Uncovering the Quality Deficiencies with Potentially Harmful Effects in Substandard and Falsified PDE-5 Inhibitors Seized by Belgian Controlling Agencies
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Physical Characteristics of the Samples
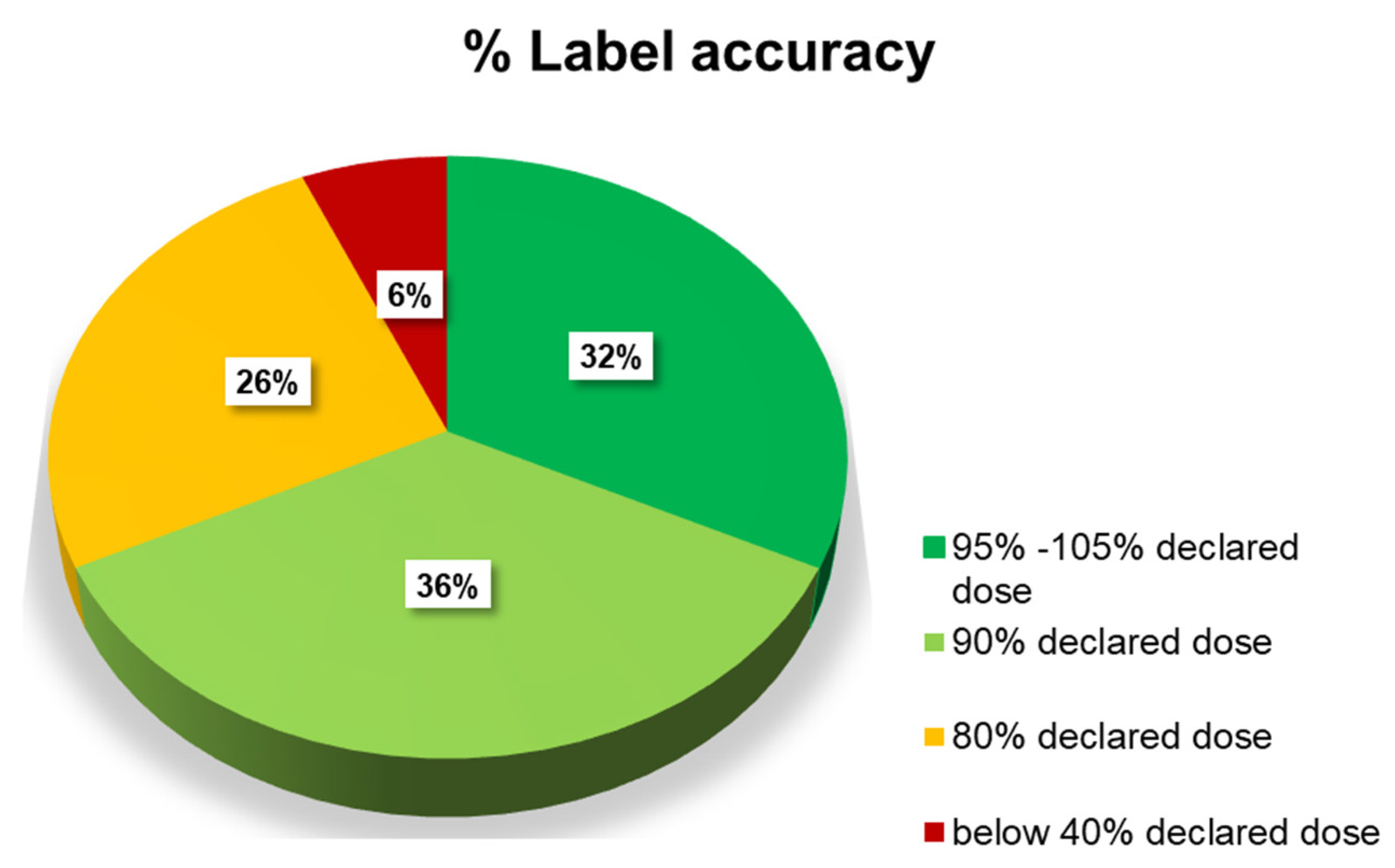
3.2. Identification and Quantification of the Detected APIs
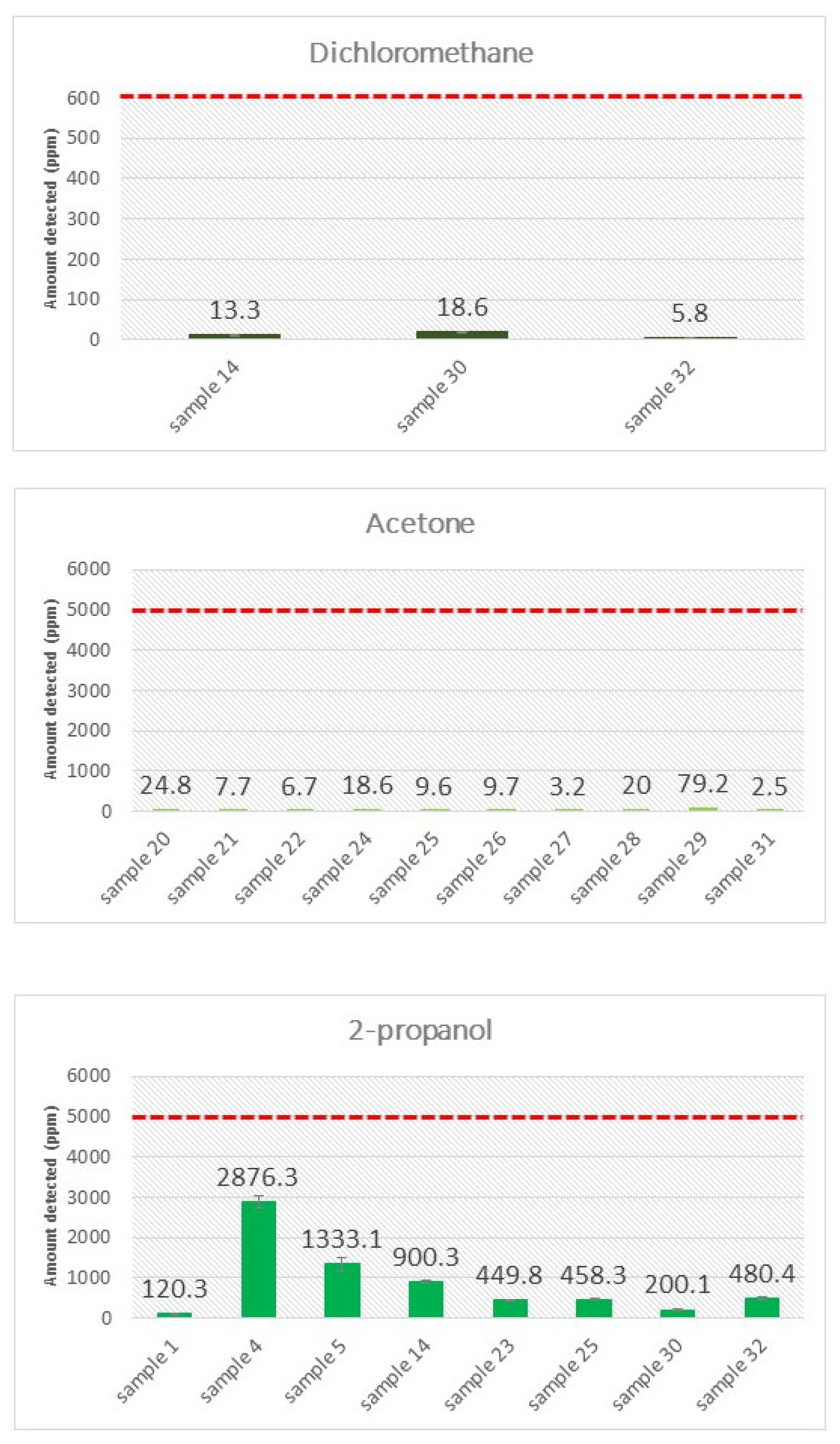
3.3. Screening and Quantification of Residual Solvents
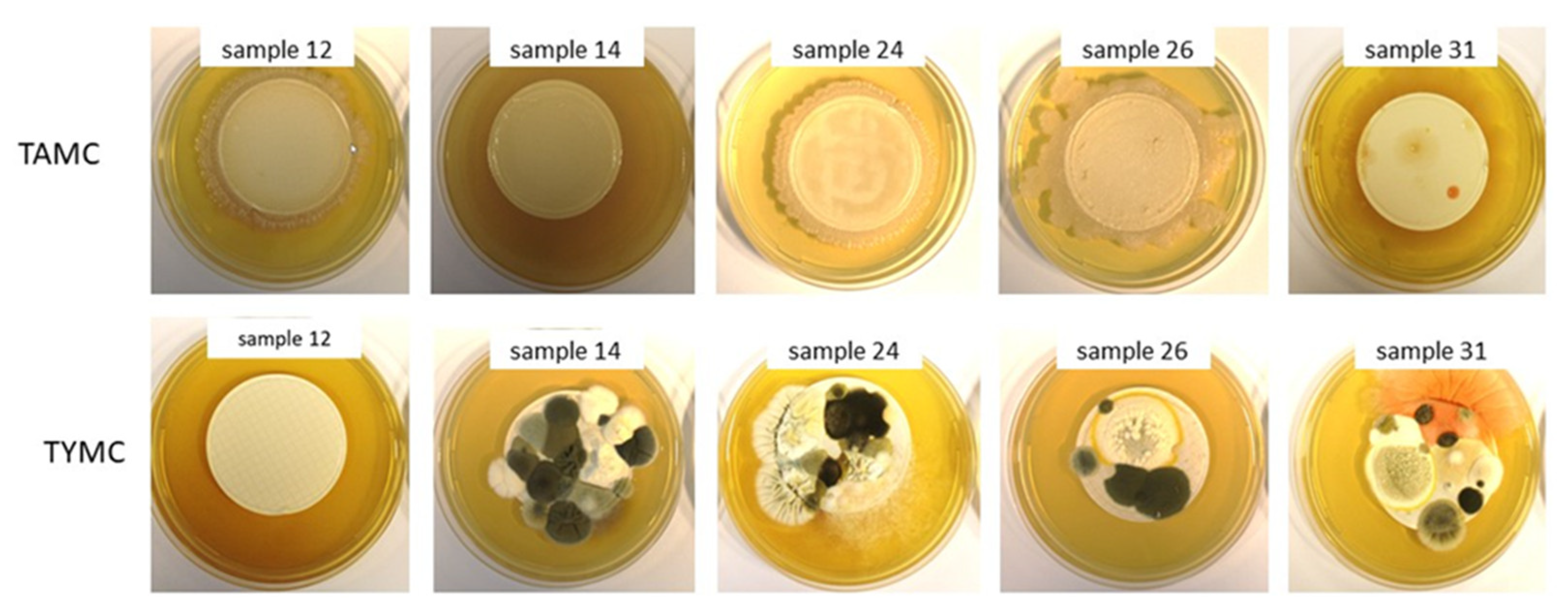
3.4. Bioburden Determination and Identification of the Microorganisms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD; EUIPO. Trade in Counterfeit Pharmaceutical Products, Illicit Trade; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- INTERPOL. USD11 Million in Illicit Mediicnes Seized in Global INTERPOL Operation. Available online: https://www.interpol.int/en/News-and-Events/News/2022/USD-11-million-in-illicit-medicines-seized-in-global-INTERPOL-operation (accessed on 18 January 2023).
- Pharmaceutical Security Institute. Available online: https://www.psi-inc.org/incident-trends (accessed on 18 January 2023).
- World Health Organization. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/handle/10665/326708 (accessed on 18 January 2023).
- Fadeyi, I.; Lalani, M.; Mailk, N.; Van Wyk, A.; Kaur, H. Quality of the antibiotics—Amoxicillin and co-trimoxazole from Ghana, Nigeria, and the United Kingdom. Am. J. Trop Med. Hyg. 2015, 92, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, H.; Allan, E.L.; Mamadu, I.; Hall, Z.; Green, M.D.; Swamidos, I.; Dwivedi, P.; Culzoni, M.J.; Fernandez, F.M.; Garcia, G.; et al. Prevalence of substandard and falsified artemisinin-based combination antimalarial medicines on Bioko Island, Equatorial Guinea. BMJ Glob. Health 2017, 2, e000409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frimpong, G.; Ofori-Kwakye, K.; Kuntworbe, N.; Buabeng, K.O.; Osei, Y.A.; Boakye-Gyasi, M.E.; Adi-Dako, O. Quality Assessment of Some Essential Children’s Medicines Sold in Licensed Outlets in Ashanti Region, Ghana. J. Trop Med. 2018, 2018, 1494957. [Google Scholar] [CrossRef] [Green Version]
- Abuye, H.; Abraham, W.; Kebede, S.; Tatiparthi, R.; Suleman, S. Physicochemical Quality Assessment of Antimalarial Medicines: Chloroquine Phosphate and Quinine Sulfate Tablets from Drug Retail Outlets of South-West Ethiopia. Infect. Drug Resist. 2020, 13, 691–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irungu, B.N.; Koech, L.C.; Ondicho, J.M.; Keter, L.K. Quality assessment of selected co-trimoxazole suspension brands marketed in Nairobi County, Kenya. PLoS ONE 2021, 16, e0257625. [Google Scholar] [CrossRef]
- Chiumia, F.K.; Nyirongo, H.M.; Kampira, E.; Muula, A.S.; Khuluza, F. Burden of and factors associated with poor quality antibiotic, antimalarial, antihypertensive and antidiabetic medicines in Malawi. PLoS ONE 2022, 17, e0279637. [Google Scholar] [CrossRef]
- Adigwe, O.P.; Onavbavba, G.; Wilson, D.O. Challenges Associated with Addressing Counterfeit Medicines in Nigeria: An Exploration of Pharmacists’ Knowledge, Practices, and Perceptions. Integr. Pharm. Res. Pract. 2022, 11, 177–186. [Google Scholar] [CrossRef]
- Deconinck, E.; Vanhee, C.; Keizers, P.; Guinot, P.; Mihailova, A.; Syversen, P.V.; Li-Ship, G.; Young, S.; Blazewicz, A.; Poplawska, M.; et al. The occurrence of non-anatomical therapeutic chemical-international nonproprietary name molecules in suspected illegal or illegally traded health products in Europe: A retrospective and prospective study. Drug Test. Anal. 2021, 13, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.L.; Chan, C.L.; Tan, B.; Lim, C.C.; Dalan, R.; Gardner, D.; Pratt, E.; Lee, M.; Lee, K.O. An unusual outbreak of hypoglycemia. N. Engl. J. Med. 2009, 360, 734–736. [Google Scholar] [CrossRef]
- Jackson, G.; Arver, S.; Banks, I.; Stecher, V.J. Counterfeit phosphodiesterase type 5 inhibitors pose significant safety risks. Int. J. Clin. Pract. 2010, 64, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Deconinck, E.; Canfyn, M.; Sacré, P.Y.; Courselle, P.; De Beer, J.O. Evaluation of the residual solvent content of counterfeit tablets and capsules. J. Pharm. Biomed. Anal. 2013, 81–82, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Veronin, M.A.; Nutan, M.T.; Dodla, U.K. Quantification of active pharmaceutical ingredient and impurities in sildenafil citrate obtained from the Internet. Ther. Adv. Drug Saf. 2014, 5, 180–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, D.B.D.J.; Caldas, E.D. GC-MS quantitative analysis of black market pharmaceutical products containing anabolic androgenic steroids seized by the Brazilian Federal Police. Forensic. Sci. Int. 2017, 275, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Krug, O.; Kamber, M.; Thevis, M. Qualitative and Semiquantitative Analysis of Doping Products Seized at the Swiss Border. Subst. Use Misuse. 2017, 52, 742–753. [Google Scholar] [CrossRef]
- Vanhee, C.; Janvier, S.; Moens, G.; Goscinny, S.; Courselle, P.; Deconinck, E. Identification of epidermal growth factor (EGF), in an unknown pharmaceutical preparation suspected to contain insulin like growth factor 1 (IGF-1). Drug Test Anal. 2017, 9, 831–837. [Google Scholar] [CrossRef]
- Janvier, S.; Cheyns, K.; Canfyn, M.; Goscinny, S.; De Spiegeleer, B.; Vanhee, C.; Deconinck, E. Impurity profiling of the most frequently encountered falsified polypeptide drugs on the Belgian market. Talanta 2018, 188, 795–807. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Kuczyńska, K.; Kosmal, W.; Markiewicz, K.; Adamowicz, P. Carfentanil—From an animal anesthetic to a deadly illicit drug. Forensic. Sci. Int. 2021, 320, 110715. [Google Scholar] [CrossRef]
- Chapman, B.P.; Lai, J.T.; Krotulski, A.J.; Fogarty, M.F.; Griswold, M.K.; Logan, B.K.; Babu, K.M. A Case of Unintentional Opioid (U-47700) Overdose in a Young Adult After Counterfeit Xanax Use. Pediatr. Emerg. Care. 2021, 37, e579–e580. [Google Scholar] [CrossRef]
- Fabresse, N.; Gheddar, L.; Kintz, P.; Knapp, A.; Larabi, I.A.; Alvarez, J.C. Analysis of pharmaceutical products and dietary supplements seized from the black market among bodybuilders. Forensic. Sci. Int. 2021, 322, 110771. [Google Scholar] [CrossRef]
- Cohen, P.A.; Travis, J.C.; Vanhee, C.; Ohana, D.; Venhuis, B.J. Nine prohibited stimulants found in sports and weight loss supplements: Deterenol, phenpromethamine (Vonedrine), oxilofrine, octodrine, beta-methylphenylethylamine (BMPEA), 1,3-dimethylamylamine (1,3-DMAA), 1,4-dimethylamylamine (1,4-DMAA), 1,3-dimethylbutylamine (1,3-DMBA) and higenamine. Clin. Toxicol. 2021, 59, 975–981. [Google Scholar] [CrossRef]
- Wang, F.; Yu, S.; Liu, K.; Chen, F.E.; Song, Z.; Zhang, X.; Xu, X.; Sun, X. Acute intraocular inflammation caused by endotoxin after intravitreal injection of counterfeit bevacizumab in Shanghai, China. Ophthalmology 2013, 120, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Pullirsch, D.; Bellemare, J.; Hackl, A.; Trottier, Y.L.; Mayrhofer, A.; Schindl, H.; Taillon, C.; Gartner, C.; Hottowy, B.; Beck, G.; et al. Microbiological contamination in counterfeit and unapproved drugs. BMC Pharmacol. Toxicol. 2014, 15, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janvier, S.; Wattijn, E.; Botteldoorn, N.; De Spiegeleer, B.; Deconinck, E.; Vanhee, C. Are injectable illegal polypeptide drugs safe? Case report demonstrating the presence of haemolytic Bacillus cereus in 2 illegal peptide drugs. Drug Test. Anal. 2018, 10, 791–795. [Google Scholar] [CrossRef]
- Tie, Y.; Adams, E.; Deconinck, E.; Vanhee, C. Substandard and falsified antimicrobials: A potential biohazard in disguise? Drug Test. Anal. 2020, 12, 285–291. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines and Health Care. Available online: https://www.edqm.eu/en/know-x (accessed on 18 January 2023).
- Vanhee, C.; Tuenter, E.; Kamugisha, A.; Canfyn, M.; Moens, G.; Courselle, P.; Pieters, L.; Deconinck, E.; Exarchou, V. Identification and Quantification Methodology for the Analysis of Suspected Illegal Dietary Supplements: Reference Standard or no Reference Standard, that’s the Question. J. Forensic. Toxicol. Pharmacol. 2018, 7, 1. [Google Scholar] [CrossRef]
- Sacré, P.Y.; Deconinck, E.; Chiap, P.; Crommen, J.; Mansion, F.; Rozet, E.; Courselle, P.; De Beer, J.O. Development and validation of a ultra-high-performance liquid chromatography-UV method for the detection and quantification of erectile dysfunction drugs and some of their analogues found in counterfeit medicines. J. Chromatogr. A 2011, 1218, 6439–6447. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 15. [Google Scholar] [CrossRef] [Green Version]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, 1. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High troughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [Green Version]
- Carroll, L.M.; Cheng, R.A.; Kovac, J. No Assembly Required: Using BTyper3 to assess the congruency of a proposed taxonomic framework for the Bacillus cereus group with historical typing methods. Front. Microbiol. 2020, 11, 580691. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; Garcia-Fernandez, A.; Larsen, M.V.; Lund, O.; Villa, L.; Aarestrup, F.M.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wattiau, P.; Klee, S.R.; Fretin, D.; Van Hessche, M.; Ménart, M.; Franz, T.; Chasseur, C.; Butaye, P.; Imberechts, H. Occurrence and genetic diversity of Bacillus anthracis strains isolated in an active wool-cleaning factory. Appl. Environ. Microbiol. 2008, 74, 4005–4011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute Document M45. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. Available online: https://clsi.org/standards/products/microbiology/documents/m45/ (accessed on 18 January 2023).
- Knisely, R.F. Selective medium for Bacillus anthracis. J. Bacteriol. 1966, 92, 784–786. [Google Scholar] [CrossRef] [Green Version]
- Belgian Centre for Pharmacotherapeutic Information. Available online: https://www.famhp.be/en/human_use/medicines/medicines/information_about_medicines/bcficbip (accessed on 18 January 2023).
- Rentz, E.D.; Lewis, L.; Mujica, O.J.; Barr, D.B.; Schier, J.G.; Weerasekera, G.; Kuklenyik, P.; McGeehin, M.; Osterloh, J.; Wamsley, J.; et al. Outbreak of acute renal failure in Panama in 2006: A case-control study. Bull. World Health Organ. 2008, 86, 749–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Available online: https://www.who.int/news/item/05-10-2022-medical-product-alert-n-6-2022-substandard-(contaminated)-paediatric-medicines (accessed on 18 January 2023).
- United States Pharmacopoeia 42; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2022.
- Japanese Pharmacopoeia, 18th ed.; Society of Japanese Pharmacopoeia: Tokyo, Japan, 2022.
- European Pharmacopoeia 10.0; Council of Europe: Strasbourg, France, 2022.
- International Conference on Harmonisation (ICH) of Technical Requirements for the Registration of Pharmaceuticals for Human Use, Q3C: Impurities: Guidelines for Residual Solvents, Step 4. 2021. Available online: https://database.ich.org/sites/default/files/ICH_Q3C-R8_Guideline_Step4_2021_0422_1.pdf (accessed on 18 January 2023).
- Patel, S.; Gupta, R.S. A phylogenomic and comparative genomic framework for resolving the polyphyly of the genus Bacillus: Proposal for six new genera of Bacillus species, Peribacillus gen. nov., Cytobacillus gen. nov., Mesobacillus gen. nov., Neobacillus gen. nov., Metabacillus gen. nov. and Alkalihalobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 406–438. [Google Scholar] [CrossRef]
- Cui, Y.; Märtlbauer, E.; Dietrich, R.; Luo, H.; Ding, S.; Zhu, K. Multifaceted toxin profile, an approach toward a better understanding of probiotic Bacillus cereus. Crit. Rev. Toxicol. 2019, 49, 342–356. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus Group: Bacillus Species with Pathogenic Potential. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Celandroni, F.; Salvetti, S.; Gueye, S.A.; Mazzantini, D.; Lupetti, A.; Senesi, S.; Ghelardi, E. Identification and Pathogenic Potential of Clinical Bacillus and Paenibacillus Isolates. PLoS ONE. 2016, 11, e0152831. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wang, H.; Gu, X.; Zheng, X.; Wang, Y.; Diao, J.; Peng, Y.; Zhang, H. Reply to Comment on “Screening and Identification of Novel Ochratoxin A-Producing Fungi from Grapes”. Toxins 2016, 8, 333”—In Reporting Ochratoxin A Production from Strains of Aspergillus, Penicillium and Talaromyces. Toxins 2017, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Egbuta, M.A.; Mwanza, M.; Babalola, O.O. Health Risks Associated with Exposure to Filamentous Fungi. Int. J. Environ. Res. Public Health 2017, 14, 719. [Google Scholar] [CrossRef] [Green Version]
- Hesse, S.E.; Luethy, P.M.; Beigel, J.H.; Zelazny, A.M. Penicillium citrinum: Opportunistic pathogen or idle bystander? A case analysis with demonstration of galactomannan cross-reactivity. Med. Mycol. Case Rep. 2017, 17, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Navale, V.; Vamkudoth, K.R.; Ajmera, S.; Dhuri, V. Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicol. Rep. 2021, 8, 1008–1030. [Google Scholar] [CrossRef] [PubMed]
- Ráduly, Z.; Szabó, L.; Madar, A.; Pócsi, I.; Csernoch, L. Toxicological and Medical Aspects of Aspergillus-Derived Mycotoxins Entering the Feed and Food Chain. Front. Microbiol. 2020, 10, 2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, G.; Susca, A. Penicillium Species and Their Associated Mycotoxins. Methods Mol. Biol. 2017, 1542, 107–119. [Google Scholar] [CrossRef]
- Deconinck, E.; Andriessens, S.; Bothy, J.L.; Courselle, P.; De Beer, J.O. Comparative dissolution study on counterfeit medicines of PDE-5 inhibitors. J. Pharm. Anal. 2014, 4, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Tie, Y.; van Loock, K.; Deconinck, E.; Adams, E. Evaluation of impurities and dissolution profiles of illegal antimicrobial drugs encountered in Belgium. Drug Test. Anal. 2020, 12, 53–66. [Google Scholar] [CrossRef]
- Rahman, M.S.; Yoshida, N.; Tsuboi, H.; Maeda, E.; Ibarra, A.V.V.; Zin, T.; Akimoto, Y.; Tanimoto, T.; Kimura, K. Patient safety and public health concerns: Poor dissolution rate of pioglitazone tablets obtained from China, Myanmar and internet sites. BMC Pharmacol. Toxicol. 2021, 22, 12. [Google Scholar] [CrossRef]
| n° | Information on the Outer Packaging or Blister | Appearance of the Tablet | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quantity | Lot Number | Mfr. | Mfg. Lic. No. | Expiration Date | Colorants | Tablet Color | Inscription on the Tablet | Scored | ||
| 1 | 100 mg sildenafil | Y | Y | Y | 1 October 2024 | Quinoline yellow, brilliant blue and titanium dioxide | green | “KGR 100” and logo “ap” on other side | N |  |
| 2 | 50 mg sildenafil | Y | Y | Y | 1 July 2023 | Quinoline yellow, brilliant blue and titanium dioxide | green | “KGR 50” and logo “ap” on other side | N |  |
| 3 | 100 mg sildenafil 50 mg tadalafil | Y | N | N | 1 July 2024 | Red oxide iron, indigo caramine, Ponceau 4R and titanium dioxide | red | n.a. | Y |  |
| 4 | 100 mg sildenafil 60 mg dapoxetine | Y | Y | N | 1 September 2023 | n.a. | green | n.a. | N |  |
| 5 | 50 mg sildenafil | Y | Y | Y | 1 April 2024 | Titanium dioxide and lake indigo carmine | blue | n.a. | Y |  |
| 6 | 200 mg sildenafil citrate | Y | Y | N | 1 February 2023 | n.a. | yellow | “200” on one side | N |  |
| 7 | 25 mg sildenafil | Y | N | N | 1 March 2023 | Indigo carmine and titanium dioxide | blue | “25” on one side | N |  |
| 8 | 100 mg sildenafil | Y | Y | N | 1 April 2024 | n.a. | blue | “100” on both sides | N |  |
| 9 | 200 mg sildenafil | Y | Y | Y | 1 March 2024 | Black oxide or iron | black | n.a. | N |  |
| 10 | 100 mg sildenafil | Y | Y | N | 1 September 2024 | n.a. | purple | “100” on one side and “F” on the other side | N | 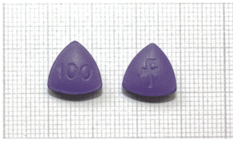 |
| 11 | 100 mg sildenafil | Y | Y | Y | 1 April 2024 | Indigo carmine | blue | “100” on both sides | N |  |
| 12 | 100 mg sildenafil | Y | Y | N | 1 October 2023 | Brilliant blue and indigo carmine | blue | “100” on one side | N |  |
| 13 | 150 mg sildenafil | Y | Y | Y | 1 April 2024 | Titanium dioxide, iron oxide red, lake of indigo carmine and lake of Ponceau 4R | red | n.a. | N |  |
| 14 | 120 mg sildenafil | Y | N | N | 1 January 2024 | n.a. | red | “120” on one side | N |  |
| 15 | 50 mg sildenafil | Y | N | N | 1 December 2023 | n.a. | blue | “50” on one side | N |  |
| 16 | 100 mg sildenafil | Y | Y | Y | 1 December 2024 | Quinoline yellow, brilliant blue and titanium dioxide | green | “KGR 100” and logo “ap” on other side | N | 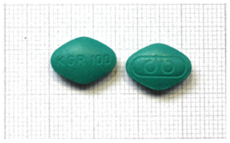 |
| 17 | 100 mg sildenafil | Y | Y | Y | 1 July 2023 | Lake of sunset yellow, lake of quinoline yellow and erythrosine | coloring depends on the flavor | “KGR 100” and logo “ap” on other side | N | 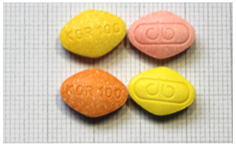 |
| 18 | 100 mg sildenafil | Y | Y | N | 1 September 2023 | n.a. | blue | “100” on both sides | N | 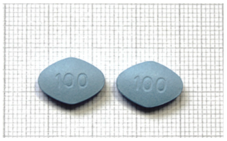 |
| 19 | 200 mg sildenafil | Y | Y | y | 1 April 2024 | Black oxide or iron | black | n.a. | N |  |
| 20 | 100 mg sildenafil | Y | Y | Y | 1 June 2024 | Quinoline yellow, sunset yellow, Ponceau 4R and brilliant blue | / | / | / |  |
| 21 | 100 mg sildenafil | Y | Y | N | 1 January 2024 | n.a. | / | / | / |  |
| 22 | 100 mg sildenafil 20 mg tadalafil | Y | N | N | 1 September 2024 | Red oxide iron, indigo caramine, Ponceau 4R and titanium dioxide | red | n.a. | Y |  |
| 23 | 100 mg sildenafil | Y | Y | N | 1 January 2025 | Indigo caramine and titanium dioxide | blue | “100” on one side | N |  |
| 24 | 100 mg sildenafil | Y | Y | N | 1 November 2023 | Indigo carmine | blue | “100” on one side | N |  |
| 25 | 25 mg sildenafil | Y | N | N | 1 April 2024 | Indigo carmine | blue | “25” on one side | N |  |
| 26 | 100 mg sildenafil | Y | Y | Y | 1 August 2024 | n.a. | white | “100” on both sides | N |  |
| 27 | 25 mg sildenafil | Y | Y | Y | 1 October 2024 | Indigo carmine | blue | “25” on one side | N |  |
| 28 | not clear | Y | Y | Y | 1 May 2023 | n.a. | red | “F” on one side | N |  |
| 29 | 25 mg sildenafil | Y | N | N | 1 September 2024 | Indigo carmine | blue | n.a. | N |  |
| 30 | 100 mg sildenafil | Y | N | N | 1 September 2024 | Indigo carmine | blue | n.a. | N |  |
| 31 | 50 mg sildenafil | Y | N | N | 1 May 2024 | Not mentioned on the blister | blue | “50” on one side | N |  |
| 32 | 100 mg sildenafil | Y | Y | Y | 1 August 2024 | Ponceau 4R, sunset yellow, titanium dioxide (marked in French) | red | n.a. | Y |  |
| n° | Quantity API Declared | API Detected | API Quantified (mg) | % Declared Dose | % MU |
|---|---|---|---|---|---|
| 1 | 100 mg sildenafil | sildenafil | 93.2 | 93.2 | 1.6 |
| 2 | 50 mg sildenafil | sildenafil | 90.5 | 90.5 | 3.8 |
| 3 | 100 mg sildenafil | sildenafil | 86.0 | 86.0 | 1.5 |
| 50 mg tadalafil | tadalafil | 19.6 | 39.1 | 0.5 | |
| 4 | 100 mg sildenafil | sildenafil | 89.11 | 94.4 | 4.7 |
| 60 mg dapoxetine | dapoxetine | 50.4 | 83.9 | 3.6 | |
| 5 | 50 mg sildenafil | sildenafil | 46.3 | 92.7 | 2.2 |
| 6 | 200 mg sildenafil citrate | sildenafil | 186.5 | 93.3 | 0.5 |
| 7 | 25 mg sildenafil | sildenafil | 23.3 | 93.0 | 0.4 |
| 8 | 100 mg sildenafil | sildenafil | 96.3 | 96.3 | 1.0 |
| 9 | 200 mg sildenafil | sildenafil | 195.5 | 97.8 | 0.5 |
| 10 | 100 mg sildenafil | sildenafil | 78.9 | 78.9 | 9.5 * |
| 11 | 100 mg sildenafil | sildenafil | 87.6 | 87.6 | 1.1 |
| 12 | 100 mg sildenafil | sildenafil | 78.3 | 78.3 | 1.5 |
| 13 | 150 mg sildenafil | sildenafil | 143.4 | 87.6 | 1.8 |
| 14 | 120 mg sildenafil | sildenafil | 105.3 | 87.8 | 6.5 * |
| 15 | 50 mg sildenafil | sildenafil | 45.1 | 90.2 | 1.8 |
| 16 | 100 mg sildenafil | sildenafil | 17.9 | 17.9 | 3.9 |
| 17 | 100 mg sildenafil | sildenafil | 83.0 | 83.0 | 1.4 |
| 18 | 100 mg sildenafil | sildenafil | 91.4 | 91.4 | 0.3 |
| 19 | 200 mg sildenafil | sildenafil | 174.4 | 87.2 | 4.4 * |
| 20 | 100 mg sildenafil | sildenafil | 93.1 | 93.1 | 0.6 |
| 21 | 100 mg sildenafil | sildenafil | 34.9 | 34.9 | 0.6 |
| 22 | 100 mg sildenafil | sildenafil | 89.4 | 89.4 | 2.9 |
| 20 mg tadalafil | tadalafil | 19.8 | 99.0 | 1.2 | |
| 23 | 100 mg sildenafil | sildenafil | 89.1 | 89.1 | 6.8 * |
| 24 | 100 mg sildenafil | sildenafil | 93.8 | 93.8 | 0.4 |
| 25 | 25 mg sildenafil | sildenafil | 21.4 | 85.5 | 7.0 |
| 26 | 100 mg sildenafil | sildenafil | 100.9 | 100.9 | 1.3 |
| 27 | 25 mg sildenafil | sildenafil | 18.7 | 74.8 | 11.1 |
| 28 | n.a. | sildenafil | 97.4 | n.a. | 8.7 * |
| 29 | 25 mg sildenafil | sildenafil | 19.6 | 78.4 | 8.7 |
| 30 | 100 mg sildenafil | sildenafil | 91.1 | 91.1 | 4.0 |
| 31 | 50 mg sildenafil | sildenafil | 39.6 | 79.2 | 15.8 * |
| 32 | 100 mg sildenafil | sildenafil | 97.4 | 97.4 | 0.4 |
| n° | Microbial Load | Identification of Microorganism | ||
|---|---|---|---|---|
| 1 | TAMC | not compliant | biofilm | Sutcliffiella cohnii and Bacillus thermoamylovorans (biofilm) |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 2 | TAMC | compliant | - | n.a. |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 3 | TAMC | compliant | - | n.a. |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 4 | TAMC | not compliant | biofilm | Bacillus mojavensis |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 5 | TAMC | compliant | - | n.a. |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 6 | TAMC | not compliant | biofilm | Bacillus cereus sensu lato |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 7 | TAMC | not compliant | biofilm | Bacillus pumilus |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 8 | TAMC | compliant | - | n.a. |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 9 | TAMC | compliant | - | n.a. |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 10 | TAMC | not compliant | biofilm | Bacillus spp. |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 11 | TAMC | compliant | ≈20 CFU/g | Micrococcus luteus |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 12 | TAMC | not compliant | biofilm | Bacillus spp. |
| TYMC | compliant | ≈20 CFU/g | ||
| VRBG | no growth | - | ||
| 13 | TAMC | not compliant | biofilm | Bacillus spp. (biofilm) and Corynebacterium ssp. |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 14 | TAMC | not compliant | biofilm | Bacteria: Bacillus cereus sensu lato and Paenibacillus amylolyticus |
| TYMC | not compliant | >200 CFU/g | ||
| VRBG | growth of fungi and molds | ≈110 CFU/g | Fungi: Alternaria triticimaculans, Penicillium citrinum, Penicillium rubens, Aspergillus versicolor, Aspergillus fumigatus and Penicillium chrysogenum | |
| 15 | TAMC | compliant | - | n.a. |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 16 | TAMC | compliant | ≈20 CFU/g | Staphylococcus hominis |
| TYMC | compliant | ≈20 CFU/g | ||
| VRBG | no growth | - | ||
| 17 | TAMC | not compliant | biofilm | Bacillus spp. and Kocuria rhizophila |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 18 | TAMC | compliant | ≈20 CFU/g | n.a. |
| TYMC | compliant | ≈60 CFU/g | ||
| VRBG | no growth | - | ||
| 19 | TAMC | compliant | ≈20 CFU/g | Bacillus beringensis |
| TYMC | compliant | ≈20 CFU/g | ||
| VRBG | no growth | - | ||
| 20 | TAMC | compliant | - | n.a. |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 21 | TAMC | compliant | - | n.a. |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 22 | TAMC | compliant | ≈20 CFU/g | Staphylococcus hominis |
| TYMC | compliant | ≈20 CFU/g | ||
| VRBG | no growth | - | ||
| 23 | TAMC | compliant | ≈20 CFU/g | Priestia megaterium and Micrococcus luteus |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 24 | TAMC | not compliant | biofilm | Bacteria: Bacillus spp., Priestia megaterium and Micrococcus luteus |
| TYMC | not compliant | >200 CFU/g | ||
| VRBG | no growth | - | Fungi: Aspergillus flavus | |
| 25 | TAMC | compliant | ≈100 CFU/g | Bacteria: Peribacillus simplex and Bevundimonas vesicularis |
| TYMC | not compliant | >200 CFU/g | ||
| VRBG | growth of molds | ≈20 CFU/g | Fungi: Aspergillus fumigatus | |
| 26 | TAMC | not compliant | biofilm | Bacillus licheniformis |
| TYMC | compliant | ≈100 CFU/g | ||
| VRBG | no growth | - | ||
| 27 | TAMC | not compliant | Biofilm | Alkalihalobacillus spp. (biofilm) and Corynebacterium liphiloflavum |
| TYMC | compliant | ≈20 CFu/g | ||
| VRBG | no growth | - | ||
| 28 | TAMC | not compliant | biofilm | Alkalihalobacillus halodurans (biofilm) and Micrococcus luteus |
| TYMC | compliant | ≈140 CFU/g | ||
| VRBG | growth of molds | ≈40 CFU/g | ||
| 29 | TAMC | compliant | ≈40 CFU/g | n.a. |
| TYMC | compliant | ≈20 CFU/g | ||
| VRBG | no growth | - | ||
| 30 | TAMC | compliant | ≈300 CFU/g | Corynebacterium spp. |
| TYMC | compliant | - | ||
| VRBG | no growth | - | ||
| 31 | TAMC | compliant | ≈80 CFU/g | Bacteria: Bacillus spp. Fungi: Penicillium frequentans and Talaromyces amestolkiae |
| TYMC | not compliant | >200 CFU/g | ||
| VRBG | growth of molds | ≈40 CFU/g | ||
| 32 | TAMC | compliant | ≈ 20 CFU/g | Bacteria: Staphylococcus haemolyticus and Kocuria atrinae Fungi: Aspergillus fumigatus Aspergillus jensenii |
| TYMC | not compliant | >200 CFU/g | ||
| VRBG | growth of molds | ≈ 40 CFU/g | ||
| Sample n° | Identified Chemical Risk a | Identified Biological Risk |
|---|---|---|
| 1 | Bacterial contamination | |
| 2 | ||
| 3 | Mixture of sildenafil and tadalafil, and amount of tadalafil found was less than 40% of the API mentioned on the package/blister | |
| 4 | Bacterial contamination | |
| 5 | ||
| 6 | Sildenafil found exceeds the recommended dosage (186.5 mg/unit) | Contamination with potential pathogenic bacteria |
| 7 | Bacterial contamination | |
| 8 | ||
| 9 | Sildenafil found exceeds the recommended dosage (195.5 mg/unit) | |
| 10 | Large variations (RSD > 5%) in API content between the different tablets in one package/blister | Bacterial contamination |
| 11 | ||
| 12 | Bacterial contamination | |
| 13 | Sildenafil found exceeds the recommended dosage (143.4 mg/unit) | Bacterial contamination |
| 14 | Large variations (RSD > 5%) in API content between the different tablets in one package/blister | Contamination with potential pathogenic bacteria and fungal contamination |
| 15 | ||
| 16 | Amount less than 20% declared dosage | |
| 17 | Bacterial contamination | |
| 18 | ||
| 19 | Sildenafil found exceeds the recommended dosage (174.4 mg/unit) and large variations (RSD > 5%) in API content between the different tablets in one package/blister | |
| 20 | ||
| 21 | Amount less than 40% declared dosage | |
| 22 | ||
| 23 | Large variations (RSD > 5%) in API content between the different tablets in one package/blister | |
| 24 | Bacterial and fungal contamination | |
| 25 | Fungal contamination | |
| 26 | Bacterial contamination | |
| 27 | Bacterial contamination | |
| 28 | Large variations (RSD > 5%) in API content between the different tablets in one package/blister | Bacterial contamination |
| 29 | ||
| 30 | ||
| 31 | Large variations (RSD > 5%) in API content between the different tablets in one package/blister | Fungal contamination |
| 32 | Fungal contamination |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanhee, C.; Jacobs, B.; Mori, M.; Kamugisha, A.; Debehault, L.; Canfyn, M.; Ceyssens, B.; Van Der Meersch, H.; van Hoorde, K.; Deconinck, E.; et al. Uncovering the Quality Deficiencies with Potentially Harmful Effects in Substandard and Falsified PDE-5 Inhibitors Seized by Belgian Controlling Agencies. Forensic Sci. 2023, 3, 426-451. https://doi.org/10.3390/forensicsci3030031
Vanhee C, Jacobs B, Mori M, Kamugisha A, Debehault L, Canfyn M, Ceyssens B, Van Der Meersch H, van Hoorde K, Deconinck E, et al. Uncovering the Quality Deficiencies with Potentially Harmful Effects in Substandard and Falsified PDE-5 Inhibitors Seized by Belgian Controlling Agencies. Forensic Sciences. 2023; 3(3):426-451. https://doi.org/10.3390/forensicsci3030031
Chicago/Turabian StyleVanhee, Celine, Bram Jacobs, Marcella Mori, Angélique Kamugisha, Loïc Debehault, Michael Canfyn, Bart Ceyssens, Hans Van Der Meersch, Koenraad van Hoorde, Eric Deconinck, and et al. 2023. "Uncovering the Quality Deficiencies with Potentially Harmful Effects in Substandard and Falsified PDE-5 Inhibitors Seized by Belgian Controlling Agencies" Forensic Sciences 3, no. 3: 426-451. https://doi.org/10.3390/forensicsci3030031
APA StyleVanhee, C., Jacobs, B., Mori, M., Kamugisha, A., Debehault, L., Canfyn, M., Ceyssens, B., Van Der Meersch, H., van Hoorde, K., Deconinck, E., & Willocx, M. (2023). Uncovering the Quality Deficiencies with Potentially Harmful Effects in Substandard and Falsified PDE-5 Inhibitors Seized by Belgian Controlling Agencies. Forensic Sciences, 3(3), 426-451. https://doi.org/10.3390/forensicsci3030031







