1. Introduction
Accurate, precise, and unbiased age-at-death estimates from human skeletal remains are crucial in biological anthropology. Numerous challenges persist in adult age estimation that complicate our ability to objectively analyze human skeletal remains in bioarchaeological and forensic contexts. The aim of any given age estimation method is to correlate biological age (using skeletal age as a proxy) with chronological age [
1]. However, biological age does not always reflect chronological age [
1,
2,
3,
4]. Individuals may experience various biological ages at any given chronological age within a population [
1]. This discrepancy between biological and chronological age is influenced by various environmental and biological factors such as genetics, body mass, and physical activity levels [
1,
5,
6]. Despite the importance that these components have to methods of age estimation, limited research has been conducted on factors that may influence the process of skeletal aging. Historically, debates about age estimation based on skeletal remains have been focused on how to improve the observation of traits and the processing of data (see Clark et al., 2022 [
7] for a review).
Estimating age-at-death in adults is challenging because age-related degenerative skeletal changes are more variable than age-related developmental changes in juveniles [
2,
8,
9]. Methods tend to underestimate age-at-death of older individuals and depend on wide age ranges that lack precision [
10,
11]. Poor precision of age estimates limits the utility of biological profiles in forensic settings [
12] and has the potential to limit demographic and other comparisons in bioarchaeological settings. This challenge can lead practitioners to narrow age ranges based on experience rather than validated methods [
13].
In forensic contexts, methods based on contemporary populations are preferred because of possible secular effects on processes of skeletal aging. Thus, recently, many scholars have refined existing methods with larger, more modern skeletal samples (e.g., [
14,
15,
16]). Limited studies have demonstrated that these revised methods are more accurate than the original methods on which they were based. Furthermore, recent scholarship in age estimation research has focused on the development of methods using novel skeletal markers of age (e.g., [
17,
18]).
Multifactorial methods of age estimation have been shown to increase accuracy and control for variation among different stages of skeletal aging at different anatomical regions within an individual [
12]. Many traditional age estimation methods incorporate few anatomical features, with no standardized manner for combining methods [
9]. Moreover, incomplete or partial skeletons are a common occurrence in both bioarchaeological and forensic settings. Having multifactorial methods increases the likelihood of being able to reliably estimate the age of those individuals. When all skeletal elements are present, multifactorial methods increase the accuracy and precision of age estimates since anatomical regions may age at different rates within the same individual [
2,
12].
Transition analysis (TA) age estimation, as described by Boldsen et al. (2002), was designed to address many of the challenges mentioned previously [
8]. Transition analysis, as a statistical approach, has been applied to other age-at-death estimation methods. However, “TA” in this study is referring to the multifactorial method of skeletal age-at-death estimation developed by Boldsen et al. (2002), which utilizes ADBOU 2.1 software to generate age estimates [
8,
19]. TA uses the pubic symphysis, the iliac auricular surface, and cranial sutures [
8,
19]. The ADBOU 2.1 computer program calculates a maximum likelihood point estimate and a 95% confidence interval for each skeleton analyzed with TA [
8,
11]. ADBOU is based on prior probability distributions and Bayesian statistical modeling, which accounts for biological sex, ancestry, and bioarcheological or forensic populations. Because TA relies upon Bayesian modeling, it decreases bias related to age mimicry [
20,
21]. Age mimicry, as originally described by Bocquet-Appel and Masset (1982) [
22], results in significant bias to age estimation by assuming that the age distribution of the sample population is the same as that of the population used to develop the methods for age estimation [
8,
9,
20]. A more recent version of TA (commonly referred to as TA3) is available for use at
https://www.statsmachine.net/software/TA3/ (accessed on 9 March 2023), but the method has not been published or validated in an academic journal as of the writing of this paper. Therefore, it is not discussed herein [
9,
23,
24,
25].
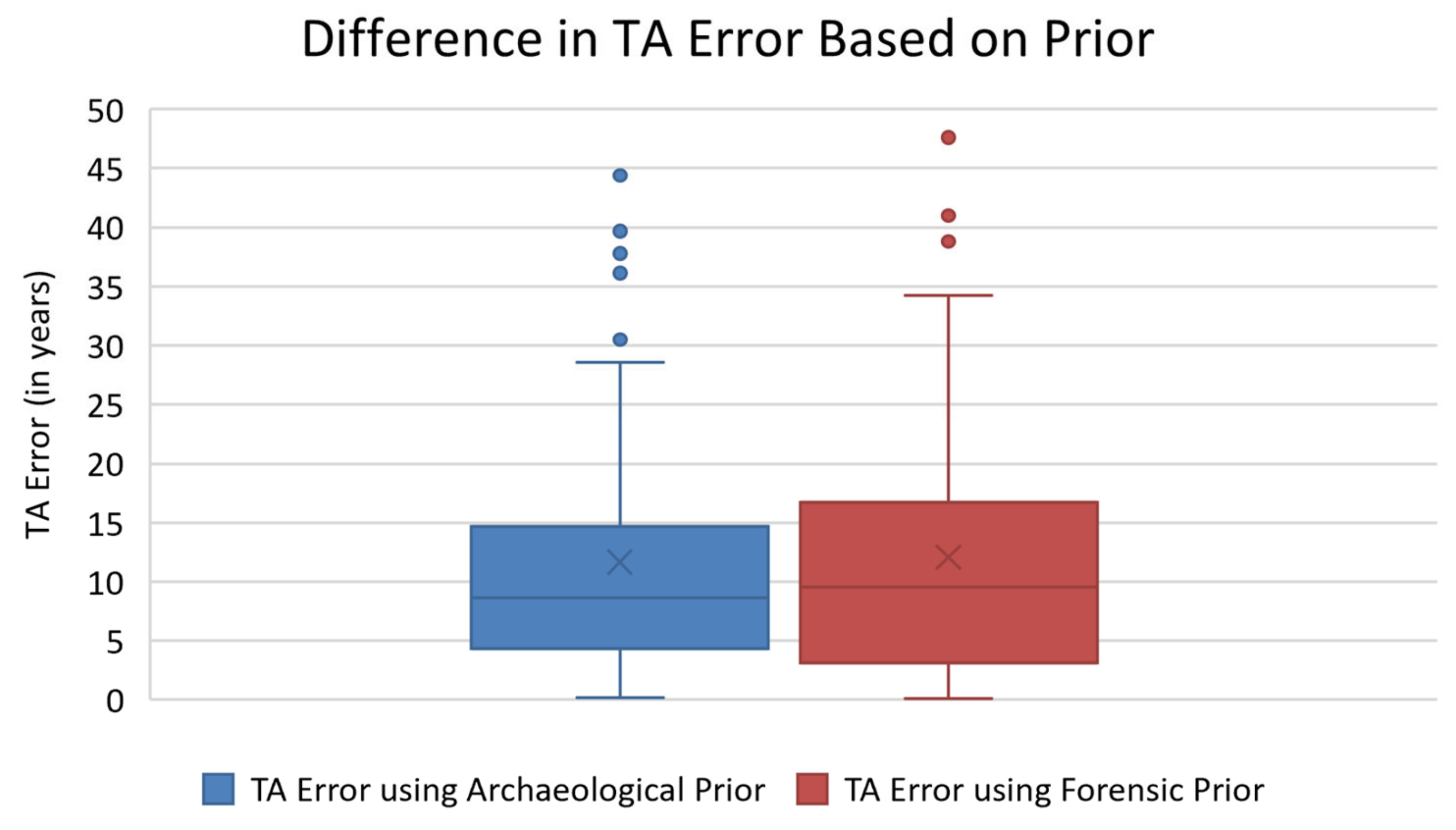
For all previously stated reasons, TA has been promoted as a more accurate method for estimating age-at-death, relative to traditional methods. Many studies have also demonstrated that variation among populations makes the informative prior distributions inappropriate for diverse target samples [
11,
19,
24,
25,
26,
27]. For instance, Xanthopoulou et al. (2018) found that TA was less accurate in a contemporary Greek skeletal assemblage compared to traditional age estimation methods [
27]. Similarly, Simon and Hubbe (2021) assessed the accuracy of TA in the Hamann–Todd Osteological Collection and found that the mean age estimate error was 11.6 years, with the errors for White individuals’ being significantly higher than for Black individuals [
25]. Simon and Hubbe (2021) argue that this trend can likely be attributed to the informative prior distribution for White individuals being less appropriate for this population [
25].
While Godde and Hens (2012) found that the target population does not need to fit perfectly with the informative prior for it to perform well [
20], it has been shown that informative prior distributions still have an advantage over uniform prior distributions, which assume equal probability of death at any age [
20,
26,
28]. Milner and Boldsen’s (2012) validation study of TA found that TA is better suited for reconstructing past demography, as opposed to individual age-at-death estimations [
11]. Therefore, TA is less reliable when aiming to obtain accurate and precise biological profiles for individual skeletons, compared to illustrating overall population trends in mortality.
These findings may also indicate a flaw in our foundational understanding of age-at-death estimation from skeletal remains. Estimation of age-at-death is reliant upon the assumption that biological age is correlated with chronological age and that degenerative changes generally occur at the same chronological age in all individuals [
1]. However, due to a myriad of factors, both environmental and biological, correlations between biological age and chronological age vary at the individual and population levels. This leads to difficulty in establishing whether age estimation methods inadequately measure biological characteristics of age in the human skeleton or, more likely, if there is significant variation at the individual and population levels which makes age estimates inaccurate and unreliable [
2].
As is important to the current discussion, people age at different rates based on several extrinsic and intrinsic factors. Biological age is more strongly associated with mortality risk and health status than chronological age [
29]. Therefore, we test the hypothesis that physiological stress will affect biological aging and, by extension, the accuracy and precision of age-at-death estimates. Physiological stress is used here as a proxy of overall health status. However, there are many components of health—not all of which can be measured from the skeleton [
30]. Physiological stress captures only one component of an individual’s or population’s overall health [
31], but has nonetheless been used as a proxy for health in studies of past populations [
30,
31]. Moreover, stress is a concept that addresses the detriments of disruptive biological and environmental events on the individual and population levels [
32]. Despite advancements in our understanding of the manifestation of physiological stress in human skeletal remains, there are still numerous challenges that must be considered when using physiological stress as a proxy for health.
Physiological stress is relevant when studying age estimation methods because it is a continuous process that affects individuals throughout the entirety of their lives. The human body is constantly responding to different stressors and using biological and environmental resources to prevent deleterious health outcomes [
33,
34], which may also influence the process of biological aging [
29]. Ultimately, prolonged exposure to physiological stress can result in an accumulated allostatic load and early signs of senescence, which may result in increased differences between the biological age and chronological age of the individual. Given that the relationship between stress, senescence, and chronological age has not been widely studied, this study uses the prevalence of osteological markers of physiological stress to evaluate whether they have a significant impact in age estimation errors, and if this should be something to be considered in future age-estimation studies. Therefore, the primary aim of this paper is to understand the behavior of calculated error in TA age estimates in a sample from the Hamann–Todd Osteological Collection in relation to the prevalence of osteological markers of physiological stress, as a proxy for how “health” may have influenced biological aging processes in this skeletal sample.
Historically, the Hamann–Todd Osteological Collection has been instrumental in developing methods to estimate skeletal age-at-death, sex, population affinity, and stature. However, such research has often neglected to acknowledge the identities of the people that compose the sample [
35], with the sample largely representing individuals from low socioeconomic classes [
35,
36,
37,
38,
39] who likely display a high prevalence of physiological stress indicators resulting from poor living conditions. Consequently, this article also emphasizes the lived experiences of the individuals that make up the skeletal sample in conjunction with exploring methodological implications for age estimation.
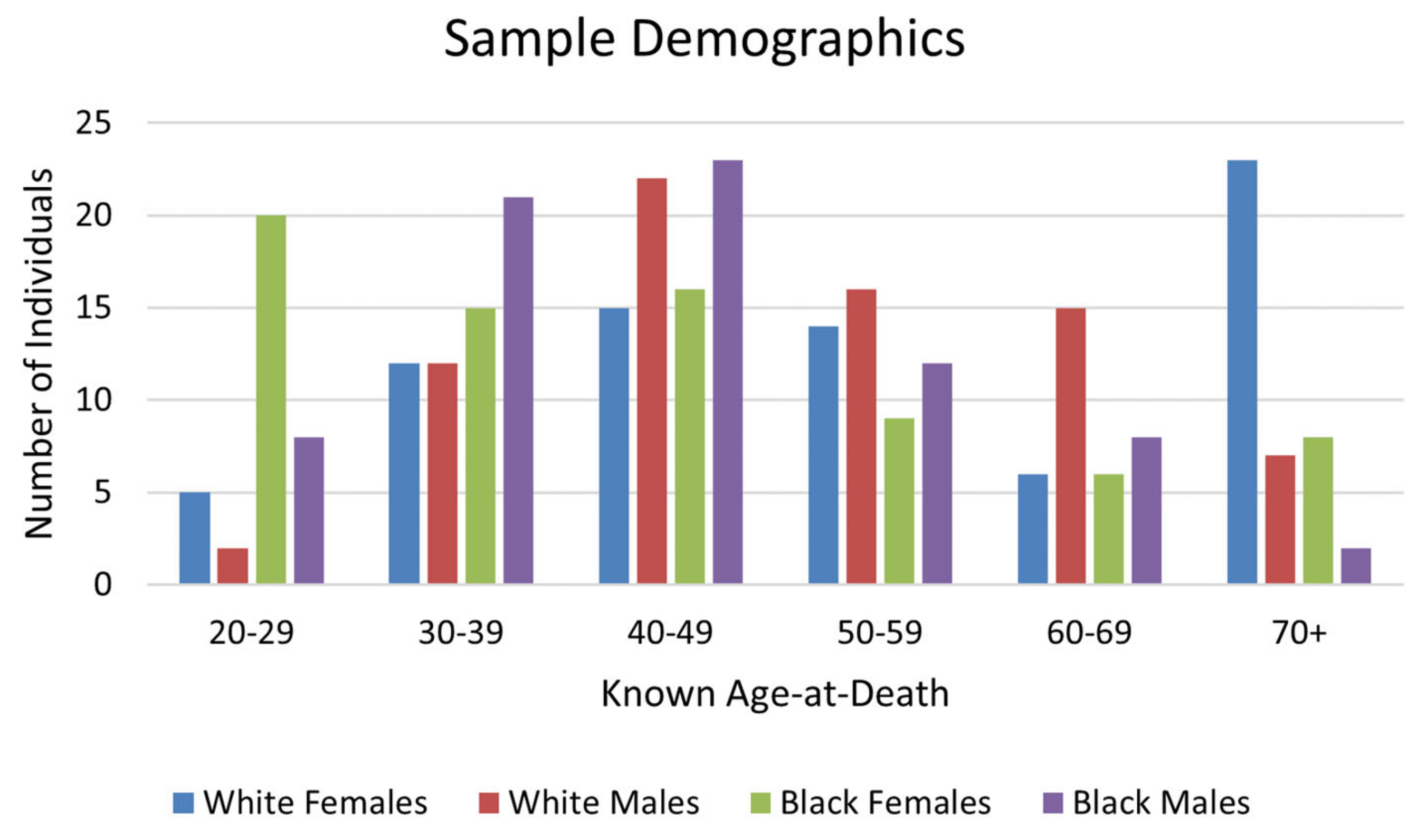
3. Results
Despite the larger sample size included here, the TA accuracy results are consistent with the findings of Simon and Hubbe (2021) [
25]. The mean absolute error in age estimation was 11.772 years, with a standard deviation of 10.572 years. Wilcoxon tests show that the mean absolute error differed significantly among the identity categories used in this study (V = 4100.5,
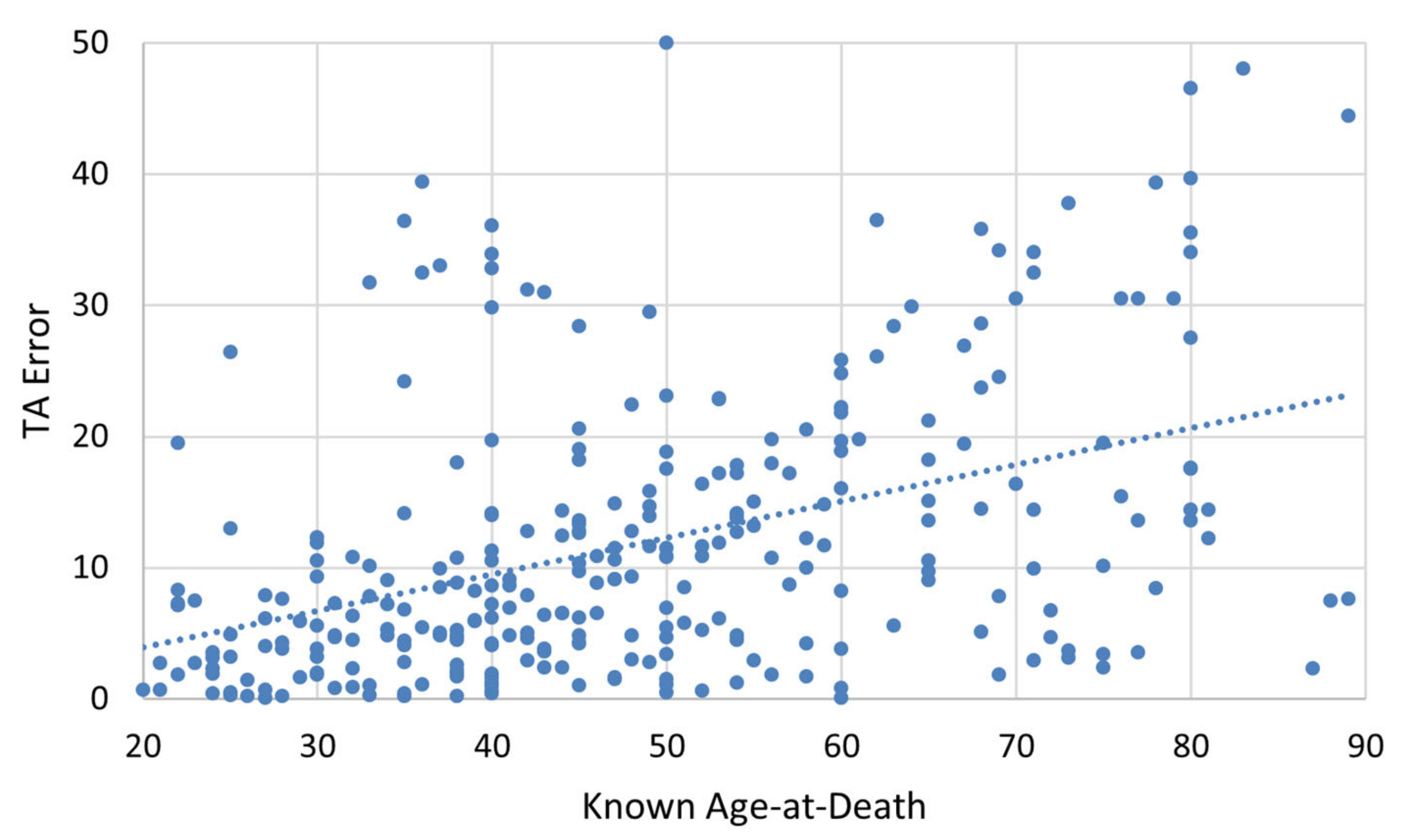
p = 0.007), with White Americans exhibiting higher TA error on average (13.673 years) compared to Black Americans (9.857 years). Spearman’s rank correlation was computed to assess the relationship between known age-at-death and absolute TA error. As expected, absolute TA error had a moderate positive correlation with advancing age-at-death (r = 0.447,
p < 0.001), with around 18% of the variance in TA error explained by age-at-death (
Figure 3).
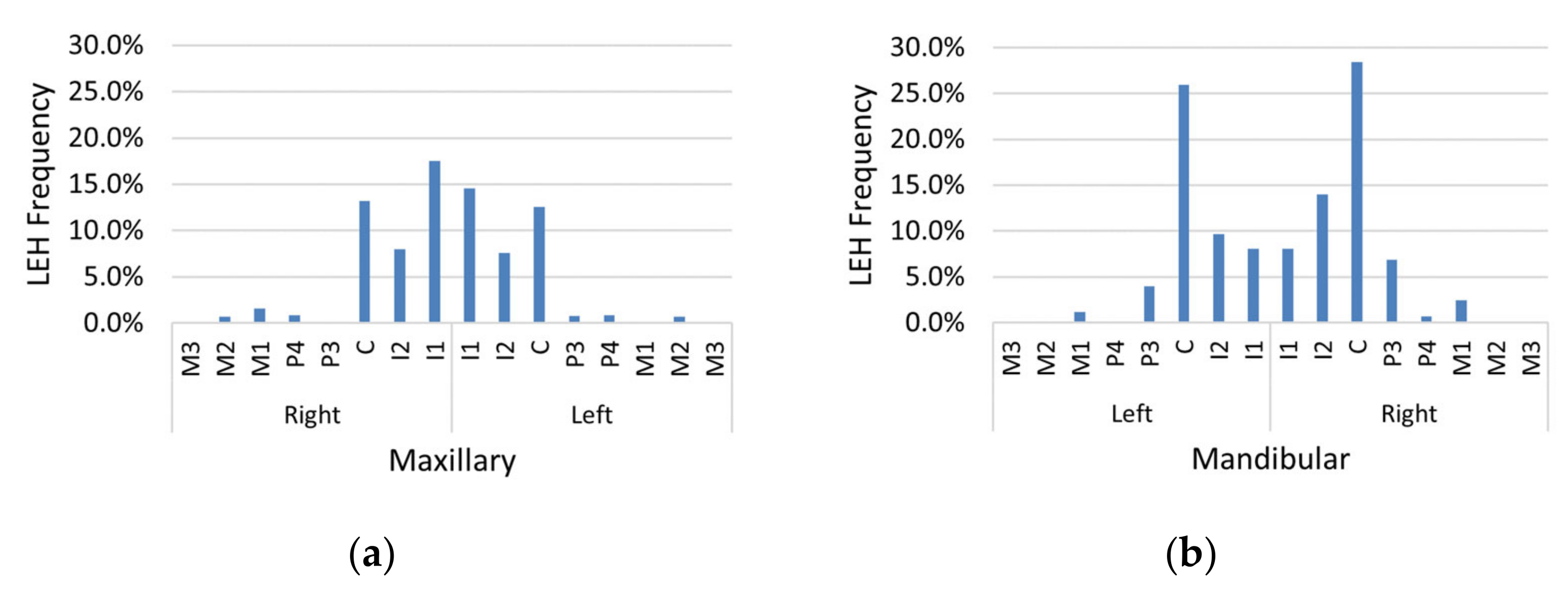
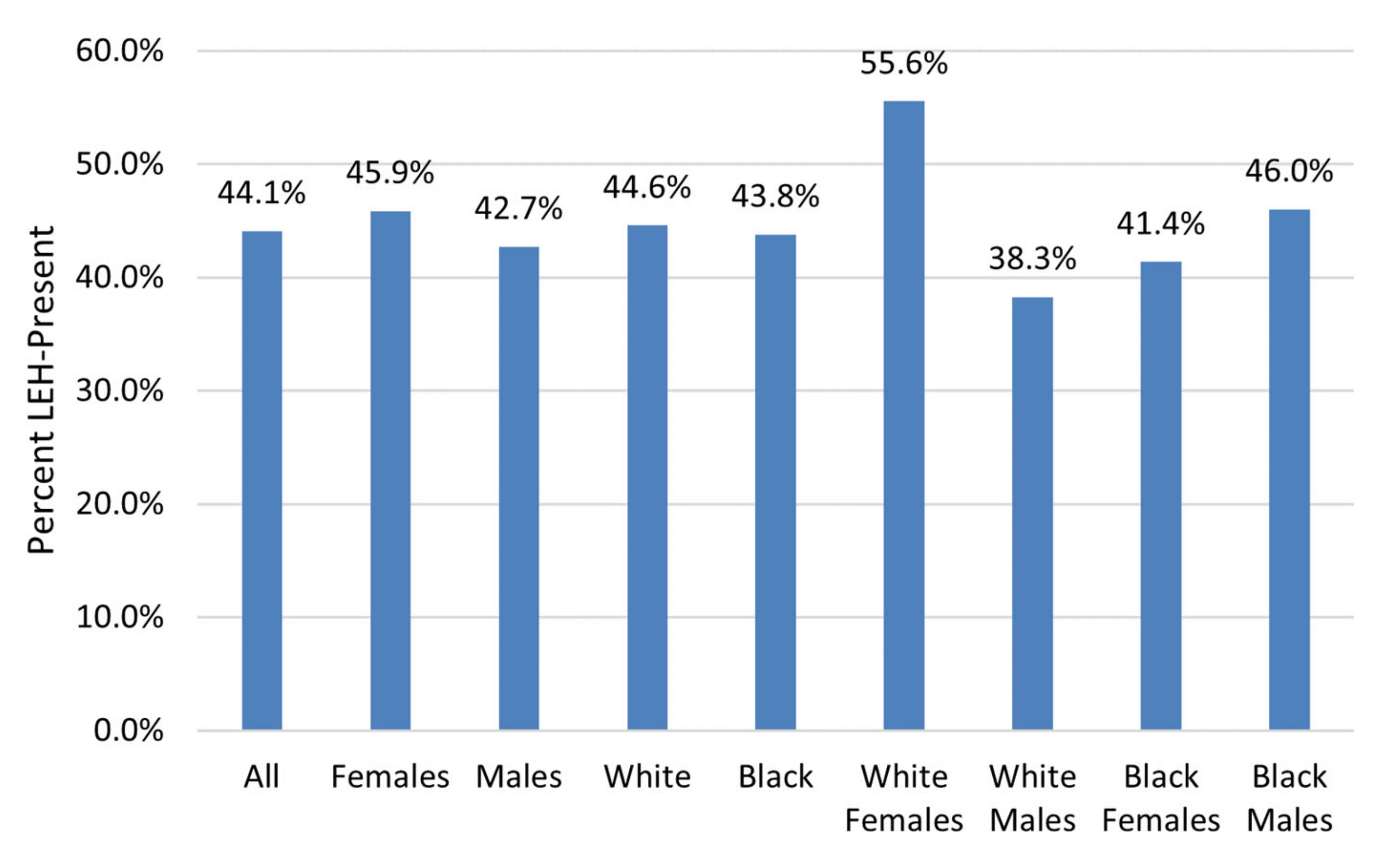
LEH prevalence was high in this sample, with 44.1% of individuals having at least one LEH (
Table 1). LEH prevalence was highest on the mandibular canines (
Figure 4). The frequency of LEH was highest for White females and lowest for White males (
Figure 5); however, chi-square testing results for differences in LEH presence among different groups showed that these differences were not statistically significant (
Table 2).
The mean TA error did not differ significantly between individuals with at least one LEH and individuals without LEH. This trend was consistent when each population group was analyzed separately (
Table 3). LEH presence was not related to known age-at-death. All Wilcoxon’s tests comparing known age-at-death for individuals with at least one LEH compared to those without LEH yielded non-significant
p-values.
Correlations between stature and TA error were non-significant for males (p = 0.382), while females displayed a weak, albeit significant, positive association, explaining just 5.4% of the variance (r = 0.232, p = 0.005). Correlations between stature and AMTL revealed a weak, but significant, positive association for males, explaining just 6.4% of the variance (r = 0.253, p = 0.002), but this was not the case for females (p = 0.748).
Males with at least one LEH had a mean stature of 1722 mm, while males without any LEH had a slightly higher mean stature of 1745 mm, although this difference was not found to be significant (
t = 1.498,
p = 0.068). For females with at least one LEH, the mean stature was 1598 mm, compared to 1644 mm for individuals without any LEH. The difference in mean stature for females with at least one LEH and those without any LEH was significant (
t = 2.412,
p = 0.009). The differences in the distribution of statures for individuals without LEH and those with at least one LEH is displayed in
Figure 6.
When analyzed by sub-group, independent
t-tests comparing statures for those with and without LEH yielded significant results for Black females and White males only (
Table 4).
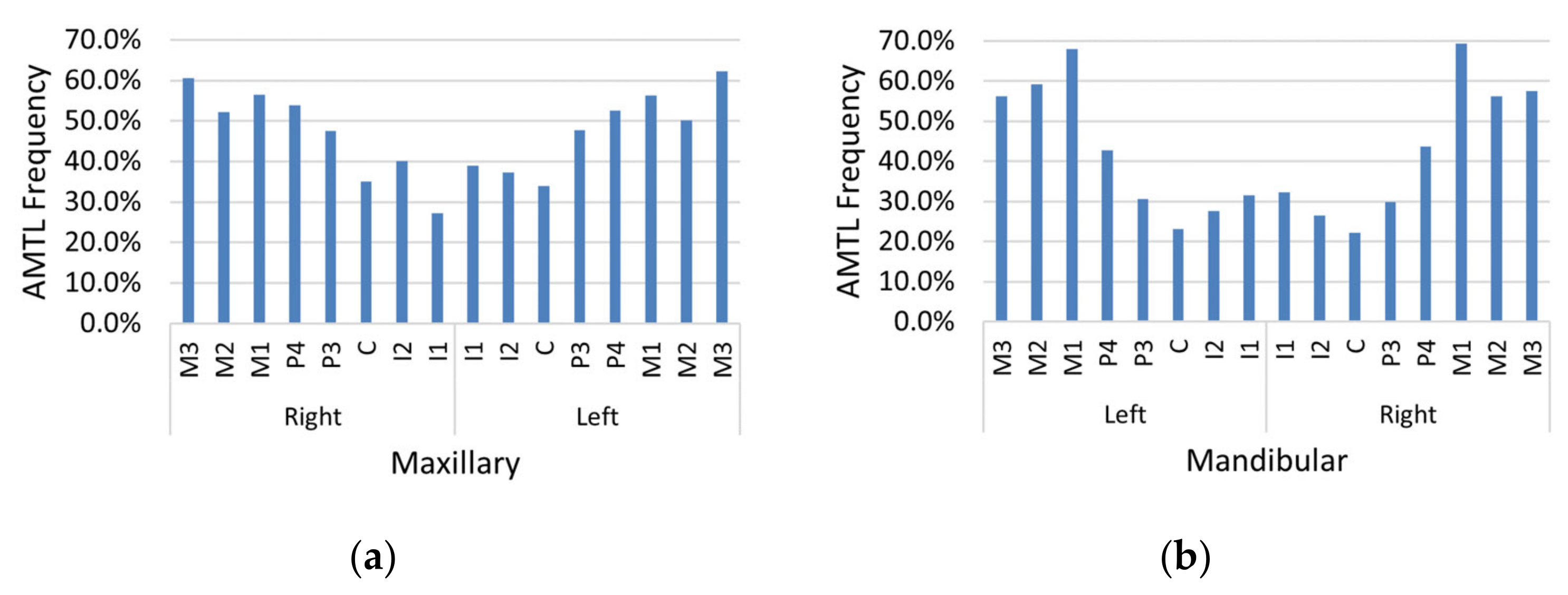
AMTL was present in nearly every individual in the sample (97.0%). Maxillary and mandibular molars were affected by AMTL most often relative to other teeth, and maxillary teeth (
Figure 7a) exhibited greater AMTL frequency compared to mandibular teeth (
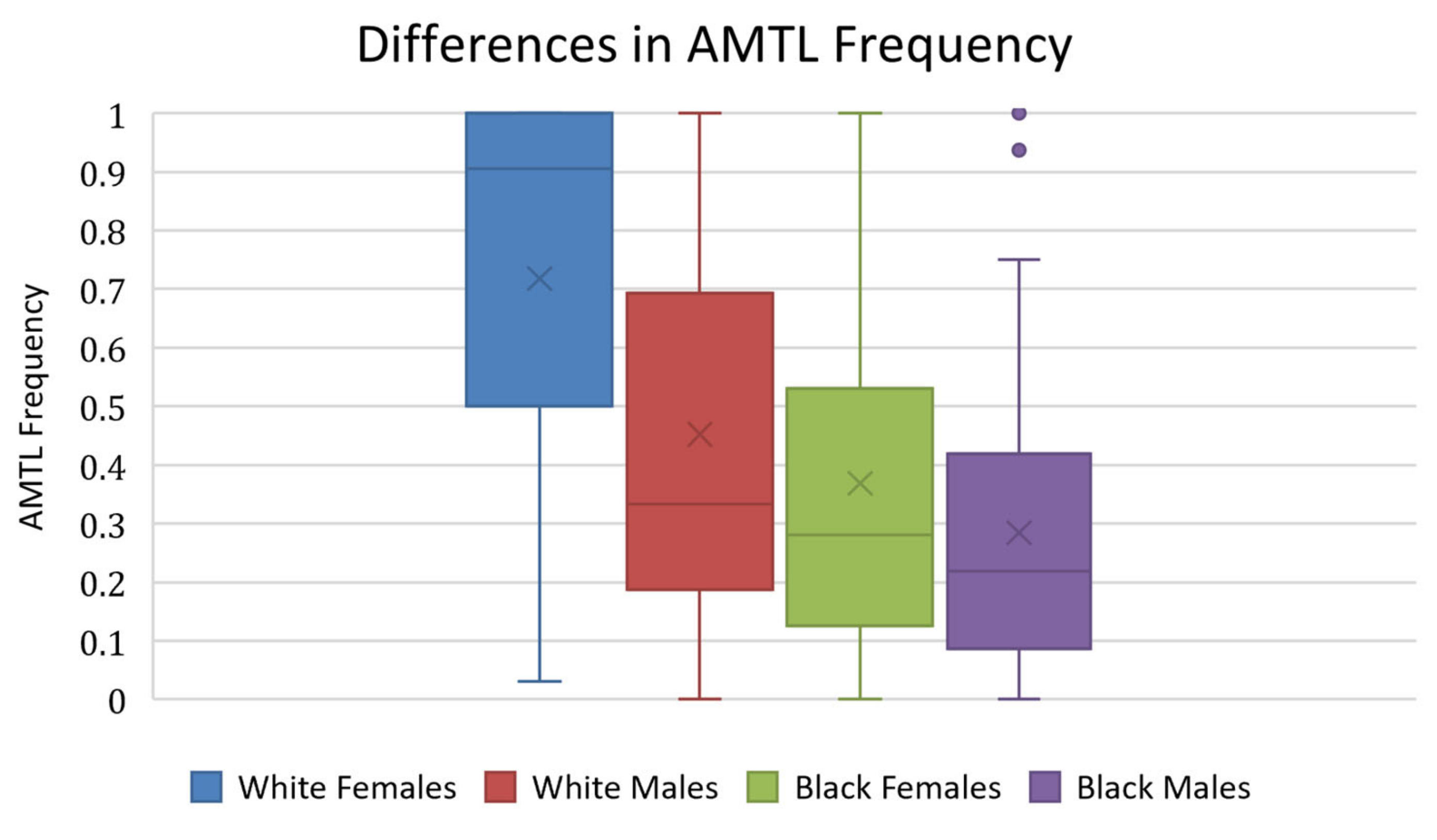
Figure 7b). The proportion of teeth affected by AMTL varied by population group (
Table 5). White females displayed the highest mean proportion of AMTL, followed by White males, Black females, and Black males, respectively (
Figure 8). Kruskal–Wallis results showed that the population differences in AMTL frequency among subgroups were significant (H = 61.21,
p < 0.001). The results of Dunn’s test to compare AMTL between pairs of sub-groups is reported in
Table 6. There were significant differences found between White females and all other subgroups (
p < 0.001).
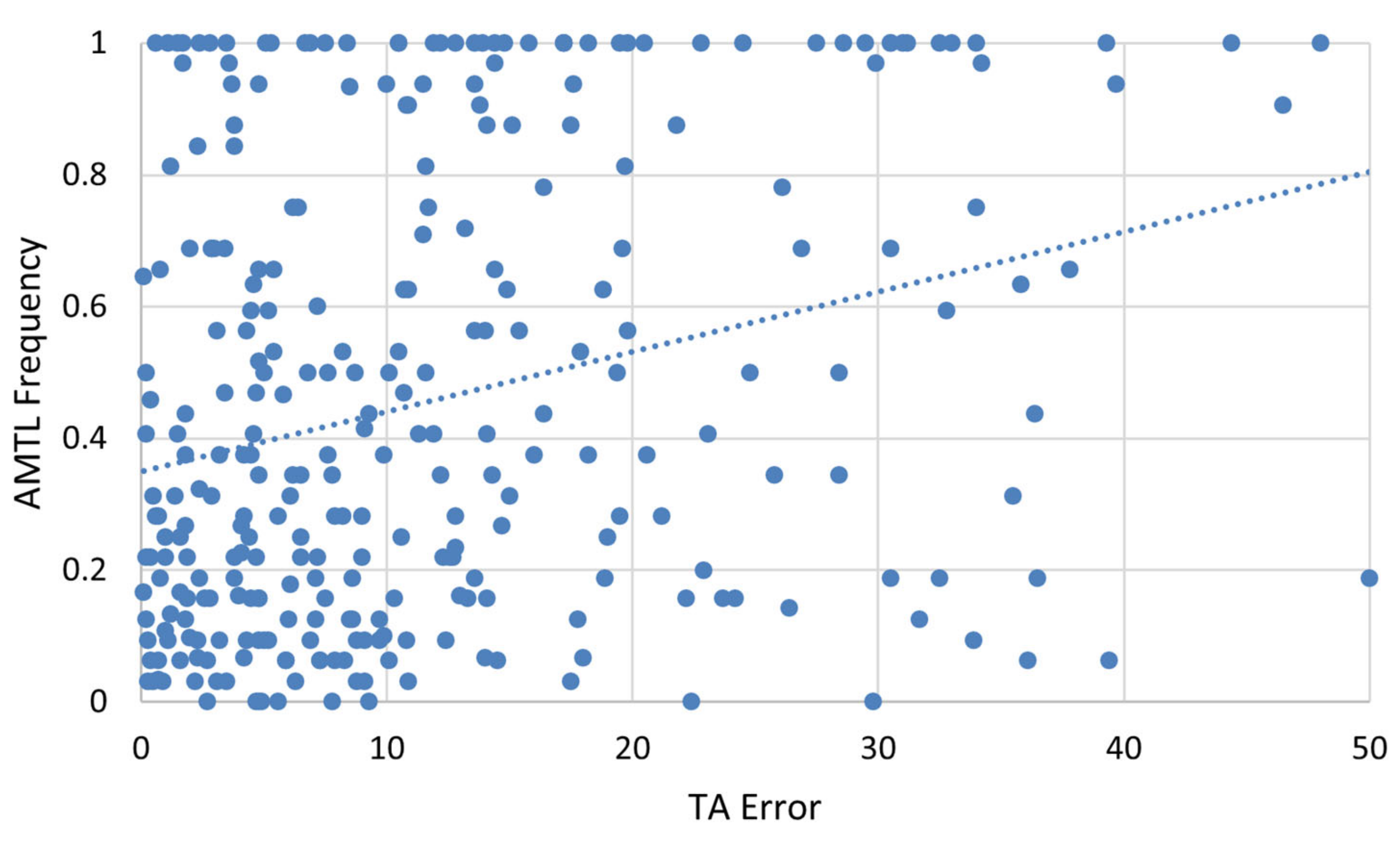
Spearman’s rank correlation was used to assess the relationship between AMTL frequency and absolute TA error. It revealed a weak, but significant, relationship between the two variables, explaining around 7.6% of the variance (
Figure 9, r = 0.276,
p < 0.001). Since AMTL and TA errors are both expected to increase with advanced age, a partial correlation was performed controlling for known age-at-death. When controlling for age-at-death, the partial correlation was not significant (r = 0.024;
p = 0.684), indicating that the apparent relationship between AMTL and TA error is mostly explained by the relationship of both variables with age-at-death.
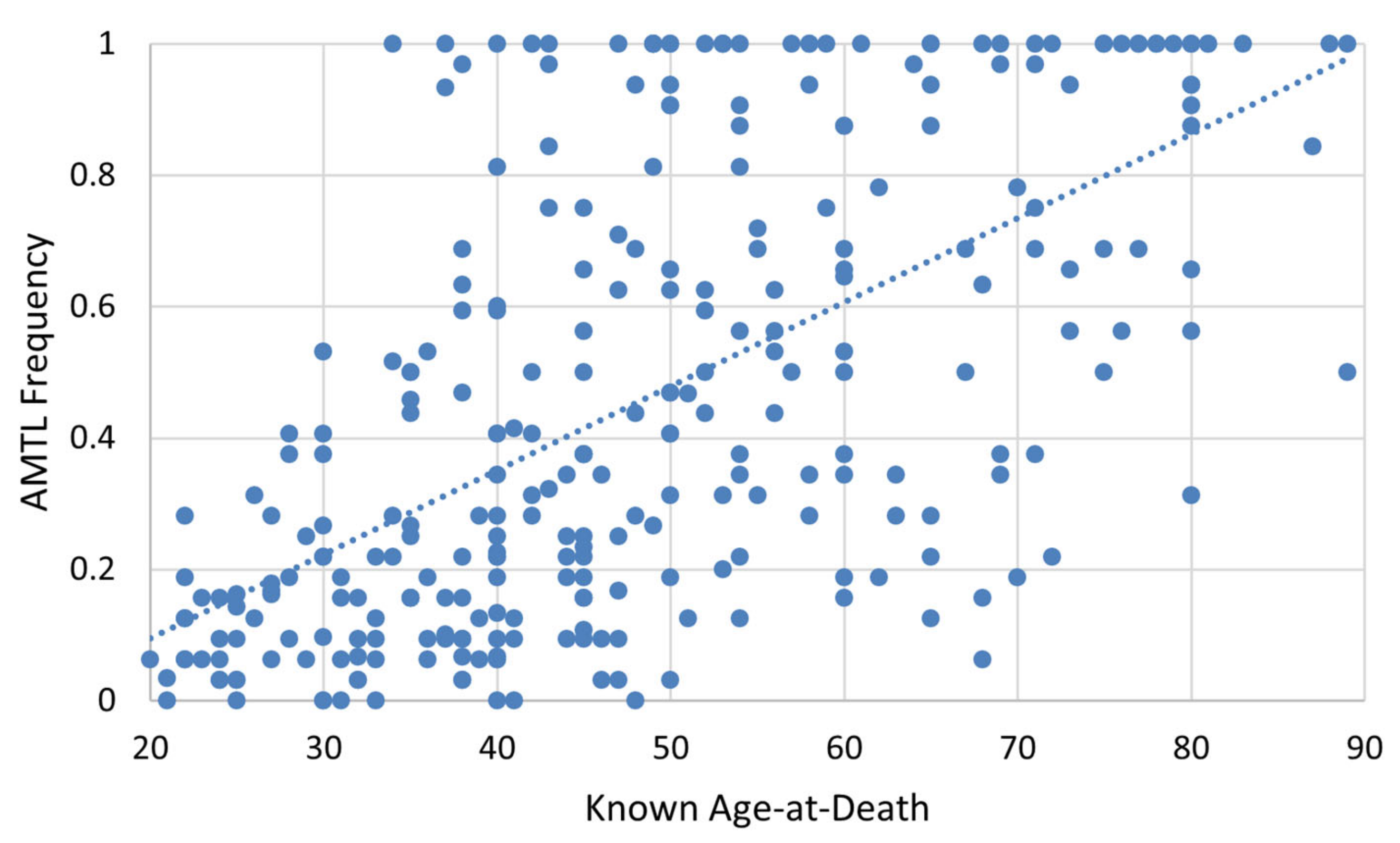
As depicted in
Figure 10, a relatively strong correlation exists between known age-at-death and AMTL frequency (r = 0.626,
p < 0.001), accounting for approximately 37% of variation in AMTL frequency. Spearman’s correlation of age-at-death and AMTL for each population group produced similar results (
Table 7).
4. Discussion
As is evident from the contextual information surrounding the acquisition of the human remains in the Hamann–Todd Osteological Collection (HTOC) and from the data presented herein, individuals in the current sample were exposed to many stressors throughout their lives. LEH incidence was high in this sample, affecting 44.1% of individuals. These data indicate that nearly one half of the people in the sample experienced a stressor that manifested in an LEH at the time the crowns of the incisors and canines were forming (approximately 6 months to 6 years) [
76]. However, this figure is much lower compared to previous analyses of enamel hypoplasia using the HTOC (e.g., [
59,
77]). These differences could reflect sampling differences or differences in methodological choices between studies. Although it has been found that individuals with LEH are more likely to die at younger ages (e.g., [
58]), there was no significant difference in age-at-death between individuals with LEH and those without LEH for this population.
Females were significantly shorter in stature if they had at least one LEH, showing possible severe and prolonged physiological stress exposure in this population that manifested in the skeleton through more than one indicator, namely, LEH presence and decreased stature. However, the same pattern was not found in males. This suggests that after experiencing stress in early childhood (i.e., when the LEH formed), females may not have been able to achieve the same catch-up growth as males in adolescence. Children between the ages of one and three years typically experience rapid musculoskeletal and brain growth. However, when faced with a significant or prolonged physiological stressor, energy is diverted from musculoskeletal growth to brain growth [
62,
63] and immune function [
78,
79], resulting in both LEH and decreased stature. This period is followed by relatively gradual linear growth until about age nine, when musculoskeletal growth accelerates for girls and peaks just prior to the onset of menstruation. Musculoskeletal growth in girls continues gradually after menarche and ceases around age 15. Moreover, the onset of menarche has been shown to be negatively correlated with prenatal and psychosocial stress, whereby females who experience more prenatal [
80] or psychosocial stressors (e.g., [
81,
82]) begin menstruation earlier and thus cease musculoskeletal growth earlier [
83]. The association between age at menarche and stress, therefore, further shortens the window females have for catch-up growth. Males, however, experience a slightly later and much longer adolescent growth spurt, making musculoskeletal gains until about ages 18–19 [
84]. Thus, males who experienced early childhood stress are generally better able to achieve catch-up growth because they have a longer window in which to achieve it [
85]. Differences in growth trajectories, therefore, may help to explain the association between LEH and stature among females in this sample.
Previous literature has documented a trend of “superior female buffering” by which females may be less sensitive to various physiological stressors than males [
86,
87]. The results presented herein are not in opposition to superior female buffering, but do not provide direct support for this theory. Based on historical documentation and previous studies of the HTOC, it is known that females in the sample population were exposed to higher rates of long-term institutionalization [
35] and had limited employment opportunities [
36], in addition to being exposed to stressors related to poverty that their male counterparts would have also experienced. Even if females in the HTOC were exposed to more severe or prolonged periods of stress compared to males, local circumstances can explain differing results between this study and others that have found evidence for superior female buffering (e.g., [
86,
87]).
Furthermore, the literature shows that females are more likely to have AMTL due to biological and cultural reasons [
70]. Biologically, females have higher rates of dental caries, most likely due to salivary flow related to hormone variation, which can result in AMTL if untreated. A substantial body of research exists on the influence of pregnancy and lactation on oral health (e.g., [
88,
89,
90]). Sex hormones are known to fluctuate during pregnancy, affecting levels of oral bacteria and increasing risk of infection, including periodontal disease [
70]. Culturally, AMTL is affected by diet, nutrition, and behavior. It is possible that dietary differences existed among populations in early 20th century Cleveland which contributed to the varying rates of AMTL seen here. Last, socioeconomic status and gender have been tied to oral health in many populations [
70]. Thus, sex-based differences in AMTL may also be reflective of the lower socioeconomic status of women in the HTOC.
With specific regard to the HTOC, the high frequency of AMTL in White females may reflect higher institutionalization rates. De la Cova (2020) found that roughly 40 percent of White females in the utilized HTOC sample were hospitalized long-term or placed in a mental health institution [
35]. Poor funding and staffing in such institutions during the early 20th century contributed to unsanitary and unsafe conditions for patients, which is evidenced by higher frequencies of hip fractures among White females in the Terry Collection, in which individuals lived in similar conditions to those in the HTOC [
35]. Overall, these observed differences in the prevalence of physiological stress markers and AMTL between males and females and White and Black Americans may reflect different biological and cultural risk factors and buffers.
Regardless of differences between subsamples, the high prevalence of AMTL in this sample demonstrates poor overall health. These findings are consistent with what is known about the socioeconomic backgrounds of the individuals that compose the HTOC. Generally, they were among the poorest of urban Cleveland [
35,
36,
37,
38,
39]. Access to resources such as medical care, job opportunities, and education would have been restricted in this setting [
36,
37], which would have influenced the overall pattern and expression of stress in these individuals.
We did not find a difference in physiological stress markers between Black and White individuals in the HTOC. However, mortality rates for tuberculosis and pneumonia among Black Americans were more than double those of White Americans in Cleveland [
91]. This reflects the greater risk of infectious disease resulting from poorer living conditions in Black communities during the late 19th and early 20th century in Cleveland. Black Americans were often excluded from jobs in industry and faced greater economic marginalization than White Americans [
36,
37]. Moreover, previous literature has shown that Reconstruction-era Black males exhibited higher rates of tuberculosis and treponematosis than White males in the HTOC [
39]. It can be concluded that although there was no difference in physiological stress markers between Black and White Americans in the HTOC, differences still existed in the lived experiences of these communities.
Although the sample studied herein demonstrates evidence of poor overall health and differences in stress marker prevalence between the subsamples, no association between age estimation error and stress markers, with the exception of AMTL, was found in the overall sample or any subsamples. Although the correlation between age estimation error and AMTL is significant, this relationship is mostly explained by age-at-death. The results presented herein provide evidence that physiological stress and health status do not significantly affect age estimation accuracy in this sample. This is an important consideration in forensic contexts when applying age estimation methods to individuals thought to have experienced moderate to severe physiological stress or poor health, as is common in forced migration and humanitarian cases [
92,
93]. In these samples, physiological stress may be an unlikely source of bias in age estimation. However, other factors, such as genetics, epigenetics, activity levels, and lifestyle, may contribute to age estimation error more significantly than the aspects of physiological stress tested in this study.
Historically, the literature focused on age-at-death estimation has emphasized refining existing methods or developing new methods using different skeletal markers of age to improve accuracy and precision. This approach fundamentally assumes that biological age correlates with chronological age and that we can improve methods by refining which skeletal markers and statistical approaches are used. Yet, some recently developed methods have shown a significant advancement in age estimation accuracy and precision over traditional age-at-death estimation methods (e.g., [
94]). An exception is the recent findings of Navega et al. (2022), which demonstrate that multifactorial methods and machine learning may lead to needed advancements in accuracy and precision [
94].
Thus, it is necessary to consider which factors may affect skeletal aging and understand to what extent and how these factors influence age estimation. This research represents progress towards this goal. Although physiological stress was not found to be a significant factor affecting skeletal aging and age estimation accuracy in this adult sample, other possible variables including, but not limited to, activity levels, genetics, and epigenetics should be investigated in the future. Further, different stress markers should be explored in this context, since different markers may represent different periods in individual lifespans or proxies for different biological systems influenced by the physical or psychological stressors experienced during life.
It must also be considered that the high age estimation error using TA in the HTOC is attributable to the general poor health of the population. In other words, it is assumed in this study that all or most individuals in the sample experienced moderate to severe physiological stress during their lifetimes. Here, we relied on comparisons between different sub-samples in the study, i.e., those without skeletal markers of physiological stress compared to those with skeletal markers of physiological stress, but found the same age estimation error rates. However, even those that did not display LEH or shorter stature may have experienced physiological stress that did not manifest in the skeleton. This point is especially relevant when considering the background of skeletal samples that are often used to develop and refine age-at-death estimation techniques in biological anthropology. Namely, many of the samples used for the development of age-at-death estimation techniques are similar in background to the HTOC, in that they often comprise individuals from lower socioeconomic backgrounds. For example, transition analysis, the accuracy of which was tested in this study, was originally developed and tested using individuals curated in the Terry Collection [
8]. These individuals would have been similarly stressed to the individuals in the HTOC in that they also represent individuals of lower socioeconomic status who lived in and around St. Louis, Missouri during similar timeframes as the HTOC (e.g., de la Cova, 2020 [
35]). Thus, further studies are needed to determine whether similar results can be observed in archaeological or modern known age-at-death skeletal samples that represent individuals of higher socioeconomic status for whom the socioeconomic context does not match that of the reference samples for which TA was initially developed. Future studies should compare these results with other collections believed to have had a higher quality of life.
The inter-relatedness of the markers of physiological stress studied herein may also provide another avenue for future investigations. Combining all three indicators of physiological stress for each individual may reveal deeper trends reflecting sociocultural structures and environment for the sample represented in the HTOC.
Improving age estimation from skeletal remains relies on building a stronger understanding of many factors that influence biological age relative to chronological age, instead of only attempting to develop new methods. One such factor that could influence processes of biological aging is overall health, estimated here using three markers of physiological stress as a proxy. While no associations were observed between TA age estimate error and the physiological stress markers in this sample, they and other factors that could influence age remain important factors for future consideration in tests of the accuracy of age-at-death estimation techniques.