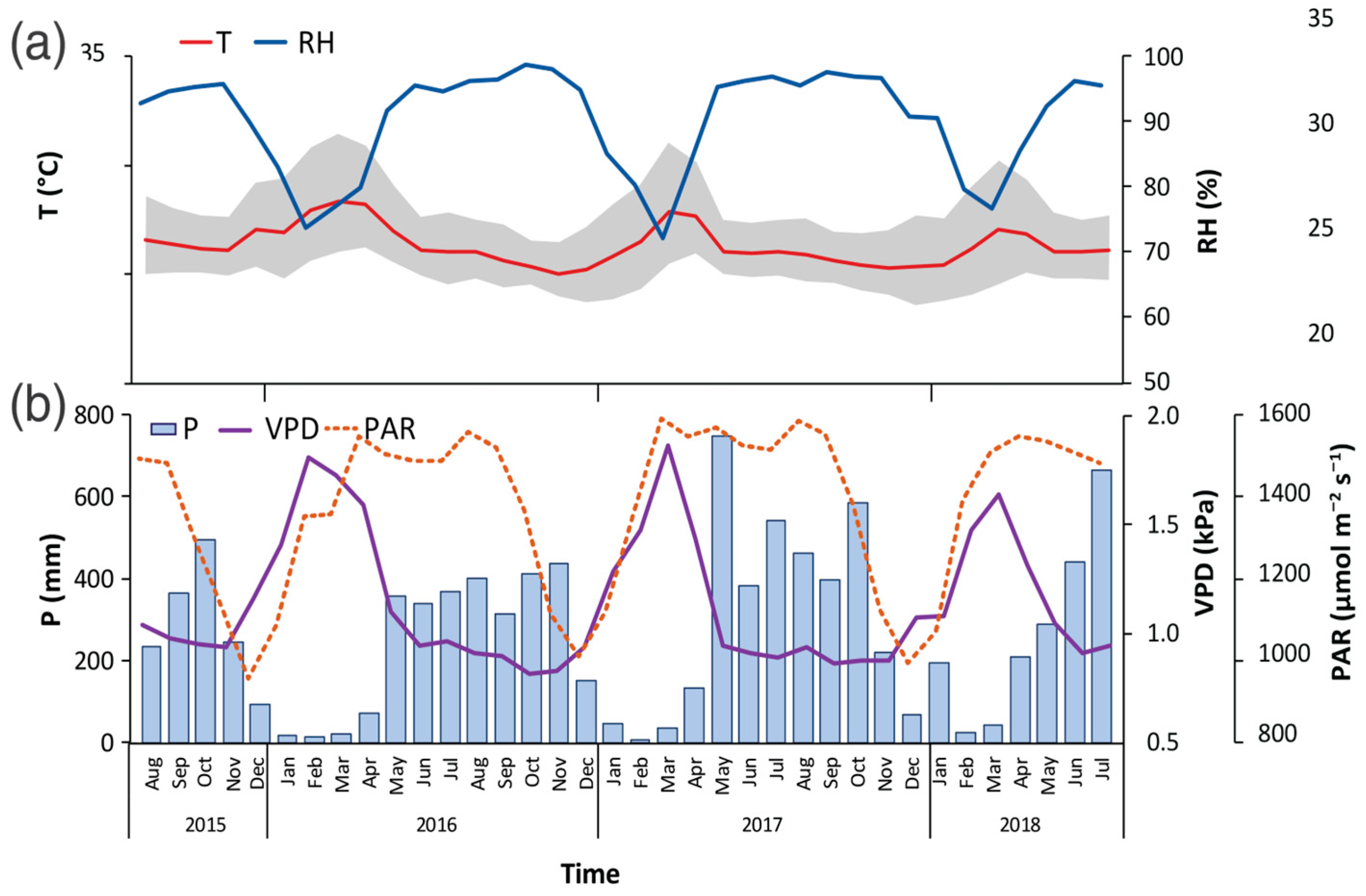
3.1. Climatic Conditions in the Study Period
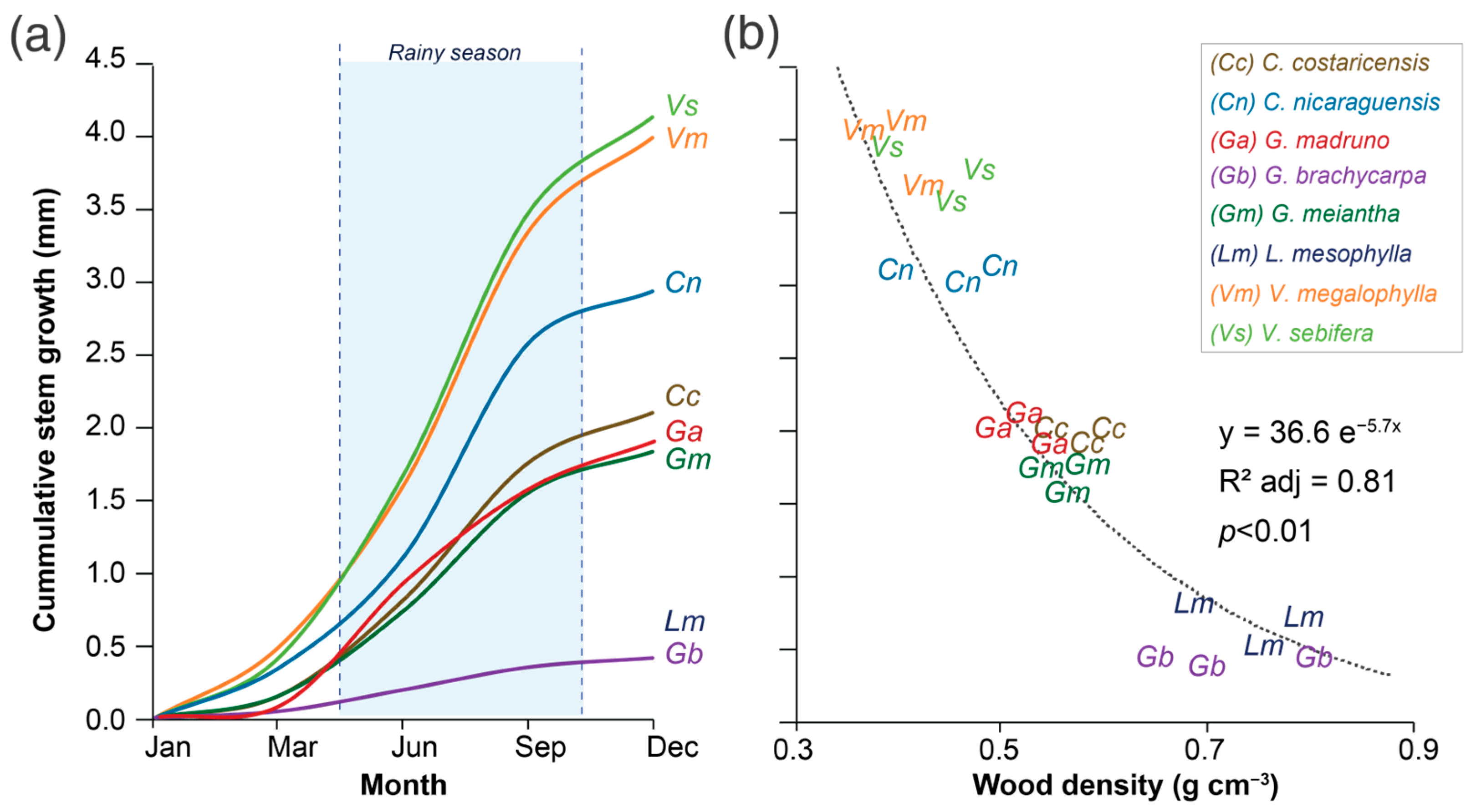
3.2. Stem Growth and Wood Density Partners
3.3. Stem Growth Rate Model Adjustment with Climatic Variables
4. Discussion
4.1. Dynamic Stem Growth Rate
4.2. Role of Wood Density in Stem Growth Rate Stationary
4.3. Equations for Estimating Growth as a Function of Environmental Variables
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bello, J.; Vallet, P.; Perot, T.; Balandier, P.; Seigner, V.; Perret, S.; Couteau, C.; Korboulewsky, N. How do mixing tree species and stand density affect seasonal radial growth during drought events? For. Ecol. Manag. 2019, 432, 436–445. [Google Scholar] [CrossRef]
- Binkley, D.; Stape, J.L.; Ryan, M.G. Thinking about efficiency of resource use in forests. For. Ecol. Manag. 2004, 193, 5–16. [Google Scholar] [CrossRef]
- Campoe, O.C.; Munhoz, J.S.; Alvares, C.A.; Carneiro, R.L.; de Mattos, E.M.; Ferez, A.P.C.; Stape, J.L. Meteorological seasonality affecting individual tree growth in forest plantations in Brazil. For. Ecol. Manag. 2016, 380, 149–160. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Powers, J.; Cochard, H.; Choat, B. Hanging by a thread? Forests and drought. Science 2020, 368, 261–266. [Google Scholar] [CrossRef]
- Franco, M.G.; Mundo, I.A.; Pastur, G.J.M.; Barrera, M.D. Radial growth responses to thinning and climate in native Nothofagus betuloides forests in Tierra del Fuego, Argentina. Dendrochronologia 2019, 57, 125625. [Google Scholar] [CrossRef]
- Güney, A.; Küppers, M.; Rathgeber, C.; Şahin, M.; Zimmermann, R. Intra-annual stem growth dynamics of Lebanon Cedar along climatic gradients. Trees 2017, 31, 587–606. [Google Scholar] [CrossRef]
- Acosta-Vargas, L.G.; Arias-Aguilar, D.; Valverde, J.C. Caracterización Florística Del Bosque Montano y Subpáramo del Volcán Irazú, Costa Rica. Acta Biológica Colomb. 2024, 29, 32–40. [Google Scholar]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Ameray, A.; Bergeron, Y.; Valeria, O.; Montoro Girona, M.; Cavard, X. Forest Carbon Management: A Review of Silvicultural Practices and Management Strategies Across Boreal, Temperate and Tropical Forests. Curr. For. Rep. 2021, 7, 245–266. [Google Scholar] [CrossRef]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef]
- Labrecque-Foy, J.-P.; Gaspard, A.; Simard, M.; Boudreau, S. Radial growth of subarctic tree and shrub species: Relationships with climate and association with the greening of the forest–tundra ecotone of subarctic Québec, Canada. Arct. Sci. 2023, 10, 201–214. [Google Scholar] [CrossRef]
- Valverde, J.C.; Arias, D.; Castillo, M.; Torres, D. Relación de la variabilidad climática con el crecimiento diamétrico de ocho especies arbóreas de bosque seco en Costa Rica. Ecosistemas 2021, 30, 2092. [Google Scholar] [CrossRef]
- Clark, D.A.; Clark, D.B. Climate-induced annual variation in canopy tree growth in a Costa Rican tropical rain forest. J. Ecol. 1994, 82, 865–872. [Google Scholar] [CrossRef]
- Banbury Morgan, R.; Herrmann, V.; Kunert, N.; Bond-Lamberty, B.; Muller-Landau, H.C.; Anderson-Teixeira, K.J. Global patterns of forest autotrophic carbon fluxes. Glob. Chang. Biol. 2021, 27, 2840–2855. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, N.; Gessler, A.; Kayler, Z.; Keel, S.G.; Badeck, F.; Barthel, M.; Boeckx, P.; Buchmann, N.; Brugnoli, E.; Esperschütz, J.; et al. Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: A review. Biogeosciences 2011, 8, 3457–3489. [Google Scholar] [CrossRef]
- Campelo, F.; Gutiérrez, E.; Ribas, M.; Sánchez-Salguero, R.; Nabais, C.; Camarero, J.J. The facultative bimodal growth pattern in Quercus ilex–a simple model to predict sub-seasonal and inter-annual growth. Dendrochronologia 2018, 49, 77–88. [Google Scholar] [CrossRef]
- Cardil, A.; Imbert, J.B.; Camarero, J.J.; Primicia, I.; Castillo, F. Temporal interactions among throughfall, type of canopy and thinning drive radial growth in an Iberian mixed pine-beech forest. Agr. Forest Meteorol. 2018, 252, 62–74. [Google Scholar] [CrossRef]
- Enquist, B.J.; Leffler, A.J. Long-term tree ring chronologies from sympatric tropical dry-forest trees: Individualistic responses to climatic variation. J. Trop. Ecol. 2001, 17, 41–60. [Google Scholar] [CrossRef]
- Fichtler, E.; Clark, D.A.; Worbes, M. Age and long-term growth of trees in an old-growth tropical rain forest, based on analyses of tree rings and 14C1. Biotropica 2003, 35, 306–317. [Google Scholar]
- Pritzkow, C.; Szota, C.; Williamson, V.; Arndt, S.K. Previous drought exposure leads to greater drought resistance in eucalypts through changes in morphology rather than physiology. Tree Physiol. 2021, 41, 1186–1198. [Google Scholar] [CrossRef]
- Maseda, P.H.; Fernández, R.J. Stay wet or else: Three ways in which plants can adjust hydraulically to their environment. J. Exp. Bot. 2006, 57, 3963–3977. [Google Scholar] [CrossRef] [PubMed]
- Peltier, D.M.P.; Ogle, K. Tree growth sensitivity to climate is temporally variable. Ecol. Lett. 2020, 23, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Gould, W.A.; Álvarez-Berríos, N.L.; Parrotta, J.A.; McGinley, K. Climate change and tropical forests. In Future Forests; Elsevier: Amsterdam, The Netherlands, 2024; pp. 203–219. [Google Scholar]
- Gunderson, C.A.; O’hara, K.H.; Campion, C.M.; Walker, A.V.; Edwards, N.T. Thermal plasticity of photosynthesis: The role of acclimation in forest responses to a warming climate. Glob. Chang. Biol. 2010, 16, 2272–2286. [Google Scholar] [CrossRef]
- Ma, F.; Xu, T.T.; Ji, M.F.; Zhao, C.M. Differential drought tolerance in tree populations from contrasting elevations. AoB PLANTS 2014, 6, plu069. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.J.; Oberbauer, S.F.; Clark, D.B.; Clark, D.A. Phenology and stem diameter increment seasonality in a Costa Rican wet tropical forest. Biotropica 2008, 40, 151–159. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Link, R.M.; Fuchs, S.; Aguilar, D.A.; Leuschner, C.; Ugalde, M.C.; Otarola, J.C.V.; Schuldt, B. Tree height predicts the shape of radial sap flow profiles of Costa-Rican tropical dry forest tree species. Agric. For. Meteorol. 2020, 287, 107913. [Google Scholar] [CrossRef]
- ASTM. Standard Test Methods for Density and Specific Gravity (Relative Density) of Wood and Wood-Based Materials (D2395-17). In Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2022; Volume 08.01, p. 13. [Google Scholar]
- Allen, R.G.; Jensen, M.E.; Wright, J.L.; Burman, R.D. Operational Estimates of Reference Evapotranspiration. Agron. J. 1989, 81, 650–662. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://R-project.org (accessed on 6 February 2023).
- Wang, K.H.; Hamzah, M.Z. Annual wood formation of tropical pioneer species related to stem diameters. J. Wood Sci. 2019, 65, 1–12. [Google Scholar] [CrossRef]
- Anderson, L.O.; Ribeiro Neto, G.; Cunha, A.P.; Fonseca, M.G.; Mendes de Moura, Y.; Dalagnol, R.; Wagner, F.H.; de Aragão, L.E.O.e.C. Vulnerability of Amazonian forests to repeated droughts. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170411. [Google Scholar] [CrossRef]
- Phillips, O.L.; Van Der Heijden, G.; Lewis, S.L.; López-González, G.; Aragão, L.E.; Lloyd, J.; Malhi, Y.; Monteagudo, A.; Almeida, S.; Dávila, E.A. Drought–mortality relationships for tropical forests. New Phytol. 2010, 187, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, W.; Gruber, A. Climatic influences on intra-annual stem radial increment of Pinus sylvestris (L.) exposed to drought. Trees 2010, 24, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Iberle, B.G.; Van Pelt, R.; Sillett, S.C. Development of mature second-growth Sequoia sempervirens forests. For. Ecol. Manag. 2020, 459, 117816. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Y.; Dong, M.; Du, E.; Zhou, Z.; Zhao, S.; Xu, H. Diverse responses of radial growth to climate across the southern part of the Asian boreal forests in northeast China. For. Ecol. Manag. 2020, 458, 117759. [Google Scholar] [CrossRef]
- Valverde, J.C.; Arias, D.; Castillo, M. Estimación del movimiento de flujo de savia en fustes y ramas de diferente grosor de Samanea saman Jacq. Espirales Rev. Multidiscip. Investig. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Schwartz, N.B.; Budsock, A.M.; Uriarte, M. Fragmentation, forest structure, and topography modulate impacts of drought in a tropical forest landscape. Ecology 2019, 100, e02677. [Google Scholar] [CrossRef]
- Vogt, D.; Vogt, K.; Gmur, S.; Scullion, J.; Suntana, A.; Daryanto, S.; Sigurðardóttir, R. Vulnerability of tropical forest ecosystems and forest dependent communities to droughts. Environ. Res. 2016, 144, 27–38. [Google Scholar] [CrossRef]
- Jiménez, M.; Navarro, F.; Sánchez-Miranda, A.; Ripoll, M. Using stem diameter variations to detect and quantify growth and relationships with climatic variables on a gradient of thinned Aleppo pines. For. Ecol. Manag. 2019, 442, 53–62. [Google Scholar] [CrossRef]
- Hu, L.; Fan, Z. Stem radial growth in response to microclimate in an Asian tropical dry karst forest. Acta Ecol. Sin. 2016, 36, 401–409. [Google Scholar] [CrossRef]
- Aldea, J.; Bravo, F.; Vázquez-Piqué, J.; Rubio-Cuadrado, A.; del Río, M. Species-specific weather response in the daily stem variation cycles of Mediterranean pine-oak mixed stands. Agric. For. Meteorol. 2018, 256, 220–230. [Google Scholar] [CrossRef]
- Vieira, S.; de Camargo, P.B.; Selhorst, D.; Da Silva, R.; Hutyra, L.; Chambers, J.Q.; Brown, I.F.; Higuchi, N.; Dos Santos, J.; Wofsy, S.C. Forest structure and carbon dynamics in Amazonian tropical rain forests. Oecologia 2004, 140, 468–479. [Google Scholar] [CrossRef] [PubMed]

| Family | Species | Type | DBH (cm) | Bark (mm) | H (m) |
|---|---|---|---|---|---|
| Clusiaceae | Garcinia madruno (Kunth) Hammel | Perennial | 21.23 (1.55) | 15.6 (8.8) | 21.39 (2.61) |
| Icacinaceae | Calatola costaricensis Standl | Perennial | 19.30 (0.69) | 10.2 (6.9) | 16.61 (1.24) |
| Lecythidaceae | Gustavia brachycarpa Pittier | Perennial | 23.19 (1.59) | 4.9 (3.2) | 23.00 (1.90) |
| Lecythidaceae | Lecythis mesophylla S.A. Mori | Perennial | 51.82 (9.49) | 20.1 (5.2) | 37.96 (3.52) |
| Malvaceae | Goethalsia meiantha (Donn. Sm.) Burret | Deciduous | 26.53 (1.53) | 4.0 (1.6) | 22.75 (2.85) |
| Meliaceae | Carapa nicaraguensis C. DC. | Perennial | 37.58 (3.37) | 20.9 (5.8) | 29.45 (2.16) |
| Myristicaceae | Virola sebifera Aubl. | Perennial | 25.96 (2.75) | 11.4 (3.9) | 23.45 (2.13) |
| Vochysiaceae | Vochysia megalophylla Stafleu | Deciduous | 75.45 (2.69) | 19.2 (7.3) | 37.34 (2.34) |
| Variable | Tree Size | Year | Species | Tree Size × Year | Tree Size × Species | Year × Species | Year × Tree Size × Species |
|---|---|---|---|---|---|---|---|
| Stem growth rate | 0.11 ns | 0.30 ns | <0.01 * | 0.12 ns | 0.44 ns | 0.91 ns | 0.92 ns |
| Wood density | 0.09 ns | 0.29 ns | <0.01 * | 0.22 ns | 0.23 ns | 0.82 ns | 0.95 ns |
| Species | Climatic Variable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| P (mm) | T (°C) | Mean PAR (µmol m−2 s−1) | Mean RH (%) | Mean VPD (kPa) | |||||
| Minimum | Mean | Maximum | Minimum | Mean | Maximum | ||||
| C. costaricensis | 0.552 ns | 0.732 ** | 0.233 ns | −0.588 * | −0.696 * | −0.516 * | 0.011 ns | 0.011 ns | 0.201 ns |
| C. nicaraguensis | 0.366 ns | 0.659 ** | 0.411 ns | −0.536 * | −0.756 * | −0.486 * | 0.095 ns | 0.028 ns | −0.200 ns |
| G. madruno | 0.220 ns | 0.601 ** | 0.332 ns | −0.613 * | −0.803 * | −0.537 * | 0.010 ns | 0.009 ns | 0.009 ns |
| G. meiantha | 0.422 ns | 0.705 ** | 0.150 ns | −0.520 * | −0.722 * | −0.499 * | 0.013 ns | 0.055 ns | −0.305 ns |
| G. brachycarpa | 0.332 ns | 0.687 ** | 0.144 ns | −0.557 * | −0.677 * | −0.600 * | 0.021 ns | 0.045 ns | 0.045 ns |
| L. mesophylla | 0.255 ns | 0.603 ** | 0.355 ns | −0.605 * | −0.775 * | −0.511 * | 0.009 ns | 0.008 ns | 0.180 ns |
| V. sebifera | 0.310 ns | 0.680 ** | 0.255 ns | −0.597 * | −0.777 * | −0.509 * | 0.012 ns | 0.029 ns | 0.029 ns |
| V. megalophylla | 0.250 ns | 0.641 ** | 0.366 ns | −0.533 * | −0.703 * | −0.544 * | 0.015 ns | 0.009 ns | 0.390 * |
| Species | Regression Model | R2 adj | RMSE | p-Value | MAPE |
|---|---|---|---|---|---|
| C. costaricensis | Sg = 1.289 + 0.001 × P − 0.053 × T | 0.68 | 1.00 | 0.013 * | 0.14 |
| C. nicaraguensis | Sg = 3.849 + 0.001 × P − 0.092 × T | 0.79 | 0.61 | 0.011 * | 0.04 |
| G. madruno | Sg = 1.463 + 0.001 × P − 0.036 × T | 0.62 | 1.18 | 0.001 ** | 0.12 |
| G. meiantha | Sg = 1.929 + 0.001 × P − 0.069 × T | 0.74 | 0.78 | 0.001 ** | 0.03 |
| G. brachycarpa | Sg = 0.206 + 0.001 × P − 0.005 × T | 0.78 | 0.73 | 0.001 ** | 0.02 |
| L. mesophylla | Sg = 0.197 + 0.001 × P − 0.005 × T | 0.72 | 0.71 | 0.002 ** | 0.07 |
| V. sebifera | Sg = 3.569 + 0.001 × P − 0.132 × T | 0.70 | 0.92 | 0.001 ** | 0.06 |
| V. megalophylla | Sg = 3.078 + 0.002 × P − 0.485 × T | 0.72 | 0.99 | 0.001 ** | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valverde, J.C.; Arias-Aguilar, D.; Castillo-Ugalde, M.; Zamora-Villalobos, N. Influence of Climatic Variables on the Stem Growth Rate in Trees of a Tropical Wet Forest. Conservation 2024, 4, 139-149. https://doi.org/10.3390/conservation4020010
Valverde JC, Arias-Aguilar D, Castillo-Ugalde M, Zamora-Villalobos N. Influence of Climatic Variables on the Stem Growth Rate in Trees of a Tropical Wet Forest. Conservation. 2024; 4(2):139-149. https://doi.org/10.3390/conservation4020010
Chicago/Turabian StyleValverde, Juan Carlos, Dagoberto Arias-Aguilar, Marvin Castillo-Ugalde, and Nelson Zamora-Villalobos. 2024. "Influence of Climatic Variables on the Stem Growth Rate in Trees of a Tropical Wet Forest" Conservation 4, no. 2: 139-149. https://doi.org/10.3390/conservation4020010
APA StyleValverde, J. C., Arias-Aguilar, D., Castillo-Ugalde, M., & Zamora-Villalobos, N. (2024). Influence of Climatic Variables on the Stem Growth Rate in Trees of a Tropical Wet Forest. Conservation, 4(2), 139-149. https://doi.org/10.3390/conservation4020010






