First-Principle Insights into Positive Triboelectrification of Polyoxymethylene Through Homolytic Bond Rupture
Abstract
1. Introduction
2. Methods
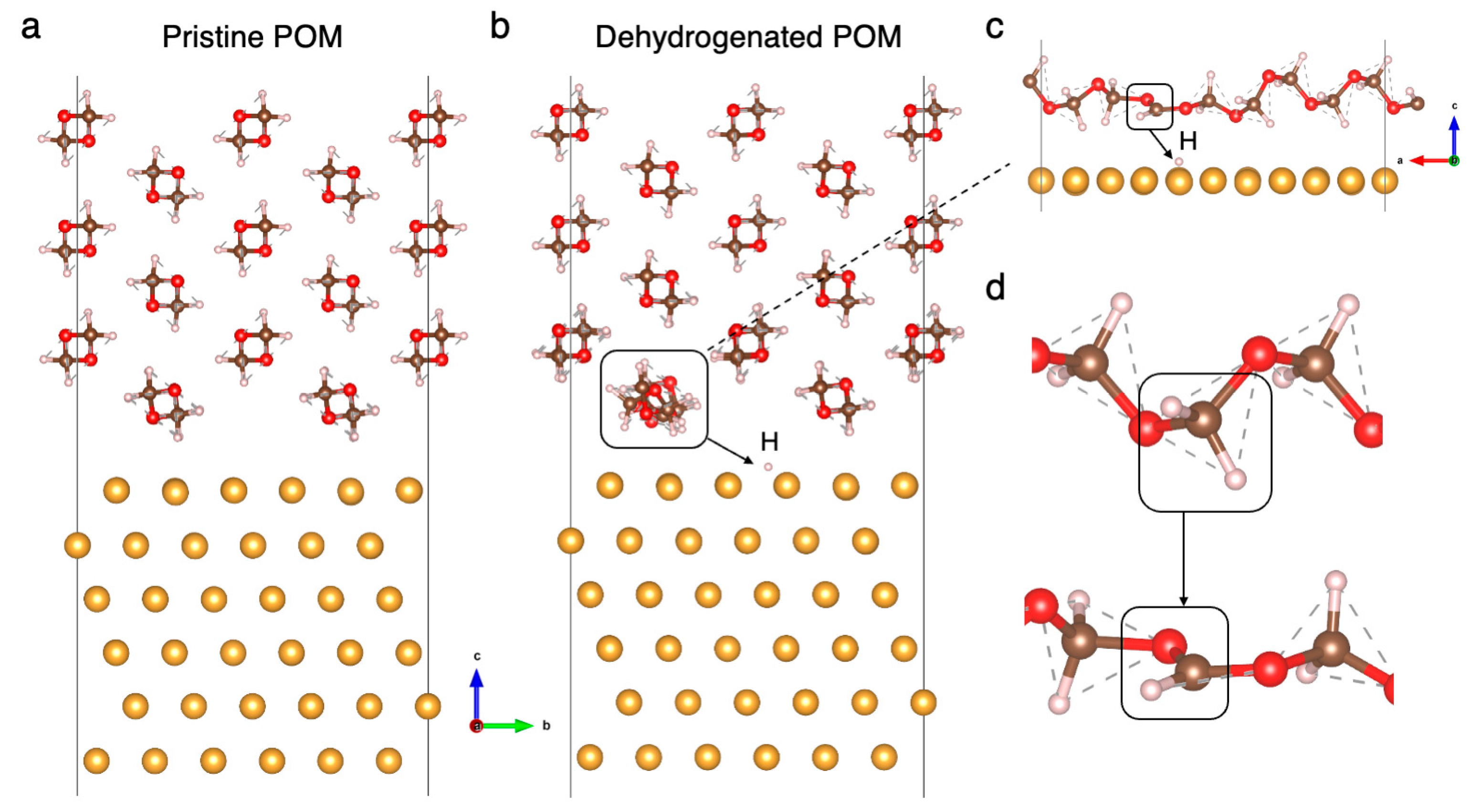
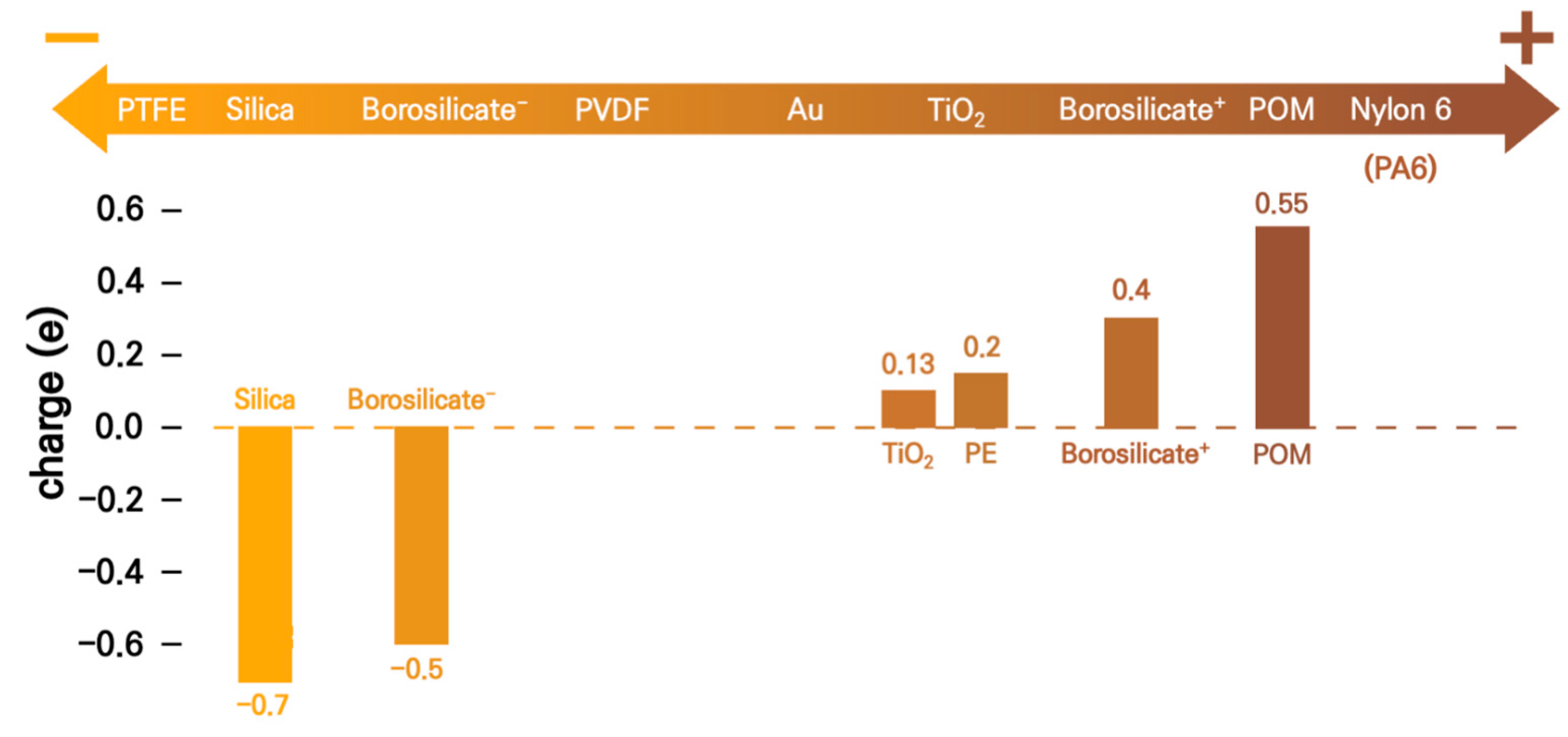
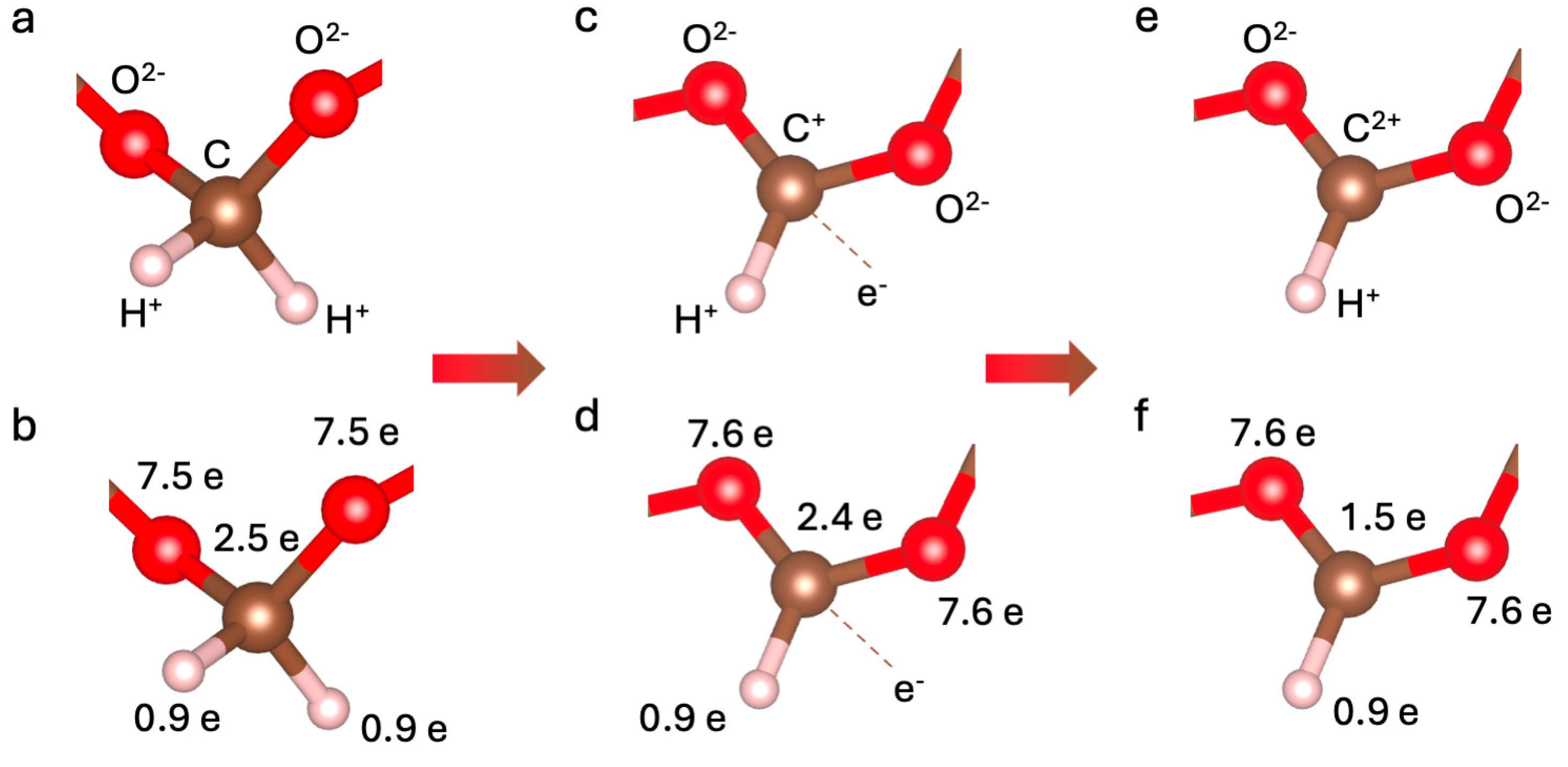
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; He, L.; Zhang, R.; Yuan, W.; Wang, J.; Hu, Y.; Zhao, Z.; Zhou, L.; Wang, J.; Wang, Z.L. Achieving Material and Energy Dual Circulations of Spent Lithium-Ion Batteries via Triboelectric Nanogenerator. Adv. Energy Mater. 2023, 13, 2301353. [Google Scholar] [CrossRef]
- Dong, K.; Wang, Z.L. Self-charging power textiles integrating energy harvesting triboelectric nanogenerators with energy storage batteries/supercapacitors. J. Semicond. 2021, 42, 101601. [Google Scholar] [CrossRef]
- Kwak, S.S.; Yoon, H.-J.; Kim, S.-W. Textile-Based Triboelectric Nanogenerators for Self-Powered Wearable Electronics. Adv. Funct. Mater. 2019, 29, 1804533. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, Y.; He, P.; Wang, G.; Xia, X.; Ding, G.; Tao, T.H. “Self-Matched” Tribo/Piezoelectric Nanogenerators Using Vapor-Induced Phase-Separated Poly(vinylidene fluoride) and Recombinant Spider Silk. Adv. Mater. 2020, 32, 1907336. [Google Scholar] [CrossRef]
- Wang, Z.L. Triboelectric Nanogenerators as New Energy Technology for Self-Powered Systems and as Active Mechanical and Chemical Sensors. ACS Nano 2013, 7, 9533–9557. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Zhang, B.; Yang, O.; Yuan, W.; He, L.; Wei, X.; Wang, J.; Wang, Z.L. Harvesting Wind Energy by a Triboelectric Nanogenerator for an Intelligent High-Speed Train System. ACS Energy Lett. 2021, 6, 1490–1499. [Google Scholar] [CrossRef]
- Cao, X.; Xiong, Y.; Sun, J.; Xie, X.; Sun, Q.; Wang, Z.L. Multidiscipline Applications of Triboelectric Nanogenerators for the Intelligent Era of Internet of Things. Nano-Micro Lett. 2022, 15, 14. [Google Scholar] [CrossRef]
- Yang, P.; Shi, Y.; Tao, X.; Liu, Z.; Dong, X.; Wang, Z.L.; Chen, X. Radical anion transfer during contact electrification and its compensation for charge loss in triboelectric nanogenerator. Matter 2023, 6, 1295–1311. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, Q.; Ouyang, H.; Li, H.; Yan, L.; Shi, B.; Li, Z. A size-unlimited surface microstructure modification method for achieving high performance triboelectric nanogenerator. Nano Energy 2016, 28, 172–178. [Google Scholar] [CrossRef]
- Gooding, D.M.; Kaufman, G.K. Tribocharging and the Triboelectric Series. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 1–14. ISBN 978-1-119-95143-8. [Google Scholar]
- Chen, Q.; Shang, H.; Cheng, B.; Lu, C.; Wang, Y.; Zhang, Y.; Shao, T. Quantifying triboelectric series of polymers based on the measurement of triboelectrification with NaCl solution. Chem. Eng. J. 2024, 488, 150871. [Google Scholar] [CrossRef]
- Diaz, A.F.; Felix-Navarro, R.M. A semi-quantitative tribo-electric series for polymeric materials: The influence of chemical structure and properties. J. Electrost. 2004, 62, 277–290. [Google Scholar] [CrossRef]
- Lacks, D.J.; Shinbrot, T. Long-standing and unresolved issues in triboelectric charging. Nat. Rev. Chem. 2019, 3, 465–476. [Google Scholar] [CrossRef]
- Li, J.; Shepelin, N.A.; Sherrell, P.C.; Ellis, A.V. Poly(dimethylsiloxane) for Triboelectricity: From Mechanisms to Practical Strategies. Chem. Mater. 2021, 33, 4304–4327. [Google Scholar] [CrossRef]
- Lapčinskis, L.; Linarts, A.; Mālnieks, K.; Kim, H.; Rubenis, K.; Pudzs, K.; Smits, K.; Kovaļovs, A.; Kalniņš, K.; Tamm, A.; et al. Triboelectrification of nanocomposites using identical polymer matrixes with different concentrations of nanoparticle fillers. J. Mater. Chem. A 2021, 9, 8984–8990. [Google Scholar] [CrossRef]
- Šutka, A.; Mālnieks, K.; Lapčinskis, L.; Kaufelde, P.; Linarts, A.; Bērziņa, A.; Zābels, R.; Jurķāns, V.; Gorņevs, I.; Blūms, J.; et al. The role of intermolecular forces in contact electrification on polymer surfaces and triboelectric nanogenerators. Energy Environ. Sci. 2019, 12, 2417–2421. [Google Scholar] [CrossRef]
- Šutka, A.; Linarts, A.; Mālnieks, K.; Stiprais, K.; Lapčinskis, L. Dramatic increase in polymer triboelectrification by transition from a glassy to rubbery state. Mater. Horiz. 2020, 7, 520–523. [Google Scholar] [CrossRef]
- Lapčinskis, L.; Mālnieks, K.; Blūms, J.; Knite, M.; Oras, S.; Käämbre, T.; Vlassov, S.; Antsov, M.; Timusk, M.; Šutka, A. The Adhesion-Enhanced Contact Electrification and Efficiency of Triboelectric Nanogenerators. Macromol. Mater. Eng. 2020, 305, 1900638. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Zi, Y.; Zhang, G.; Wang, Z.L. Excluding Contact Electrification in Surface Potential Measurement Using Kelvin Probe Force Microscopy. ACS Nano 2016, 10, 2528–2535. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, B.; Wang, A.C.; Cai, W.; Zi, Y.; Feng, P.; Wang, Z.L. Effects of Metal Work Function and Contact Potential Difference on Electron Thermionic Emission in Contact Electrification. Adv. Funct. Mater. 2019, 29, 1903142. [Google Scholar] [CrossRef]
- Lin, S.; Xu, L.; Zhu, L.; Chen, X.; Wang, Z.L. Electron Transfer in Nanoscale Contact Electrification: Photon Excitation Effect. Adv. Mater. 2019, 31, 1901418. [Google Scholar] [CrossRef]
- Lin, S.; Xu, L.; Xu, C.; Chen, X.; Wang, A.C.; Zhang, B.; Lin, P.; Yang, Y.; Zhao, H.; Wang, Z.L. Electron Transfer in Nanoscale Contact Electrification: Effect of Temperature in the Metal–Dielectric Case. Adv. Mater. 2019, 31, 1808197. [Google Scholar] [CrossRef] [PubMed]
- Fatti, G.; Ciniero, A.; Ko, H.; Lee, H.U.; Na, Y.; Jeong, C.K.; Lee, S.-G.; Kwak, D.; Park, K.-I.; Cho, S.B.; et al. Rational Design Strategy for Triboelectric Nanogenerators Based on Electron Back Flow and Ionic Defects: The Case of Polytetrafluoroethylene. Adv. Electron. Mater. 2023, 9, 2300333. [Google Scholar] [CrossRef]
- Fu, H.; Gong, J.; Cao, J.; Zhang, Z.; Long, Z.; Yang, B.; Chen, J.; Chen, Y.; Tao, X. Understanding contact electrification via direct covalent bond cleavage of polymer chains for ultrahigh electrostatic charge density. Energy Environ. Sci. 2024, 17, 3776–3787. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Carazzolo, G.; Mammi, M. Crystal structure of a new form of polyoxymethylene. J. Polym. Sci. A 1963, 1, 965–983. [Google Scholar] [CrossRef]
- Fatti, G.; Righi, M.C.; Dini, D.; Ciniero, A. Ab Initio Study of Polytetrafluoroethylene Defluorination for Tribocharging Applications. ACS Appl. Polym. Mater. 2020, 2, 5129–5134. [Google Scholar] [CrossRef]
- Ong, S.P.; Richards, W.D.; Jain, A.; Hautier, G.; Kocher, M.; Cholia, S.; Gunter, D.; Chevrier, V.L.; Persson, K.A.; Ceder, G. Python Materials Genomics (pymatgen): A robust, open-source python library for materials analysis. Comput. Mater. Sci. 2013, 68, 314–319. [Google Scholar] [CrossRef]
- Fatti, G.; Kim, H.; Sohn, C.; Park, M.; Lim, Y.; Li, Z.; Park, K.-I.; Szlufarska, I.; Ko, H.; Jeong, C.K.; et al. Uncertainty and Irreproducibility of Triboelectricity Based on Interface Mechanochemistry. Phys. Rev. Lett. 2023, 131, 166201. [Google Scholar] [CrossRef] [PubMed]
- Archodoulaki, V.-M.; Lüftl, S.; Koch, T.; Seidler, S. Property changes in polyoxymethylene (POM) resulting from processing, ageing and recycling. Polym. Degrad. Stab. 2007, 92, 2181–2189. [Google Scholar] [CrossRef]
- Fatti, G.; Restuccia, P.; Calandra, C.; Righi, M.C. Phosphorus Adsorption on Fe(110): An ab Initio Comparative Study of Iron Passivation by Different Adsorbates. J. Phys. Chem. C 2018, 122, 28105–28112. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. Improved grid-based algorithm for Bader charge allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 84204. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatti, G.; Ko, H.; Cho, S.B. First-Principle Insights into Positive Triboelectrification of Polyoxymethylene Through Homolytic Bond Rupture. Nanoenergy Adv. 2025, 5, 1. https://doi.org/10.3390/nanoenergyadv5010001
Fatti G, Ko H, Cho SB. First-Principle Insights into Positive Triboelectrification of Polyoxymethylene Through Homolytic Bond Rupture. Nanoenergy Advances. 2025; 5(1):1. https://doi.org/10.3390/nanoenergyadv5010001
Chicago/Turabian StyleFatti, Giulio, Hyunseok Ko, and Sung Beom Cho. 2025. "First-Principle Insights into Positive Triboelectrification of Polyoxymethylene Through Homolytic Bond Rupture" Nanoenergy Advances 5, no. 1: 1. https://doi.org/10.3390/nanoenergyadv5010001
APA StyleFatti, G., Ko, H., & Cho, S. B. (2025). First-Principle Insights into Positive Triboelectrification of Polyoxymethylene Through Homolytic Bond Rupture. Nanoenergy Advances, 5(1), 1. https://doi.org/10.3390/nanoenergyadv5010001





