Abstract
Reducing the large amounts of carbon dioxide emitted during cement processing is crucial to control the adverse effects of greenhouse gases. This study provides a promising alternative technology to reduce such carbon dioxide emissions and investigate physical and mechanical characteristics of alkali-activated materials with rice husk ash (RHA). To this end, compressive strength, drying shrinkage, and water penetration resistance of mortar made with RHA, blast furnace slag (BFS), and alkaline activator (sodium carbonate, NC) are investigated. Two RHA particle sizes of 45 and 150 µm types are used, thereby varying the RHA replacement ratio of 0, 7.5, 15.0 wt.%. Based on adiabatic hydration temperature, Archimedes porosity, pH, ignition loss, scanning electron microscopy, and energy-dispersive X-ray spectroscopy and X-ray diffraction results of paste, the effect of RHA on mechanical characteristics is examined. Experimental investigation reveals that compressive strengths of mortar sample made with the RHA replacement ratio of 15 wt.% to BFS were recorded between 48 and 51 MPa. When the RHA replacement ratio of 15 wt.% 150 µm was used, the length change was 1147 × 10−6 and the moisture penetration depth was less than 11 mm. Notably, water penetration resistance significantly improves with increasing RHA content; however, at high replacement ratios, the particle-size effect is not prominent. Furthermore, increasing the RHA replacement ratio decreases the porosity but increases the ignition loss and produces C-S-H gel.
1. Introduction
Cement used in construction and civil engineering structures emits a significant amount of CO2 during the manufacturing process, accounting for approximately 8% of global CO2 emissions [1]. Therefore, identifying a material that can replace cement and reduce greenhouse gases is an urgent task [2,3].
BFS and fly ash, which are produced as industrial by-products, do not undergo hydration reactions on their own but react with alkaline activators such as calcium hydroxide, caustic soda, and sodium silicate. If used alone or in combination, the amount of cement required can be actively reduced. Therefore, alkaline-activated materials have recently been highlighted as substitutes for cement [4,5].
RHA is an agricultural by-product produced in large quantities worldwide and contains carbon, silica, and low contents of alkaline components [6,7]. Additionally, it could be used to produce high-purity amorphous silica by performing appropriate washing and calcination processes [8,9,10].
From the construction materials perspective, the amorphous silica present in RHA hydrates much faster than commonly used BFS and fly ash; therefore, it is considered an attractive material for actively utilizing this pozzolanic reaction. Recently, several studies have reported the effective utilization of RHA, including soil stabilization [11,12,13,14], supplementary cementitious material (SCMs) and filler [15,16,17,18,19,20,21,22,23,24], geopolymer composite [25,26,27,28], phase-changing material (PCM) [29,30,31], zeolite [32], masonry block [33], and glass filler [34,35] for polymer [36].
As a characteristic of cement containing RHA, if the replacement ratio of cement with RHA is high, the initial fluidity decreases, which is reported to be a disadvantage of RHA. However, if cement was replaced by RHA that is less than 30 wt.%, along with the use of a high-performance water-reducing agent, the compressive strength increases, and drying shrinkage is reduced [37]. It can be concluded that pore structure is densified and durability is improved by the hydration of calcium silicate generated by the pozzolanic reaction from the silica contained in the RHA [38]. Thus, by actively promoting the pozzolanic reaction of RHA and using it as a substitute for cement, it is possible to create a construction material with high durability.
Meanwhile, the authors conducted a study on the durability of alkali-activated binder using BFS as the binder and NC as the alkaline activator [39]. In the previous papers of previous authors, a compressive strength of 50 MPa or more was recorded when RHA was replaced with BFS [40]. However, it is considered that high strength does not necessarily guarantee high durability. In addition to compressive strength, it is reported that the decrease in dimensional stability and water permeability [39] indicates that water easily evaporates and penetrates, and the decrease in durability due to rapid water movement leads to a shortened life of long-term building structures. Therefore, if the performance shortcomings of the alkali-activated binder are not overcome, its applicability in future engineering applications will be limited. Additionally, there have been few studies on the effect of RHA quality on the durability of alkaline-activated materials made of NC and BFS, such as compressive strength development, drying shrinkage characteristics, and water-penetration resistance. It is unclear whether the replacement ratio and particle size of RHA positively affect the durability of these alkaline-active materials.
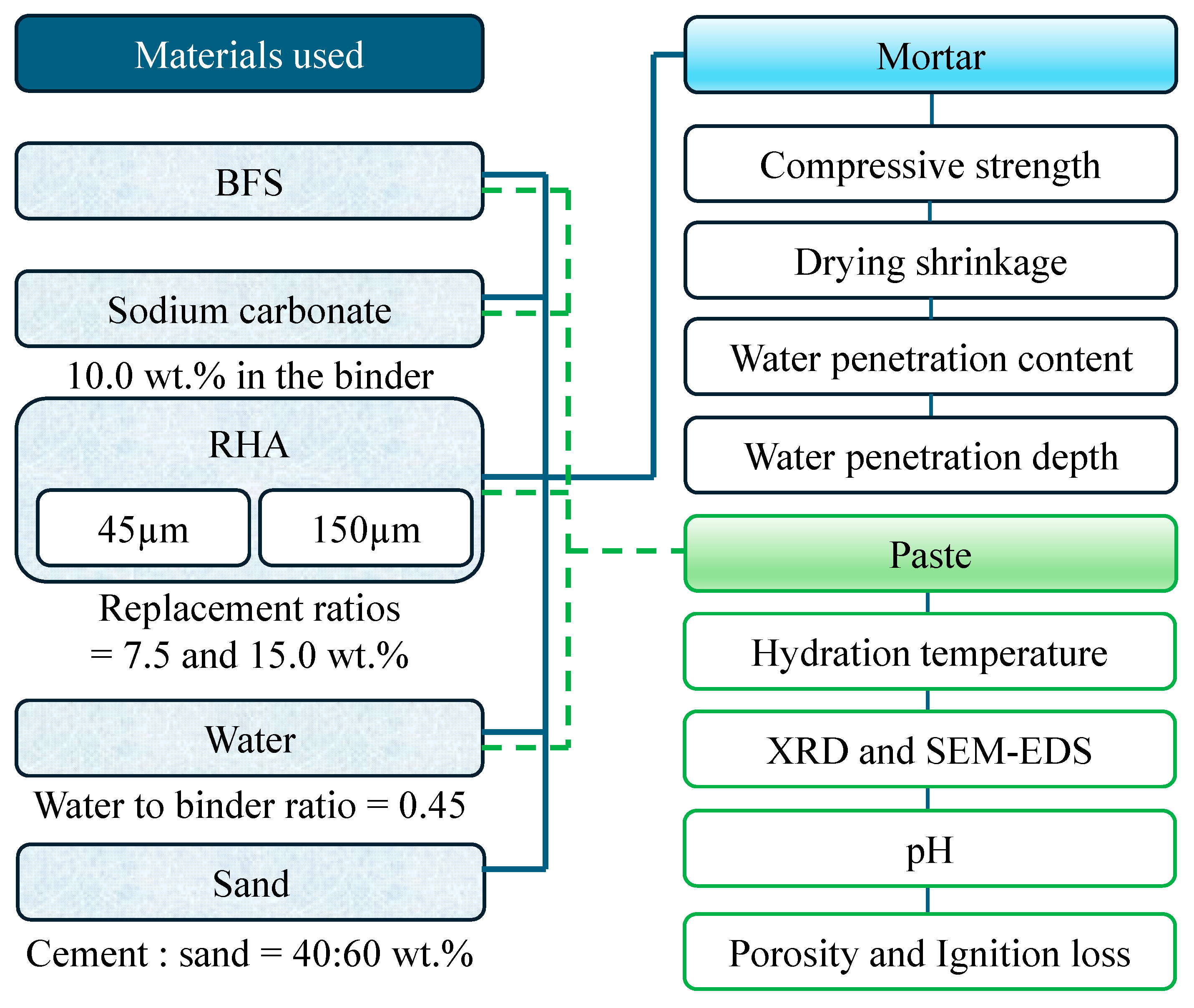
In this study, the compressive strength, drying shrinkage, and water-penetration resistance of mortar incorporating RHA and BFS, as binders, and NC, as the alkaline activator, were investigated. The detailed test procedure can be seen Figure 1. Two RHA particle sizes and three levels of RHA replacement ratios were used in the BFS. The effect of RHA factors on compressive strength development, drying shrinkage, and water penetration depth were investigated using mortar-mixed silica sand. In addition, hydration temperature, Archimedes porosity, pH, ignition loss, and X-ray diffraction, scanning electron microscopy, and energy-dispersive X-ray spectroscopy analyses (SEM–EDS) using paste were performed. This study provides valuable insight for expanding the scope of engineering to actively utilize agricultural and industrial by-products, such as RHA and BFS, as construction materials to reduce greenhouse gas emissions.
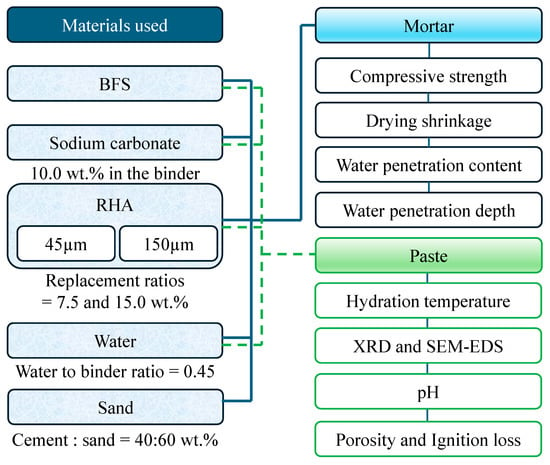
Figure 1.
Experimental plan.
2. Materials and Methods
2.1. Materials Used
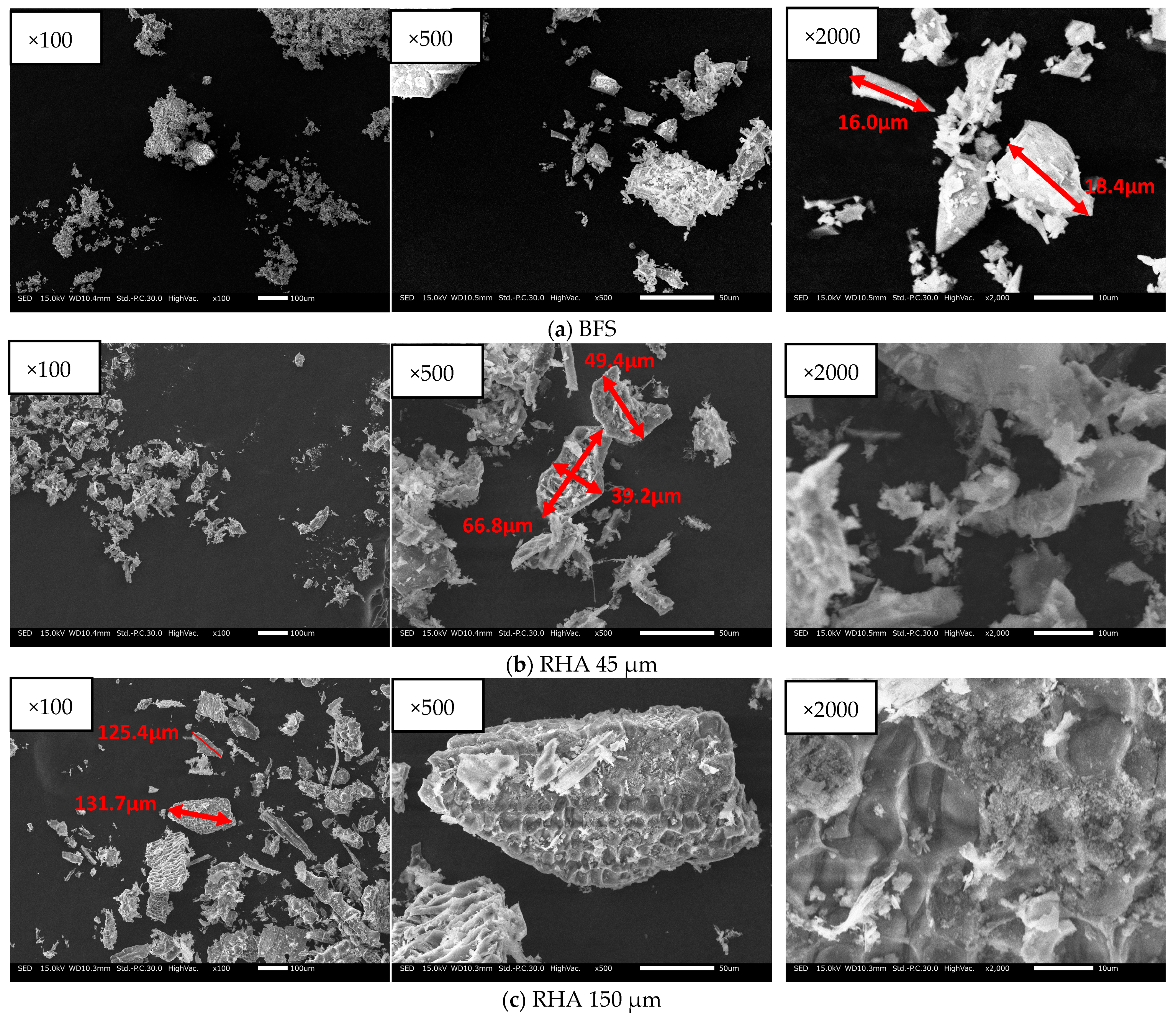
BFS is a by-product of iron and the steel-making process, and RHA is a silica-rich agricultural by-product that has removed carbon. In the experiments, BFS was used; Blaine fineness: 4340 cm2/g; SO3 content: 2.14%; density: 2.89 g/cm3. SEM and SEM–EDS analysis and XRD results of each material with BFS, and 45 µm and 150 µm RHA are show in Figure 2, Figure 3 and Figure 4. As shown in Figure 1, the BFS used in this study is distributed with particles of approximately 18.4 µm or less and has a slightly angular shape. RHA 45 is composed of particles of 66.8 µm or less and many small particles are also distributed. As shown in Figure 2b, RHA 45 µm (×2000), it is confirmed that there are many pores within the particles of the RHA. RHA 150 is composed of particles of 131 µm or less and many small particles are also distributed. As given in Figure 2c, RHA 150 µm (×2000), it is confirmed that the surface of the RHA is made in a honeycomb shape.
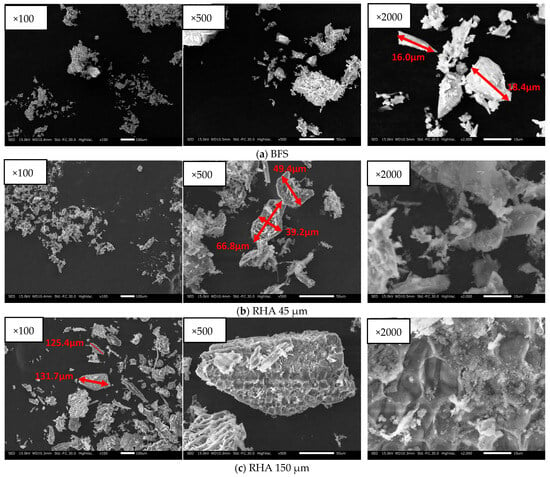
Figure 2.
SEM image of solid precursors used.
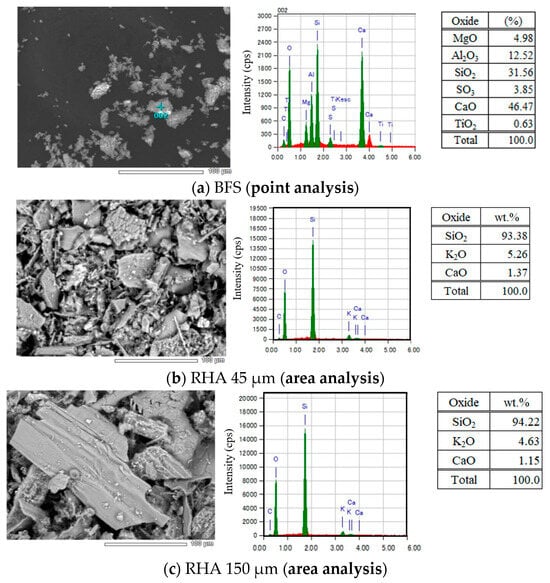
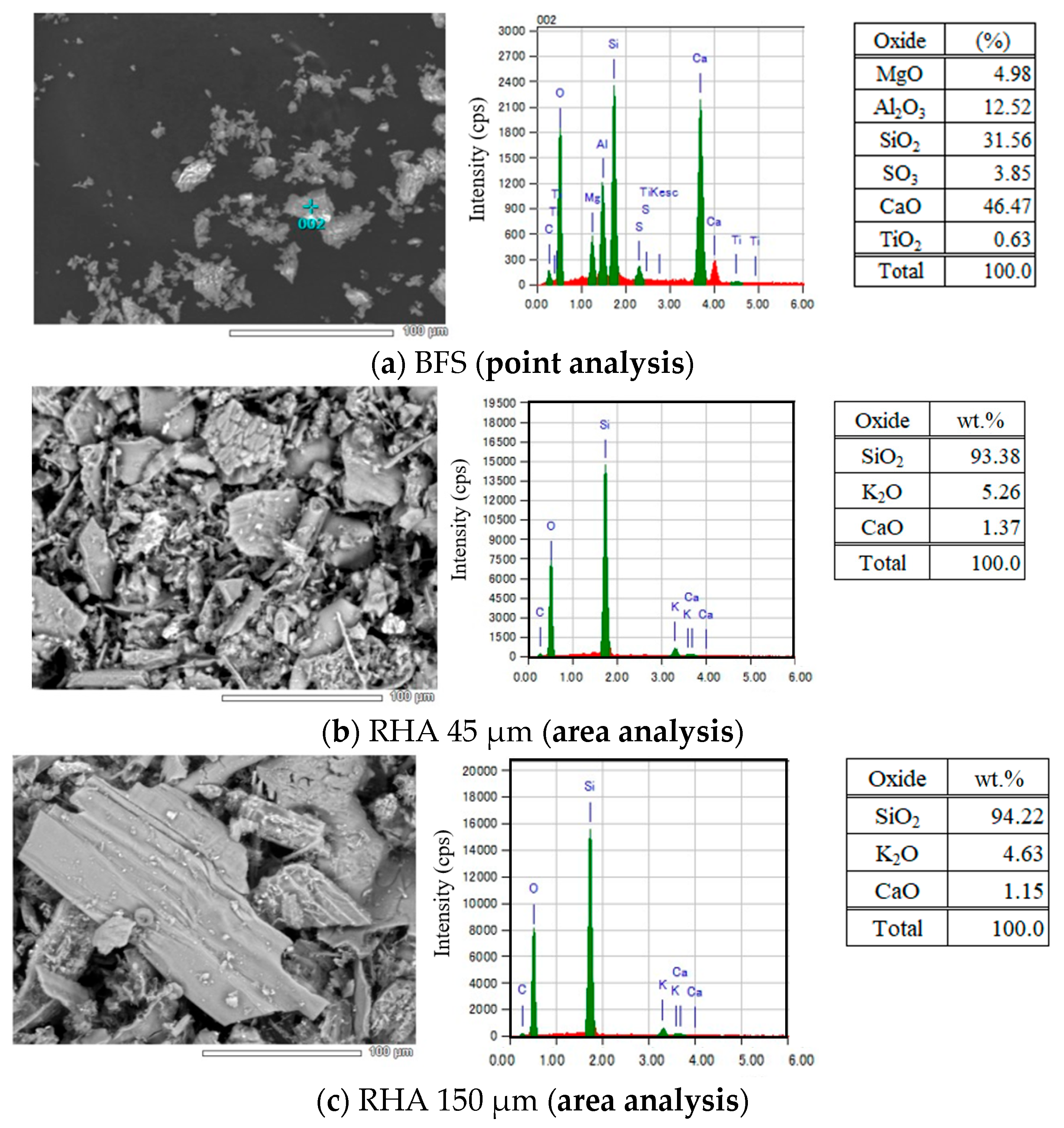
Figure 3.
SEM–EDS analysis of solid precursors used.
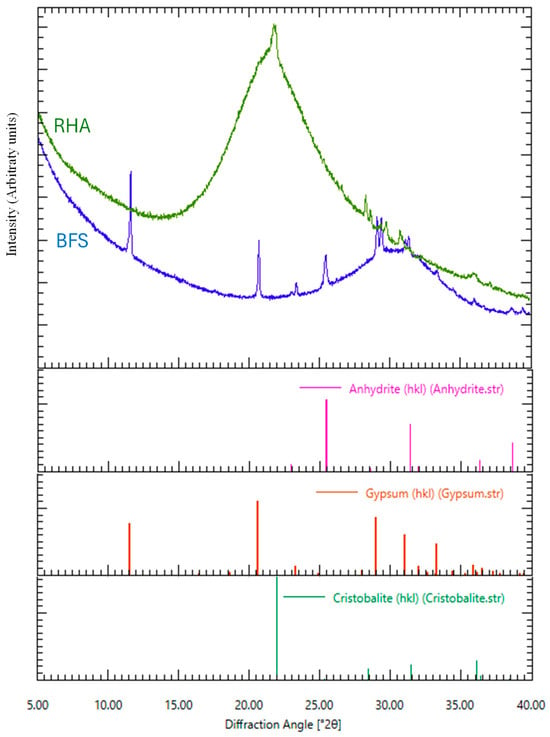
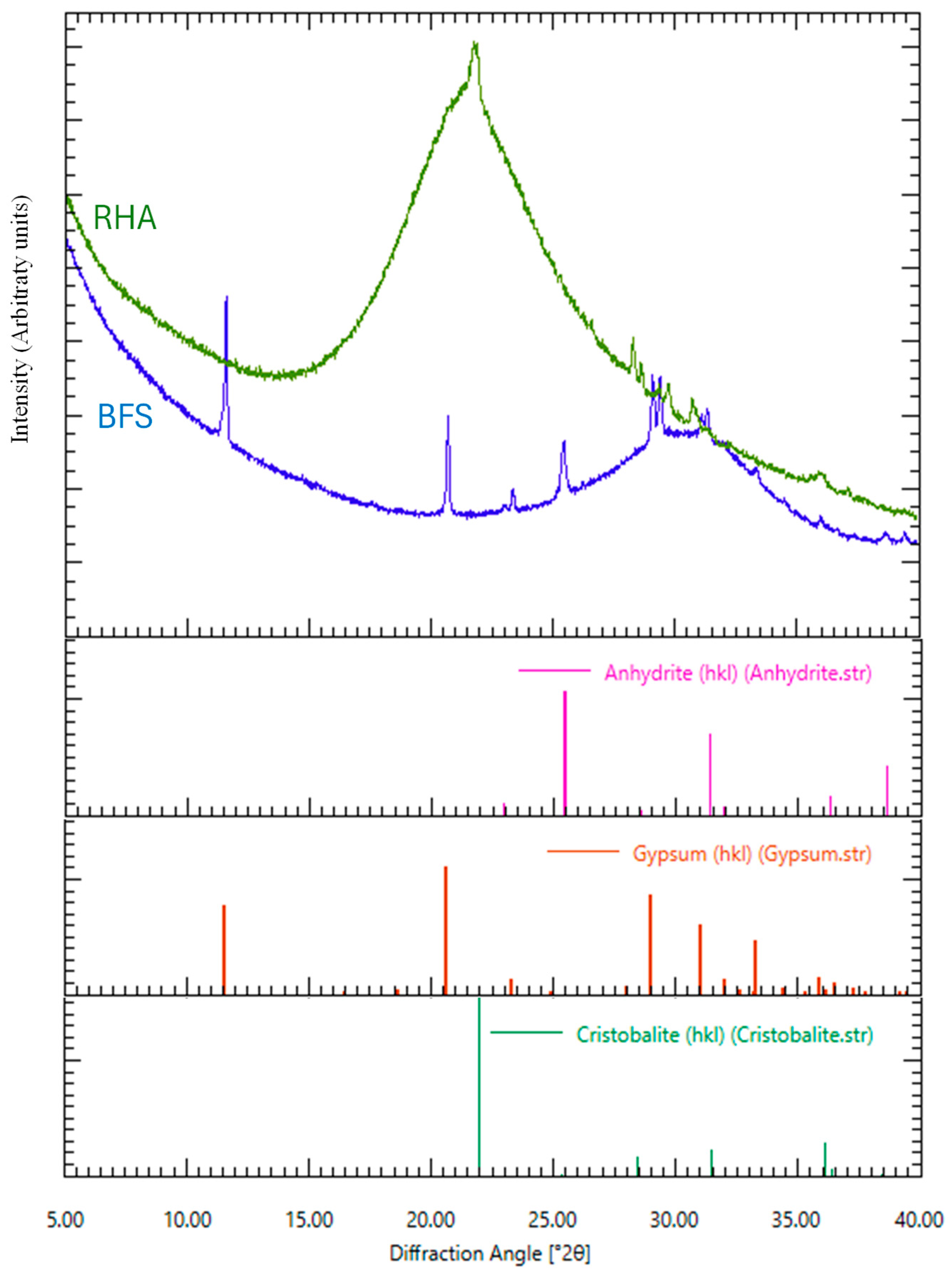
Figure 4.
X-ray diffraction analysis of RHA 45 µm and BFS as raw materials.
As given in Figure 3, from the results of the chemical composition of BFS analyzed by SEM–EDS, the contents of Al2O3, SiO2, and CaO in BFS were 12.52, 31.56, and 46.47 wt.%, respectively. MgO and SO3 were also detected, and their amounts were 4.98 and 3.85 wt.%, respectively. In addition, from the result of XRD analysis of BFS, an amorphous peak was confirmed around 30°θ, and peaks of gypsum and anhydrite were confirmed.
From the results of chemical composition of two types of RHA with different sizes of 45 µm and 150 µm analyzed by SEM–EDS, the 45 µm RHA contained SiO2, K2O, and CaO, and their amounts were 93.38, 5.26, and 1.37 wt.%, respectively. It was also confirmed that the 150 µm RHA also contained SiO2, K2O, and CaO, and their contents were 94.22, 4.63, and 1.15 wt.%. As a result of XRD analysis at 45 µm RHA, the peak of cristobalite was detected at around 24°θ. Therefore, it is considered that not only amorphous silica but also a trace amount of crystalline phase exists in the 45 µm RHA.
In addition, silica sand, which is 2.65 g/cm3 of surface dry density and 0.42% of water absorption of the sand, was used. In addition, taking into consideration the effect of RHA particle size on the physical properties of the mortar, two types of 45 and 150 µm sieves of RHA particle size were prepared in the experiment. To create RHA of each size, the RHA was ground using an electric grinder mill of 2-layer blades, and the entire RHA was passed through each sieve and was not discarded during powder preparation. As an alkaline activator to activate the hydration of BFS, NC (Na2CO3 regent, purity < 95.0%) was used from Hayashi Pure Chemical Ind. Ltd., Osaka, Japan. To control the flow ability and air contents, two types of the chemical admixtures in the experiment were used, which are high-range water-reducing agent and air-entraining agent (HP-8: Polycarboxylate type from Takemoto Oil and Fat Co., Ltd., Gamagori, Aichi, Japan; NO.21: Polycarboxylate salt from Kao Global Chemicals, Co., Ltd., Tokyo, Japan). The air contents of all the mortar samples investigated using these chemical admixtures ranged between 3.0 and 6.0%.
2.2. Mixture Proportions and Specimen Preparation
The mixture proportions of the paste or mortar samples are listed in Table 1 and Table 2. Five types of mixtures were obtained. The water-to-binder ratio (W/B = water/(BFS + NC + RHA)) was 0.45 and an NC of 10 wt.% was set in the binder. Two types of RHA were added to the mortar at replacement levels of 0, 7.5, and 15.0 wt.% to BFS. This RHA maximum replacement ratio was set to obtain the flowability (<160 mm based on flow test of mortar). The binder to sand ratio was set at 40:60.

Table 1.
Mixture proportion of mortar.

Table 2.
Mixture proportion of paste.
The materials were mixed for 4 min and then cast with 40 mm × 40 mm × 160 mm prisms to make the mortar test specimens. After 3 d or 4 d, the samples were de-molded and cured at 20 °C in water for 28 d.
2.3. Testing Methods
2.3.1. Mortar
- (1)
- Compressive Strength
The effect of particle size and replacement ratio of RHA on compressive strength was investigated. In this test, a prism mold with dimensions of 40 mm × 40 mm × 160 mm was used. After mixing and casting, the mortar samples were sealed to prevent moisture from evaporating, and placed at 20 ± 2 °C in the experimental room for 3 or 4 d. Then, the samples were de-molded and cured at 20 °C in water until testing. The compressive strength of the mortar test specimens was tested in accordance with JIS R 5201 [41].
- (2)
- Drying Shrinkage
After casting, the mortar samples investigated were sealed and cured in a laboratory at 20 °C until 7 days had passed, and then they were placed in the curing room in conditions of 20 ± 2 °C and relative humidity 50 ± 10%. The length change and mass loss were tested by JIS A 1129 [42]. Length measurements were conducted using a digital micrometer (1/1000 mm). The length change and mass loss were calculated before and after the drying period progressed.
- (3)
- Water penetration depth
The water penetration depth was measured according to JSCE-G 582-2018 [43]. The mortar was cast in a 50 × 100 mm plastic cylinder mold and sealed. Then, they were placed in a curing room at 20 ± 2 °C until 28 days had passed, the samples were dried at 40 °C until 56 days. After that, all mortar samples were placed in the experimental room for 6 h at 20 °C and taped on the sides of the mortar samples, they were submerged in water 10 mm from the bottom surface. After 5, 24, and 48 h, they were split using the compression testing machine, and the water penetration depths were measured by spraying a color-differentiating water indicator according to the NDIS 3423 method. The weight of the mortar sample investigated after water immersion was also recorded.
2.3.2. Paste
- (1)
- Hydration temperature
The hydration temperature of two types of samples, paste samples with P-Control, P45 µm-15 wt.%, and P150 µm-15 wt.%, were measured based on results of compressive strength at early stages. As described in the literature [44], an insulation box (material: Styrofoam, sizes: 300 × 300 × 400, paste sample size; cylinder 50 mm × 100 placed in the center) was manufactured to measure the simple hydration temperature with a data log (TDS-302, Tokyo Measuring Instruments Laboratory Co., Ltd., Tokyo, Japan). After mixing, the paste sample was placed in the insulation box and then, a type-T thermocouple was inserted at the center of the paste sample. In addition, the interval time of the test was set at 30 min.
- (2)
- pH Value
Paste samples were prepared for this test. These paste samples were mixed for approximately 5 min using a hand mixer and then placed in a plastic cylinder mold with dimensions of 200 mm × 10 mm. The paste samples were mixed and then slowly rotated to minimize the segregation of water from the paste. After rotating for 4 d, they were cast and stored in a container at 20 °C in water. At 28 d age, the samples were placed in an oven at 40 °C and dried for 3 d. Then, the paste was pulverized to less than 150 µm. The appropriate liquid to powder ratio was 2, as reported in the literature [45]. Here, distilled water was used and the liquid to powder ratio was set at 10 [39]. A total of 1 g of powder sample and 10 mL of distilled water were added in a 15 mL plastic container. After distilled water and powder was mixed for approximately 2 min, the pH was measured after 5 min using a pH meter.(DKK-TOA Co., HM-30P, DKK-TOA Co., Tokyo, Japan).
- (3)
- Archimedes porosity
To investigate the variation in the porosity of the paste due to the use of different materials, the Archimedes porosity (porosity) was examined. After the pastes were made with different materials in the same manner as that for the pH test, hydration of the paste was stopped using ethanol. All the pasted samples investigated were placed at 40 °C for 3 d to dry the ethanol. After crushing the paste into about 5mm size, about 10 g were weighed, then the paste sample was placed in a vacuum container containing water, and the paste was immersed using a vacuum pump. After that, the surface of the paste was wiped with a paper towel, and the weight in the dry state was measured. Then, the paste sample was placed in a container containing water, and the weight of the paste in the state of water in cement paste was measured [39].
- (4)
- Ignition Loss
The ignition loss of paste samples was measured to identify the change in hydration products. A total of 1 to 2 g of paste was placed in a furnace for 40 min at 350 °C using electric furnace (PETIT, Shirota Electric Furnace Co., Ltd., Tokyo, Japan). The ignition loss was calculated using the following formula [39].
where
- m40 is the weight of paste at 40 °C;
- m350 is the weight of paste at 350 °C.
- (5)
- X-ray Diffraction (XRD) Analysis
Using paste samples under 150 µm after drying at 40 °C for 72 h, the hydration products were analyzed by a Smart Lab X-ray diffractometer (Rigaku Corporation, Tokyo, Japan). A copper-target X-ray tube (Cu Kα) was used as the radiation source, with the scanning range set to 5–40° (2θ), a scanning rate of 10.1°/min, and a step size of 0.01°. The phases present in the samples were identified by analyzing the diffraction peaks in the patterns using XRD data analysis software (5.4), using profex version 5.4 software [46]. Hydration products of X-ray diffraction pattern of paste were compared to the Crystallography Open Database (COD).
- (6)
- SEM–EDS Analysis
SEM–EDS analysis was performed to investigate the effect of RHA on microstructure. For SEM–EDS analysis, paste samples cured in a sealed container at 20 °C for 21 days were used. After casting, hydration of the paste sample was stopped using iso-propanol and placed in vacuum chamber for 24 h and polished using sandpaper #1000 before analysis. Carbon-coating equipment was not used. The microstructure was analyzed by a JSM-IT300LA (InTouchScope™, JEOL Ltd., Tokyo, Japan) and was obtained by scanning the surface with a 15 kV electron beam, collecting secondary electron signals. In addition, chemical composition of each paste sample was measured using backscattered electron condition, with a 20 kV electron beam, and a working distance of 10.4 to 10.5mm under low-vacuum condition.
3. Results
3.1. Compressive Strength Results
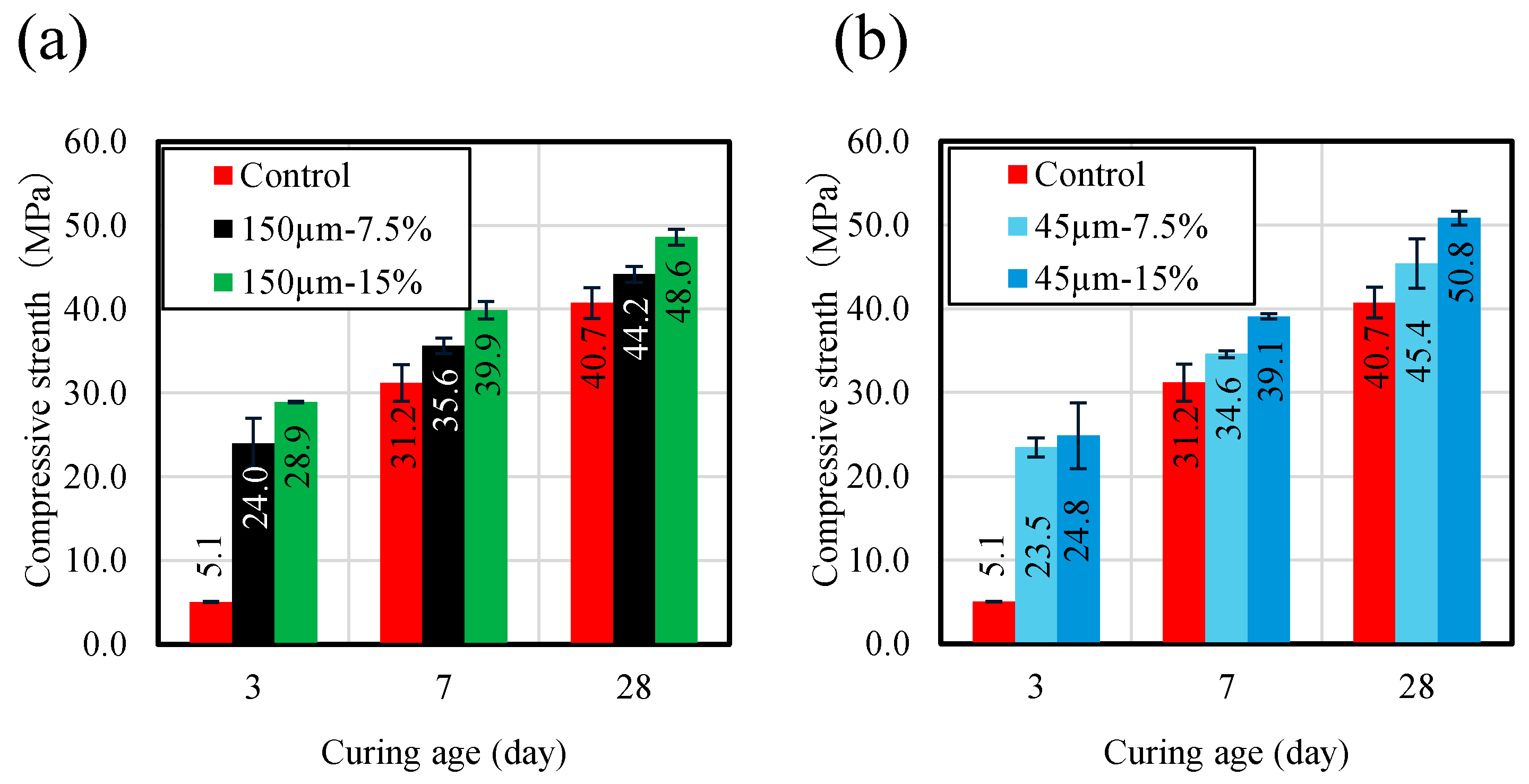
Figure 5 shows compressive strength results for RHA blended mortar samples with three replacement ratios of 7.5 and 15.0 wt.%, and maximum particle sizes of 45 and 150 µm, compared with the control sample without RHA. Regarding the effect of RHA replacement ratios with respect to BFS on compressive strength, all samples increased with increasing curing age, as shown in Figure 5. Compressive strength increased with increasing the RHA replacement ratio before 7 d of curing, and this increase could be due to the promotion of the pozzolanic reaction of RHA. Moreover, the hydration reaction of BFS is slightly slow, but it can be concluded that the hydration reaction within the binder is accelerated as part of the BFS is replaced by RHA. For the effect of the RHA particle size on compressive strength of the mortar sample, at 28 d, the compressive strengths of the mortar samples made with RHA 45 µm and 150 µm replacement ratio of 15 wt.% were recorded at 51.6 and 48.6 MPa, respectively. This suggests that a smaller particle size of RHA has a positive effect on mortar compressive strength at 28 d.
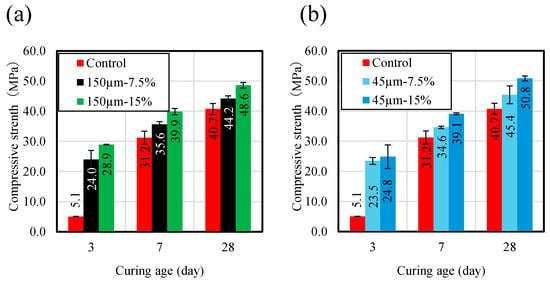
Figure 5.
Compressive strength (MPa) of mortar samples. (a) RHA150 µm. (b) RHA45 µm.
3.2. Hydration Temperature
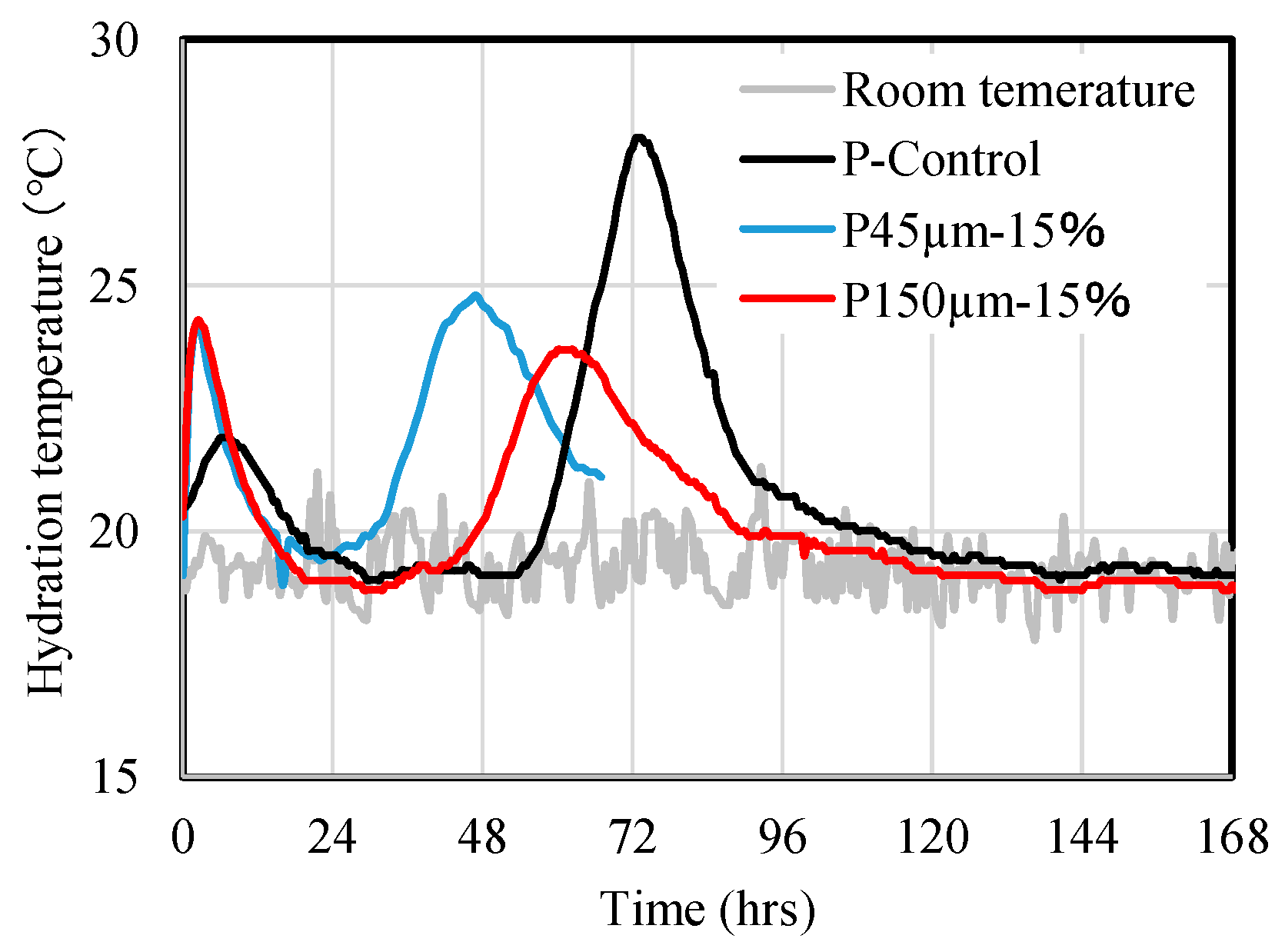
Figure 6 shows the results of hydration temperature measurements in the adiabatic state of the paste, with and without RHA. Two peaks were observed for P-Control sample. The first peak appeared between 6 and 12 h, and the second peak appeared at approximately 72 h. Meanwhile, for the paste samples of P45 µm-15 wt.% and P150 µm-15 wt.%, first peak was confirmed at approximately 2 h to 3 h and second peak is observed at 48 h for 45 µm-15 wt.%, and at 67 h for 150 µm-15 wt.%. This suggests that when BFS was replaced by the RHA, the appearance of first and second exothermic peaks was shortened, implying acceleration of hydration in the paste.
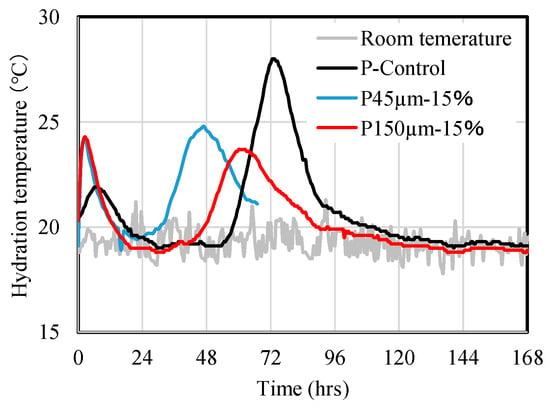
Figure 6.
Results of the hydration measurement of the paste P-Control, P45 µm-15% (RHA 45 µm replacement ratio 15 wt.%), and P150 µm-15% (RHA 150 µm replacement ratio 15 wt.%).
3.3. pH, Porosity, and Ignition Loss Results
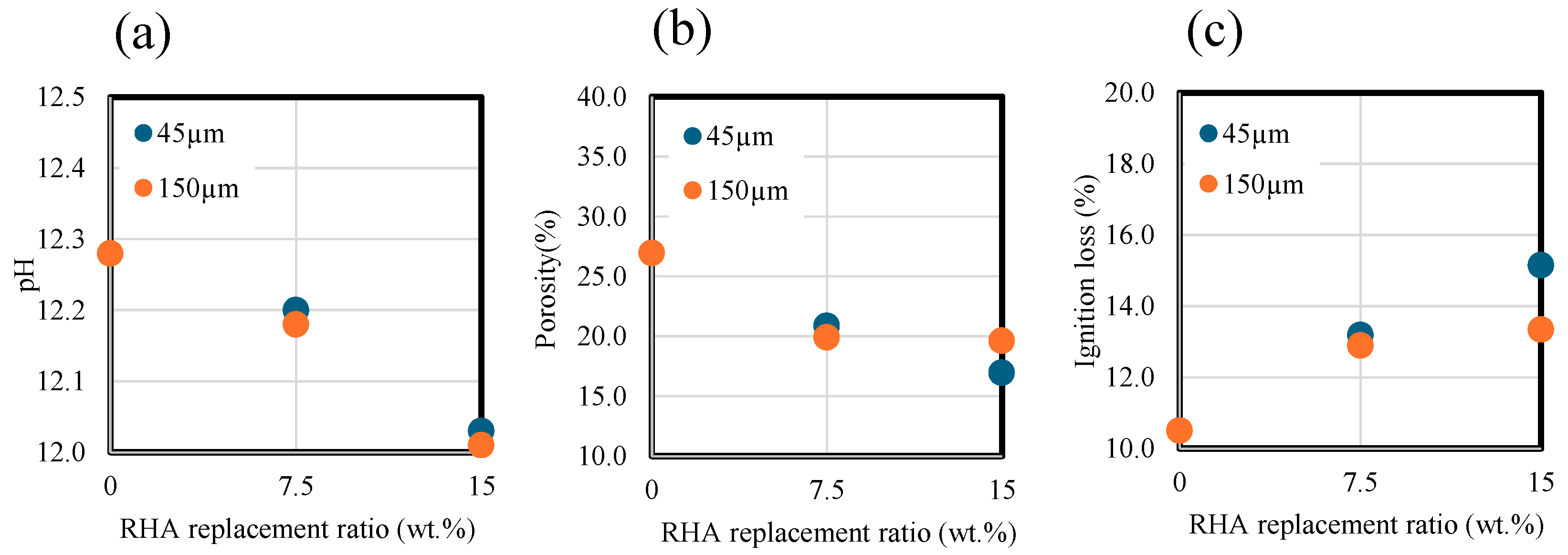
Figure 7 and Table 3 show the effect of RHA replacement ratio and particle size on the pH of the liquid phase. As shown in the figure, the higher the RHA replacement ratio with respect to the BFS, the lower the pH value due to pozzolanic reaction of RHA, which reacts with calcium ion and produces C-S-H with low CaO/SiO2. Moreover, there was no significant change in pH with different RHA sizes.
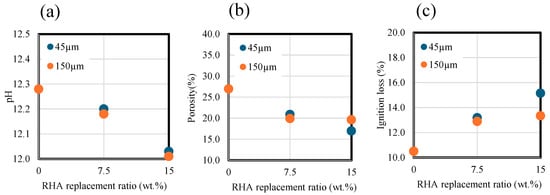
Figure 7.
pH, porosity, and ignition loss results. (a) pH. (b) Porosity. (c) Ignition loss.

Table 3.
pH, porosity, and ignition loss results.
Figure 7b,c show the effects of RHA replacement ratios and particle size on the paste porosity and ignition loss. As shown in the figure, as the RHA replacement ratio increases, the porosity decreases. At a replacement ratio of 15 wt.% of RHA for the BFS, the smaller the RHA size, the greater the decrease in porosity. Figure 7c shows ignition losses of pastes ranging from 40 to 350 °C. Consequently, at an RHA replacement ratio of 15 wt.% with a particle size of 45 µm, the paste ignition loss was higher than that of the paste with a particle size of 150 µm, which is related to the acceleration of pozzolan reaction. These results indicate that replacement ratio for BFS slag has a significant effect on the decrease in porosity and the increase in ignition loss, and RHA particle size is thought to partially contribute to pore densification and hydration generation.
3.4. XRD Analysis
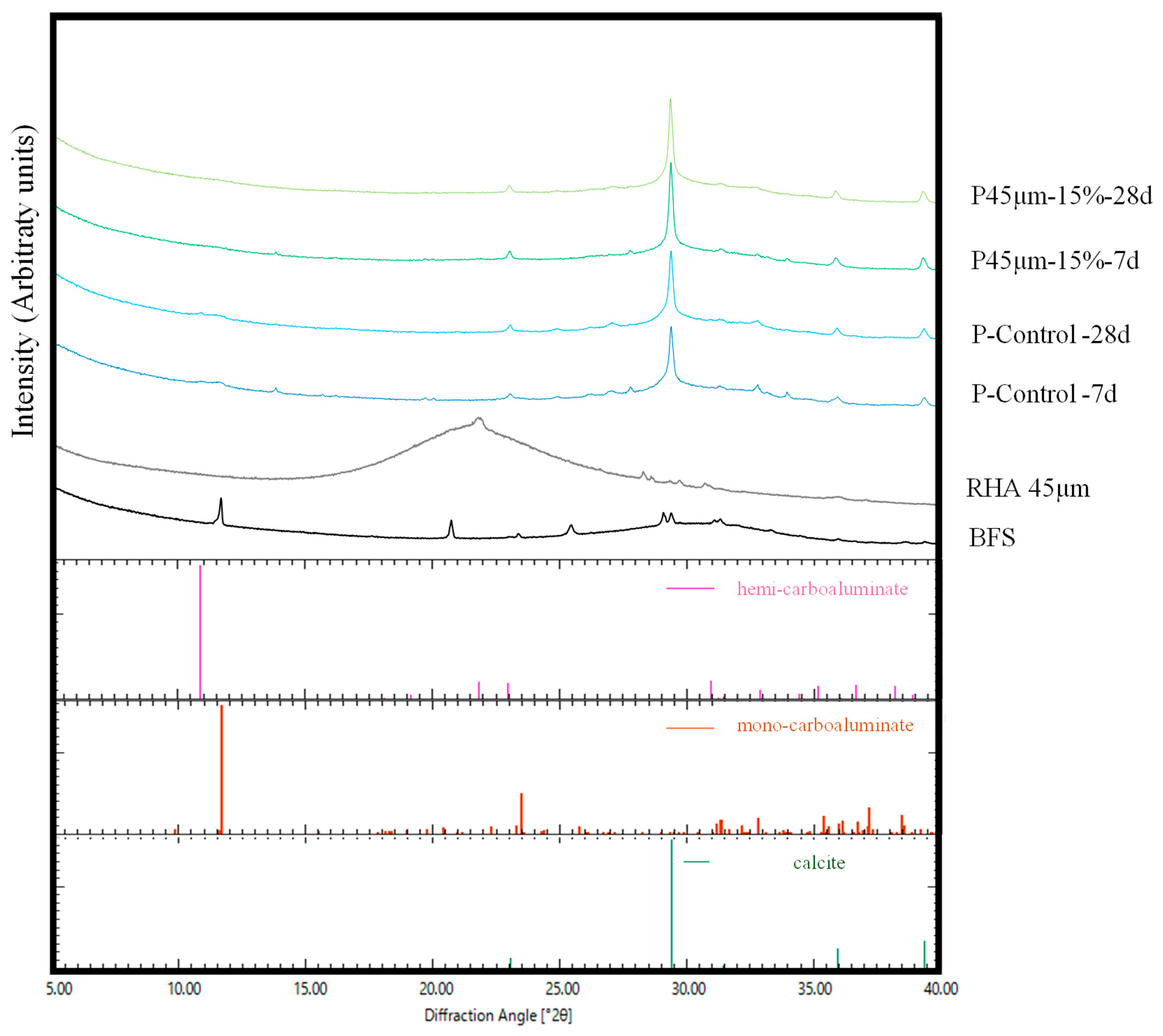
XRD analysis was performed to study the relationship between the compressive strength development of mortars containing RHA and the hydration products. In a previous study, Yoshida et al. studied the effects of calcium hydroxide and NC, and the hydration reaction of BFS using calorimetry, differential thermal analysis, and XRD analysis [47]. They reported that when 4 to 8 wt.% NC was added to BFS, mono-carbonate portlandite, limestone, and C-S-H were formed [47]. Hydration products with hemi-carbonate, hydrotalcite, and calcite were confirmed, as reported in the literature [48]. In this study, peaks considered as calcite, mono-carbonate, and hemi-carbonate were detected in the P-Control sample at 7 d or 28 d, as shown in Figure 8. In contrast to P-Control, the presence of mono and hemi-carbonate peaks in the range of 10–14° (2θ) was not confirmed in the P45 µm at 7 d or 28 d. This could be because the RHA replacement ratio increased and the overall BFS amount decreased.
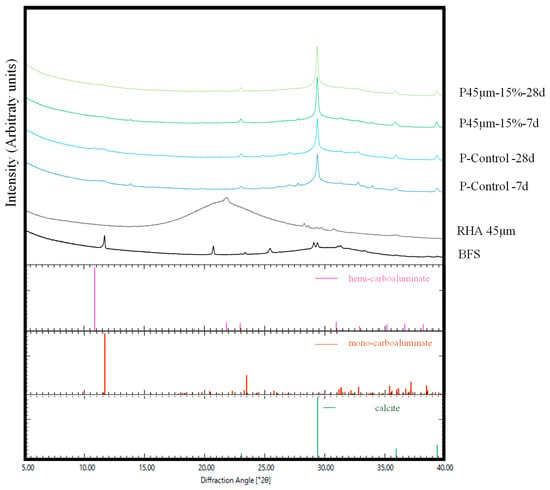
Figure 8.
XRD pattern of paste.
3.5. SEM–EDS Analysis
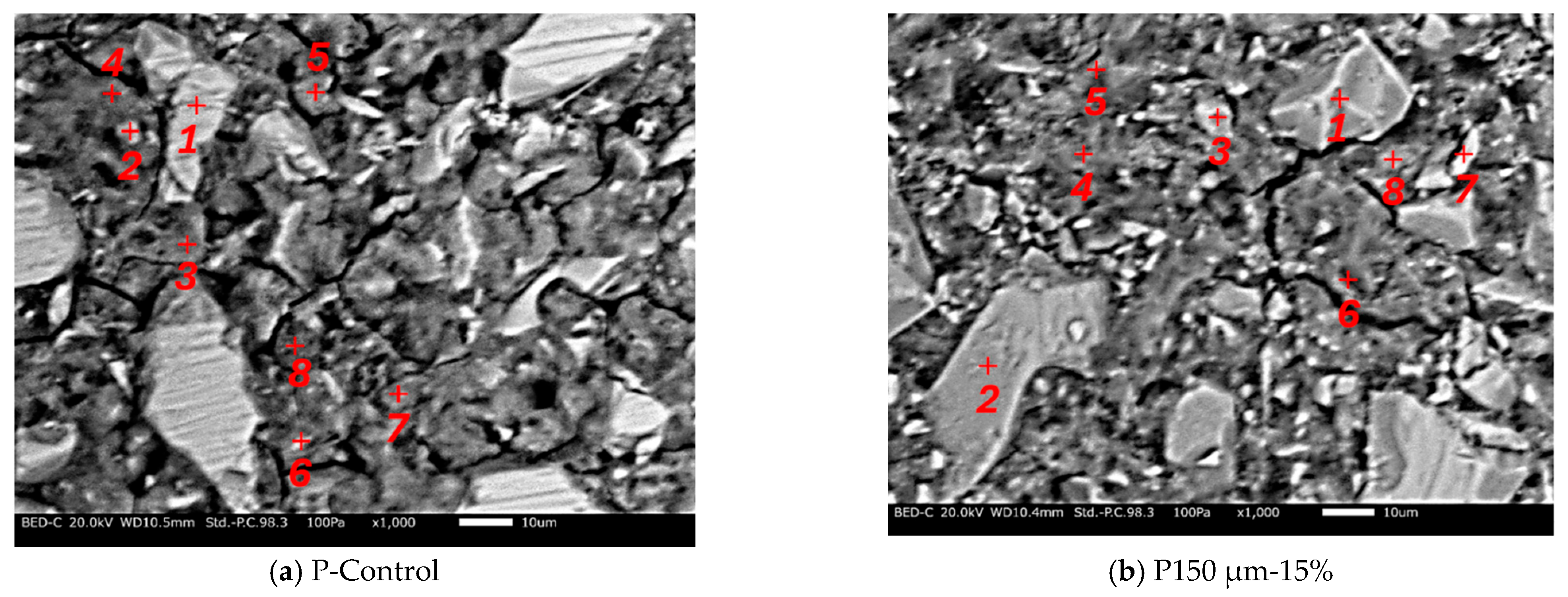
For the SEM analysis, no carbon coating was conducted. The analysis results are shown in Figure 9. It was found that micro cracks were confirmed in both samples and there are many black parts, except for the micro cracks, which implies the presence of pores. Microstructure analysis by SEM indicates that RHA may cause a smaller volume of pores, which can be filled by the hydration product of RHA. In the case of Figure 9a, the hydration products expected to be un-hydrated BFS (location 1), C-S-H (locations 3, 4, 5, 6, 8), and calcite (locations 2, 7) were confirmed. In the case of Figure 9b, the hydration products of un-hydrated BFS (locations 1, 2, 3, 6), RHA (location 7), and C-S-H (locations 4, 5, 8) were confirmed. Table 4 and Table 5 show the SEM–EDS results. As a result of confirming the CaO/SiO2 ratio of the hydration products confirmed as C-S-H from each chemical composition, the CaO/SiO2 ratios of the paste without RHA and the paste with RHA were in the ranges of 1.18 to 1.28 and 0.56 to 1.08, respectively. From these results, it was confirmed that the CaO/SiO2 ratio of C-S-H decreased when BFS was replaced by RHA, which is mainly due to the pozzolanic reaction of RHA. It means that the amorphous silica in the RHA reacts with calcium ion from the BFS, resulting in C-S-H production. Therefore, the pore structure from the production of this hydration products was densified. However, further investigation is needed to clarify the effect of the presence of C-S-H on the pore diameter due to the use of RHA.
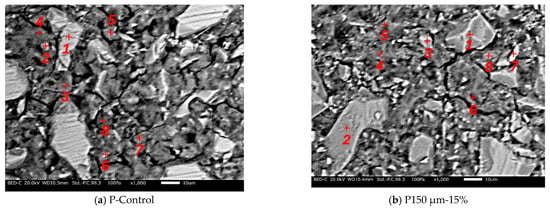
Figure 9.
SEM analysis.

Table 4.
SEM–EDS analysis results of P-Control paste. ✓ symbol represents each material and the hydration products.

Table 5.
SEM–EDS analysis results of P150 µm-15% paste sample. ✓ symbol represents each material and the hydration products.
3.6. Drying Shrinkage
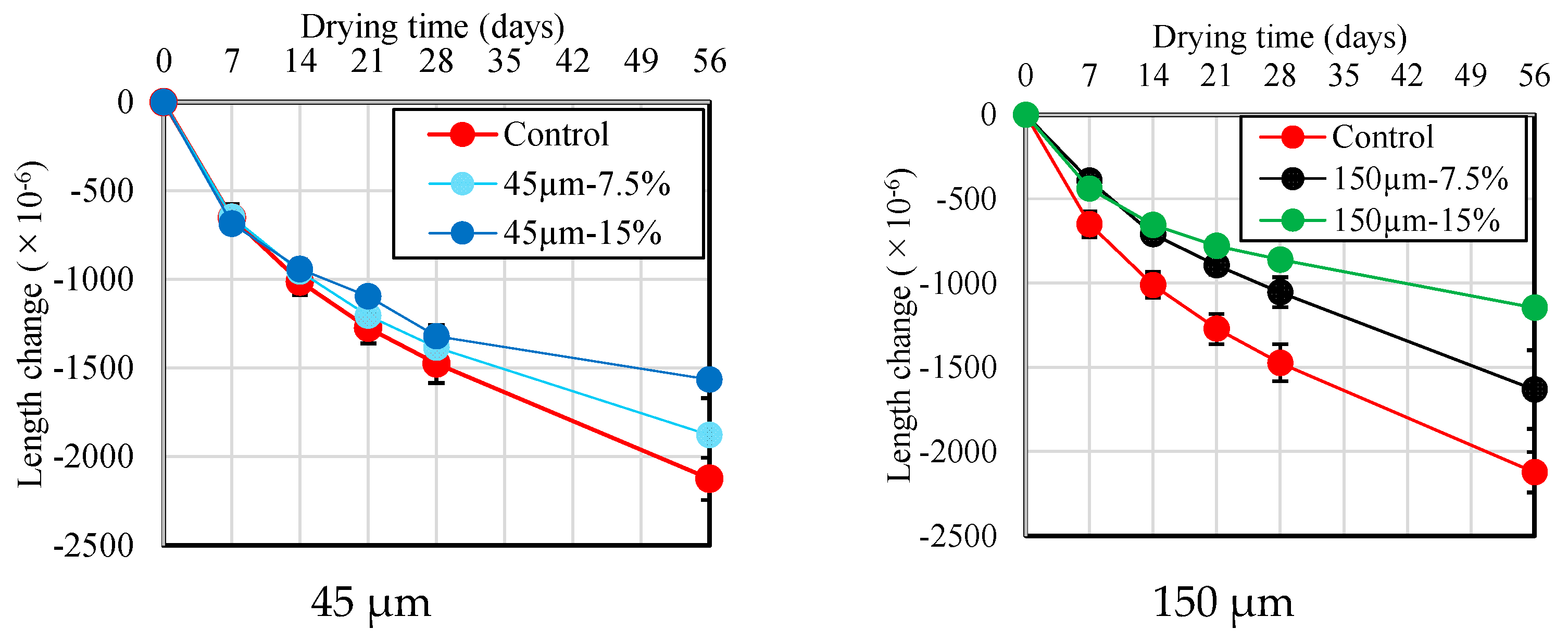
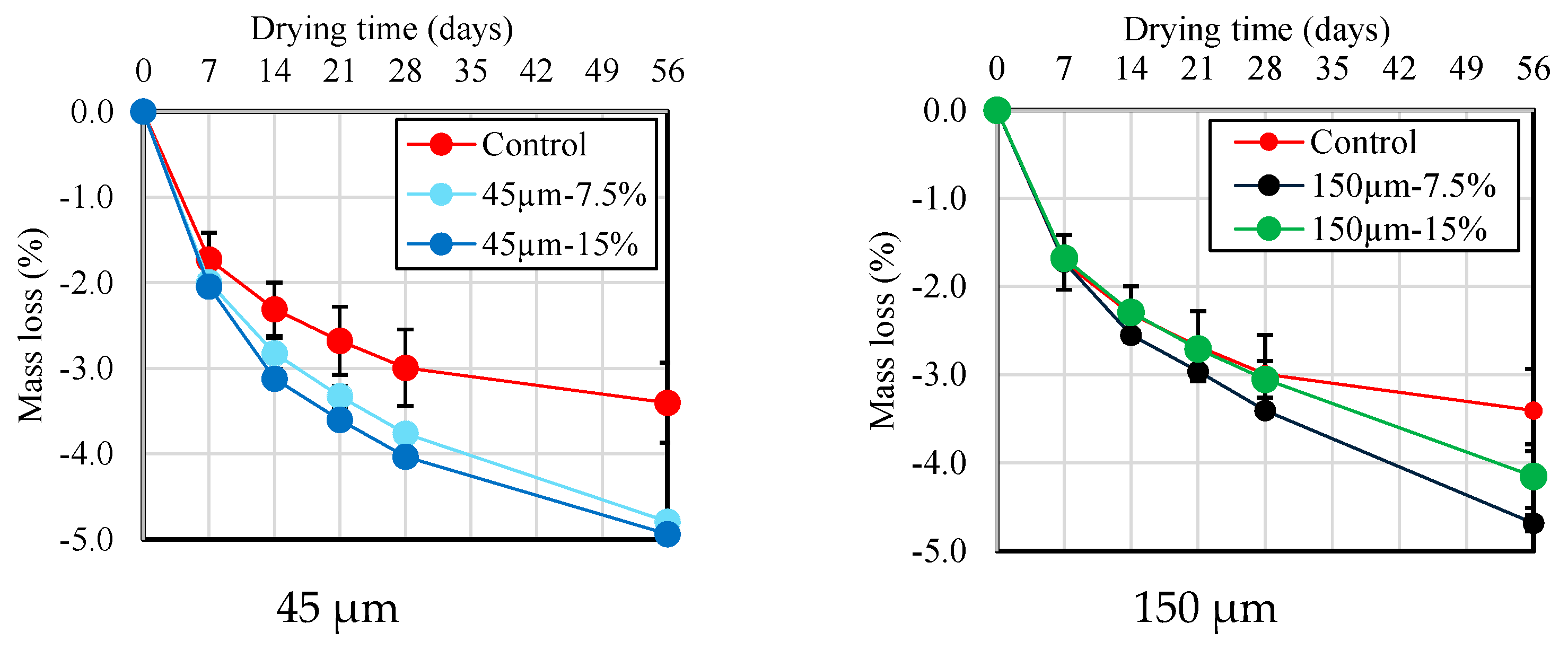
Figure 10 and Figure 11 show the results of the change in length and mass loss according to the RHA replacement ratio for BFS until 56 d of age. As shown in Figure 10, there is no or little significant difference in drying shrinkage across different RHA replacement ratios and particle sizes for up to 7 d after drying. However, at the 14 d mark, the length change becomes smaller as the RHA replacement ratio increases. This is thought to be because the pozzolanic reaction is activated using RHA, which densifies the pore structure and complicates the path through which moisture can evaporate. Furthermore, as shown in Figure 11, compared with the mortar sample without RHA, a slight increase in mass loss was detected as the RHA replacement ratio to BFS slag increased. These results confirm that length change and mortar mass reduction using RHA 150 µm particle size are lower than the results obtained from the RHA 45 µm particle size. From these results, it is suggested that the RHA replacement ratio and the use of large-sized RHA particles have a positive effect on the improvement in the dimensional stability of the mortar during the drying process, and that RHA helps enhance dimensional stability, which has been pointed out as the biggest problem with alkaline-activated materials.
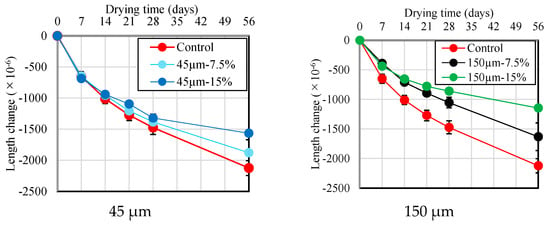
Figure 10.
Length change of mortar samples with different replacement ratio and size of RHA.
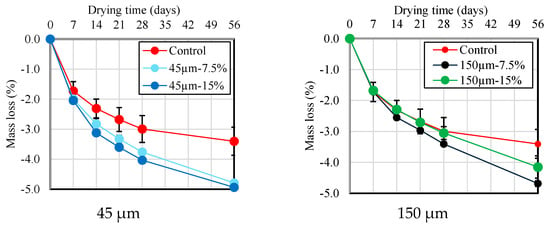
Figure 11.
Mass loss of mortar samples with different replacement ratio and size of RHA.
3.7. Water Absorption Contents and Coefficient
Figure 12 shows the measurements of water that penetrated the specimen during the water-penetration test. The weight of each sample was measured at 0 h, 5 h, 24 h, and 48 h of immersion, and water absorption content was calculated using the following formula.
As shown in Figure 12a,b, the water absorption contents in mortar samples increased with increasing immersion time. Moreover, as shown in Figure 12b, the water absorption contents of the control samples made with mortar without RHA was approximately 22.5 g at 48 h, and mortar samples at replacement ratios of 7.5 and 15 wt.% were 18.8 and 9.5 g at 48 h, respectively. These results indicate that the RHA replacement ratio of 15 wt.% as an alkaline-active binder can inhibit the penetration of moisture from the outside.
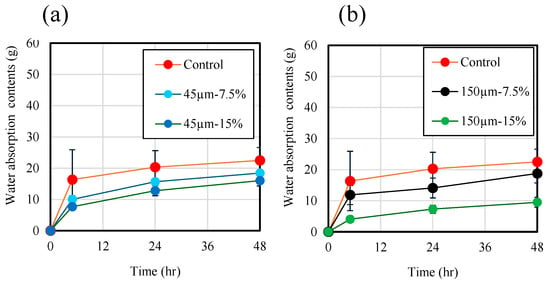
Figure 12.
Water penetration contents of mortar samples. (a) RHA45µm. (b) RHA150µm.
Figure 12.
Water penetration contents of mortar samples. (a) RHA45µm. (b) RHA150µm.
3.8. Water Penetration Depth
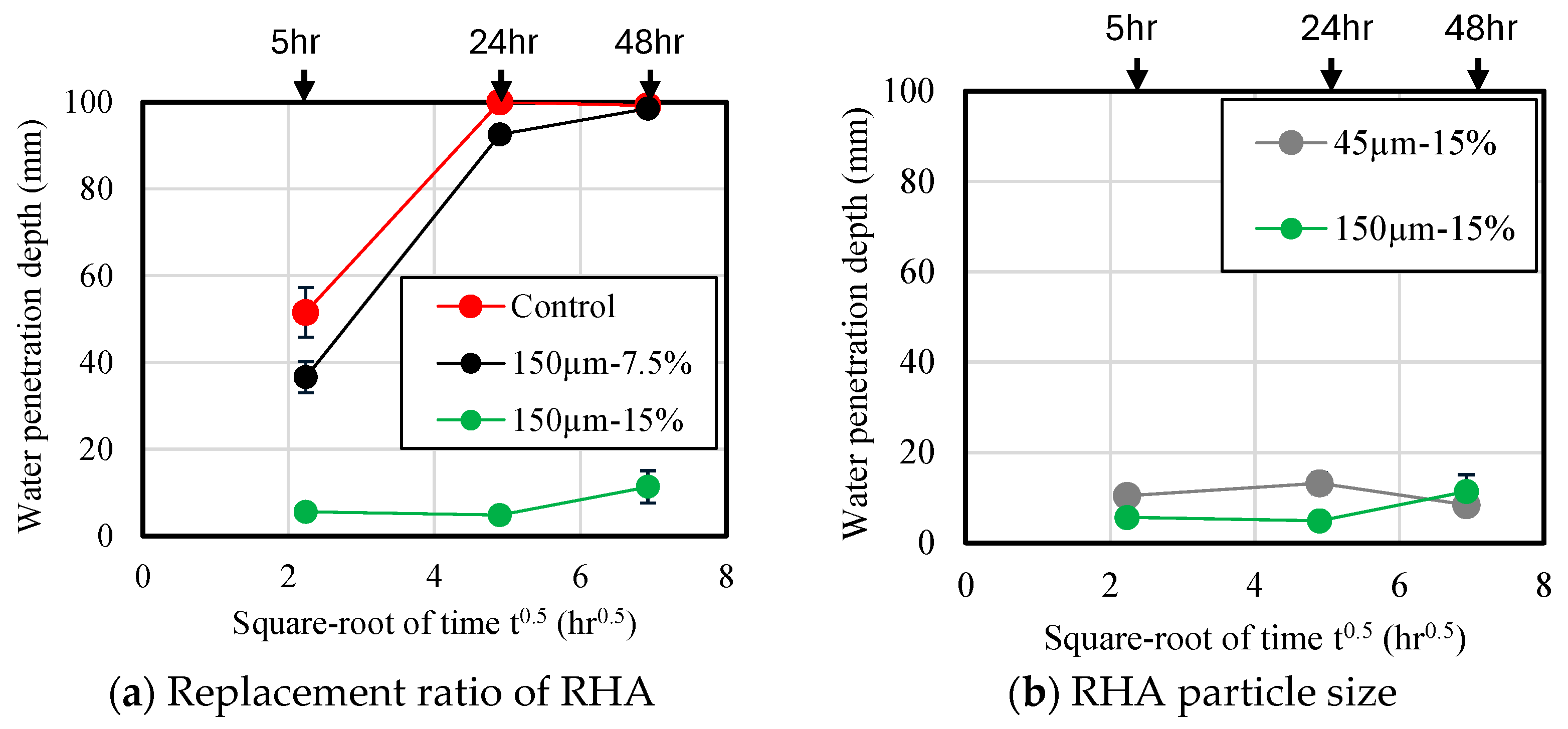
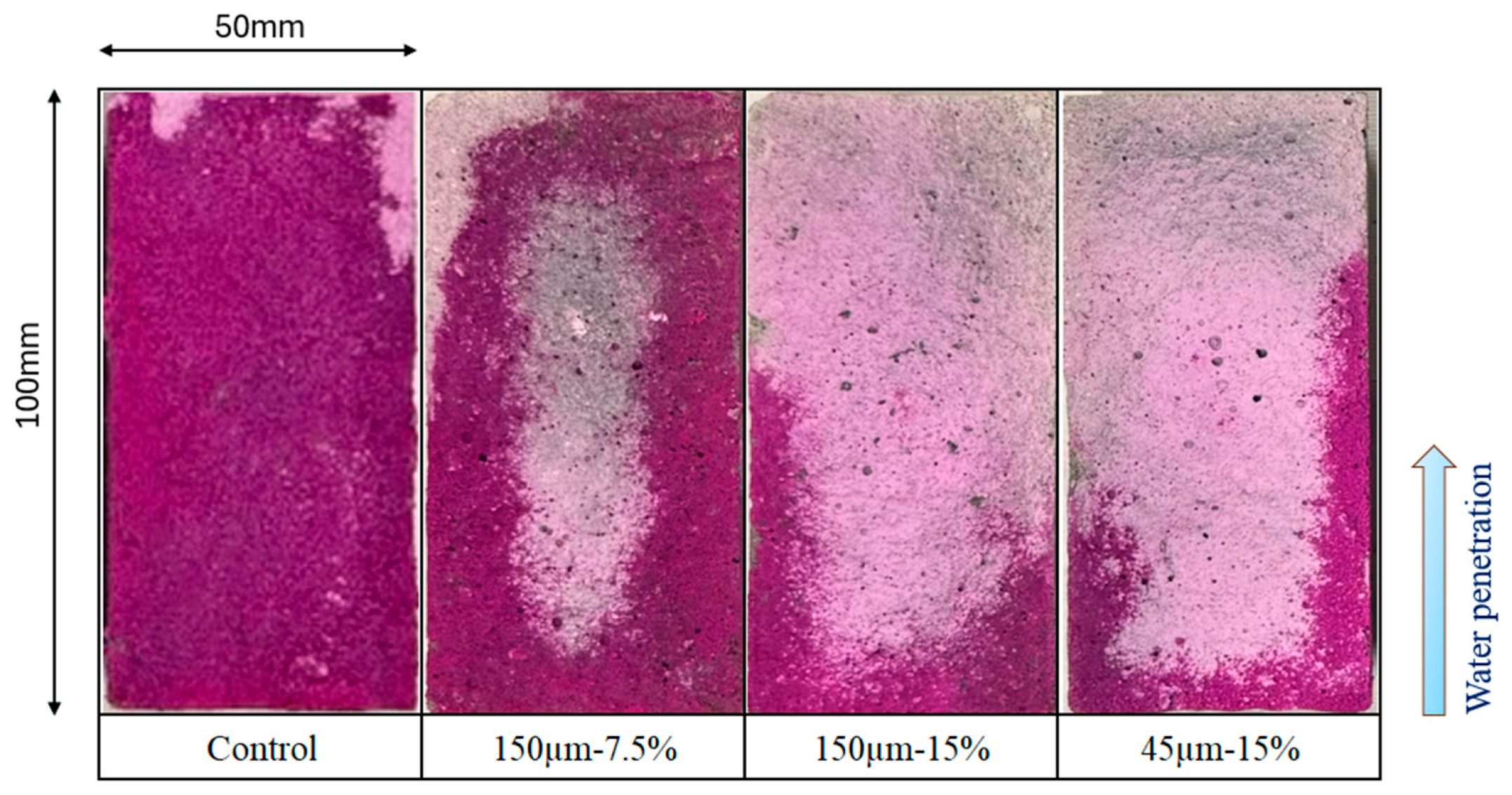
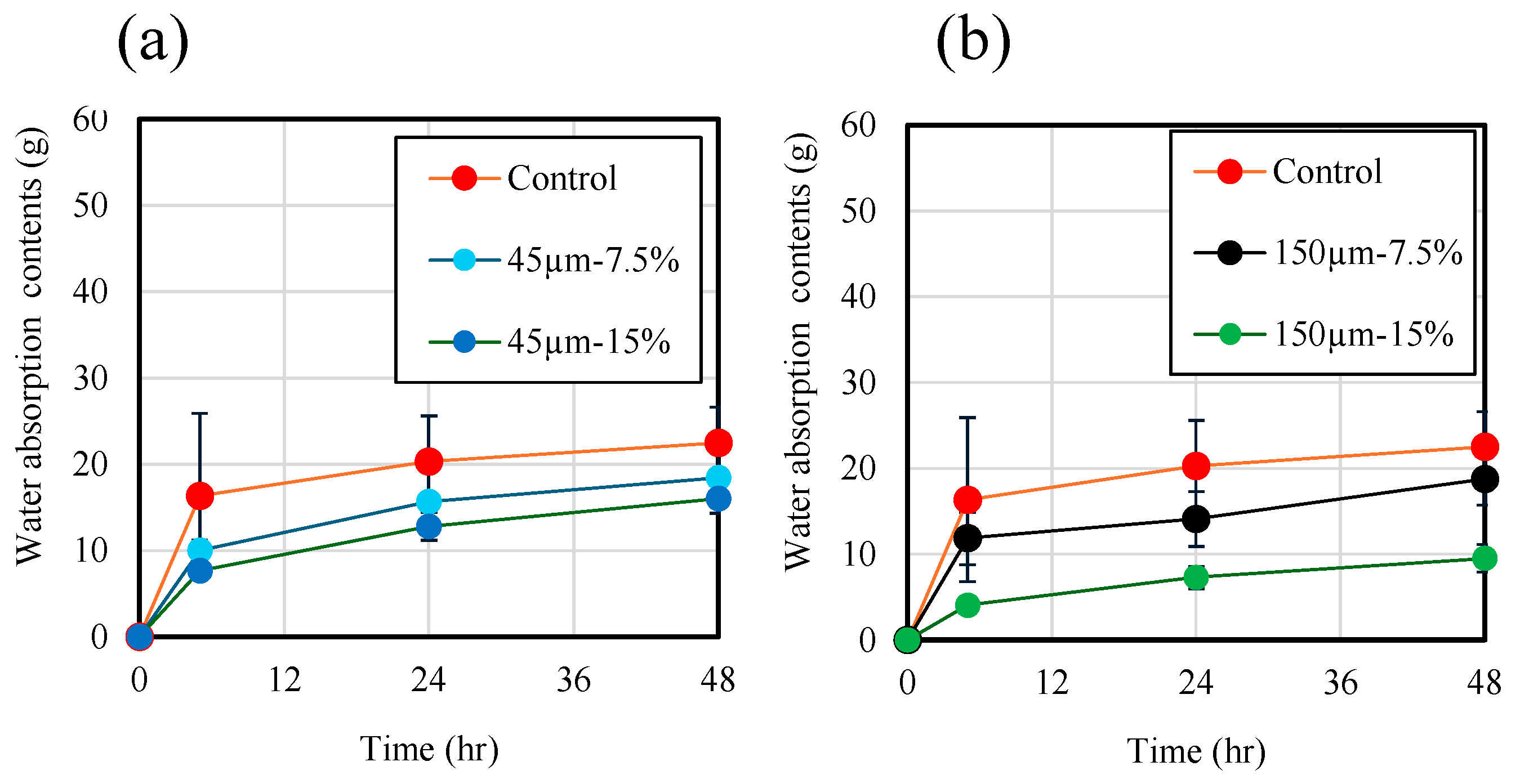
Figure 13 shows the results of measuring water penetration depth of the mortar samples. Each material was sealed and cured for 28 d, then stored in a 40 °C oven dryer for 28 d. Then, all specimens were cut into through a splitting tensile strength test. Immediately after cutting, water penetration depth of the mortar was measured using developer spray. The water penetration depth is plotted as the square root of immersion time. As shown in Figure 13a, the water penetration depth of the mortar sample without RHA was measured to be approximately 52 mm after 5 h of immersion, indicating that water entered the mortar at a high rate during the early stage of immersion. When the immersion was performed for approximately 24 h, water penetrated approximately 100 mm. This is consistent with the results of a previous study [21]. Additionally, as the replacement ratio of RHA increased, the water penetration depth decreased. At a replacement ratio of 15 wt.% with RHA 150 µm, water penetration depth based on immersion time at 48 h was found to be 89% lower than that of the control sample. Figure 13b shows the effect of RHA with 45 µm and 150 µm on water penetration depth of mortar. Consequently, despite the increase in the immersion time of the samples, there was no significant increase in the water penetration depth of each sample, and the penetration depth after 48 h was less than 11 mm. Therefore, when the RHA replacement rate is high, there is no clear difference in water penetration depth across samples with larger RHA particles. Figure 14 shows the cross-section of each sample after an immersion time of 48 h. The purple color in the figure indicates the depth of water penetration. In the case of the samples with the control sample and 7.5% RHA replacement in the BFS, almost all cross-sections of the samples were purple, which confirms that a large amount of water penetrated the test specimen. On the other hand, in the case of the mortar sample containing 15 wt.% of 150 µm RHA, it is confirmed that water partially penetrated the side and bottom surfaces of the test piece. From these results, it can be concluded that when BFS was replaced by 15 wt.% of RHA 45 µm and 150 µm, moisture is less likely to penetrate compared to control sample without RHA, and that mixing RHA helps improve the decrease in water-penetration resistance, which has been previously reported as a disadvantage of alkaline-activated materials.
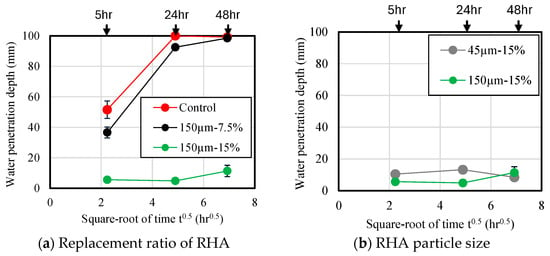
Figure 13.
Water penetration depth.
Figure 14.
Cross-sections of the chosen tested sample after 48 h.
4. Discussion
4.1. Effect of RHA on Mechanical Properties
The results of measuring the compressive strength show that when BFS was partially replaced with RHA, the compressive strength within 7 days of curing was increased. A similar trend was confirmed in the results of measuring the hydration temperature. From the test results obtained, it was confirmed that the appearance of the hydration temperature peak of paste with RHA was accelerated. Therefore, RHA can be used as a reinforcing admixture for the initial strength of the alkaline-activated material. In addition, replacement of BFS with RHA had a positive effect on the microstructure given that (i) pH and porosity decreased; (ii) mono-carbonate, hemi-carbonate, and calcium carbonate were generated; and (iii) the ignition loss estimated by the decomposition temperature of C-S-H between 40 °C and 350 °C increased. These results imply that RHA contributes to the increase in compressive strength.
4.2. Effect of RHA on Drying Shrinkage
From the test results obtained of drying shrinkage, when BFS is replaced by RHA, the length change of mortar was decreased. This improvement in dimensional stability is due to two factors. First, the rapid pozzolanic reaction of RHA consumes free water, resulting in the reduction in the amount of water evaporation in the mortar. When RHA is added, the initial compressive strength within 7 days of curing increases. In other words, the pore structure inside the mortar becomes dense, making it difficult for water to evaporate from the inside to the outside, and the additional pozzolanic reaction of RHA can make the passage through which water can evaporate in an irregular manner, leading to a decrease in drying shrinkage. The second reason is that RHA has many nano-sized pores [37]. In general, if there are more pores of larger size, the easier it is for water to evaporate, but if there are more small pores, the more difficult it is for water to evaporate. Therefore, when RHA containing many nano-sized small pores is added, the evaporation rate of water can be slowed down, and the presence of these small pores contributes to increasing the drying shrinkage resistance.
4.3. Effect of RHA on Water-Penetration Resistance
The water penetration depth results of the control sample without RHA indicate that the water penetration depth is significantly increased with an increase in immersion time. This means that this alkali-activated binder may result in lower resistance to the water penetration test, which agrees with results from previous studies [39]. However, there is no clear explanation for the poor water resistance of the alkali-activated binder. Previous studies [49] have shown that surface damage takes place in alkali-activated concrete using NC and BFS. They reported that the use of a large amount of BFS deteriorates the quality of the surface layer of alkali-activated concrete.
Therefore, mortars made with such a large amount of BFS have a reduced surface quality, which may lead to an increase in water penetration depth. On the other hand, in the case of mortars made with the RHA 150 um replacement ratio of 15 wt.%, the water penetration depth was lower than that of the control mortar sample, which is due to the densification of the pore structure due to the pozzolanic reaction of RHA. This suggests that the use of RHA can enhance the reduction in water-penetration resistance of mortars made with BFS and NC.
5. Conclusions
This study evaluated the effects of particle size and replacement ratio of RHA on the compressive strength, drying shrinkage, and water-penetration resistance of alkali-activated materials composed of NC and BFS using mortar. Hydration temperature, porosity, ignition loss, pH, XRD, and SEM–EDS analyses using paste were performed to investigate the relationship between test results and the microstructure of mortar. Based on the experimental results, the following conclusions, which are useful for improving the design of alkali-activated materials to enhance their durability, are drawn.
(a) As a result of measuring the compressive strength of mortar using different RHA replacement ratios and particle sizes for BFS, the compressive strength within 7 d of curing increased as the RHA replacement ratio increased. Therefore, it is suggested that a change in the RHA replacement ratio is effective in improving the compressive strength at an early stage. After 28 d of curing, the smaller the size of the RHA, the slightly higher the compressive strength.
(b) As a result of measuring the adiabatic hydration temperature of the paste, it was confirmed that the hydration temperature peak appeared earlier in the paste containing 15 wt.% of RHA with a size of 150 µm or less than that observed in the sample without RHA. Therefore, incorporating RHA can promote the hydration reaction of NC and alkaline-activated materials composed of BFS.
(c) By measuring the pH and porosity of the paste after 28 d of curing, it was confirmed that the pH and porosity decreased as the replacement ratio of RHA increased. Therefore, it can be concluded that RHA helps densify the pore structures of NC and BFS. From XRD and loss on ignition analyses, it was confirmed that peaks of mono-carbonate, hemi-carbonate, calcite, and C-S-H were detected, and the loss on ignition was confirmed to increase.
(d) From the results of SEM–EDS analysis of the hydration products of paste samples made with RHA replacement ratio of 15 wt.%, replacement of RHA may make C-S-H gel with a low CaO/SiO2 ratio in the paste sample, and, consequently, the pore structure can be filled with the hydration products of RHA, resulting in rapid compressive strength development.
(e) The effect of the replacement ratio and particle size of RHA on the drying shrinkage of BFS confirmed that the variations in length decreased as the replacement ratio of RHA increased. This contributes to the improvement in the dimensional stability of the alkali-activated binder. Regarding the effect of particle size, the lowest length change was observed when BFS was replaced by 15 wt.% of RHA 150 µm.
(f) Water penetration depth measurements show that water penetration depth decreased as the replacement ratio of RHA increased. Therefore, the use of RHA with an appropriate replacement ratio can improve the water-penetration resistance of alkaline-activated materials. When RHA with different sizes was used to replace 15 wt.% of the BFS, no significant difference in water penetration depth was observed.
(g) An increase in drying shrinkage ability and a decrease in water-penetration resistance are major engineering tasks for the application range of alkali-activated materials. This problem may be overcome if BFS is replaced by RHA. Therefore, this study is a new idea from both environmental and engineering points of view.
Author Contributions
Investigation: S.N.; writing—original draft: S.N.; data curation: S.N. and W.Z.; review and editing: S.N. and W.Z.; project administration: S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Obayashi Foundation (2023-Kenkyu-62-84).
Data Availability Statement
The data presented in this study are available on request.
Acknowledgments
Some parts of the experiment were conducted by Atsushi Hirooka at Nishinippon Institute of Technology, for whom I express my gratitude. We would like to thank Yuya Hashimoto (Nippon Steel Slag Products Co., Ltd.) for supplying the blast furnace slag powder for this research. The authors also gratefully acknowledge the supply of anti-foaming agent from Kao Chemicals. We also would like to thank Kazo Matsui as technical staff at Nishinippon Institute of Technology, for guidance in using the SEM–EDS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fapohunda, C.; Akinbile, B.; Shittu, A. Structure and properties of mortar and concrete with rice husk ash as partial replacement of ordinary Portland cement—A review. Int. J. Sustain. Built Environ. 2017, 6, 675–692. [Google Scholar]
- Thomas, B.S.; Yang, J.; Mo, K.H.; Abdalla, J.A.; Hawileh, R.A.; Ariyachandra, E. Biomass ashes from agricultural wastes as supplementary cementitious materials or aggregate replacement in cement/geopolymer concrete: A comprehensive review. J. Build. Eng. 2021, 40, 102332. [Google Scholar]
- Blesson, S.; Rao, A.U. Agro-industrial-based wastes as supplementary cementitious or alkali-activated binder material: A comprehensive review. Innov. Infrastruct. Solut. 2023, 8, 125. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, C.; Na, S.; Jin, Y.; Kang, W.; Zhu, J.; Zhang, W.; Bian, Y.; Shah, S.P. A study of blast furnace slag on the mechanical properties improvement and microstructure of hemihydrate phosphogypsum pretreated by calcium hydroxide. Case Stud. Constr. Mater. 2025, 22, e04121. [Google Scholar]
- Na, S.; Lee, W.; Song, M. Hardening Properties of Foamed Concrete with Circulating Fluidized Bed Boiler Ash, Blast Furnace Slag, and Desulfurization Gypsum as the Binder. Open J. Civ. Eng. 2021, 11, 301–316. [Google Scholar] [CrossRef]
- Glushankova, I.; Ketov, A.; Krasnovskikh, M.; Rudakova, L.; Vaisman, I. Rice Hulls as a Renewable Complex Material Resource. Resources 2023, 7, 31. [Google Scholar]
- Freire, A.L.; Moura-Nickel, C.D.; Scaratti, G.; De Rossi, A.; Araújo, M.H.; Júnior, A.D.N.; Rodrigues, A.E.; Castellón, E.R.; Moreira, R.d.F.P.M. Geopolymers produced with fly ash and rice husk ash applied to CO2 capture. J. Clean. Prod. 2020, 273, 122917. [Google Scholar]
- Umeda, J.; Kondoh, K. High-purification of amorphous silica originated from rice husks by combination of polysaccharide hydrolysis and metallic impurities removal. Ind. Crops Prod. 2010, 32, 539–544. [Google Scholar]
- Umeda, J.; Takada, R.; Michiura, Y.; Kondoh, K. High-Purity Amorphous Silica Originated in Rice Husks of Agricultural Waste and Utilization of Concrete Admixture. J. Smart Process. 2014, 3, 323–327. [Google Scholar]
- Bakar, R.A.; Yahya, R.; Gan, S.N. Production of High Purity Amorphous Silica from Rice Husk. Procedia Chem. 2016, 19, 189–195. [Google Scholar]
- Manaviparast, H.R.; Cristelo, N.; Pereira, E.; Miranda, T. A Comprehensive Review on Clay Soil Stabilization Using Rice Husk Ash and Lime Sludge. Appl. Sci. 2025, 15, 2376. [Google Scholar] [CrossRef]
- Atef, A.; Hossain, Z. Sustainable Soil Reinforcement by Maximizing Geotechnical Performance with Rice Husk Ash in Subgrade Layers. Materials 2025, 18, 873. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Ouyang, M.; Bao, X.; Zhang, Q.; Bi, Z.; Yan, H.; Li, S.; Shi, Y. Performance Evaluation of Stabilized Soils with Selected, Common Waste Materials of Rice Husk Ash, Steel Slag and Iron Tailing Powder. Materials 2025, 18, 346. [Google Scholar] [CrossRef] [PubMed]
- Adajar, M.A.; Tan, J.; Ang, A.B.; Sy, V.P. Shear Strength and Durability of Expansive Soil Treated with Recycled Gypsum and Rice Husk Ash. Appl. Sci. 2024, 14, 3540. [Google Scholar] [CrossRef]
- Barragán-Ramírez, R.; González-Hernández, A.; Bautista-Ruiz, J. Michel Ospina and Willian Aperador Chaparro, Enhancing Concrete Durability and Strength with Fly Ash, Steel Slag, and Rice Husk Ash for Marine Environments. Materials 2024, 17, 3001. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Z.; Yang, X.; Zhang, L.; Li, C.; He, C.; Xu, W. The Influence of Rice Husk Ash Incorporation on the Properties of Cement-Based Materials. Materials 2025, 18, 460. [Google Scholar] [CrossRef]
- Alsaed, M.M.; Al Mufti, R.L. The Effects of Rice Husk Ash as Bio-Cementitious Material, in Concrete. Constr. Mater. 2024, 4, 629–639. [Google Scholar] [CrossRef]
- Marangu, J.M.; Sharma, M.; Kafodya, I.; Mutai, V.K.; Latif, E.; Novelli, V.I.; Ashish, D.K.; Maddalena, R. Deepankar Kumar Ashish and Riccardo Maddalena, Durability of Ternary Blended Concrete Incorporating Rice Husk Ash and Calcined Clay. Buildings 2024, 14, 1201. [Google Scholar] [CrossRef]
- Thiedeitz, M.; Schmidt, W.; Kränkel, T. Performance of Rice Husk Ash as Supplementary Cementitious Material after Production in the Field and in the Lab. Materials 2020, 13, 4319. [Google Scholar] [CrossRef]
- Alharthai, M.; Onyelowe, K.C.; Ali, T.; Qureshi, M.Z.; Rezzoug, A.; Deifalla, A.; Alharthi, K. Enhancing concrete strength and durability through incorporation of rice husk ash and high recycled ag-gregate. Case Stud. Constr. Mater. 2025, 22, e04152. [Google Scholar] [CrossRef]
- Memon, M.J.; Jhatial, A.A.; Murtaza, A.; Raza, M.S.; Phulpoto, K.B. Production of eco-friendly concrete incorporating rice husk ash and polypropylene fibres. Environ. Sci. Pollut. Res. 2021, 28, 39168–39184. [Google Scholar]
- Kang, S.-H.; Hong, S.-G.; Moon, J. The use of rice husk ash as reactive filler in ultra-high performance concrete. Cem. Concr. Res. 2018, 115, 389–400. [Google Scholar]
- Alam, M.J.; Biswas, M.; Mia, M.B.; Alam, S.; Hossain, M.M. The Influence of Rice Husk Ash on Mechanical Properties of the Mortar and Concrete: A Critical Review. Open J. Civ. Eng. 2024, 14, 2024. [Google Scholar] [CrossRef]
- Demis, S.; Tapali, J.G.; Papadakis, V.G. An Investigation of the Effectiveness of the Utilisation of Biomass Ashes as Pozzolanic Materials. Constr. Build. Mater. 2014, 68, 291–300. [Google Scholar]
- Basri, M.S.M.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Optimization of Adhesion Strength and Microstructure Properties by Using Response Surface Methodology in Enhancing the Rice Husk Ash-Based Geopolymer Composite Coating. Polymers 2020, 12, 2709. [Google Scholar] [CrossRef]
- Abdullah, M.N.; Khan, T.; Sebaey, T.A. Thermal Properties and Drying Shrinkage Performance of Palm Kernel Shell Ash and Rice Husk Ash-Based Geopolymer Concrete. Materials 2024, 17, 1298. [Google Scholar] [CrossRef]
- Hossain, S.S.; Roy, P.K.; Bae, C.J. Utilization of waste rice husk ash for sustainable geopolymer: A review. Constr. Build. Mater. 2021, 310, 125218. [Google Scholar]
- Mohd Basri, M.S.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Rice-Husk-Ash-Based Geopolymer Coating: Fire-Retardant, Opti-mize Composition, Microstructural, Thermal and Element Characteristics Analysis. Polymers 2021, 13, 3747. [Google Scholar] [CrossRef]
- Hong, Z.; Wang, S.; Ying, H.; Lu, Z.; Liu, B.; Xu, J. Synergistic Freeze-Resistant Strategy of Multi-Stage PCM Concrete Incorporated with Rice Husk Ash and Fly Ash. Buildings 2024, 14, 2604. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Jia, W.; Ying, H.; Lu, Z.; Hong, Z. The Effect of RHA as a Supplementary Cementitious Material on the Performance of PCM Aggregate Concrete. Buildings 2024, 14, 2150. [Google Scholar] [CrossRef]
- Xu, H.; Qu, W. Development of a Controlled Low-Strength Material Containing Paraffin–Rice Husk Ash Composite Phase Change Material. Coatings 2024, 14, 1173. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; Marinangeli, L.; Tangari, A.C.; Rosatelli, G.; Ciulla, M.; di Profio, P. Rice Husk as Raw Material in Synthesis of NaA (LTA) Zeolite. Molecules 2024, 29, 4396. [Google Scholar] [CrossRef] [PubMed]
- Onyenokporo, N.C.; Taki, A.; Montalvo, L.Z.; Oyinlola, M.A. Exploring the Impact of Rice Husk Ash Masonry Blocks on Building Energy Performance. Buildings 2024, 14, 1290. [Google Scholar] [CrossRef]
- Wahab, R.A.A.; Zaid, M.H.M.; Aziz, S.H.A.; Matori, K.A.; Fen, Y.W.; Yaakob, Y. Effects of Sintering Temperature Variation on Synthesis of Glass-Ceramic Phosphor Using Rice Husk Ash as Silica Source. Materials 2020, 13, 5413. [Google Scholar] [CrossRef]
- Gonçalves, J.; da Silva, G.; Lima, L.; Morgado, D.; Nalin, M.; Armas, L.E.G.; Valsecchi, C.; Menezes, J.W. Production of Transparent Soda-Lime Glass from Rice Husk Containing Iron and Manganese Impurities. Ceramics 2020, 3, 494–506. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Satyanarayana, K.G.; Pramada, P.N.; Raghavan, P.; Gupta, T.N. Processing, properties and applications of reactive silica from rice husk—An overview. J. Mater. Sci. 2003, 38, 3159–3168. [Google Scholar]
- Na, S.; Kanazawa, T.; Kondo, K.; Umeda, J. Study on application of cement using nano-structured silica particles derived from rice husk to low-alkaline cement. In Proceedings of the 71st Cement Technology Conference, Tokyo, Japan, 27–29 May 2017. [Google Scholar]
- Zhao, X.; Wu, S.; Xu, X.; Wang, L.; Sun, S.; Xiong, G. Research on the effect of different content of rice husk ash on the microstructure of concrete. Ferroelectrics 2024, 13, 1731–1741. [Google Scholar] [CrossRef]
- Na, S.; Zhang, W.; Ichikawa, Y.; Komatsu, M.; Takemura, A. Fundamental Study on Alkali-Activated Slag System with Sodium Carbonate or Calcium Hydroxide. J. Mater. Sci. Chem. Eng. 2024, 12, 55–70. [Google Scholar]
- Na, S.; Zhang, W.; Kitagawa, M.; Hirooka, A.; Komatsu, M. Optimization Using Central Composite Design of the Response Surface Methodology for the Compressive Strength of Alkali-Activated Material from Rice Husk Ash. Constr. Mater. 2025, 5, 5. [Google Scholar] [CrossRef]
- Japan Industrial Standards Committee. Physical Testing Methods for Cement; Japan Industrial Standards Committee: Tokyo, Japan, 2015. [Google Scholar]
- Japan Industrial Standards Committee. Methods of Measurement for Length Change of Mortar and Concrete—Part 3: Method with Dial Gauge; Japan Industrial Standards Committee: Tokyo, Japan, 2010. [Google Scholar]
- JSCE-G 582-2018; Test Method for Water Penetration Rate Coefficient of Concrete Subjected to Water in Short Term. Japan Society of Civil Engineers: Tokyo, Japan, 2018; Volume 56. [CrossRef]
- Park, S.-J.; Jeon, S.-H.; Kim, K.-N.; Song, M.-S. Hydration Characteristics and Synthesis of Hauyne-Belite Cement as Low Temperature Sintering Cementitious Materials. J. Korean Ceram. Soc. 2018, 55, 224–229. [Google Scholar]
- Yousuf, S.; Shafigh, P.; Ibrahim, Z. The pH of Cement-based Materials: A Review. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 5. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program Bgmn. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [PubMed]
- Yoshida, R.; Atarashi, D.; Itoh, T.; Osaki, M. Sodium carbonate and calcium nitrite in combination hydration reaction analysis of blast furnace slag. Cem. Sci. Concr. Technol. 2022, 76, 108–114. [Google Scholar]
- Miyahara, S.; Ogino, M.; Owaki, E.; Sakai, E. Carbonation behavior of pastes containing blast furnace slag powder. Cem. Chem. 2020, 74, 59–66. [Google Scholar]
- Owaki, E.; Okamoto, R.; Matsumoto, J.; Watanabe, S. Development of Concrete Containing High-volume Supplementary Cementitious Materials and Its Applications. Concr. J. 2019, 57, 71–74. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).