The Impact of Weather on the Spread of COVID-19: The Case of the Two Largest Cities in Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Statistical Analysis
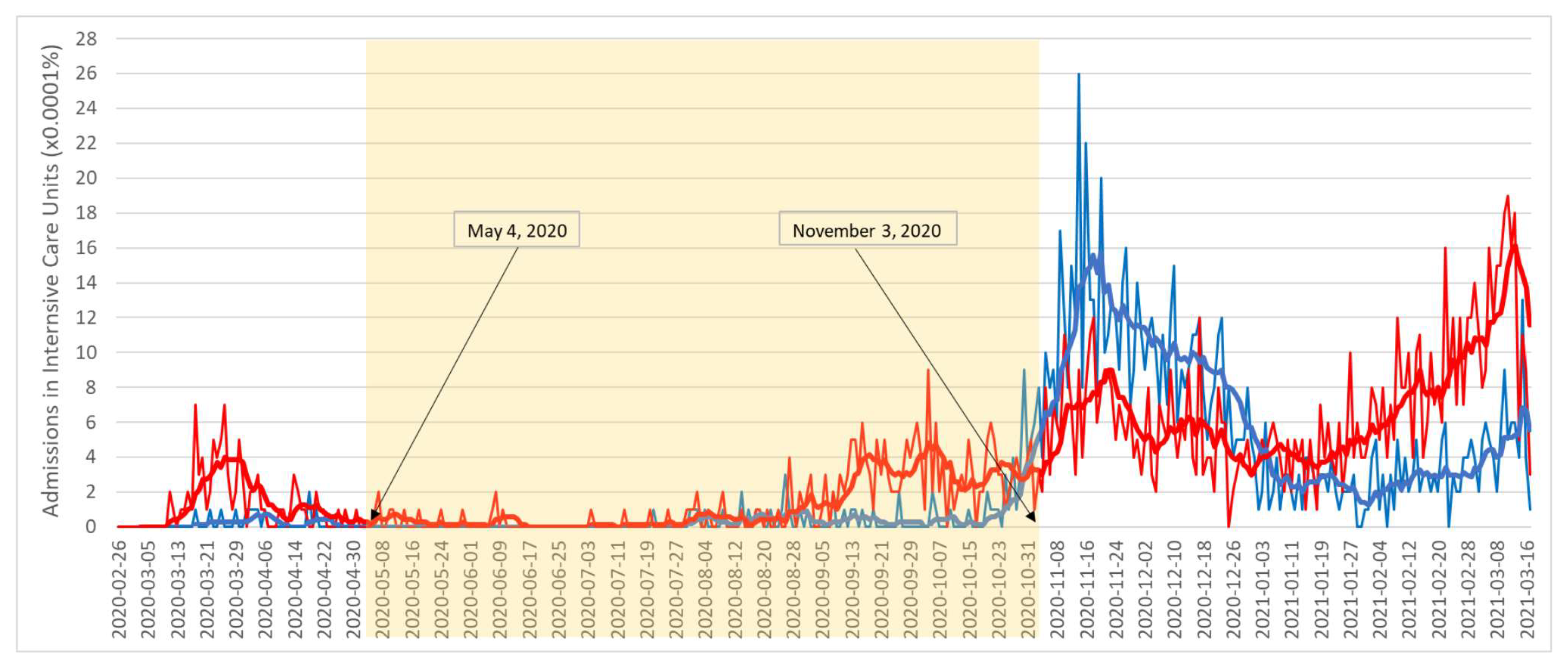
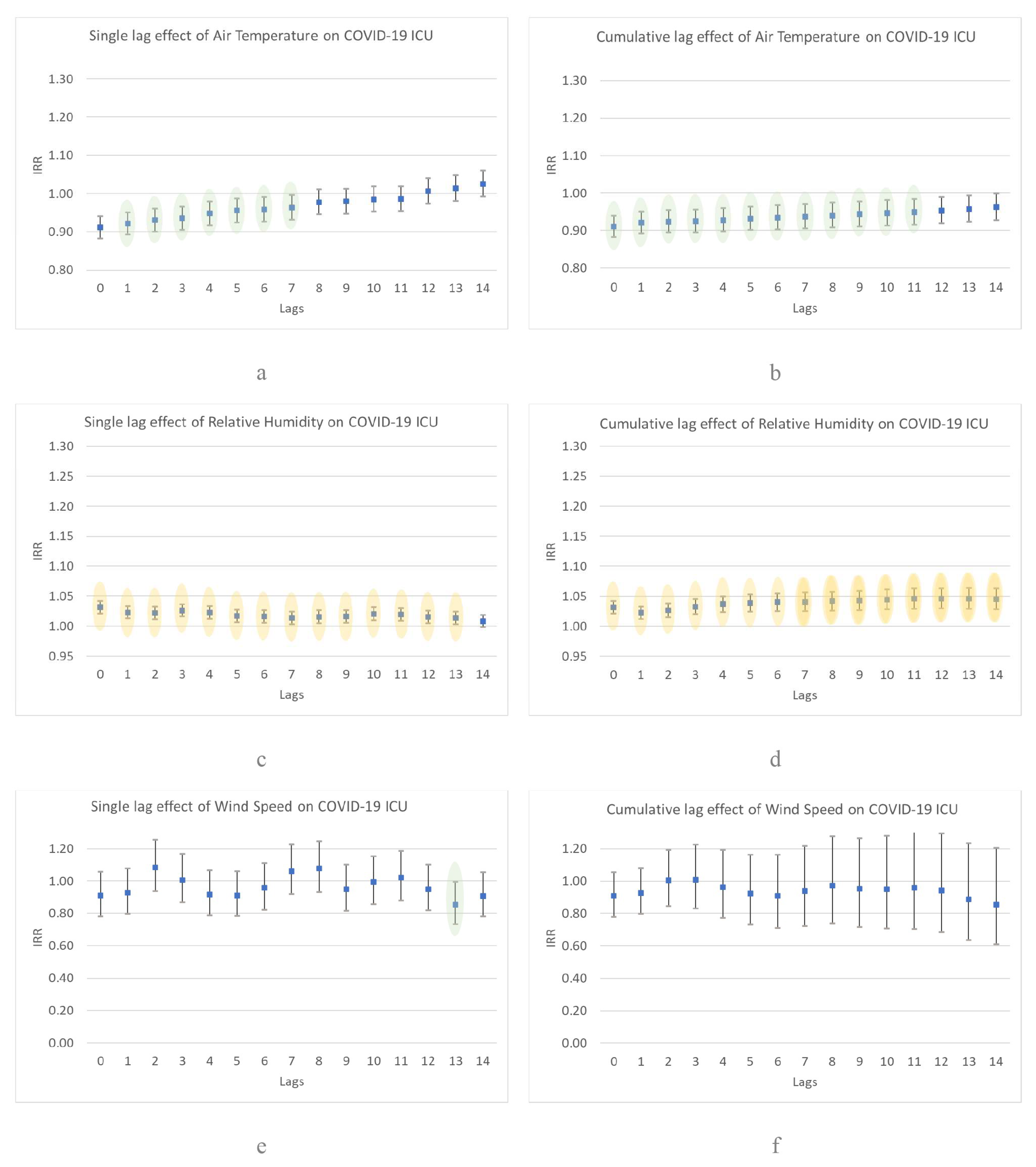
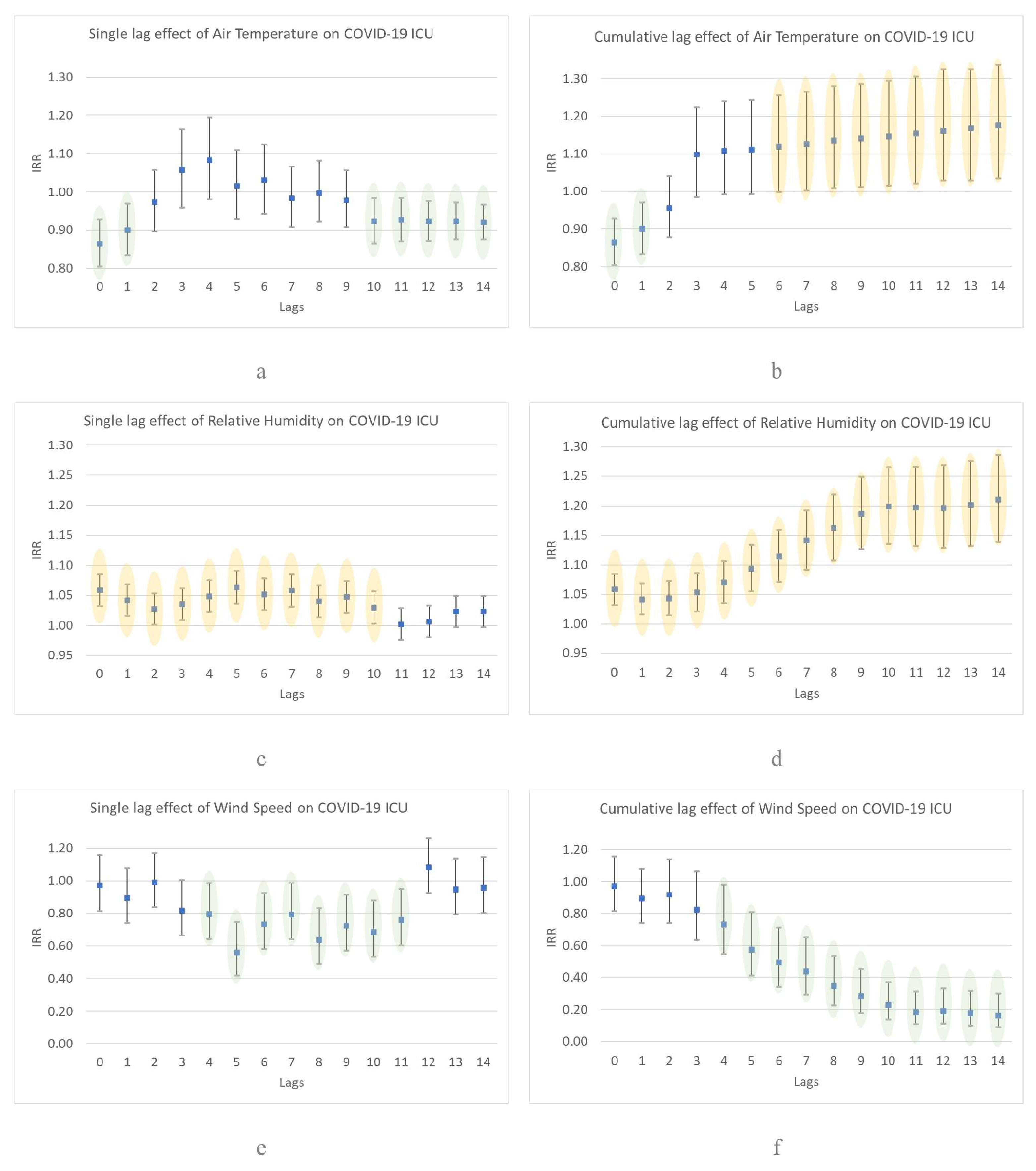
3. Results
4. Discussion
4.1. Comparison of Study Results with Another Researchs
4.2. Case Study and Justification of Results
- -
- R.U. of the Central Sector of Athens: cases: 266, deaths: 23, and admissions to ICU: 16;
- -
- R.U. of Thessaloniki: cases: 99, deaths: 0, and admissions to ICU: 1.2nd Stage 1 August 2020–3 November 2020
- -
- R.U. of the Central Sector of Athens: 8974 cases, 188 deaths, and 135 admissions to ICU;
- -
- R.U. of Thessaloniki: 3654 cases, 58 deaths, and 39 admissions to ICU.
4.3. Strength and Limitations
- -
- The study covered a long period of six months (4 May–3 November) and was characterized by a large seasonal range (summer–autumn) with isolated fluctuations in meteorological (temperature, humidity, wind speed) and epidemiological data (e.g., COVID-19 ICU admissions). A period when restrictive measures were gradually being lifted, the tourist season was opening in Greece, the population was mobile, and commercial and tourist activities were returning.
- -
- We used the number of daily COVID-19 ICU admissions, in contrast to other studies that were based on daily numbers of cases and deaths. An increase or decrease in the number of COVID-19 ICU admissions is associated with an exacerbation or de-escalation of the disease. Therefore, this number is an important indicator of the epidemiological evolution of the disease. Deaths or admissions to Intensive Care Units (ICUs) are more reliable epidemiological data to capture the true picture of the pandemic than daily cases, as the number of asymptomatic patients contributing to the transmission of the disease is difficult to identify [56].
- -
- The incubation period and the median time from the onset of symptoms to ICU admission were considered to study the effect of meteorological parameters up to 14 days before ICU admission for COVID-19.
- -
- -
- Data such as length of stay, number of direct ICU admissions, and number of patients admitted to the ICU from other hospital departments (e.g., Emergency Department) or after hospitalization were unavailable.
- -
- Other meteorological parameters, such as air pollution, UV radiation, precipitation, and other factors, may affect SARS-CoV-2 transmission.
- -
- Public health policies for surveillance and containment, social isolation strategies, human behavior, such as adherence to hygiene measures, personal hygiene, use of disinfectants, resistance to the virus, mobility, and population density.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carfì, A.; Bernabei, R.; Landi, F.; for the Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603. [Google Scholar] [CrossRef] [PubMed]
- del Rio, C.; Collins, L.F.; Malani, P. Long-Term Health Consequences of COVID-19. JAMA 2020, 324, 1723. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Rothman-Ostrow, P.; Osman, A.Y.; Arruda, L.B.; Macfarlane-Berry, L.; Elton, L.; Thomason, M.J.; Yeboah-Manu, D.; Ansumana, R.; Kapata, N.; et al. COVID-19—Zoonosis or Emerging Infectious Disease? Front. Public Health 2020, 8, 596944. [Google Scholar] [CrossRef] [PubMed]
- Dhand, R.; Li, J. Coughs and Sneezes: Their Role in Transmission of Respiratory Viral Infections, Including SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 202, 651–659. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- WHO Coronavirus Disease (COVID-19). How Long Does It Take to Develop Symptoms? Updated 13 May 2021. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19 (accessed on 12 May 2021).
- Mousavizadeh, L.; Ghasemi, S. Genotype and Phenotype of COVID-19: Their Roles in Pathogenesis. J. Microbiol. Immunol. Infect. 2021, 54, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Docea, A.; Tsatsakis, A.; Albulescu, D.; Cristea, O.; Zlatian, O.; Vinceti, M.; Moschos, S.; Tsoukalas, D.; Goumenou, M.; Drakoulis, N.; et al. A New Threat from an Old Enemy: Re-emergence of Coronavirus (Review). Int. J. Mol. Med. 2020, 45, 1631–1643. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.D.; Talwar, A.; Lee, J.T. A Proposed Framework and Timeline of the Spectrum of Disease Due to SARS-CoV-2 Infection: Illness Beyond Acute Infection and Public Health Implications. JAMA 2020, 324, 2251. [Google Scholar] [CrossRef]
- Beyer, D.K.; Forero, A. Mechanisms of Antiviral Immune Evasion of SARS-CoV-2. J. Mol. Biol. 2022, 434, 167265. [Google Scholar] [CrossRef]
- Escalera, A.; Gonzalez-Reiche, A.S.; Aslam, S.; Mena, I.; Laporte, M.; Pearl, R.L.; Fossati, A.; Rathnasinghe, R.; Alshammary, H.; Van De Guchte, A.; et al. Mutations in SARS-CoV-2 Variants of Concern Link to Increased Spike Cleavage and Virus Transmission. Cell Host Microbe 2022, 30, 373–387.e7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta Variant of SARS-CoV-2: A Comparative Computational Study of Spike Protein. J. Med. Virol. 2022, 94, 1641–1649. [Google Scholar] [CrossRef]
- Mecenas, P.; Bastos, R.T.D.R.M.; Vallinoto, A.C.R.; Normando, D. Effects of Temperature and Humidity on the Spread of COVID-19: A Systematic Review. PLoS ONE 2020, 15, e0238339. [Google Scholar] [CrossRef]
- EODY. Daily Report of Epidemiological Surveillance of New Coronavirus Infection (COVID-19). Available online: https://eody.gov.gr/wp-content/uploads/2021/03/covid-gr-daily-report-20210316.pdf (accessed on 16 March 2021).
- Anwar, A.; Anwar, S.; Ayub, M.; Nawaz, F.; Hyder, S.; Khan, N.; Malik, I. Climate Change and Infectious Diseases: Evidence from Highly Vulnerable Countries. Iran. J. Public Health 2019, 48, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Bezirtzoglou, C.; Dekas, K.; Charvalos, E. Climate Changes, Environment and Infection: Facts, Scenarios and Growing Awareness from the Public Health Community within Europe. Anaerobe 2011, 17, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.S.; Samuel, M.D. Climate Warming and Disease Risks for Terrestrial and Marine Biota. Science 2002, 296, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Kovats, R.S.; Bouma, M.J.; Hajat, S.; Worrall, E.; Haines, A. El Niño and Health. Lancet 2003, 362, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Karande, S. An Observational Study to Detect Leptospirosis in Mumbai, India, 2000. Arch. Dis. Child 2003, 88, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza Virus Transmission Is Dependent on Relative Humidity and Temperature. PLoS Pathog. 2007, 3, e151. [Google Scholar] [CrossRef] [PubMed]
- Shaman, J.; Kohn, M. Absolute Humidity Modulates Influenza Survival, Transmission, and Seasonality. Proc. Natl. Acad. Sci. USA 2009, 106, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Tamerius, J.D.; Shaman, J.; Alonso, W.J.; Bloom-Feshbach, K.; Uejio, C.K.; Comrie, A.; Viboud, C. Environmental Predictors of Seasonal Influenza Epidemics across Temperate and Tropical Climates. PLoS Pathog. 2013, 9, e1003194. [Google Scholar] [CrossRef]
- Tan, J.; Mu, L.; Huang, J.; Yu, S.; Chen, B.; Yin, J. An Initial Investigation of the Association between the SARS Outbreak and Weather: With the View of the Environmental Temperature and Its Variation. J. Epidemiol. Community Health 2005, 59, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Peiris, J.S.M.; Lam, S.Y.; Poon, L.L.M.; Yuen, K.Y.; Seto, W.H. The Effects of Temperature and Relative Humidity on the Viability of the SARS Coronavirus. Adv. Virol. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gardner, E.G.; Kelton, D.; Poljak, Z.; Van Kerkhove, M.; von Dobschuetz, S.; Greer, A.L. A Case-Crossover Analysis of the Impact of Weather on Primary Cases of Middle East Respiratory Syndrome. BMC Infect. Dis. 2019, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Moazeni, M.; Rahimi, M.; Ebrahimi, A. What Are the Effects of Climate Variables on COVID-19 Pandemic? A Systematic Review and Current Update. Adv. Biomed. Res. 2023, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jing, W.; Liu, J.; Ma, Q.; Yuan, J.; Wang, Y.; Du, M.; Liu, M. Effects of Temperature and Humidity on the Daily New Cases and New Deaths of COVID-19 in 166 Countries. Sci. Total Environ. 2020, 729, 139051. [Google Scholar] [CrossRef]
- Nottmeyer, L.; Armstrong, B.; Lowe, R.; Abbott, S.; Meakin, S.; O’Reilly, K.M.; Von Borries, R.; Schneider, R.; Royé, D.; Hashizume, M.; et al. The Association of COVID-19 Incidence with Temperature, Humidity, and UV Radiation—A Global Multi-City Analysis. Sci. Total Environ. 2023, 854, 158636. [Google Scholar] [CrossRef]
- Feurer, D.; Riffe, T.; Kniffka, M.S.; Acosta, E.; Armstrong, B.; Mistry, M.; Lowe, R.; Royé, D.; Hashizume, M.; Madaniyazi, L.; et al. Meteorological Factors, Population Immunity, and COVID-19 Incidence: A Global Multi-City Analysis. Environ. Epidemiol. 2024, 8, e338. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, I.A.; Tella, S.A.; Oyesiku, K.O.; Oseni, I.O. Spatio-Temporal Analysis of Meteorological Factors in Abating the Spread of COVID-19 in Africa. Heliyon 2020, 6, e04749. [Google Scholar] [CrossRef] [PubMed]
- Koanda, O.; Yonaba, R.; Tazen, F.; Karoui, H.; Sidibé, M.L.; Lèye, B.; Diop, M.; Andrianisa, H.A.; Karambiri, H. Climate and COVID-19 Transmission: A Cross-Sectional Study in Africa. Sci. Rep. 2023, 13, 18702. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Gasparrini, A.; Hajat, S.; Smeeth, L.; Armstrong, B. Time series regression studies in environmental epidemiology. Int. J. Epidemiol. 2013, 42, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Xiao, S.; Shi, R.; Ward, M.P.; Chen, Y.; Tu, W.; Su, Q.; Wang, W.; Wang, X.; Zhang, Z. COVID-19 Transmission in Mainland China Is Associated with Temperature and Humidity: A Time-Series Analysis. Sci. Total Environ. 2020, 728, 138778. [Google Scholar] [CrossRef]
- Zomuanpuii, R.; Lalrohlua, P.C.; Lalbiakmawia, B.; Chawngthu, Z.; Hmar, L.; Roy, S.; Laldinmawii, G.; Zohmingthanga, J.; Kumar, N.S.; Zothanzama, J.; et al. Meteorological Impact on the Incidence of COVID-19 and SARS-CoV-2 Variants in Mizoram, Northeast India. Environ. Sustain. 2023, 6, 455–464. [Google Scholar] [CrossRef]
- Batool, H.; Tian, L. Correlation Determination between COVID-19 and Weather Parameters Using Time Series Forecasting: A Case Study in Pakistan. Math. Probl. Eng. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Alkhowailed, M.; Shariq, A.; Alqossayir, F.; Alzahrani, O.A.; Rasheed, Z.; Al Abdulmonem, W. Impact of Meteorological Parameters on COVID-19 Pandemic: A Comprehensive Study from Saudi Arabia. Inform. Med. Unlocked 2020, 20, 100418. [Google Scholar] [CrossRef] [PubMed]
- Şahin, M. Impact of Weather on COVID-19 Pandemic in Turkey. Sci. Total Environ. 2020, 728, 138810. [Google Scholar] [CrossRef]
- Hussain, M.R.A.; Shaba, S.S.E.; Bunthen, E.; Eity, K.F.; Roshid, M.M.; Kuddus, M.A. The Dynamics of COVID-19 in Hiroshima Prefecture Compared to Japan and Its Association With Meteorological Factors: A Comparative Analysis. Cureus 2024, 16, e57708. [Google Scholar] [CrossRef]
- Coccia, M. How do low wind speeds and high levels of air pollution support the spread of COVID-19? Atmos. Pollut. Res. 2021, 12, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Planella-Morató, J.; Pelegrí, J.L.; Martín-Rey, M.; Olivé Abelló, A.; Vallès, X.; Roca, J.; Rodrigo, C.; Estrada, O.; Vallès-Casanova, I. Environmental Predictors of SARS-CoV-2 Infection Incidence in Catalonia (Northwestern Mediterranean). Front. Public Health 2024, 12, 1430902. [Google Scholar] [CrossRef]
- Colston, J.M.; Hinson, P.; Nguyen, N.-L.H.; Chen, Y.T.; Badr, H.S.; Kerr, G.H.; Gardner, L.M.; Martin, D.N.; Quispe, A.M.; Schiaffino, F.; et al. Effects of Hydrometeorological and Other Factors on SARS-CoV-2 Reproduction Number in Three Contiguous Countries of Tropical Andean South America: A Spatiotemporally Disaggregated Time Series Analysis. IJID Reg. 2023, 6, 29–41. [Google Scholar] [CrossRef]
- Rosario, D.K.A.; Mutz, Y.S.; Bernardes, P.C.; Conte-Junior, C.A. Relationship between COVID-19 and Weather: Case Study in a Tropical Country. Int. J. Hyg. Environ. Health 2020, 229, 113587. [Google Scholar] [CrossRef] [PubMed]
- Livadiotis, G. Statistical Analysis of the Impact of Environmental Temperature on the Exponential Growth Rate of Cases Infected by COVID-19. PLoS ONE 2020, 15, e0233875. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.R.M.T.; Hasanuzzaman, M.; Shammi, M.; Salam, R.; Bodrud-Doza, M.; Rahman, M.M.; Mannan, M.A.; Huq, S. Are Meteorological Factors Enhancing COVID-19 Transmission in Bangladesh? Novel Findings from a Compound Poisson Generalized Linear Modeling Approach. Environ. Sci. Pollut. Res. 2021, 28, 11245–11258. [Google Scholar] [CrossRef] [PubMed]
- Sangkham, S.; Thongtip, S.; Vongruang, P. Influence of Air Pollution and Meteorological Factors on the Spread of COVID-19 in the Bangkok Metropolitan Region and Air Quality during the Outbreak. Environ. Res. 2021, 197, 111104. [Google Scholar] [CrossRef]
- Susanna, D.; Saputra, Y.A.; Poddar, S. Analysis of the Effect of Wind Speed in Increasing the COVID-19 Cases in Jakarta. F1000Res 2023, 12, 145. [Google Scholar] [CrossRef]
- Ng, K.Y.; Hasan, M.Z.; Rahman, A. Examining the Roles of Meteorological Variables in COVID-19 Spread in Malaysia. Aerobiologia 2024, 40, 129–144. [Google Scholar] [CrossRef]
- Haq, F.U.; Abduljaleel, Y.; Ahmad, I. Effect of Temperature on Fast Transmission of COVID-19 in Low per Capita GDP Asian Countries. Sci. Rep. 2023, 13, 21165. [Google Scholar] [CrossRef]
- Maleki, A.; Aboubakri, O.; Rezaee, R.; Alahmad, B.; Sera, F. Seasonal Variation of COVID-19 Incidence and Role of Land Surface and Air Temperatures: A Case Study in the West of Iran. Int. J. Environ. Health Res. 2024, 34, 1342–1354. [Google Scholar] [CrossRef] [PubMed]
- Asem, N.; Ramadan, A.; Hassany, M.; Ghazy, R.M.; Abdallah, M.; Ibrahim, M.; Gamal, E.M.; Hassan, S.; Kamal, N.; Zaid, H. Pattern and Determinants of COVID-19 Infection and Mortality across Countries: An Ecological Study. Heliyon 2021, 7, e07504. [Google Scholar] [CrossRef] [PubMed]
- Mejdoubi, M.; Kyndt, X.; Djennaoui, M. ICU Admissions and In-Hospital Deaths Linked to COVID-19 in the Paris Region Are Correlated with Previously Observed Ambient Temperature. PLoS ONE 2020, 15, e0242268. [Google Scholar] [CrossRef]
- d’Albis, H.; Coulibaly, D.; Roumagnac, A.; de Carvalho Filho, E.; Bertrand, R. Quantification of the Effects of Climatic Conditions on French Hospital Admissions and Deaths Induced by SARS-CoV-2. Sci. Rep. 2021, 11, 21812. [Google Scholar] [CrossRef]
- Paireau, J.; Charpignon, M.-L.; Larrieu, S.; Calba, C.; Hozé, N.; Boëlle, P.-Y.; Thiebaut, R.; Prague, M.; Cauchemez, S. Impact of Non-Pharmaceutical Interventions, Weather, Vaccination, and Variants on COVID-19 Transmission across Departments in France. BMC Infect. Dis. 2023, 23, 190. [Google Scholar] [CrossRef] [PubMed]
- Valero, C.; Barba, R.; Riancho, J.A.; Santurtún, A. Influence of Meteorological Factors on the Severity of COVID-19 in Spain: Observational Ecological Study. Med. Clin. 2023, 160, 327–332. [Google Scholar] [CrossRef]
- Hachim, M.Y.; Hachim, I.Y.; Naeem, K.; Hannawi, H.; Al Salmi, I.; Hannawi, S. Higher Temperatures, Higher Solar Radiation, and Less Humidity Is Associated with Poor Clinical and Laboratory Outcomes in COVID-19 Patients. Front. Public Health 2021, 9, 618828. [Google Scholar] [CrossRef] [PubMed]
- Oliveiros, B.; Caramelo, L.; Ferreira, N.C.; Caramelo, F. Role of Temperature and Humidity in the Modulation of the Doubling Time of Covid-19 Cases. JBGSR 2021, 6. [Google Scholar] [CrossRef]
- Paraskevis, D.; Kostaki, E.G.; Alygizakis, N.; Thomaidis, N.S.; Cartalis, C.; Tsiodras, S.; Dimopoulos, M.A. A Review of the Impact of Weather and Climate Variables to COVID-19: In the Absence of Public Health Measures High Temperatures Cannot Probably Mitigate Outbreaks. Sci. Total Environ. 2021, 768, 144578. [Google Scholar] [CrossRef] [PubMed]
- Menhat, M.; Ariffin, E.H.; Dong, W.S.; Zakaria, J.; Ismailluddin, A.; Shafril, H.A.M.; Muhammad, M.; Othman, A.R.; Kanesan, T.; Ramli, S.P.; et al. Rain, Rain, Go Away, Come Again Another Day: Do Climate Variations Enhance the Spread of COVID-19? Glob. Health 2024, 20, 43. [Google Scholar] [CrossRef]
- Sun, Z.; Thilakavathy, K.; Kumar, S.S.; He, G.; Liu, S.V. Potential Factors Influencing Repeated SARS Outbreaks in China. Int. J. Environ. Res. Public Health 2020, 17, 1633. [Google Scholar] [CrossRef]
- Aboubakr, H.A.; Sharafeldin, T.A.; Goyal, S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transbounding Emerg. Dis. 2021, 68, 296–312. [Google Scholar] [CrossRef]
- De Ángel Solá, D.E.; Wang, L.; Vázquez, M.; Méndez-Lázaro, P.A. Weathering the pandemic: How the Caribbean Basin can use viral and environmental patterns to predict, prepare, and respond to COVID-19. J. Med. Virol. 2020, 92, 1460–1468. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- Ahmadi, M.; Sharifi, A.; Dorosti, S.; Jafarzadeh Ghoushchi, S.; Ghanbari, N. Investigation of effective climatology parameters on COVID-19 outbreak in Iran. Sci. Total Environ. 2020, 729, 138705. [Google Scholar] [CrossRef]
- Kaye, A.D.; Okeagu, C.N.; Pham, A.D.; Silva, R.A.; Hurley, J.J.; Arron, B.L.; Sarfraz, N.; Lee, H.N.; Ghali, G.E.; Gamble, J.W.; et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: International perspectives. Best. Pract. Res. Clin. Anaesthesiol. 2021, 35, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Clouston, S.A.P.; Morozova, O.; Meliker, J.R. A wind speed threshold for increased outdoor transmission of coronavirus: An ecological study. BMC Infect. Dis. 2021, 21, 1194. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020, 729, 138474. [Google Scholar] [CrossRef]
- Menebo, M.M. Temperature and precipitation associate with COVID-19 new daily cases: A correlation study between weather and COVID-19 pandemic in Oslo, Norway. Sci. Total Environ. 2020, 737, 139659. [Google Scholar] [CrossRef]
- Vandelli, V.; Palandri, L.; Coratza, P.; Rizzi, C.; Ghinoi, A.; Righi, E.; Soldati, M. Conditioning factors in the spreading of COVID-19—Does geography matter? Heliyon 2024, 10, e25810. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.P.; Flaxman, S.; Gallinat, A.S.; Kinosian, S.P.; Stemkovski, M.; Unwin, H.J.T.; Watson, O.J.; Whittaker, C.; Cattarino, L.; Dorigatti, I.; et al. Temperature and population density influence SARS-CoV-2 transmission in the absence of nonpharmaceutical interventions. Proc. Natl. Acad. Sci. USA 2021, 118, e2019284118. [Google Scholar] [CrossRef] [PubMed]
- Tantrakarnapa, K.; Bhopdhornangkul, B.; Nakhaapakorn, K. Influencing factors of COVID-19 spreading: A case study of Thailand. J. Public Health 2022, 30, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Hellenic National Meteorological Service Climatic Data by City. Available online: www.emy.gr (accessed on 4 March 2021).
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Routledge: London, UK, 2019; ISBN 978-0-203-75373-6. [Google Scholar]
- Cardoso, F.S.; Papoila, A.L.; Machado, R.S.; Fidalgo, P. Age, Sex, and Comorbidities Predict ICU Admission or Mortality in Cases with SARS-CoV2 Infection: A Population-Based Cohort Study. Crit. Care 2020, 24, 465. [Google Scholar] [CrossRef]
- Rainer, L.; Bachner, F.; Eglau, K.; Ostermann, H.; Siebert, U.; Zuba, M. Comorbidities and COVID-19 Hospitalization, ICU Admission and Hospital Mortality in Austria: A Retrospective Cohort Study. Wien. Klin. Wochenschr. 2022, 134, 856–867. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tounta, D.D.; Nastos, P.T.; Paraskevis, D.N.; Sarantopoulos, A.D. The Impact of Weather on the Spread of COVID-19: The Case of the Two Largest Cities in Greece. Geographies 2025, 5, 5. https://doi.org/10.3390/geographies5010005
Tounta DD, Nastos PT, Paraskevis DN, Sarantopoulos AD. The Impact of Weather on the Spread of COVID-19: The Case of the Two Largest Cities in Greece. Geographies. 2025; 5(1):5. https://doi.org/10.3390/geographies5010005
Chicago/Turabian StyleTounta, Despoina D., Panagiotis T. Nastos, Dimitrios N. Paraskevis, and Athanasios D. Sarantopoulos. 2025. "The Impact of Weather on the Spread of COVID-19: The Case of the Two Largest Cities in Greece" Geographies 5, no. 1: 5. https://doi.org/10.3390/geographies5010005
APA StyleTounta, D. D., Nastos, P. T., Paraskevis, D. N., & Sarantopoulos, A. D. (2025). The Impact of Weather on the Spread of COVID-19: The Case of the Two Largest Cities in Greece. Geographies, 5(1), 5. https://doi.org/10.3390/geographies5010005







