Abstract
Reptiles live in a range of different habitats from tropical forests to temperate zones where the climate may change on seasonal or a daily basis. The thermal environment is a major determinant of how efficiently they can achieve optimum or preferred body temperatures and, in terms of physiologically optimum body temperatures, these may not be possible in a natural environment. In this paper, null models have been employed to evaluate thermoregulatory efficiency in Hermann’s tortoise, Testudo hermanni, in high summer in central Montenegro when temperatures change on a daily basis. The study area is defined as a low-cost thermal environment and thus we assumed that tortoises should be able to achieve an efficient level of thermoregulation. However, the results varied and depended on where the tortoises operated and the weather conditions. High levels of efficiency were found during sunny weather in areas with abundant patches of shade and sunlit areas. These reflected the temperatures of models placed in these areas and in females during cooler cloudy weather when thermoregulatory effort increased. Model temperatures placed in partially shaded sunlit areas were in better agreement with tortoise body temperatures than models in other areas. Tortoise body temperatures were in closer agreement with set point temperatures than any of the null models placed in either open sunny, shaded or partially shaded areas, indicating that tortoise movement was non-random and due to active thermoregulation.
1. Introduction
The key role of temperature together with humidity in the biology and ecology of reptiles is well known; most physiological functions are temperature dependent, including reproduction, feeding and digestion, locomotory movement, sensing the immediate environment, securing prey and avoidance of predators (e.g., [1,2,3,4]). However, to attain optimum body temperatures, a reptile must efficiently harness heat from the environment, which depends on the thermal quality of the habitat. This may change on a daily or seasonal basis from an environment that is thermally tolerable to thermally challenging. This is particularly important in the temperate zones where cool spring or autumn weather may impact on a reptile’s capacity for heat uptake, even in small lizards [5]. For example, in a low-quality thermal habitat with low temperatures and limited basking sites, the energy costs of locomotory movement to access basking sites along with potential high predation risk may constrain their ability for heat uptake. Theoretically, if both the energy and ecological costs of thermoregulation outweigh the physiological benefits, a reptile should thermoregulate less precisely [1,2]. More recent research has indicated increased thermoregulatory effort as the thermal environment cools [6]. Studies on how different species of reptile respond to changes in the thermal environment provide additional insight into the range of thermoregulatory behaviours.
Most of the research on reptile thermal ecology, including theoretical conceptions, has involved lizards. Fewer studies have examined chelonians [7], but they are of special interest because of their semi-spherical shape that, due to differences in surface area to mass geometry, slows rates of heat gain and heat loss compared to similar sized lizards and snakes. This will increase basking duration to achieve optimum body temperatures, potentially delaying and reducing time available for other activities. Additionally, chelonians move very slowly compared to typical lizards or snakes [8] and, as a consequence of higher rates of thermal inertia, they risk overheating, especially in large individuals or species living in open habitats if cover is limited [9,10,11]. However, the dermal armour of chelonians reduces risk of predation when they are foraging or thermoregulating. This is important in herbivorous species that may be constrained to forage widely and feed in open locations where predation risk especially for non-armoured reptiles is greater.
Hermann’s tortoise Testudo hermanni boettgeri (Figure 1) is a small European terrestrial chelonian that is primarily a vegetarian selecting mainly legumes [12]. During periods of very hot weather, especially during high summer, they must balance feeding budgets with the risks of overheating when entering open clearings in the search for food. Previous studies in this species have indicated daily maximum field body temperatures of 34 °C when climatic conditions permit, but the results are lower during cooler weather [13,14,15,16]. These studies showed that the seasonal effects of temperature and their impact on different size classes limited the tortoises’ ability to achieve optimum body temperatures. They also showed the different priorities of males and the physically larger females [15]. These priorities interact with other ecological demands such as predation, feeding and locomotory movement and mate-seeking among others. In this study, we use non-thermoregulating null models to enable greater insight into the impact of differing temperature levels and variation in the thermal environment of this species.
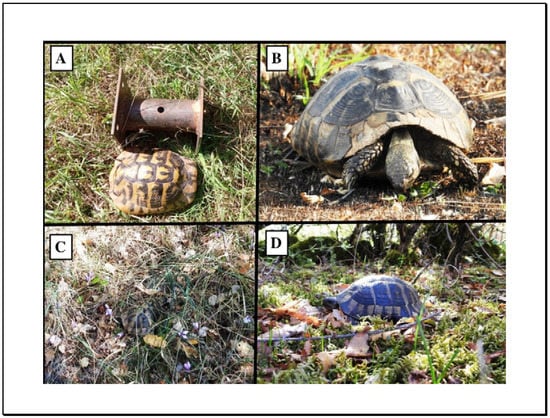
Figure 1.
Examples of T. hermanni (A) shown with null model (metal cylinder) for size comparison, (B) feeding in partial sunlit area, (C) resting in shade and (D) basking in a partially shaded area.
Null models are defined as physical models that take a variety of forms including metal cylinders, aluminium casts or clay models painted and shaped to represent the approximate thermal properties of the species in question [17,18,19,20,21]. Their use has been subject to debate with evidence suggesting that model type is less important than earlier thought [18,21]. They are perceived to approximate a single measure of the position of a reptile in the field environment [22]. Frequently used are hollow-walled metal tubes [21,23] that have low heat capacity and respond readily to changes in radiation levels, enabling predictions of the amounts of time it would take for a reptile’s body temperature to exceed or fail to achieve physiological optimum or threshold temperatures with reasonable accuracy. However, their rates of change are invariably slower than real reptiles due to blood shunt mechanisms that reptiles use to accelerate rates of heating or slow rates of heat loss [11,19]. They are invariably used in combination with estimates of set point temperatures to estimate the extent of thermoregulation [2]. Set point temperatures are the body temperatures that reptiles select in laboratory heat gradients, defined as cost free thermal environments, where there are no predators or the need to search for food; hence, there are little or no restrictions on achieving preferred body temperatures. In this paper, we have compared data from null models, tortoise body temperatures and set point temperatures to examine the thermal ecology of T. hermanni. Using this approach gave us a unique opportunity to add to data from earlier field studies on the thermal ecology of Hermann´s tortoise in Montenegro. The study area represents a low-cost thermal environment [1] consisting of abundant open sunny clearings, dappled sunlight and shaded areas where a heliothermic reptile under appropriate conditions should be able to achieve close to optimum body temperatures.
In this study we asked the following questions:
- (1)
- How effective is T. hermanni as a thermoregulator in attaining preferred body temperatures during different weather conditions and seasons? For example, when the environment begins to cool, do they increase thermoregulatory efforts or remain thermally passive and accept lower body temperatures? This is important because of the influence of body temperature levels on physiological processes.
- (2)
- What would be the thermal consequences for tortoises to remain in shaded areas avoiding open sunlit clearings or patches? Would they be capable of achieving preferred temperatures and, if so, how frequently?
- (3)
- What would be the thermal consequences for a tortoise to forage in open sunlit areas during hot weather for long periods?
- (4)
- Are there thermal consequences due to size differences in different individuals, for example between males and larger females?
- (5)
- We assumed that a tortoise could equilibrate body temperature in dappled sunlit areas by either being half in sun and half in shade or spending 50% of its time within sun and shade. To what extent do tortoise body temperatures agree with model temperatures in this type of habitat?
2. Methods
2.1. Species and Study Area
The eastern subspecies of Hermann’s tortoise, Testudo hermanni boettgeri, is the only terrestrial tortoise in a Mediterranean habitat in Montenegro (Figure 1). The study site was at Kadića boan (Danilovgrad municipality), located in central Montenegro: 345,547.00 m E and 4,716,026.00 m N with average elevation of 132 m above sea level (Figure 2). Research was carried out on an area of 5.70 ha. It is rocky terrain is described as an open-type thermophilic deciduous thicket with Quercus trojana, Q. cerris, Fraxinus ornus, Pistacia terebinthus, Punica granatum, Ruscuss acculeatus, Asparagus acutifolia, Carpinus betulus and Cornus masas the dominant vegetation. Less common was Cotinus coggygria, Paliurus spina-christi and Quercus pubescens. The climate is sub-Mediterranean with warm summers and average annual temperature 15 °C [24]. Survey procedure was carried out during a 15-day period from 10:00 h until approximately 17:00 h.
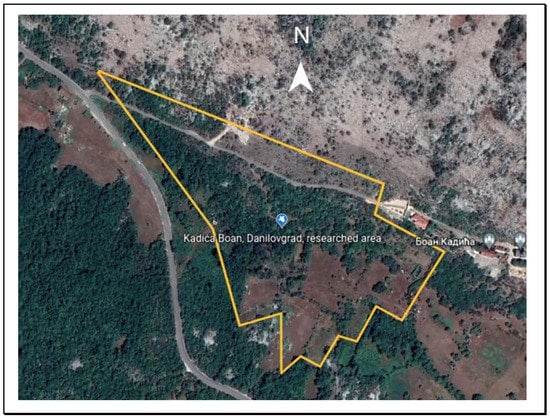
Figure 2.
Map of the study area. Open area was mostly in the southern end and wooded (darker) areas to the north (Google Earth).
Field data were gathered from August 2021 until September 2022 by walking different daily routes through the habitat. When a tortoise was located, measurements of both the body temperature of the tortoise (at the cloaca) and the corresponding model temperatures were taken using a digital thermometer (Model TFA Dostmann, (Wertheim-Reicholzheim, Germany)) with an approximate error of ±0.5 °C. The models consisted of five metal cylinders filled with water placed each day in habitat types representing different thermal environments: full shade, half or partial shaded areas and open sunny locations. The models situated in the shade microhabitats represent the coolest areas and the lowest rates of heat gain; those situated in full sunny areas represent the hottest area with greatest rates of heat gain; the models in partially sunlit areas represent both high rates of heat gain if a tortoise was situated in a sunlit patch and easy access for cooling in shaded patches. Figure 3 gives examples of these areas. Due to changes in the position of the sun as the day progressed, the cylinders were, by necessity, moved but always to locations in the same microhabitat they were originally placed. This ensured they reflected the approximate temperatures in different microhabitats. Thermometer probes were inserted into the centre of the water filled cylinder approximately every 10 min or when a tortoise was located. All models were the same dimensions with lengths of 130 mm and diameter of 57 mm. They showed good general agreement in size with an average adult tortoise (see Figure 1).

Figure 3.
Examples of key areas for thermoregulation in a low-cost thermal environment: (A) open sunlit area where maximum temperatures were found; (B) tortoise moving through partial shaded/sunlit area; (C,D) show examples of low-cost habitat in terms of thermoregulation for a reptile with small distances between sunlit and shaded areas.
When captured, each tortoise was given a unique mark [25,26]. Sex was determined by tail length and shell shape, specifically plastron concavity in males, which is flatter in females [27]. Size of the SCL (straight line carapace length) was used for maturity determination for adults: 130 mm SCL for males and 150 mm SCL for females, and all individuals with a lower value than the above were classified as juveniles.
2.2. Statistic Analysis
Our primary objective was to examine for evidence of thermoregulation and how tortoises responded to different levels of heat in their environment. To measure thermoregulatory efficiency, a modification of the methods described in [2]. Ref. [28] has been used which gives an equation of the form:
where E is the measure of thermoregulatory efficiency derived from the proportion of model temperatures (P)Tm that lie within Tset, (P)Tm = Tset), divided by the proportion of tortoise body temperatures Tb that lie within Tset, (P) Tb = Tset). The value for Tset is derived from the set point temperature range of 29–32 °C for T. hermanni selected in a laboratory heat gradient [29]. The closer E is to 1, the more efficient the tortoise is in achieving perfect thermoregulatory efficiency. No thermoregulatory efficiency is indicated when E = 0.
E = 1 − ((P)Tm = Tset)/((P)Tb = Tset)
Tests for differences in medians and variances between model and tortoise body temperatures during both sunny and cloudy weather were by a non-parametric Kruskal–Wallis test for multiple comparisons with post hoc by a Dunn’s test. This compares medians of the ranked data using a χ2 statistic and tests the null hypothesis that H0: = H1 = … = Hk. Variances of the model and body temperatures were determined by Levene’s test for homogeneity of multiple variances using the F-distribution. In all tests, alpha was set at 0.05 with the null hypothesis homogeneity of variances H0: σ21 = … = σ2k. ANOVA was used for all other analyses after testing for normality using the Anderson–Darling test. Statistics were carried out using Minitab V17 and internet sites Statistics Kingdom and Social Science Statistics.
3. Results
3.1. Tortoise Numbers, Sex Ratios and Their Sizes
Total number of tortoises measured was 129, composed of 21 females and 33 males during cloudy weather and 22 females and 53 males when the weather was sunny. However, due to a series of fires and subsequent injuries to several tortoises (9 males and 10 females), not all tortoises were measured for body mass. This reduced the body mass sample to 77 males and 37 females, giving a total of n = 114. Females were found to be heavier (mean = 828.8 ± 328.5, range 115–1446 g) than males (mean = 565.7 ± 226.7, range 140–1128 g) with the difference significant F(1,112) = 24.7, p < 0.0001.
3.2. Body Temperatures
The Anderson–Darling tests of male and female body temperatures during cloudy and sunny weather gave normal distributions in all 4 data sets, a2 values from 0.21 to 0.4, p values from 0.33 to 0.82 and hence comparisons were made by ANOVA. Male body temperatures (mean = 29.8 ± 2.6 °C, n = 86) and female (mean = 29.3 ± 2.7 °C, n = 43) body temperatures were not significantly different during either sunny, F = 1.3, p = 0.26 or, when the weather was cloudy (males 28.5 ± 2.4 °C versus females 28.6 ± 2.6 °C), F = 0.03, p = 0.86 (question 4). We therefore pooled the data for comparison of cloudy and sunny weather. This indicated body temperatures during sunny weather (mean = 30.4 ± 2.5, n = 75) were higher than during cloudy weather (mean = 28.6 ± 2.5) and the differences were significant F (1,127) = 17.8, p < 0.0001. Figure 4 shows body temperature distributions during cloudy and sunny weather in relation to set point temperature.
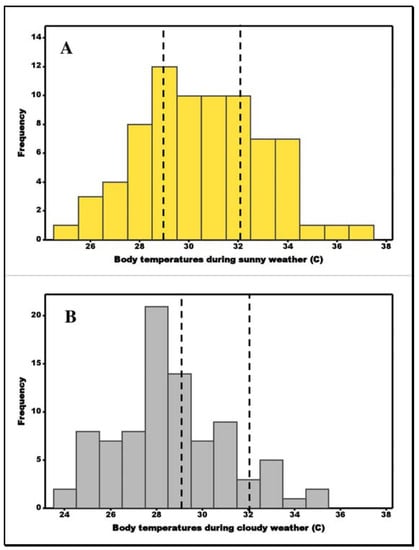
Figure 4.
Body temperatures (males + females) during (A) sunny and (B) cloudy weather. Broken vertical lines represent set point temperature range for T. hermanni.
3.3. Comparison of Model Temperatures
The highest median temperature was recorded in Model Sun (median = 44.2 °C), followed by the two Partial Shade models (medians = 33.1 & 32.4 °C), with the lowest temperatures in the shaded models (medians = 30.4 & 30.3 °C). The Kruskal–Wallis H-test indicated significant differences between models (χ2 (4 d.f.) = 394.5, p < 0.001). The post-hoc Dunn’s indicated that the mean ranks of Model Sun were significantly different from all other models with the two partial shade models significantly different from the two shade models. The Levene’s test indicated significant differences in model variances (F = 41.39, p < 0.0001) with the Tukey HSD post hoc indicating greater Model Sun variance in comparison to all other models. These results confirmed our assumption that the thermal environment was heterogeneous and thus presented the tortoises with a wide range of thermal constraints and opportunities including dangerously high temperatures (question 2) and a necessity for efficient thermoregulation.
Body temperatures were significantly higher than Model Shade temperatures (F1,341 = 68.05, p < 0.0001), significantly lower than Model Sun temperatures (F1,214 = 130.5, p < 0.0001), but in agreement with Model Partial Shade (F1,341 = 0.72, p = 0.4). These results answer questions 2, 3 and 5.
3.4. Testing for Efficiency of Thermoregulation
Males. During sunny weather, 31 of 53 (58.5%) male body temperatures were within the set point range compared to 47 of 211 (22.3%) of the corresponding model temperatures. This gives an E-score of 0.38. In cooler cloudy weather, 10 of 33 (30.3%) male tortoise body temperatures were within the set point range compared to 28 of 132 (21.2%) of the corresponding model temperatures. This gives an E-score of 0.31. Male thermoregulatory efficiency therefore increased by 28.2% when the weather became sunny and warmer.
Females. During sunny weather, 12 of 22 (54.5%) female body temperatures were within the set point range compared to 39 of 88 (44.3%) of the corresponding model temperatures, giving an E-score of 0.19. When the weather was cloudy, the figure was 10 of 21 (47.6%) body temperatures and 24 of 84 (28.6%) of the model temperatures within set point range. This gives an E-score of 0.41, which is an increase of 0.22 compared to sunny weather. Females therefore increased thermoregulatory effort as the weather became cooler (question 1).
In open areas during sunny weather, the female E-score was 1 indicating perfect thermoregulation, while that of males was 0.97, thus indicating the tortoises were avoiding dangerously high temperatures. Figure 5 shows model temperature distributions in open sunny habitats during both cloudy and sunny weather with the set point ranges inserted. These results suggest that a tortoise, during sunny weather, must limit time in open habitats for foraging or feeding due to the risk of overheating and hence requires finely tuned thermoregulation (question 3). In partial sunlit areas, E increased from 0.49, during overcast weather (pooled m + f), to 0.80 when the weather was hot and sunny, suggesting that the body temperatures of those in sunlit and shaded areas of the environment were often in close agreement with the microhabitat temperatures, enabling high levels of thermoregulatory efficiency.
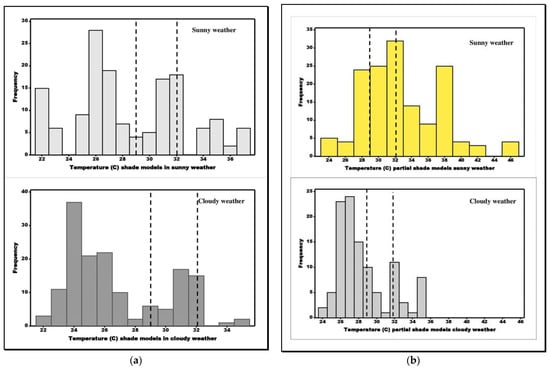
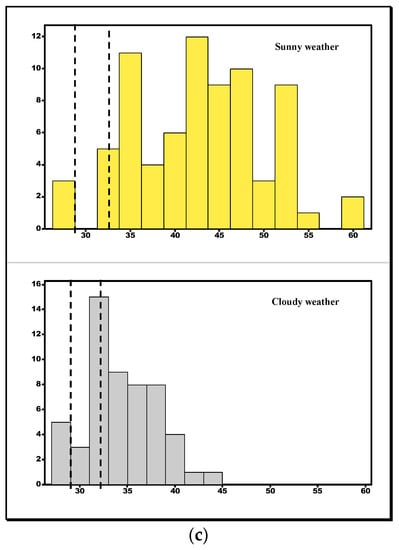
Figure 5.
Histograms of model temperatures placed in (a) shaded locations, (b) partially shaded locations, and (c) sunny locations. Vertical broken lines represent set point body temperature ranges.
4. Discussion
4.1. General Considerations
The results of this study have shown varying levels of thermoregulatory efficiency (E) that depended on weather and the thermal properties of microhabitats. During hot sunny conditions, due to very high temperatures in open areas, tortoises thermoregulated very efficiently by operating in partially sunlit areas where small patches of sunlight and shade enabled efficient thermoregulation. This applies to both sexes, despite differences in body mass and slower heating rates (question 4). This result was in good agreement with earlier studies of T. hermanni [14,15], although lower body temperatures in females were found during the cooler autumn weather [15]. The results therefore showed flexibility of thermoregulation that responded to weather changes, with females increasing E-values during cloudy weather [6] (question 1). The lower E-values in the smaller sized males could be attributed to higher levels of activity [15]. Efficient thermoregulation in partial sunlight areas with high levels of vegetation cover and abundant sunlit patches (Figure 4) provide refuges, basking sites and food plants [30], and avoid the dangerously high body temperatures in open areas. These observations are in general agreement with thermoregulatory studies on lizards where low thermoregulatory costs were found in landscapes with an abundance of optimal microclimates, reducing the distance between sun and shade [31], which is a characteristic of woodland areas in our study (Figure 3).
The dermal armour of terrestrial chelonians as a defense mechanism might suggest that achieving optimum body temperatures may have different ecological functions than, for example, the muscular energy needed for flight in many lizards and snakes. Herbivorous lizards and chelonians are highly dependent on temperature and resident bacterial populations to ferment plant polymers for digestion [31,32,33,34], and a need for extensive foraging for food may explain why adult tortoises were found to exceed set point temperatures during sunny weather (18 of 74 (24.7%) of body temperatures). Fifty-three percent of these tortoises were active or feeding in open locations but it is not clear if this was body temperature selection or whether the tortoises were simply tolerating higher activity body temperatures for physiological reasons (e.g., digestion). Similar body temperature levels were found during sunny weather in southern Croatia, Budva and Sutomore when 18.0%, 26.3% and 26.7% of adults, respectively, had body temperatures that exceeded the set point range [14,15,35]. The numbers of juveniles located in these sites was low (eighteen individuals) but six were found to have body temperatures that exceeded maximum set point temperatures and 50% of these were during sunny weather. Smaller sized (body mass 18–82 g) tortoises have low thermal inertia and, due to slow growth, this condition will be present for around 12–14 years.
This might raise the question of how laboratory heat gradient studies of chelonians and other reptiles, particularly herbivorous species, should be interpreted in respect to field data. If body temperatures higher than set point body temperatures are tolerated rather than selected in natural environments, this would agree with studies of lizards where body temperatures are frequently above set point temperatures when the environment becomes hot. This has been attributed to time constraints to complete foraging activities and is likely an ecological necessity [36,37,38]. In T. hermanni boettgeri, dietary selection was strongly for legumes [12,30,39]; if widely dispersed in open areas, tortoises would be constrained to forage during hot weather. Females frequently excavate nests on open clearings for egg-laying where appropriate incubation temperatures for embryonic development are found. However, calculations based on field observations have shown that a nesting tortoise will risk overheating if this is attempted in open locations in hot weather [40]. These critical aspects of the T. hermanni lifestyle suggest that the capacity to operate at higher than set point range temperatures will be adaptive and support our observations of increased thermoregulatory effort during hot sunny weather in high quality microhabitats such as partially sunlit areas in woodland. This avoids the high-energy cost of basking compared to shuttling behaviour between the closely situated sunny and shaded patches [41].
A useful comparison with T. hermanni boettgeri thermal ecology is found in the thermoregulatory strategy of the sympatric Pseudopus apodus. This large, snake-like, heavily armoured lizard with bony elements within the scales [42,43] feeds mostly on small invertebrates such as snails and slugs that they hunt during both warm and sunny or cool and overcast weather and even light rain [44]. Set point temperature found for P. apodus in a thermal gradient ranged from 24–35 °C [42], which is much wider than in T. hermanni boettgeri. This lizard basks during mornings and calculations have shown hourly increases in field body temperature of adults increasing at a mean rate of ~5–6 °C h−1, compared to T. hermanni boettgeri where the mean rate was ~3.0 °C h−1. This is likely an artefact of a much higher skin-surface-to-body-mass geometry in the snake-like P. apodus, but maximum field Tbs were 3–4 °C lower ([44] and unpublished data), despite opportunities for further higher body temperatures under the same weather conditions. Dietary differences could partly explain the differences since the carnivorous diet in P. apodus provides more energy and easier digestion than equivalent plant food mass. This suggests that both species have evolved a balance between body temperatures in the field and other ecological activities, e.g., foraging, but also physiological factors [45]. The lower field body temperatures in P. apodus are likely a balance between locating slow moving prey, often in cooler, moist weather, against the physiological costs of higher temperatures.
4.2. Concluding Remarks
Activity levels and microhabitat utilization are key factors in determining patterns of distribution in T. hermanni [30,46] and are a critical aspect in the avoidance of excess heat, as has been previously shown in other chelonians including giant tortoises [10,11]. The extent of how hot the thermal environment becomes for T. hermanni boettgeri can be seen in the open area model data during sunny weather that regularly exceeded the critical thermal maximum body temperature in T. hermanni of 39–42 °C [47]. Our findings demonstrate the challenges and benefits of thermoregulation during times when the environment becomes high or low cost; for example, too hot or too cold [48,49]. The predicted increases in temperatures due to global warming will potentially not only impact directly on ecosystems but will also have serious side effects such as increasing the risk of fires. These already occur in the Danilovgrad municipality [50], including a major fire in the study area during August 2021. Indeed, the Danilovgrad Protection Service recorded and extinguished 271 fires on low and medium vegetation and, between January and September 2022, when a total of 299 fires were recorded. If climate warming increases frequency of serious fires, then subsequent major habitat changes can be expected due to, among other things, vapour pressure deficit and habitat drying. Understanding the interactions of reptiles with their thermal environments enables more precise predictions of how reptiles will respond to such habitat changes and degradations due to climate warming [31,51], as already demonstrated in other reptiles including species from Europe [52,53].
Our data describing aspects T. hermanni boettgeri thermal ecology and the comparisons with sympatric P. apodus illustrate the complex way in which reptiles interact with their environment and the importance of understanding this complexity. Future field work in 2023 in the study locality will employ a more synthetic, integrative approach, including a comparative study with sympatric P. apodus. These new data will involve using an increased number of null models for T. hermanni boettgeri, along with null models for P. apodus that will reflect the lizard’s morphometry and thermal properties. We expect these new data to increase our understanding of the complex of factors that two reptile species with different life histories employ to harness heat energy from the environment. Such data would assist conservation efforts to mitigate potential global warming effects [1,4,52,53,54,55] and preserve current habitats.
Author Contributions
Conceptualization: R.M., A.V. and V.P.; methodology: R.M.; formal analysis: R.M.; Field work/data collection A.V.; writing original draft preparation: R.M., A.V., V.P.; writing, review and editing: all authors; supervision: R.M., V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require ethical approval since no experimental procedures were carried out.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are shown graphically in the figures and not publicly available due to ongoing longitudinal analysis.
Acknowledgments
The authors greatly appreciate comments by three anonymous referees that improved on the original submitted draft.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huey, R.B.; Slatkin, M. Costs and benefits of lizard thermoregulation. Q. Rev. Biol. 1976, 51, 363–384. [Google Scholar] [CrossRef] [PubMed]
- Hertz, P.E.; Huey, R.B.; Stevenson, R.D. Evaluating temperature regulation by field-active ectotherms: The fallacy of the inappropriate question. Am. Nat. 1993, 142, 796–818. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, D.; Hertz, P.E.; Castilla, A.M. Thermoregulation in a lacertid lizard: The relative contributions of distinct behavioral mechanisms. Ecology 1996, 77, 1818–1830. [Google Scholar] [CrossRef]
- Castilla, A.M.; Van Damme, R.; Bauwens, D. Field body temperatures, mechanisms of thermoregulation and evolution of thermal characteristics in lacertid lizards. Nat. Croat. 1999, 8, 253–274. [Google Scholar]
- Grbac, I.; Bauwens, D. Constraints on temperature regulation in two sympatric Podacis lizards during autumn. Copeia 2001, 1, 178–186. [Google Scholar] [CrossRef]
- Blouin-Demers, G.; Nadeau, P. The cost-benefit model of thermoregulation does not predict lizard thermoregulatory behavior. Ecology 2005, 86, 560–566. [Google Scholar] [CrossRef]
- Dubois, Y.; Blouin-Demers, G.; Shipley, B.; Thomas, D. Thermoregulation and habitat selection in wood turtles Glyptemys insculpta: Chasing the sun slowly. J. Anim. Ecol. 2009, 78, 1023–1032. [Google Scholar] [CrossRef]
- Jayes, A.S.; Alexander, R.M. The gaits of chelonians: Walking techniques for very low speeds. J. Zool. 1980, 191, 353–378. [Google Scholar] [CrossRef]
- Branch, W.R. Preliminary observations on the ecology of the angulate tortoise (Chersina angulata) in Eastern Cape Province, South Africa. Amphibia-Reptilia 1984, 5, 43–55. [Google Scholar] [CrossRef]
- Swingland, I.R.; Frazier, J.G. The conflict between feeding and overheating in the Aldabran giant tortoise. In A Handbook of Biotelemetry and Radio Tracking; Amlaner, C.J., MacDonald, D.W., Eds.; Pergamon Press: Oxford, UK, 1979; pp. 611–615. [Google Scholar]
- Moulherat, S.; Delmas, V.; Slimani, T.; El Mouden, E.; Louzizi, T.; Lagarde, F.; Bonnet, X. How far can a tortoise walk in open habitat before overheating? Implications for conservation. J.Nat. Conserv. 2014, 22, 186–192. [Google Scholar] [CrossRef]
- Meek, R. Nutritional selection in Hermann`s tortoise Testudo hermanni, in Montenegro and Croatia. BCG Testudo 2010, 7, 88–95. [Google Scholar]
- Pulford, E.; Hailey, A.; Stubbs, D. Thermal relations of Testudo hermanni robertmertensi in S. France. Amphibia-Reptilia 1984, 5, 37–41. [Google Scholar]
- Meek, R. Thermoregulatory behaviour in a population of Hermann’s tortoise (Testudo hermanni) in southern Yugoslavia. Br. J. Herpetol. 1984, 6, 387–391. [Google Scholar]
- Meek, R. The thermal ecology of Hermann’s tortoise (Testudo hermanni) in summer and autumn in Yugoslavia. J. Zool. 1988, 215, 99–111. [Google Scholar] [CrossRef]
- Filippi, E.; Rugerio, L.; Capula, M.; Burke, R.L.; Luiselli, L. Population and Thermal Ecology of Testudo hermanni hermanni in the Tolfa Mountains of Central Italy. Chelonian Conserv. Biol. 2010, 9, 54–60. [Google Scholar] [CrossRef]
- Zimmerman, L.C.; O’Connor, M.P.; Kemp, S.A.; Spotila, S.R. Thermoregulation by desert tortoises (Gopherus agassizii) at the Desert Tortoise Conservation Center, Las Vegas, Nevada: Preliminary results. In Proceedings of the Symposium-Desert Tortoise Council, Acton, CA, USA, 1992; pp. 103–117. [Google Scholar]
- Vitt, L.J.; Sartorius, S.S. HOBO’s, Tidbits and lizard models: The utility of electronic devices in field studies of ectotherm thermoregulation. Funct. Ecol. 1999, 13, 670–674. [Google Scholar] [CrossRef]
- Meek, R. Thermoregulation and activity patterns in captive water dragons Physignathus cocincinus, in a naturalistic environment. Herpetol. J. 1999, 9, 137–146. [Google Scholar]
- Meek, R.; Jolley, E.; de Silva, A.; Goonewardene, S.; Drake, J.; Chalalochani, H.M.N.; Liyanage, P.; Abeysekera, T.S.; Mayadunna, M.D.I.P.K.; Somathilaka, S.A.U.S.; et al. Altitudinal differences in thermoregulatory behaviour in Calotes versicolor in the Knuckles region, Sri Lanka. The diversity of the Dumbara Mountains: The Knuckles Massif, Sri Lanka: With special reference to its herpetofauna. Lyriocephalus 2005, 6, 83–93. [Google Scholar]
- Shine, R.; Kearney, M. Field studies of reptile thermoregulation: How well do physical models predict operative temperatures? Funct. Ecol. 2001, 15, 282–288. [Google Scholar]
- Bakken, G.S. Measurement and Application of Operative and Standard Operative Temperature in Ecology. Am. Zool. 1992, 32, 194–216. Available online: https://www.jstor.org/stable/3883758 (accessed on 5 September 2022). [CrossRef]
- Meek, R.; Avery, R.A. Basking in the Australian Water Dragon Physignathus lesueurii; why do alpha males not respond to operative temperatures in the same way as adults and sub-adults? Amphibia-Reptilia 2008, 29, 257–262. [Google Scholar]
- Burić, M.; Milenković, M.; Ducic, M. The Specificities of the Climate of Danilovgrad (Montenegro). Glas. Srp. Geogr. Drus. 2019, 99, 19–28. Available online: http://www.doiserbia.nb.rs/Article.aspx?ID=0350-35931901019B#.Y8BKv33MKpo (accessed on 5 September 2022). [CrossRef]
- Cagle, F.R. A system of marking turtles for future identification. Copeia 1939, 1939, 170–173. [Google Scholar] [CrossRef]
- Stubbs, D.; Hailey, A.; Pulford, E.; Tyler, W. Population ecology of European tortoises: Review of field techniques. Amphibia-Reptilia 1984, 5, 57–68. [Google Scholar] [CrossRef]
- Willemsen, R.E.; Hailey, A. Sexual dimorphism of body size and shell shape in European tortoises. J. Zool. 2003, 260, 353–365. [Google Scholar] [CrossRef]
- Van Damme, R.; Bauwens, D.; Castilla, A.M.; Verheyen, R.F. Altitudinal variation of the thermal biology and running performance in the lizard Podarcis tiliguerta. Oecologia 1989, 80, 516–524. [Google Scholar] [CrossRef]
- Cherchi, M. Termoregulazione in Testudo hermanni. Bolletino Musei Inst. Biol. Univ. Genova 1956, 28, 9–87. [Google Scholar]
- Sears, M.W.; Angilletta, M.J.; Schuler, M.S.; Borchert, J.D.; Dilliplane, K.F.; Stegman, M.; Rusch, T.; Mitchell, W.A. Configuration of the thermal landscape determines thermoregulatory performance of ectotherms. Proc. Natl. Acad. Sci. USA 2016, 113, 10595–10600. [Google Scholar] [CrossRef]
- Troyer, K. Small differences in daytime body temperature affect digestion of natural food in a herbivorous lizard (Iguana iguana). Comp. Biochem. Physiol. 1987, 87A, 623–626. [Google Scholar] [CrossRef]
- Bjorndal, K.A. Fermentation in Reptiles and Amphibians. In Gastrointestinal Microbiology; Springer: Boston, MA, USA, 1997; pp. 199–230. [Google Scholar]
- Niu, C.; Zhang, T.; Sun, R. Food consumption and growth of juvenile Chinese soft-shelled turtles (Pelodiscus sinensis) in relation to body weight and water temperature. Asiat. Herpetol. Res. 1999, 8, 81–84. [Google Scholar]
- Mackie, R.I.; Rycyk, M.; Ruemmler, R.L.; Aminov, R.; Wikelski, M. Biochemical and microbiological evidence for fermentative digestion in free-living land iguanas (Conolophus pallidus) and marine iguanas (Amblyrhynchus cristatus) on the Galapagos archipelago. Physiol. Biochem. Zool. 2004, 77, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Meek, R.; Inskeep, R. Aspects of the field biology of a population of Hermanns tortoise (Testudo hermanni) in southern Yugoslavia. Br. J. Herpetol. 1981, 6, 159–164. [Google Scholar]
- DeWitt, C.B. Precision of thermoregulation and its relation to environmental factors in the desert iguana, Dipsosaurus dorsalis. Physiol. Zool. 1967, 40, 49–66. [Google Scholar] [CrossRef]
- Grant, B.W.; Dunham, A.E. Thermally imposed time constraints on the activity of the desert lizard Sceloporus merriami. Ecology 1988, 69, 167. [Google Scholar] [CrossRef]
- Grant, B.W.; Dunham, A.E. Elevational covariation in environmental constraints and life histories of the desert lizard Sceloporus Merriami. Ecology 1990, 71, 1765–1776. [Google Scholar] [CrossRef]
- Haxhiu, I. Results of studies on the chelonians of Albania. Chelonian Conserv. Biol. 1995, 1, 324–326. [Google Scholar]
- Meek, R. Thermal loads experienced by a nesting female Testudo hermanni. Amphibia-Reptilia 1988, 9, 311–312. [Google Scholar] [CrossRef]
- Basson, C.H.; Levy, O.; Angilletta, M.J.; Clusella-Trullas, S. Lizards paid a greater opportunity cost to thermoregulate in a less heterogeneous environment. Funct. Ecol. 2017, 31, 856–865. [Google Scholar] [CrossRef]
- Hailey, A. Thermoregulation and activity metabolism in the armoured anguid Ophisaurus apodus. Br. J. Herpetol. 1984, 6, 391–398. [Google Scholar]
- Hailey, A.; Theophilidis, G. Cardiac response to stress and activity in the armoured legless lizard (Ophisaurus apodus): Comparison with snake and tortoise. Comp. Biochem. Physiol. 1987, 88, 201–206. [Google Scholar] [CrossRef]
- Meek, R. Field body temperature of the glass lizard Ophisaurus apodus in Yugoslavia. Amphib.-Reptil. 1986, 7, 43–49. [Google Scholar] [CrossRef]
- Christian, K.A.; Bedford, G.S.; Schultz, T.J. Energetic consequences of metabolic depression in tropical and temperate-zone lizards. Aust. J. Zool. 1999, 47, 133–141. [Google Scholar] [CrossRef]
- Rugiero, L.; Luiselli, L. Ecological modelling of habitat use and the annual activity patterns in an urban population of the tortoise, Testudo hermanni. Ital. J. Zool. 2006, 73, 219–225. [Google Scholar] [CrossRef]
- Cherchi, M. Uteriori richerchi sulla termoregulazione in Testudo hermanni. Bolletino Musei Inst. Biol. Univ. Genova 1960, 30, 35–60. (In Italian) [Google Scholar]
- Del Vecchio, S.; Burke, R.L.; Rugiero, L.; Capula, M.; Luiselli, L. The turtle is in the details: Microhabitat choice by Testudo hermanni is based on microscale plant distribution. Anim. Biol. 2011, 61, 249–261. [Google Scholar] [CrossRef]
- Stojadinović, D.; Milošević, Đ.; Sretić, K.; Cvetković, M.; Jovanović, T.; Jovanović, B.; Crnobrnja-Isailović, J.M. Activity patterns and habitat preference of eastern Hermann’s tortoise (Testudo hermanni boettgeri) in Serbia. Turk. J. Zool. 2017, 41, 1036–1044. [Google Scholar] [CrossRef]
- Vujović, A.; Iković, V.; Golubović, A.; Đorđević, S.; Pešić, V.; Tomović, L.J. Effects of fires and roadkills on the isolated population of Testudo hermanni Gmelin, 1789 (Reptilia: Testudinidae) in central Montenegro. Acta Zool. Bulg. 2015, 67, 75–84. [Google Scholar]
- Huey, R.B.; Kearney, M.R.; Krockenberger, A.; Holtum, A.M.; Jess, M.; Williams, S.E. Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2012, 367, 1665–1679. [Google Scholar] [CrossRef]
- Park Williams, A.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang 2013, 3, 292–297. [Google Scholar] [CrossRef]
- Luiselli, L.; Vignoli, L.; Rugiero, L.; Meek, R. Declining occupancy rates in the hibernacula of aspic vipers (Vipera aspis) in Italy and France; evidence for climatic effects? Herpetol. J. 2018, 28, 137–142. [Google Scholar]
- Kearney, M.; Shine, R.; Porter, W.P. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc. Natl. Acad. Sci. USA 2009, 106, 3835–3840. [Google Scholar] [CrossRef] [PubMed]
- Westerling, A.L. Increasing western US forest wildfire activity: Sensitivity to changes in the timing of spring. Phil. Trans. R. Soc. B 2016, 371, 20150178. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).