Systematic Evaluation of Sea Stars of the Genus Heliaster from the Southeastern Pacific and Redescription of Heliaster helianthus
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Material Examined
2.3. Comparative Material Examined
2.4. Morphological Measurements
2.5. Molecular Protocol for Gene Amplification and Sequencing
2.6. Phylogenetic Analysis
2.7. Species Delimitation
3. Results
3.1. Taxonomic Account
3.2. New Diagnosis
3.3. Redescription
3.4. Distribution
3.5. Habitat
3.6. Neotype Designation and Type Locality
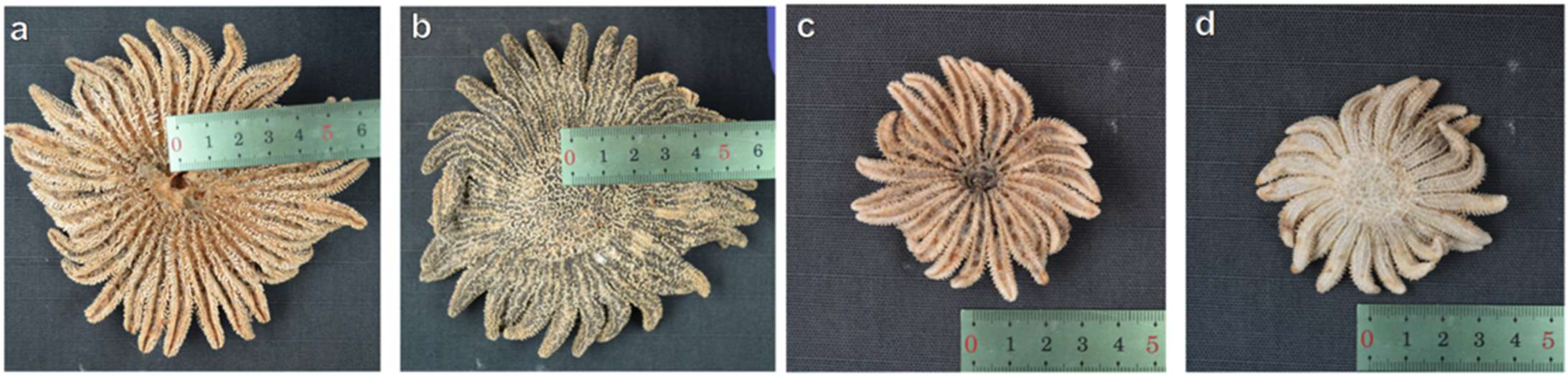
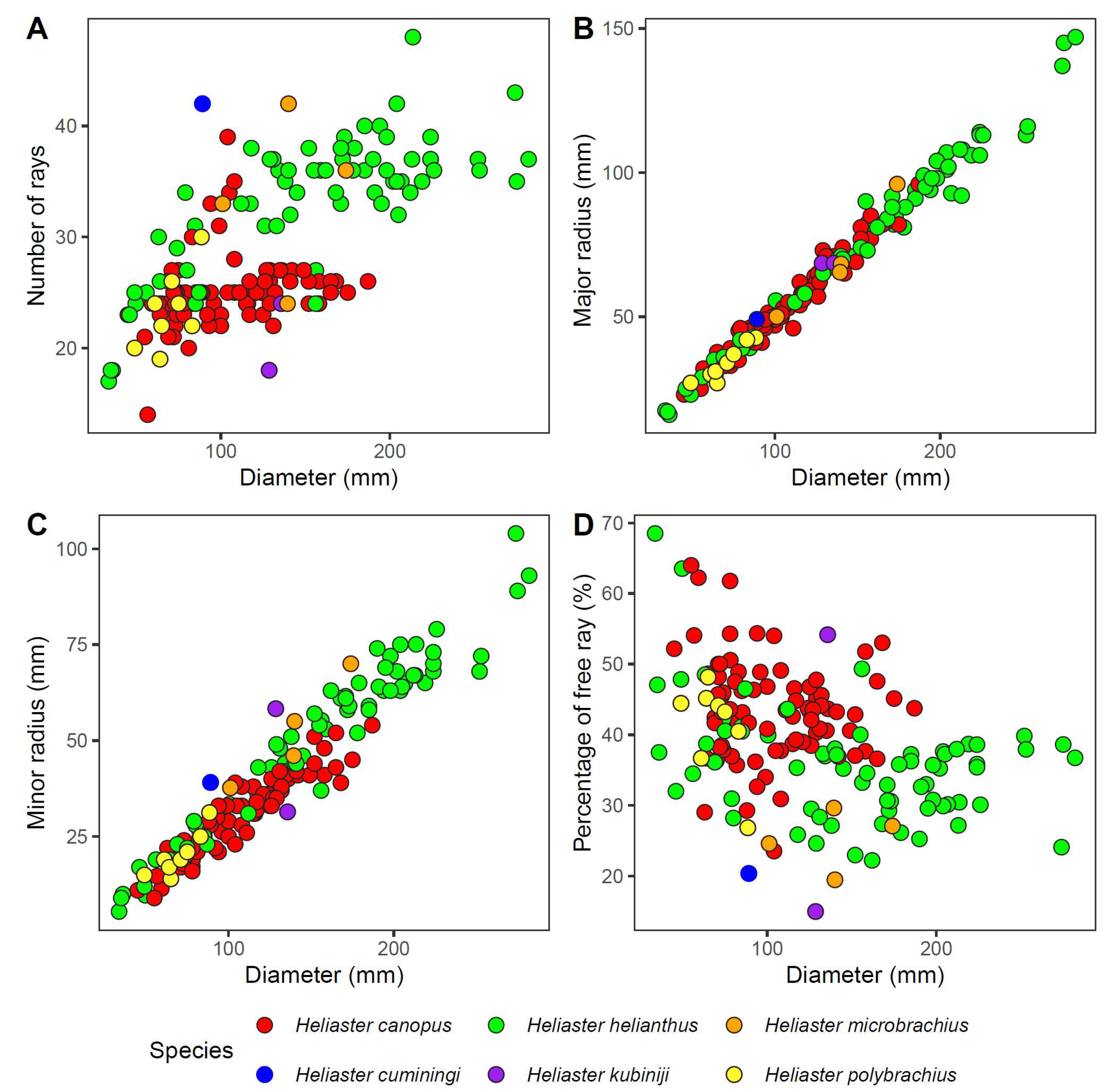
3.7. Morphological Variability
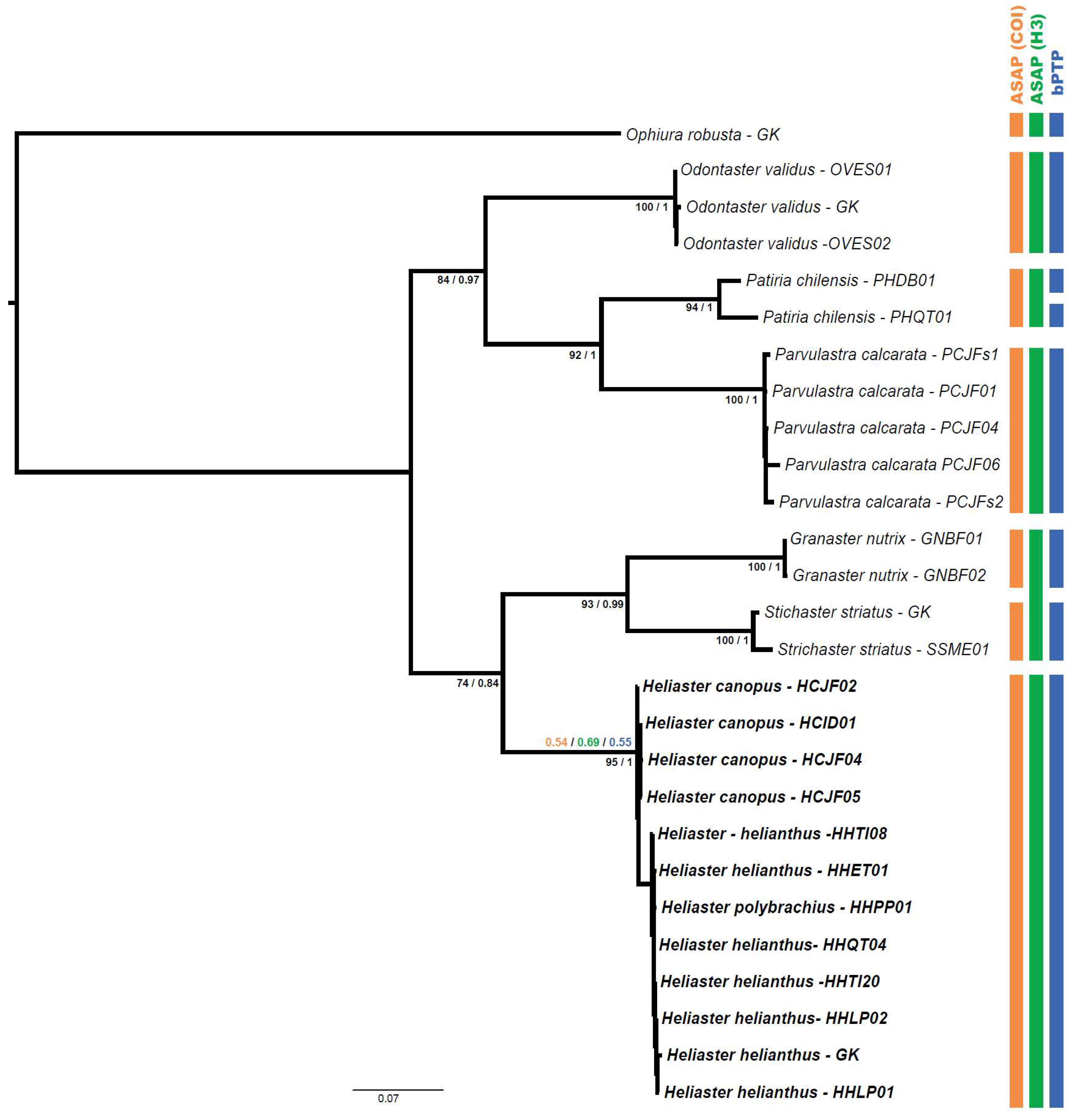
3.8. Phylogeny and Species Delimitation
4. Discussion
4.1. Morphology
4.2. Species Delimitation and Phylogeny
4.3. Distribution
4.4. Biogeographic and Evolutionary Implications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Code | Locality | Collection | Latitude | Longitude | COI | H3 |
|---|---|---|---|---|---|---|---|
| Heliaster polybrachius | HPPP01 | Pucusana, Perú | 2013 | −12.4799 | −76.8009 | PX112071 | PX136096 |
| Heliaster canopus | HCID01 | San ambrosio Island | 2018 | −26.3380 | −79.8732 | PX112064 | PX136089 |
| Heliaster canopus | HCJF01 | Robinson Crusoe Island | 2009 | −33.6213 | −78.8453 | ||
| Heliaster canopus | HCJF02 | Robinson Crusoe Island | 2009 | −33.6361 | −78.8301 | PX112065 | PX136090 |
| Heliaster canopus | HCJF04 | Robinson Crusoe Island | 2009 | −33.6420 | −78.8247 | PX112066 | PX136091 |
| Heliaster canopus | HCJF05 | Robinson Crusoe Island | 2009 | −33.6420 | −78.8247 | PX112067 | PX136092 |
| Heliaster canopus | HCJF07 | Robinson Crusoe Island | 2009 | −33.6213 | −78.8453 | ||
| Heliaster canopus | HCJF08 | Robinson Crusoe Island | 2025 | −33.6405 | −78.8143 | ||
| Heliaster canopus | HCJF09 | Robinson Crusoe Island | 2025 | −33.6405 | −78.8143 | ||
| Heliaster canopus | HCJF10 | Robinson Crusoe Island | 2025 | −33.6405 | −78.8143 | ||
| Heliaster canopus | HCJF11 | Robinson Crusoe Island | 2025 | −33.6405 | −78.8143 | ||
| Heliaster canopus | HCJF12 | Robinson Crusoe Island | 2025 | −33.6405 | −78.8143 | ||
| Heliaster canopus | HCJF13 | Robinson Crusoe Island | 2025 | −33.6361 | −78.8301 | ||
| Heliaster canopus | HCJF14 | Robinson Crusoe Island | 2025 | −33.6420 | −78.8247 | ||
| Heliaster canopus | HCJF15 | Robinson Crusoe Island | 2025 | −33.6420 | −78.8247 | ||
| Heliaster helianthus | HHLP01 | Lobitos, Perú | 2014 | −4.4526 | −81.2891 | PX112069 | PX136094 |
| Heliaster helianthus | HHLP02 | Lobitos, Perú | 2014 | −4.4526 | −81.2891 | PX112070 | PX136095 |
| Heliaster helianthus | HHTI08 | Tres Islas, Iquique | 2014 | −20.3149 | −70.1383 | PX112073 | PX136098 |
| Heliaster helianthus | HHTI20 | Tres Islas, Iquique | 2014 | −20.3149 | −70.1383 | PX112074 | PX136099 |
| Heliaster helianthus | HHLM01 | Los Molles, Chile | 2025 | −32.2398 | −71.5111 | ||
| Heliaster helianthus | HHQT04 | Quintay, Chile | 2018 | −33.1911 | −71.7025 | PX112072 | PX136097 |
| Heliaster helianthus | MNHNCL EQUI-17521 * | Quintay, Chile | 2022 | −33.1828 | −71.6856 | ||
| Heliaster helianthus | HHAL01 | Algarrobo, Chile | 2023 | −33.3580 | −71.6644 | ||
| Heliaster helianthus | HHET01 | El Caleuche, El Tabo | 2019 | −33.4567 | −71.6716 | PX112068 | PX136093 |
| Heliaster helianthus | HHET02 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET03 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET04 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET05 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET06 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET07 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET08 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET09 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET10 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET11 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET12 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET13 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET14 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET15 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET16 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET17 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET18 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET19 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET20 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET21 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET22 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET23 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET24 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET25 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET26 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHET27 | El Caleuche, El Tabo | 2025 | −33.4567 | −71.6716 | ||
| Heliaster helianthus | HHLC01 | Las Cruces, Chile | 2025 | −33.5005 | −71.6351 | ||
| Heliaster helianthus | HHLC02 | Las Cruces, Chile | 2025 | −33.5005 | −71.6351 | ||
| Heliaster helianthus | HHLB01 | La Boca, Chile | 2025 | −33.9197 | −71.8492 | ||
| Stichaster striatus | SSME 01 | Mejillones | 2013 | −23.0988 | −70.4601 | PX112084 | PX136109 |
| Granaster nutrix | GNBF01 | Bahía Fildes, Antártica | 2022 | −62.1995 | −58.9592 | ||
| Granaster nutrix | GNBF02 | Bahía Fildes, Antártica | 2022 | −62.1995 | −58.9592 | ||
| Odontaster validus | OVES01 | Escudero, Antártica | 2022 | −62.1995 | −58.9592 | PX112075 | PX136100 |
| Odontaster validus | OVES02 | Escudero, Antártica | 2022 | −62.1995 | −58.9592 | PX112076 | PX136101 |
| Patiria chilensis | PHB01 | Desembocadura, Biobio | 2020 | −36.8125 | −73.1749 | PX112082 | PX136107 |
| Patiria chilensis | PHQT01 | Quintay | 2018 | −33.1911 | −71.7025 | PX112083 | PX136108 |
| Parvulastra calcarata | PCJF01 | Robinson Crusoe Island | 2009 | −33.6361 | −78.8301 | PX112077 | PX136102 |
| Parvulastra calcarata | PCJF04 | Robinson Crusoe Island | 2009 | −33.6361 | −78.8301 | PX112078 | PX136103 |
| Parvulastra calcarata | PCJF06 | Robinson Crusoe Island | 2009 | −33.6361 | −78.8301 | PX112079 | PX136104 |
| Parvulastra calcarata | PCJFS1 | Robinson Crusoe Island | 2019 | −33.6308 | −78.8797 | PX112080 | PX136105 |
| Parvulastra calcarata | PCJFS2 | Robinson Crusoe Island | 2019 | −33.6308 | −78.8797 | PX112081 | PX136106 |
| Code | Species | N° of Rays | R (mm) | r (mm) | Free Rays (%) | Maximum Diameter (mm) |
|---|---|---|---|---|---|---|
| HHPP01 | H. polybrachius | 30 | 37.27 | 29.45 | 20.98 | 88.48 |
| HCID01 | H. canopus | 14 | 32.00 | 14.70 | 54.06 | 56.70 |
| HCJF01 | H. canopus | 27 | 36.95 | 21.92 | 40.68 | 74.84 |
| HCJF02 | H. canopus | 25 | 44.46 | 25.27 | 43.16 | 85.27 |
| HCJF08 | H. canopus | 23 | 45.13 | 17.25 | 61.78 | 78.02 |
| HCJF09 | H. canopus | 24 | 37.69 | 19.36 | 48.63 | 65.01 |
| HCJF10 | H. canopus | 25 | 38.06 | 21.96 | 42.30 | 75.18 |
| HCJF11 | H. canopus | 24 | 30.33 | 11.45 | 62.25 | 59.30 |
| HCJF12 | H. canopus | 24 | 42.39 | 24.69 | 41.76 | 82.48 |
| HCJF13 | H. canopus | 24 | 51.36 | 26.26 | 48.87 | 95.86 |
| HCJF14 | H. canopus | 26 | 67.16 | 40.09 | 40.31 | 128.95 |
| HCJF15 | H. canopus | 23 | 36.74 | 19.04 | 48.18 | 71.26 |
| HHME01 | H. helianthus | 17 | 17.41 | 5.48 | 68.52 | 33.68 |
| MNHNCL EQUI-17521 * | H. helianthus | 33 | 91.72 | 61.54 | 32.90 | 170.93 |
| HHLM01 | H. helianthus | 35 | 92.82 | 64.84 | 30.14 | 206.75 |
| HHAL01 | H. helianthus | 48 | 107.93 | 75.08 | 30.44 | 213.56 |
| HHET02 | H. helianthus | 31 | 39.15 | 23.05 | 41.12 | 84.70 |
| HHET03 | H. helianthus | 36 | 69.90 | 44.05 | 36.98 | 134.10 |
| HHET04 | H. helianthus | 37 | 82.45 | 55.05 | 33.23 | 156.20 |
| HHET05 | H. helianthus | 31 | 61.00 | 43.00 | 29.51 | 126.00 |
| HHET06 | H. helianthus | 37 | 82.00 | 58.00 | 29.27 | 172.01 |
| HHET07 | H. helianthus | 34 | 71.00 | 46.00 | 35.21 | 145.10 |
| HHET08 | H. helianthus | 31 | 67 | 42.00 | 37.31 | 133.00 |
| HHET09 | H. helianthus | 35 | 145.00 | 89.00 | 38.62 | 275.00 |
| HHET10 | H. helianthus | 36 | 94.00 | 59.00 | 37.23 | 185.00 |
| HHET11 | H. helianthus | 37 | 147.00 | 93.00 | 36.73 | 282.00 |
| HHET12 | H. helianthus | 37 | 113.00 | 68.00 | 39.82 | 252.10 |
| HHET13 | H. helianthus | 39 | 85.00 | 59.00 | 30.59 | 173.20 |
| HHET14 | H. helianthus | 42 | 101.00 | 63.00 | 37.62 | 204.00 |
| HHET15 | H. helianthus | 35 | 106.00 | 65.00 | 38.68 | 219.00 |
| HHET16 | H. helianthus | 39 | 106.00 | 68.00 | 35.85 | 224.10 |
| HHET17 | H. helianthus | 40 | 91.00 | 58.00 | 36.26 | 185.20 |
| HHET18 | H. helianthus | 36 | 81.00 | 52.00 | 35.80 | 178.10 |
| HHET19 | H. helianthus | 37 | 92.00 | 67.00 | 27.17 | 213.00 |
| HHET20 | H. helianthus | 36 | 81.00 | 53.00 | 34.57 | 159.10 |
| HHET21 | H. helianthus | 40 | 94.00 | 63.00 | 32.98 | 194.10 |
| HHET22 | H. helianthus | 36 | 116.00 | 72.00 | 37.93 | 253.00 |
| HHET23 | H. helianthus | 37 | 114.00 | 70.00 | 38.60 | 224.20 |
| HHET24 | H. helianthus | 35 | 105.00 | 68.00 | 35.24 | 202.00 |
| HHET25 | H. helianthus | 35 | 107.00 | 75.00 | 29.91 | 204.00 |
| HHET26 | H. helianthus | 39 | 104.00 | 72.00 | 30.77 | 198.00 |
| HHET27 | H. helianthus | 37 | 113.00 | 73.00 | 35.40 | 224.00 |
| HHLC01 | H. helianthus | 24 | 26.60 | 9.7.00 | 63.53 | 49.66 |
| HHLC02 | H. helianthus | 33 | 55.60 | 33.4.00 | 39.93 | 100.50 |
| HHLB01 | H. helianthus | 30 | 35.05 | 18.05 | 48.50 | 63.20 |
| Code | Species | Locality | Latitude | Longitude | N° of Rays | R (mm) | r (mm) | Free Rays (%) | Max. Diameter (mm) |
|---|---|---|---|---|---|---|---|---|---|
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 24 | 85 | 41 | 51.76 | 158 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 26 | 83 | 39 | 53.01 | 168 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 26 | 77 | 48 | 37.66 | 158 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 23 | 33 | 21 | 36.36 | 70 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 27 | 71 | 40 | 43.66 | 136 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 25 | 82 | 45 | 45.12 | 175 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 26 | 82 | 52 | 36.59 | 165 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 26 | 81 | 51 | 37.04 | 152 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 25 | 60 | 34 | 43.33 | 125 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 26 | 96 | 54 | 43.75 | 187 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 25 | 62 | 38 | 38.71 | 115 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 27 | 65 | 41 | 36.92 | 142 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 23 | 47 | 25 | 46.81 | 100 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 27 | 73 | 40 | 45.21 | 129 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 28 | 53 | 33 | 37.74 | 108 |
| MNHNCL-EQUI-15416 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 25 | 64 | 36 | 43.75 | 124 |
| MNHNCL-EQUI-15407 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 22 | 49 | 29 | 40.82 | 100 |
| MNHNCL-EQUI-15407 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 25 | 68 | 37 | 45.59 | 132 |
| MNHNCL-EQUI-15407 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 25 | 82 | 43 | 47.56 | 165 |
| MNHNCL-EQUI-15415 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 24 | 58 | 31 | 46.55 | 116 |
| MNHNCL-EQUI-15415 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 24 | 54 | 31 | 42.59 | 115 |
| MNHNCL-EQUI-15415 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 27 | 69 | 41 | 40.58 | 135 |
| MNHNCL-EQUI-15415 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 23 | 58 | 32 | 44.83 | 117 |
| MNHNCL-EQUI-15406 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 25 | 46 | 21 | 54.35 | 94 |
| MNHNCL-EQUI-15406 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 25 | 55 | 28 | 49.09 | 108 |
| MNHNCL-EQUI-15406 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 25 | 50 | 23 | 54.00 | 104 |
| MNHNCL-EQUI-15406 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 25 | 46 | 26 | 43.48 | 111 |
| MNHNCL-EQUI-15406 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 23 | 62 | 33 | 46.77 | 125 |
| MNHNCL-EQUI-15372 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 23 | 34 | 17 | 50.00 | 71 |
| MNHNCL-EQUI-15372 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 22 | 47 | 30 | 36.17 | 93 |
| MNHNCL-EQUI-15408 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 27 | 69 | 41 | 40.58 | 149 |
| MNHNCL-EQUI-15408 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 25 | 65 | 40 | 38.46 | 126 |
| MNHNCL-EQUI-15408 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 25 | 48 | 28 | 41.67 | 89 |
| MNHNCL-EQUI-15408 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 25 | 68 | 38 | 44.12 | 132 |
| MNHNCL-EQUI-15405 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 34 | 53 | 33 | 37.74 | 105 |
| MNHNCL-EQUI-15405 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 30 | 45 | 23 | 48.89 | 83 |
| MNHNCL-EQUI-15405 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 35 | 55 | 38 | 30.91 | 108 |
| MNHNCL-EQUI-15405 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 39 | 51 | 39 | 23.53 | 104 |
| MNHNCL-EQUI-15405 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 31 | 50 | 33 | 34.00 | 99 |
| MNHNCL-EQUI-15405 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 33 | 49 | 33 | 32.65 | 94 |
| MNHNCL-EQUI-15940 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 23 | 41 | 22 | 46.34 | 92 |
| MNHNCL-EQUI-15940 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 22 | 33 | 18 | 45.45 | 73 |
| MNHNCL-EQUI-15611 | H. canopus | Alejandro Selkirk Island | −33.7000 | −79.0000 | 21 | 36 | 18 | 50.00 | 72 |
| MNHNCL-EQUI-15611 | H. canopus | Alejandro Selkirk Island | −33.7000 | −79.0000 | 21 | 33 | 19 | 42.42 | 69 |
| MNHNCL-EQUI-15611 | H. canopus | Alejandro Selkirk Island | −33.7000 | −79.0000 | 24 | 36 | 21 | 41.67 | 69 |
| MNHNCL-EQUI-15611 | H. canopus | Alejandro Selkirk Island | −33.7000 | −79.0000 | 23 | 37 | 20 | 45.95 | 74 |
| MNHNCL-EQUI-15675 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 24 | 77 | 44 | 42.86 | 152 |
| MNHNCL-EQUI-15675 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 25 | 59 | 36 | 38.98 | 121 |
| MNHNCL-EQUI-15675 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 27 | 62 | 35 | 43.55 | 127 |
| MNHNCL-EQUI-15222 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 24 | 42 | 27 | 35.71 | 82 |
| MNHNCL-EQUI-15222 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 20 | 40 | 21 | 47.50 | 81 |
| MNHNCL-EQUI-15222 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 25 | 46 | 29 | 36.96 | 79 |
| MNHNCL-EQUI-15222 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 25 | 37 | 23 | 37.84 | 74 |
| MNHNCL-EQUI-15222 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 27 | 35 | 19 | 45.71 | 71 |
| MNHNCL-EQUI-15260 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 24 | 37 | 21 | 43.24 | 73 |
| MNHNCL-EQUI-15260 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 26 | 74 | 42 | 43.24 | 141 |
| MNHNCL-EQUI-15260 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 24 | 67 | 35 | 47.76 | 129 |
| MNHNCL-EQUI-15590 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 22 | 71 | 42 | 40.85 | 131 |
| MNHNCL-EQUI-15590 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 26 | 56 | 34 | 39.29 | 117 |
| MNHNCL-EQUI-15590 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 25 | 39 | 24 | 38.46 | 73 |
| MNHNCL-EQUI-15590 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 25 | 34 | 21 | 38.24 | 72 |
| MNHNCL-EQUI-15590 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 25 | 41 | 29 | 29.27 | 88 |
| MNHNCL-EQUI-15248 | H. canopus | San Ambrosio Island | −26.3439 | −79.8714 | 23 | 23 | 11 | 52.17 | 45 |
| MNHNCL-EQUI-15409 | H. canopus | Robinson Crusoe Island | −33.6167 | −78.8667 | 23 | 35 | 16 | 54.29 | 78 |
| MNHNCL-EQUI-15410 | H. canopus | Santa Clara Island | −33.7066 | −78.9393 | 26 | 57 | 33 | 42.11 | 126 |
| MNHNCL-EQUI-15612 | H. canopus | San Ambrosio Island | −26.3439 | −79.8714 | 24 | 31 | 22 | 29.03 | 63 |
| MNHNCL-EQUI-15748 | H. canopus | Juan Fernandez Archipelago | −33.6167 | −78.8667 | 24 | 39 | 22 | 43.59 | 78 |
| MNHNCL-EQUI-15910 | H. canopus | Alejandro Selkirk Island | −33.7000 | −79.0000 | 21 | 25 | 9 | 64.00 | 55 |
| MNHNCL-EQUI-15936 | H. canopus | Alejandro Selkirk Island | −33.7000 | −79.0000 | 23 | 30 | 19 | 36.67 | 64 |
| MNHNCL-EQUI-15116 | H. helianthus | ND | ND | ND | 36 | 90 | 54 | 40.00 | 155 |
| MNHNCL-EQUI-15116 | H. helianthus | ND | ND | ND | 34 | 84 | 61 | 27.38 | 168 |
| MNHNCL-EQUI-15188 | H. helianthus | Iquique | −21.0906 | −70.1356 | 35 | 70 | 51 | 27.14 | 138 |
| MNHNCL-EQUI-15188 | H. helianthus | Iquique | −21.0906 | −70.1356 | 33 | 55 | 31 | 43.64 | 112 |
| MNHNCL-EQUI-15379 | H. helianthus | Las Cruces | −33.5012 | −71.6362 | 38 | 88 | 65 | 26.14 | 179 |
| MNHNCL-EQUI-15379 | H. helianthus | Las Cruces | −33.5012 | −71.6362 | 37 | 99 | 74 | 25.25 | 190 |
| MNHNCL-EQUI-15109 | H. helianthus | Cobija, Tocopilla | −22.5500 | −70.2667 | 32 | 102 | 64 | 37.25 | 205 |
| MNHNCL-EQUI-15109 | H. helianthus | Cobija, Tocopilla | −22.5500 | −70.2667 | 34 | 108 | 67 | 37.96 | 212 |
| MNHNCL-EQUI-15106 | H. helianthus | Los Vilos, Coquimbo | −31.9090 | −71.5175 | 38 | 74 | 57 | 22.97 | 152 |
| MNHNCL-EQUI-15106 | H. helianthus | Los Vilos, Coquimbo | −31.9090 | −71.5175 | 34 | 95 | 64 | 32.63 | 191 |
| MNHNCL-EQUI-15108 | H. helianthus | Cobija, Tocopilla | −22.5500 | −70.2667 | 26 | 36 | 23 | 36.11 | 69 |
| MNHNCL-EQUI-15108 | H. helianthus | Cobija, Tocopilla | −22.5500 | −70.2667 | 27 | 39 | 28 | 28.21 | 80 |
| MNHNCL-EQUI-15679 | H. helianthus | Iquique | −20.4764 | −70.1864 | 25 | 29 | 19 | 34.48 | 56 |
| MNHNCL-EQUI-15679 | H. helianthus | Iquique | −20.4764 | −70.1864 | 25 | 23 | 12 | 47.83 | 49 |
| MNHNCL-EQUI-15218 | H. helianthus | Santa Clara Island | −33.7066 | −78.9393 | 24 | 42 | 25 | 40.48 | 85 |
| MNHNCL-EQUI-15218 | H. helianthus | Santa Clara Island | −33.7066 | −78.9393 | 25 | 43 | 23 | 46.51 | 87 |
| MNHNCL-EQUI-15218 | H. helianthus | Santa Clara Island | −33.7066 | −78.9393 | 24 | 73 | 37 | 49.32 | 156 |
| MNHNCL-EQUI-15239 | H. helianthus | Alejandro Selkirk Island | −33.7000 | −79.0000 | 24 | 37 | 22 | 40.54 | 75 |
| MNHNCL-EQUI-15739 | H. helianthus | Las Cruces | −33.5012 | −71.6362 | 26 | 31 | 19 | 38.71 | 64 |
| MNHNCL-EQUI-15021 | H. helianthus | El Tabo | −33.4500 | −71.6333 | 23 | 25 | 17 | 32.00 | 46 |
| MNHNCL-EQUI-15096 | H. helianthus | Iquique | −20.2194 | −70.1719 | 34 | 42 | 29 | 30.95 | 79 |
| MNHNCL-EQUI-15103 | H. helianthus | Los Vilos, Coquimbo | −31.9090 | −71.5175 | 36 | 98 | 63 | 35.71 | 198 |
| MNHNCL-EQUI-15104 | H. helianthus | Los Vilos, Coquimbo | −31.9090 | −71.5175 | 37 | 67 | 48 | 28.36 | 131 |
| MNHNCL-EQUI-15105 | H. helianthus | Los Vilos, Coquimbo | −31.9090 | −71.5175 | 38 | 88 | 61 | 30.68 | 171 |
| MNHNCL-EQUI-15107 | H. helianthus | Los Vilos, Coquimbo | −31.9090 | −71.5175 | 33 | 98 | 69 | 29.59 | 195 |
| MNHNCL-EQUI-15119 | H. helianthus | Quintero | −32.7667 | −71.5333 | 36 | 81 | 63 | 22.22 | 162 |
| MNHNCL-EQUI-15120 | H. helianthus | Quintero | −32.7667 | −71.5333 | 36 | 113 | 79 | 30.09 | 226 |
| MNHNCL-EQUI-15121 | H. helianthus | Quintero | −32.7667 | −71.5333 | 37 | 65 | 49 | 24.62 | 129 |
| MNHNCL-EQUI-15122 | H. helianthus | ND | ND | ND | 43 | 137 | 104 | 24.09 | 274 |
| MNHNCL-EQUI-15176 | H. helianthus | ND | ND | ND | 38 | 58 | 43 | 25.86 | 118 |
| MNHNCL-EQUI-15188 | H. helianthus | Iquique | −21.0906 | −70.1356 | 36 | 71 | 44 | 38.03 | 140 |
| MNHNCL-EQUI-15278 | H. helianthus | El Tabo | −33.4500 | −71.6333 | 18 | 16 | 10 | 37.50 | 36 |
| MNHNCL-EQUI-15374 | H. helianthus | Quintero | −32.7667 | −71.5333 | 32 | 70 | 44 | 37.14 | 141 |
| MNHNCL-EQUI-15594 | H. helianthus | Isla Negra | −33.4380 | −71.6917 | 18 | 17 | 9 | 47.06 | 35 |
| MNHNCL-EQUI-15371 | H. polybrachius | Robinson Crusoe Island | −33.6167 | −78.8667 | 22 | 42 | 25 | 40.48 | 83 |
| MNHNCL-EQUI-15371 | H. polybrachius | Robinson Crusoe Island | −33.6167 | −78.8667 | 24 | 30 | 19 | 36.67 | 61 |
| MNHNCL-EQUI-15368 | H. polybrachius | San Ambrosio Island | −26.3439 | −79.8714 | 20 | 27 | 15 | 44.44 | 49 |
| MNHNCL-EQUI-15368 | H. polybrachius | San Ambrosio Island | −26.3439 | −79.8714 | 22 | 27 | 14 | 48.15 | 65 |
| MNHNCL-EQUI-15370 | H. polybrachius | Robinson Crusoe Island | −33.6167 | −78.8667 | 19 | 31 | 17 | 45.16 | 64 |
| MNHNCL-EQUI-15373 | H. polybrachius | Robinson Crusoe Island | −33.6167 | −78.8667 | 26 | 34 | 19 | 44.12 | 71 |
| MNHNCL-EQUI-15399 | H. polybrachius | Alejandro Selkirk Island | −33.7000 | −79.0000 | 24 | 37 | 21 | 43.24 | 75 |
| Code | Species | Locality | N° of Rays | R (mm) | r (mm) | Free Rays (%) | Diameter (mm) |
|---|---|---|---|---|---|---|---|
| USNM-E03771 | H. canopus | Juan Fernandez Is., Chile | 24 | 46.15 | 24.82 | 46.22 | 84.92 |
| USNME-3554 | H. helianthus | Salaverry, Peru | 29 | 35.06 | 24.16 | 31.09 | 74.03 |
| USNME-032619 | H. kubiniji | 18 | 68.57 | 58.30 | 14.98 | 128.57 | |
| USNM-2017 | H. microbrachius | Baja California, Mexico | 36 | 96.00 | 70.00 | 27.08 | 174.00 |
| USNM-E02624 | H. microbrachius | 42 | 68.30 | 55.00 | 19.47 | 140.00 | |
| USNME-6767 | H. microbrachius | Perlas Archipelago, Panama | 24 | 65.45 | 46.06 | 29.63 | 139.39 |
| MNHN-IE-2014-424-01 | H. canopus | Juan Fernandez Is., Chile | 23 | 35.14 | 20.27 | 42.32 | 70.95 |
| MNHN-IE-2014-424-02 | H. canopus | Juan Fernandez Is., Chile | 24 | 38.44 | 19.01 | 50.55 | 78.17 |
| MNHN-IE-2017-1389 | H. canopus | Chile | 24 | 36.15 | 20.00 | 44.67 | 70.00 |
| MNHN-IE-2017-669 | H. helianthus | Caldera, Chile | 33 | 56.25 | 40.00 | 28.89 | 117.50 |
| MNHN-IE-2017-1831 | H. cuminingi | Perú | 42 | 49.09 | 39.09 | 20.37 | 89.09 |
| MNHN-IE-2017-1350 | H. kubiniji | Acapulco, Mexico | 24 | 68.57 | 31.43 | 54.16 | 135.71 |
| MNHN-IE-2017-665 | H. microbrachius | California, EEUU | 33 | 50.00 | 37.70 | 24.60 | 101.10 |
References
- Mah, C.; Foltz, D. Molecular phylogeny of the Forcipulatacea (Asteroidea: Echinodermata): Systematics and biogeography. Zool. J. Linn. Soc. 2011, 162, 646–660. [Google Scholar] [CrossRef]
- Perrier, E. Étude sur la Repartition Géographique des Astérides; Second Series; Nouvelles Archives du Muséum d’Histoire Naturelle: Paris, France, 1878; Volume 1, pp. 1–108. [Google Scholar]
- Clark, H.L. The starfishes of the genus Heliaster. Bull. Mus. Comp. Zool. Harv. 1907, 51, 25–76. [Google Scholar]
- Castilla, J.C.; Navarrete, S.A.; Manzur, T.; Barahona, M. Heliaster helianthus; The Johns Hopkins University Press: Baltimore, MD, USA, 2013; pp. 153–160. [Google Scholar]
- Tokeshi, M. Development of a foraging model for a field population of the South American sun-star Heliaster helianthus. J. Anim. Ecol. 1989, 58, 189–206. [Google Scholar] [CrossRef]
- Perrier, E. Révision de la collection de Stellérides du Muséum d’Histoire Naturelle de Paris. Arch. Zool. Exp. Gen. 1875, 4, 265–450. [Google Scholar]
- Alvarado, J.J.; Solís-Marín, F.A. Echinoderm research and diversity in Latin America. In Echinoderm Research and Diversity in Latin America; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–9. [Google Scholar] [CrossRef]
- de Lamarck, J.B.P.A. Asterie. In Histoire Naturelle des Animaux sans Vertèbres; Verdière: Paris, France, 1816; Volume 2, pp. 547–568. [Google Scholar]
- Rozbaczylo, N.; Castilla, J.C. Invertebrados marinos del archipiélago de Juan Fernández. In Islas Oceánicas Chilenas: Conocimiento Científico y Necesidades de Investigaciones; Ediciones Universidad Católica de Chile: Santiago, Chile, 1987; pp. 167–189. [Google Scholar]
- Martínez, A.; Merino-Yunnissi, C.; Mutschke, E. A new catalogue for the echinoderms housed in the collection of Invertebrate Zoology Department at the National Museum of Natural History, Chile. Publicación Ocas. Mus. Nac. Hist. Nat. 2018, 68, 1–88. [Google Scholar]
- Ramírez, M.E.; Osorio, C. Patrones de distribución de macroalgas y macroinvertebrados intermareales de la isla Robinson Crusoe, archipiélago de Juan Fernández, Chile. Investig. Mar. 2000, 28, 1–13. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, M.C.; Andreu-Cazenave, M.; Ruz, C.S.; Ruano-Chamorro, C.; Ramírez, F.; González, C.; Fernández, M. Initial assessment of coastal benthic communities in the Marine Parks at Robinson Crusoe Island. Lat. Am. J. Aquat. Res. 2014, 42, 918–936. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.A.X.; Robertson, J. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Ballesteros, E.; Caselle, J.E.; Gaymer, C.F.; Palma, A.T.; Petit, I.; Varas, E.; Wilson, A.M.; Sala, E. Marine biodiversity in Juan Fernández and Desventuradas Islands, Chile: Global endemism hotspots. PLoS ONE 2016, 11, e0145059. [Google Scholar] [CrossRef]
- Delrieu-Trottin, E.; Hartmann-Salvo, H.; Saenz-Agudelo, P.; Landaeta, M.F.; Pérez-Matus, A. DNA reconciles morphology and colouration in the drunk blenny genus Scartichthys (Teleostei: Blenniidae) and provides insights into their evolutionary history. J. Fish Biol. 2022, 100, 507–518. [Google Scholar] [CrossRef]
- Pardo-Gandarillas, M.C.; Carrasco, S.A.; Varela, A.I.; Ibáñez, C.M. Systematic and biogeography of two sympatric octopuses from the remote Juan Fernández Archipelago, South Pacific Ocean. Rev. Fish Biol. Fish. 2024, 34, 1685–1706. [Google Scholar] [CrossRef]
- Stuessy, T.F.; Foland, K.A.; Sutter, J.F.; Sanders, R.W.; Silva, M. Botanical and geological significance of potassium-argon dates from the Juan Fernandez Islands. Science 1984, 225, 49–51. [Google Scholar] [CrossRef]
- Haye, P.A.; Segovia, N.I.; Muñoz-Herrera, N.C.; Gálvez, F.E.; Martínez, A.; Meynard, A.; Faugeron, S. Phylogeographic structure in benthic marine invertebrates of the southeast Pacific coast of Chile with differing dispersal potential. PLoS ONE 2014, 9, e88613. [Google Scholar] [CrossRef]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef]
- Hoareau, T.B.; Boissin, E. Design of phylum-specific hybrid primers for DNA barcoding: Addressing the need for efficient COI amplification in the Echinodermata. Mol. Ecol. Resour. 2010, 10, 960–967. [Google Scholar] [CrossRef]
- Foltz, D.W.; Mah, C.L. Recent relaxation of purifying selection on the tandem-repetitive early-stage histone H3 gene in brooding sea stars. Mar. Genom. 2009, 2, 113–118. [Google Scholar] [CrossRef]
- Filatov, D.A. ProSeq: A software for preparation and evolutionary analysis of DNA sequence data sets. Mol. Ecol. Notes 2002, 2, 621–624. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; Von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Suchard, M. Tracer V1.6—MCMC Trace Analysis Package; Institute of Evolutionary Biology, Department of Computer Science: Edinburgh, UK, 2013. [Google Scholar]
- Rambaut, A. FigTree. Tree Figure Drawing Tool. Edinburgh, UK. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 20 August 2025).
- Zhang, J.J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Manzur, T.; Barahona, M.; Navarrete, S.A. Ontogenetic changes in habitat use and diet of the sea-star Heliaster helianthus on the coast of central Chile. J. Mar. Biol. Assoc. U. K. 2010, 90, 537–546. [Google Scholar] [CrossRef]
- Cornejo, C.F.; Vargas, T.; Curaz, S.; Sellanes, J.; Ibáñez, C.M. La regla de islas y el tamaño corporal del poliplacóforo Plaxiphora mercatoris en Rapa Nui. Rev. Biol. Mar. Oceanogr. 2022, 57, 112–118. [Google Scholar] [CrossRef]
- Foltz, D.W.; Bolton, M.T.; Kelley, S.P.; Kelley, B.D.; Nguyen, A.T. Combined mitochondrial and nuclear sequences support the monophyly of forcipulatacean sea stars. Mol. Phylogenet. Evol. 2007, 43, 627–634. [Google Scholar] [CrossRef]
- Navarrete, S.A.; Manzur, T. Individual- and population-level responses of a keystone predator to geographic variation in prey. Ecology 2008, 89, 2005–2018. [Google Scholar] [CrossRef]
- Hormazabal, S.; Combes, V.; Morales, C.E.; Correa-Ramirez, M.A.; Di Lorenzo, E.; Nuñez, S. Intrathermocline eddies in the coastal transition zone off central Chile (31–41°S). J. Geophys. Res. Ocean. 2013, 118, 4811–4821. [Google Scholar] [CrossRef]





| No. of Rays | Percentage of Free Rays | Distribution | ||||
|---|---|---|---|---|---|---|
| H. helianthus | 30 or more | 30% or more | Tropical, subtropical and temperate west coasts of South America | Mexico, Peru, Chile | West coast of South America | Paita, Callao, Caldera, Valparaiso. |
| H. canopus | 28 or less | 40–70% | Juan Fernandez Archipelago, Chile | Eastern Islands | Juan Fernandez Archipelago, Chile | Juan Fernandez Archipelago, Chile |
| H. cuminigi | 30 or more | more than 20% | Galapagos Islands, Ecuador | Nicaragua | Galapagos Islands, Ecuador | Zorritos, Paita, Peru. Galapagos Islands, Ecuador |
| H. solaris | 28 or less | 50–70% | Galapagos Islands, Ecuador | Costa Rica | Galapagos Islands, Ecuador | Galapagos Islands, Ecuador |
| H. polybrachius | 30 or more | more than 20% | Tropical coast of Peru | Mexico, Ecuador, Peru | West coast of tropical South America | - |
| H. microbrachius | 30 or more | 30% or less | West coast of Mexico and Central America | Mexico, Panama, Peru | West coast of Mexico and Central America | Margarita Bay, La Paz, Cape San Lucas, Mazatlan, Acapulco, Hawaiian Islands. |
| References | [3] | [3] | [4] | [7] | [3] | [2] |
| Species | COI | H3 |
|---|---|---|
| Heliaster helianthus | JQ918254 | JQ918206 |
| Stichaster striatus | JX130053 | EU707677 |
| Odontaster validus | GQ294396 | EU707663 |
| Ophiura robusta | MG935300 | KP113641 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalán, J.; Ibáñez, C.M.; Carrasco, S.A.; Sellanes, J.; Díaz, A.; Pardo-Gandarillas, M.C. Systematic Evaluation of Sea Stars of the Genus Heliaster from the Southeastern Pacific and Redescription of Heliaster helianthus. Taxonomy 2025, 5, 59. https://doi.org/10.3390/taxonomy5040059
Catalán J, Ibáñez CM, Carrasco SA, Sellanes J, Díaz A, Pardo-Gandarillas MC. Systematic Evaluation of Sea Stars of the Genus Heliaster from the Southeastern Pacific and Redescription of Heliaster helianthus. Taxonomy. 2025; 5(4):59. https://doi.org/10.3390/taxonomy5040059
Chicago/Turabian StyleCatalán, Jennifer, Christian M. Ibáñez, Sergio A. Carrasco, Javier Sellanes, Angie Díaz, and M. Cecilia Pardo-Gandarillas. 2025. "Systematic Evaluation of Sea Stars of the Genus Heliaster from the Southeastern Pacific and Redescription of Heliaster helianthus" Taxonomy 5, no. 4: 59. https://doi.org/10.3390/taxonomy5040059
APA StyleCatalán, J., Ibáñez, C. M., Carrasco, S. A., Sellanes, J., Díaz, A., & Pardo-Gandarillas, M. C. (2025). Systematic Evaluation of Sea Stars of the Genus Heliaster from the Southeastern Pacific and Redescription of Heliaster helianthus. Taxonomy, 5(4), 59. https://doi.org/10.3390/taxonomy5040059










