Materials and Processing of Lithium-Ion Battery Cathodes
Abstract
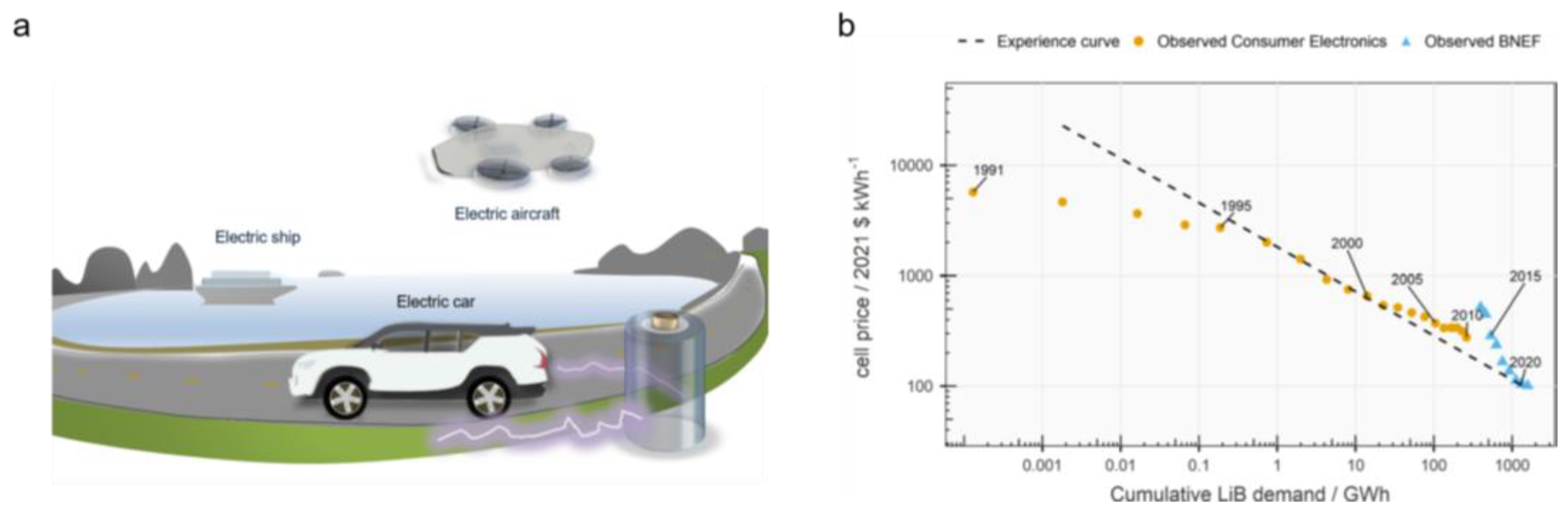
1. Introduction
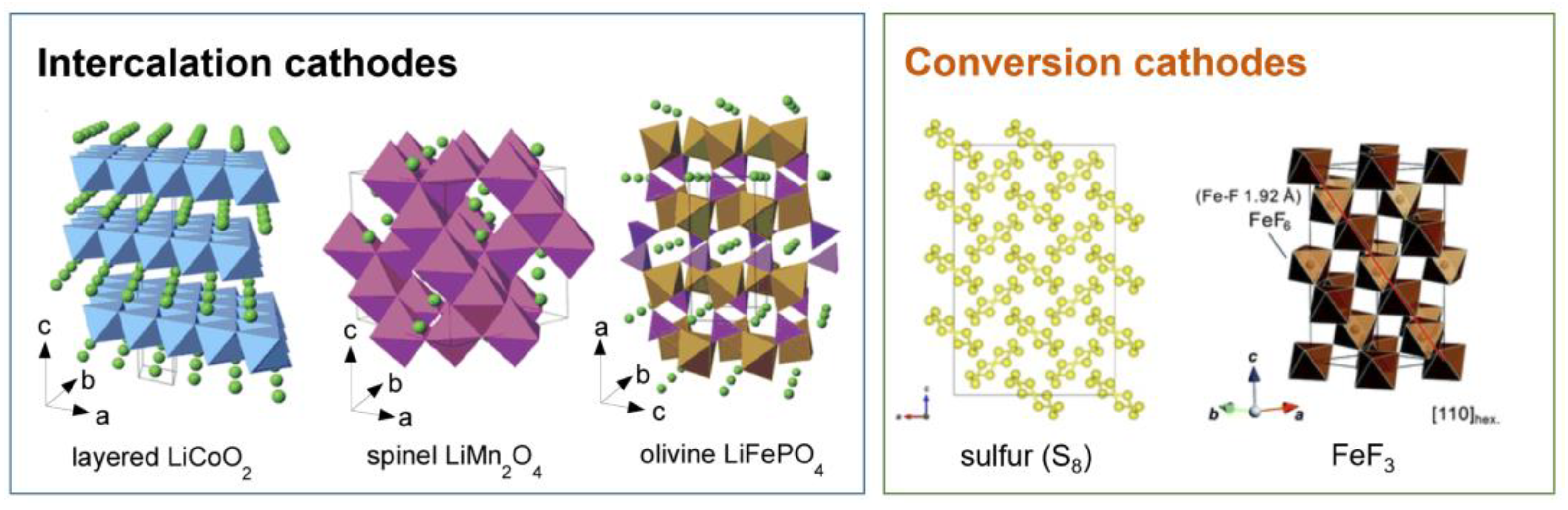
2. Cathode Materials
2.1. Intercalation Cathodes
2.2. Conversion Cathodes
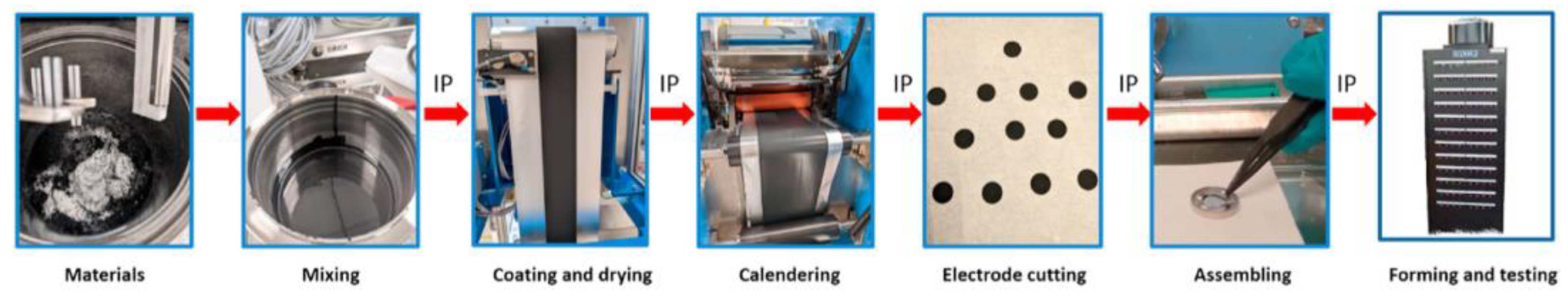
3. Electrode Processing
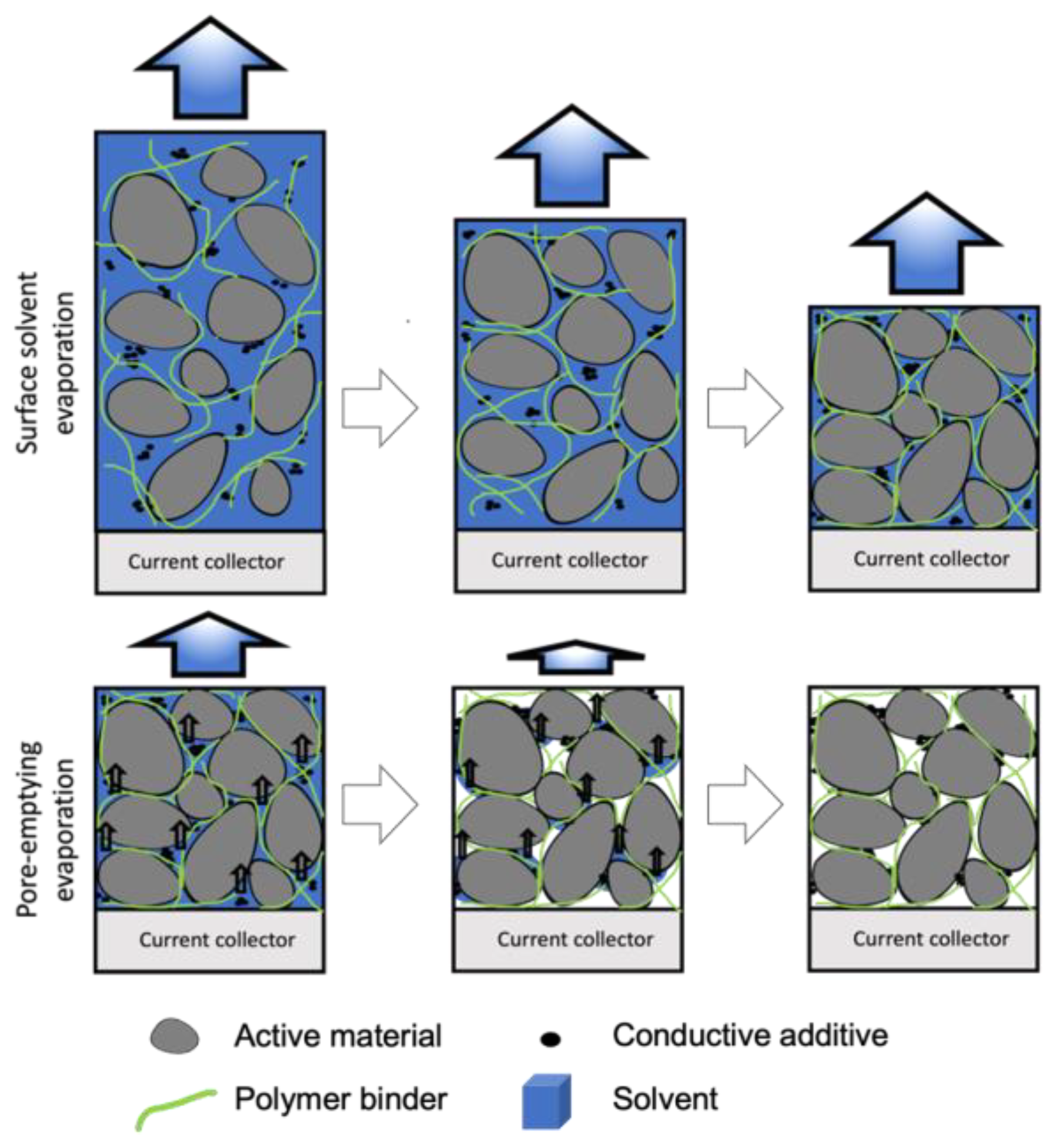
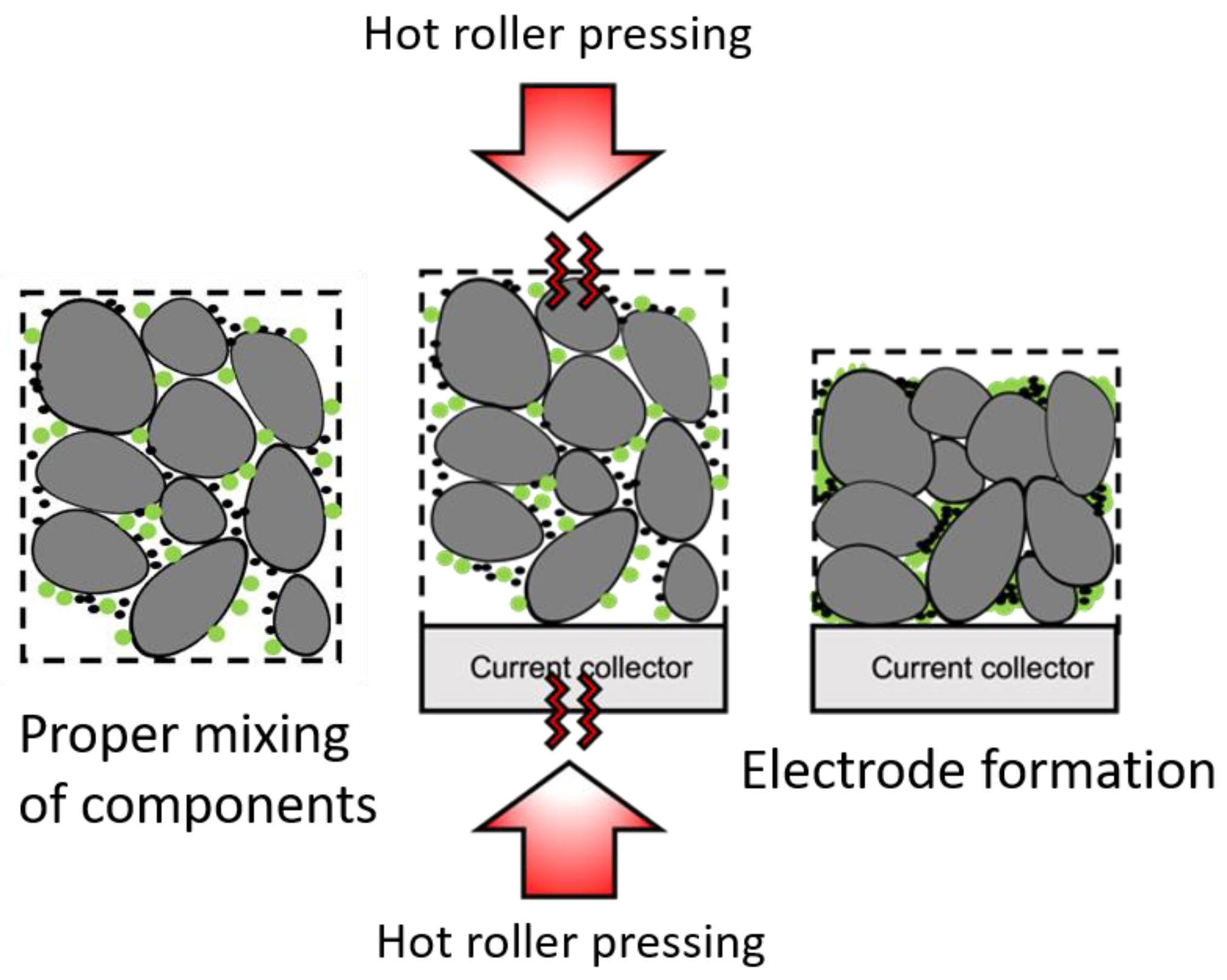
3.1. Drying Process
3.2. The Role of Binder
3.3. Dry Electrode Process
4. Key Parameters for Cathode Electrodes
4.1. Defect and Crystalinity
4.2. Particle Size and Distribution
4.3. Tortuosity
4.4. Electrode Architecture
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boudet, H.S. Public perceptions of and responses to new energy technologies. Nat. Energy 2019, 4, 446–455. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z. Amine, 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Shamim, N.; Crawford, A.; Huang, Q.; Vartanian, C.K.; Viswanathan, V.V.; Paiss, M.D.; Alam, M.J.E.; Reed, D.M.; Sprenkle, V.L. Li-ion battery technology for grid application. J. Power Sources 2021, 511, 230419. [Google Scholar] [CrossRef]
- Fu, W.; Turcheniuk, K.; Naumov, O.; Mysyk, R.; Wang, F.; Liu, M.; Kim, D.; Ren, X.; Magasinski, A.; Yu, M.; et al. Materials and technologies for multifunctional, flexible or integrated supercapacitors and batteries. Mater. Today 2021, 48, 176–197. [Google Scholar] [CrossRef]
- Nykvist, B.; Nilsson, M. Rapidly falling costs of battery packs for electric vehicles. Nat. Clim. Chang. 2015, 5, 329–332. [Google Scholar] [CrossRef]
- Frith, J.T.; Lacey, M.J.; Ulissi, U. A non-academic perspective on the future of lithium-based batteries. Nat. Commun. 2023, 14, 420. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Bondarev, D.; Amatucci, G.G.; Yushin, G. Battery materials for low-cost electric transportation. Mater. Today 2021, 42, 57–72. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Wu, F.; Yushin, G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ. Sci. 2017, 10, 435–459. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.; Zou, J.; Gao, P.; Niu, X.; Li, H.; Chen, L. Li-free Cathode Materials for High Energy Density Lithium Batteries. Joule 2019, 3, 2086–2102. [Google Scholar] [CrossRef]
- Fu, W.; Kim, D.; Wang, F.; Yushin, G. Stabilizing cathodes and interphases for next-generation Li-ion batteries. J. Power Sources 2023, 561, 232738. [Google Scholar] [CrossRef]
- Li, J.; Fleetwood, J.; Hawley, W.B.; Kays, W. From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300. [Google Scholar] [CrossRef]
- Gonçalves, R.; Lanceros-Méndez, S.; Costa, C.M. Electrode fabrication process and its influence in lithium-ion battery performance: State of the art and future trends. Electrochem. Commun. 2022, 135, 107210. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Kirsch, D.; Hu, L. Thick Electrode Batteries: Principles, Opportunities, and Challenges. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Arnot, D.J.; Mayilvahanan, K.S.; Hui, Z.; Takeuchi, K.J.; Marschilok, A.C.; Bock, D.C.; Wang, L.; West, A.C.; Takeuchi, E.S. Thick Electrode Design for Facile Electron and Ion Transport: Architectures, Advanced Characterization, and Modeling. Accounts Mater. Res. 2022, 3, 472–483. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Groult, H. Comparative Issues of Cathode Materials for Li-Ion Batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef]
- Jung, S.C.; Han, Y.-K. Monoclinic sulfur cathode utilizing carbon for high-performance lithium–sulfur batteries. J. Power Sources 2016, 325, 495–500. [Google Scholar]
- Yabuuchi, N.; Sugano, M.; Yamakawa, Y.; Nakai, I.; Sakamoto, K.; Muramatsu, H.; Komaba, S. Effect of heat-treatment process on FeF3 nanocomposite electrodes for rechargeable Li batteries. J. Mater. Chem. 2011, 21, 10035–10041. [Google Scholar] [CrossRef]
- Muralidharan, N.; Self, E.C.; Nanda, J.; Belharouak, I. Next-Generation Cobalt-Free Cathodes—A Prospective Solution to the Battery Industry’s Cobalt Problem. Transit. Met. Oxides Electrochem. Energy Storage 2022, 12, 33–53. [Google Scholar]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- Ciez, R.E.; Whitacre, J.F. Examining different recycling processes for lithium-ion batteries. Nat. Sustain. 2019, 2, 148–156. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Mohanty, D.; Li, J.; Nagpure, S.C.; Wood, D.L.; Daniel, C. Understanding the structure and structural degradation mechanisms in high-voltage, lithium-manganese–rich lithium-ion battery cathode oxides: A review of materials diagnostics. MRS Energy Sustain. 2015, 2, E15. [Google Scholar] [CrossRef]
- Ling, J.; Karuppiah, C.; Krishnan, S.G.; Reddy, M.V.; Misnon, I.I.; Ab Rahim, M.H.; Yang, C.-C.; Jose, R. Phosphate Polyanion Materials as High-Voltage Lithium-Ion Battery Cathode: A Review. Energy Fuels 2021, 35, 10428–10450. [Google Scholar] [CrossRef]
- Zhang, S.S. Problems and their origins of Ni-rich layered oxide cathode materials. Energy Storage Mater. 2020, 24, 247–254. [Google Scholar] [CrossRef]
- Jiang, M.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. A Review of Degradation Mechanisms and Recent Achievements for Ni-Rich Cathode-Based Li-Ion Batteries. Adv. Energy Mater. 2021, 11, 2103005. [Google Scholar] [CrossRef]
- Zhao, W.; Zou, L.; Zhang, L.; Fan, X.; Zhang, H.; Pagani, F.; Brack, E.; Seidl, L.; Ou, X.; Egorov, K.; et al. Assessing Long-Term Cycling Stability of Single-Crystal Versus Polycrystalline Nickel-Rich NCM in Pouch Cells with 6 mAh cm−2 Electrodes. Small 2022, 18, 2107357. [Google Scholar] [CrossRef]
- Park, G.-T.; Namkoong, B.; Kim, S.-B.; Liu, J.; Yoon, C.S.; Sun, Y.-K. Introducing high-valence elements into cobalt-free layered cathodes for practical lithium-ion batteries. Nat. Energy 2022, 7, 946–954. [Google Scholar] [CrossRef]
- Olbrich, L.F.; Xiao, A.W.; Pasta, M. Conversion-type fluoride cathodes: Current state of the art. Curr. Opin. Electrochem. 2021, 30, 100779. [Google Scholar] [CrossRef]
- Xu, J.; Ma, J.; Fan, Q.; Guo, S.; Dou, S. Recent Progress in the Design of Advanced Cathode Materials and Battery Models for High-Performance Lithium-X (X = O2, S, Se, Te, I2, Br2). Batter. Adv. Mater. 2017, 29, 1606454. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide Shuttle Study in the Li/S Battery System. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Edström, K.; Gustafsson, T.; Thomas, J. The cathode–electrolyte interface in the Li-ion battery. Electrochimica Acta 2004, 50, 397–403. [Google Scholar]
- Wu, H.; Chan, G.; Choi, J.W.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; Cui, Y. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nature Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Román-Ramírez, L.A.; Apachitei, G.; Faraji-Niri, M.; Lain, M.; Widanage, W.D.; Marco, J. Understanding the effect of coating-drying operating variables on electrode physical and electrochemical properties of lithium-ion batteries. J. Power Sources 2021, 516, 230689. [Google Scholar] [CrossRef]
- Defraeye, T. Advanced computational modelling for drying processes—A review. Appl. Energy 2014, 131, 323–344. [Google Scholar] [CrossRef]
- Westphal, B.G.; Kwade, A. Critical electrode properties and drying conditions causing component segregation in graphitic anodes for lithium-ion batteries. J. Energy Storage 2018, 18, 509–517. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Courtier, N.E.; Zhang, Z.; Liu, K.; Bailey, J.J.; Boyce, A.M.; Richardson, G.; Shearing, P.R.; Kendrick, E.; Brett, D.J.L. A Review of Lithium-Ion Battery Electrode Drying: Mechanisms and Metrology. Adv. Energy Mater. 2021, 12, 2102233. [Google Scholar] [CrossRef]
- Susarla, N.; Ahmed, S.; Dees, D.W. Modeling and analysis of solvent removal during Li-ion battery electrode drying. J. Power Sources 2018, 378, 660–670. [Google Scholar] [CrossRef]
- Stein, M.; Mistry, A.; Mukherjee, P.P. Mechanistic Understanding of the Role of Evaporation in Electrode Processing. J. Electrochem. Soc. 2017, 164, A1616–A1627. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, X.; Yu, G. Material and Structural Design of Novel Binder Systems for High-Energy, High-Power Lithium-Ion Batteries. Accounts Chem. Res. 2017, 50, 2642–2652. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liang, Z.; Kang, Y.; Zhou, Y.; Li, Y.; He, X.; Wang, L.; Mai, W.; Wang, X.; Zhou, G.; et al. Rational design of functional binder systems for high-energy lithium-based rechargeable batteries. Energy Storage Mater. 2021, 35, 353–377. [Google Scholar] [CrossRef]
- Chou, S.-L.; Pan, Y.; Wang, J.-Z.; Liu, H.K.; Dou, S.X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef]
- Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S. Exploring Chemical, Mechanical, and Electrical Functionalities of Binders for Advanced Energy-Storage Devices. Chem. Rev. 2018, 118, 8936–8982. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, C.; Pastel, G.; Li, Y.; Song, J.; Mi, R.; Kong, W.; Liu, B.; Jiang, Y.; Yang, K.; et al. Conductive Cellulose Nanofiber Enabled Thick Electrode for Compact and Flexible Energy Storage Devices. Adv. Energy Mater. 2018, 8, 1802398. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A Major Constituent of Brown Algae for Use in High-Capacity Li-Ion Batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef]
- Pieczonka, N.P.W.; Borgel, V.; Ziv, B.; Leifer, N.; Dargel, V.; Aurbach, D.; Kim, J.-H.; Liu, Z.; Huang, X.; Krachkovskiy, S.A.; et al. Lithium Polyacrylate (LiPAA) as an Advanced Binder and a Passivating Agent for High-Voltage Li-Ion Batteries. Adv. Energy Mater. 2015, 5, 1501008. [Google Scholar] [CrossRef]
- Le, A.V.; Wang, M.; Noelle, D.J.; Shi, Y.; Meng, Y.S.; Wu, D.; Fan, J.; Qiao, Y. Using high-HFP-content cathode binder for mitigation of heat generation of lithium-ion battery. Int. J. Energy Res. 2017, 41, 2430–2438. [Google Scholar] [CrossRef]
- Mu, P.; Zhang, H.; Jiang, H.; Dong, T.; Zhang, S.; Wang, C.; Li, J.; Ma, Y.; Dong, S.; Cui, G. Bioinspired Antiaging Binder Additive Addressing the Challenge of Chemical Degradation of Electrolyte at Cathode/Electrolyte Interphase. J. Am. Chem. Soc. 2021, 143, 18041–18051. [Google Scholar] [CrossRef] [PubMed]
- Maazi, S.; Navarchian, A.H.; Khosravi, M.; Chen, P. Effect of poly (vinylidene fluoride)/poly (vinyl acetate) blend composition as cathode binder on electrochemical performances of aqueous Li-ion battery. Solid State Ionics 2018, 320, 84–91. [Google Scholar] [CrossRef]
- Liu, S.; Zhong, H.; Zhang, C.; Yan, X.; Zhao, X.; Zhang, L. Improving the processability and cycling stability of nano-LiFePO4cathode by using PVDF/TX binary binder. Compos. Interfaces 2019, 26, 1013–1024. [Google Scholar] [CrossRef]
- Ahn, J.; Im, H.-G.; Lee, Y.; Lee, D.; Jang, H.; Oh, Y.; Chung, K.; Park, T.; Um, M.-K.; Yi, J.W.; et al. A novel organosilicon-type binder for LiCoO2 cathode in Li-ion batteries. Energy Storage Mater. 2022, 49, 58–66. [Google Scholar] [CrossRef]
- Xu, J.; Chou, S.-L.; Gu, Q.-f.; Liu, H.-K.; Dou, S.-X. The effect of different binders on electrochemical properties of LiNi1/3Mn1/3Co1/3O2 cathode material in lithium ion batteries. J. Power Sources 2013, 225, 172–178. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.; Yin, J.; Wang, H.; Yuan, X.; Li, Q. Effect of different binders on electrochemical properties of LiFePO4/C cathode material in lithium ion batteries. Chem. Eng. J. 2014, 237, 497–502. [Google Scholar] [CrossRef]
- Das, P.; Elizalde-Segovia, R.; Zayat, B.; Salamat, C.Z.; Pace, G.; Zhai, K.; Vincent, R.C.; Dunn, B.S.; Segalman, R.A.; Tolbert, S.H.; et al. Enhancing the Ionic Conductivity of Poly(3,4-propylenedioxythiophenes) with Oligoether Side Chains for Use as Conductive Cathode Binders in Lithium-Ion Batteries. Chem. Mater. 2022, 34, 2672–2686. [Google Scholar] [CrossRef]
- Pham, H.Q.; Kim, G.; Jung, H.M.; Song, S.-W. Fluorinated Polyimide as a Novel High-Voltage Binder for High-Capacity Cathode of Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1704690. [Google Scholar] [CrossRef]
- Dong, T.; Zhang, H.; Ma, Y.; Zhang, J.; Du, X.; Lu, C.; Shangguan, X.; Li, J.; Zhang, M.; Yang, J.; et al. A well-designed water-soluble binder enlightening the 5 V-class LiNi0.5Mn1.5O4 cathodes. J. Mater. Chem. A 2019, 7, 24594–24601. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, K.; Ma, J.; Xu, G.; Dong, S.; Chen, B.; Li, J.; Chen, Z.; Zhou, X.; Cui, G. A biomass based free radical scavenger binder endowing a compatible cathode interface for 5 V lithium-ion batteries. Energy Environ. Sci. 2019, 12, 273–280. [Google Scholar] [CrossRef]
- Ryou, M.-H.; Hong, S.; Winter, M.; Lee, H.; Choi, J.W. Improved cycle lives of LiMn2O4 cathodes in lithium ion batteries by an alginate biopolymer from seaweed. J. Mater. Chem. A 2013, 1, 15224–15229. [Google Scholar] [CrossRef]
- Brilloni, A.; Poli, F.; Spina, G.E.; Samorì, C.; Guidi, E.; Gualandi, C.; Maisuradze, M.; Giorgetti, M.; Soavi, F. Easy recovery of Li-ion cathode powders by the use of water-processable binders. Electrochimica Acta 2022, 418, 140376. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.-T.; Liu, J.; Deng, Y.-P.; Wu, Z.-G.; Yin, Z.-W.; Guo, D.; Huang, L.; Sun, S.-G. Suppressing the voltage-fading of layered lithium-rich cathode materials via an aqueous binder for Li-ion batteries. Chem. Commun. 2016, 52, 4683–4686. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Pang, Q.; Chen, X.; Dong, J.; Zhao, Y.; Lian, R.; Zhang, D.; Liu, B.; Chen, G.; Wei, Y. Lithium poly-acrylic acid as a fast Li+ transport media and a highly stable aqueous binder for Li3V2(PO4)3 cathode electrodes. J. Mater. Chem. A 2018, 6, 23357–23365. [Google Scholar] [CrossRef]
- Xia, J.; Wang, Z.; Rodrig, N.D.; Nan, B.; Zhang, J.; Zhang, W.; Lucht, B.L.; Yang, C.; Wang, C. Super-Reversible CuF(2) Cathodes Enabled by Cu(2+) -Coordinated Alginate. Adv. Mater. 2022, 34, e2205229. [Google Scholar] [CrossRef]
- Kim, S.; Cho, M.; Lee, Y. Multifunctional Chitosan–rGO Network Binder for Enhancing the Cycle Stability of Li–S Batteries. Adv. Funct. Mater. 2020, 30, 1907680. [Google Scholar] [CrossRef]
- Du, Z.; Rollag, K.M.; Li, J.; An, S.J.; Wood, M.; Sheng, Y.; Mukherjee, P.P.; Daniel, C.; Wood, D.L. Enabling aqueous processing for crack-free thick electrodes. J. Power Sources 2017, 354, 200–206. [Google Scholar] [CrossRef]
- Hawley, W.B.; Parejiya, A.; Bai, Y.; Meyer, H.M.; Wood, D.L.; Li, J. Lithium and transition metal dissolution due to aqueous processing in lithium-ion battery cathode active materials. J. Power Sources 2020, 466, 228315. [Google Scholar] [CrossRef]
- Loeffler, N.; Kim, G.T.; Mueller, F.; Diemant, T.; Kim, J.K.; Behm, R.J.; Passerini, S. In Situ Coating of Li[Ni0.33 Mn0.33 Co0.33 ]O2 Particles to Enable Aqueous Electrode Processing. ChemSusChem 2016, 9, 1112–1117. [Google Scholar] [CrossRef]
- Kuenzel, M.; Bresser, D.; Diemant, T.; Carvalho, D.V.; Kim, G.T.; Behm, R.J.; Passerini, S. Complementary Strategies Toward the Aqueous Processing of High-Voltage LiNi(0.5) Mn(1.5) O(4) Lithium-Ion Cathodes. ChemSusChem 2018, 11, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Sahore, R.; Wood, D.L.; Kukay, A.; Grady, K.M.; Li, J. Belharouak, Towards Understanding of Cracking during Drying of Thick Aqueous-Processed LiNi0.8Mn0.1Co0.1O2 Cathodes. ACS Sustain. Chem. Eng. 2020, 8, 3162–3169. [Google Scholar] [CrossRef]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage—The transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef]
- Lux, S.F.; Schappacher, F.; Balducci, A.; Passerini, S.; Winter, M. Low Cost, Environmentally Benign Binders for Lithium-Ion Batteries. J. Electrochem. Soc. 2010, 157, A320–A325. [Google Scholar] [CrossRef]
- Komaba, S.; Shimomura, K.; Yabuuchi, N.; Ozeki, T.; Yui, H.; Konno, K. Study on Polymer Binders for High-Capacity SiO Negative Electrode of Li-Ion Batteries. J. Phys. Chem. C 2011, 115, 13487–13495. [Google Scholar] [CrossRef]
- Bauer, W.; Çetinel, F.A.; Müller, M.; Kaufmann, U. Effects of pH control by acid addition at the aqueous processing of cathodes for lithium ion batteries. Electrochim. Acta 2019, 317, 112–119. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.-Z.; Yuan, H.; Hu, J.-K.; Huang, J.-Q.; Zhang, Q. Dry electrode technology, the rising star in solid-state battery industrialization. Matter 2022, 5, 876–898. [Google Scholar] [CrossRef]
- Liu, J.; Ludwig, B.; Liu, Y.; Zheng, Z.; Wang, F.; Tang, M.; Wang, J.; Wang, J.; Pan, H.; Wang, Y. Scalable Dry Printing Manufacturing to Enable Long-Life and High Energy Lithium-Ion Batteries. Adv. Mater. Technol. 2017, 2, 1700106. [Google Scholar] [CrossRef]
- Ludwig, B.; Liu, J.; Chen, I.M.; Liu, Y.; Shou, W.; Wang, Y.; Pan, H. Understanding Interfacial-Energy-Driven Dry Powder Mixing for Solvent-Free Additive Manufacturing of Li-Ion Battery Electrodes. Adv. Mater. Interfaces 2017, 4, 1700570. [Google Scholar] [CrossRef]
- Ludwig, B.; Zheng, Z.; Shou, W.; Wang, Y.; Pan, H. Solvent-Free Manufacturing of Electrodes for Lithium-ion Batteries. Sci. Rep. 2016, 6, 23150. [Google Scholar] [CrossRef]
- de la Torre-Gamarra, C.; Sotomayor, M.E.; Sanchez, J.-Y.; Levenfeld, B.; Várez, A.; Laïk, B.; Pereira-Ramos, J.-P. High mass loading additive-free LiFePO4 cathodes with 500 μm thickness for high areal capacity Li-ion batteries. J. Power Sources 2020, 458, 228033. [Google Scholar] [CrossRef]
- Maurel, A.; Grugeon, S.; Fleutot, B.; Courty, M.; Prashantha, K.; Tortajada, H.; Armand, M.; Panier, S.; Dupont, L. Three-Dimensional Printing of a LiFePO(4)/Graphite Battery Cell via Fused Deposition Modeling. Sci. Rep. 2019, 9, 18031. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Hippauf, F.; Schumm, B.; Doerfler, S.; Althues, H.; Fujiki, S.; Shiratsuchi, T.; Tsujimura, T.; Aihara, Y.; Kaskel, S. Overcoming binder limitations of sheet-type solid-state cathodes using a solvent-free dry-film approach. Energy Storage Mater. 2019, 21, 390–398. [Google Scholar] [CrossRef]
- Jiang, T.; He, P.; Liang, Y.; Fan, L.-Z. All-dry synthesis of self-supporting thin Li10GeP2S12 membrane and interface engineering for solid state lithium metal batteries. Chem. Eng. J. 2021, 421, 129965. [Google Scholar] [CrossRef]
- Nakamura, H.; Kawaguchi, T.; Masuyama, T.; Sakuda, A.; Saito, T.; Kuratani, K.; Ohsaki, S.; Watano, S. Dry coating of active material particles with sulfide solid electrolytes for an all-solid-state lithium battery. J. Power Sources 2019, 448, 227579. [Google Scholar] [CrossRef]
- Zheng, C.; Tang, S.; Wen, F.; Peng, J.; Yang, W.; Lv, Z.; Wu, Y.; Tang, W.; Gong, Z.; Yang, Y. Reinforced cathode-garnet interface for high-capacity all-solid-state batteries. Mater. Futur. 2022, 1, 045103. [Google Scholar] [CrossRef]
- Patry, G.; Romagny, A.; Martinet, S.; Froelich, D. Cost modeling of lithium-ion battery cells for automotive applications. Energy Sci. Eng. 2015, 3, 71–82. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Zhang, H.; Judez, X.; Adenusi, H.; Armand, M.; Passerini, S.; Figgemeier, E. Production of high-energy Li-ion batteries comprising silicon-containing anodes and insertion-type cathodes. Nat. Commun. 2021, 12, 5459. [Google Scholar] [CrossRef]
- Heubner, C.; Nikolowski, K.; Reuber, S.; Schneider, M.; Wolter, M.; Michaelis, A. Recent Insights into Rate Performance Limitations of Li-ion Batteries. Batter. Supercaps 2021, 4, 268–285. [Google Scholar] [CrossRef]
- Bo, Z.; Cheng, X.; Yang, H.; Guo, X.; Yan, J.; Cen, K.; Han, Z.; Dai, L. Ultrathick MoS2 Films with Exceptionally High Volumetric Capacitance. Adv. Energy Mater. 2022, 12, 2103394. [Google Scholar] [CrossRef]
- Lu, X.; Daemi, S.R.; Bertei, A.; Kok, M.D.R.; O’Regan, K.B.; Rasha, L.; Park, J.; Hinds, G.; Kendrick, E.; Brett, D.J.L.; et al. Microstructural Evolution of Battery Electrodes During Calendering. Joule 2020, 4, 2746–2768. [Google Scholar] [CrossRef]
- Meyer, C.; Bockholt, H.; Haselrieder, W.; Kwade, A. Characterization of the calendering process for compaction of electrodes for lithium-ion batteries. J. Mater. Process. Technol. 2017, 249, 172–178. [Google Scholar] [CrossRef]
- Shodiev, A.; Chouchane, M.; Gaberscek, M.; Arcelus, O.; Xu, J.; Oularbi, H.; Yu, J.; Li, J.; Morcrette, M.; Franco, A.A. Deconvoluting the benefits of porosity distribution in layered electrodes on the electrochemical performance of Li-ion batteries. Energy Storage Mater. 2022, 47, 462–471. [Google Scholar] [CrossRef]
- Boyce, A.M.; Lu, X.; Brett, D.J.; Shearing, P.R. Exploring the influence of porosity and thickness on lithium-ion battery electrodes using an image-based model. J. Power Sources 2022, 542, 231779. [Google Scholar] [CrossRef]
- Bläubaum, L.; Röder, F.; Nowak, C.; Chan, H.S.; Kwade, A.; Krewer, U. Impact of Particle Size Distribution on Performance of Lithium-Ion Batteries. Chemelectrochem 2020, 7, 4755–4766. [Google Scholar] [CrossRef]
- Qiu, B.; Zhang, M.; Wu, L.; Wang, J.; Xia, Y.; Qian, D.; Liu, H.; Hy, S.; Chen, Y.; An, K.; et al. Gas–solid interfacial modification of oxygen activity in layered oxide cathodes for lithium-ion batteries. Nat. Commun. 2016, 7, 12108. [Google Scholar] [CrossRef]
- Sun, H.H.; Ryu, H.-H.; Kim, U.-H.; Weeks, J.A.; Heller, A.; Sun, Y.-K.; Mullins, C.B. Beyond Doping and Coating: Prospective Strategies for Stable High-Capacity Layered Ni-Rich Cathodes. ACS Energy Lett. 2020, 5, 1136–1146. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, K.; Lu, L. Cycling effects on surface morphology, nanomechanical and interfacial reliability of LiMn2O4 cathode in thin film lithium ion batteries. Electrochimica Acta 2012, 68, 52–59. [Google Scholar] [CrossRef]
- Wang, R.; Chen, X.; Huang, Z.; Yang, J.; Liu, F.; Chu, M.; Liu, T.; Wang, C.; Zhu, W.; Li, S.; et al. Twin boundary defect engineering improves lithium-ion diffusion for fast-charging spinel cathode materials. Nat. Commun. 2021, 12, 3085. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Tang, Z.-K.; Devaraj, A.; Chen, G.; Amine, K.; Zhang, J.-G.; Liu, L.-M.; Wang, C. Injection of oxygen vacancies in the bulk lattice of layered cathodes. Nat. Nanotechnol. 2019, 14, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Zhang, S.; Di, A.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Challenges and Strategies towards Single-Crystalline Ni-Rich Layered Cathodes. Adv. Energy Mater. 2022, 12, 2201510. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, R.; Zhou, K.; Gao, Z.; He, W.; Zhang, L.; Han, C.; Kang, F.; Li, B. A Comparative Investigation of Single Crystal and Polycrystalline Ni-Rich NCMs as Cathodes for Lithium-Ion Batteries. Energy Environ. Mater. 2022. ahead of print. [Google Scholar] [CrossRef]
- Trevisanello, E.; Ruess, R.; Conforto, G.; Richter, F.H.; Janek, J. Polycrystalline and Single Crystalline NCM Cathode Materials—Quantifying Particle Cracking, Active Surface Area, and Lithium Diffusion. Adv. Energy Mater. 2021, 11, 2003400. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Namkoong, B.; Kim, J.-H.; Belharouak, I.; Yoon, C.S.; Sun, Y.-K. Capacity Fading Mechanisms in Ni-Rich Single-Crystal NCM Cathodes. ACS Energy Lett. 2021, 6, 2726–2734. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Yang, Y.; Mu, L.; Wei, C.; Yu, X.; Pianetta, P.; Zhao, K.; Cloetens, P.; Lin, F.; et al. Machine-learning-revealed statistics of the particle-carbon/binder detachment in lithium-ion battery cathodes. Nat. Commun. 2020, 11, 2310. [Google Scholar] [CrossRef]
- Lim, J.; Li, Y.; Alsem, D.H.; So, H.; Lee, S.C.; Bai, P.; Cogswell, D.A.; Liu, X.; Jin, N.; Yu, Y.-S.; et al. Origin and hysteresis of lithium compositional spatiodynamics within battery primary particles. Science 2016, 353, 566–571. [Google Scholar] [CrossRef]
- Wood, M.; Li, J.; Du, Z.; Daniel, C.; Dunlop, A.R.; Polzin, B.J.; Jansen, A.N.; Krumdick, G.K.; Wood, D.L. Impact of secondary particle size and two-layer architectures on the high-rate performance of thick electrodes in lithium-ion battery pouch cells. J. Power Sources 2021, 515, 230429. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, J.; Sun, K.; Wang, Z. Balancing particle properties for practical lithium-ion batteries. Particuology 2022, 61, 18–29. [Google Scholar] [CrossRef]
- Röder, F.; Sonntag, S.; Schröder, D.; Krewer, U. Simulating the Impact of Particle Size Distribution on the Performance of Graphite Electrodes in Lithium-Ion Batteries. Energy Technol. 2016, 4, 1588–1597. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, X.; Tan, C.; Heenan, T.M.M.; Lagnoni, M.; O'Regan, K.; Daemi, S.; Bertei, A.; Jones, H.G.; Hinds, G.; et al. Multi-length scale microstructural design of lithium-ion battery electrodes for improved discharge rate performance. Energy Environ. Sci. 2021, 14, 5929–5946. [Google Scholar] [CrossRef]
- Zan, G.; Qian, G.; Gul, S.; Li, J.; Matusik, K.; Wang, Y.; Lewis, S.; Yun, W.; Pianetta, P.; Vine, D.J.; et al. In situ visualization of multicomponents coevolution in a battery pouch cell. Proc. Natl. Acad. Sci. USA 2022, 119, e2203199119. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Liang, J.; Cui, C.; Zhai, T.; Li, H. Dynamic Investigation of Battery Materials via Advanced Visualization: From Particle, Electrode to Cell Level. Adv. Mater. 2022, 34, 2200777. [Google Scholar] [CrossRef] [PubMed]
- Park, G.-T.; Ryu, H.-H.; Noh, T.-C.; Kang, G.-C.; Sun, Y.-K. Microstructure-optimized concentration-gradient NCM cathode for long-life Li-ion batteries. Mater. Today 2021, 52, 9–18. [Google Scholar] [CrossRef]
- Wu, S.; Yu, B.; Wu, Z.; Fang, S.; Shi, B.; Yang, J. Effect of particle size distribution on the electrochemical performance of micro-sized silicon-based negative materials. RSC Adv. 2018, 8, 8544–8551. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.H. Enhancing electrode wettability in lithium-ion battery via particle-size ratio control. Appl. Mater. Today 2021, 22, 100976. [Google Scholar] [CrossRef]
- Parmananda, M.; Norris, C.; Roberts, S.A.; Mukherjee, P.P. Probing the Role of Multi-scale Heterogeneity in Graphite Electrodes for Extreme Fast Charging. ACS Appl. Mater. Interfaces 2022, 14, 18335–18352. [Google Scholar] [CrossRef]
- Li, J.; Sharma, N.; Jiang, Z.; Yang, Y.; Monaco, F.; Xu, Z.; Hou, D.; Ratner, D.; Pianetta, P.; Cloetens, P.; et al. Dynamics of particle network in composite battery cathodes. Science 2022, 376, 517–521. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Villanova, J.; Su, Z.; Tucoulou, R.; Fleutot, B.; Delobel, B.; Delacourt, C.; Demortière, A. 3D Quantification of Microstructural Properties of LiNi0.5Mn0.3Co0.2O2 High-Energy Density Electrodes by X-ray Holographic Nano-Tomography. Adv. Energy Mater. 2021, 11, 2003529. [Google Scholar] [CrossRef]
- Song, J.; Park, J.; Appiah, W.A.; Kim, S.-S.; Munakata, H.; Kanamura, K.; Ryou, M.-H.; Lee, Y.M. 3D electrochemical model for a Single Secondary Particle and its application for operando analysis. Nano Energy 2019, 62, 810–817. [Google Scholar] [CrossRef]
- Sarawutanukul, S.; Tomon, C.; Phattharasupakun, N.; Duangdangchote, S.; Duriyasart, F.; Chiochan, P.; Sawangphruk, M. Optimization of the Electrode Properties for High-Performance Ni-Rich Li-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 30643–30652. [Google Scholar] [CrossRef]
- Mayer, J.K.; Almar, L.; Asylbekov, E.; Haselrieder, W.; Kwade, A.; Weber, A.; Nirschl, H. Influence of the Carbon Black Dispersing Process on the Microstructure and Performance of Li-Ion Battery Cathodes. Energy Technol. 2019, 8, 1900161. [Google Scholar] [CrossRef]
- Chauhan, A.; Asylbekov, E.; Kespe, S.; Nirschl, H. Influence of carbon binder domain on the performance of lithium-ion batteries: Impact of size and fractal dimension. Electrochem. Sci. Adv. 2022, 3, e2100151. [Google Scholar] [CrossRef]
- Ngandjong, A.C.; Lombardo, T.; Primo, E.N.; Chouchane, M.; Shodiev, A.; Arcelus, O.; Franco, A.A. Investigating electrode calendering and its impact on electrochemical performance by means of a new discrete element method model: Towards a digital twin of Li-Ion battery manufacturing. J. Power Sources 2021, 485, 229320. [Google Scholar] [CrossRef]
- Song, K.; Li, W.; Chen, Z.; Wu, X.; Zhou, Q.; Snyder, K.; Zhang, L. An effective approach to improve electrochemical performance of thick electrodes. Ionics 2021, 27, 1261–1270. [Google Scholar] [CrossRef]
- Ju, Z.; Zhang, X.; Wu, J.; King, S.T.; Chang, C.-C.; Yan, S.; Xue, Y.; Takeuchi, K.J.; Marschilok, A.C.; Wang, L.; et al. Tortuosity Engineering for Improved Charge Storage Kinetics in High-Areal-Capacity Battery Electrodes. Nano Lett. 2022, 22, 6700–6708. [Google Scholar] [CrossRef] [PubMed]
- Ebner, M.; Chung, D.-W.; García, R.E.; Wood, V. Tortuosity Anisotropy in Lithium-Ion Battery Electrodes. Adv. Energy Mater. 2014, 4, 1301278. [Google Scholar] [CrossRef]
- Kikukawa, H.; Honkura, K.; Koyama, M. Influence of inter-particle resistance between active materials on the discharge characteristics of the positive electrode of lithium ion batteries. Electrochim. Acta 2018, 278, 385–395. [Google Scholar] [CrossRef]
- Xiong, R.; Zhang, Y.; Wang, Y.; Song, L.; Li, M.; Yang, H.; Huang, Z.; Li, D.; Zhou, H. Scalable Manufacture of High-Performance Battery Electrodes Enabled by a Template-Free Method. Small Methods 2021, 5, 2100280. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, C.; Fan, L.; Zhang, N.; Sun, K. Iron fluoride vertical nanosheets array modified with graphene quantum dots as long-life cathode for lithium ion batteries. Chem. Eng. J. 2019, 371, 245–251. [Google Scholar] [CrossRef]
- Zhang, X.; Hui, Z.; King, S.T.; Wu, J.; Ju, Z.; Takeuchi, K.J.; Marschilok, A.C.; West, A.C.; Takeuchi, E.S.; Wang, L.; et al. Gradient Architecture Design in Scalable Porous Battery Electrodes. Nano Lett. 2022, 22, 2521–2528. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Bertei, A.; Finegan, D.P.; Tan, C.; Daemi, S.R.; Weaving, J.S.; O’Regan, K.B.; Heenan, T.M.M.; Hinds, G.; Kendrick, E.; et al. 3D microstructure design of lithium-ion battery electrodes assisted by X-ray nano-computed tomography and modelling. Nat. Commun. 2020, 11, 2079. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Slater, P.R.; Kendrick, E. Insights into architecture, design and manufacture of electrodes for lithium-ion batteries. Mater. Des. 2022, 223, 111208. [Google Scholar] [CrossRef]
- Thorat, I.V.; Stephenson, D.E.; Zacharias, N.A.; Zaghib, K.; Harb, J.N.; Wheeler, D.R. Quantifying tortuosity in porous Li-ion battery materials. J. Power Sources 2009, 188, 592–600. [Google Scholar] [CrossRef]
- Landesfeind, J.; Hattendorff, J.; Ehrl, A.; Wall, W.A.; Gasteiger, H.A. Tortuosity Determination of Battery Electrodes and Separators by Impedance Spectroscopy. J. Electrochem. Soc. 2016, 163, A1373. [Google Scholar] [CrossRef]
- Parikh, D.; Christensen, T.; Li, J. Correlating the influence of porosity, tortuosity, and mass loading on the energy density of LiNi0.6Mn0.2Co0.2O2 cathodes under extreme fast charging (XFC) conditions. J. Power Sources 2020, 474, 228601. [Google Scholar] [CrossRef]
- Bae, C.-J.; Erdonmez, C.K.; Halloran, J.W.; Chiang, Y.-M. Design of Battery Electrodes with Dual-Scale Porosity to Minimize Tortuosity and Maximize Performance. Adv. Mater. 2013, 25, 1254–1258. [Google Scholar] [CrossRef]
- Elango, R.; Nadeina, A.; Cadiou, F.; de Andrade, V.; Demortière, A.; Morcrette, M.; Seznec, V. Impact of electrode porosity architecture on electrochemical performances of 1 mm-thick LiFePO4 binder-free Li-ion electrodes fabricated by Spark Plasma Sintering. J. Power Sources 2021, 488, 229402. [Google Scholar] [CrossRef]
- Kim, Y.; Drews, A.; Chandrasekaran, R.; Miller, T.; Sakamoto, J. Improving Li-ion battery charge rate acceptance through highly ordered hierarchical electrode design. Ionics 2018, 24, 2935–2943. [Google Scholar] [CrossRef]
- Huang, C.; Grant, P.S. Coral-like directional porosity lithium ion battery cathodes by ice templating. J. Mater. Chem. A 2018, 6, 14689–14699. [Google Scholar] [CrossRef]
- Huang, C.; Dontigny, M.; Zaghib, K.; Grant, P.S. Low-tortuosity and graded lithium ion battery cathodes by ice templating. J. Mater. Chem. A 2019, 7, 21421–21431. [Google Scholar] [CrossRef]
- Hyun, G.; Cao, S.; Ham, Y.; Youn, D.-Y.; Kim, I.-D.; Chen, X.; Jeon, S. Three-Dimensional, Submicron Porous Electrode with a Density Gradient to Enhance Charge Carrier Transport. ACS Nano 2022, 16, 9762–9771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hui, Z.; King, S.; Wang, L.; Ju, Z.; Wu, J.; Takeuchi, K.J.; Marschilok, A.C.; West, A.C.; Takeuchi, E.S.; et al. Tunable Porous Electrode Architectures for Enhanced Li-Ion Storage Kinetics in Thick Electrodes. Nano Lett. 2021, 21, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Yari, S.; Hamed, H.; D’haen, J.; Van Bael, M.K.; Renner, F.U.; Hardy, A.; Safari, M. Constructive versus Destructive Heterogeneity in Porous Electrodes of Lithium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 11820–11829. [Google Scholar] [CrossRef]
- Gao, H.; Wu, Q.; Hu, Y.; Zheng, J.P.; Amine, K.; Chen, Z. Revealing the Rate-Limiting Li-Ion Diffusion Pathway in Ultrathick Electrodes for Li-Ion Batteries. J. Phys. Chem. Lett. 2018, 9, 5100–5104. [Google Scholar] [CrossRef]
- Park, J.; Jeon, C.; Kim, W.; Bong, S.-J.; Jeong, S.; Kim, H.-J. Challenges, laser processing and electrochemical characteristics on application of ultra-thick electrode for high-energy lithium-ion battery. J. Power Sources 2021, 482, 228948. [Google Scholar] [CrossRef]
- Lu, L.-L.; Lu, Y.-Y.; Zhu, Z.-X.; Shao, J.-X.; Yao, H.-B.; Wang, S.; Zhang, T.-W.; Ni, Y.; Wang, X.-X.; Yu, S.-H. Extremely fast-charging lithium ion battery enabled by dual-gradient structure design. Sci. Adv. 2022, 8, eabm6624. [Google Scholar] [CrossRef]
- Wu, J.; Ju, Z.; Zhang, X.; Xu, X.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S.; Yu, G. Low-Tortuosity Thick Electrodes with Active Materials Gradient Design for Enhanced Energy Storage. ACS Nano 2022, 16, 4805–4812. [Google Scholar] [CrossRef]
- Ma, Y. Computer Simulation of Cathode Materials for Lithium Ion and Lithium Batteries: A Review. Energy Environ. Mater. 2018, 1, 148–173. [Google Scholar] [CrossRef]
- Lv, C.; Zhou, X.; Zhong, L.; Yan, C.; Srinivasan, M.; Seh, Z.W.; Liu, C.; Pan, H.; Li, S.; Wen, Y.; et al. Machine Learning: An Advanced Platform for Materials Development and State Prediction in Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2101474. [Google Scholar] [CrossRef]
- Liu, K.; Wei, Z.; Zhang, C.; Shang, Y.; Teodorescu, R.; Han, Q.-L. Towards Long Lifetime Battery: AI-Based Manufacturing and Management. IEEE/CAA J. Autom. Sin. 2022, 9, 1139–1165. [Google Scholar] [CrossRef]


| Binder | Solvent | Cathode | Cycle Number | Retention | Ref. |
|---|---|---|---|---|---|
| PVDF | NMP | NCM111 | 200 @0.5C | 86.3% | [56] |
| PVDF-HFP | NMP | NCM523 | 330 @1C | 50% | [51] |
| Organosilicon binder | NMP | LCO | 100 @0.5C | 92% | [55] |
| PMMA | NMP | LFP | 120 @1C | 86.3% | [57] |
| (Hex:OE)PProDOTs | NMP | NCA | 200 @C | 87.9% | [58] |
| Fluorinated Polyimide | NMP | NCM | 100 @0.2C | 89% | [59] |
| PVDF-PS | NMP | NCM532 | 400 @1C | 90% | [52] |
| Cellulose nanofibers | water | LFP | 150 @2mA cm−2 | 90% | [48] |
| P(MVE-LMA) | water | LNMO | 400 @1C | 92% | [60] |
| Lignin | alkaline water | LNMO | 1000 @1C | 94.1% | [61] |
| Alginate | water | LMO | 120 @1C | 97.7% | [62] |
| Pullulan | water | NCM523 | 500 @0.33C, 1C | 83.33% | [63] |
| Guar gum | water | LNMO | 250 @20 mA g−1 | 95.2% | [64] |
| Li-PAA | water | LVP | 300 @1C | 97.4% | [65] |
| Sodium alginate | water | CuF2 | 50 @0.05C | 68.23% | [66] |
| rGO-Chitosan | aqueous acetic acid (1.5%) | S | 1000 @1C | 84% | [67] |
| CMC/acrylic emulsion | water/isopropyl alcohol | NCM523 | 100 @0.33C | 97.3% | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.; Wang, Y.; Kong, K.; Kim, D.; Wang, F.; Yushin, G. Materials and Processing of Lithium-Ion Battery Cathodes. Nanoenergy Adv. 2023, 3, 138-154. https://doi.org/10.3390/nanoenergyadv3020008
Fu W, Wang Y, Kong K, Kim D, Wang F, Yushin G. Materials and Processing of Lithium-Ion Battery Cathodes. Nanoenergy Advances. 2023; 3(2):138-154. https://doi.org/10.3390/nanoenergyadv3020008
Chicago/Turabian StyleFu, Wenbin, Yice Wang, Kanglin Kong, Doyoub Kim, Fujia Wang, and Gleb Yushin. 2023. "Materials and Processing of Lithium-Ion Battery Cathodes" Nanoenergy Advances 3, no. 2: 138-154. https://doi.org/10.3390/nanoenergyadv3020008
APA StyleFu, W., Wang, Y., Kong, K., Kim, D., Wang, F., & Yushin, G. (2023). Materials and Processing of Lithium-Ion Battery Cathodes. Nanoenergy Advances, 3(2), 138-154. https://doi.org/10.3390/nanoenergyadv3020008







