Optimal Temporal Windows for Mapping Fynbos Seep Wetlands Using Unmanned Aerial Vehicle Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. The Dominant Wetland Species
2.3. Reconnaisance and Acquisition of UAV Data
2.4. Processing UAV Data
2.5. Creating Spectral Indices
2.6. Classification of the Wetland Species
2.7. Statistical Analysis
3. Results and Discussion
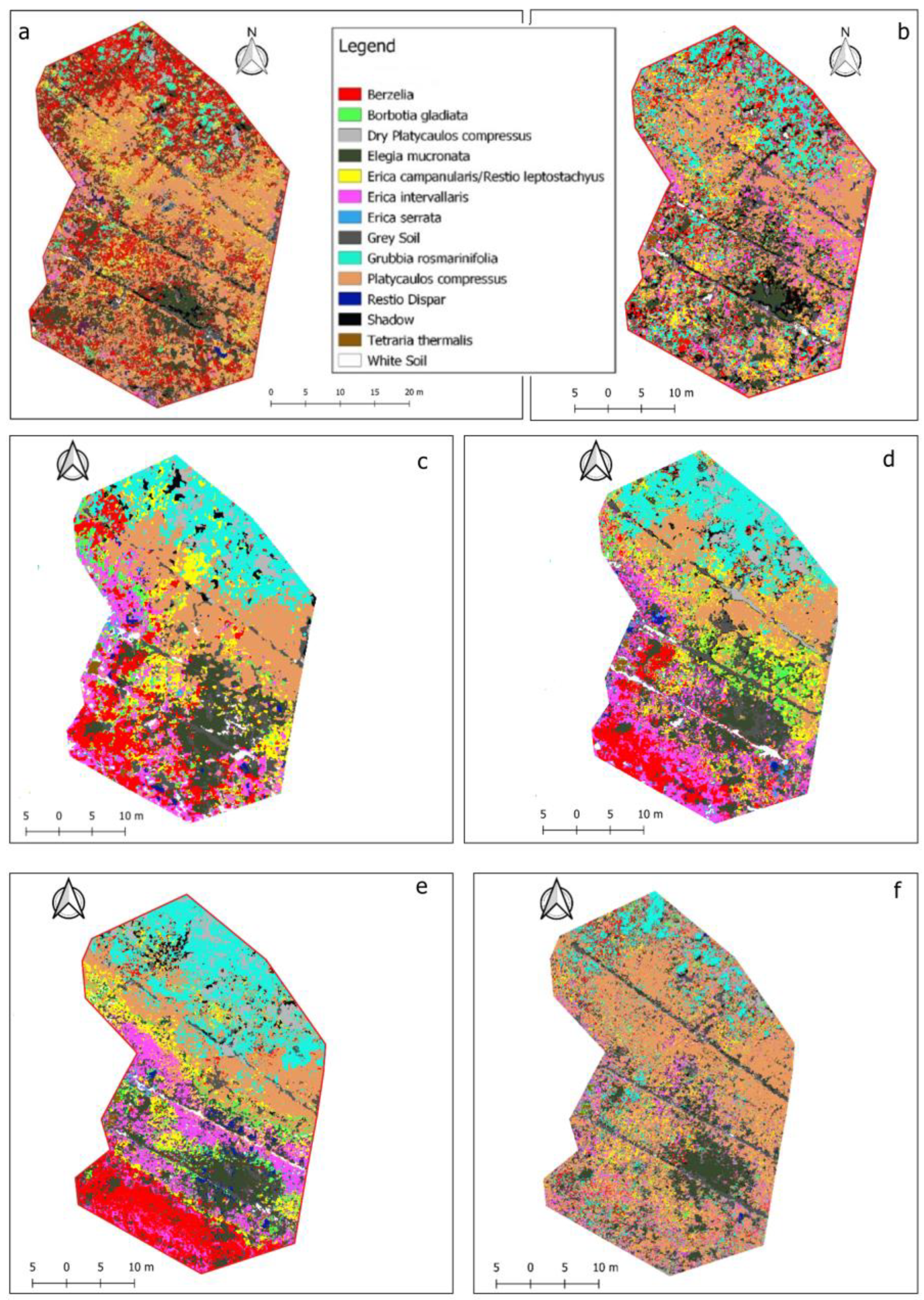
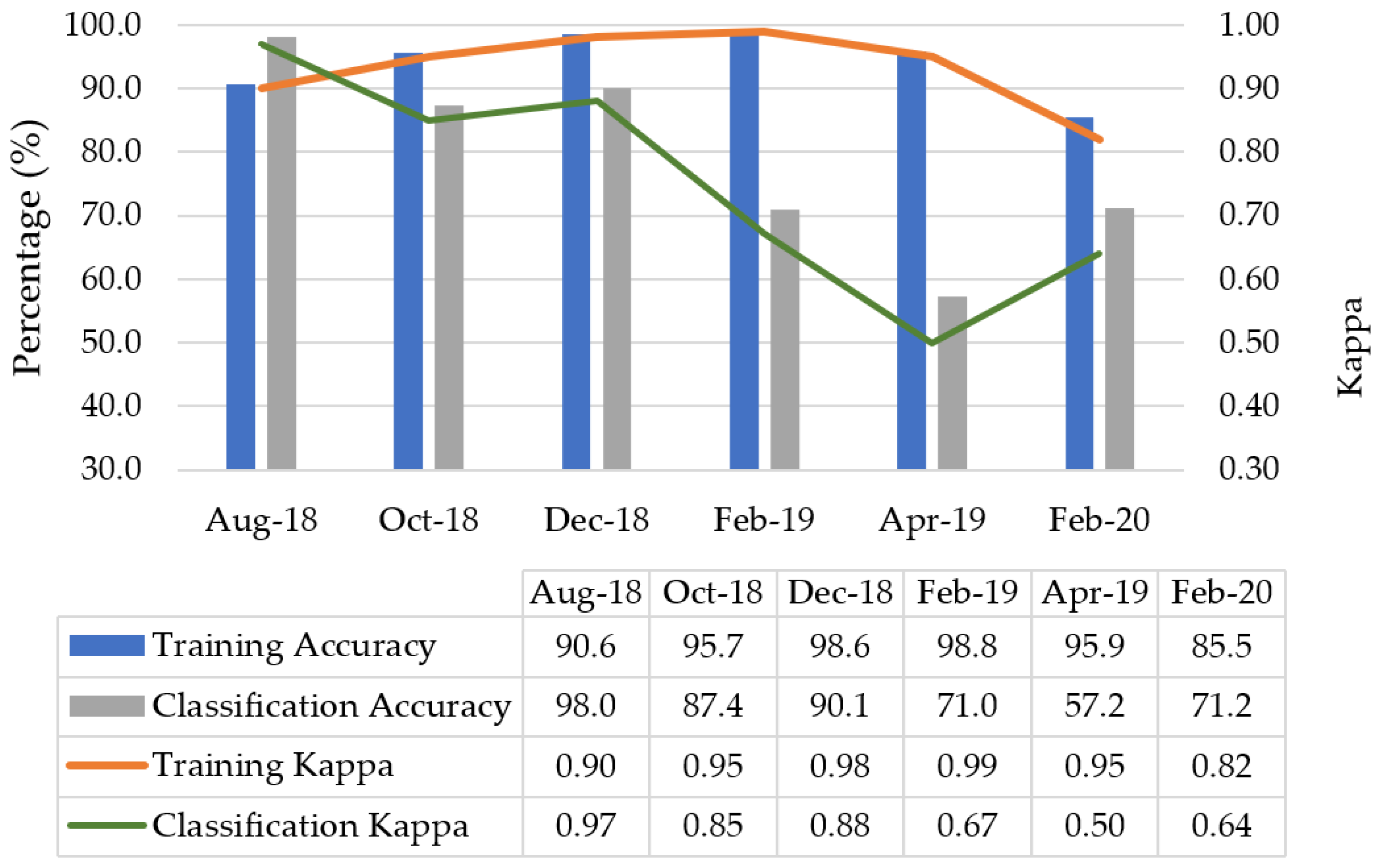
3.1. Seasonal Classification Results
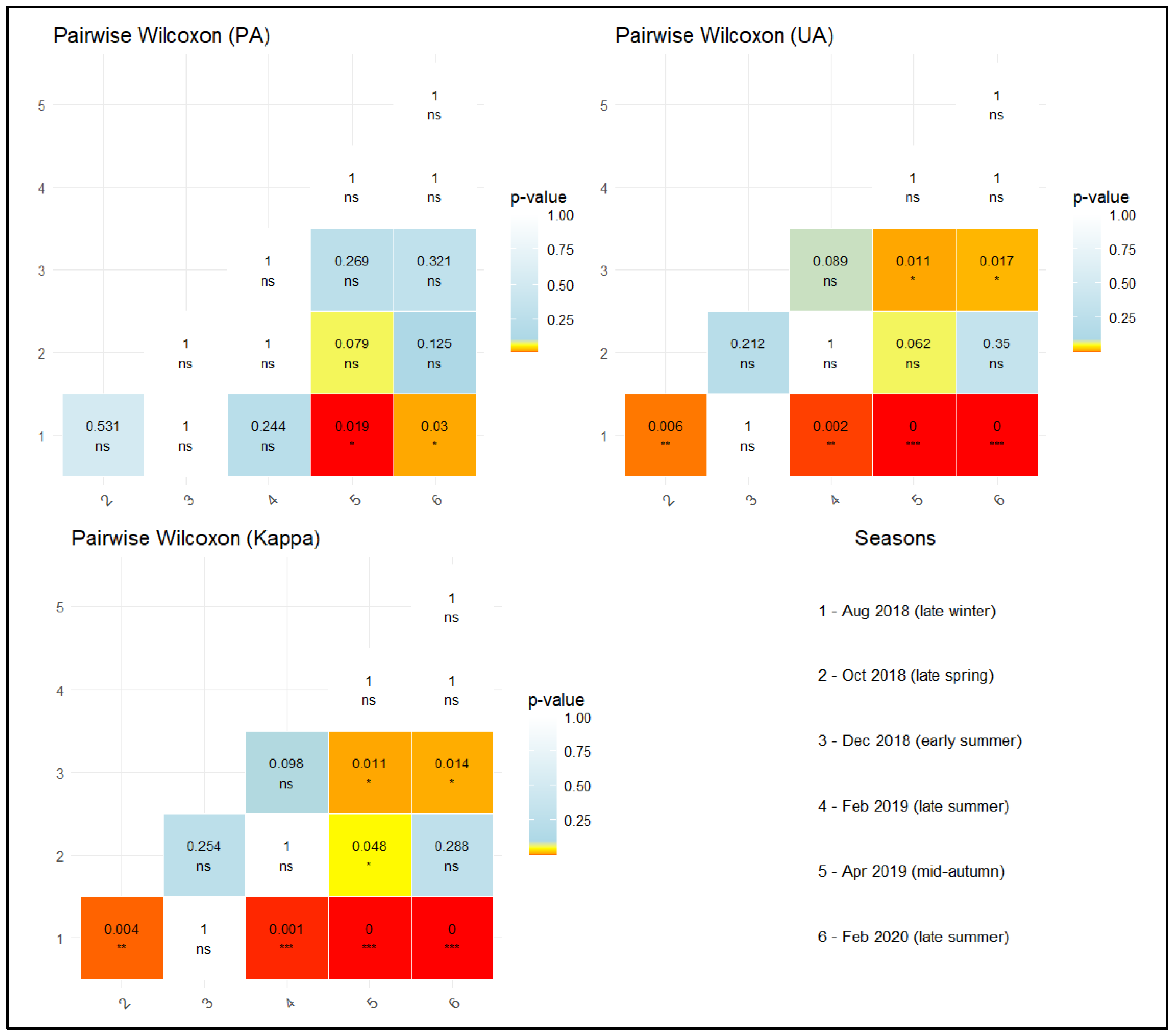
3.2. Temporal Insights into Species-Specific Classification Accuracy
3.3. Cross-Sensor Evaluation of Seasonal Species Classification Accuracy
3.4. Optimizing Remote Sensing Data Collection in CFR Seep Wetlands
4. Conclusions
4.1. Seasonal Classification Results
4.2. Temporal Insights into Species-Specific Classification Accuracy
4.3. Cross-Sensor Evaluation of Seasonal Species Classification Accuracy
4.4. Future Work: Leveraging Emerging Technologies for Improved Wetland Vegetation Mapping
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esterhuizen, N.; Forrester, J.; Esler, K.J.; Wigley-Coetzee, C.; Morcillo, R.J.; Kleinert, A.; Pérez-Fernández, M.; Valentine, A.J. Nitrogen and Phosphorus Influence Acacia Saligna Invasiveness in the Fynbos Biome. Plant Ecol. 2020, 221, 309–320. [Google Scholar] [CrossRef]
- Kraaij, T.; Baard, J.A.; Rikhotso, D.R.; Cole, N.S.; Van Wilgen, B.W. Assessing the Effectiveness of Invasive Alien Plant Management in a Large Fynbos Protected Area. Bothalia 2017, 47, a2105. [Google Scholar] [CrossRef]
- Hall, S.A.; Bastos, R.; Vicente, J.; Vaz, A.S.; Honrado, J.P.; Holmes, P.M.; Gaertner, M.; Esler, K.J.; Cabral, J.A. A Dynamic Modeling Tool to Anticipate the Effectiveness of Invasive Plant Control and Restoration Recovery Trajectories in South African Fynbos. Restor. Ecol. 2021, 29, e13324. [Google Scholar] [CrossRef]
- Cardoso, A.W.; Brodrick, P.G.; Wilson, A.M.; Slingsby, J.A.; Forbes, C.J.; Thornton, M.; Hestir, E.L. Increasing Data Access in Multi-Sensor Airborne Campaigns: Lessons from BioSCape in South Africa. In Proceedings of the IGARSS 2024—2024 IEEE International Geoscience and Remote Sensing Symposium, Athens, Greece, 7–12 July 2024; pp. 2898–2901. [Google Scholar]
- Thamaga, K.H.; Dube, T.; Shoko, C. Evaluating the Impact of Land Use and Land Cover Change on Unprotected Wetland Ecosystems in the Arid-Tropical Areas of South Africa Using the Landsat Dataset and Support Vector Machine. Geocarto Int. 2022, 37, 10344–10365. [Google Scholar] [CrossRef]
- Xulu, S.; Mbatha, N.; Peerbhay, K. Burned Area Mapping over the Southern Cape Forestry Region, South Africa Using Sentinel Data within GEE Cloud Platform. ISPRS Int. J. Geoinf. 2021, 10, 511. [Google Scholar] [CrossRef]
- van Blerk, J.J.; West, A.G.; Smit, J.; Altwegg, R.; Hoffman, M.T. UAVs Improve Detection of Seasonal Growth Responses During Post-Fire Shrubland Recovery. Landsc. Ecol. 2022, 37, 3179–3199. [Google Scholar] [CrossRef]
- Jovanovic, N.; Garcia, C.L.; Bugan, R.D.H.; Teich, I.; Rodriguez, C.M.G. Validation of Remotely-Sensed Evapotranspiration and NDWI Using Ground Measurements at Riverlands, South Africa. Water SA 2014, 40, 211–220. [Google Scholar] [CrossRef]
- Skowno, A.L.; Jewitt, D.; Slingsby, J.A. Rates and Patterns of Habitat Loss across South Africa’s Vegetation Biomes. S. Afr. J. Sci. 2021, 117, 1–6. [Google Scholar] [CrossRef]
- Chiloane, C.; Dube, T.; Shoko, C. Multispectral Remote Sensing of Potential Groundwater Dependent Vegetation in the Greater Floristic Region of the Western Cape, South Africa. S. Afr. Geogr. J. 2023, 105, 481–499. [Google Scholar] [CrossRef]
- Duncan, P.; Podest, E.; Esler, K.J.; Geerts, S.; Lyons, C. Mapping Invasive Herbaceous Plant Species with Sentinel-2 Satellite Imagery: Echium Plantagineum in a Mediterranean Shrubland as a Case Study. Geomatics 2023, 3, 328–344. [Google Scholar] [CrossRef]
- Slingsby, J.A.; Wilson, A.M.; Maitner, B.; Moncrieff, G.R. Regional Ecological Forecasting across Scales: A Manifesto for a Biodiversity Hotspot. Methods Ecol. Evol. 2023, 14, 757–770. [Google Scholar] [CrossRef]
- van Blerk, J.J.; Slingsby, J.A.; West, A.G. Unpacking Satellite Pixels: UAVs Reveal Fine-Scale Drivers of Land Surface Phenology in a Winter Rainfall Shrubland. Environ. Res. Lett. 2024, 19, 084008. [Google Scholar] [CrossRef]
- van Deventer, H.; Naidoo, L.; Apleni, P.; le Roux, J.; Blaauw, C.; Nel, W.; Tema, H. Remote Sensing Monitoring of Soil Moisture for South African. Water Wheel 2025, 24, 28–31. [Google Scholar]
- Musungu, K.; Dube, T.; Smit, J.; Shoko, M. Using UAV Multispectral Photography to Discriminate Plant Species in a Seep Wetland of the Fynbos Biome. Wetl. Ecol. Manag. 2024, 32, 207–227. [Google Scholar] [CrossRef]
- Walmsley, R. A Description of the Wetlands Research Programme; Foundation for Research Development: Pretoria, South Africa, 1988; Volume 145. [Google Scholar]
- Liu, H.; Liao, T.; Wang, Y.; Qian, X.; Liu, X.; Li, C.; Li, S.; Guan, Z.; Zhu, L.; Zhou, X.; et al. Fine-Grained Wetland Classification for National Wetland Reserves Using Multi-Source Remote Sensing Data and Pixel Information Expert Engine (PIE-Engine). GIsci. Remote Sens. 2023, 60, 2286746. [Google Scholar] [CrossRef]
- McDonald, A.; Gillespie, J. Beyond the Selective Regulation of Wetlands: Towards a Rights of Wetlands. Aust. Geogr. 2024, 55, 329–344. [Google Scholar] [CrossRef]
- Grenfell, M.C.; Abrahams, E.; Fisher, R.-M. Analysis and Conceptual Geospatial Modelling of the Intermediary Role of Wetlands in Drylands in Post-Fire Material Flux Dynamics, Silvermine River Catchment, Cape Town. Wetl. Ecol. Manag. 2022, 30, 623–645. [Google Scholar] [CrossRef]
- van Blerk, J.J.; Cramer, M.D.; West, A.G.; Musker, S.D.; Verboom, G.A. The Contribution of Hydric Habitats to the Richness of the Cape Fynbos Flora. Divers. Distrib. 2025, 31, e13962. [Google Scholar] [CrossRef]
- Chi, J.; Afandi, S.H.M.; Matthew, N.K.A. Mapping Hotspots and Emerging Trends in Global Wetlands Research: A Scientometric Analysis (2002–2022). J. Environ. Earth Sci. 2025, 7, 101–116. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Z.; Zhang, Y.; Sun, T.; Wang, W.; Zhang, Z. Spatiotemporal Evolution and Driving Mechanisms of Ecological Risk in the Yuncheng Salt Lake Wetland, China. Water 2025, 17, 524. [Google Scholar] [CrossRef]
- Kaiss, R.; Benjouid, Z.; Snoussi, N.; El Mountassir, E.K.; Nabil, N.; Halim, A.; Zarouali, S.S. Impact of Climate Change on Water Resources and Ecological Sustainability in Morocco: A 1990–2022 Analysis. Res. Ecol. 2025, 7, 53–70. [Google Scholar] [CrossRef]
- Sadiki, M.; van Deventer, H.; Hansen, C. Assessing the Extent to Which African Wetland Inventories Can Report to the Global Targets on Biodiversity, Including Goal A of the Global Biodiversity Framework. Afr. J. Aquat. Sci. 2024, 49, 254–261. [Google Scholar] [CrossRef]
- Servat, G.P.; Alcocer, R.; Larico, M.V.; Olarte, M.E.; Linares-Palomino, R.; Alonso, A.; Ledesma, K. The Effects of Area and Habitat Heterogeneity on Bird Richness and Composition in High Elevation Wetlands (“Bofedales”) of the Central Andes of Peru. Wetlands 2018, 38, 1133–1145. [Google Scholar] [CrossRef]
- Stephenson, P.J.; Ntiamoa-Baidu, Y.; Simaika, J.P. The Use of Traditional and Modern Tools for Monitoring Wetlands Biodiversity in Africa: Challenges and Opportunities. Front. Environ. Sci. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Dube, T.; Musasa, T. Wetland Vegetation Species Diversity and Productivity Assessment: Implications on Ecosystem Services. In Revealing Ecosystem Services Through Geospatial Technologies; Mutanga, O., Pandey, P.C., Das, S., Chatterjee, U., Eds.; Spinger Nature: Cham, Switzerland, 2025; pp. 171–184. [Google Scholar]
- Faelga, R.A.; Assiri, M.; Silvestri, S. UAV Photogrammetry-Based Leaf Area Index for Above-Ground Biomass Estimation in Wetlands. IEEE J. Sel. Top Appl. Earth Obs. Remote Sens. 2025, 18, 13844–13861. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, L.; Drahota, J.; Woldt, W.; Varner, D.; Bishop, A.; LaGrange, T.; Neale, C.M.U.; Tang, Z. Combining Multi-View UAV Photogrammetry, Thermal Imaging, and Computer Vision Can Derive Cost-Effective Ecological Indicators for Habitat Assessment. Remote Sens. 2024, 16, 1081. [Google Scholar] [CrossRef]
- Fromm, L.T.; Smith, L.C.; Kyzivat, E.D. Wetland Vegetation Mapping Improved by Phenological Leveraging of Multitemporal Nanosatellite Images. Geocarto Int. 2025, 40, 2452252. [Google Scholar] [CrossRef]
- Hill, W.; Unwin, M.; Eckersley, S.; Bernadini, N.; Turconi, A.; Da Silva Curiel, A.; Cardellach, E.; Pirat, C. HydroSwarm: Using a Cooperative Swarm of CubeSats to Enhance GNSS-R Capabilities for Surface Soil Moisture and Inundation Measurements. In Proceedings of the Small Satellites Systems and Services Symposium (4S 2024), Palma de Mallorca, Spain, 27–31 May 2024; Petrozzi-Ilstad, M., Ed.; SPIE: Bellingham, WA, USA, 2025; p. 113. [Google Scholar]
- Alizadeh Moghaddam, S.H.; Gazor, S.; Homayouni, S.; Karami, F. Multiscale Deformable DenseNet for Wetland Mapping Using Hyperspectral Images. IEEE Geosci. Remote Sens. Lett. 2025, 22, 1–5. [Google Scholar] [CrossRef]
- Magidi, J.; Bangira, T.; Kelepile, M.; Shoko, M. Land Use and Land Cover Changes in Notwane Watershed, Botswana, Using Extreme Gradient Boost (XGBoost) Machine Learning Algorithm. Afr. Geogr. Rev. 2024, 44, 497–517. [Google Scholar] [CrossRef]
- Solórzano, J.V.; Peralta-Carreta, C.; Gallardo-Cruz, J.A. Mapping Tropical Forested Wetlands Biomass with LiDAR: A Machine Learning Comparison. Remote Sens. 2025, 17, 1076. [Google Scholar] [CrossRef]
- Paudel, A.; Richardson, M.; King, D. Multispectral and LiDAR-Derived Vegetation Indicators of Water Table Dynamics in Forested Wetlands. Can. J. Remote Sens. 2025, 51, 2471502. [Google Scholar] [CrossRef]
- Deng, Y.; Shi, R.; Zhang, C.; Wang, X.; Liu, C.; Gao, W. Wetland Vegetation Species Classification Using Optical and SAR Remote Sensing Images: A Case Study of Chongming Island, Shanghai, China. Chin. Geogr. Sci. 2025, 35, 510–527. [Google Scholar] [CrossRef]
- Varugu, B.K.; Jones, C.E.; Oliver-Cabrera, T.; Simard, M.; Jensen, D.J. Study of Hydrologic Connectivity and Tidal Influence on Water Flow Within Louisiana Coastal Wetlands Using Rapid-Repeat Interferometric Synthetic Aperture Radar. Remote Sens. 2025, 17, 459. [Google Scholar] [CrossRef]
- Taddeo, S.; Dronova, I.; Depsky, N. Spectral Vegetation Indices of Wetland Greenness: Responses to Vegetation Structure, Composition, and Spatial Distribution. Remote Sens. Environ. 2019, 234, 111467. [Google Scholar] [CrossRef]
- Miller, G.J.; Dronova, I.; Oikawa, P.Y.; Knox, S.H.; Windham-Myers, L.; Shahan, J.; Stuart-Haëntjens, E. The Potential of Satellite Remote Sensing Time Series to Uncover Wetland Phenology under Unique Challenges of Tidal Setting. Remote Sens. 2021, 13, 3589. [Google Scholar] [CrossRef]
- Borges, J.; Higginbottom, T.P.; Symeonakis, E.; Jones, M. Sentinel-1 and Sentinel-2 Data for Savannah Land Cover Mapping: Optimising the Combination of Sensors and Seasons. Remote Sens. 2020, 12, 3862. [Google Scholar] [CrossRef]
- Pargieła, K. Optimising UAV Data Acquisition and Processing for Photogrammetry: A Review. Geomat. Environ. Eng. 2023, 17, 29–59. [Google Scholar] [CrossRef]
- Surasinghe, T.D.; Singh, K.K.; Smart, L.S. Leveraging Phenology to Assess Seasonal Variations of Plant Communities for Mapping Dynamic Ecosystems. Remote Sens. 2025, 17, 1778. [Google Scholar] [CrossRef]
- Im, J.; Jensen, J.R. Hyperspectral Remote Sensing of Vegetation. Geogr. Compass 2008, 2, 1943–1961. [Google Scholar] [CrossRef]
- Conrad, C.; Dech, S.; Dubovyk, O.; Fritsch, S.; Klein, D.; Löw, F.; Schorcht, G.; Zeidler, J. Derivation of Temporal Windows for Accurate Crop Discrimination in Heterogeneous Croplands of Uzbekistan Using Multitemporal RapidEye Images. Comput. Electron. Agric. 2014, 103, 63–74. [Google Scholar] [CrossRef]
- Jönsson, P.; Cai, Z.; Melaas, E.; Friedl, M.; Eklundh, L. A Method for Robust Estimation of Vegetation Seasonality from Landsat and Sentinel-2 Time Series Data. Remote Sens. 2018, 10, 635. [Google Scholar] [CrossRef]
- Kang, X.; Hao, Y.; Cui, X.; Chen, H.; Huang, S.; Du, Y.; Li, W.; Kardol, P.; Xiao, X.; Cui, L. Variability and Changes in Climate, Phenology, and Gross Primary Production of an Alpine Wetland Ecosystem. Remote Sens. 2016, 8, 391. [Google Scholar] [CrossRef]
- Ai, J.; Gao, W.; Gao, Z.; Shi, R.; Zhang, C. Phenology-Based Spartina Alterniflora Mapping in Coastal Wetland of the Yangtze Estuary Using Time Series of GaoFen Satellite No. 1 Wide Field of View Imagery. J. Appl. Remote Sens. 2017, 11, 026020. [Google Scholar] [CrossRef]
- Fan, C.; Yang, J.; Zhao, G.; Dai, J.; Zhu, M.; Dong, J.; Liu, R.; Zhang, G. Mapping Phenology of Complicated Wetland Landscapes through Harmonizing Landsat and Sentinel-2 Imagery. Remote Sens. 2023, 15, 2413. [Google Scholar] [CrossRef]
- Larkin, D.J. Wetland Heterogeneity. In The Wetland Book; Springer: Dordrecht, The Netherlands, 2018; pp. 177–182. ISBN 9789048196593. [Google Scholar]
- Nhamo, L.; Magidi, J.; Dickens, C. Determining Wetland Spatial Extent and Seasonal Variations of the Inundated Area Using Multispectral Remote Sensing. Water SA 2017, 43, 543–552. [Google Scholar] [CrossRef]
- Torbick, N.; Salas, W. Mapping Agricultural Wetlands in the Sacramento Valley, USA with Satellite Remote Sensing. Wetl. Ecol. Manag. 2015, 23, 79–94. [Google Scholar] [CrossRef]
- Dronova, I.; Taddeo, S.; Harris, K. Plant Diversity Reduces Satellite-Observed Phenological Variability in Wetlands at a National Scale. Sci. Adv. 2022, 8, 8214. [Google Scholar] [CrossRef]
- Kovács, G.M.; Horion, S.; Fensholt, R. Characterizing Ecosystem Change in Wetlands Using Dense Earth Observation Time Series. Remote Sens. Environ. 2022, 281, 113267. [Google Scholar] [CrossRef]
- Van Deventer, H. Remote Sensing of Wetland Tree Species in the ISimangaliso Wetland Park, KwaZulu-Natal, South Africa; University of KwaZulu-Natal: Pietermaritzburg, South Africa, 2016. [Google Scholar]
- van Deventer, H.; Cho, M.A.; Mutanga, O. Multi-Season RapidEye Imagery Improves the Classification of Wetland and Dryland Communities in a Subtropical Coastal Region. ISPRS J. Photogramm. Remote Sens. 2019, 157, 171–187. [Google Scholar] [CrossRef]
- Musungu, K.; Shoko, M.; Smit, J. Determining the Spectral Characteristics of Fynbos Wetland Vegetation Species Using Unmanned Aerial Vehicle Data. Geomatics 2025, 5, 17. [Google Scholar] [CrossRef]
- Mu, X.; Hu, M.; Song, W.; Ruan, G.; Ge, Y.; Wang, J.; Huang, S.; Yan, G. Evaluation of Sampling Methods for Validation of Remotely Sensed Fractional Vegetation Cover. Remote Sens. 2015, 7, 16164–16182. [Google Scholar] [CrossRef]
- Shetty, S.; Gupta, P.K.; Belgiu, M.; Srivastav, S.K. Assessing the Effect of Training Sampling Design on the Performance of Machine Learning Classifiers for Land Cover Mapping Using Multi-Temporal Remote Sensing Data and Google Earth Engine. Remote Sens. 2021, 13, 1433. [Google Scholar] [CrossRef]
- Cao, S.; Danielson, B.; Clare, S.; Koenig, S.; Campos-Vargas, C.; Sanchez-Azofeifa, A. Radiometric Calibration Assessments for UAS-Borne Multispectral Cameras: Laboratory and Field Protocols. ISPRS J. Photogramm. Remote Sens. 2019, 149, 132–145. [Google Scholar] [CrossRef]
- Daniels, L.; Eeckhout, E.; Wieme, J.; Dejaegher, Y.; Audenaert, K.; Maes, W.H. Identifying the Optimal Radiometric Calibration Method for UAV-Based Multispectral Imaging. Remote Sens. 2023, 15, 2909. [Google Scholar] [CrossRef]
- Guo, Y.; Senthilnath, J.; Wu, W.; Zhang, X.; Zeng, Z.; Huang, H. Radiometric Calibration for Multispectral Camera of Different Imaging Conditions Mounted on a UAV Platform. Sustainability 2019, 11, 978. [Google Scholar] [CrossRef]
- Tmušić, G.; Manfreda, S.; Aasen, H.; James, M.R.; Gonçalves, G.; Ben-Dor, E.; Brook, A.; Polinova, M.; Arranz, J.J.; Mészáros, J.; et al. Current Practices in UAS-Based Environmental Monitoring. Remote Sens. 2020, 12, 1001. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, Y.; An, Z.; Zhang, H.; Han, Y.; Zhao, Z.; Li, F.; Zhang, C.; Hou, C. Assessing Radiometric Calibration Methods for Multispectral UAV Imagery and the Influence of Illumination, Flight Altitude and Flight Time on Reflectance, Vegetation Index and Inversion of Winter Wheat AGB and LAI. Comput. Electron. Agric. 2024, 219, 108821. [Google Scholar] [CrossRef]
- Solymosi, K.; Kövér, G.; Romvári, R. The Progression of Vegetation Indices: A Short Overview. Acta Agrar. Kaposváriensis 2019, 23, 75–90. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Bhagat, V.; Kada, A.; Kumar, S. Analysis of Remote Sensing Based Vegetation Indices (VIs) for Unmanned Aerial System (UAS): A Review. Remote Sens. Land 2020, 3, 58–73. [Google Scholar] [CrossRef]
- Giovos, R.; Tassopoulos, D.; Kalivas, D.; Lougkos, N.; Priovolou, A. Remote Sensing Vegetation Indices in Viticulture: A Critical Review. Agriculture 2021, 11, 457. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, Y.; Gowda, P.H.; Dong, J.; Zhang, G.; Kakani, V.G.; Wagle, P.; Chen, L.; Flynn, K.C.; Jiang, W. Application of the Water-Related Spectral Reflectance Indices: A Review. Ecol. Indic. 2019, 98, 68–79. [Google Scholar] [CrossRef]
- Poley, L.; McDermid, G. A Systematic Review of the Factors Influencing the Estimation of Vegetation Aboveground Biomass Using Unmanned Aerial Systems. Remote Sens. 2020, 12, 1052. [Google Scholar] [CrossRef]
- Walsh, O.S.; Shafian, S.; Marshall, J.M.; Jackson, C.; McClintick-Chess, J.R.; Blanscet, S.M.; Swoboda, K.; Thompson, C.; Belmont, K.M.; Walsh, W.L. Assessment of UAV Based Vegetation Indices for Nitrogen Concentration Estimation in Spring Wheat. Adv. Remote Sens. 2018, 7, 71–90. [Google Scholar] [CrossRef]
- Guirado, E.; Tabik, S.; Alcaraz-Segura, D.; Cabello, J.; Herrera, F. Deep-Learning Versus OBIA for Scattered Shrub Detection with Google Earth Imagery: Ziziphus Lotus as Case Study. Remote Sens. 2017, 9, 1220. [Google Scholar] [CrossRef]
- Dronova, I. Object-Based Image Analysis in Wetland Research: A Review. Remote Sens. 2015, 7, 6380–6413. [Google Scholar] [CrossRef]
- Veljanovski, T.; Kanjir, U.; Oštir, K. Object-Based Image Analysis of Remote Sensing Data. Geod. Vestn. 2011, 55, 641–688. [Google Scholar] [CrossRef]
- Slagter, B.; Tsendbazar, N.E.; Vollrath, A.; Reiche, J. Mapping Wetland Characteristics Using Temporally Dense Sentinel-1 and Sentinel-2 Data: A Case Study in the St. Lucia Wetlands, South Africa. Int. J. Appl. Earth Obs. Geoinf. 2020, 86, 102009. [Google Scholar] [CrossRef]
- van Deventer, H.; Linström, A.; Naidoo, L.; Job, N.; Sieben, E.J.J.; Cho, M.A. Comparison between Sentinel-2 and WorldView-3 Sensors in Mapping Wetland Vegetation Communities of the Grassland Biome of South Africa, for Monitoring under Climate Change. Remote Sens. Appl. 2022, 28, 100875. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Hao, Y.Y.; Wang, Y.C.; Zhou, S.Q.; Wu, W.B.; Yuan, Q.; Gao, Y.; Guo, H.Q.; Cai, X.X.; Zhao, B. Coastal Wetland Vegetation Classification Using Pixel-Based, Object-Based and Deep Learning Methods Based on RGB-UAV. Land 2022, 11, 2039. [Google Scholar] [CrossRef]
- Simioni, J.P.D.; Guasselli, L.A. Dual-Season Comparison of OBIA and Pixel-Based Approaches for Coastal Wetland Classification. Rev. Bras. Recur. Hidr. 2024, 29, e5. [Google Scholar] [CrossRef]
- Tonyaloğlu, E.E.; Erdoğan, N.; Çavdar, B.; Kurtşan, K.; Nurlu, E. Comparison of Pixel and Object Based Classification Methods on Rapideye Satellite Image. Turk. J. For. Sci. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Dervisoglu, A.; Bilgilioglu, B.B.; Yağmur, N. Comparison of Pixel-Based and Object-Based Classification Methods in Determination of Wetland Coastline. Int. J. Environ. Geoinform. 2020, 7, 213–220. [Google Scholar] [CrossRef]
- Islam, M.K.; Simic Milas, A.; Abeysinghe, T.; Tian, Q. Integrating UAV-Derived Information and WorldView-3 Imagery for Mapping Wetland Plants in the Old Woman Creek Estuary, USA. Remote Sens. 2023, 15, 1090. [Google Scholar] [CrossRef]
- Abeysinghe, T.; Simic Milas, A.; Arend, K.; Hohman, B.; Reil, P.; Gregory, A.; Vázquez-Ortega, A. Mapping Invasive Phragmites Australis in the Old Woman Creek Estuary Using UAV Remote Sensing and Machine Learning Classifiers. Remote Sens. 2019, 11, 1380. [Google Scholar] [CrossRef]
- De Giglio, M.; Greggio, N.; Goffo, F.; Merloni, N.; Dubbini, M.; Barbarella, M. Comparison of Pixel- and Object-Based Classification Methods of Unmanned Aerial Vehicle Data Applied to Coastal Dune Vegetation Communities: Casal Borsetti Case Study. Remote Sens. 2019, 11, 1416. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Probst, P.; Wright, M.N.; Boulesteix, A. Hyperparameters and Tuning Strategies for Random Forest. WIREs Data Min. Knowl. Discov. 2019, 9, e1301. [Google Scholar] [CrossRef]
- Speiser, J.L.; Miller, M.E.; Tooze, J.; Ip, E. A Comparison of Random Forest Variable Selection Methods for Classification Prediction Modeling. Expert Syst. Appl. 2019, 134, 93–101. [Google Scholar] [CrossRef]
- Berhane, T.M.; Costa, H.; Lane, C.R.; Anenkhonov, O.A.; Chepinoga, V.V.; Autrey, B.C. The Influence of Region of Interest Heterogeneity on Classification Accuracy in Wetland Systems. Remote Sens. 2019, 11, 551. [Google Scholar] [CrossRef]
- Deshambo, A.; Stewart, E.C.; Ulik, S.; Williams, J.M. Delineating Wetland Vegetation Species Using a UAV-Mounted Multi-Spectral Camera and Computer-Aided Classification. Proc. Wis. Space Conf. 2023, 1, 1. [Google Scholar] [CrossRef]
- Martínez Prentice, R.; Villoslada Peciña, M.; Ward, R.D.; Bergamo, T.F.; Joyce, C.B.; Sepp, K. Machine Learning Classification and Accuracy Assessment from High-Resolution Images of Coastal Wetlands. Remote Sens. 2021, 13, 3669. [Google Scholar] [CrossRef]
- Okolie, C.; Adeleke, A.; Mills, J.; Smit, J.; Maduako, I.; Bagheri, H.; Komar, T.; Wang, S. Assessment of Explainable Tree-Based Ensemble Algorithms for the Enhancement of Copernicus Digital Elevation Model in Agricultural Lands. Int. J. Image Data Fusion 2024, 15, 430–460. [Google Scholar] [CrossRef]
- Breiman, L. Bagging Predictors. Mach. Learn. 1996, 24, 123–140. [Google Scholar] [CrossRef]
- Eisavi, V.; Homayouni, S.; Yazdi, A.M.; Alimohammadi, A. Land Cover Mapping Based on Random Forest Classification of Multitemporal Spectral and Thermal Images. Environ. Monit. Assess 2015, 187, 291. [Google Scholar] [CrossRef]
- Ghasemian, N.; Akhoondzadeh, M. Introducing Two Random Forest Based Methods for Cloud Detection in Remote Sensing Images. Adv. Space Res. 2018, 62, 288–303. [Google Scholar] [CrossRef]
- Karasiak, N. Dzetsaka QGIS Classification Plugin 2016. Available online: https://github.com/nkarasiak/dzetsaka (accessed on 18 August 2025).
- Sovann, C.; Olin, S.; Mansourian, A.; Sakhoeun, S.; Prey, S.; Kok, S.; Tagesson, T. Importance of Spectral Information, Seasonality, and Topography on Land Cover Classification of Tropical Land Cover Mapping. Remote Sens. 2025, 17, 1551. [Google Scholar] [CrossRef]
- Congedo, L. Semi-Automatic Classification Plugin: A Python Tool for the Download and Processing of Remote Sensing Images in QGIS. J. Open Source Softw. 2021, 6, 3172. [Google Scholar] [CrossRef]
- Kim, S.; Lee, C.W.; Park, H.-J.; Hwang, J.E.; Park, H.B.; Yoon, Y.-J.; Kim, Y.-J. UAV Telephotography Elucidates Floristic Variability and Beta Diversity of Island Cliffs Under Grazing Interventions. Sci. Rep. 2024, 14, 15465. [Google Scholar] [CrossRef]
- Surasinghe, T.D.; Chen, Y.; Singh, K.K. Restored Wetlands Show Rapid Vegetation Recovery and Substantial Surface-water Expansion. Restor. Ecol. 2025, 33, e70046. [Google Scholar] [CrossRef]
- Jenačković, D.D.; Lakušić, D.; Zlatković, I.; Jušković, M.; Ranđelović, N.V. Emergent Wetland Vegetation Data Recording: Does an Optimal Period Exist? Appl. Veg. Sci. 2019, 22, 200–212. [Google Scholar] [CrossRef]
- Piaser, E.; Villa, P. Evaluating Capabilities of Machine Learning Algorithms for Aquatic Vegetation Classification in Temperate Wetlands Using Multi-Temporal Sentinel-2 Data. Int. J. Appl. Earth Obs. Geoinf. 2023, 117, 103202. [Google Scholar] [CrossRef]
- Rupasinghe, P.A.; Chow-Fraser, P. Identification of Most Spectrally Distinguishable Phenological Stage of Invasive Phramites Australis in Lake Erie Wetlands (Canada) for Accurate Mapping Using Multispectral Satellite Imagery. Wetl. Ecol. Manag. 2019, 27, 513–538. [Google Scholar] [CrossRef]
- Dong, D.; Wang, C.; Yan, J.; He, Q.; Zeng, J.; We, Z. Combing Sentinel-1 and Sentinel-2 Image Time Series for Invasive Spartina Alterniflora Mapping on Google Earth Engine: A Case Study in Zhangjiang Estuary. J. Appl. Remote Sens. 2020, 14, 044504. [Google Scholar]
- Lu, B.; Francescutto, L.; Howie, S.; Lin, H.; Wu, Q.; Hedley, N.; Jamali, A.; McDonald, I. Exploring the Concept of Digital Twins of Wetlands for Supporting Ecosystem Monitoring and Management. Big Earth Data 2025, 1–31. [Google Scholar] [CrossRef]
- Liu, Y.; He, K.; Qin, F. Remote Sensing Big Data Analysis of the Lower Yellow River Ecological Environment Based on Internet of Things. J. Sens. 2021, 2021, 1059517. [Google Scholar] [CrossRef]
- Sharma, L.K.; Naik, R. Future Technologies for Wetland Studies. In Conservation of Saline Wetland Ecosystems; Springer Nature: Singapore, 2024; pp. 175–215. [Google Scholar]
- Cardoso, A.W.; Hestir, E.L.; Slingsby, J.A.; Forbes, C.J.; Moncrieff, G.R.; Turner, W.; Skowno, A.L.; Nesslage, J.; Brodrick, P.G.; Gaddis, K.D.; et al. BioSCape Combines Local Knowledge and Remote-Sensing Technology for Inclusive Biodiversity Science. Nat. Rev. Biodivers. 2025, 1, 2–4. [Google Scholar] [CrossRef]
- Cardoso, A.W.; Hestir, E.L.; Slingsby, J.A.; Forbes, C.J.; Moncrieff, G.R.; Turner, W.; Skowno, A.L.; Nesslage, J.; Brodrick, P.G.; Gaddis, K.D.; et al. The Biodiversity Survey of the Cape (BioSCape), Integrating Remote Sensing with Biodiversity Science. npj Biodivers. 2025, 4, 2. [Google Scholar] [CrossRef]


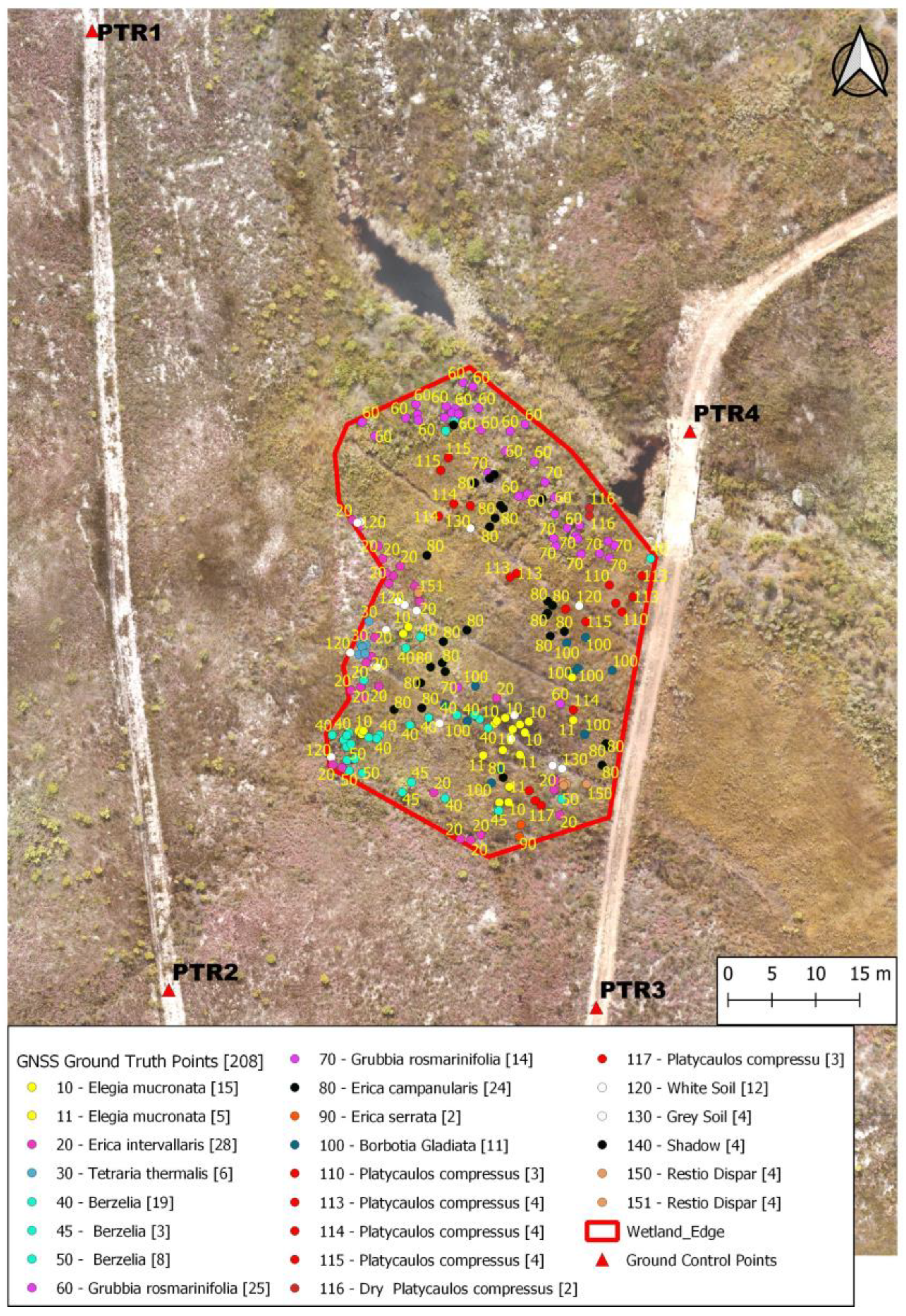



| Plant Name | Leaves | Soil Moisture | Average Height (m) |
|---|---|---|---|
| Berzelia lanuginosa | Small and narrow in whorls | Seasonally inundated | 1.5 m |
| Bobartia gladiata | Rigid ensiform | Seasonally inundated | 0.8 m |
| Elegia mucronata | Stout erect sheaths | Seasonally inundated | 2.0 m |
| Erica campanularis | Small needle-like | Seasonally inundated | 0.7 m |
| Erica intervallaris | Incurved, erect squarrose | Seasonally inundated | 0.7 m |
| Erica serrata | Serrated edges | Seasonally inundated | 0.7 m |
| Grubbia rosmarinifolia | Glossy narrow lanceolate | Permanently Inundated | 1.3 m |
| Platycaulos compressus | Long and narrow | Permanently Inundated | 0.5 m |
| Restio dispar | Reed-like tufts | Seasonally inundated | 1.0 m |
| Restio leptostachyus | Feathery plume-like spikelets | Seasonally inundated | 0.5 m |
| Tetraria thermalis | Drooping sword-shaped | Seasonally inundated | 0.4 m |
| Bands | Centre Wavelength (nm) | Bandwidth (nm) |
|---|---|---|
| Green | 550 | 40 |
| Red | 660 | 40 |
| Red Edge | 735 | 10 |
| Near-Infrared | 790 | 40 |
| Bands | Centre Wavelength (nm) | Bandwidth (nm) |
|---|---|---|
| Blue | 475 | 40 |
| Green | 560 | 40 |
| Red | 668 | 40 |
| Red Edge | 717 | 10 |
| Near-Infrared | 840 | 40 |
| Date | Time | Sensor | Season |
|---|---|---|---|
| 31 August 2018 | 11 h 15 | Parrot Sequoia | Late Winter |
| 4 October 2018 | 10 h 45 | Parrot Sequoia | Late Spring |
| 10 December 2018 | 14 h 57 | Parrot Sequoia | Early Summer |
| 8 February 2019 | 13 h 02 | Parrot Sequoia | Late Summer |
| 26 April 2019 | 11 h 34 | Micasense RedEdge-M | Mid-Autumn |
| 22 February 2020 | 13 h 03 | Micasense RedEdge-M | Late Summer |
| August 2018 (Late Winter)-Overall Accuracy [%] = 98.0 and Kappa = 0.97 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | B | BG | DPC | EM | EC | EI | ES | GR | PC | RD | TT |
| PA [%] | 98.1 | 93.5 | 94.5 | 99.4 | 96.9 | 79.9 | 100.0 | 100.0 | 100.0 | 75.4 | 44.7 |
| UA [%] | 98.9 | 94.4 | 97.5 | 99.5 | 95.7 | 98.7 | 95.0 | 93.2 | 98.0 | 100.0 | 83.3 |
| Kappa | 0.99 | 0.94 | 0.97 | 0.99 | 0.95 | 0.99 | 0.95 | 0.93 | 0.97 | 1.00 | 0.83 |
| October 2018 (late spring)-Overall Accuracy [%] = 87.4 and Kappa = 0.85 | |||||||||||
| PA [%] | 85.1 | 83.8 | 88.0 | 94.7 | 88.3 | 89.7 | 35.4 | 74.8 | 93.0 | 47.3 | 87.2 |
| UA [%] | 66.7 | 85.2 | 92.9 | 92.8 | 73.2 | 83.5 | 57.5 | 90.2 | 96.9 | 60.7 | 82.7 |
| Kappa | 0.63 | 0.85 | 0.93 | 0.92 | 0.72 | 0.82 | 0.57 | 0.88 | 0.96 | 0.60 | 0.83 |
| December 2018 (early summer)-Overall Accuracy [%] = 90.1 and Kappa = 0.88 | |||||||||||
| PA [%] | 100.0 | 74.7 | 99.5 | 100.0 | 94.7 | 82.7 | 32.9 | 70.5 | 98.3 | 49.9 | 100.0 |
| UA [%] | 44.1 | 100.0 | 100.0 | 97.4 | 87.5 | 77.8 | 100.0 | 100.0 | 93.1 | 100.0 | 100.0 |
| Kappa | 0.42 | 1.00 | 1.00 | 0.97 | 0.87 | 0.76 | 1.00 | 1.00 | 0.92 | 1.00 | 1.00 |
| February 2019 (late summer)-Overall Accuracy [%] = 71.0 and Kappa = 0.67 | |||||||||||
| PA [%] | 69.7 | 65.7 | 61.6 | 86.4 | 71.2 | 77.0 | 25.9 | 86.1 | 77.2 | 21.3 | 100.0 |
| UA [%] | 64.7 | 87.0 | 23.2 | 63.9 | 27.1 | 73.4 | 87.5 | 85.0 | 93.9 | 55.4 | 94.1 |
| Kappa | 0.61 | 0.86 | 0.22 | 0.61 | 0.24 | 0.70 | 0.87 | 0.83 | 0.92 | 0.55 | 0.94 |
| April 2019 (mid autumn)-Overall Accuracy [%] = 57.2 and Kappa = 0.50 | |||||||||||
| PA [%] | 55.6 | 84.9 | 79.1 | 76.5 | 38.8 | 35.3 | 5.1 | 67.2 | 60.7 | 26.1 | 71.5 |
| UA [%] | 28.8 | 61.4 | 24.4 | 79.9 | 32.5 | 31.0 | 44.4 | 75.6 | 92.0 | 52.4 | 23.5 |
| Kappa | 0.24 | 0.60 | 0.23 | 0.77 | 0.28 | 0.23 | 0.43 | 0.70 | 0.89 | 0.51 | 0.23 |
| February 2020 (late summer)-Overall Accuracy [%] = 71.2 and Kappa = 0.64 | |||||||||||
| PA [%] | 54.7 | 34.4 | 77.3 | 75.2 | 59.0 | 45.0 | 32.5 | 81.8 | 86.7 | 18.5 | 53.2 |
| UA [%] | 72.5 | 68.8 | 70.8 | 66.2 | 49.3 | 36.9 | 66.0 | 79.3 | 84.0 | 68.6 | 32.5 |
| Kappa | 0.70 | 0.68 | 0.70 | 0.61 | 0.47 | 0.32 | 0.65 | 0.77 | 0.74 | 0.68 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musungu, K.; Shoko, M.; Smit, J. Optimal Temporal Windows for Mapping Fynbos Seep Wetlands Using Unmanned Aerial Vehicle Data. Geographies 2025, 5, 60. https://doi.org/10.3390/geographies5040060
Musungu K, Shoko M, Smit J. Optimal Temporal Windows for Mapping Fynbos Seep Wetlands Using Unmanned Aerial Vehicle Data. Geographies. 2025; 5(4):60. https://doi.org/10.3390/geographies5040060
Chicago/Turabian StyleMusungu, Kevin, Moreblessings Shoko, and Julian Smit. 2025. "Optimal Temporal Windows for Mapping Fynbos Seep Wetlands Using Unmanned Aerial Vehicle Data" Geographies 5, no. 4: 60. https://doi.org/10.3390/geographies5040060
APA StyleMusungu, K., Shoko, M., & Smit, J. (2025). Optimal Temporal Windows for Mapping Fynbos Seep Wetlands Using Unmanned Aerial Vehicle Data. Geographies, 5(4), 60. https://doi.org/10.3390/geographies5040060








