Multivalent Metal-Ion Batteries: Unlocking the Future of Post-Lithium Energy Storage
Abstract
1. Introduction
2. Zinc-Ion Batteries (ZIBs)
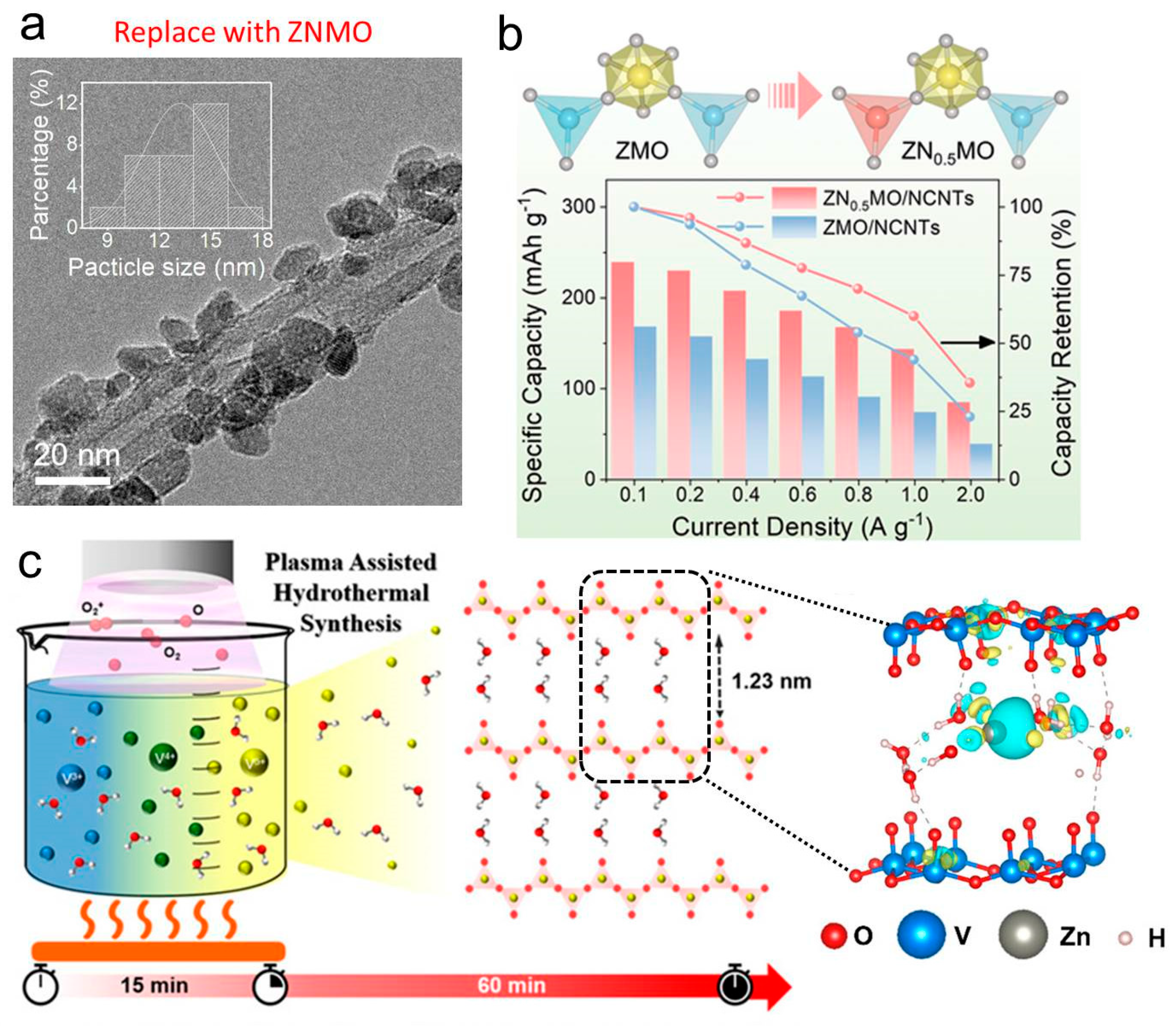
2.1. Emerging Synthesis Strategies for High-Performance ZIB Materials
2.2. Anode Interface Engineering and Electrolyte Additives

2.3. Inorganic Cathode Modification Strategies
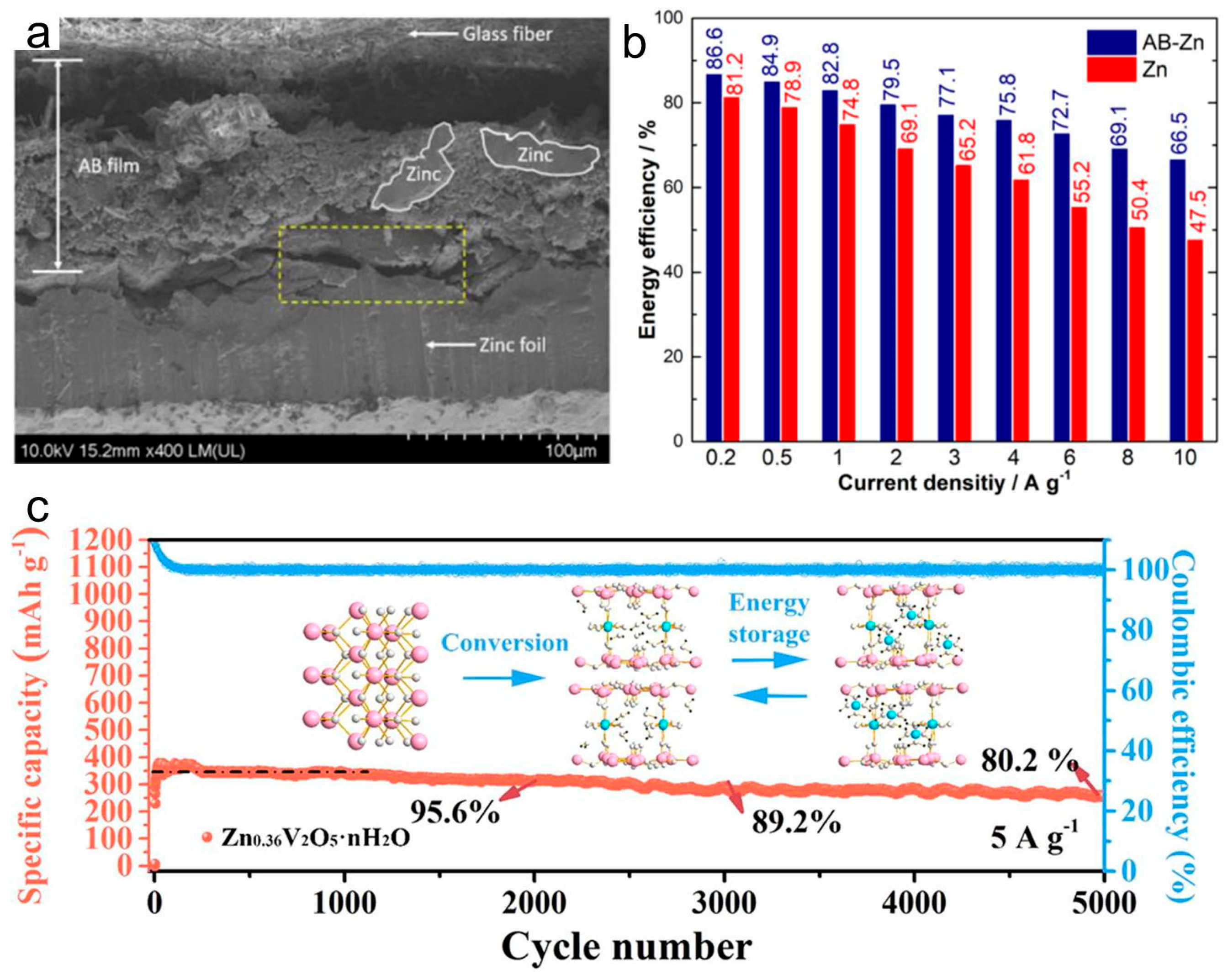
2.4. Advanced Organic/Polymer Cathodes, Flexible and Self-Powered Architectures for ZIBs

3. Magnesium-Ion Batteries (MIBs)
3.1. Anode Innovations and Protective Strategies
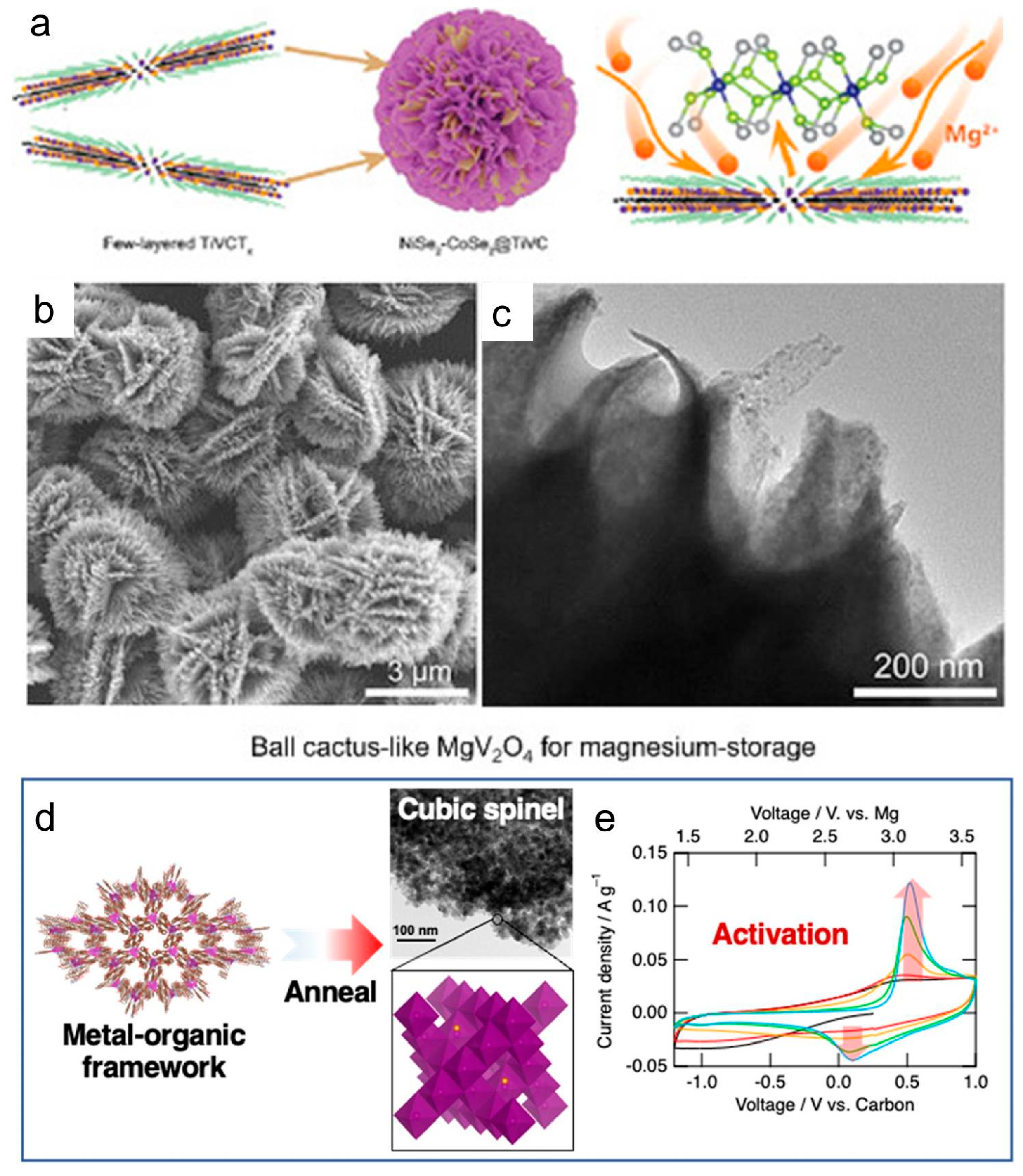
3.2. Advanced Cathode Materials and Structural Engineering
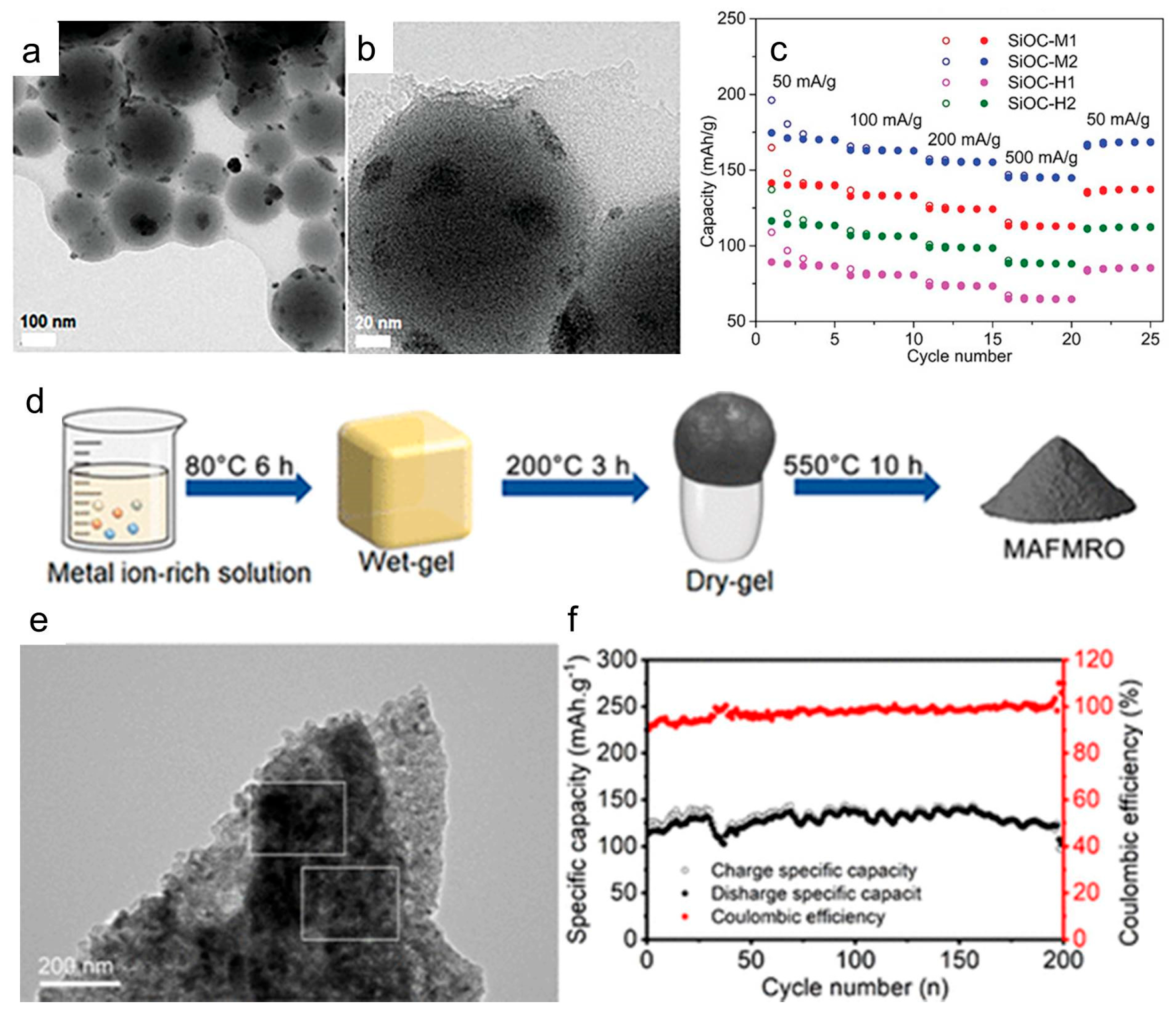
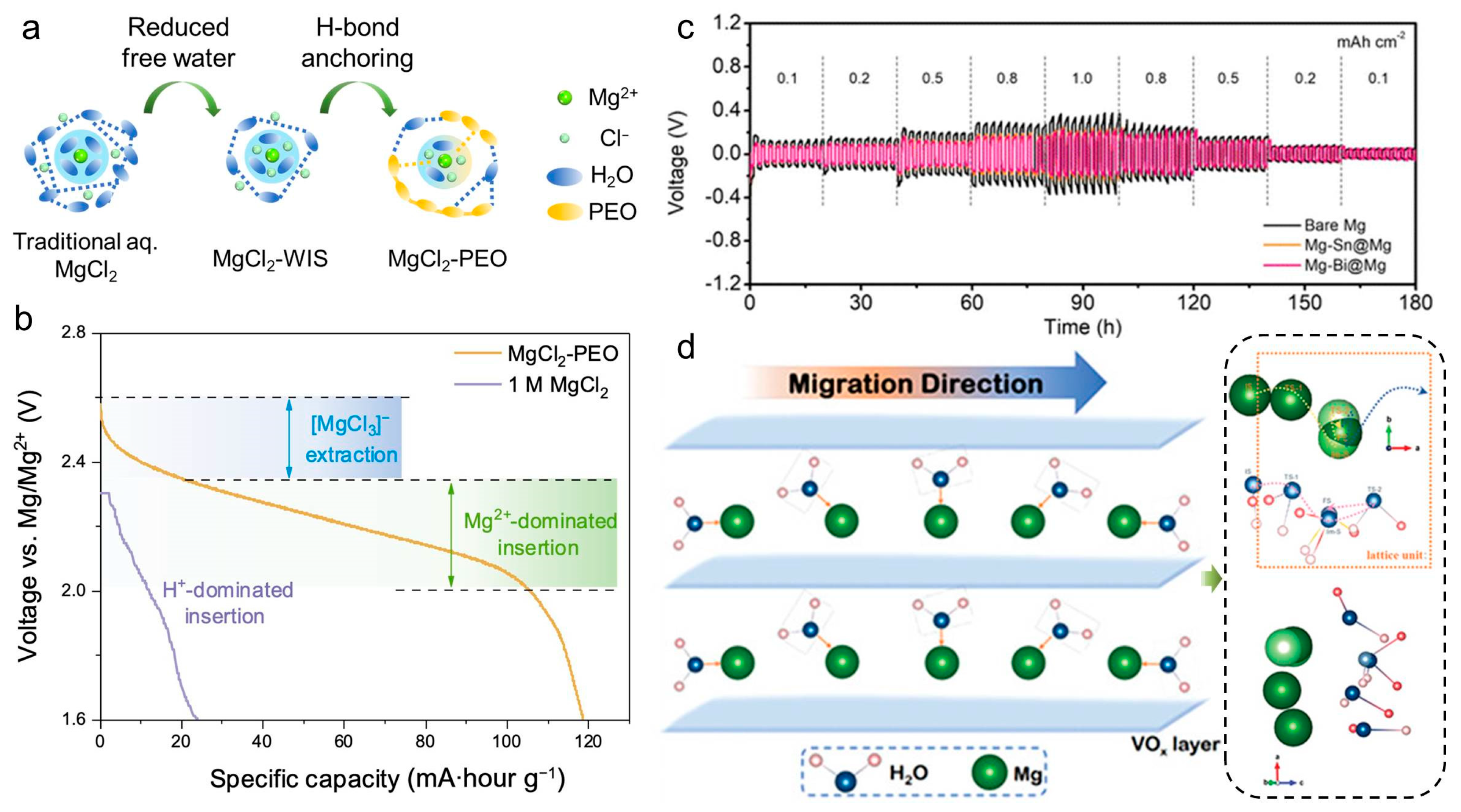
3.3. Electrolyte and Ionic Transport
3.4. Interfacial Engineering and Performance Enhancement
4. Calcium-Ion Batteries (CIBs)
4.1. Advances in Anode Design and Interface Engineering

4.2. Polyanionic and Framework-Based Cathodes
4.3. Electrolyte Design and Solvation Engineering
4.4. Organic and Hybrid Electrode Architectures

5. Aluminum-Ion Batteries (AIBs)
5.1. Electrolyte Optimization and Anode-Free Strategies
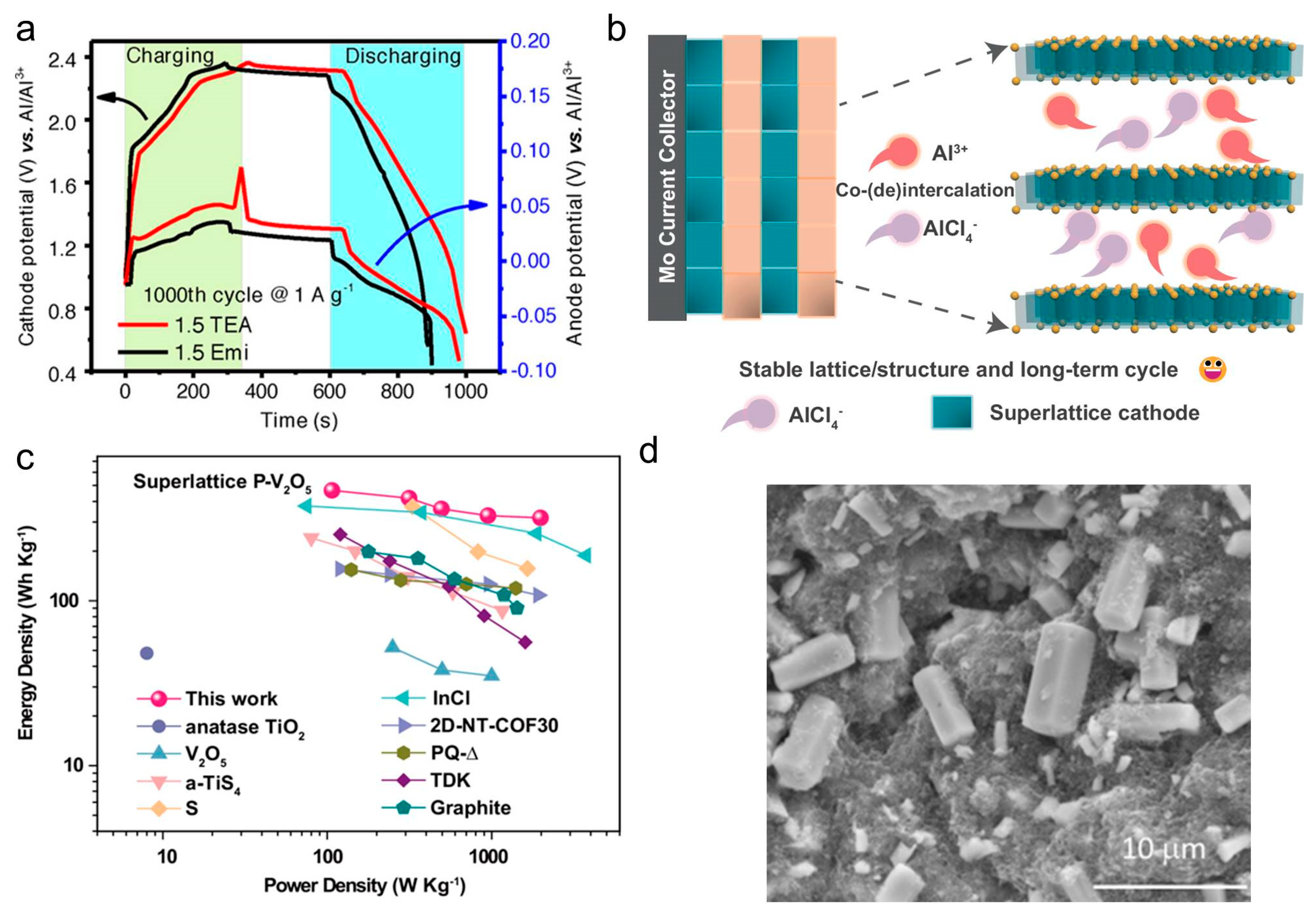
5.2. Advanced Cathode Architectures and Doping Strategies
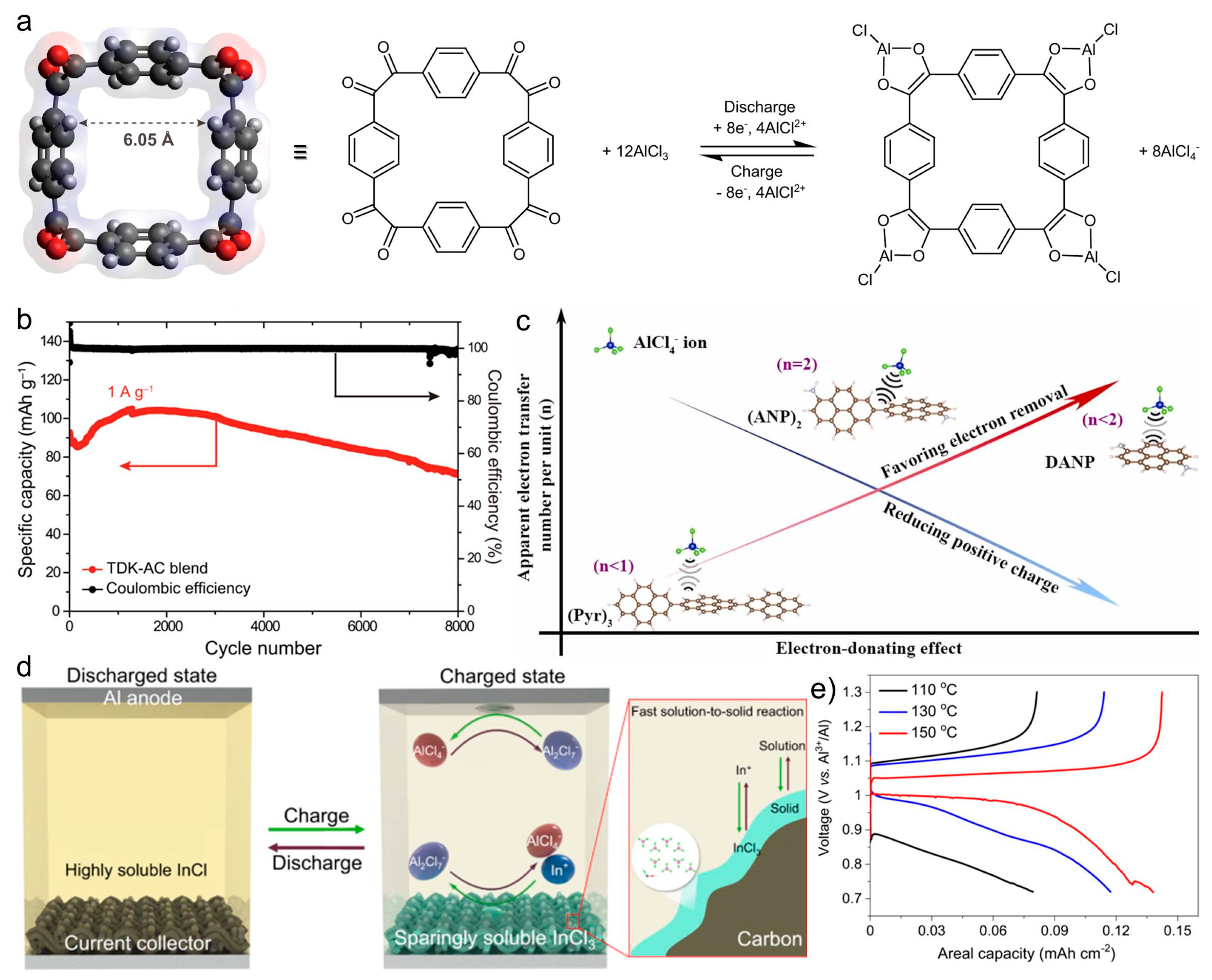
5.3. Organic Cathode Development and Molecular Engineering
5.4. Sulfur-/Molton-Salts-Based Systems and Chalcogen-Aluminum-Ion Batteries
6. Challenges and Future Perspectives
6.1. Material Innovation: Discovering Optimal Host Structures
6.2. Electrolyte Engineering: Ensuring Stability and Compatibility
6.3. Interfacial Optimization: Enhancing Stability at the Electrolyte-Electrode Interface
6.4. Innovative Device Architecture and Recyle: Bridging Lab and Real-World Applications
6.5. Data-Driven Discovery: Accelerating Innovation Through Computational Methods
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LIBs | lithium-ion batteries |
| MMIBs | multivalent metal-ion batteries |
| ZIBs | zinc-ion batteries |
| SEI | solid-electrolyte interphase |
| ZMO | ZnMn2O4 |
| PAHT | plasma-assisted hydrothermal |
| WiVO | water-intercalated vanadium oxide |
| CaV4O9 | calcium vanadate |
| DFT | Density functional theory |
| Zn(OTf)2 | zinc trifluoromethanesulfonate |
| CPANZ | cyclization of polyacrylonitrile, resulting in a pyridine nitrogen-rich, Zn2+-conductive coating |
| NH4Cl | ammonium chloride |
| ZnCl2 | zinc chloride |
| ZnSO4 | zinc sulfate |
| GQDs | graphene quantum dots |
| ZP | Zn3(PO4)2.4H2O |
| V2O5 | vanadium pentoxide |
| MnO2 | manganese dioxide |
| BMO | bi-doped α-MnO2 |
| VO2 | vanadium dioxide |
| KVO | potassium vanadate |
| AB-Zn | acetylene black-modified Zn foil anode |
| PPy | polypyrrole |
| ZVO | ZnδV2O5.nH2O |
| Aza-CMP | aza-fused π-conjugated microporous polymer |
| PPPA | poly(phenazine-alt-pyromellitic anhydride |
| IoT | Internet of Things |
| HCHATN | hexacyanohexaazatrinaphthylene |
| SiOC/Sn | tin-containing silicon oxycarbide |
| MOFs | metal–organic frameworks |
| RGO@CNT | reduced graphene oxide/carbon nanotube |
| GO | graphene oxide |
| WIS | water-in-salt |
| QSMB | quasi-solid-state Mg-ion batteries |
| PTCDA | perylene-3,4,9,10-tetracarboxylic dianhydride |
| PEO | polyethylene oxide |
| MVOH | Mg0.75V10O24.4H2O |
| NTO | Na2Ti3O7 |
| TMDs | transition metal dichalcogenides |
| CuHCF | copper hexacyanoferrate |
| PBAs | Prussian blue analogues |
| QVO | Quinoline-modified V2O5 |
| DIB | dual-ion battery |
| PAQS | poly(anthraquinonyl sulfide) |
| PTCDI | 3,4,9,10-perylenetetracarboxylic diimide |
| PT | 5,7,12,14-pentacenetetrone |
| COFs | covalent organic frameworks |
| PANI | polyaniline |
| PAHs | polycyclic aromatic hydrocarbons |
| GY | graphyne |
| ANP | 1-aminopyrene |
| NQ | Naphthoquinone |
| InCl3 | indium chloride |
References
- Li, J.; Fleetwood, J.; Hawley, W.B.; Kays, W. From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar] [CrossRef]
- Yoshino, A. The Birth of the Lithium-Ion Battery. Angew. Chem. Int. Edn. 2012, 51, 5798–5800. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Lakshmi, K.C.S.; Lin, T.-W. Interfacial Tuning of Polymeric Composite Materials for High-Performance Energy Devices. Batteries 2023, 9, 487. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Seetha Lakshmi, K.C. Beyond lithium-ion: Emerging frontiers in next-generation battery technologies. Front. Batter. Electrochem. 2024, 3, 1377192. [Google Scholar] [CrossRef]
- Liang, Y.; Dong, H.; Aurbach, D.; Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 2020, 5, 646–656. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; An, Z.; Zhang, M.; Wang, W.; Guo, Q.; Li, Y.; Han, S.; Zhang, L. Toward the next generation of sustainable aluminum-ion batteries: A review. Green Chem. 2025, 27, 352–378. [Google Scholar] [CrossRef]
- Guduru, R.K.; Icaza, J.C. A brief review on multivalent intercalation batteries with aqueous electrolytes. Nanomaterials 2016, 6, 41. [Google Scholar] [CrossRef]
- Huang, T.; Xue, X.; Zhang, Y.; Miao, Y.; Xiao, B.; Qi, J.; Wei, F.; Sui, Y. A review of metal sulfide cathode materials for non-aqueous multivalent ion (Mg2+, Ca2+, Al3+) batteries. J. Energy Storage 2024, 79, 110172. [Google Scholar] [CrossRef]
- Mahmood, A.; Bai, Z.; Wang, T.; Lei, Y.; Wang, S.; Sun, B.; Khan, H.; Khan, K.; Sun, K.; Wang, G. Enabling high-performance multivalent metal-ion batteries: Current advances and future prospects. Chem. Soc. Rev. 2025, 54, 2369–2435. [Google Scholar] [CrossRef]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, X.; Yang, J.; Li, X.; Liu, Z.; Loh, X.J.; Wang, J. Aqueous Rechargeable Multivalent Metal-Ion Batteries: Advances and Challenges. Adv. Energy Mater. 2021, 11, 2100608. [Google Scholar] [CrossRef]
- Tang, M.; Liu, Q.; Zou, X.; Zhang, B.; An, L. High-Energy-Density Aqueous Zinc-Ion Batteries: Recent Progress, Design Strategies, Challenges, and Perspectives. Adv. Mater. 2025, 2501361. [Google Scholar] [CrossRef]
- Ho, V.-C.; Lim, H.; Kim, M.J.; Mun, J. Improving the Performance of Aqueous Zinc-ion Batteries by Inhibiting Zinc Dendrite Growth: Recent Progress. Chem. Asian J. 2022, 17, e202200289. [Google Scholar] [CrossRef]
- Schroeder, M.A.; Ma, L.; Pastel, G.; Xu, K. The mystery and promise of multivalent metal-ion batteries. Curr. Opin. Electrochem. 2021, 29, 100819. [Google Scholar] [CrossRef]
- Fu, N.; Xu, Y.-T.; Zhang, S.; Deng, Q.; Liu, J.; Zhou, C.-J.; Wu, X.-W.; Guo, Y.-G.; Zeng, X.-X. Electrode materials for aqueous multivalent metal-ion batteries: Current status and future prospect. J. Energy Chem. 2022, 67, 563–584. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, D.; Chen, L.; Liu, G.; Ding, Y.; Cao, Y.; Chen, Z. Emerging Intercalation Cathode Materials for Multivalent Metal-Ion Batteries: Status and Challenges. Small Struct. 2021, 2, 2100082. [Google Scholar] [CrossRef]
- Bao, H.; Guo, H.; Zhang, X.; Tian, Z.; Huang, J.; Liu, T.; Lai, F. Anti-Freezing Electrolytes in Aqueous Multivalent Metal-Ion Batteries: Progress, Challenges, and Optimization Strategies. Chem. Rec. 2024, 24, e202300212. [Google Scholar] [CrossRef]
- Zhang, S.; Long, T.; Zhang, H.-Z.; Zhao, Q.-Y.; Zhang, F.; Wu, X.-W.; Zeng, X.-X. Electrolytes for Multivalent Metal-Ion Batteries: Current Status and Future Prospect. ChemSusChem 2022, 15, e202200999. [Google Scholar] [CrossRef]
- Zhang, Q.; He, L.; Liu, A.; Han, W.; Li, A.; Zhao, Z.; Zhu, X.; Tan, G. High-performance mg-ion battery materials: Recent progress and future perspectives. J. Energy Storage 2025, 132, 117900. [Google Scholar] [CrossRef]
- Gummow, R.J.; Vamvounis, G.; Kannan, M.B.; He, Y. Calcium-Ion Batteries: Current State-of-the-Art and Future Perspectives. Adv. Mater. 2018, 30, 1801702. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, J.; Tian, J.; Niu, Z. Recent Progress in the Electrolytes of Aqueous Zinc-Ion Batteries. Chem. Eur. J. 2019, 25, 14480–14494. [Google Scholar] [CrossRef]
- Farooq, A.; Zhao, R.; Han, X.; Yang, J.; Hu, Z.; Wu, C.; Bai, Y. Towards Superior Aqueous Zinc-Ion Batteries: The Insights of Artificial Protective Interfaces. ChemSusChem 2024, 17, e202301942. [Google Scholar] [CrossRef]
- Ye, X.; Xiao, X.; Wu, Z.; Zhan, Y.; Wu, X.; Liu, S. Recent advances in rechargeable aqueous magnesium-ion batteries. J. Mater. Chem. A 2024, 12, 23337–23363. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Wang, Y.; Pan, T.; Pang, H. Research progress on modification of cathodes for aqueous zinc ion batteries. Mater. Chem. Front. 2024, 8, 3702–3723. [Google Scholar] [CrossRef]
- Khan, H.; Zhao, C.; Khan, K.; Tareen, A.K.; Shahzad, A.; Langford, S.J.; Liu, H.; Mahmood, A.; Wang, G. Challenges and Design Strategies for Stable Zinc Anodes in Rechargeable Zinc Batteries. Small 2025, 21, 2504170. [Google Scholar] [CrossRef]
- He, Z.; Huang, W.; Xiong, F.; Tan, S.; Wu, T.; Wang, R.; Ducati, C.; De Volder, M.; An, Q. Organic solid–electrolyte interface layers for Zn metal anodes. Chem. Commun. 2024, 60, 6847–6859. [Google Scholar] [CrossRef]
- Li, C.; Ni, C.; Huang, X.-L.; Yao, L.; Yang, L.; Zhang, L.; Zhu, K.; Liu, H.-K.; Wang, Y.-X. Recent advances and perspectives of electrolyte additives for enhanced anode reversibility in aqueous zinc-ion batteries. Chem. Commun. 2025, 61, 10643–10667. [Google Scholar] [CrossRef]
- Ullah, B.; Wang, T.; Cai, R.; Feng, Y.; Ming, X.; Hassanzadeh-Aghdam, M.K.; Zeng, L.; Xi, K.; Tian, L.; Shen, G. Design Principles of Flexible Substrates and Polymer Electrolytes for Flexible Zinc Ion Batteries. Small 2025, 21, 2501671. [Google Scholar] [CrossRef]
- Man, Y.; Jaumaux, P.; Xu, Y.; Fei, Y.; Mo, X.; Wang, G.; Zhou, X. Research development on electrolytes for magnesium-ion batteries. Sci. Bull. 2023, 68, 1819–1842. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Zhang, Z.; Aurbach, D. Alloy Anode Materials for Rechargeable Mg Ion Batteries. Adv. Energy Mater. 2020, 10, 2000697. [Google Scholar] [CrossRef]
- Ji, B.; He, H.; Yao, W.; Tang, Y. Recent Advances and Perspectives on Calcium-Ion Storage: Key Materials and Devices. Adv. Mater. 2021, 33, 2005501. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Xiao, Y.; Ma, L.; Zhi, C.; Chen, S. Recent Advances in Electrolytes for “Beyond Aqueous” Zinc-Ion Batteries. Adv. Mater. 2022, 34, 2106409. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, B.-H.; Huang, J.; Xiao, K.; Liu, Z.-Q. Microstructure Strain of ZnMn2O4 Spinel by Regulation of Tetrahedral Sites for High-Performance Aqueous Zinc-Ion Battery. Adv. Funct. Mater. 2024, 34, 2405680. [Google Scholar] [CrossRef]
- Choi, S.; Kim, S.-W.; Hwang, C.; Park, J.-H.; Wang, P.C.; Ang, E.Y.M.; Lee, S.Y.; Park, S.; Abou-Elanwar, A.M.; Yun, S.W.; et al. Plasma Assisted Hydrothermal Synthesis of 2D & 3D Water Intercalated V2O5 Nanosheet Clusters for High Performing Aqueous Zinc Ion Battery. Small Struct. 2025, 6, 2500269. [Google Scholar] [CrossRef]
- Du, Y.; Wang, X.; Zhang, Y.; Zhang, H.; Man, J.; Liu, K.; Sun, J. High mass loading CaV4O9 microflowers with amorphous phase transformation as cathode for aqueous zinc-ion battery. Chem. Eng. J. 2022, 434, 134642. [Google Scholar] [CrossRef]
- Upreti, B.B.; Kamboj, N.; Dey, R.S. Advancing Zinc Anodes: Strategies for Enhanced Performance in Aqueous Zinc-Ion Batteries. Small 2025, 21, 2408138. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Du, H.; Liu, Y.; Wu, X.; Yin, C.; Wang, D.; Wu, X.; He, Z.; Wu, X. A Stable Fluoride-Based Interphase for a Long Cycle Zn Metal Anode in an Aqueous Zinc Ion Battery. J. Mater. Chem. A 2022, 10, 14399–14410. [Google Scholar] [CrossRef]
- Kim, Y.; Park, Y.; Kim, M.; Lee, J.; Kim, K.J.; Choi, J.W. Corrosion as the origin of limited lifetime of vanadium oxide-based aqueous zinc ion batteries. Nat. Commun. 2022, 13, 2371. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Jeoun, Y.; Kim, J.H.; Shim, J.; Ahn, K.-S.; Yu, S.-H.; Sung, Y.-E. Selective Ion Transport Layer for Stable Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2310884. [Google Scholar] [CrossRef]
- Liu, Z.; Li, G.; Xi, M.; Huang, Y.; Li, H.; Jin, H.; Ding, J.; Zhang, S.; Zhang, C.; Guo, Z. Interfacial Engineering of Zn Metal via a Localized Conjugated Layer for Highly Reversible Aqueous Zinc Ion Battery. Angew. Chem. Int. Edn. 2024, 63, e202319091. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, Y.; Guo, S.; Cao, X.; Pan, A.; Fang, G.; Zhou, J.; Liang, S. Fundamentals and perspectives in developing zinc-ion battery electrolytes: A comprehensive review. Energy Environ. Sci. 2020, 13, 4625–4665. [Google Scholar] [CrossRef]
- Xue, T.; Guan, J.; Mu, Y.; Han, M.; Yang, C.; Zang, L.; Zeng, L. Quasi-Solid-State Electrolytes: Bridging the gap between solid and liquid electrolytes for zinc-ion batteries. Chem. Eng. J. 2025, 514, 162994. [Google Scholar] [CrossRef]
- Lee, S.; Huh, S.-H.; Lee, Y.-H.; Kim, S.H.; Bae, J.-S.; Ahn, K.-S.; Huh, J.; Sung, Y.-E.; Yu, S.-H. Exploring the effects of biomolecular additive on performance of aqueous zinc metal batteries. Chem. Eng. J. 2025, 515, 163465. [Google Scholar] [CrossRef]
- Hou, S.; Luo, J.; Gong, W.; Xie, Y.; Zhou, X.; Yue, F.; Shen, J.; Li, C.; Wei, L.; Xu, F.; et al. High-Entropy Multiple-Anion Aqueous Electrolytes for Long-Life Zn-Metal Anodes. ACS Nano 2024, 18, 31524–31536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, R.; Li, S.; Liu, C.; Li, H.; Zou, G.; Hu, J.; Hou, H.; Ji, X. Graphene quantum dots enable dendrite-free zinc ion battery. Nano Energy 2022, 92, 106752. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.; Heo, K.; Lim, J.H.; Yashiro, H.; Myung, S.T. Nature of Zinc-Derived Dendrite and Its Suppression in Mildly Acidic Aqueous Zinc-Ion Battery. Adv. Energy Mater. 2023, 13, 2203189. [Google Scholar] [CrossRef]
- Ye, B.; Wu, F.; Zhao, R.; Zhu, H.; Lv, M.; Han, X.; Chen, T.; Wang, X.; Bai, Y.; Wu, C. Electrolyte Regulation toward Cathodes with Enhanced-Performance in Aqueous Zinc Ion Batteries. Adv. Mater. 2025, 37, 2501538. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, M.; Liu, R.; Xiao, H.; Liu, Y.; Wang, X.; Huang, Y.; Yuan, G. Molecular tailoring of MnO2 by bismuth doping to achieve aqueous zinc-ion battery with capacitor-level durability. Energy Storage Mater. 2022, 48, 212–222. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Q.; Shi, J.; Wang, F.; Wang, S.; Chen, Z.; Yue, Y.; Gao, Y. Layered Topological Insulator MnBi2Te4 as a Cathode for a High Rate Performance Aqueous Zinc-Ion Battery. ACS Nano 2024, 18, 5981–5990. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.H.; Yang, C.Q.; Guo, X.Z.; Yu, Y.X.; Yang, Y. Rational Regulation of Optimal Oxygen Vacancy Concentrations on VO2 for Superior Aqueous Zinc-Ion Battery Cathodes. ACS Appl. Mater. Interfaces 2024, 16, 40903–40913. [Google Scholar] [CrossRef]
- Qiu, N.; Yang, Z.; Xue, R.; Wang, Y.; Zhu, Y.; Liu, W. Toward a High-Performance Aqueous Zinc Ion Battery: Potassium Vanadate Nanobelts and Carbon Enhanced Zinc Foil. Nano Lett. 2021, 21, 2738–2744. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, J.; Liu, Y.; Jiang, H.; Hu, T.; Cui, M.; Tian, F.; Meng, C.; Zhang, Y. Polypyrrole-intercalation tuning lamellar structure of V2O5·nH2O boosts fast zinc-ion kinetics for aqueous zinc-ion battery. J. Power Sources 2022, 536, 231489. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, F.; Sun, X.; Hou, T.; Xu, Z.; Na, Y.; An, Q.; Chen, Z.; Cai, S.; Zheng, C. High-capacity zinc vanadium oxides with long-term cyclability enabled by in-situ electrochemical oxidation as zinc-ion battery cathode. Chem. Eng. J. 2022, 445, 136714. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Z.; Chen, H.; Pu, X.; Chen, Z. MXene-Based Materials for Multivalent Metal-Ion Batteries. Batteries 2023, 9, 174. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, M.; Wen, L.; Zeng, W.; Ge, B.; Zhang, C.; Yue, Y.; Wang, S. A New (De)Intercalation MXene/Bi Cathode for Ultrastable Aqueous Zinc-Ion Battery. Adv. Funct. Mater. 2023, 34, 2214506. [Google Scholar] [CrossRef]
- Tie, Z.; Niu, Z. Design Strategies for High-Performance Aqueous Zn/Organic Batteries. Angew. Chem. Int. Edn. 2020, 59, 21293–21303. [Google Scholar] [CrossRef]
- Li, L.; Shi, Y.; Jia, S.; Wang, C.; Liu, W.; Zhang, D. Research progress of polymer material in zinc ion battery. J. Energy Storage 2024, 97, 112819. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, K.; Gao, Y.; Wang, C. Challenges and Perspectives of Organic Multivalent Metal-Ion Batteries. Adv. Mater. 2022, 34, 2200662. [Google Scholar] [CrossRef]
- Li, Z.; Tan, J.; Zhu, X.; Xie, S.; Fang, H.; Ye, M.; Shen, J. High capacity and long-life aqueous zinc-ion battery enabled by improving active sites utilization and protons insertion in polymer cathode. Energy Storage Mater. 2022, 51, 294–305. [Google Scholar] [CrossRef]
- Ye, F.; Liu, Q.; Dong, H.; Guan, K.; Chen, Z.; Ju, N.; Hu, L. Organic Zinc-Ion Battery: Planar, π-Conjugated Quinone-Based Polymer Endows Ultrafast Ion Diffusion Kinetics. Angew. Chem. Int. Edn. 2022, 61, e202214244. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Guo, J.; Lai, F.; Zhao, F.; Jiao, Y.; Brett, D.J.L.; Liu, T.; He, G.; Parkin, I.P. Insights on Flexible Zinc-Ion Batteries from Lab Research to Commercialization. Adv. Mater. 2021, 33, 2007548. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Yang, X.-Y.; Liu, T.; Zhang, X.-B. Flexible 1D Batteries: Recent Progress and Prospects. Adv. Mater. 2020, 32, 1901961. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, D.; Liu, N.; Liu, Z.; Ren, Z.; Yin, J.; Jia, P.; Lu, W.; Gao, Y. A Zinc-Ion Battery-Type Self-Powered Pressure Sensor with Long Service Life. Adv. Mater. 2022, 34, 2205369. [Google Scholar] [CrossRef]
- De, P.; Pumera, M. Aqueous Multivalent Metal-ion Batteries: Toward 3D-printed Architectures. Small 2024, 20, 2404227. [Google Scholar] [CrossRef]
- Ren, Y.; Meng, F.; Zhang, S.; Ping, B.; Li, H.; Yin, B.; Ma, T. CNT@MnO2 composite ink toward a flexible 3D printed micro-zinc-ion battery. Carbon Energy 2022, 4, 446–457. [Google Scholar] [CrossRef]
- Yang, M.; Wang, D.; Ling, Y.; Guo, X.; Chen, W. Emerging Advanced Photo-Rechargeable Batteries. Adv. Funct. sMater. 2024, 34, 2410398. [Google Scholar] [CrossRef]
- Chen, X.; Su, H.; Liu, X.; Su, L.; Lan, L.; Yang, B.; Liu, Q. Introduction of Electron-Withdrawing Groups in Organic Cathodes for Enhancing the Performance of Air-Charging Aqueous Zinc-Ion Batteries. Energy Fuels 2025, 39, 14910–14920. [Google Scholar] [CrossRef]
- Mittal, N.; Ojanguren, A.; Kundu, D.; Lizundia, E.; Niederberger, M. Bottom-Up Design of a Green and Transient Zinc-Ion Battery with Ultralong Lifespan. Small 2023, 19, 2206249. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Hao, Y.; Jia, H.; Shen, X.; Qu, B.; Huang, G.; Zhou, X.; Wang, J.; Xu, C.; et al. Challenges and Progress in Rechargeable Magnesium-Ion Batteries: Materials, Interfaces, and Devices. Adv. Funct. Mater. 2024, 34, 2410406. [Google Scholar] [CrossRef]
- Wei, C.; Tan, L.; Zhang, Y.; Wang, Z.; Feng, J.; Qian, Y. Towards better Mg metal anodes in rechargeable Mg batteries: Challenges, strategies, and perspectives. Energy Storage Mater. 2022, 52, 299–319. [Google Scholar] [CrossRef]
- Medina, A.; Pérez-Vicente, C.; Alcántara, R. Advancing towards a Practical Magnesium Ion Battery. Materials 2021, 14, 7488. [Google Scholar] [CrossRef]
- Lee, E.-S.; Huh, S.-H.; Lee, S.-H.; Yu, S.-H. On the Road to Stable Electrochemical Metal Deposition in Multivalent Batteries. ACS Sust. Chem. Eng. 2023, 11, 2014–2032. [Google Scholar] [CrossRef]
- Kwak, J.H.; Jeoun, Y.; Oh, S.H.; Yu, S.; Lim, J.-H.; Sung, Y.-E.; Yu, S.-H.; Lim, H.-D. Operando Visualization of Morphological Evolution in Mg Metal Anode: Insight into Dendrite Suppression for Stable Mg Metal Batteries. ACS Energy Lett. 2022, 7, 162–170. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Ye, J.; Wen, J.; Wang, J.; Huang, G.; Wang, J. A High Voltage Magnesium Ion Battery Based on Na3V2(PO4)3@C Cathode and Bi Nanowire Anode. Langmuir ACS J. Surf. Colloids 2025, 41, 18919–18928. [Google Scholar] [CrossRef]
- Guo, W.; Icin, O.; Vakifahmetoglu, C.; Kober, D.; Gurlo, A.; Bekheet, M.F. Magnesium-Ion Battery Anode from Polymer-Derived SiOC Nanobeads. Adv. Funct. Mater. 2023, 33, 2304933. [Google Scholar] [CrossRef]
- Guo, Z.; Wei, W.; Shi, J.; Wang, P.; Ye, Z.; Mi, L. NiS2 nanoparticles by the NaCl-assisted less-liquid reaction system for the magnesium-ion battery cathode. Nanoscale 2023, 15, 1702–1708. [Google Scholar] [CrossRef]
- Wang, L.; Ng, A.; Family, R.; Detsi, E.; Pikul, J. Liquid eutectic gallium–indium as a magnesium-ion battery anode with ultralong cycle life enabled by liquid–solid phase transformation during (de)magnesiation at room temperature. J. Mater. Chem. A 2024, 12, 27435–27442. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X. Minireview on Metal–Chalcogenide Cathode Materials With Dual-Redox Centers for Magnesium-Metal-Anode-Based Batteries: Recent Progress and Future Directions. Energy Fuels 2023, 37, 18441–18455. [Google Scholar] [CrossRef]
- Yan, X.; Luo, S.-h.; Liu, Q.; Sun, Q.; Yan, S.-x.; Wang, Q.; Zhang, Y.; Liu, X. Insertion-type cathode materials in magnesium ion batteries: Research progress and application potential. Mater. Today Energy 2025, 53, 102001. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Fichtner, M. Beyond Intercalation Chemistry for Rechargeable Mg Batteries: A Short Review and Perspective. Front. Chem. 2019, 6, 656. [Google Scholar] [CrossRef]
- Shi, M.; Li, T.; Shang, H.; Zhang, D.; Qi, H.; Huang, T.; Xie, Z.; Qi, J.; Wei, F.; Meng, Q.; et al. A critical review of inorganic cathode materials for rechargeable magnesium ion batteries. J. Energy Storage 2023, 68, 107765. [Google Scholar] [CrossRef]
- Mei, L.; Xu, J.; Wei, Z.; Liu, H.; Li, Y.; Ma, J.; Dou, S. Chevrel Phase Mo6T8 (T = S, Se) as Electrodes for Advanced Energy Storage. Small 2017, 13, 1701441. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Kang, H.; Jin, T.; Jiao, L. Design, synthesis, and energy-related applications of metal sulfides. Mater. Horiz. 2016, 3, 402–421. [Google Scholar] [CrossRef]
- Kong, L.; Liu, M.; Huang, H.; Xu, Y.; Bu, X.-H. Metal/Covalent-Organic Framework Based Cathodes for Metal-Ion Batteries. Adv. Energy Mater. 2022, 12, 2100172. [Google Scholar] [CrossRef]
- Fei, Y.; Man, Y.; Sun, J.; Du, Y.; Chen, B.; Bao, J.; Zhou, X. Implanting CuS Quantum Dots into Carbon Nanorods for Efficient Magnesium-Ion Batteries. Small 2023, 19, 2301954. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xiao, H.; Yu, H.; Zhang, J.; Yang, Y.; Zhang, P.; Chen, Y.; Gao, X.; Chen, X.; Cui, L. Anionic TeO32− doped CoS nanomaterials for high-performance magnesium-ion battery cathode materials. J. Colloid Interface Sci. 2025, 700, 138330. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, T.; Miao, J.; Ning, W.; Sun, Y.; Qiu, F.; Shi, X.; Miao, S. Mg(Al, Fe, Mn, REE)2O4 Spinel Prepared from Pelagic REE-Rich Clays and Application as Magnesium-Ion-Battery Cathodes. ACS Appl. Mater. Interfaces 2024, 16, 60122–60131. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.M.; Yuan, Z.; Xu, H.; Li, D.; Li, Y.; Han, W.; Wang, L. TiVCTx MXene/Chalcogenide Heterostructure-Based High-Performance Magnesium-Ion Battery as Flexible Integrated Units. Small 2022, 18, e2202313. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Gao, H.; Shao, Y.; Liu, Y.; Zhu, Y.; Zhang, J.; Li, L. VS4 anchored on Ti3C2 MXene as a high-performance cathode material for magnesium ion battery. J. Power Sources 2022, 518, 230731. [Google Scholar] [CrossRef]
- Ding, Q.; Han, T.; Zhou, T.; Lin, X.; Liu, J. A Temperature-Tolerant Magnesium-Ion Battery Using Ball Cactus-like MgV2O4 as High-Performance Cathode. Chem. Eur. J. 2024, 30, e202302978. [Google Scholar] [CrossRef]
- Dong, C.; Kobayashi, H.; Honma, I. Self-activation effect in bimetallic MgMn2O4 and boosting its electrochemical performance using metal-organic framework template for magnesium-ion battery cathodes. Mater. Today Energy 2022, 30, 101143. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Liu, G.; Zhang, T.; Zhang, C.; Zhang, Y.; Feng, Y.; Chi, Q. Improvements in the Magnesium Ion Transport Properties of Graphene/CNT-Wrapped TiO2-B Nanoflowers by Nickel Doping. Small 2024, 20, 2304969. [Google Scholar] [CrossRef]
- Mohtadi, R.; Mizuno, F. Magnesium batteries: Current state of the art, issues and future perspectives. Beilstein J. Nanotechnol. 2014, 5, 1291–1311. [Google Scholar] [CrossRef]
- Smith, M.B.; Becker, W.E. The constitution of the grignard reagent—III: The reaction between R2Mg and MgX2 in tetrahydrofuran. Tetrahedron 1967, 23, 4215–4227. [Google Scholar] [CrossRef]
- Xu, R.; Gao, X.; Chen, Y.; Peng, C.; Zhang, Z.; Wang, C.; Sun, H.; Chen, X.; Cui, L. Research advances of the electrolytes for rechargeable magnesium ion batteries. Mater. Today Phys. 2023, 36, 101186. [Google Scholar] [CrossRef]
- Wally, N.K.; Sheha, E.; Morad, I.M.; El-Desoky, M.M. Combined Effects of LiCl Addition and Solvent-in-Water Approaches on Aqueous Magnesium-Ion Battery Performance. ACS Appl. Energy Mater. 2025, 8, 4441–4455. [Google Scholar] [CrossRef]
- Li, X.; Suo, C.; Zhang, B.; Hu, B.; Huang, J.; Zhao, Y.; Zhang, W.; Ji, X.; Li, W. Free water based high capacity aqueous magnesium ion battery with nano-amorphized cathodic V6O13@Mg-MnO2 in the presence of Cl. Chem. Eng. J. 2025, 510, 161725. [Google Scholar] [CrossRef]
- Yang, T.; Ma, F.; Zhang, X.; Yang, Y.n.; Lv, J.; Jin, Z.; Wang, L.; Gao, H.; Wang, X.; Chen, N. High-rate aqueous magnesium ion battery enabled by Li/Mg hybrid superconcentrated electrolyte. Appl. Surf. Sci. 2024, 660, 159995. [Google Scholar] [CrossRef]
- Leong, K.W.; Pan, W.; Yi, X.; Luo, S.; Zhao, X.; Zhang, Y.; Wang, Y.; Mao, J.; Chen, Y.; Xuan, J.; et al. Next-generation magnesium-ion batteries: The quasi-solid-state approach to multivalent metal ion storage. Sci. Adv. 2023, 9, eadh1181. [Google Scholar] [CrossRef]
- Karlsmo, M.; Hosaka, T.; Johansson, P. Le Chatelier’s principle enables stable and sustainable aqueous sodium/magnesium-ion batteries. J. Mater. Chem. A 2024, 12, 4029–4036. [Google Scholar] [CrossRef]
- Liang, Z.; Ban, C. Strategies to Enable Reversible Magnesium Electrochemistry: From Electrolytes to Artificial Solid–Electrolyte Interphases. Angew. Chem. Int. Edn. 2021, 60, 11036–11047. [Google Scholar] [CrossRef]
- Hardin, N.Z.; Woodley, C.P.; McDonald, K.D.; Bartlett, B.M. Triiodide Anion as a Magnesium-ion Transporter for Low Overpotential Battery Cycling in Iodine-Containing Mg(TFSI)2 Electrolyte. ACS Appl. Mater. Interfaces 2024, 16, 14883–14889. [Google Scholar] [CrossRef]
- Chai, X.; Xin, Y.; He, B.; Zhang, F.; Xie, H.; Tian, H. High-efficiency electrodeposition of magnesium alloy-based anodes for ultra-stable rechargeable magnesium-ion batteries. Nanoscale 2024, 16, 9123–9135. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Wang, D.; Chen, A.; Li, X.; Huang, Z.; Liang, G.; Wang, Y.; Zhi, C. Tellurium: A High-Performance Cathode for Magnesium Ion Batteries Based on a Conversion Mechanism. ACS Nano 2022, 16, 5349–5357. [Google Scholar] [CrossRef]
- Ma, X.-F.; Zhao, B.-Q.; Liu, H.; Tan, J.; Li, H.-Y.; Zhang, X.; Diao, J.; Yue, J.; Huang, G.; Wang, J.; et al. H2O-Mg2+ Waltz-Like Shuttle Enables High-Capacity and Ultralong-Life Magnesium-Ion Batteries. Adv. Sci. 2024, 11, 2401005. [Google Scholar] [CrossRef]
- Fan, X.; Gaddam, R.R.; Kumar, N.A.; Zhao, X.S. A Hybrid Mg2+/Li+ Battery Based on Interlayer-Expanded MoS2/Graphene Cathode. Adv. Energy Mater. 2017, 7, 1700317. [Google Scholar] [CrossRef]
- Walter, M.; Kravchyk, K.V.; Ibáñez, M.; Kovalenko, M.V. Efficient and Inexpensive Sodium–Magnesium Hybrid Battery. Chem. Mater. 2015, 27, 7452–7458. [Google Scholar] [CrossRef]
- Yan, L.; Yang, W.; Yu, H.; Zhang, L.; Shu, J. Recent progress in rechargeable calcium-ion batteries for high-efficiency energy storage. Energy Storage Mater. 2023, 60, 102822. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, C.; Tan, C.; Zhang, D. A Comprehensive Review of Anode Materials in Rechargeable Calcium-Ion Batteries. Chem. Eur. J. 2025, 31, e202500071. [Google Scholar] [CrossRef]
- Deng, X.; Li, L.; Zhang, G.; Zhao, X.; Hao, J.; Han, C.; Li, B. Anode chemistry in calcium ion batteries: A review. Energy Storage Mater. 2022, 53, 467–481. [Google Scholar] [CrossRef]
- Tinker, H.R.; Howard, C.A.; Zhou, M.; Xu, Y. Exploring anodes for calcium-ion batteries. Mater. Adv. 2023, 4, 2028–2041. [Google Scholar] [CrossRef]
- Wang, L.; Riedel, S.; Zhao-Karger, Z. Challenges and Progress in Anode-Electrolyte Interfaces for Rechargeable Divalent Metal Batteries. Adv. Energy Mater. 2024, 14, 2402157. [Google Scholar] [CrossRef]
- Zuo, C.; Shao, Y.; Li, M.; Zhang, W.; Zhu, D.; Tang, W.; Hu, J.; Liu, P.; Xiong, F.; An, Q. Layered Na2Ti3O7 as an Ultrastable Intercalation-Type Anode for Non-Aqueous Calcium-Ion Batteries. ACS Appl. Mater. Interfaces 2024, 16, 33733–33739. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Yu, R.; Qiao, F.; Wang, J.; Wang, J.; An, Q. Molecular Engineering to Construct MoS2 with Expanded Interlayer Spacing and Enriched 1T Phase for “Rocking-Chair” Aqueous Calcium-Ion Pouch Cells. ACS Nano 2024, 18, 35286–35295. [Google Scholar] [CrossRef]
- Pan, H.; Wang, C.; Qiu, M.; Wang, Y.; Han, C.; Nan, D. Mo Doping and Electrochemical Activation Co-Induced Vanadium Composite as High-Rate and Long-Life Anode for Ca-Ion Batteries. Energy Environ. Mater. 2024, 7, e12690. [Google Scholar] [CrossRef]
- Kim, S.; Yin, L.; Bak, S.M.; Fister, T.T.; Park, H.; Parajuli, P.; Gim, J.; Yang, Z.; Klie, R.F.; Zapol, P.; et al. Investigation of Ca Insertion into α-MoO3 Nanoparticles for High Capacity Ca-Ion Cathodes. Nano Lett. 2022, 22, 2228–2235. [Google Scholar] [CrossRef]
- Hua, Y.; Ma, Y.; Qi, Q.; Xu, Z.-L. Cathode materials for non-aqueous calcium rechargeable batteries. Nanoscale 2024, 16, 17683–17698. [Google Scholar] [CrossRef]
- Chando, P.; Chen, S.H.; Shellhamer, J.M.; Wall, E.; Wang, X.; Schuarca, R.L.; Smeu, M.; Hosein, I.D. Exploring Calcium Manganese Oxide as a Promising Cathode Material for Calcium-Ion Batteries. Chem. Mater. 2023, 35, 8371–8381. [Google Scholar] [CrossRef]
- Jin, S.; Yu, R.; Wang, J.; Cui, L.; Huang, M.; Zhang, L.; An, Q. Self-Adaptive Electrochemistry of Phosphate Cathodes toward Improved Calcium Storage. ACS Nano 2024, 18, 28246–28257. [Google Scholar] [CrossRef]
- Tekliye, D.B.; Kumar, A.; Weihang, X.; Mercy, T.D.; Canepa, P.; Sai Gautam, G. Exploration of NaSICON Frameworks as Calcium-Ion Battery Electrodes. Chem. Mater. 2022, 34, 10133–10143. [Google Scholar] [CrossRef]
- Du, C.-Y.; Zhang, Z.-H.; Li, X.-L.; Luo, R.-J.; Ma, C.; Bao, J.; Zeng, J.; Xu, X.; Wang, F.; Zhou, Y.-N. Enhancing structural and cycle stability of Prussian blue cathode materials for calcium-ion batteries by introducing divalent Fe. Chem. Eng. J. 2023, 451, 138650. [Google Scholar] [CrossRef]
- Adil, M.; Sarkar, A.; Sau, S.; Muthuraj, D.; Mitra, S. Non-aqueous rechargeable calcium-ion batteries based on high voltage zirconium-doped ammonium vanadium oxide cathode. J. Power Sources 2022, 541, 231669. [Google Scholar] [CrossRef]
- Zhao, X.; Li, L.; Zhang, G.; Yi, Y.; Yang, T.; Han, C.; Li, B. Discovering the Order-Disorder Transition in Quinoline Intercalated Vanadium Oxide with Superior Calcium Storage via Polyhedral Distortion. Small Methods 2024, 8, 2400097. [Google Scholar] [CrossRef]
- Li, Z.; Cui, S.; Häcker, J.; Nojabaee, M.; Fichtner, M.; Cui, G.; Zhao-Karger, Z. Calcium Chemistry as A New Member of Post-Lithium Battery Family: What Can We Learn from Sodium and Magnesium Systems. Angew. Chem. Int. Edn. 2025, 64, e202415942. [Google Scholar] [CrossRef]
- Arroyo-de Dompablo, M.E.; Ponrouch, A.; Johansson, P.; Palacín, M.R. Achievements, Challenges, and Prospects of Calcium Batteries. Chem. Rev. 2020, 120, 6331–6357. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, L.; Sun, X.; Liu, T.L. Progress and prospects of electrolyte chemistry of calcium batteries. Chem. Sci. 2022, 13, 5797–5812. [Google Scholar] [CrossRef]
- Melemed, A.M.; Khurram, A.; Gallant, B.M. Current Understanding of Nonaqueous Electrolytes for Calcium-Based Batteries. Batter. Supercaps 2020, 3, 570–580. [Google Scholar] [CrossRef]
- Shinde, S.S.; Wagh, N.K.; Kim, S.-H.; Lee, J.-H. Li, Na, K, Mg, Zn, Al, and Ca Anode Interface Chemistries Developed by Solid-State Electrolytes. Adv. Sci. 2023, 10, 2304235. [Google Scholar] [CrossRef]
- Zeng, F.; Li, S.; Hu, S.; Qiu, M.; Zhang, G.; Li, M.; Chang, C.; Wang, H.; Xu, M.; Zheng, L.; et al. Weak Solvation Effect Enhancing Capacity and Rate Performance of Vanadium-Based Calcium Ion Batteries: A Strategy Guided by Donor Number. Adv. Funct. Mater. 2024, 34, 2302397. [Google Scholar] [CrossRef]
- Li, J.; Han, C.; Ou, X.; Tang, Y. Concentrated Electrolyte for High-Performance Ca-Ion Battery Based on Organic Anode and Graphite Cathode. Angew. Chem. Int. Edn. 2022, 61, e202116668. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, J.; Wang, L.; Jiao, Z.; Xiao, M.; Huang, Q.-a.; Liu, M.; Shao, Q.; Sun, X.; Zhang, J. Prussian blue and its analogues as cathode materials for Na-, K-, Mg-, Ca-, Zn- and Al-ion batteries. Nano Energy 2022, 99, 107424. [Google Scholar] [CrossRef]
- Streng, R.L.; Reiser, S.; Wager, S.; Pommer, N.; Bandarenka, A.S. A Fast and Highly Stable Aqueous Calcium-Ion Battery for Sustainable Energy Storage. ChemSusChem 2025, 18, e202401469. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, X.; Feng, W.; Ponrouch, A.; Poizot, P.; Armand, M.; Zhou, Z.; Zhang, H. Carbonyl-based organic electrode materials spanning from nonaqueous rechargeable lithium to calcium batteries. Energy Environ. Mater. 2025, 18, 7756–7791. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Y.; Wang, D.; Li, C.; Han, Y.; Shi, Z.; Feng, S. Poly(Anthraquinonyl Sulfide)/CNT Composites as High-Rate-Performance Cathodes for Nonaqueous Rechargeable Calcium-Ion Batteries. Adv. Sci. 2022, 9, 2200397. [Google Scholar] [CrossRef]
- Jao, W.-Y.; Tai, C.-W.; Chang, C.-C.; Hu, C.-C. Non-aqueous calcium-based dual-ion batteries with an organic electrode of high-rate performance. Energy Storage Mater. 2023, 63, 102990. [Google Scholar] [CrossRef]
- Qiao, F.; Wang, J.; Yu, R.; Huang, M.; Zhang, L.; Yang, W.; Wang, H.; Wu, J.; Zhang, L.; Jiang, Y.; et al. Aromatic Organic Small-Molecule Material with (020) Crystal Plane Activation for Wide-Temperature and 68000 Cycle Aqueous Calcium-Ion Batteries. ACS Nano 2023, 17, 23046–23056. [Google Scholar] [CrossRef]
- Han, C.; Li, H.; Li, Y.; Zhu, J.; Zhi, C. Proton-assisted calcium-ion storage in aromatic organic molecular crystal with coplanar stacked structure. Nat. Commun. 2021, 12, 2400. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Y.-L.; Ren, S.; Li, C.; Chen, X.-B.; Li, Z.; Han, Y.; Shi, Z.; Feng, S. Covalent Organic Framework with Multiple Redox Active Sites for High-Performance Aqueous Calcium Ion Batteries. J. Am. Chem. Soc. 2023, 145, 17309–17320. [Google Scholar] [CrossRef]
- Luo, P.; Zhu, D.; Zuo, C.; Zhang, W.; Li, M.; Chao, F.; Yu, G.; Zhong, W.; An, Q. Synergistic enhanced kinetics and dual active sites in PANI@V2O5 nanoflower for superior Ca2+ ion storage. Chem. Eng. J. 2024, 482, 149177. [Google Scholar] [CrossRef]
- Liu, Y.; He, B.; Pu, J.; Yu, M.; Zhang, Y.; Meng, C.; Zhang, Q.; Wu, J.; Wei, L.; Pan, Z. High-energy fiber-shaped calcium-ion batteries enable integrated wearable electronics for human body monitoring. J. Energy Chem. 2024, 99, 661–670. [Google Scholar] [CrossRef]
- Yuan, X.; Lin, Z.; Duan, Y.; Chen, Z.; Fu, L.; Chen, Y.; Liu, L.; Yuan, X.; Wu, Y. Research Progress, Challenges, and Prospects of High Energy Density Aqueous Aluminum-Ion Batteries: A Mini-Review. Batter. Supercaps 2024, 7, e202400263. [Google Scholar] [CrossRef]
- Jia, B.-E.; Thang, A.Q.; Yan, C.; Liu, C.; Lv, C.; Zhu, Q.; Xu, J.; Chen, J.; Pan, H.; Yan, Q. Rechargeable Aqueous Aluminum-Ion Battery: Progress and Outlook. Small 2022, 18, 2107773. [Google Scholar] [CrossRef]
- Raj, M.R.; Zaghib, K.; Lee, G. Advanced aqueous electrolytes for aluminum-ion batteries: Challenges and opportunities. Energy Storage Mater. 2025, 78, 104211. [Google Scholar] [CrossRef]
- Melethil, K.; Kumar, M.S.; Wu, C.-M.; Shen, H.-H.; Vedhanarayanan, B.; Lin, T.-W. Recent Progress of 2D Layered Materials in Water-in-Salt/Deep Eutectic Solvent-Based Liquid Electrolytes for Supercapacitors. Nanomaterials 2023, 13, 1257. [Google Scholar] [CrossRef]
- Ma, D.; Yuan, D.; Ponce de León, C.; Jiang, Z.; Xia, X.; Pan, J. Current Progress and Future Perspectives of Electrolytes for Rechargeable Aluminum-Ion Batteries. Energy Environ. Mater. 2023, 6, e12301. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.; Hu, Y.; Yan, W.; Tie, Z.; Jin, Z. Initial-anode-free aluminum ion batteries: In-depth monitoring and mechanism studies. Energy Storage Mater. 2022, 44, 461–468. [Google Scholar] [CrossRef]
- Almodóvar, P.; Rey-Raap, N.; Flores-López, S.L.; Sotillo, B.; Santos, L.C.D.; Tinoco, M.; Ramírez-Castellanos, J.; Álvarez-Serrano, I.; López, M.a.L.; Cameán, I.; et al. Enhancing Aluminium-Ion Battery Performance With Carbon Xerogel Cathodes. Batter. Supercaps 2024, 7, e202400114. [Google Scholar] [CrossRef]
- Mukundan, C.; Eckert, M.; Drillet, J.-F. Impact of Aluminium Electrode Potential during Charging on Aluminium-Ion Battery Performance with TEA-AlCl3 Electrolyte. Batter. Supercaps 2023, 6, e202300042. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, W.; Jin, H.; Wan, J. Research Progress of Cathode Materials for Rechargeable Aluminum Batteries in AlCl3/[EMIm]Cl and Other Electrolyte Systems. ChemistrySelect 2023, 8, e202204575. [Google Scholar] [CrossRef]
- Mukundan, C.; Drillet, J.-F. Mitigating the Effect of High Overpotential during Al Deposition on Aluminium-Graphite Battery Performance. Batter. Supercaps 2025, 8, e202400718. [Google Scholar] [CrossRef]
- Yang, X.; Huang, C. Progress in improving the performance of inorganic cathodes for aluminium-ion batteries. J. Energy Storage 2024, 78, 110069. [Google Scholar] [CrossRef]
- Yang, C.; Liang, Z.; Dong, B.; Guo, Y.; Xie, W.; Chen, M.; Zhang, K.; Zhou, L. Heterostructure Engineering for Aluminum-Ion Batteries: Mechanism, Challenge, and Perspective. Small 2024, 20, 2405495. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Pan, W.; Wang, M.; Zhang, H.; Zhang, D.; Zhang, D. Application of expanded graphite-based materials for rechargeable batteries beyond lithium-ions. Nanoscale 2021, 13, 19291–19305. [Google Scholar] [CrossRef]
- Kong, D.; Cai, T.; Fan, H.; Hu, H.; Wang, X.; Cui, Y.; Wang, D.; Wang, Y.; Hu, H.; Wu, M.; et al. Polycyclic Aromatic Hydrocarbons as a New Class of Promising Cathode Materials for Aluminum-Ion Batteries. Angew. Chem. Int. Edn. 2022, 61, e202114681. [Google Scholar] [CrossRef]
- Thanwisai, P.; Chaiyapo, N.; Phuenhinlad, P.; Kanaphan, Y.; Nash, J.; Chotsuwan, C.; Klamchuen, A.; Wang, Y.; Nann, T.; Meethong, N. Mesoporous and defective activated carbon cathode for AlCl4− anion storage in non-aqueous aluminium-ion batteries. Carbon 2022, 191, 195–204. [Google Scholar] [CrossRef]
- Xu, H.; Chen, H.; Gao, C. Advanced Graphene Materials for Sodium/Potassium/Aluminum-Ion Batteries. ACS Mater. Lett. 2021, 3, 1221–1237. [Google Scholar] [CrossRef]
- Abhijitha, V.G.; Mishra, S.B.; Ramaprabhu, S.; Nanda, B.R.K. Design of an aluminium ion battery with a graphyne host: Lowest volume expansion, high stability and low diffusion barriers. Nanoscale Adv. 2022, 4, 3870–3882. [Google Scholar] [CrossRef] [PubMed]
- Abhijitha, V.G.; Mishra, S.B.; Nanda, B.R.K. Si2BN nanosheets as anchoring cathode material for realizing high capacity Al-ion battery. J. Energy Storage 2024, 77, 109913. [Google Scholar] [CrossRef]
- Cui, F.; Li, J.; Lai, C.; Li, C.; Sun, C.; Du, K.; Wang, J.; Li, H.; Huang, A.; Peng, S.; et al. Superlattice cathodes endow cation and anion co-intercalation for high-energy-density aluminium batteries. Nat. Commun. 2024, 15, 8108. [Google Scholar] [CrossRef]
- Almodóvar, P.; Giraldo, D.; Díaz-Guerra, C.; Ramírez-Castellanos, J.; González Calbet, J.M.; Chacón, J.; López, M.L. h-MoO3/AlCl3-Urea/Al: High performance and low-cost rechargeable Al-ion battery. J. Power Sources 2021, 516, 230656. [Google Scholar] [CrossRef]
- Luo, Z.; Peng, X.; Wang, L.; Luo, B. Insights into Mechanistic Aspect of Organic Materials for Aluminum-Ion Batteries. ChemSusChem 2025, 18, e202401397. [Google Scholar] [CrossRef]
- Huang, Z.; Du, X.; Ma, M.; Wang, S.; Xie, Y.; Meng, Y.; You, W.; Xiong, L. Organic Cathode Materials for Rechargeable Aluminum-Ion Batteries. ChemSusChem 2023, 16, e202202358. [Google Scholar] [CrossRef]
- Yoo, D.-J.; Heeney, M.; Glöcklhofer, F.; Choi, J.W. Tetradiketone macrocycle for divalent aluminium ion batteries. Nat. Commun. 2021, 12, 2386. [Google Scholar] [CrossRef]
- Canever, N.; Nann, T. Unraveling the multivalent aluminium-ion redox mechanism in 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA). Phys. Chem. Chem. Phys. 2022, 24, 5886–5893. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Tang, C.; Lei, C.; Nanjundan, A.K.; Chen, S.; Ahmed, N.; Rakov, D.; Du, A.; Huang, X.; Yu, C. Enabling two-electron redox chemistry of P-type organic cathode for high-capacity aluminium-ion batteries. Nano Energy 2022, 102, 107727. [Google Scholar] [CrossRef]
- Wei, G.; Qiao, J.; Li, X.; Dou, A.; Hu, S.; Xie, W.; Luo, Z.; Yang, J. High performance rechargeable aluminium ion batteries enabled by strategy of covalent organic frame material. Chem. Eng. J. 2025, 511, 161845. [Google Scholar] [CrossRef]
- Xu, X.; Wei, G.; Xu, W.; Li, X.; Zhang, X.; Hu, S.; Yang, J.; Qiao, J. Asymmetric 1, 2-naphthoquinone cathode material with enhanced capacity for rechargeable aluminium ion batteries. J. Energy Storage 2024, 81, 110454. [Google Scholar] [CrossRef]
- Wang, X.; Xi, Z.; Zhao, Q. Progress on aqueous rechargeable aluminium metal batteries. Ind. Chem. Mater. 2025, 3, 7–30. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; He, S.; He, D.; Shang, Y.; Yu, H. Molten salts for rechargeable batteries. Mater. Today 2022, 60, 128–157. [Google Scholar] [CrossRef]
- Meng, J.; Hong, X.; Xiao, Z.; Xu, L.; Zhu, L.; Jia, Y.; Liu, F.; Mai, L.; Pang, Q. Rapid-charging aluminium-sulfur batteries operated at 85 °C with a quaternary molten salt electrolyte. Nat. Commun. 2024, 15, 596. [Google Scholar] [CrossRef]
- Meng, J.; Yao, X.; Hong, X.; Zhu, L.; Xiao, Z.; Jia, Y.; Liu, F.; Song, H.; Zhao, Y.; Pang, Q. A solution-to-solid conversion chemistry enables ultrafast-charging and long-lived molten salt aluminium batteries. Nat. Commun. 2023, 14, 3909. [Google Scholar] [CrossRef]
- Tong, X.; Song, Y.; Zhang, M.; Chen, Y.; Liu, Y.; Chen, J.; Wang, W.; Zhou, C.; Liu, F.; Meng, J. Carbon-Supported In2O3 Cathode with a Solution-to-Solid Conversion Chemistry Enables Fast-Charging and Durable Aluminum Battery. ACS Appl. Energy Mater. 2025, 8, 3553–3562. [Google Scholar] [CrossRef]
- Pang, Q.; Meng, J.; Gupta, S.; Hong, X.; Kwok, C.Y.; Zhao, J.; Jin, Y.; Xu, L.; Karahan, O.; Wang, Z.; et al. Fast-charging aluminium–chalcogen batteries resistant to dendritic shorting. Nature 2022, 608, 704–711. [Google Scholar] [CrossRef]
- Sun, H.; Chen, B.; Tan, M.; Li, Y.; Lang, X.; Jiang, Q. Rational design and construction of aqueous multivalent metal ion battery systems. J. Mater. Chem. A 2025, 13, 17159–17196. [Google Scholar] [CrossRef]
- Sonar, V.A.; Kulkarni, A.A.; Sonar, P.; Dubal, D.P. Covalent Organic Frameworks (COFs): A New Class of Materials for Multivalent Metal-Ion Energy Storage Systems. Batter. Supercaps 2024, 8, e202400537. [Google Scholar] [CrossRef]
- Lei, Z.; Zhan, J.; Tang, L.; Zhang, Y.; Wang, Y. Recent Development of Metallic (1T) Phase of Molybdenum Disulfide for Energy Conversion and Storage. Adv. Energy Mater. 2018, 8, 1703482. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Yang, F.; Liu, S.; Zhang, S.; Mao, J.; Guo, Z. Electrolyte Engineering Enables High Performance Zinc-Ion Batteries. Small 2022, 18, 2107033. [Google Scholar] [CrossRef]
- Park, S.K.; Dose, W.M.; Boruah, B.D.; De Volder, M. In Situ and Operando Analyses of Reaction Mechanisms in Vanadium Oxides for Li-, Na-, Zn-, and Mg-Ions Batteries. Adv. Mater. Tech. 2022, 7, 2100799. [Google Scholar] [CrossRef]
- Guo, M.; Yuan, C.; Zhang, T.; Yu, X. Solid-State Electrolytes for Rechargeable Magnesium-Ion Batteries: From Structure to Mechanism. Small 2022, 18, 2106981. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and future lithium-ion battery manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef]
- Ju, Z.; Zheng, T.; Zhang, B.; Yu, G. Interfacial chemistry in multivalent aqueous batteries: Fundamentals, challenges, and advances. Chem. Soc. Rev. 2024, 53, 8980–9028. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.; Zhang, L.; Liu, Q.; Wang, Y.; Lin, Y.; Xu, C.; Guo, J.; Cheali, P.; Xia, X. Progress, challenges, and prospects of spent lithium-ion batteries recycling: A review. J. Energy Chem. 2024, 89, 144–171. [Google Scholar] [CrossRef]
- Gomez-Flores, A.; Park, H.; Hong, G.; Nam, H.; Gomez-Flores, J.; Kang, S.; Heyes, G.W.; Leal Filho, L.d.S.; Kim, H.; Lee, J.M.; et al. Flotation separation of lithium–ion battery electrodes predicted by a long short-term memory network using data from physicochemical kinetic simulations and experiments. J. Ind. Inf. Integr. 2024, 42, 100697. [Google Scholar] [CrossRef]
- Pastel, G.R.; Pollard, T.P.; Borodin, O.; Schroeder, M.A. From Ab Initio to Instrumentation: A Field Guide to Characterizing Multivalent Liquid Electrolytes. Chem. Rev. 2025, 125, 3059–3164. [Google Scholar] [CrossRef]
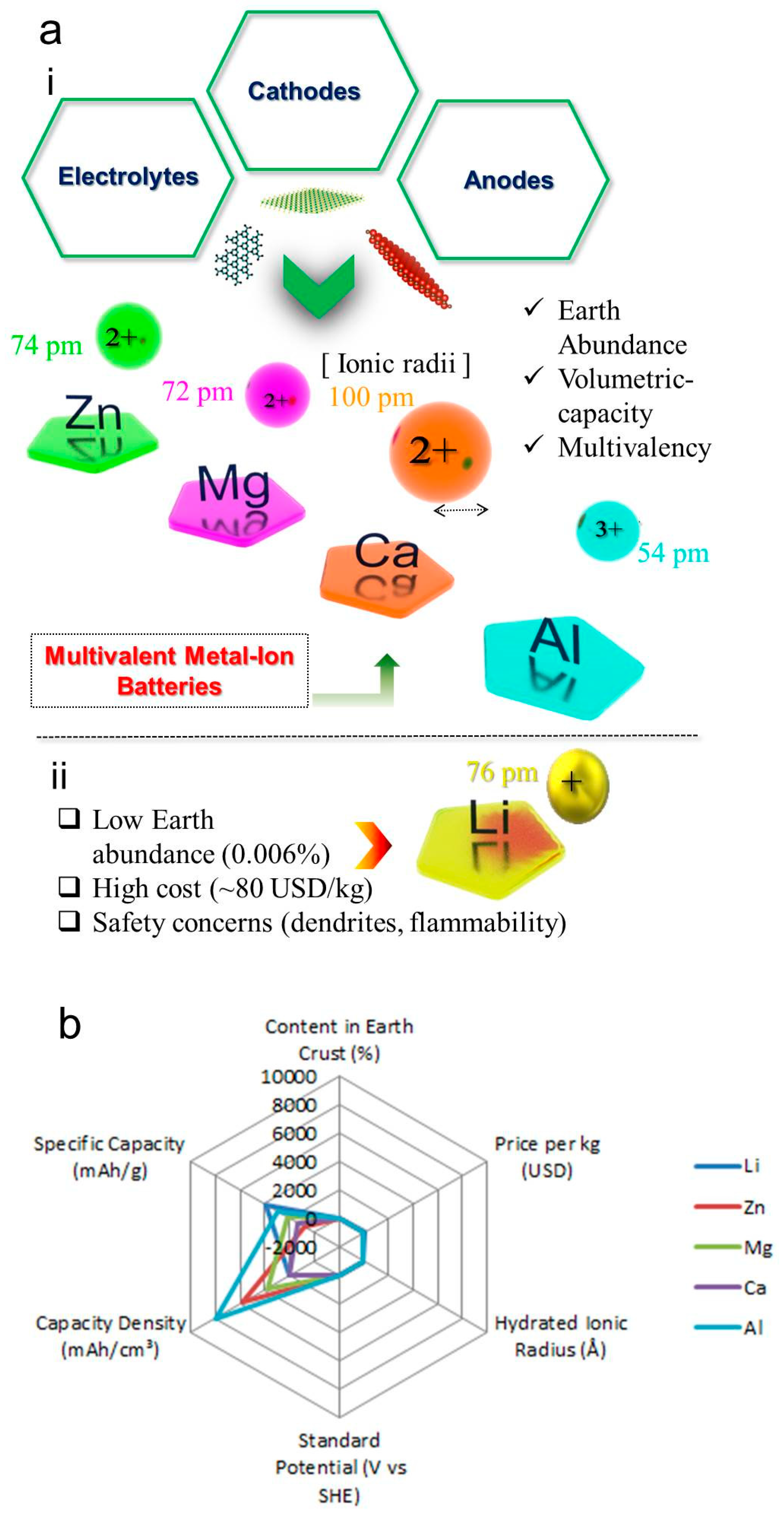
| Property | Li | Mg | Ca | Al | Zn |
|---|---|---|---|---|---|
| Gravimetric Capacity (mAh/g) | >3500 | ~2000 | ~1200 | ~2900 | ~820 |
| Volumetric Capacity (mAh/cm3) | ~2000 | ~3800 | ~2000 | ~8000 | ~5800 |
| Cycle Life | 500–2000 cycles | 100–500 cycles | 100–500 cycles | 100–500 cycles | 300–1000 cycles |
| Operating Temperature | −20 °C to 60 °C | −10 °C to 50 °C | −10 °C to 50 °C | −20 °C to 60 °C | −0 °C to 40 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vedhanarayanan, B.; Nagaraj, J.; Arjunan, K.; Lakshmi, K.C.S. Multivalent Metal-Ion Batteries: Unlocking the Future of Post-Lithium Energy Storage. Nanoenergy Adv. 2025, 5, 13. https://doi.org/10.3390/nanoenergyadv5040013
Vedhanarayanan B, Nagaraj J, Arjunan K, Lakshmi KCS. Multivalent Metal-Ion Batteries: Unlocking the Future of Post-Lithium Energy Storage. Nanoenergy Advances. 2025; 5(4):13. https://doi.org/10.3390/nanoenergyadv5040013
Chicago/Turabian StyleVedhanarayanan, Balaraman, Jagadesh Nagaraj, Kishorekumar Arjunan, and K. C. Seetha Lakshmi. 2025. "Multivalent Metal-Ion Batteries: Unlocking the Future of Post-Lithium Energy Storage" Nanoenergy Advances 5, no. 4: 13. https://doi.org/10.3390/nanoenergyadv5040013
APA StyleVedhanarayanan, B., Nagaraj, J., Arjunan, K., & Lakshmi, K. C. S. (2025). Multivalent Metal-Ion Batteries: Unlocking the Future of Post-Lithium Energy Storage. Nanoenergy Advances, 5(4), 13. https://doi.org/10.3390/nanoenergyadv5040013









