Using Botanical Gardens as Butterfly Gardens: Insights from a Pilot Project in the Gran Sasso and Monti Della Laga National Park (Italy)
Abstract
1. Introduction
- (1)
- Setting up gardens with suitable host plants can be an effective tool to restore the habitat of some species and thus to mitigate the loss of habitats in agricultural environments [70].
- (2)
- (3)
- Butterfly gardens can be an attraction for the public, as they are perceived as an important opportunity for mental refreshment [73]. Butterfly gardens can therefore play an important social role as recreational spaces.
- (4)
- Butterfly gardens can be an important tool in education programs and to raise awareness of environmental problems [74].
- (5)
- Butterfly gardens may allow people to practice nature photography and other outdoor activities in safe and easy-to-reach areas. Moreover, promoting leisure activities in these spaces may contribute to diverting people away from more natural and fragile contexts.
2. Characteristics of the Selected Site
3. The Butterfly Community of the Garden
4. Actions Performed and Planned
5. Conclusions and Perspectives
- (1)
- There are already numerous species of plants that attract butterflies and that can provide them with food and refuge throughout the year.
- (2)
- There is a small pond from which the butterflies can take water and mineral salts, especially during the most arid period. This is a common situation in botanical gardens that almost always have fountains, ponds, basins for aquatic plants, etc., and may be especially important in regions with dry climates.
- (3)
- The architectural structure of the garden, with many protected places, allows butterflies to be not disturbed by wind. Trees, shrubs, hedges, buildings, or other windbreak structures are commonly found in botanical gardens. Trees, shrubs, and hedges are also important because they provide larvae and pupae with shelter in winter and shade in summer.
- (4)
- The place is already open to the public.
- (5)
- The already existing paths are perfectly suitable for butterfly watching.
- (6)
- No maintenance operations other than those already in place are required. In general, butterfly gardens require professional construction and maintenance techniques, which makes their installation in school gardens difficult, a reason that leads to the proposal of creating virtual butterfly gardens through augmented reality systems [106].
- (1)
- Increase in visibility of the park among the population, given the considerable attraction exerted by butterflies.
- (2)
- Promotion of environmental awareness, scientific dissemination, and citizen science projects, which help to increase positive synergies with local cultural agencies, such as schools and universities.
- (3)
- Promotion of nature observation activities in an accessible and safe context. Natural areas cannot be fully accessible to people with mobility difficulties. A properly equipped butterfly garden can be an important place for social inclusion.
- (4)
- Concentration of tourist activities in an attractive area, but of lesser conservation value within the protected area (this is exactly the case of the San Colombo Botanical Garden, which is immediately out of the protected area). Ecotourism can lead, as a negative consequence, to forms of disturbance. For example, nature photography enthusiasts can be a source of disturbance in the best-preserved sites. A butterfly garden can be useful to “divert” these forms of disturbance from the most valuable contexts to a site specifically designed to encourage nature observation and photography activities, thanks to the considerable variety and abundance of butterflies and the ease with which they can be observed and photographed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MEA. Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; 135p. [Google Scholar]
- Borges, P.A.; Gabriel, R.; Fattorini, S. Biodiversity erosion: Causes and consequences. In Life on Land; Encyclopedia of the UN Sustainable Development Goals; Filho, W.L., Azul, A.M., Brandli, L., Lange Salvia, A., Wall, T., Eds.; Springer: Cham, Switzerland, 2020; pp. 81–90. [Google Scholar]
- Cardoso, P.; Leather, S.R. Predicting a global insect apocalypse. Insect Conserv. Divers. 2019, 12, 263–267. [Google Scholar]
- Habel, J.C.; Gossner, M.M.; Schmitt, T. Just beautiful?! What determines butterfly species for nature conservation. Biodivers. Conserv. 2021, 30, 2481–2493. [Google Scholar] [CrossRef]
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists’ warning to humanity on insect extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Harvey, J.A.; Heinen, R.; Armbrecht, I.; Basset, Y.; Baxter-Gilbert, J.H.; Bezemer, T.M.; Böhm, M.; Bommarco, R.; Borges, P.A.V.; Cardoso, P.; et al. International scientists formulate a roadmap for insect conservation and recovery. Nat. Ecol. Evol. 2020, 4, 174–176. [Google Scholar] [CrossRef]
- Samways, M.J.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Solutions for humanity on how to conserve insects. Biol. Conserv. 2020, 242, 108427. [Google Scholar]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar]
- Tsafack, N.; Fattorini, S.; Boieiro, M.; Rigal, F.; Ros-Prieto, A.; Ferreira, M.T.; Borges, P.A.V. The role of small lowland patches of exotic forests as refuges of rare endemic Azorean arthropods. Diversity 2021, 13, 443. [Google Scholar] [CrossRef]
- Stork, N.E. How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar]
- IPBES. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; 56p. [Google Scholar]
- Dunn, R.R. Modern insect extinctions, the neglected majority. Conserv. Biol. 2005, 19, 1030–1036. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Riddle, B.R.; Whittaker, R.J.; Brown, J.H. Biogeography, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2010; 878p. [Google Scholar]
- Lomolino, M.V. Conservation biogeography. In Frontiers of Biogeography: New Directions in the Geography of Nature; Lomolino, M.V., Heaney, L.R., Eds.; Sinauer Associates: Sunderland, MA, USA, 2004; pp. 293–296. [Google Scholar]
- Whittaker, R.J.; Araujo, M.B.; Jepson, P.; Ladle, R.J.; Watson, J.E.M.; Willis, K.J. Conservation biogeography: Assessment and prospect. Divers. Distrib. 2005, 11, 3–23. [Google Scholar] [CrossRef]
- Possingham, H.P.; Grantham, H.; Rondinini, C. How can you conserve species that haven’t been found? J. Biogeogr. 2007, 34, 758–759. [Google Scholar] [CrossRef]
- Cardoso, P.; Erwin, T.L.; Borges, P.A.V.; New, T.R. The seven impediments in invertebrate conservation and how to overcome them. Biol. Conserv. 2011, 144, 2647–2655. [Google Scholar]
- Weeks, P.J.D.; Gaston, K.J. Image analysis, neural networks, and the taxonomic impediment to biodiversity studies. Biodivers. Conserv. 1997, 6, 263–274. [Google Scholar]
- Arbuckle, T.; Schröder, S.; Steinhage, V.; Wittmann, D. Biodiversity informatics in action: Identification and monitoring of bee species using ABIS. In Proceedings of the 15th International Symposium Informatics for Environmental Protection; ETH: Zürich, Switzerland, 2001; pp. 425–430. [Google Scholar]
- Martineau, M.; Conte, D.; Raveaux, R.; Arnault, I.; Munier, D.; Venturini, G. A survey on image-based insect classification. Pattern Recognit. 2017, 65, 273–284. [Google Scholar]
- Valan, M.; Makonyi, K.; Maki, A.; Vondráček, D.; Ronquist, F. Automated taxonomic identification of insects with expert-level accuracy using effective feature transfer from convolutional networks. Syst. Biol. 2019, 68, 876–895. [Google Scholar] [PubMed]
- Brito, D. Overcoming the Linnean shortfall: Data deficiency and biological survey priorities. Basic Appl. Ecol. 2010, 11, 709–713. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2022-1. Available online: https://www.iucnredlist.org (accessed on 29 November 2022).
- Van Klink, R.; August, T.; Bas, Y.; Bodesheim, P.; Bonn, A.; Fossoy, F.; Hoye, T.T.; Jongejans, E.; Menz, M.H.M.; Miraldo, A.; et al. Emerging technologies revolutionise insect ecology and monitoring. Trends Ecol. Evol. 2022, 37, 872–885. [Google Scholar] [CrossRef]
- Bini, L.M.; Diniz-Filho, J.A.F.; Rangel, T.F.L.V.B.; Bastos, R.P.; Pinto, M.P. Challenging Wallacean and Linnean shortfalls: Knowledge gradients and conservation planning in a biodiversity hotspot. Divers. Distrib. 2006, 12, 475–482. [Google Scholar]
- Hortal, J.; de Bello, F.; Diniz-Filho, J.A.F.; Lewinsohn, T.M.; Lobo, J.M.; Ladle, R.J. Seven shortfalls that beset large scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 523–549. [Google Scholar] [CrossRef]
- Sanderson, C.; Braby, M.F.; Bond, S. Butterflies Australia: A national citizen science database for monitoring changes in the distribution and abundance of Australian butterflies. Austral Entomol. 2021, 60, 111–127. [Google Scholar]
- Barua, M.; Gurdak, D.J.; Ahmed, R.A.; Tamuly, J. Selecting flagships for invertebrate conservation. Biodivers. Conserv. 2012, 21, 1457–1476. [Google Scholar] [CrossRef]
- Lorenz, A.R.; Libarkin, J.C.; Ording, G.J. Disgust in response to some arthropods aligns with disgust provoked by pathogens. Glob. Ecol. Conserv. 2014, 2, 248–254. [Google Scholar] [CrossRef]
- Govorushko, S. Human-Insect Interactions; CRC Press: Boca Raton, FI, USA, 2018; 442p. [Google Scholar]
- Sumner, S.; Law, G.; Cini, A. Why we love bees and hate wasps. Ecol. Entomol. 2018, 43, 836–845. [Google Scholar] [CrossRef]
- Bowen-Jones, E.; Entwistle, A. Identifying appropriate flagship species: The importance of culture and local contexts. Oryx 2002, 36, 189–195. [Google Scholar]
- Clucas, B.; McHugh, K.; Caro, T. Flagship species on covers of US conservation and nature magazines. Biodivers. Conserv. 2008, 17, 1517–1528. [Google Scholar]
- Entwistle, A. Flagships for the future? Oryx 2000, 34, 239–240. [Google Scholar] [CrossRef]
- Veríssimo, D.; Fraser, I.; Groombridge, J.; Bristol, R.; MacMillan, D.C. Birds as tourism flagship species: A case study of tropical islands. Anim. Conserv. 2009, 12, 549–558. [Google Scholar]
- Schlegel, J.; Breuer, G.; Rupf, R. Local insects as flagship species to promote nature conservation? A survey among primary school children on their attitudes toward invertebrates. Anthrozoös 2015, 28, 229–245. [Google Scholar]
- Qian, J.; Zhuang, H.; Yang, W.; Chen, Y.; Chen, S.; Qu, Y.; Zhang, Y.; Yang, Y.; Wang, Y. Selecting flagship species to solve a biodiversity conservation conundrum. Plant Divers. 2020, 42, 488–491. [Google Scholar]
- Preston, S.D.; Liao, J.D.; Toombs, T.P.; Romero-Canyas, R.; Speiser, J.; Seifert, C.M. A case study of a conservation flagship species: The monarch butterfly. Biodivers. Conserv. 2021, 30, 2057–2207. [Google Scholar] [CrossRef]
- Berenbaum, M. Insect conservation and the Entomological Society of America. Am. Entomol. 2008, 54, 117–120. [Google Scholar] [CrossRef]
- Vane-Wright, R.I. Butterflies, worldviews, biodiversity, general systems theory, and taxonomy. In Report on Insect Inventory Project in Tropical Asia (TAIIV); Yata, O., Ed.; Kyushu University: Fukuoka, Japan, 2008; pp. 1–20. [Google Scholar]
- Oberhauser, K.; Guiney, M. Insects as flagship conservation species. Terr. Arthropod Rev. 2009, 1, 111–123. [Google Scholar] [CrossRef]
- Kakehashi, E.; Muramatsu, K.; Hibino, H. Computational color combination analysis of Papilionidae butterflies as aesthetic objects. Color Res. Appl. 2020, 45, 65–84. [Google Scholar] [CrossRef]
- Smart, P. The Illustrated Encyclopedia of the Butterfly World; Chartwell Books: London, UK, 1977; 274p. [Google Scholar]
- Nazari, V. Chasing butterflies in medieval Europe. J. Lepid. Soc. 2014, 68, 223–231. [Google Scholar]
- Kritsky, G.; Smith, J.J. Insect biodiversity in culture and art. In Insect Biodiversity, Science and Society; Foottit, R.G., Adler, P.H., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2018; Volume 2, pp. 869–898. [Google Scholar]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar]
- Agrawal, A.A. Monarchs and Milkweed: A Migrating Butterfly, a Poisonous Plant, and Their Remarkable Story of Coevolution; Princeton University Press: Princeton, NJ, USA, 2017; 296p. [Google Scholar]
- Chowdhury, S.; Fuller, R.A.; Dingle, H.; Chapman, J.W.; Zalucki, M.P. Migration in butterflies: A global overview. Biol Rev. 2021, 96, 1462–1483. [Google Scholar] [CrossRef]
- Urquhart, F.A.; Urquhart, N.R. The overwintering site of the eastern population of the monarch butterfly (Danaus plexippus; Danaidae) in southern Mexico. J. Lepid. Soc. 1976, 30, 53–158. [Google Scholar]
- Gustafsson, K.M.; Agrawal, A.A.; Lewenstein, B.V.; Wolf, S.A. The Monarch butterfly through time and space: The social construction of an icon. Bioscience 2015, 65, 612–622. [Google Scholar]
- Van Tongeren, E.; Sistri, G.; Zingaro, V.; Cini, A.; Dapporto, L.; Portera, M. Assessing the Aesthetic Attractivity of European Butterflies: A Web-Based Survey Protocol. Available online: https://ecoevorxiv.org/repository/view/4655/ (accessed on 29 November 2022).
- Ajilvsgi, G. Butterfly Gardening for Texas; Louise Lindsey Merrick Natural Environment Series Book 46; Texas A&M University Press: College Station, TX, USA, 2013; 448p. [Google Scholar]
- Kline, C. Butterfly Gardening with Native Plants: How to Attract and Identify Butterflies; Simon and Schuster: New York, NY, USA, 2015; 144p. [Google Scholar]
- Samuels, T.M. Butterfly Gardening Using Southern Native Plants; CreateSpace: Scotts Valley, CA, USA, 2015; 312p. [Google Scholar]
- Steel, J. Butterfly Gardening: How to Encourage Butterflies to Your Garden (Gardening with Nature Series); Brambleby Books: Taunton, MA, USA, 2015; 80p. [Google Scholar]
- Tekulsky, M. The Art of Butterfly Gardening: How to Make Your Backyard into a Beautiful Home for Butterflies; Skyhorse: New York, NY, USA, 2015; 208p. [Google Scholar]
- Xerces Society. Gardening for Butterflies: How You Can Attract and Protect Beautiful, Beneficial Insects; Timber Press: Portland, OR, USA, 2016; 288p. [Google Scholar]
- Hurwitz, J. Butterfly Gardening: The North American Butterfly Association Guide; Princeton University Press: Princeton, NJ, USA, 2018; 288p. [Google Scholar]
- McAtee, J.M. Florida Gardening for Butterflies; CreateSpace: Scotts Valley, CA, USA, 2018; 126p. [Google Scholar]
- Dziedzic, B. Raising Butterflies in the Garden; Firefly Books: Buffalo, NY, USA, 2019; 336p. [Google Scholar]
- Butterfly Gardening. Available online: https://www.thebutterflysite.com/gardening.shtml (accessed on 29 November 2022).
- How to Make a Butterfly Garden. Available online: https://www.thespruce.com/how-to-make-a-butterfly-garden-4427931 (accessed on 29 November 2022).
- Butterfly Gardening and Habitat Program. Available online: https://nababutterfly.com/start-butterfly-garden/ (accessed on 29 November 2022).
- Butterfly Gardening. Available online: https://butterflywebsite.com/butterflygardening.cfm (accessed on 29 November 2022).
- Gardening. Available online: https://butterfly-conservation.org/how-you-can-help/get-involved/gardening (accessed on 29 November 2022).
- How to Make Butterfly Gardens. Available online: https://entomology.ca.uky.edu/ef006 (accessed on 29 November 2022).
- Butterfly Gardening. Available online: https://www.monarchwatch.org/garden/ (accessed on 29 November 2022).
- Butterfly Gardening. Available online: https://www.missouribotanicalgarden.org/visit/family-of-attractions/butterfly-house/butterflies-and-plants/butterfly-gardening.aspx (accessed on 29 November 2022).
- Butterfly Gardening. Available online: https://agrilifeextension.tamu.edu/solutions/butterfly-gardening/ (accessed on 29 November 2022).
- Cutting, B.T.; Tallamy, D.W. An evaluation of butterfly gardens for restoring habitat for the Monarch butterfly (Lepidoptera: Danaidae). Environ. Entomol. 2015, 44, 1328–1335. [Google Scholar] [PubMed]
- Mathew, G.; Anto, M. In situ conservation of butterflies through establishment of butterfly gardens: A case study at Peechi, Kerala, India. Curr. Sci. 2007, 93, 337–347. [Google Scholar]
- Penn, J.; Penn, H.; Hu, W. Public knowledge of Monarchs and support for butterfly conservation. Sustainability 2018, 10, 807. [Google Scholar] [CrossRef]
- Pals, R.; Steg, L.; Siero, F.W.; van der Zee, K.I. Development of the PRCQ: A measure of perceived restorative characteristics of zoo attractions. J. Environ. Psychol. 2009, 29, 441–449. [Google Scholar]
- Clayburn, J.; Koptur, S.; O’Brien, G.; Whelan, K.R.T. The Schaus Swallowtail habitat enhancement project: An applied service-learning project continuum from Biscayne National Park to Miami-Dade county public schools. Southeast. Nat. 2017, 16, 26–46. [Google Scholar]
- Mathew, G.; George, E.; Anto, M. Role of butterfly gardens in promoting biodiversity conservation. In ENVIS Bulletin: Arthropods and Their Conservation in India (Insects & Spiders); Wildlife Institute of India: Dehradun, India, 2011; Volume 14, pp. 87–97. [Google Scholar]
- Stewart, A.B.; Sritongchuay, T.; Teartisup, P.; Kaewsomboon, S.; Bumrungsri, S. Habitat and landscape factors influence pollinators in a tropical megacity, Bangkok, Thailand. PeerJ 2018, 6, e5335l. [Google Scholar] [CrossRef]
- Giovanetti, M.; Giuliani, C.; Boff, S.; Fico, G.; Lupi, D. A botanic garden as a tool to combine public perception of nature and life-science investigations on native/exotic plants interactions with local pollinators. PLoS ONE 2020, 15, e0228965. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.S. Botanic gardens science for conservation and global change. Trends Plant Sci. 2009, 14, 608–613. [Google Scholar]
- Baldock, K.C.R.; Goddard, M.A.; Hicks, D.M.; Kunin, W.E.; Mitschunas, N.; Morse, H.; Osgathorpe, L.M.; Potts, S.G.; Robertson, K.M.; Scott, A.V.; et al. A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat. Ecol. Evol. 2019, 3, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Prudic, K.L.; Cruz, T.M.P.; Winzer, J.I.B.; Oliver, J.C.; Melkonoff, N.A.; Verbais, H.; Hogan, A. Botanical gardens are local hotspots for urban butterflies in arid environments. Insects 2022, 13, 865. [Google Scholar] [PubMed]
- Samways, M.J. Insect Conservation: A Global Synthesis; CABI: Wallingford, UK, 2020; 540p. [Google Scholar]
- Chowdhury, S.; Jennions, M.D.; Zalucki, M.P.; Maron, M.; Watson, J.E.M.; Fuller, R.A. Protected areas and the future of insect conservation. Trends Ecol. Evol. 2023, 38, 85–95. [Google Scholar]
- Govorushko, S.M.; Nowicki, P. Lessons from insect conservation in Russia. J. Insect Conserv. 2019, 23, 1–14. [Google Scholar] [CrossRef]
- Wang, W.-L.; Suman, D.O.; Zhang, H.-H.; Xu, Z.-B.; Ma, F.-Z.; Hu, S.-J. Butterfly conservation in China: From science to action. Insects 2020, 11, 661. [Google Scholar] [PubMed]
- Romo, H.; Munguira, M.L.; García-Barros, E. Area selection for the conservation of butterflies in the Iberian Peninsula and Balearic Islands. Anim. Biodivers. Conserv. 2007, 30, 7–27. [Google Scholar]
- Fajardo, J.; Lessmann, J.; Bonaccorso, E.; Devenish, C.; Muñoz, J. Combined use of systematic conservation planning, species distribution modelling, and connectivity analysis reveals severe conservation gaps in a megadiverse country (Peru). PLoS ONE 2014, 9, e114367. [Google Scholar] [CrossRef]
- Girardello, M.; Griggio, M.; Whittingham, M.J.; Rushton, S.P. Identifying important areas for butterfly conservation in Italy. Anim. Conserv. 2009, 12, 20–28. [Google Scholar] [CrossRef]
- Sistri, G.; Menchetti, M.; Santini, L.; Pasquali, L.; Sapienti, S.; Cini, A.; Platania, L.; Balletto, E.; Barbero, F.; Bonelli, S.; et al. The isolated Erebia pandrose Apennine population is genetically unique and endangered by climate change. Insect Conserv. Divers. 2022, 15, 136–148. [Google Scholar] [CrossRef]
- Bonifacino, M.; Pasquali, L.; Sistri, G.; Menchetti, M.; Santini, L.; Corbella, C.; Bonelli, S.; Balletto, E.; Vila, R.; Dincă, V.; et al. Climate change may cause the extinction of the butterfly Lasiommata petropolitana in the Apennines. J. Insect Conserv. 2022, 26, 959–972. [Google Scholar]
- QGIS Development Team, 2009. QGIS Geographic Information System. Open Source Geospatial Foundation. Available online: http://qgis.org (accessed on 17 December 2022).
- Pollard, E.; Yates, T.J. Monitoring Butterflies for Ecology and Conservation; Chapman & Hall: London, UK, 1993; 274p. [Google Scholar]
- European Butterfly Monitoring Scheme—eBMS. Available online: https://butterfly-monitoring.net/ (accessed on 29 November 2022).
- Balletto, E.; Cassulo, L.-A.; Bonelli, S. An annotated checklist of the Italian butterflies and skippers (Papilionoidea, Hesperiioidea). Zootaxa 2014, 3853, 1–114. [Google Scholar]
- Dapporto, L.; Menchetti, M.; Vodă, R.; Corbella, C.; Cuvelier, S.; Djemadi, I.; Gascoigne-Pees, M.; Hinojosa, J.C.; Ting Lam, N.; Serracanta, M.; et al. The atlas of mitochondrial genetic diversity for Western Palaearctic butterflies. Glob. Ecol. Biogeog. 2022, 31, 2184–2190. [Google Scholar]
- Conti, F.; Bartolucci, F.; Tinti, D.; Manzi, A. Guida fotografica alle piante del Parco Nazionale del Gran Sasso e Monti della Laga. Compendio della flora vascolare; Ente Parco Nazionale del Gran Sasso e Monti della Laga: Assergi, Italy, 2018; 934p. [Google Scholar]
- Acta Plantarum. Available online: https://www.actaplantarum.org/ (accessed on 21 December 2022).
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 31 August 2021).
- Balletto, E.; Bonelli, S.; Barbero, F.; Casacci, L.P.; Sbordoni, V.; Dapporto, L.; Scalercio, S.; Zilli, A.; Battistoni, A.; Teofili, C.; et al. Lista Rossa IUCN delle Farfalle Italiane—Ropaloceri; Comitato Italiano IUCNe Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Roma, Italy, 2015; 45p. [Google Scholar]
- Majewska, A.A.; Altizer, S. Planting gardens to support insect pollinators. Conserv. Biol. 2020, 34, 15–25. [Google Scholar] [CrossRef]
- Kurylo, J.S.; Threlfall, C.G.; Parris, K.M.; Ossola, A.; Williams, N.S.G.; Evans, K.L. Butterfly richness and abundance along a gradient of imperviousness and the importance of matrix quality. Ecol. Appl. 2020, 30, e02144. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, D.; Dietz, T.; Rockwood, L.R. Determining the effect of urbanization on generalist butterfly species diversity in butterfly gardens. Urban Ecosyst. 2007, 10, 427–439. [Google Scholar]
- Scali, V. Imaginal diapause and gonadal maturation of Maniola jurtina (Lepidoptera: Satyridae) from Tuscany. J. Anim. Ecol. 1971, 40, 467–472. [Google Scholar]
- EC Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. L. 1992, 206, pp. 7–50. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31992L0043 (accessed on 19 December 2022).
- Bryant, S.; Thomas, C.; Bale, J. Nettle-feeding nymphalid butterflies: Temperature, development and distribution. Ecol. Entomol. 1997, 22, 390–398. [Google Scholar] [CrossRef]
- Tarng, W.; Ou, K.-L.; Yu, C.-S.; Liou, F.-L.; Liou, H.-H. Development of a virtual butterfly ecological system based on augmented reality and mobile learning technologies. Virtual Real. 2015, 19, 253–266. [Google Scholar]
- Fattorini, S.; Mantoni, C.; De Simoni, L.; Galassi, D.M.P. Island biogeography of insect conservation in urban green spaces. Environ. Conserv. 2018, 45, 1–10. [Google Scholar]
- Fattorini, S. Ecologia Urbana; Ediesse: Roma, Italy, 2019; 304p. [Google Scholar]
- Levy, J.M.; Connor, E.F. Are gardens effective in butterfly conservation? A case study with the pipevine swallowtail, Battus philenor. J. Insect Conserv. 2004, 8, 323–330. [Google Scholar]
- Mathew, G. Butterfly gardens and ecotourisms. In Ecotourism Development and Management; Hosetti, B.B., Ed.; Pointer Publishers: Jaipur, India, 2007; pp. 172–177. [Google Scholar]
- iNaturalist. Available online: https://www.inaturalist.org/ (accessed on 29 November 2021).
- Dorward, L.J.; Mittermeier, J.C.; Sandbrook, C.; Spooner, F. Pokémon Go: Benefits, costs, and lessons for the conservation movement. Conserv. Lett. 2017, 10, 160–165. [Google Scholar] [CrossRef]
- Fattorini, S.; Gabriel, R.; Arroz, A.M.; Amorim, I.R.; Borges, P.A.V.; Cafaro, P. Children’s preferences for less diverse greenspaces do not disprove biophilia. Proc. Natl. Acad. Sci. USA 2017, 114, E7215. [Google Scholar]
- Balmford, A.; Clegg, L.; Coulson, T.; Taylor, J. Why conservationists should heed Pokémon. Science 2002, 295, 2367. [Google Scholar] [CrossRef]
- Monarchs Need Milkweed. Available online: https://www.saveourmonarchs.org/why-milkweed.html (accessed on 29 November 2021).
- Milkweed for Monarchs. Available online: https://www.nwf.org/Garden-for-Wildlife/About/Native-Plants/Milkweed (accessed on 29 November 2021).
- About Milkweed and Monarchs. Available online: https://www.naturewatch.ca/milkweedwatch/about-milkweed-and-monarchs/ (accessed on 29 November 2021).
- Il Giardino delle Farfalle. Available online: https://giardinofarfalle.it/chi-siamo-1 (accessed on 20 January 2023).
- Un Giardino delle Farfalle All’Oasi Le Cesine. Available online: https://www.wwf.it/pandanews/animali/curiosita/un-giardino-delle-farfalle-alloasi-le-cesine/ (accessed on 20 January 2023).
- Il Giardino delle Farfalle. Available online: https://www.oasilipumassaciuccoli.org/il-giardino-delle-farfalle/ (accessed on 20 January 2023).
- Il Parco. Available online: https://vulci.it/il-parco/ (accessed on 20 January 2023).
- Giardino delle Farfalle. Available online: https://www.comune.cremona.it/giardino-delle-farfalle (accessed on 20 January 2023).
- Goals & Objectives. Available online: https://www.life4pollinators.eu/en/Goals%20%26%20Objectives (accessed on 20 January 2023).
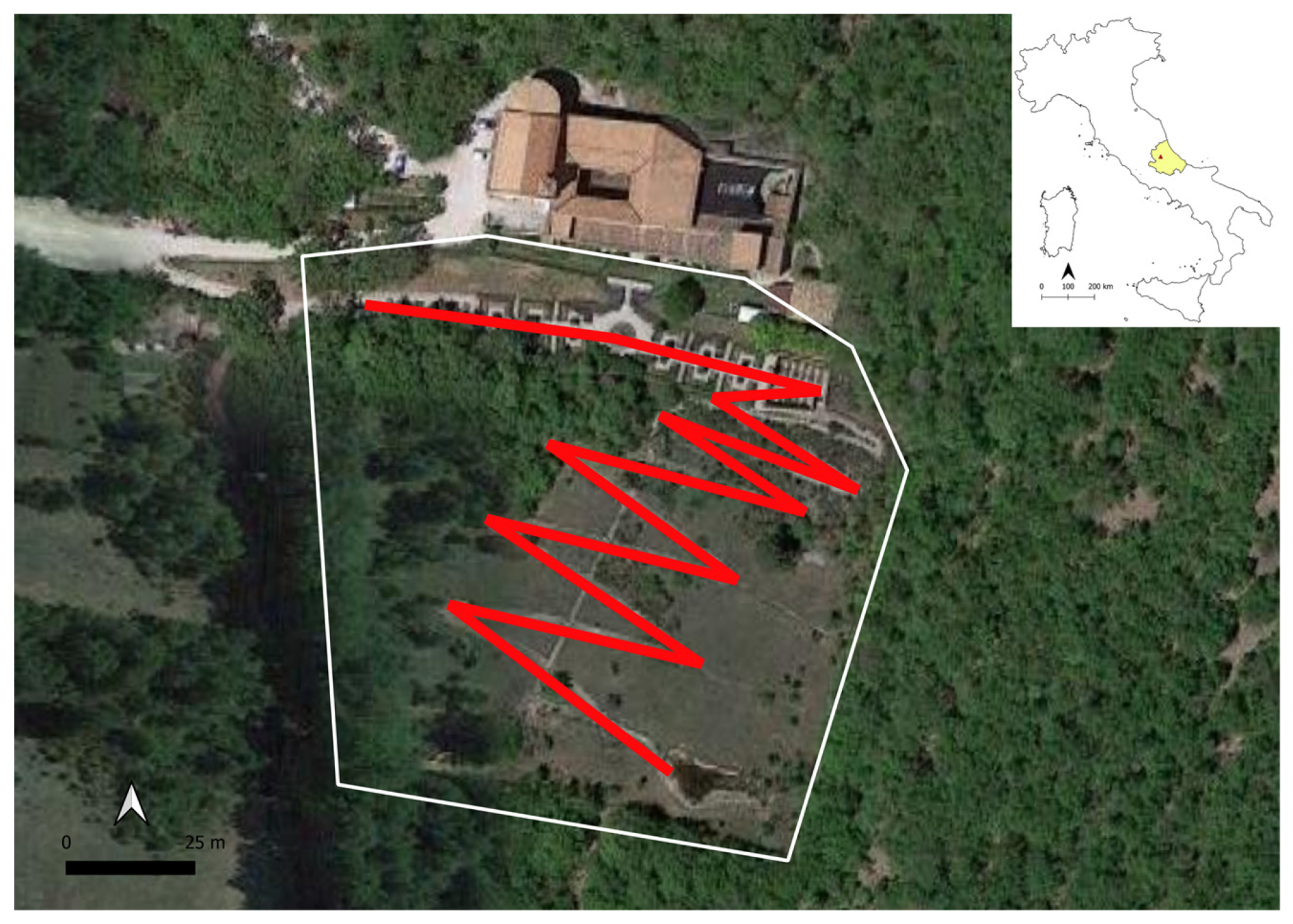

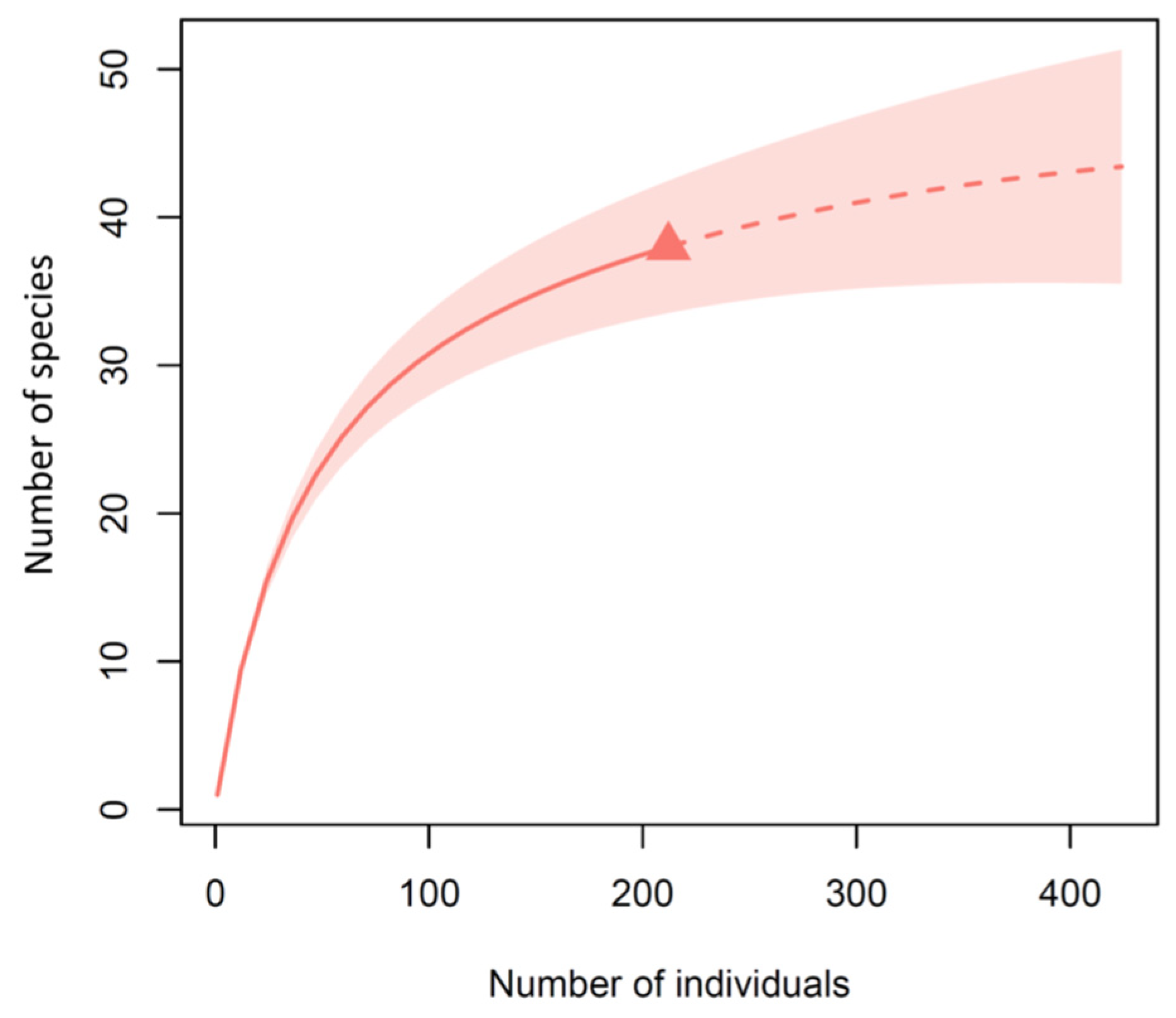

| Species | Number of Observed Individuals |
|---|---|
| Argynnis paphia (Linné, 1758) | 10 |
| Aricia agestis (Denis & Schiffermüller, 1775) | 7 |
| Brintesia circe (Fabricius, 1775) | 5 |
| Callophrys rubi (Linné, 1758) | 1 |
| Coenonympha pamphilus (Linné, 1758) | 2 |
| Colias crocea (Geoffroy, 1785) | 3 |
| Cupido minimus (Fuessly, 1775) | 2 |
| Cyaniris semiargus (Rottemburg, 1775) | 2 |
| Fabriciana niobe (Linné, 1758) | 1 |
| Gonepteryx cleopatra (Linné, 1767) | 2 |
| Gonepteryx rhamni (Linné, 1758) | 3 |
| Hipparchia semele (Linné, 1758) | 1 |
| Hyponephele lycaon (Küns, 1774) | 5 |
| Iphiclides podalirius (Linné, 1758) | 9 |
| Issoria lathonia (Linné, 1758) | 1 |
| Lampides boeticus (Linné, 1767) | 1 |
| Lasiommata maera (Linné, 1758) | 3 |
| Lasiommata megera (Linné, 1767) | 1 |
| Leptidea sinapis complex | 3 |
| Limenitis reducta Staudinger, 1901 | 8 |
| Maniola jurtina (Linné, 1758) | 9 |
| Melanargia galathea (Linné, 1758) | 24 |
| Melitaea didyma (Esper, 1778) | 6 |
| Melitaea phoebe (Denis & Schiffermüller, 1775) * | 1 |
| Ochlodes sylvanus (Esper, 1777) | 8 |
| Papilio machaon Linné, 1758 * | 1 |
| Pieris brassicae (Linné, 1758) | 15 |
| Pieris ergane (Geyer, 1828) | 5 |
| Pieris napi (Linné, 1758) | 5 |
| Pieris rapae (Linné, 1758) | 9 |
| Polygonia c-album (Linné, 1758) | 1 |
| Polyommatus dolus virgilius (Oberthür, 1910) | 25 |
| Polyommatus eros (Ochsenheimer, 1808) | 1 |
| Polyommatus icarus (Rottemburg, 1775) | 4 |
| Polyommatus thersites (Cantener, 1835) | 5 |
| Pontia edusa (Fabricius, 1777) | 1 |
| Satyrium ilicis (Esper, 1779) | 12 |
| Satyrus ferula (Fabricius, 1793) | 2 |
| Thymelicus sp. | 3 |
| Vanessa cardui (Linné, 1758) | 7 |
| Total | 214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattorini, S.; Mantoni, C.; Dapporto, L.; Davini, G.; Di Biase, L. Using Botanical Gardens as Butterfly Gardens: Insights from a Pilot Project in the Gran Sasso and Monti Della Laga National Park (Italy). Conservation 2023, 3, 109-126. https://doi.org/10.3390/conservation3010010
Fattorini S, Mantoni C, Dapporto L, Davini G, Di Biase L. Using Botanical Gardens as Butterfly Gardens: Insights from a Pilot Project in the Gran Sasso and Monti Della Laga National Park (Italy). Conservation. 2023; 3(1):109-126. https://doi.org/10.3390/conservation3010010
Chicago/Turabian StyleFattorini, Simone, Cristina Mantoni, Leonardo Dapporto, Giorgio Davini, and Letizia Di Biase. 2023. "Using Botanical Gardens as Butterfly Gardens: Insights from a Pilot Project in the Gran Sasso and Monti Della Laga National Park (Italy)" Conservation 3, no. 1: 109-126. https://doi.org/10.3390/conservation3010010
APA StyleFattorini, S., Mantoni, C., Dapporto, L., Davini, G., & Di Biase, L. (2023). Using Botanical Gardens as Butterfly Gardens: Insights from a Pilot Project in the Gran Sasso and Monti Della Laga National Park (Italy). Conservation, 3(1), 109-126. https://doi.org/10.3390/conservation3010010







