Measuring Physical and Chemical Properties of Single Nanofibers for Energy Applications—Possibilities and Limits
Abstract
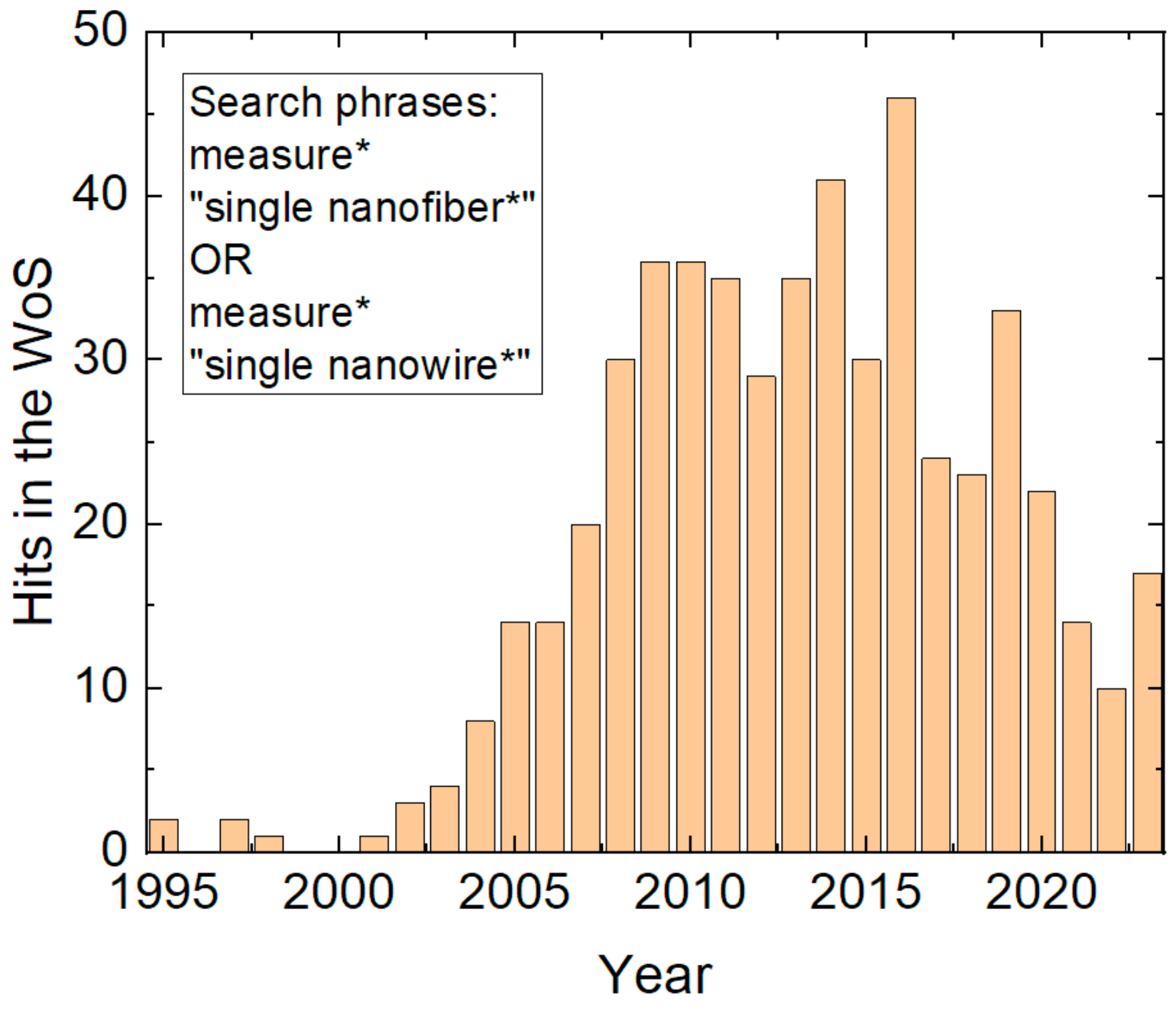
1. Introduction
2. Nanofibers in Energy Applications
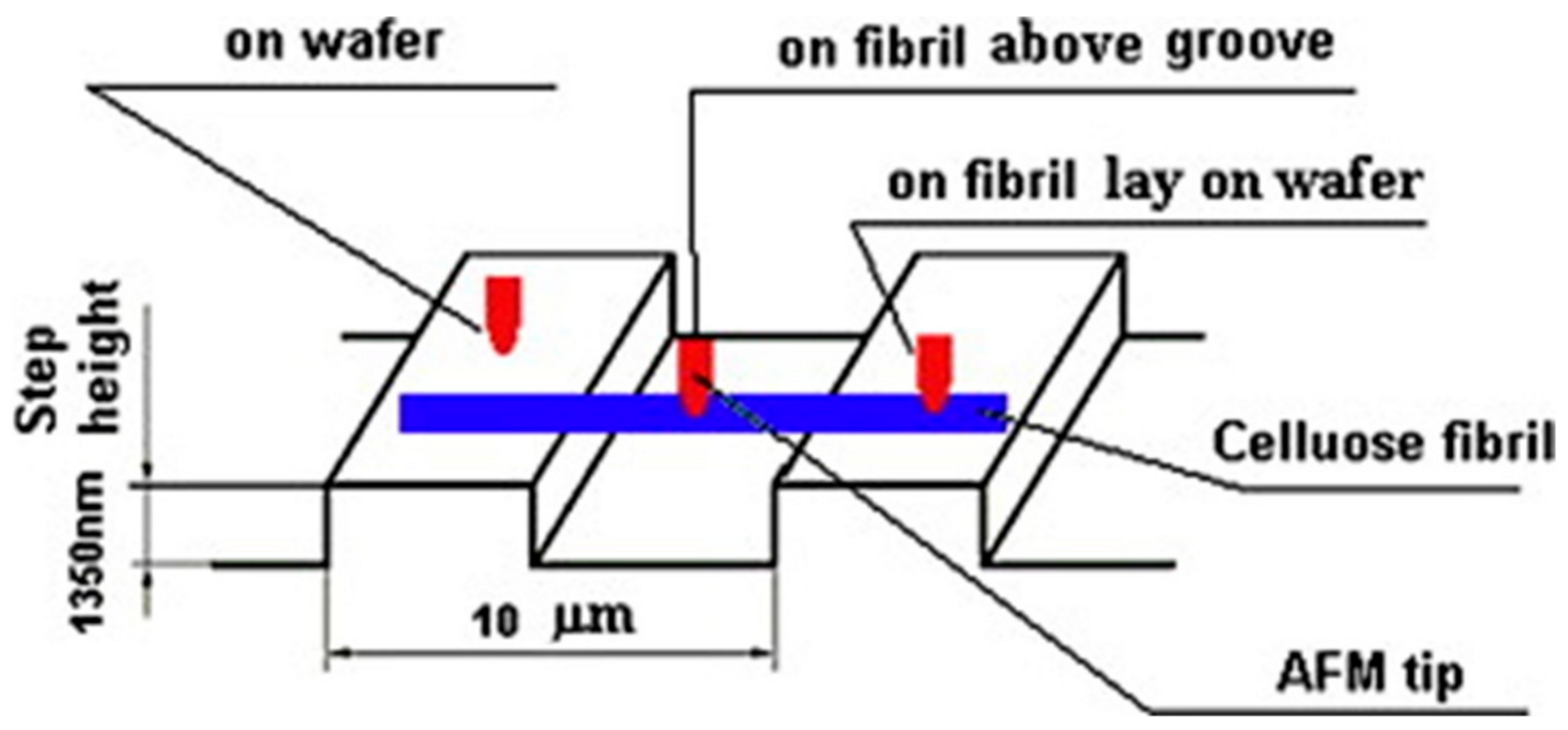
3. Measuring the Morphology of Single Nanofibers
4. Other AFM Techniques to Investigate Single Nanofibers
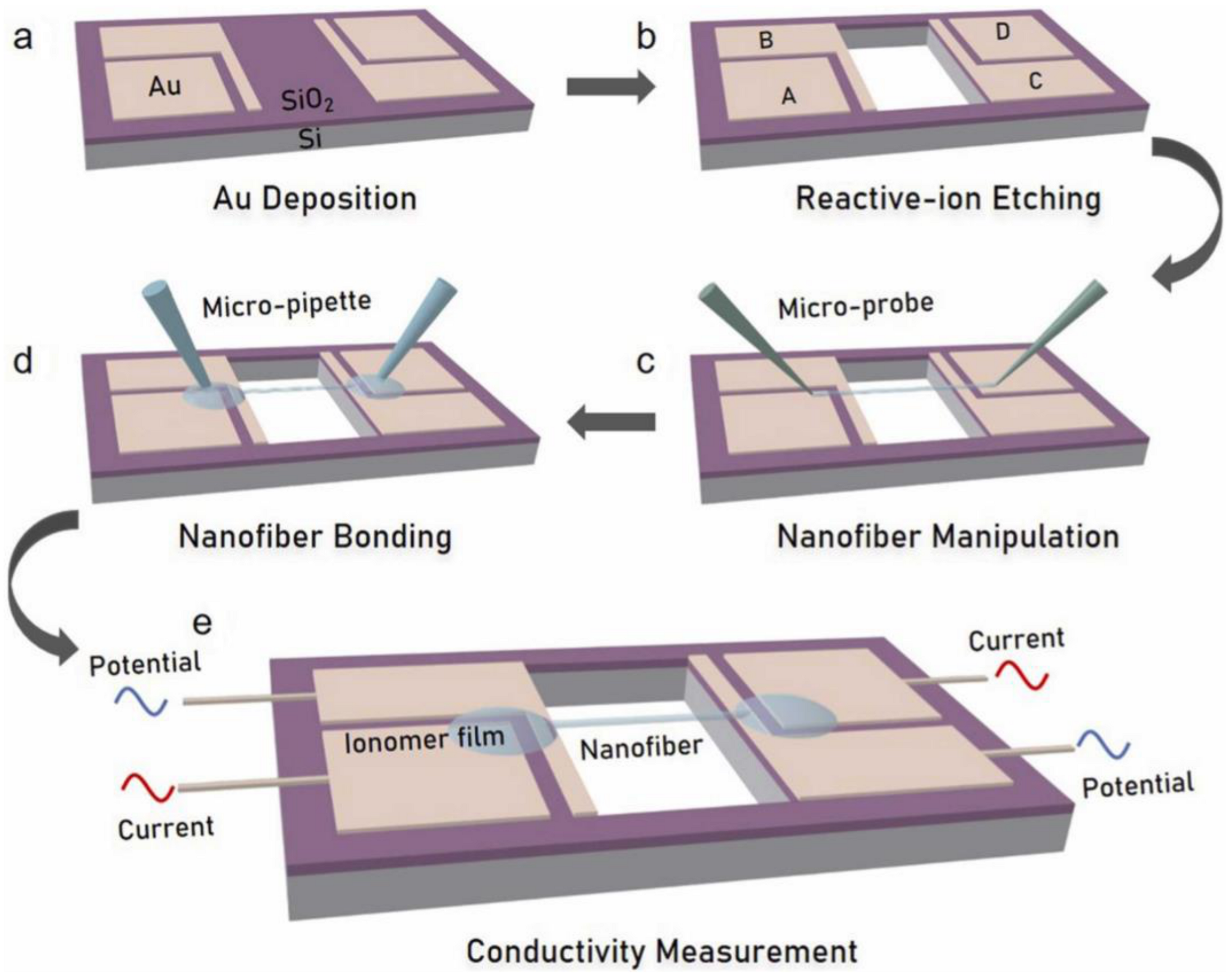
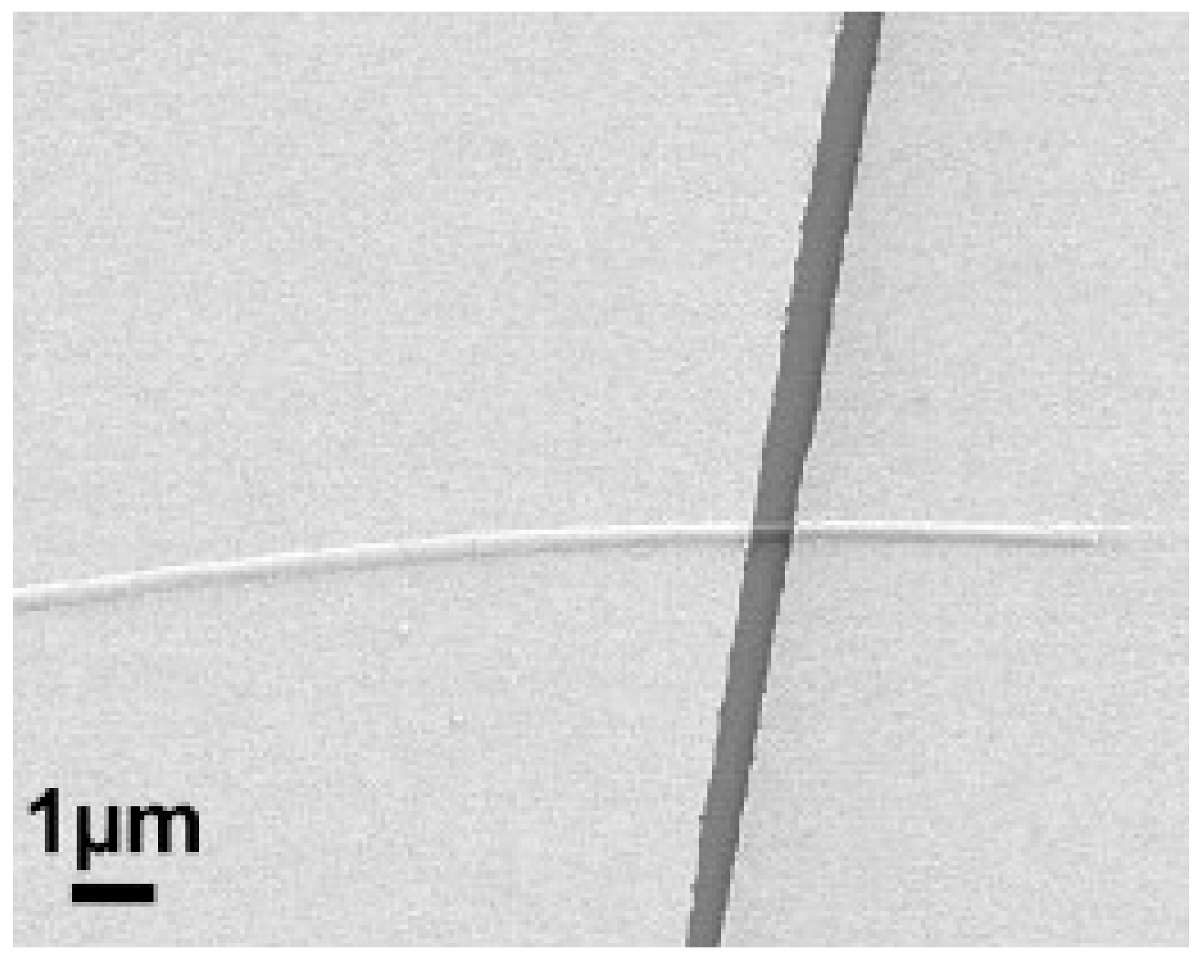
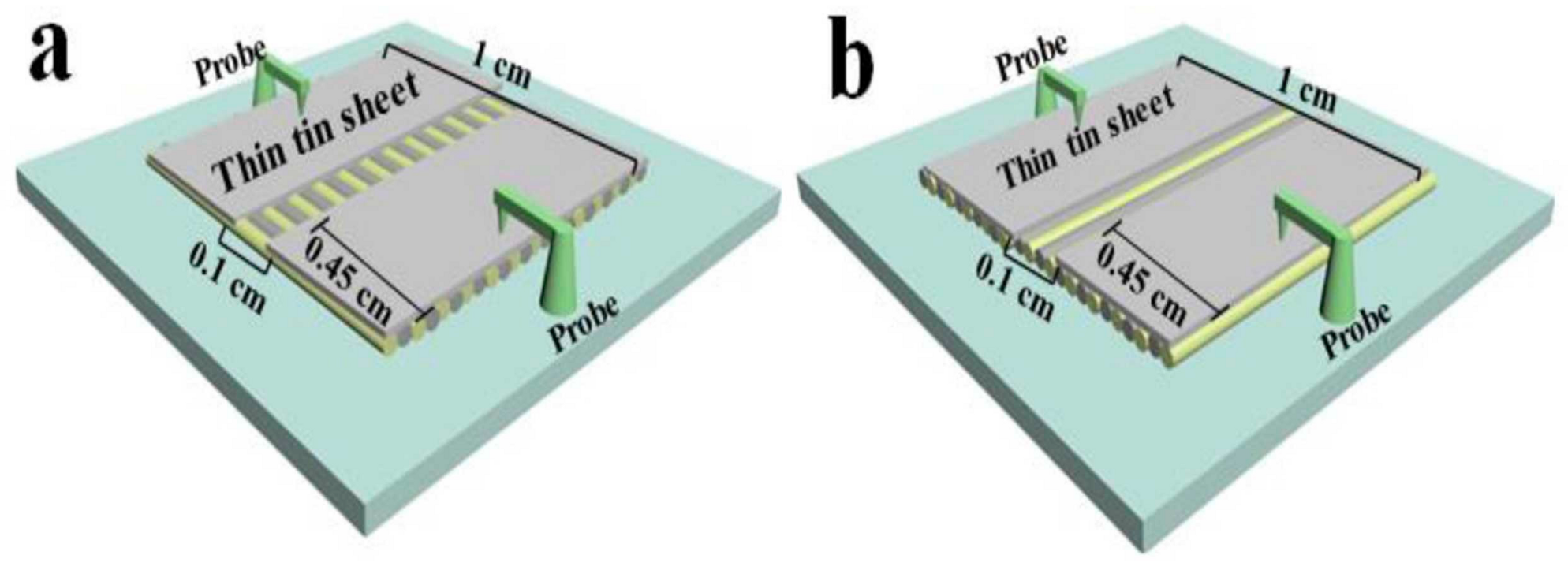
5. Conductivity Measurements of Single Nanofibers
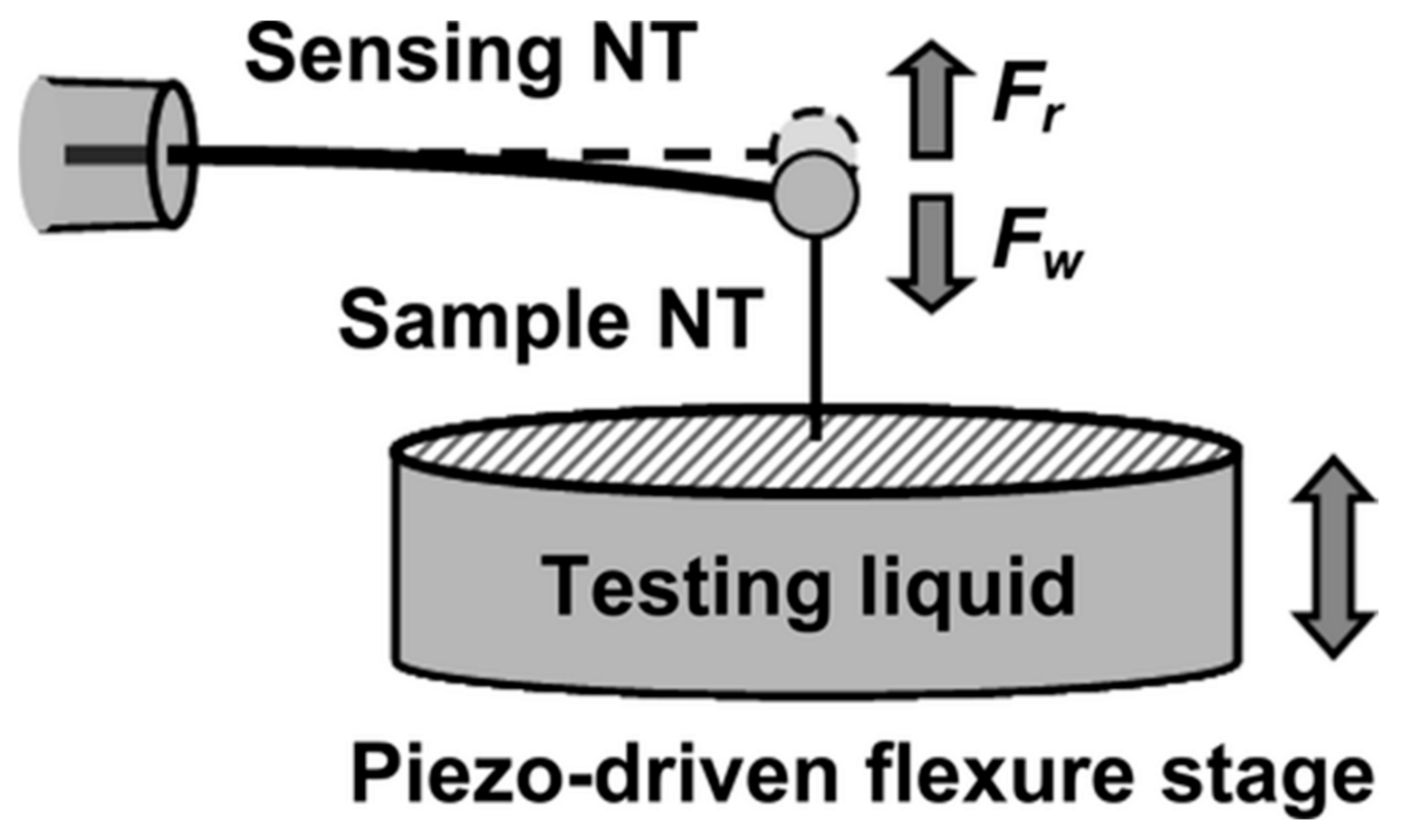
6. Measuring the Wettability of Single Nanofibers
7. Measuring the Mechanical Properties of Single Nanofibers
8. Other Microscopic Techniques to Investigate Single Nanofibers
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.Q.; Wu, S.S.; Wang, J.; Yu, A.; Wei, G. Carbon nanofiber-based functional nanomaterials for sensor applications. Nanomaterials 2019, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.-T.; Choi, S.-J.; Kim, N.-H.; Jang, J.-S.; Kim, I.-D. Catalyst-decorated hollow WO3 nanotubes using layer-by-layer self-assembly on polymeric nanofiber templates and their application in exhaled breath sensor. Sens. Act. B Chem. 2016, 223, 301–310. [Google Scholar] [CrossRef]
- Wang, Q.N.; Yildiz, O.; Li, A.; Aly, K.; Qiu, Y.P.; Jiang, Q.R.; Pui, D.Y.H.; Chen, S.-C.; Bradford, P.D. High temperature carbon nanotube—Nanofiber hybrid filters. Sep. Purif. Technol. 2020, 236, 116255. [Google Scholar] [CrossRef]
- Yalcinkaya, F. A review on advanced nanofiber technology for membrane distillation. J. Eng. Fibers Fabr. 2019, 14, 1558925018824901. [Google Scholar] [CrossRef]
- Grothe, T.; Böhm, T.; Habashy, K.; Abdullaeva, O.S.; Zablocki, J.; Lützen, A.; Dedek, K.; Schiek, M.; Ehrmann, A. Optical index matching, flexible electrospun substrates for seamless organic photocapacitive sensors. Phys. Stat. Sol. B 2021, 258, 2170021. [Google Scholar] [CrossRef]
- Sakamoto, H.; Fujiwara, I.; Takamura, E.; Suye, S.-i. Nanofiber-guided orientation of electrospun carbon nanotubes and fabrication of aligned CNT electrodes for biodevice applications. Mater. Chem. Phys. 2020, 245, 122745. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Le, T.-H.; Kim, S.; Park, G.S.; Yang, K.S.; Yoon, H.S. Single-walled carbon nanotube-in-binary-polymer nanofiber structures and their use as carbon precursors for electrochemical applications. J. Phys. Chem. C 2018, 122, 4189–4198. [Google Scholar] [CrossRef]
- Banitaba, S.N.; Ehrmann, A. Application of electrospun nanofibers for fabrication of versatile and highly efficient electrochemical devices: A review. Polymers 2021, 13, 1741. [Google Scholar] [CrossRef]
- Prabu, G.T.V.; Dhurai, B. A novel profiled multi-pin electrospinning system for nanofiber production and encapsulation of nanoparticles into nanofibers. Sci. Rep. 2020, 10, 4302. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Toriello, M.; Afsari, M.; Shon, H.K.; Tijing, L.D. Progress on the fabrication and application of electrospun nanofiber composites. Membranes 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Moulefera, I.; Trabelsi, M.; Mamun, A.; Sabantina, L. Electrospun carbon nanofibers from biomass and biomass blends—Current trends. Polymers 2021, 13, 1071. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent advances in carbon nanofibers and their applications—A review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Mamun, A.; Kiari, M.; Sabantina, L. A recent review of electrospun porous carbon nanofiber mats for energy storage and generation applications. Membranes 2023, 13, 830. [Google Scholar] [CrossRef]
- Surovcík, J.; Medvecká, V.; Gregus, J.; Gregor, M.; Roch, T.; Annusová, A.; Durina, P.; Vojteková, T. Characterization of TiO2 nanofibers with enhanced photocatalytic properties prepared by plasma assisted calcination. Ceram. Int. 2022, 48, 37322–37332. [Google Scholar] [CrossRef]
- Khalili, S.; Chenari, H.M. Successful electrospinning fabrication of ZrO2 nanofibers: A detailed physical—Chemical characterization study. J. Alloys Comp. 2020, 828, 154414. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Recent developments in electrospun ZnO nanofibers: A short review. J. Eng. Fibers Fabr. 2020, 15, 1558925019899682. [Google Scholar] [CrossRef]
- Liu, X.Y.; Jiang, Y.; Song, X.P.; Qin, C.R.; Wang, S.F.; Li, K.C. A bio-mechanical process for cellulose nanofiber production—Towards a greener and energy conservation solution. Carbohydr. Polym. 2019, 208, 191–199. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Espinosa, E.; Bascón-Villegas, I.; Pérez-Rodríguez, F.; Carrasco, E.; Rodríguez, A. Production of cellulose nanofibers from olive tree harvest—A residue with wide applications. Agronomy 2020, 10, 696. [Google Scholar] [CrossRef]
- Fahmi Supian, M.A.; Mohd Amin, K.N.; Jamari, S.S.; Mohamad, S. Production of cellulose nanofiber (CNF) from empty fruit bunch (EFB) via mechanical method. J. Environ. Chem. Eng. 2020, 8, 103024. [Google Scholar]
- Zhou, X.X.; Liu, B.; Chen, Y.; Guo, L.; Wei, G. Carbon nanofiber-based three-dimensional nanomaterials for energy and environmental applications. Mater. Adv. 2020, 1, 2163–2181. [Google Scholar] [CrossRef]
- Joshi, J.S.; Langwald, S.V.; Ehrmann, A.; Sabantina, L. Algal-based biopolymers for batteries and biofuel application in comparison with bacterial biopolymers—A review. Polymers 2024, 16, 610. [Google Scholar] [CrossRef] [PubMed]
- Khademolqorani, S.; Banitaba, S.N.; Gupta, A.; Poursharifi, N.; Ghaffari, A.A.; Jadhav, V.V.; Ul Arifeen, W.; Sing, M.; Borah, M.; Chamanehpour, E.; et al. Application scopes of miniaturized MXene-functionalized electrospun nanofibers-based electrochemical energy devices. Small 2024, 20, 2309572. [Google Scholar] [CrossRef]
- Dahal, B.; Mukhiya, T.; Ojha, G.P.; Muthurasu, A.; Chae, S.H.; Kim, T.; Kang, D.W.; Kim, H.Y. In-built fabrication of MOF assimilated B/N co-doped 3D porous carbon nanofiber network as a binder-free electrode for supercapacitors. Electrochim. Acta 2019, 301, 209–219. [Google Scholar] [CrossRef]
- Lei, W.; Zhang, H.J.; Liu, D.Z.; Lin, L.X. Fabrication of nitrogen and sulfur co-doped carbon nanofibers with three-dimensional architecture for high performance supercapacitors. Appl. Surf. Sci. 2019, 495, 143572. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liu, Q.; Wang, L.; Yang, X.F.; Yang, W.Y.; Zheng, J.J.; Hou, H.L. Advanced supercapacitors based on porous hollow carbon nanofiber electrodes with high specific capacitance and large energy density. ACS Appl. Mater. Interfaces 2020, 12, 4777–4786. [Google Scholar] [CrossRef]
- Lin, C.H.; Tsai, C.H.; Tseng, F.G.; Chen, I.C.; Hsieh, C.K. Electrochemical pulse deposition of Ni nanoparticles on the 3D graphene network to synthesize vertical CNFs as the full-carbon hybrid nanoarchitecture for supercapacitors. Mater. Lett. 2017, 192, 40–43. [Google Scholar] [CrossRef]
- Nie, G.D.; Zhao, X.W.; Luan, Y.X.; Jiang, J.M.; Kou, Z.K.; Wang, J. Key issues facing electrospun carbon nanofibers in energy applications: On-going approaches and challenges. Nanoscale 2020, 12, 13225–13248. [Google Scholar] [CrossRef] [PubMed]
- Massaglia, G.; Margaria, V.; Sacco, A.; Castellino, M.; Chiodoni, A.; Pirri, F.C.; Quaglio, M. N-doped carbon nanofibers as catalyst layer at cathode in single chamber microbial fuel cells. Int. J. Hydrog. Energy 2019, 44, 4442–4449. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, Y.D.; Wang, Z.M.; Lei, Y.P.; Wang, B.; Wu, N.; Han, C.; Gou, Y.Z. Three-dimensional (3D) interconnected networks fabricated via in-situ growth of N-doped graphene/carbon nanotubes on Co-containing carbon nanofibers for enhanced oxygen reduction. Nano Res. 2016, 9, 317–328. [Google Scholar] [CrossRef]
- Liu, Y.X.; Si, L.; Du, Y.C.; Zhou, X.S.; Dai, Z.H.; Bao, J.C. Strongly bonded selenium/microporous carbon nanofibers composite as a high-performance cathode for lithium-selenium batteries. J. Phys. Chem. C 2015, 119, 27316–27321. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Yang, Z.Z.; Gu, L.; Chen, Q.W.; Yu, Y. Sodium-ion batteries: Improving the rate capability of 3D interconnected carbon nanofibers thin film by boron, nitrogen dual-doping. Adv. Sci. 2017, 4, 1600468. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.J.; Chi, Q.G.; Feng, Y.; Zhang, Y.; Zhang, T.D.; Zhang, C.H.; Chen, Q.G.; Lei, Q.Q. High energy storage density and efficiency in aligned nanofiber filled nanocomposites with multilayer structure. Compos. B Eng. 2020, 198, 108206. [Google Scholar] [CrossRef]
- Yan, Y.H.; Liu, X.Y.; Yan, J.; Guan, C.; Wang, J. Electrospun nanofibers for new generation flexible energy storage. Energy Environ. Mater. 2021, 4, 502–521. [Google Scholar] [CrossRef]
- Gu, H.H.; Huang, Y.P.; Zuo, L.Z.; Fan, W.; Liu, T.X. Graphene sheets wrapped carbon nanofibers as a highly conductive three-dimensional framework for perpendicularly anchoring of MoS2: Advanced electrocatalysts for hydrogen evolution reaction. Electrochim. Acta 2016, 219, 604–613. [Google Scholar] [CrossRef]
- Shao, Y.; Luo, C.; Deng, B.-w.; Yin, B.; Yang, M.-b. Flexible porous silicone rubber-nanofiber nanocomposites generated by supercritical carbon dioxide foaming for harvesting mechanical energy. Nano Energy 2020, 67, 104290. [Google Scholar] [CrossRef]
- Shi, L.; Jin, H.; Dong, S.R.; Huang, S.Y.; Kuang, H.Z.; Xu, H.S.; Chen, J.K.; Xuan, W.P.; Zhang, S.M.; Li, S.J.; et al. High-performance triboelectric nanogenerator based on electrospun PVDF-graphene nanosheet composite nanofibers for energy harvesting. Nano Energy 2021, 80, 105599. [Google Scholar] [CrossRef]
- Guan, X.Y.; Xu, B.G.; Wu, M.J.; Jing, T.T.; Yang, Y.J.; Gao, Y.Y. Breathable, washable and wearable woven-structured triboelectric nanogenerators utilizing electrospun nanofibers for biomechanical energy harvesting and self-powered sensing. Nano Energy 2021, 80, 105549. [Google Scholar] [CrossRef]
- Babu, A.; Aazem, I.; Walden, R.; Bairagi, S.; Mulvihill, D.M.; Pillai, S.C. Electrospun nanofiber based TENGs for wearable electronics and self-powered sensing. Chem. Eng. J. 2023, 452, 139060. [Google Scholar] [CrossRef]
- Jiang, F.; Zhou, X.R.; Lv, J.; Chen, J.; Chen, J.T.; Kongcharoen, H.; Zhang, Y.H.; Lee, P.S. Stretchable, breathable, and stable lead-free perovskite/polymer nanofiber composite for hybrid triboelectric and piezoelectric energy harvesting. Adv. Mater. 2022, 34, 2200042. [Google Scholar] [CrossRef]
- He, X.Y.; Gu, J.T.; Hao, Y.N.; Zheng, M.R.; Wang, L.M.; Yu, J.Y.; Qin, Q.H. Continuous manufacture of stretchable and integratable thermoelectric nanofiber yarn for human body energy harvesting and self-powered motion detection. Chem. Eng. J. 2022, 450, 137937. [Google Scholar] [CrossRef]
- Xie, L.; Zhou, S.; Liu, J.R.; Qiu, B.L.; Liu, T.Y.; Liang, Q.R.; Zheng, X.Z.; Li, B.; Zeng, J.; Yan, M.; et al. Sequential superassembly of nanofiber arrays to carbonaceous ordered mesoporous nanowires and their heterostructure membranes for osmotic energy conversion. J. Am. Chem. Soc. 2021, 143, 6922–6932. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.D.; Xin, W.W.; Kong, X.-Y.; Chen, J.J.; Qian, Y.C.; Sun, Y.; Zhao, X.L.; Chen, W.P.; Jiang, L.; Wen, L.P. Enhanced ion transport by graphene oxide/cellulose nanofibers assembled membranes for high-performance osmotic energy harvesting. Mater. Horiz. 2020, 7, 2702–2709. [Google Scholar] [CrossRef]
- Mamun, A.; Klöcker, M.; Blachowicz, T.; Sabantina, L. Investigation of the morphological structure of needle-free electrospun magnetic nanofiber mats. Magnetochemistry 2022, 8, 25. [Google Scholar] [CrossRef]
- Mamun, A.; Blachowicz, T.; Sabantina, L. Electrospun nanofiber mats for filtering applications—Technology, structure and materials. Polymers 2021, 13, 1368. [Google Scholar] [CrossRef] [PubMed]
- Nishiuchi, H.; Tonami, H. Control of mat thickness in electrospinning with transparent conductive glass collector. Polym. Eng. Sci. 2022, 62, 2252–2259. [Google Scholar] [CrossRef]
- Sharif, N.; Khoshnoudi-Nia, S.; Jafari, S.M. Confocal laser scanning microscopy (CLSM) of nanoencapsulated food ingredients. In Characterization of Nanoencapsulated Food Ingredients; Academic Press Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–158. [Google Scholar]
- Gallegos-Cerda, S.D.; Hernández-Verela, J.D.; Chanona-Pérez, J.J.; Tamayo, B.A.; Méndez Méndez, J.V. Super-resolution microscopy and their applications in food materials: Beyond the resolution limits of fluorescence microscopy. Food Bioprocess Technol. 2022, 16, 268–288. [Google Scholar] [CrossRef]
- Wortmann, M.; Westphal, M.; Kaltschmidt, B.; Klöcker, M.; Layland, A.S.; Brockhagen, B.; Hütten, A.; Frese, N.; Ehrmann, A. Nanofibers are a matter of perspective: Effects of methodology and subjectivity on diameter measurements. Nanoscale Adv. 2023, 5, 5900–5906. [Google Scholar] [CrossRef]
- Serag, E.; Abd El-Aziz, A.M.; El-Maghraby, A.; Taha, N.A. Electrospun non-wovens potential wound dressing material based on polyacrylonitrile/chicken feathers keratin nanofiber. Sci. Rep. 2022, 12, 15460. [Google Scholar] [CrossRef]
- Rahmani, F.; Ziyadi, H.; Baghali, M.; Luo, H.R.; Ramakrishna, S. Electrospun PVP/PVA nanofiber mat as a novel potential transdermal drug-delivery system for buprenorphine: A solution needed for pain management. Appl. Sci. 2021, 11, 2779. [Google Scholar] [CrossRef]
- Pham Le, Q.; Uspenskaya, M.V.; Olekhnovich, R.O.; Baranov, M.A. The mechanical properties of PVC nanofiber mats obtained by electrospinning. Fibers 2021, 9, 2. [Google Scholar] [CrossRef]
- Vafaye, S.E.; Rahman, A.; Safaeian, S.; Adabi, M. An electrochemical aptasensor based on electrospun carbon nanofiber mat and gold nanoparticles for the sensitive detection of Penicillin in milk. J. Food Meas. Charact. 2021, 15, 876–882. [Google Scholar] [CrossRef]
- Xie, W.H.; Shi, Y.L.; Wang, Y.X.; Zheng, Y.L.; Liu, H.; Hu, Q.; Wie, S.Y.; Gu, H.B.; Guo, Z.H. Electrospun iron/cobalt alloy nanoparticles on carbon nanofibers towards exhaustive electrocatalytic degradation of tetracycline in wastewater. Chem. Eng. J. 2021, 405, 126585. [Google Scholar] [CrossRef]
- Baek, S.H.; Roh, J.H.; Park, C.Y.; Kim, M.W.; Shi, R.J.; Kailasa, S.K.; Park, T.J. Cu-nanoflower decorated gold nanoparticles-graphene oxide nanofiber as electrochemical biosensor for glucose detection. Mater. Sci. Eng. C 2020, 107, 110273. [Google Scholar] [CrossRef]
- Zainab, G.; Babar, A.A.; Ali, N.; Aboalhassan, A.A.; Wang, X.F.; Yu, J.Y.; Ding, B. Electrospun carbon nanofibers with multi-aperture/opening porous hierarchical structure for efficient CO2 adsorption. J. Coll. Interface Sci. 2020, 561, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Conlee, B.; Tallerine, I.; Kennedy, W.J.; Naraghi, M. A novel path towards synthesis of nitrogen-rich porous carbon nanofibers for high performance supercapacitors. Chem. Eng. J. 2020, 399, 125788. [Google Scholar] [CrossRef]
- Saha, D.; Gismondi, P.; Kolasinski, K.W.; Shumlas, S.L.; Rangan, S.; Eslami, B.; McConnell, A.; Bui, T.V.; Cunfer, K. Fabrication of electrospun nanofiber composite of g-C3N4 and Au nanoparticles as plasmonic photocatalyst. Surf. Interfaces 2021, 26, 101367. [Google Scholar] [CrossRef]
- Ju, Z.S.; Li, P.; Zhao, X.N.; Ma, J.G.; Xu, H.Y.; Liu, Y.C. Flexible TiN/Co@Carbon nanofiber mats for high-performance electromagnetic interference shielding and Joule heating applications. Carbon 2022, 196, 612–620. [Google Scholar] [CrossRef]
- Mousa, H.M.; Hussein, K.H.; Sayed, M.M.; Abd El-Rahman, M.K.; Woo, H.-M. Development and characterization of cellulose/iron acetate nanofibers for bone tissue engineering applications. Polymers 2021, 13, 1339. [Google Scholar] [CrossRef]
- Wabah, J.A.; Mamun, S.A. Polyacrylonitrile nanofiber mats containing titania/AgNP composite nanoparticles for antibacterial applications. Mater. Res. Expr. 2020, 7, 015416. [Google Scholar]
- Wortmann, M.; Layland, A.S.; Frese, N.; Kahmann, U.; Grothe, T.; Storck, J.L.; Blachowicz, T.; Grzybowski, J.; Hüsgen, B.; Ehrmann, A. On the reliability of highly magnified micrographs for structural analysis in materials science. Sci. Rep. 2020, 10, 14708. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Wang, K.; Yu, D.-G.; Yang, Y.Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater. Sci. Eng. C 2020, 111, 110805. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Cui, J.X.; Qu, Q.L.; Ma, W.J.; Li, F.H.; Du, W.H.; Liu, K.M.; Zhang, Q.; He, S.J.; Huang, C.B. Free-standing porous carbon nanofiber membranes obtained by one-step carbonization and activation for high-performance supercapacitors. Microporous Mesoporous Mater. 2022, 329, 111545. [Google Scholar] [CrossRef]
- Chlanda, A.; Kijenska-Gawronska, E.; Zdunek, J.; Swieszkowski, W. Internal nanocrystalline structure and stiffness alterations of electrospun polycaprolactone-based mats after six months of in vitro degradation. An atomic force microscopy assay. J. Mech. Behav. Biomed. Mater. 2020, 101, 103437. [Google Scholar] [CrossRef]
- Wang, D.; Yue, Y.Y.; Wang, Q.X.; Cheng, W.L.; Han, G.P. Preparation of cellulose acetate-polyacrylonitrile composite nanofibers by multi-fluid mixing electrospinning method: Morphology, wettability, and mechanical properties. Appl. Surf. Sci. 2020, 510, 145462. [Google Scholar] [CrossRef]
- Joshi, J.; Homburg, S.V.; Ehrmann, A. Atomic force microscopy (AFM) on biopolymers and hydrogels for biotechnological applications—Possibilities and limits. Polymers 2022, 14, 1267. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.M.; Cai, Z.Y.; Hu, Z.J.; Zhu, P. Fabrication and characterization of polyacrylonitrile and polyethylene glycol composite nanofibers by electrospinning. J. Energy Storage 2022, 53, 105171. [Google Scholar] [CrossRef]
- Cuong, N.T.; Barrau, S.; Dufay, M.; Tabary, N.; Da Costa, A.; Ferri, A.; Lazzaroni, R.; Raquez, J.-M.; Leclère, P. On the Nanoscale Mapping of the Mechanical and Piezoelectric Properties of Poly(L-Lactic Acid) Electrospun Nanofibers. Appl. Sci. 2020, 10, 652. [Google Scholar] [CrossRef]
- Guo, Y.H.; Guo, Y.C.; He, W.D.; Zhao, Y.B.; Shen, R.Q.; Liu, J.X.; Wang, J. PET/TPU nanofiber composite filters with high interfacial adhesion strength based on one-step co-electrospinning. Powder Technol. 2021, 387, 136–145. [Google Scholar] [CrossRef]
- Vokoun, D.; Samal, S.; Stachiv, I. Magnetic force microscopy in physics and biomedical applications. Magnetochemistry 2022, 8, 42. [Google Scholar] [CrossRef]
- Winkler, R.; Ciria, M.; Ahmad, M.; Plank, H.; Marcuello, C. A review of the current state of magnetic force microscopy to unravel the magnetic properties of nanomaterials applied in biological systems and future directions for quantum technologies. Nanomaterials 2023, 13, 2585. [Google Scholar] [CrossRef]
- Ehrmann, A.; Blachowicz, T. Magnetic force microscopy on nanofibers—Limits and possible approaches for randomly oriented nanofiber mats. Magnetochemistry 2021, 7, 143. [Google Scholar] [CrossRef]
- Weiss, R.; Ehrmann, A. Preliminary report on MFM measurements on magnetic nanofiber mats. Commun. Dev. Assem. Text. Prod. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Brocks, O.; Stasiak, A.; Biedinger, J.; Wortmann, M.; Blachowicz, T.; Kaschuba, R.; Ehrmann, A. MOKE and MFM on magnetically coated nanofiber mats: Transferring well-known methods to uncommon samples. Appl. Res. 2023, 2, e202200113. [Google Scholar] [CrossRef]
- Berganza, E.; Jaarar, M.; Bran, C.; Fernández-Roldán, J.A.; Chubykalo-Fesenko, O.; Vázquez, M.; Asenjo, A. Multisegmented nanowires: A step towards the control of the domain wall configuration. Sci. Rep. 2017, 7, 11576. [Google Scholar] [CrossRef] [PubMed]
- Bran, C.; Fernandez-Roldan, J.A.; Palmero, E.M.; Berganza, E.; Guzman, J.; del Real, R.P.; Asenjo, A.; Fraile Rodríguez, A.; Foerster, M.; Aballe, L.; et al. Direct observation of transverse and vortex metastable magnetic domains in cylindrical nanowires. Phys. Rev. B 2017, 96, 125415. [Google Scholar] [CrossRef]
- Askey, J.; Hunt, M.O.; Langbein, W.; Ladak, S. Use of two-photon lithography with a negative resist and processing to realize cylindrical magnetic nanowires. Nanomaterials 2020, 10, 429. [Google Scholar] [CrossRef]
- Nasirpouri, F.; Nogaret, A.; Bending, S.J. Effect of size and configuration on the magnetization of nickel dot arrays. IEEE Trans. Magn. 2011, 47, 4695–4700. [Google Scholar] [CrossRef]
- Nasirpouri, F.; Peighambari-Sattari, S.-M.; Bran, C.; Palmero, E.M.; Berganza Eguiarte, E.; Vazquez, M.; Patsopoulos, A.; Kechrakos, D. Geometrically designed domain wall trap in tri-segmented nickel magnetic nanowires for spintronics devices. Sci. Rep. 2019, 9, 9010. [Google Scholar] [CrossRef]
- Corte-León, H.; Rodríguez, L.A.; Pancaldi, M.; Gatel, C.; Cox, D.; Snoeck, E.; Antonov, V.; Vavassori, P.; Kazakova, O. Magnetic imaging using geometrically constrained nano-domain walls. Nanoscale 2019, 11, 4478–4488. [Google Scholar] [CrossRef]
- Melitz, W.; Shen, J.; Kummel, A.C.; Lee, S.Y. Kelvin probe force microscopy and its application. Surf. Sci. Rep. 2011, 66, 1–27. [Google Scholar] [CrossRef]
- Wu, M.-C.; Liao, H.-C.; Cho, Y.-C.; Tóth, T.; Chen, Y.-F.; Su, W.-F.; Kordás, K. Photo-Kelvin probe force microscopy for photocatalytic performance characterization of single filament of TiO2 nanofiber photocatalysts. J. Mater. Chem. A 2013, 1, 5715–5720. [Google Scholar] [CrossRef]
- Liscio, A.; Palermo, V.; Samori, P. Probing local surface potential of quasi-one-dimensional systems: A KPFM study of P3HT nanofibers. Adv. Funct. Mater. 2008, 18, 907–914. [Google Scholar] [CrossRef]
- Liscio, A.; Palermo, V.; Samori, P. Nanoscale quantitative measurement of the potential of charged nanostructures by electrostatic and kelvin probe force microscopy: Unraveling electronic processes in complex materials. Acc. Chem. Res. 2010, 43, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Soergel, E. Piezoresponse force microscopy (PFM). J. Phys. D Appl. Phys. 2011, 44, 464003. [Google Scholar] [CrossRef]
- Liu, X.; Kuang, X.L.; Xu, S.X.; Wang, X.H. High-sensitivity piezoresponse force microscopy studies of single polyvinylidene fluoride nanofibers. Mater. Lett. 2017, 191, 189–192. [Google Scholar] [CrossRef]
- Zhu, Q.F.; Pan, K.; Xie, S.H.; Liu, Y.Y.; Li, J.Y. Nanomechanics of multiferroic composite nanofibers via local excitation piezoresponse force microscopy. J. Mech. Phys. Solids 2019, 126, 76–86. [Google Scholar] [CrossRef]
- Xie, S.H.; Gannepalli, A.; Chen, Q.N.; Liu, Y.M.; Zhou, Y.C.; Proksch, R.; Li, J.Y. High resolution quantitative piezoresponse force microscopy of BiFeO3 nanofibers with dramatically enhanced sensitivity. Nanoscale 2012, 4, 408–413. [Google Scholar] [CrossRef]
- Zheng, T.; Yue, Z.L.; Wallace, G.G.; Du, Y.; Martins, P.; Lanceros-Mendez, S.; Higgins, M.J. Local probing of magnetoelectric properties of PVDF/Fe3O4 electrospun nanofibers by piezoresponse force microscopy. Nanotechnology 2017, 28, 065707. [Google Scholar] [CrossRef]
- Gomès, S.; Assy, A.; Chapuis, P.-O. Scanning thermal microscopy: A review. Phys. Stat. Sol. A 2015, 212, 477–494. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.K.; Hui, F.; Lanza, M.; Borca-Tasciuc, T.; Munoz, R. A review on principles and applications of scanning thermal microscopy (SThM). Adv. Funct. Mater. 2020, 30, 1900892. [Google Scholar] [CrossRef]
- Moradi, A.; Szewczyk, P.K.; Stachewicz, U. Bridging a gap in thermal conductivity and heat transfer in hybrid fibers and yarns via polyimide and silicon nitride composites. Small 2023, 19, 2305104. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Szewczyk, P.K.; Roszko, A.; Fornalik-Wajs, E.; Stachewicz, U. Unraveling the impact of boron nitride and silicon nitride nanoparticles on thermoplastic polyurethane fibers and mats for advanced heat management. ACS Appl. Mater. Interfaces 2024, 16, 41475–41486. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.S.; Gonzalez-Munoz, S.; Griffiths, I.; Holdway, P.; Evers, K.; Luanwuthi, S.; Maciejewska, B.M.; Kolosov, O.; Grobert, N. Thermal conductivity of carbon/boron nitride heteronanotube and boron nitride nanotube buckypapers: Implications for thermal management composites. ACS Appl. Nano Mater. 2023, 6, 15374–15384. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, I.S.; Vishnevskiy, A.S.; Seregin, D.S.; Rezvanov, A.A.; Schneider, D.; Sigov, A.S.; Vorotilov, K.A.; Baklanov, M.R. Evaluation of mechanical properties of porous OSG films by PFQNM AFM and benchmarking with traditional instrumentation. Langmuir 2020, 36, 9377–9387. [Google Scholar] [CrossRef]
- Abdelhady, H.G.; Abdel-Salam, H.A.; Niazy, E.M.; Mueller, A.; Quast, M.J.; Effat, A.M.; Elbehairi, S.-E. Spatiotemporal PFQNM visualization of the effect of suicide dendriplexes on dividing HeLa cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2365–2371. [Google Scholar] [CrossRef]
- Papi, M.; Paoletti, P.; Geraghty, B.; Akhtar, R. Nanoscale characterization of the biomechanical properties of collagen fibrils in the sclera. Appl. Phys. Lett. 2014, 104, 103703. [Google Scholar] [CrossRef]
- Li, J.; Mathew, A.P. Effect of decoration route on the nanomechanical, adhesive, and force response of nanocelluloses—An in-situ force spectroscopy study. PLoS ONE 2022, 18, e0279919. [Google Scholar] [CrossRef]
- Mendes, A.C.; Sevilla Moreno, J.; Hanif, M.; Douglas, T.E.L.; Chen, M.L.; Chronakis, I.S. Morphological, mechanical and mucoadhesive properties of electrospun chitosan/phospholipid hybrid nanofibers. Int. J. Mol. Sci. 2018, 19, 2266. [Google Scholar] [CrossRef]
- Liu, L.L.; Chen, S.X.; Xu, A.C.; Cai, G.M. Manufacturing high sensitive strain sensor of polyurethane nanofiber mat/AgNWs by simple dip-dry method. Fibers Polym. 2020, 21, 359–365. [Google Scholar] [CrossRef]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Huang, J.; Zhang, X.; Zhang, J.B. Direct measurement of proton conductivity of a single ionomer nanofiber. Nano Energy 2022, 102, 107738. [Google Scholar] [CrossRef]
- Sengupta, D.; Chen, S.-H.; Michael, A.; Kwok, C.Y.; Lim, S.; Pei, Y.T.; Prakash Kottapalli, A.G. Single and bundled carbon nanofibers as ultralightweight and flexible piezoresistive sensors. npj Flex. Electron. 2020, 4, 9. [Google Scholar] [CrossRef]
- Mondal, K.; Maitra, T.; Srivastava, A.K.; Pawar, G.; McMurtrey, M.D.; Sharma, A. Particle size effect on enhanced graphitization and electrical conductivity of suspended gold/carbon composite nanofibers. Ind. Eng. Chem. Res. 2020, 59, 1944–1952. [Google Scholar] [CrossRef]
- Serrano-Garcia, W.; Ramakrishna, S.; Thomas, S.W. Electrospinning technique for fabrication of coaxial nanofibers of semiconductive polymers. Polymers 2022, 14, 5073. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, T.-H.; Choi, J.H.; Yu, W.-R. Improved electrical conductivity of poly(ethylene oxide) nanofibers using multi-walled carbon nanotubes. AIP Adv. 2018, 8, 035024. [Google Scholar] [CrossRef]
- Koderman Podborsek, G.; Zupancic, S.; Kaufman, R.; Surca, A.K.; Marsel, A.; Pavlixic, A.; Hodnik, N.; Drazic, G.; Bele, M. Microstructure and electrical conductivity of electrospun titanium oxynitride carbon composite nanofibers. Nanomaterials 2022, 12, 2177. [Google Scholar] [CrossRef]
- Henrichsen, H.H.; Kjelstrup-Hansen, J.; Engstrom, D.; Clausen, C.H.; Boggild, P.; Rubahn, H.-G. Electrical conductivity of organic single-nanofiber devices with different contact materials. Org. Electron. 2007, 8, 540–544. [Google Scholar] [CrossRef]
- Qi, H.N.; Wang, G.Y.; Hu, Y.L.; Shao, H.; Ma, Q.L.; Li, D.; Yu, W.S.; Chang, L.M.; Zhang, X.J.; Dong, X.T. Conjugate electro-spinning towards Janus nanofibers array synchronously endowed with conductive anisotropy, magnetism and luminescence. Mater. Today Comm. 2022, 33, 104765. [Google Scholar] [CrossRef]
- El-Ghazali, S.; Kobayashi, H.; Khatri, M.; Phan, D.-N.; Khatri, Z.; Mahar, S.K.; Kobayashi, S.; Kim, I.-S. Preparation of a Cage-Type Polyglycolic Acid/Collagen Nanofiber Blend with Improved Surface Wettability and Handling Properties for Potential Biomedical Applications. Polymers 2021, 13, 3458. [Google Scholar] [CrossRef]
- Shi, T.T.; Liu, Y.; Wang, D.H.; Xia, D.; Li, B.; Xu, R.D.; Li, N.; Liang, C.Y.; Chen, M.L. Spatially engineering tri-layer nanofiber dressings featuring asymmetric wettability for wound healing. Nano Mater. Sci. 2024, in press. [Google Scholar] [CrossRef]
- Schoolaert, E.; Cossu, L.; Becelaere, J.; van Guyse, J.F.R.; Tigrine, A.; Vergaelen, M.; Hoogenboom, R.; de Clerck, K. Nanofibers with a tunable wettability by electrospinning and physical crosslinking of poly(2-n-propyl-2-oxazoline). Mater. Des. 2020, 192, 108747. [Google Scholar] [CrossRef]
- Jalali, S.; Kruppke, I.; Enghardt, S.; Wiesmann, H.-P.; Kruppke, B. Silica nanofibers with enhanced wettability and mechanical strength for bone tissue engineering: Electrospinning without polymer carrier and subsequent heat treatment. Macromol. Mater. Eng. 2024, 309, 2300169. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, R.; Sun, Y.; Lin, D.D.; Sun, Z.Q.; Pan, W.; Downs, P. Biomimetic nanofiber patterns with controlled wettability. Soft Matter 2008, 4, 2429–2433. [Google Scholar] [CrossRef]
- Bagrov, D.; Perunova, S.; Pavlova, E.; Klinov, D. Wetting of electrospun nylon-11 fibers and mats. RSC Adv. 2021, 11, 11373–11379. [Google Scholar] [CrossRef]
- Andrade, K.L.; Faita, F.L.; do Nascimento, R.M.; Sousa Cunha, R.; Bresolin, D.; Diz Acosta, E.; Machado, R.A.F. Wettability tuning of natural rubber/polyvinylpyrrolidone electrospun nonwoven mats. Surf. Interfaces 2022, 32, 102129. [Google Scholar] [CrossRef]
- Alam, A.K.M.M.; Ewaldz, E.; Xiang, C.H.; Qu, W.D.; Bai, X.L. Tunable wettability of biodegradable multilayer sandwich-structured electrospun nanofibrous membranes. Polymers 2020, 12, 2092. [Google Scholar] [CrossRef]
- Heinz, M.; Chowdhury, I.U.; Stephan, P.; Gambaryan-Roisman, T. Water drops on nanofiber-coated substrates: Influence of wall temperature and coating thickness on hydrodynamics and wall heat flux distribution. Int. J. Heat Mass Transf. 2024, 222, 125117. [Google Scholar] [CrossRef]
- Shi, J.; Li, S.-F.; Feng, K.; Han, S.-Y.; Hu, T.-G.; Wu, H. Improving the viability of probiotics under harsh conditions by the formation of biofilm on electrospun nanofiber mat. Foods 2022, 11, 1203. [Google Scholar] [CrossRef]
- Nitti, P.; Gallo, N.; Palazzo, B.; Sannino, A.; Polini, A.; Verri, T.; Barca, A.; Gervaso, F. Effect of L-Arginine treatment on the in vitro stability of electrospun aligned chitosan nanofiber mats. Polym. Test. 2020, 91, 106758. [Google Scholar] [CrossRef]
- Bekou, S.; Mattia, D. Wetting of nanotubes. Curr. Opin. Colloid Interface Sci. 2011, 16, 259–265. [Google Scholar] [CrossRef]
- Neimark, A.V. Thermodynamic equilibrium and stability of liquid films and droplets on fibers. J. Adhes. Sci. Technol. 1999, 13, 1137–1154. [Google Scholar] [CrossRef]
- Barber, A.H.; Cohen, S.R.; Wagner, H.D. External and internal wetting of carbon nanotubes with organic liquids. Phys. Rev. B 2005, 71, 115443. [Google Scholar] [CrossRef]
- Gambaryan-Roisman, T. Liquids on porous layers: Wetting, imbibition and transport processes. Curr. Opin. Colloid Interface Sci. 2014, 19, 320–335. [Google Scholar] [CrossRef]
- Yum, K.S.; Yu, M.-F. Measurement of wetting properties of individual boron nitride nanotubes with the wilhelmy method using a nanotube-based force sensor. Nano Lett. 2006, 6, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Stachewicz, U.; Barber, A.H. Enhanced wetting behavior at electrospun polyamide nanofiber surfaces. Langmuir 2011, 27, 3024–3029. [Google Scholar] [CrossRef]
- Wang, J.; Anthony, D.B.; Fuentes, C.A.; De Luca, H.G.; Zhang, D.X.; Bismarck, A.; van Vuure, A.W.; Shaffer, M.S.P.; Seveno, D. Wettability of carbon nanotube-grafted carbon fibers and their interfacial properties in polypropylene thermoplastic composite. Compos. Part A: Appl. Sci. Manuf. 2022, 159, 106993. [Google Scholar] [CrossRef]
- Barber, A.H.; Cohen, S.R.; Wagner, H.D. Static and dynamic wetting measurements of single carbon nanotubes. Phys. Rev. Lett. 2004, 92, 186103. [Google Scholar] [CrossRef] [PubMed]
- Stachewicz, U.; Stone, C.A.; Willis, C.R.; Barber, A.H. Charge assisted tailoring of chemical functionality at electrospun nanofiber surfaces. J. Mater. Chem. 2012, 22, 22935–22941. [Google Scholar] [CrossRef]
- Stachewicz, U.; Bailey, R.J.; Zhang, H.; Stone, C.A.; Willis, C.R.; Barber, A.H. Wetting hierarchy in oleophobic 3D electrospun nanofiber networks. ACS Appl. Mater. Interfaces 2015, 7, 16645–16652. [Google Scholar] [CrossRef]
- Yazdanpanah, M.M.; Hosseini, M.; Pabba, S.; Berry, S.M.; Dobrokhotov, V.V.; Safir, A.; Keynton, R.S.; Cohn, R.W. Micro-Wilhelmy and related liquid property measurements using constant-diameter nanoneedle-tipped atomic force microscope probes. Langmuir 2008, 24, 13753–13764. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Qin, X.-H. Effects of the stabilization temperature on the structure and properties of polyacrylonitrile-based stabilized electrospun nanofiber microyarns. J. Therm. Anal. Calorim. 2013, 116, 303–308. [Google Scholar] [CrossRef]
- Duan, G.G.; Fang, H.; Huang, C.B.; Jiang, S.H.; Hou, H.Q. Microstructures and mechanical properties of aligned electrospun carbon nanofibers from binary composites of polyacrylonitrile and polyamic acid. J. Mater. Sci. 2018, 53, 15096–15106. [Google Scholar] [CrossRef]
- Storck, J.L.; Wortmann, M.; Brockhagen, B.; Frese, N.; Diestelhorst, E.; Grothe, T.; Hellert, C.; Ehrmann, A. Comparative study of metal substrates for improved carbonization of electrospun PAN nanofibers. Polymers 2022, 14, 721. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.P.S.; Goh, C.N.; Sow, C.H.; Lim, C.T. Tensile test of a single nanofiber using an atomic force microscope tip. Appl. Phys. Lett. 2005, 86, 073115. [Google Scholar] [CrossRef]
- Zou, Y.; Jiang, S.H.; Hu, X.W.; Xu, W.H.; Chen, Z.G.; Liu, K.M.; Hou, H.Q. Influence of pre-oxidation on mechanical properties of single electrospun polyacrylonitrile nanofiber. Mater. Today Comm. 2021, 26, 102069. [Google Scholar] [CrossRef]
- Hwang, K.Y.; Kim, S.-D.; Kim, Y.-W.; Yu, W.-R. Mechanical characterization of nanofibers using a nanomanipulator and atomic force microscope cantilever in a scanning electron microscope. Polym. Test. 2010, 29, 375–380. [Google Scholar] [CrossRef]
- Alharbi, N.; Daraei, A.; Lee, H.S.; Guthold, M. The effect of molecular weight and fiber diameter on the mechanical properties of single, electrospun PCL nanofibers. Mater. Today Comm. 2023, 35, 105773. [Google Scholar] [CrossRef]
- Sharpe, J.M.; Lee, H.S.; Hall, A.R.; Bonin, K.; Guthold, M. Mechanical properties of electrospun, blended fibrinogen: Pcl nanofibers. Nanomaterials 2020, 10, 1843. [Google Scholar] [CrossRef]
- Baker, S.R.; Banerjee, S.; Bonin, K.; Guthold, M. Determining the mechanical properties of electrospun poly-ε-caprolactone (PCL) nanofibers using AFM and a novel fiber anchoring technique. Mater. Sci. Eng. C 2016, 59, 203–212. [Google Scholar] [CrossRef]
- Parvej, M.S.; Wang, X.N.; Jiang, L. AFM based nanomechanical characterization of cellulose nanofibril. J. Comp. Mater. 2020, 54, 4487–4493. [Google Scholar] [CrossRef]
- Bidhar, S.; Goss, V.; Chen, W.-Y.; Stanishevsky, A.; Li, M.M.; Kuksenko, S.; Calviani, M.; Zwaska, R. Production and qualification of an electrospun ceramic nanofiber material as a candidate future high power target. Phys. Rev. Accel. Beams 2021, 24, 123001. [Google Scholar] [CrossRef]
- Bulbul, Y.E.; Uzunoglu, T.; Dilsiz, N.; Yildirim, E.; Ates, H. Investigation of nanomechanical and morphological properties of silane-modified halloysite clay nanotubes reinforced polycaprolactone bio-composite nanofibers by atomic force microscopy. Polym. Test. 2020, 92, 106877. [Google Scholar] [CrossRef]
- Cheng, Q.Z.; Wang, S.Q. A method for testing the elastic modulus of single cellulose fibrils via atomic force microscopy. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1838–1843. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A.; Chliveros, G.; Malamou, A. Overcoming challenges and limitations regarding the atomic force microscopy imaging and mechanical characterization of nanofibers. Fibers 2023, 11, 83. [Google Scholar] [CrossRef]
- Biagi, G.; Holmgaard, T.; Skovsen, E. Near-field electrospinning of dielectric-loaded surface plasmon polariton waveguides. Opt. Express 2013, 21, 4355–4360. [Google Scholar] [CrossRef] [PubMed]
- Caldiroli, A.; Cappelletti, S.; Birarda, G.; Redaelli, A.; Riboldi, S.A.; Stani, C.; Vaccari, L.; Piccirilli, F. Infrared nanospectroscopy depth-dependent study of modern materials: Morpho-chemical analysis of polyurethane/fibroin binary meshes. Analyst 2023, 48, 3584–3593. [Google Scholar] [CrossRef]
- Beermann, J.; Bozhevolnyi, S.I.; Balzer, F.; Rubahn, H.-G. Two-photon near-field mapping of local molecular orientations in hexaphenyl nanofibers. Laser Phys. Lett. 2005, 2, 480–484. [Google Scholar] [CrossRef]
- Richard-Lacroix, M.; Pellerin, C. Orientation and structure of single electrospun nanofibers of poly(ethylene terephthalate) by confocal raman spectroscopy. Macromolecules 2012, 45, 1946–1953. [Google Scholar] [CrossRef]
- Bellan, L.M.; Craighead, H.G. Molecular orientation in individual electrospun nanofibers measured via polarized Raman spectroscopy. Polymer 2008, 49, 3125–3129. [Google Scholar] [CrossRef]
- Sfakis, L.; Sharikova, A.; Tuschel, D.; Costa, F.X.; Larsen, M.; Khmaladze, A.; Castracane, J. Core/shell nanofiber characterization by Raman scanning microscopy. Biomed. Opt. Express 2017, 8, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Kharinthsev, S.S.; Saparina, S.V.; Fishman, A.I.; Stolov, A.A.; Li, J. Spectrally resolving coherent TERS spectroscopy of electrically biased carbon-coated fibers. J. Phys. Chem. C 2020, 124, 14752–14758. [Google Scholar] [CrossRef]
- Chaunchaiyakul, S.; Yano, T.; Khoklang, K.; Krukowski, P.; Akai-Kasaya, M.; Saito, A.; Kuwahara, Y. Nanoscale analysis of multiwalled carbon nanotube by tip-enhanced Raman spectroscopy. Carbon 2016, 99, 642–648. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blachowicz, T.; Mpofu, N.S.; Ehrmann, A. Measuring Physical and Chemical Properties of Single Nanofibers for Energy Applications—Possibilities and Limits. Nanoenergy Adv. 2024, 4, 300-317. https://doi.org/10.3390/nanoenergyadv4040018
Blachowicz T, Mpofu NS, Ehrmann A. Measuring Physical and Chemical Properties of Single Nanofibers for Energy Applications—Possibilities and Limits. Nanoenergy Advances. 2024; 4(4):300-317. https://doi.org/10.3390/nanoenergyadv4040018
Chicago/Turabian StyleBlachowicz, Tomasz, Nonsikelelo Sheron Mpofu, and Andrea Ehrmann. 2024. "Measuring Physical and Chemical Properties of Single Nanofibers for Energy Applications—Possibilities and Limits" Nanoenergy Advances 4, no. 4: 300-317. https://doi.org/10.3390/nanoenergyadv4040018
APA StyleBlachowicz, T., Mpofu, N. S., & Ehrmann, A. (2024). Measuring Physical and Chemical Properties of Single Nanofibers for Energy Applications—Possibilities and Limits. Nanoenergy Advances, 4(4), 300-317. https://doi.org/10.3390/nanoenergyadv4040018









