A Historical Review of Impressed Current Cathodic Protection of Steel in Concrete
Abstract
:1. Introduction
2. Historical Development
3. Research Projects
4. Anode Development
- Resistant to attack by the acids formed by the anodic reactions for the required design life.
- Compatible with the concrete it is bonded to or embedded in.
- Electrically conductive across the anode/concrete interface, i.e., capable of converting electrons to ions and vice versa so that current flows from the anode through the concrete to the steel.
- Mesh or grid systems with cementitious overlays;
- Ribbon or strip systems grouted into slots or channels in the concrete cover;
- Discrete, probe or point anodes, grouted into holes drilled in the concrete;
- Coating anodes applied to the concrete surface.
5. Development of Control and Monitoring Parameters
6. Design, Anode and Cathode Current Densities
- Overall size for even current spread and controllability; experience has shown that anode zones of 50 to 100 m2 of concrete surface area are usually suitable, as discussed in Chess and Broomfield [52]. However, much smaller zones are used on some structures, and much larger zone can also be suitable, e.g., bridge or multistorey car park decks or soffits.
- Direct current (DC) output levels of the power supply; these are typically 1 or 10 amps, therefore limiting the zone to 50 m2 of steel at a design current density of 20 mA/m2 for a 1 amp unit or at the other extreme 1000 m2 at 10 mA/m2 for a 10 amp unit.
- Variations in steel density; the soffit of a beam may have far more steel than the sides so the soffit may be one zone and the sides another.
- Exposure conditions, the most extreme being piers in marine situations where there can be fully immersed tidal and splash zones which may require different anodes and even different control criteria [64].
7. The Development of Guidance Documents and Standards
8. Issues with the Application of Impressed Current Cathodic Protection (ICCP)
- The anode must bond to the concrete and current must flow evenly from anode to steel at a sufficiently low resistance.
- There must be no electrical short circuits between anode and steel.
- The reinforcement and all other embedded metal must be electrically connected together. (continuity and stray current).
- There must be no adverse reactions in the concrete such as alkali aggregate reaction or significant loss of bond between steel and concrete.
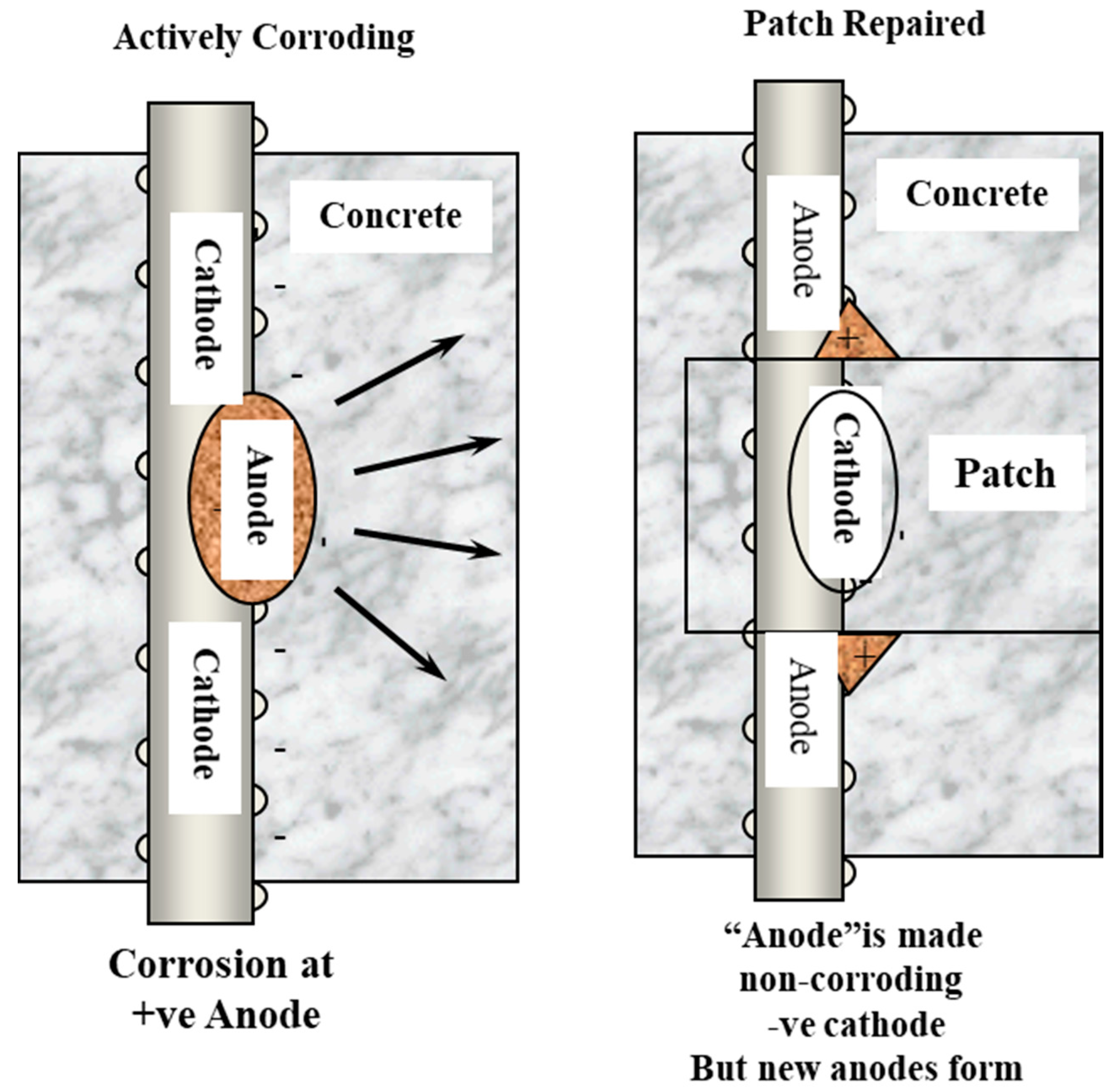
- The variations in resistivity of new and previous repair patches must allow current to pass evenly to the steel in order to achieve protection.
9. International Expansion and Case Histories
10. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Broomfield, J.P. Corrosion of Steel in Concrete-Understanding, Investigation and Repair, 2nd ed.; Taylor and Francis: London, UK, 2007. [Google Scholar]
- Davy, H. On the corrosion of copper sheeting by seawater, and on methods of preventing this effect, and on their application to ships of war and other ships. Phil. Trans. 1824, 114, 151–246. [Google Scholar]
- Corrosion Doctors. Available online: https://corrosion-doctors.org/Corrosion-History/CP-History.htm (accessed on 28 July 2020).
- Unz, M. Cathodic protection of prestressed concrete pipe. Corrosion 1955, 11, 80. [Google Scholar] [CrossRef]
- Heuze, B. Cathodic protection of steel in prestressed concrete. Mater. Prot. 1965, 4, 57–62. [Google Scholar]
- Vrable, J.B. Cathodic Protection for Reinforced Concrete Bridge Decks; Laboratory Phase, Report 180, National Cooperative Highway Research Programme; Transportation Research Board: Washington, DC, USA, 1977. [Google Scholar]
- Stratfull, R.F. Progress report on inhibiting the corrosion of steel in a reinforced concrete bridge. Corrosion 1959, 15, 331t–334t. [Google Scholar] [CrossRef]
- Stratfull, R.F. Experimental cathodic protection of a bridge deck. Transp. Res. Rec. 1974, 500, 1–15. [Google Scholar]
- Stratfull, R.F. Eleven years of success for coke breeze c.p. pavement. In Proceedings of the NACE Conference on Cathodic Protection of Reinforced Concrete Bridge Decks, San Antonio, TX, USA, 12 February 1985; pp. 66–81. [Google Scholar]
- Broomfield, J.P. Field survey of cathodic protection on North American bridges. Mater. Perform. 1992, 31, 28–33. [Google Scholar]
- Perenchio, W.F.; Landgren, J.R.; West, R.E.; Clear, K.C. Cathodic Protection of Concrete Bridge Substructures. In TRB National Cooperative Highway Research Program Report 278; Transportation Research Board: Washington, DC, USA, 1985. [Google Scholar]
- OECD. Durability of Concrete Road Bridges; Organisation for Economic Cooperation and Development: Paris, France, 1989. [Google Scholar]
- Page, C.L.; Treadaway, K.W.J. Aspects of the electrochemistry of steel in concrete. Nature 1982, 297, 109–115. [Google Scholar] [CrossRef]
- Broomfield, J.P. Physical and chemical repair and rehabilitation techniques. In Corrosion of Steel in Concrete, 2nd ed.; Taylor and Francis: London, UK, 2007; pp. 112–139. [Google Scholar]
- Sergi, G.; Seneviratne, A.M.G.; Maleki, M.T.; Sadegzadeh, M.; Page, C.L. Control of reinforcement corrosion by surface treatment of concrete. Proc. Instn. Civ. Engrs. Struct. Build. 2000, 140, 85–100. [Google Scholar] [CrossRef]
- Virmani, Y.P.; Clemena, G.G. Corrosion Protection–Concrete Bridges, FHWA-RD-98-088; Federal Highway Administration: McLean, VA, USA, 1998. [Google Scholar]
- Wallbank, E.J. The Performance of Concrete in Bridges. In Department of Transport (UK) Report; HMSO: London, UK, 1989. [Google Scholar]
- Broomfield, J.P.; Langford, P.E.; McAnoy, R. Cathodic Protection for Reinforced Concrete: Its Application to Buildings and Marine Structures. In Corrosion of Metals in Concrete; NACE: San Francisco, CA, USA, 1987. [Google Scholar]
- Glass, G.K.; Green, W.K.; Chadwick, J.R. Long Term Performance of Cathodic Protection Systems on Reinforced Concrete Structures. In Proc. UK Corrosion 91, Manchester; Inst. Corrosion: Northampton, UK, 1991. [Google Scholar]
- Geoghegan, M.P.; Das, S.C.; Broomfield, J.P. Conductive Coatings in Relation–Cathodic Protection; Taylor Woodrow Construction Ltd.: Southall, UK, 1985. [Google Scholar]
- McKenzie, M. Cathodic protection of reinforced concrete bridges. In Proc. Protection of Concrete, Dundee; E&FN Spon: London, UK, 1990; pp. 641–651. [Google Scholar]
- Gower, M.; Beamish, S. Cathodic Protection on the Midland Links Viaduct. Constr. Repair 1995, 9, 10–13. [Google Scholar]
- Blankvoll, A. History of the GimsØystaumen Bridge Repair project. In Proc. Repair of Concrete Structures; Norwegian Road Research Laboratory: Oslo, Norway, 1997; pp. 1–7. [Google Scholar]
- Escola, L.; Huura, J.; Vaelitalo, S. Cathodic protection with conductive primer as anode: Practical Experiences from Hessund Bridge. In Proc. Repair of Concrete Structures; Norwegian Road Research laboratory: Oslo, Norway, 1997; pp. 349–356. [Google Scholar]
- Broomfield, J.P.; Tinnea, J.S. Cathodic Protection of Reinforced Concrete Bridge Components, Strategic Highway Research Program SHRP-C-92-1; Transportation Research Board: Washington, DC, USA, 1992. [Google Scholar]
- Manning, D.G. Ontario’s Bridge Deck Rehabilitation Manual. In Proceedings Conference of Cathodic Protection of Reinforced Concrete in Bridges and Decks; NACE: Houston, TX, USA; San Antonio, TX, USA, 1987. [Google Scholar]
- Schell, H.C.; Manning, D.; Pianca, F. A decade of bridge deck cathodic protection in Ontario. In NACE Symposium on Corrosion of Metals in Concrete; NACE: Houston, TX, USA, 1987; pp. 65–78. [Google Scholar]
- Clemena, G.G. A Slotted Cathodic Protection System for Bridge Decks. In Proceedings Conference of Cathodic Protection of Reinforced Concrete in Bridges and Decks; American Association of State Highway Officials; Federal Highways Administration and National Association of Corrosion Engineers: Washington, DC, USA, 1985; pp. 89–103. [Google Scholar]
- Bennett, J.E.; Clear, K.C. Cathodic Protection of Steel in Concrete: A State-of-the-Art Report SHRP-S-337; Strategic Highway Research Program; Transportation Research Boards: Washington, DC, USA, 1993. [Google Scholar]
- Martin, B.L.; Bennett, J.E. An Activated Titanium Mesh Anode for the Cathodic Protection of Reinforcing Steel in Concrete. In Corrosion of Metals in Concrete; NACE: Houston, TX, USA, 1987; pp. 255–259. [Google Scholar]
- Bennett, J.; Turk, T.; Firlotte, C.; Sexton, M. Testing old cathodic protection on Ohio Bridge Decks. Mater. Perform. 2012, 51, 31–37. [Google Scholar]
- Kessler, R.J.; Lasa, I.R.; Powers, R.G. Polypropylene Fiber Reinforced Concrete in Cathodic Protection Applications. In Proc. NACE Corrosion 2004; Paper 04341; NACE International: Houston, TX, USA, 2004. [Google Scholar]
- Kendell, K.; Pithouse, K.B. The Raychem Flexible Anode System. In Corrosion in Concrete-Practical Aspects of Control by Cathodic Protection; Paper No. 6; Global Corrosion Consultants: Telford, UK, 1987. [Google Scholar]
- Eastwood, B.J.; Christensen, P.A.; Armstrong, R.D.; Bates, N.R. Electrochemical oxidation of a carbon black loaded polymer electrode in aqueous electrolytes. J. Solid State Electrochem. 1999, 3, 179–186. [Google Scholar] [CrossRef]
- Mietz, J.; Fischer, J.; Isecke, B. Cathodic protection of steel-reinforced concrete structures–results from 15 years’ experience. Mater. Perform. 2001, 40, 22–25. [Google Scholar]
- Bennett, J.E. An Activated Titanium Mesh Anode for Cathodic Protection of Steel in Concrete. UK Corros. 1987, 87, 93–114. [Google Scholar]
- Hayfield, P.C.S.; Hill, A. Ebonex® Discrete Anodes in the Cathodic Protection of Rebar in concrete: Performance of Ebofix Grouts. Int. J. Restor. Build. Monum. 2000, 6, 655–668. [Google Scholar]
- Sergi, G.; Simpson, D.; Hayfield, P.C.S. Long Term Behaviour of Ceramic Tubular Shaped Anodes for Cathodic Protection Applications. In NACE Corrosion Conference Rehabilitation of Reinforced Concrete Structures by Electrochemical and Other Techniques and Corrosion Protection Strategies for Construction; Paper No. 08305; NACE International: Houston TX, USA, 2008. [Google Scholar]
- Drewett, J.; Bott, N. The Refurbishment of Bideford Longbridge Proc Concrete Solutions; Mechtcherine, G., Dresden, S., Eds.; CRC Press: London, UK, 2012; pp. 15–18. [Google Scholar]
- Apostolos, J.A. Cathodic Protection Using Metallized Zinc. Mater. Perform. 1987, 26, 22–28. [Google Scholar]
- Funahashi, M.; Young, W.T. Field Evaluation of a New Aluminum Alloy as a Sacrificial Anode for Steel in Concretefhwa-RD-98-058; Federal Highway Administration: McLean, VA, USA, 1998. [Google Scholar]
- Lehman, J.A. Cathodic Protection for concrete structures other than bridge decks. In Proceedings Conference of Cathodic Protection of Reinforced Concrete in Bridges and Decks; NACE International: Houston, TX, USA, 1985; pp. 150–155. [Google Scholar]
- Brown, R.P. Cathodic protection of reinforced concrete using conductive coatings anodes. In Proceedings Conference of Cathodic Protection of Reinforced Concrete in Bridges and Decks; NACE International: Houston, TX, USA, 1985; pp. 156–163. [Google Scholar]
- Le Marquand, K.; Davies, K. Pier Road and Sand Street multi-storey car parks, Jersey-repair and cathodic protection systems. In Concrete Solutions, Proceedings of the 2nd International Conference, St Malo, France, 27–29 June 2006; Paper No 44; BRE Press: Watford, UK, 2006; pp. 360–366. [Google Scholar]
- Christodoulou, C.; Das, S.C.; Sharifi, A.; Goodier, C. Cathodic protection on the UK’s Midland Links motorway viaducts. Proc. ICE Bridge Eng. 2014, 167, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Pruckner, F.; Broomfield, J.P. New anode for cathodic protection of car parks. Concr. Eng. Int. 2006, 10, 54–57. [Google Scholar]
- De Peuter, F. New Conductive overlay for cathodic protection of reinforced concrete. In Structural Faults 1; Engineering Technics Press: Edinburgh, Scotland, 1993; pp. 119–128. [Google Scholar]
- Drewett, J. Cathodic protection at London Berth, Jersey in 1999. In Concrete Solutions, Proceedings of the 2nd International Conference Paper, St Malo, France, 27–29 June 2006; BRE Press: Watford, UK, 2006; Volume 35, pp. 287–294. [Google Scholar]
- Westhof, L. Field experience with a conductive cement-based composite as anode for the cathodic protection of reinforced concrete structures. In Proceedings of the 2nd International Conference on Concrete Solutions, St Malo, France, 27–29 June 2006; pp. 400–409, Paper No 46. [Google Scholar]
- Broomfield, J.P.; El Belbol, S. Impressed Current Anodes for the Cathodic Protection of Atmospherically Exposed Reinforced Concrete; Technical Note No: 11; Corrosion Prevention Association: Bordon, UK, 2019. [Google Scholar]
- Broomfield, J.P. Budget Cost and Anode Performance Information for Impressed Current Cathodic Protection of Reinforced Concrete Highway Bridges; Technical Notes No: 12; Technical Notes No: 12; Corrosion Prevention Association: Bordon, UK, 2019. [Google Scholar]
- Chess, P.M.; Broomfield, J.P. Cathodic Protection of Steel in Concrete and Masonry, 2nd ed.; CRC Press: London, UK, 2014. [Google Scholar]
- Broomfield, J.P. An overview of cathodic protection criteria for steel in atmospherically exposed concrete. Corros. Eng. Sci. Technol. 2020, 55, 303–310. [Google Scholar] [CrossRef]
- Bennett, J.; Turk, T. Technical Alert-Criteria for the Cathodic Protection of Reinforced Concrete Bridge Elements; SHRP Report SHRP-S-359; Transportation Research Board: Washington, DC, USA, 1994. [Google Scholar]
- Ansuini, F.J.; Dimond, J.R. Long-term field tests of reference electrodes for concrete-ten year results. In NACE Corrosion 2001; Paper No. 1296; NACI International: Houston, TX, USA, 2001. [Google Scholar]
- NACE. Reference Electrodes for Atmospherically Exposed Reinforced Concrete Structures, Publication 11100; NACE International: Houston, TX, USA, 2018. [Google Scholar]
- Concrete Society. Cathodic Protection of Steel in Concrete Including Model Specification; Technical Report 73; Concrete Society: Camberley, UK, 2011. [Google Scholar]
- Pedeferri, P. Principles of Cathodic Protection and Cathodic Prevention in Atmospherically Exposed Concrete Structures. In Corrosion of Reinforcement in Concrete-Monitoring, Prevention and Rehabilitation; European Federation of Corrosion Publication 25; Publisher Institute of Metal: London, UK, 1997; pp. 161–171. [Google Scholar]
- ISO. Cathodic Protection of Steel in Concrete; ISO: Geneva, Switzerland, 1997. [Google Scholar]
- Broomfield, J.P. A Case History of Cathodic Protection of a Highway Structure in the UK. In Proceedings of the Corrosion 2004; Paper 04344; NACE International: Houston, TX, USA, 2004. [Google Scholar]
- Hassanein, A.M.G.; Glass, G.K.; Buenfeld, N.R. Protection current distribution in reinforced concrete cathodic protection systems. Cem. Concr. Compos. 2002, 24, 159–167. [Google Scholar] [CrossRef]
- Bartholomew, J.; Bennett, J.; Turk, T.; Hartt, W.; Lankard, D.R.; Sagues, A.A. Control Criteria and Materials Performance Studies for Cathodic Protection of Reinforced Concrete; SHRP-S-670; Strategic Highway Research Program: Washington, DC, USA, 1993. [Google Scholar]
- Glass, G.K.; Buenfeld, N. Theoretical Basis for the Design of a Reinforced Concrete Cathodic Protection System. Brit. Corr. J. 1996, 32, 179–184. [Google Scholar] [CrossRef]
- Glass, G.K.; Hassanein, A.M.; Buenfeld, N.R. CP Criteria for Reinforced Concrete in Marine Exposure Zones. J. Mater. Civ. Eng. 2000, 12, 164–171. [Google Scholar] [CrossRef]
- Pedeferri, P. Cathodic protection and cathodic prevention. Constr. Build. Mater. 1996, 10, 391–402. [Google Scholar] [CrossRef]
- Callon, R. Cathodic protection of concrete caissons and deck of a ship repair yard. In NACE Corrosion 2016; Paper No. 7175; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Task Force 29 Report-Guide Specification for Cathodic Protection of Concrete Bridge Decks; AASHTO: Washington, DC, USA, 1994.
- Bennett, J.E.; Bushman, J.B.; Clear, K.C.; Kamp, R.N.; Swiat, W.J. Cathodic Protection of Concrete Bridges: A Manual of Practice, SHRP-S-372; National Research Council: Washington, DC, USA, 1993. [Google Scholar]
- Concrete Society. Technical Report 36 Cathodic Protection of Reinforced Concrete; Concrete Society: Camberley, UK, 1993. [Google Scholar]
- Concrete Society. Technical Report 37 Cathodic Protection of Reinforced Concrete: Model Specification; Concrete Society: Camberley, UK, 1989. [Google Scholar]
- CD 370 Cathodic Protection for Use in Reinforced Concrete Highway Structures Revision 1 (Formerly BA 83/02); Crown Copyright; Highways England: Guildford, UK, 2020.
- NACE SP 0290-2019 Impressed Current Cathodic Protection of Steel in Atmospherically Exposed Concrete Structures; NACE International: Houston, TX, USA, 2019.
- NACE. Cathodic Protection of Reinforcing Steel in Buried or Submerged Concrete Structures; NACE SP0408; NACE International: Houston, TX, USA, 2019. [Google Scholar]
- NACE. Sacrificial Cathodic Protection of Reinforcing Steel in Atmospherically Exposed Concrete Structures; SP0216-2016-SG.; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- NACE. Criteria for Evaluation of Cathodic Protection Methods for Steel in Existing Concrete Structures—A State-of-the-Art Report; TR 21463-2020; NACE International: Houston, TX, USA, 2020. [Google Scholar]
- Broomfield, J.P. An overview of US and European specifications and related documents for electrochemical treatments of reinforced concrete. Corros. Mater. 2006, 31, 8–12. [Google Scholar]
- Australian Standards. AS 2832.5-2008 Cathodic Protection of Metals-Steel in Concrete Structures; Australian Standards: Sydney, Australia, 2008. [Google Scholar]
- SABIC. Engineering Standard L02-E02 Cathodic Protection of Steel in Concrete; Saudi Arabia Basic Industries Corporation: Riyadh, Saudi Arabia, 2016. [Google Scholar]
- EN 15257:2006 Cathodic Protection–Competence Levels and Certification of Cathodic Protection Personnel; European Committee for Standardization: Brussels, Belgium, 2006.
- ISO 15257:2017 Cathodic Protection–Competence Levels of Cathodic Protection Persons–Basis for Certification Scheme; ISO: Geneva, Switzerland, 2017.
- NACE. Testing of Embeddable Impressed Current Anodes for Use in Cathodic Protection of Atmospherically Exposed Steel-Reinforced Concrete, NACE Standard TM0294-2016; NACE International: Houston TX, USA, 2016. [Google Scholar]
- ISO. Accelerated Life Test Method of Mixed Metal Oxide Anodes for Cathodic Protection-Part 1: Application–Concrete; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- NACE. Test Procedures for Organic-Based Conductive Coating Anodes for Use on Concrete Structures; NACE Standard test Method TM 0105-2018(21247); NACE International: Houston, TX, USA, 2018. [Google Scholar]
- American Welding Society. Specification for Thermal Spraying Zinc Anodes on Reinforced Concrete; AWS/ANSI Standard AWS C2.20/C2.20M:2016; American Welding Society: Miami, FL, USA, 2016. [Google Scholar]
- NACE. SP0390-2009 Maintenance and Rehabilitation Considerations for Corrosion Control of Atmospherically Exposed Existing Reinforced Concrete Structures (21044-SG); NACE International: Houston, TX, USA, 2009. [Google Scholar]
- British Standards Institution. Products and systems, for the protection and repair of concrete structures. In Definitions, Requirements, Quality Control and Evaluation of Conformity, Part 9: General Principles for Use of Products and Systems; British Standards Institution: London, UK, 2008. [Google Scholar]
- ACI. 222R-19 Guide–Protection of Reinforcing Steel in Concrete against Corrosion; American Concrete Institute: Farmington Hills, MI, USA, 2019. [Google Scholar]
- Available online: https://projects.bre.co.uk/rebarcorrosioncost/ (accessed on 28 September 2020).
- Chaudhary, Z. Cathodic Prevention of New Seawater Concrete Structures in Petrochemical Plants. In NACE Corrosion 2002, Paper 02260; NACE International: Houston, TX, USA, 2002. [Google Scholar]
- NACE Publication 01110 (2010), Stray-Current-Induced Corrosion in Reinforced and Prestressed Concrete Structures (24241-SG); NACE International: Houston, TX, USA, 2010.
- NACE SP21427-2019 Detection and Mitigation of Stray-Current Corrosion of Reinforced and Prestressed Concrete Structures; NACE International: Houston, TX, USA, 2019.
- Wyatt, B.S.; Schols, A.J.H.; Leppard, N.W.; John, D.G. Repair and cathodic protection of reinforced concrete substructures of four wharves at Mina Zayed, Abu Dhabi Part II, Concrete Repairs and application of cathodic protection system. In Proceedings of UK Corrosion 91, Manchester, UK, 22–25 October 1991; Institute of Corrosion: Northampton, UK, 1991. [Google Scholar]
- Bennett, J.E. Measurement of Steel Discontinuity. In Eltech Research Corp Interoffice Memo; Eltech: Cleveleand, OH, USA, 1990. [Google Scholar]
- Sergi, G.; Page, C.L. The Effects of Cathodic Protection on Alkali-Silica Reaction in Reinforced Concrete; Report 62; Transport Research Laboratory: Cowthorne, UK, 1994. [Google Scholar]
- Locke, C.E.; Deghanian, C.; Gibbs, L. Effect of Impressed Current on Bond Strength between Steel Rebar and Concrete. In NACE Corrosion 83; Paper 178; NACE International: Anaheim, CA, USA, 1983. [Google Scholar]
- SHRP-S-337 Cathodic Protection of Reinforced Concrete a State-of-the-Art Review; Strategic Highway Research Program; National Academies of Science: Washington, DC, USA, 1993; Available online: http://onlinepubs.trb.org/onlinepubs/shrp/SHRP-S-337.pdf (accessed on 25 November 2020).
- Virmani, Y.P.; Jones, W.R. Analysis of Concrete Cores from a Cathodically Protected Bridge Deck. Public Roads 1988, 51, 123–127. [Google Scholar]
- Buenfeld, N.R.; Broomfield, J.P. Influence of Electrochemical Chloride Extraction on the Bond between Steel and Concrete. Mag. Concr. Res. 2000, 52, 79–91. [Google Scholar] [CrossRef]
- NACE Publication 01102, State-of-the-Art Report Criteria for Cathodic Protection of Prestressed Concrete Structures (24217-SG); NACE International: Houston, TX, USA, 2002.
- Hartt, W.; Poeydomenge, A.; Stauder, A.L.; Scannell, W. Long Term Effects of Cathodic Protection on Prestressed Concrete Bridge Components; FHWA Report FHWA-RD-98-075; Federal Highway Administration: McLean, VA, USA, 1998. [Google Scholar]
- Pedeferri, P. Cathodic protection of new concrete constructions. In Proceedings of the International Conference on Structural improvement through Corrosion Protection of Reinforced Concrete, London, UK, 2–3 June 1992; IBC Technical Services Ltd.: London, UK, 1992. [Google Scholar]
- Broomfield, J.P. Acceptable Electrical Resistivities of Concrete Repairs for Cathodic Protection Systems; Technical Note 19; Corrosion Prevention Association: Bordon, UK, 2018. [Google Scholar]
- Polder, R.; Andrade, C.; Elsener, B.; Vennesland, O.; Gulikers, J.; Weidert, R.; Raupach, M. RILEM TC 154-EMC Electrochemical Techniques for Measuring Metallic Corrosion: Test Methods for On-Site Measurement of Resistivity of Concrete. Mater. Struct. 2000, 33, 603–611. [Google Scholar] [CrossRef]
- Russell, H.G. Concrete Bridge Deck Performance: A Synthesis of Highway Practice; NCHRP Synthesis report 333; Transportation Research Board: Washington, DC, USA, 2004. [Google Scholar]
- Sohanghpurwalla, A. A Synthesis of Highway Practice; NCHRP Synthesis Report 398; Transportation Research Board: Washington, DC, USA, 2009. [Google Scholar]
- Bottenberg, R. Cathodic Protection of Historic Bridges-Technology helps preserve legacies on the Oregon Coast Highway. Concr. Int. 2008, 30, 37–41. [Google Scholar]
- Gagne, M.; Martin, C. Protecting Reinforced concrete structures with external zinc anodes. Mater. Perform. 2020, 59, 36–38. [Google Scholar]
- Covino, B.S.; Cramer, S.D.; Bullard, S.J.; Holcomb, G.R.; Collins, W.K.; McGill, G.E. Thermal Spray Anodes for Impressed Current Cathodic Protection of Reinforced Concrete Structures. Mater. Perform. 1999, 38, 27–33. [Google Scholar]
- Ngala, V.; Broomfield, J.; El-Belbol, S. Impressed Current Anodes for Cathodic Protection; Technical Note 11; Corrosion Prevention Association: Bordon, UK, 2018. [Google Scholar]
- Girard, R.J. Experience with Cathodic Protection in Missouri. In Transportation Research Record 1113; Transportation Rsearch Board: Washington, DC, USA, 1987. [Google Scholar]
- Kessler, R.J.; Powers, R.G.; Lasa, I.R. An Update on the Long Term Use of Cathodic Protection of Steel Reinforced Concrete Marine Structures. In NACE Corrosion Conference; Paper No. 02254; NACE International: Houston, TX, USA, 2002. [Google Scholar]
- Akhoondan, M.; Pastor, J.; Clark, B.; Bell, G. Cathodic protection retrofit for rehabilitation of aging reinforced concrete municipal water infrastructure: A case study. In NACE Corrosion 2019; Paper 13002; NACE International: Houston, TX, USA, 2019. [Google Scholar]
- Polder, R.B.; Worm, D.; Courage, W.; Leegwater, G. Performance and working life of cathodic protection systems for concrete structures. In Concrete Solutions; Grantham, M., Mechtcherine, V., Schneck, U., Eds.; CRC Press: Dresden, Germany; Taylor & Francis: London, UK, 2011; pp. 157–162. [Google Scholar]
- Polder, R.B.; Peelen, W.H.A. Experience and Recent Innovations in Cathodic Protection of Steel in Concrete; Taylor and Francis: London, UK, 2014. [Google Scholar]
- Haldemann, C.H.; Schreyer, A. Ten Years of Cathodic Protection in Concrete in Switzerland. In Corrosion of Reinforcement in Concrete-Monitoring, Prevention and Rehabilitation European Federation of Corrosion Publication; Number 25; Institute of Materials: London, UK, 1997; pp. 184–197. [Google Scholar]
- Wyatt, B.S.; Lothian, A.M. Dunoon RO/RO Facility Cathodic Protection Engineering and Economic Factors in Selection Installation and Operation. In NACE Corrosion 89; Paper Number 378; NACE International: Houston, TX, USA, 1989. [Google Scholar]
- Rasheeduzzafar, F.H.; Algahtani, A.; Alsaadoun, S. Exposure site studies on the effect of cement composition on corrosion of reinforcing steel in concrete. Arab. J. Sci. Eng. 1989, 14, 235–248. [Google Scholar]
- Callon, R. Cathodic Protection at Durrat Al Bahrain. In NACE Corrosion Conference Rehabilitation of Reinforced Concrete Structures by Electrochemical and Other Techniques and Corrosion Protection Strategies for Construction; Paper No. 08303; NACE International: Houston, TX, USA, 2008. [Google Scholar]
- Chaudhary, Z.; Bairamov, A.K.; Fernandez, R.; Al-Mutlaq, F. Cathodic Protection Of Reinforced Concrete Seawater Structures in Petrochemical Plants. Mater. Perform. 2004, 43, 26–29. [Google Scholar]
- Broomfield, J.P.; Wyatt, B.S. Cathodic Protection of Steel in Concrete–the International Perspective; Technical Note No. 3; Corrosion Prevention Association: Bordon, UK, 2018. [Google Scholar]
- Broomfield, J.; Jacobs, W.R.; Mandeno, W.L.; Bruce, S.M. Cathodic Protection of the National War Memorial Carillon Tower, Wellington, New Zealand. Build. Pathol. 2002, 3, 288. [Google Scholar]
- Tettamenti, M.; Rossini, A.; Cheaitani, A. Cathodic Prevention and protection of Concrete Elements at the Sydney Opera House. Mater. Perform. 1997, 36, 21–25. [Google Scholar]
- Chaudhary, Z.; Al-Mutlaq, F.M.; Al-Beed, A. Condition Assessment and Cathodic Protection of a Reinforced Concrete Cooling Tower. In NACE Corrosion Conference Rehabilitation of Reinforced Concrete Structures by Electrochemical and Other Techniques and Corrosion Protection Strategies for Construction; Paper No. 08301; NACE International: Houston, TX, USA, 2008. [Google Scholar]
- Matthews, S.L.; Morlidge, J.R. What’s wrong: Concrete repair, solution or problem. In Concrete Solutions, Proceedings of the 2nd International Conference, St Malo, France, 27–29 June 2006; BRE Press: Watford, UK, 2006. [Google Scholar]
- Tilly, G. Past performance of concrete repairs. In Concrete Solutions, Proceedings of the 2nd International Conference, St Malo, France, 27–29 June 2006; BRE Press: Watford, UK, 2006. [Google Scholar]
- Broomfield, J.P. Impressed current protection for atmospherically exposed reinforced concrete structures. Mater. Perform. 2020, 59, 72. [Google Scholar]
- Dodds, W.; Christodoulou, C.; Goodier, C. Hybrid anode concrete corrosion protection–independent study. Proc. ICE Constr. Mater. 2018, 171, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Dodds, W.; Christodoulou, C.; Goodier, C.; Atkins, C.; Preston, J. Discussion Hybrid anode concrete corrosion protection–independent study. Proc. ICE Constr. Mater. 2018, 171, 265–270. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broomfield, J.P. A Historical Review of Impressed Current Cathodic Protection of Steel in Concrete. Constr. Mater. 2021, 1, 1-21. https://doi.org/10.3390/constrmater1010001
Broomfield JP. A Historical Review of Impressed Current Cathodic Protection of Steel in Concrete. Construction Materials. 2021; 1(1):1-21. https://doi.org/10.3390/constrmater1010001
Chicago/Turabian StyleBroomfield, John P. 2021. "A Historical Review of Impressed Current Cathodic Protection of Steel in Concrete" Construction Materials 1, no. 1: 1-21. https://doi.org/10.3390/constrmater1010001



