A New Peyritschiella Species (Laboulbeniales, Ascomycota) on Staphylinidae (Coleoptera, Insecta) from the Tropical Montane Cloud Forest of Mexico †
Abstract
1. Introduction
2. Materials and Methods
- Host examination and Thalli removal
- Morphological characterization
3. Results
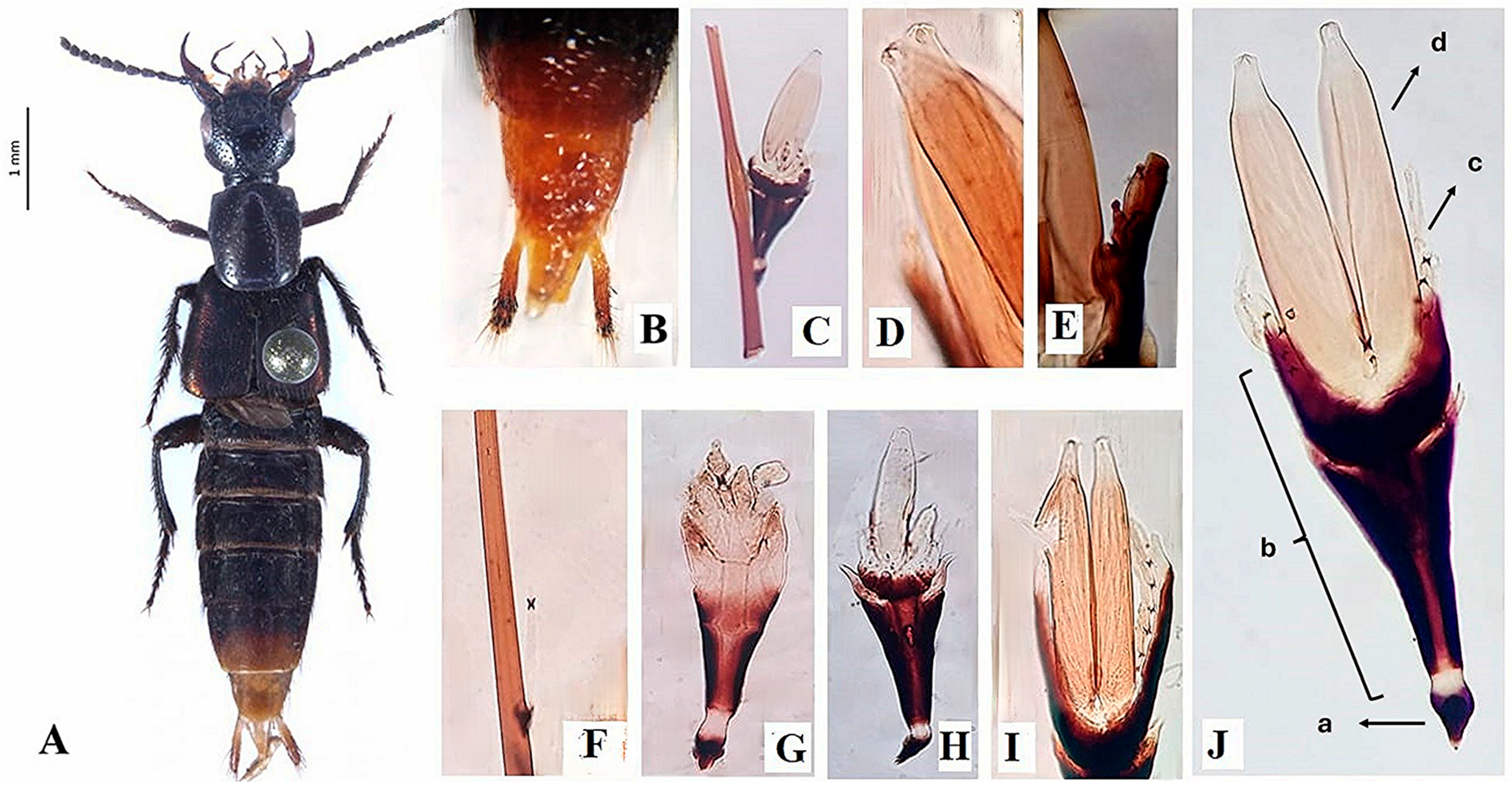
- Diagnosis. Three-leveled receptacle, subtriangular and flattened; hyaline appendages; constricted at the base with an evident dark-brown coloration; symmetrical or almost symmetrical perithecia, presence of papillae on the apical area of the perithecia; hyaline, bicellular ascospores (Figure 1).
- Etymology. The term styngeti corresponds to the genus in which the host is taxonomically located (Styngetus).
- Holotype. Mexico • Hidalgo, Molango, Acautitlán; tropical montane cloud forest; 1715 m.a.s.l.; N 20° 45′38.4″, W 88°42′50.7″; squid-baited pitfall trap; 15 to 24 May 2011; J. Márquez, ENCB-L16 ELOP02 (Figure 1J).
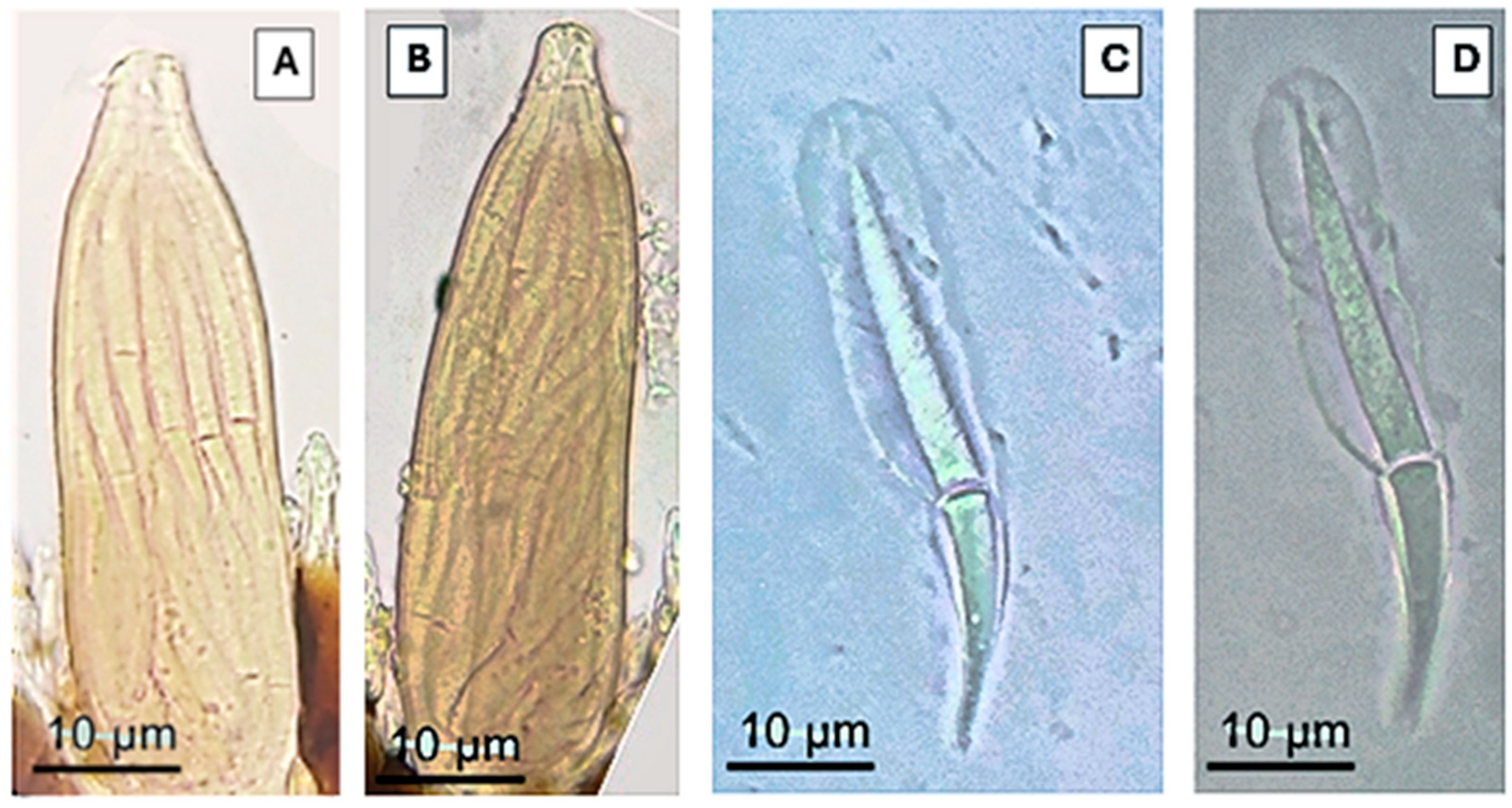
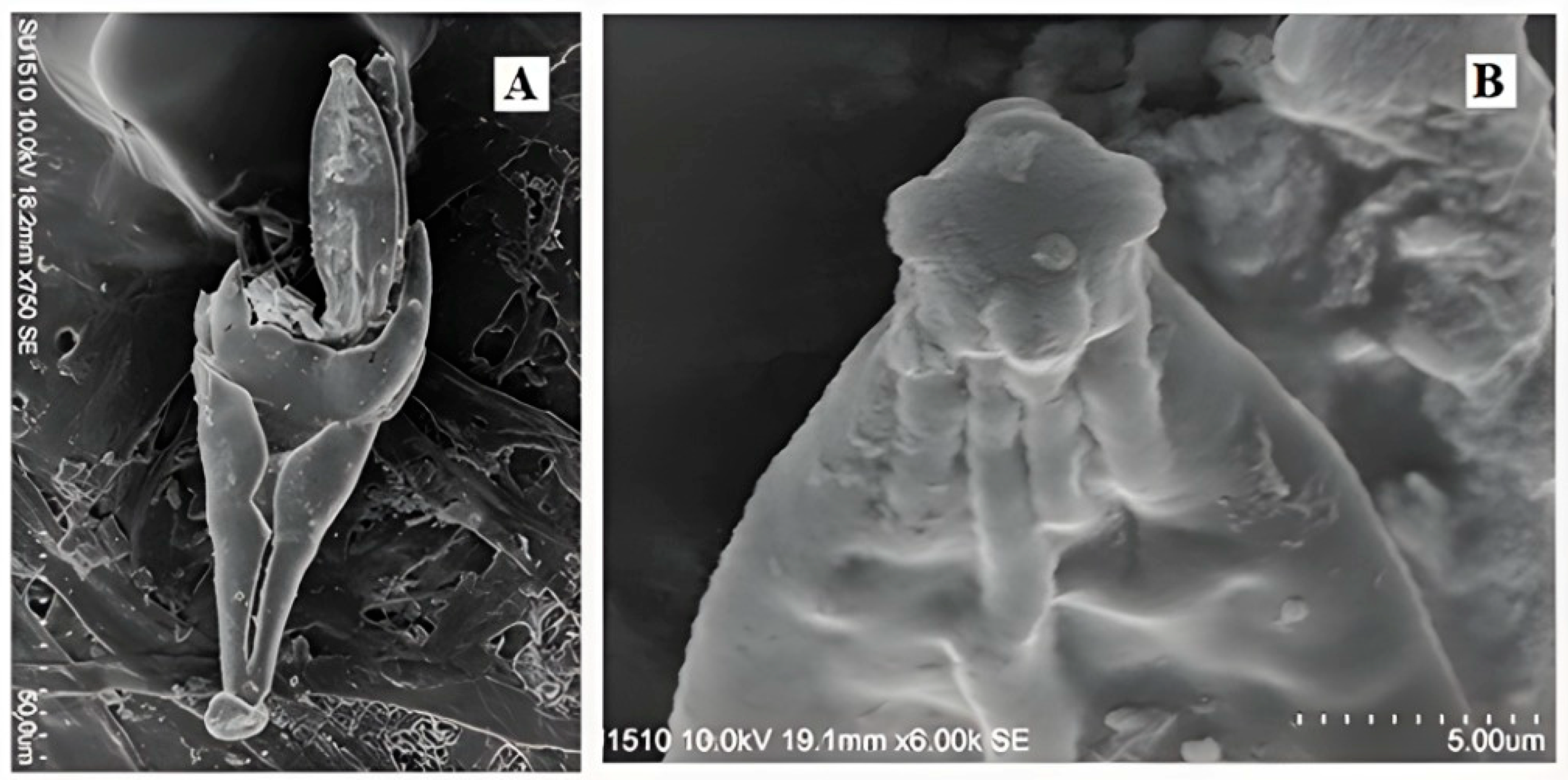
- Description. Thallus 69–280 × 20–64 µm (Figure 1J). Receptacle 50–100 × 20–64 µm, subtriangular and flattened, narrow at the base, wider under the perithecia, of superficial insertion. Receptacle cells are covered by a dark brown color, the last row of a cell arranged in transversal form and curved inwards, building a margin that longitudinally covers the basal area of the perithecia (Figure 1J(b)). Foot cell 4–20 × 9–16 µm, dark colored (Figure 1J(a)). Hyaline appendages, constricted at the base with an evident dark-brown coloration, arranged to the external sides of the perithecia over the longitudinal margin of the receptacle, 10–80 × 2–5 µm (Figure 1J(c)); compound antheridia, arranged laterally over the appendages, only observed in one 15–20 × 6–9 µm thallus (Figure 1E). Perithecia 15–150 × 5–37 µm, located apically (Figure 2 and Figure 3A,B), symmetrical or almost symmetrical, arranged one beside the other, presence of four papillae, cruciform, on the apical area of the perithecia (Figure 1D and Figure 4B); hyaline, bicellular ascospores 40–56 × 4–5 µm, fusiform, septate and covered with a mucilage which helps adhesion to the host (Figure 1I and Figure 3C,D).
- Other Specimens examined of Styngetus deyrollei. Hidalgo, Tlanchinol, camino a Apantazol, El Pozo; tropical montane cloud forest, 1391 m.a.s.l.; squid-baited pitfall trap No. 2; 1 to 22 October 2011; J. Márquez.; Zacualtipán; squid-baited pitfall trap No. 2; 20 September to 4 October 2011.; Zacualtipán; tropical montane cloud forest; N 20°38′44.5″, W 98° 36′7.2″; squid-baited pitfall trap No. 6; 14 to 28 April 2011; MA.; Zacualtipán, camino a Tizapán; tropical montane cloud forest; N 20°38′44.5″, W 98°36′7.2″; squid-baited pitfall trap; 14 to 28 May 2011; J. Márquez.; Molango, Acuatitlán; tropical montane cloud forest; 1715 m.a.s.l.; N 20°45′38.4″, W 88°42′50.7″; flight interception trap No. 1; 15 to 29 April 2011; J. Márquez.; La Misión, Los Naranjos-Palo Hueco; tropical montane cloud forest; 1715 m.a.s.l.; N 21°08′06″, W 99°05′02″; 10 to 24 March 2011; J. Márquez.; La Misión, Las Pilas; tropical montane cloud forest; flight interception trap; 12 to 19 July 2018; A. Lora and J. Márquez.; Tlanchinol, camino a Apantazol, El Pozo; tropical montane cloud forest; 1391 m.a.s.l.; N 20°59′6.73″, W 98°37′39.86″; flight interception trap No, 1; 16 to 30 April 2011; J. Márquez.; Tlanchinol; tropical montane cloud forest; 1391 m.a.s.l.; N 20°59′6.73″, W 98°37′39.86″;squid-baited pitfall trap No. 2; 16 to 30 April 2011.; Molango, Acuatitlán; tropical montane forest; 1715 m.a.s.l.; N20°45′38.4″, W 88°42′50.7″; squid-baited pitfall trap No. 4; 15 to 29 April 2011; J. Márquez.; Tlanchinol, camino a Apantazol, El Pozo; tropical montane cloud forest; 1391 m.a.s.l.; N 20°59′6.73″, W 98°32′39.86″; squid-baited pitfall trap; 16 to 30 April 2011; J. Márquez.; La Misión, Lagunita de Pilas; tropical montane cloud forest; squid-baited pitfall trap No. 2; 17 February to 17 March 2019; A, Lora.; Zacualtipán, camino a Tizapán; tropical montane cloud forest; 1790 m.a.s.l.; N 20°38′44.5″, W 98°36′7.2″; squid-baited pitfall trap; 14 to 28 April 2011; J. Márquez.; La Misión, Lagunita de Pilas; tropical montane cloud forest; 1770 m.a.s.l.; N 21°7′38″, W 99°6′53″; dung-baited pitfall trap; 11 June to 10 July 2019; J. A. Lora.; Tenango de Doria, La Viejita; tropical montane cloud forest; 1163 m.a.s.l.; N 22°47′58″, W 57°66′07″; squid-baited pitfall trap; 12 August 2010; C. Cornejo.; Tlanchinol, camino a Apantazol, El Pozo; tropical montane cloud forest; 1391 m.a.s.l.; N 20°59′6.732″, W 98°37′39.86″; squid-baited pitfall trap No. 1; 16 to 30 April 2011; J. Márquez. • Puebla, Zacapoaxtla, Apulco, Cascada La Gloria; tropical montane cloud forest; squid-baited pitfall trap; 18 May to 28 May 2021; J. Márquez and J. Asiain. • San Luis Potosí, Las Pozas de James; tropical montane cloud forest; squid-baited pitfall trap; 17 July 2007; J. Asiain, APMP, IAA, MY, J. Márquez. (ENCB).
- Host. Obligate ectoparasite of Styngetus deyrollei, specifically located on the stylus on both males and females (Figure 1A); host with predaceous habits in the tropical montane cloud forest. Other rove beetles’ specimens were revised (Philonthus, Oligotergus, Platydracus, Chroaptomus), none of them showed infection by this new species.
- Distribution. Distributed in the states of Hidalgo, Puebla and San Luis Potosí. It is a new species for the worldwide mycobiota.
- Notes.Peyritschiella styngeti sp. nov. is characterized by the presence of appendages with a dark constriction at the base (Figure 1I), two perithecia with four papillae on the apical area, cruciform aspect (Figure 1D and Figure 4B), bilateral symmetry, and a receptacle conformed by three highly pigmented levels, making it look like it is only one level (Figure 1J). This species is highly specific because it is only found on S. deyrolley’s (Figure 1A) stylus (Figure 1B). The characteristics, as mentioned earlier, differentiate this species from other taxonomically close ones like P. cafiana (Thaxt.) I.I. Tav. 1985 [18], which presents three well-defined leveled receptacle and short appendages. Furthermore, the pigmented area in this same structure is only observed on the foot cell and the adjacent area, and although both species present two lobulated perithecia, the melanization pattern is a very visible characteristic that differentiates it from P. styngeti.

4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CC-UAEH | Coleoptera Collection of Universidad Autónoma del Estado de Hidalgo |
| ENCB | Escuela Nacional de Ciencias Biológicas (IPN) |
| IPN | Instituto Politécnico Nacional |
Appendix A
| Genus | Host | The Host’s Family | Vegetation | Reference |
|---|---|---|---|---|
| Order: Herpomycetales | ||||
| Herpomyces paranensis Thaxt. | Blabera | Blaberidae (Blattodea) | Tropical Forest | [1,13,14,15] |
| Herpomyces periplanetae Thaxt. | Periplaneta | Blattidae (Blattodea) | Tropical Forest | [1,13,14,15] |
| Herpomyces platyzosteriae Thaxt. | Platyzosteria ingens Scudder | Blaberidae (Blattodea) | Tropical Forest | [1,13,14,15] |
| Order: Laboulbeniales | ||||
| Ceratomyces ansatus Thaxt. | Tropisternus | Hydrophilidae (Coleoptera) | Tropical forest Temperate Forest Boreal Forest | [1,13,14,15,18] |
| Ceratomyces confusus Thaxt. | Tropisternus glaber Herbst T. nimbatus Fabricius | Hydrophilidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15,18] |
| Ceratomyces filiformis Thaxt. | Tropisternus Pleurohomus obscurus Sharp | Hydrophilidae (Coleoptera) Dryopidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Ceratomyces mexicanus Thaxt. | Tropisternus nitidus Sharp T. chalybeus Cast | Hydrophilidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15,16] |
| Ceratomyces miriabilis Thaxt. | Tropisternus T. xantophus Sharp | Hydrophilidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Ceratomyces spiniger Thaxt. | Tropisternus apicipalpis Chevrolat | Hydrophilidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15,16] |
| Dimeromyces forficulae Thaxt. | Doru lineare Eschscholtz | Forficulidae (Dermaptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15,16] |
| Dimeromyces parasii Thaxt. | Parasitus | Parasitidae (Mesostigmata) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15,16] |
| Dixomyces clivinae (Thaxt.) I.I. Tav. | Clivina dentifermorata Putz Clivina | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15,16] |
| Dixomyces pallescens (Thaxt.) I.I. Tav. | Clivina | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [16] |
| Eucantharomyces casnoniae Thaxt. | Casnonia subdistincta Chaudoir | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15,16] |
| Eucantharomyces diaphorii Thaxt. | Diaphorus tenuicornis Chaudoir | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15,16] |
| Homaromyces epieri R.K. Benj. | Unespecified | Histeridae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [16] |
| Laboulbenia arietina Thaxt. | Disonychia | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia armata Thaxt. | Oedionychus sublineatus Jacoby | Chrysomelidae (Coleoptera) | Tropical Montane Cloud Forest | [1,13,14,15] |
| Laboulbenia barbata Thaxt. | Morion georgiae Palisot | Leiodidae (Coleoptera) | Tropical Montane Cloud Forest | [1,13,14,15,16] |
| Laboulbenia brachini Thaxt. | Brachinus elongatus Tourn B. mexicanus Dejean B. rhytiderus Chaudoir | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia bruchii (Speg.) Thaxt. | Lema albini Kraatz L. sallei Jacoby L. dimidiaticornis Jacoby | Chrysomelidae (Coleoptera) | Oak Forest Pine Tree-Oak Forest | [1,13,14,15] |
| Laboulbenia catascopi Thaxt. | Catoscopus Pinacodera atrata Chevrolat Coptodera arcuata Chevrolat Colpodes auratus Chaudoir | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia decipiens Thaxt. | Galerita nigra Chevrolat | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia diabrotica Thaxt. | Diabrotica fairmairei Baly | Chrysomelidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia disonichae Thaxt. | Disonycha figurata Jacoby | Chrysomelidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia egae Thaxt. | Ega | Carabidae (Coleoptera) | Temperate Forest | [1,13,14,15] |
| Laboulbenia elongata Thaxt. | Colpodes | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Coniferous Tree Forest | [1,13,14,15] |
| Laboulbenia erecta Thaxt. | Colpodes agilis Chaudoir C. evanescens Bates | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Coniferous Tree Forest | [1,13,14,15] |
| Laboulbenia flaccida Thaxt. | Casonia subdistincta Chaudoir | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia flagellata Peyr. | Onypterigia pusilla Chaudoir | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia galeritae Thaxt. | Galerita forreri Bates G. mexicana Chaudoir | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia guerinii Thaxt. | Gyretes G. boreandri Chevrolat G. immarginatus Chevrolat G. leionatus Duby | Gyrinidae (Coleoptera) | Tropical Forest Temperate Forest | [1,13,14,15] |
| Laboulbenia gyrinidarum Thaxt. | Gyrinus | Gyrinidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia homophoetae Speg. | Asphaera transversofasciata Jacoby Oedionychus sublineata Jacoby Systema littera Linneus | Chrysomelidae (Coleoptera) | Tropical Forest Subtropical Forest Temperate Forest Deciduous Forest | [1,13,14,15] |
| Laboulbenia mexicana Thaxt. | Galerita G. mexicana Chaudoir G. aequinoctialis Chaudoir G. nigra Chevrolat | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia minima Thaxt. | Callida | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Tropical Montane Cloud Forest | [1,13,14,15] |
| Laboulbenia morionis Thaxt. | Morion georgiae Palisot M. monillicornis Latreille Moriosomus sylvestris Motschulsky | Carabidae (Coleoptera) | Tropical Montane Cloud Forest | [1,13,14,15,16] |
| Laboulbenia pachytelis Thaxt. | Pachyteles mexicanus Chaudoir P. longicornis Chaudoir P. seriatoporus Chaudoir | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Tropical Montane Cloud Forest | [1,13,14,15] |
| Laboulbenia pallescens Thaxt. | Clivina dilutipennis Putzcys | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia parvula Thaxt. | Pelmatellus obtusus Bates | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest Tropical Montane Cloud Forest | [1,13,14,15] |
| Laboulbenia pheropsophi Thaxt. | Pheropsophus aequinoctialis Linnaeus P. biplagiatus Chaudoir | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest Tropical Montane Cloud Forest | [1,13,14,15] |
| Laboulbenia polyphaga Thaxt. | Phlaeotheratus quadricollis Chaudoir Stenognathus quadricollis Chaudoir | Carabidae (Coleoptera) | Tropical Forest Pine tree-Oak Forest Tropical Montane Cloud Forest | [1,13,14,15] |
| Laboulbenia pygmaea Thaxt. | Galerita | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia sbordonii W. Rossi & Cesari | Mexaphaenops intermedius Barr | Carabidae (Coleoptera) | Temperate Forest Coniferous Tree Forest | [10] |
| Laboulbenia texana Thaxt. | Brachinus lateralis Dejean | Carabidae (Coleoptera) | Pine Tree-Oak Forest Tropical Forest | [1,13,14,15] |
| Laboulbenia variabilis Thaxt. | Poecilus mexicanus Chaudoir | Carabidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia vulgaris Peyr. | Bembidion mexicanum Dejean | Carabidae (Coleoptera) | Coniferous Tree Forest | [1,13,14,15] |
| Limnaiomyces tropisterni Thaxt. | Tropisternus | Hydrophilidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Prolixandromyces corniculatus R.K. Benj. | Velia | Veliidae (Hemiptera) | Tropical Forest Temperate Forest Boreal Forest | [11,12] |
| Prolixandromyces veliae R.K. Benj. | Velia | Veliidae (Hemiptera) | Tropical Forest Temperate Forest Boreal Forest | [11,12,16] |
| Rhachomyces magrinii W. Rossi & M. Leonardi | Mexaphaenops elegans Barr | Carabidae (Coleoptera) | Temperate Forest Coniferous Tree Forest | [19] |
| Rhachomyces mateui Balazuc | Xendromius brachinoides Mateu | Hydrophilidae (Coleoptera) | Tropical Forest Temperate Forest Boreal Forest | [9,16] |
| Rhachomyces quetzalcoatl Balazuc | Paratrechus mexicanus Putzcys | Carabidae (Coleoptera) | Coniferous Forest | [9,16] |
| Rhachomyces velatus Thaxt. | Gynandropus mexicanus Putzcys Colpodes agilis Chaudoir | Carabidae (Coleoptera) | Coniferous Forest Tropical Forest | [1,13,14,15,16] |
| Rhachomyces zuphii Thaxt. | Zuphioides mexicanum Chaudoir | Carabidae (Coleoptera) | Coniferous Forest | [1,13,14,15,16] |
| Rhizopodomyces basifurcatus R.K. Benj. | Hebrus sp. | Hebridae (Hemiptera) | Tropical Forest Temperate Forest Boreal Forest | [11,12,16] |
| Rhizopodomyces merragate Thaxt. | Hebrus bilineatus Champion | Hebridae (Hemiptera) | Tropical Forest Temperate Forest Boreal Forest | [11,12,16] |
| Rhizopodomyces mexicanus R.K. Benj. | Hebrus bilineatus Champion | Hebridae (Hemiptera) | Tropical Forest Temperate Forest Boreal Forest | [11,12,16] |
| Rhizopodomyces polhemi R.K. Benj. | Hebrus sp. | Hebridae (Hemiptera) | Tropical Forest Temperate Forest Boreal Forest | [11,12,16] |
| Rickia apiculifera Thaxt. | Chondrocephalus debilis Bates Other unspecified species of passalids | Passalidae (Coleoptera) | Coniferous Forest | [1,13,14,15,16] |
| Rickia bifida Thaxt. | Passalus P. punctiger Lepeletier & Audinet-Serville | Passalidae (Coleoptera) | Coniferous Forest | [1,13,14,15,16] |
| Rickia furcata Thaxt. | Euzercon | Euzerconidae (Mesostigmata) | Tropical Forest Coniferous Forest Deciduous Forest | [1,13,14,15,16] |
| Rickia parasiti Thaxt. | Parasitus | Parasitidae (Mesostigmata) | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15,16] |
| Rickia passalina Thaxt. | Chondrocephalus debilis Bates passalids | Passalidae (Coleoptera) | Coniferous Forest | [1,13,14,15,16] |
| Stigmatomyces benjaminii W. Rosii & A. Weir | Spilochroa polita Malloch | Heleomyzidae (Diptera) | Temperate Forest Tropical Montane Cloud Forest | [17] |
| Stigmatomyces indentatus Thaxt. | Psilopa | Veliidae (Hemiptera) | Coniferous Forest Tropical Forest Deciduous Forest | [1,13,14,15] |
| Stigmatomyces inflatus Thaxt. | Sapromyza | Lauxaniidae (Diptera) | Coniferous Forest Tropical Forest Deciduous Forest | [1,13,14,15] |
| Stigmatomyces limnophorae Thaxt. | Onesia | Calliphoridae (Diptera) | Pine Tree-Oak Forest Tropical Forest Deciduous Forest | [1,13,14,15] |
| Stigmatomyces limosinae Thaxt. | Limosina | Sphaeroceridae (Diptera) | Pine Tree-Oak Forest Tropical Forest Deciduous Forest | [1,13,14,15] |
Appendix B
| Species | Host | Vegetation | Reference |
|---|---|---|---|
| Balazucia bilateralis R.K. Benj. | Phloeonomus sp. | Tropical Forest Temperate Forest Boreal Forest | [16] |
| Corethromyces brasilianus Thaxt. | Cryptobium flohri Sharp C. venustum Sharp C. similipenne Bernhauer | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15,16] |
| Laboulbenia cristata Thaxt. | Paederus sp. P. erythroderus Erichson | Tropical Forest Temperate Forest Boreal Forest | [1,13,14,15] |
| Laboulbenia philonthi Thaxt. | Philonthus incertus Solsky P. furvus var. flohrii Sharp | Tropical Forest Temperate Forest Boreal Forest Tropical Montane Cloud Forest | [1,13,14,15] |
| Mimeomyces quedionuchi Thaxt. | Quedius sp. | Deciduous Forest Coniferous Forest | [16] |
| Peyritschiella exilis (Thaxt.) I.I. Tav. | Belonuchus rufipennis Erichson Belonuchus oxyporinus Sharp | Deciduous Forest Tropical Montane Cloud Forest | [1,13,14,15,16] |
| Peyritschiella mexicana (Thaxt.) I.I. Tav. | Philonthus atriceps Sharp | Tropical Forest Temperate Forest Boreal Forest Tropical Montane Cloud Forest | [1,13,14,15,16] |
| Peyritschiella princeps (Thaxt.) I.I. Tav. | Philonthus sp. Quediomacrus puniceipennis Solsky | Tropical Forest Temperate Forest Boreal Forest Tropical Cloud Forest | [1,13,14,15,16] |
| Peyritschiella vulgata (Thaxt.) I.I. Tav. | Philonthus flavolimbatus Erichson | Tropical Forest Temperate Forest Boreal Forest Tropical Cloud Forest | [1,13,14,15,16] |
| Sphaleromyces quedionuchi Thaxt. | Quedionuchus impuctus Sharp | Deciduous Forest | [1,13,14,15] |
| Peyritschiella styngeti (E.L. Ortiz-Pacheco, Raymundo, Baut.-Hern.) | Styngetus deyrollei Solsky | Tropical Montane Cloud Forest | This paper |
References
- Thaxter, R. On some North American species of Laboulbeniaceae. Proc. Am. Acad. Arts Sci. 1890, 25, 5–14. Available online: https://www.biodiversitylibrary.org/part/246995 (accessed on 10 July 2025). [CrossRef]
- Thaxter, R. Contribution Towards Monograph of the Laboulbeniaceae; Reimpresion 1971; Bibliotheca mycologica; J. Cramer: Lehre, Germany, 1971; pp. 1896–1931. [Google Scholar]
- Thaxter, R. Contribution towards a monograph of the Laboulbeniaceae, Part V. Proc. Mem. Amer. Acad. Arts Sci. 1931, 16, 1–435. [Google Scholar] [CrossRef]
- Healewaters, D.; Blackwell, M.; Pfister, D.H. Laboulbeniomycetes: Intimate fungal associates of arthropods. Annu. Rev. Entomol. 2021, 66, 257–276. [Google Scholar] [CrossRef]
- Van der Linde, E.J.; Rhong, I.H. New and interesting records of South African fungi. XV. Two new Laboulbeniales records from South Africa. Afr. J. Bot. 1997, 63, 109–110. [Google Scholar] [CrossRef]
- Santamaría, S. Los Laboulbeniales, un grupo enigmático de hongos parásitos de insectos. Lazarroa 2001, 22, 3–19. [Google Scholar]
- Lindroth, C.H. Notes on the ecology of Laboulbeniaceae infesting carabid beetles. Sven. Bot. Tidskr. 1948, 42, 34–41. [Google Scholar]
- Peyritsch, J. Über Vorkommen und Biologie von Laboulbeniaceen. Sitzungsber. Kaiserl. Akad. Wiss. Math. Naturiwss. Cl. Abt. 1875, 72, 377–385. [Google Scholar]
- Luna-Zendejas, H.; Pérez-Silva, E.; Reyes-Castillo, P. The Laboulbeniales of Mexico and a study on three new records of Rickia parasiting beetles (Passalidae). Rev. Mex. Mic. 1988, 4, 303–316. [Google Scholar]
- Cavara, F. Funghetti parassiti raccolti nella Repubblica Argentina dal Dr. Carlo Spegazzini. Malpighia 1899, 13, 177–193. [Google Scholar]
- Balazuc, J. Recherches sur les Laboulbeniomycetes. II. Description de cinq especes nouvelles de Rhachomyces, parasites de Coleoptères Carabiques. Rev. Mycol. 1973, 38, 218–227. [Google Scholar]
- Rossi, W.; Cesari, G. Laboulbeniales (Ascomycetes) parassite di Coleotteri cavernicoli del Messico. In Problemi Attuali di Scienza e di Cultura, Sezione Missioni ed Esplorazioni, Section 1; Accademia Nazionale dei Lincei: Rome, Italy, 1997; Volume 171, p. 373. [Google Scholar]
- Benjamin, R.K. Laboulbeniales on semiaquatic Hemiptera, III. Rhizopodomyces. Aliso 1979, 9, 379–409. [Google Scholar] [CrossRef]
- Benjamin, R.K. Laboulbeniales on semiaquatic Hemiptera, IV. Addenda to Prolixandromyces. Aliso 1981, 10, 1–17. [Google Scholar] [CrossRef]
- Thaxter, R. New species of Laboulbeniaceae from various localities. Proc. Am. Acad. Arts Sci. 1893, 10, 156–188. [Google Scholar] [CrossRef]
- Thaxter, R. Preliminary diagnoses of new species of Laboulbeniaceae, VI. Proc. Am. Acad. Arts Sci. 1905, 41, 303–318. [Google Scholar] [CrossRef]
- Thaxter, R. Laboulbeniales parasitic on Chrysomelidae. Proc. Am. Acad. Arts Sci. 1914, 50, 17–50. [Google Scholar] [CrossRef]
- Tavares, I.I. Laboulbeniales (Fungi, Ascomycota); Mycologia memoir No. 9. J. Cramer; The New York Botanical Garden: Braunschweig, Germany, 1985; 627p. [Google Scholar]
- Rossi, W.; Weir, A. New Species of Stigmatomyces from various continents. Mycologia 2007, 99, 139–143. [Google Scholar] [CrossRef]
- Kaishian, P.J. Insects and their Laboulbeniales (Ascomycota, Fungi) of Lake Eustis and Emeralda Marsh Conservation Area: A case study on urbanization and diversity. Ecol. Evol. 2021, 11, 16618–16633. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Hyde, K.D.; Wang, S.; Sun, Y.-R.; Suwannarach, N.; Sysouphanthong, P.; Abdel-Wahab, M.A.; Abdel-Aziz, F.A.; Abeywickrama, P.D.; Abreu, V.P.; et al. Fungal diversity notes 1512–1610: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2023, 117, 1–272. [Google Scholar] [CrossRef]
- Index Fungorum. 2025. Available online: http://www.indexfungorum.org/Names/Names.asp (accessed on 14 July 2025).
- Solsky, S. Deux Staphylins nouveaux du Mexique. Hor. Ent. Ross. BHL 1866, 4, 314–316. [Google Scholar]
- Thaxter, R. Contribution towards a monograph of the Laboulbeniaceae. Part II. Mem. Amer. Acad. Arts Sci. 1908, 13, 217–469. [Google Scholar] [CrossRef]
- Gravenhorst, J.L.C. Monographia Coleopterorum Micropterorum; Typis Henrici Dieterich: Gottingae, Germany, 1806; pp. 1–236. [Google Scholar]
- Fabricius, J.C. Systema Eleutheratorum Secundum Ordines, Genera, Species: Adiectis Synonymis, Locis, Observationibus, Descriptionibus; Kiliae: Imprensis Bibliopolii Academici novi1801; Volume II, 687p. Available online: https://www.biodiversitylibrary.org/page/63318329 (accessed on 15 July 2025).
- Sharp, D. Biologia Centrali-Americana. Insecta. Coleoptera; R.H. Porter: London, UK, 1885; Volume I, Part 2: Staphylinidae; pp. 1–824. [Google Scholar]
- Solsky, S. Études sur les Staphylinides du Mexique. Hor. Ent. Ross 1868, 5, 3–112. [Google Scholar]
- Erichson, W.F. Genera et Species Staphylinorum Insectorum Coleopterorum Familiae; F.H. Morin: Berlin, Germany, 1840; pp. 401–954, pls. 1–5. [Google Scholar]
- Thaxter, R. Contribution towards a monograph of the Laboulbeniaceae. Mem. Amer. Acad. Arts Sci. 1895, 12, 187–429. Available online: https://www.jstor.org/stable/41550031 (accessed on 29 July 2025). [CrossRef]
- Frank, J.H. The Parasites of Staphylinidae (Coleoptera) are a Contribution Towards an Encyclopedia of the Staphylinidae; Encyclopedia of Staphylinidae. Bulletin Agricultural Experiment Stations; University of Florida: Gainesville, FL, USA, 1982; 118p. [Google Scholar]
- Newton, A.F. StaphBase. Offline database catalog. (Consulted August 2025).
- Navarrete-Heredia, J.L.; Newton, A.F. Biodiversidad de Staphylinidae (Insecta: Coleoptera) en México. Rev. Mex. Biodivers. 2014, 85 (Suppl. S1), S332–S338. [Google Scholar] [CrossRef]
- Rossi, W.; Weir, A. New species of Cucujomyces (Laboulbeniales) on Chilean Leiodidae. Aliso 2008, 26, 9–14. [Google Scholar] [CrossRef]
- Thaxter, R. Preliminary diagnoses of new species of Laboulbeniaceae. Proc. Amer. Acad. Arts Sci. 1900, 35, 429–442. [Google Scholar] [CrossRef]
- Navarrete-Heredia, J.L.; Newton, A.; Thayer, M.; Ashe, J.; Chandler, D. Guía Ilustrada para los Géneros de Staphylinidae (Coleoptera) de México; Universidad de Guadalajara y CONABIO: Guadalajara, Mexico, 2002; 401p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Pacheco, E.L.; Raymundo, T.; Bautista-Hernández, S.; Márquez, J.; Asiain, J. A New Peyritschiella Species (Laboulbeniales, Ascomycota) on Staphylinidae (Coleoptera, Insecta) from the Tropical Montane Cloud Forest of Mexico. Taxonomy 2025, 5, 53. https://doi.org/10.3390/taxonomy5040053
Ortiz-Pacheco EL, Raymundo T, Bautista-Hernández S, Márquez J, Asiain J. A New Peyritschiella Species (Laboulbeniales, Ascomycota) on Staphylinidae (Coleoptera, Insecta) from the Tropical Montane Cloud Forest of Mexico. Taxonomy. 2025; 5(4):53. https://doi.org/10.3390/taxonomy5040053
Chicago/Turabian StyleOrtiz-Pacheco, Ericka Lorena, Tania Raymundo, Silvia Bautista-Hernández, Juan Márquez, and Julieta Asiain. 2025. "A New Peyritschiella Species (Laboulbeniales, Ascomycota) on Staphylinidae (Coleoptera, Insecta) from the Tropical Montane Cloud Forest of Mexico" Taxonomy 5, no. 4: 53. https://doi.org/10.3390/taxonomy5040053
APA StyleOrtiz-Pacheco, E. L., Raymundo, T., Bautista-Hernández, S., Márquez, J., & Asiain, J. (2025). A New Peyritschiella Species (Laboulbeniales, Ascomycota) on Staphylinidae (Coleoptera, Insecta) from the Tropical Montane Cloud Forest of Mexico. Taxonomy, 5(4), 53. https://doi.org/10.3390/taxonomy5040053






