A New Species of Krameropteris (Dennstaedtiaceae) from Mid-Cretaceous Myanmar Amber †
Abstract
1. Introduction
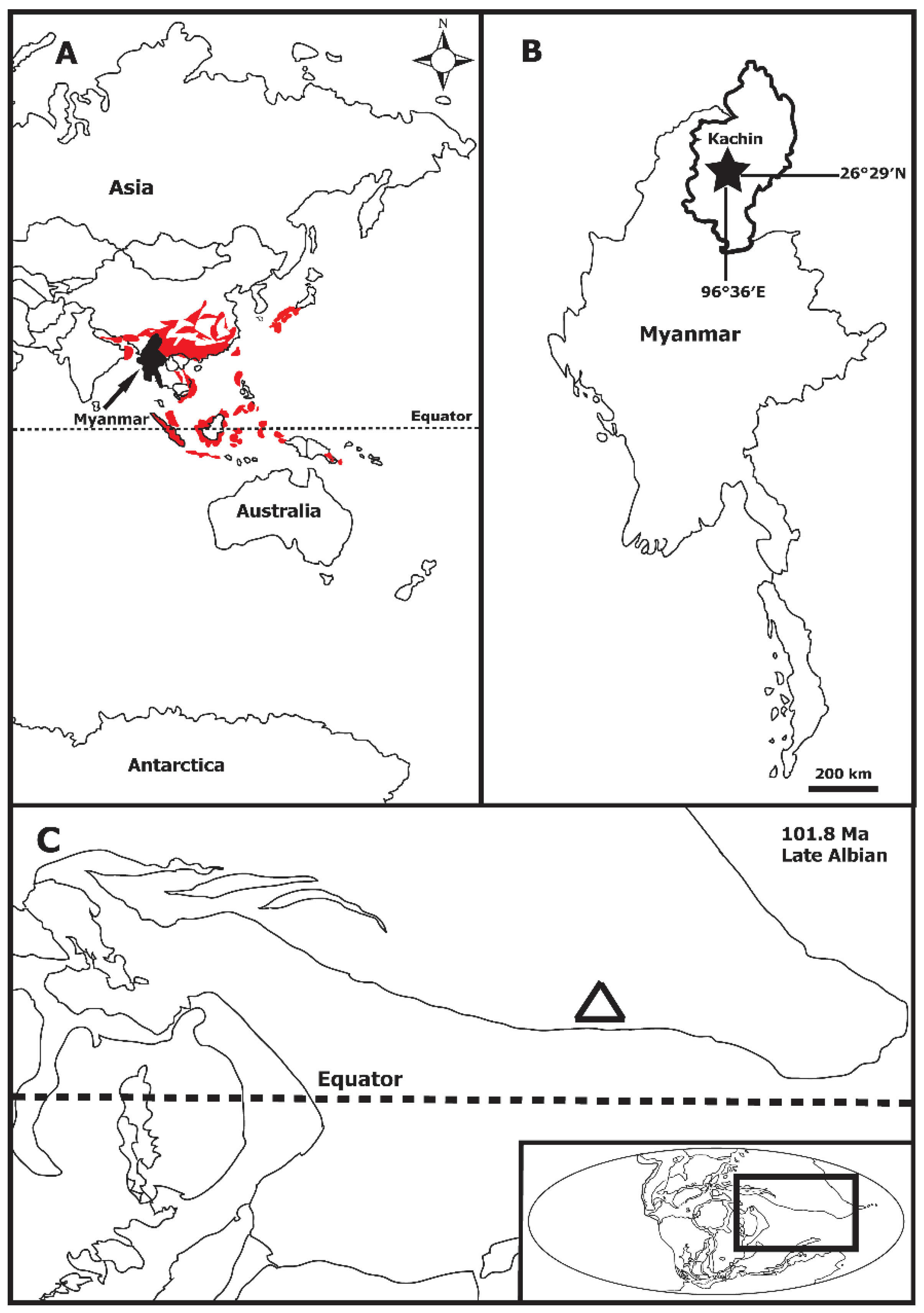
2. Materials and Methods
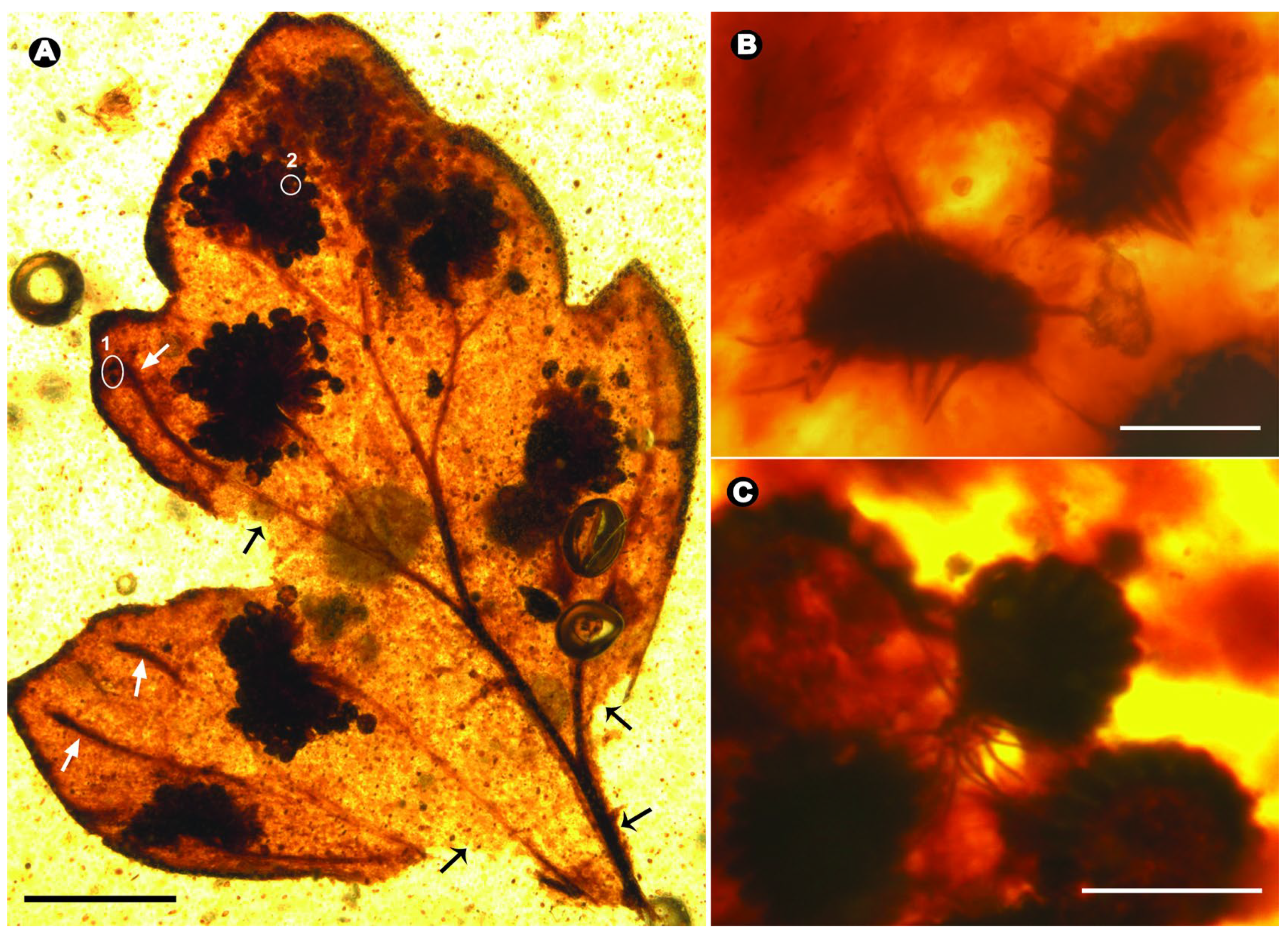
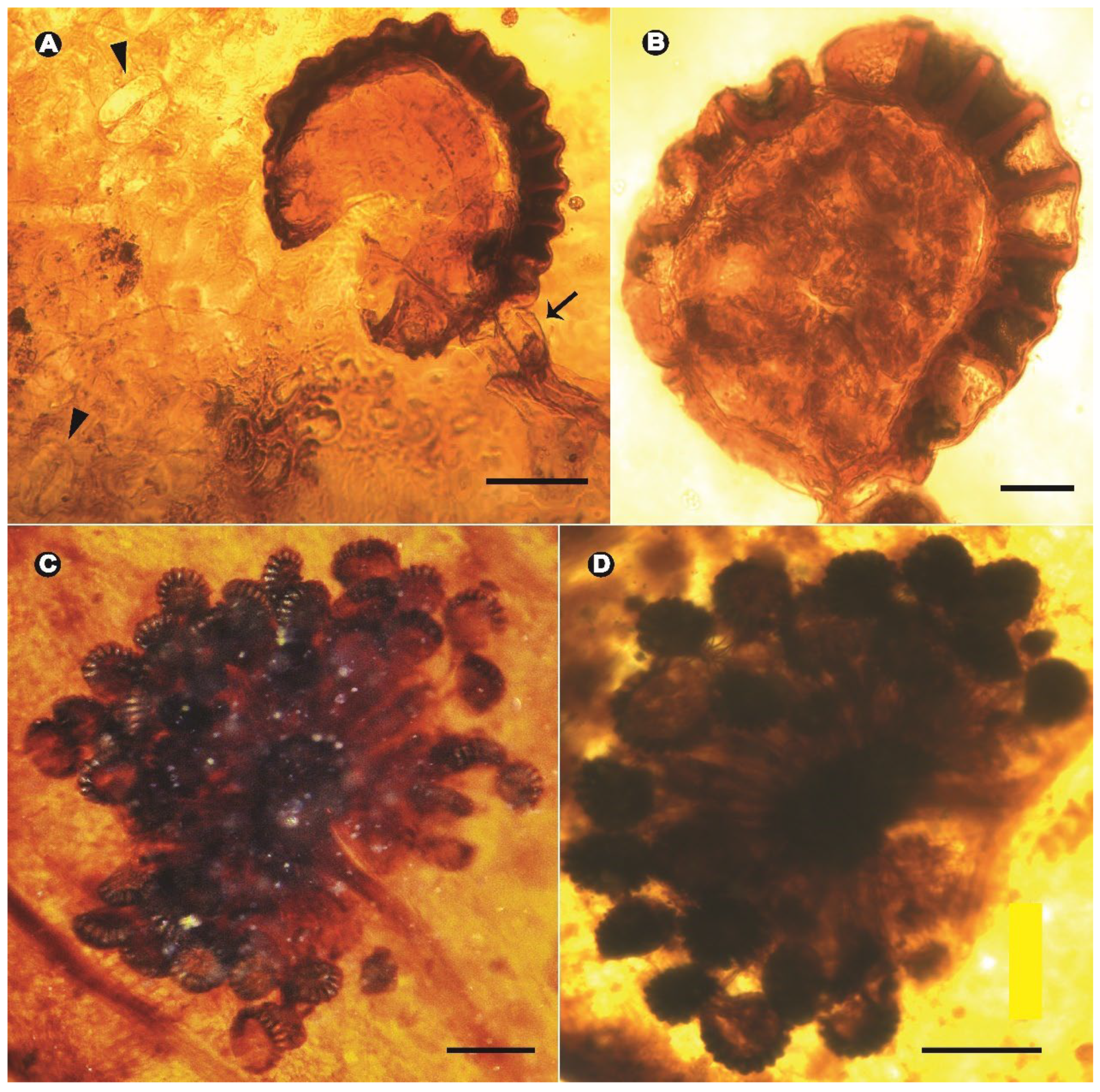
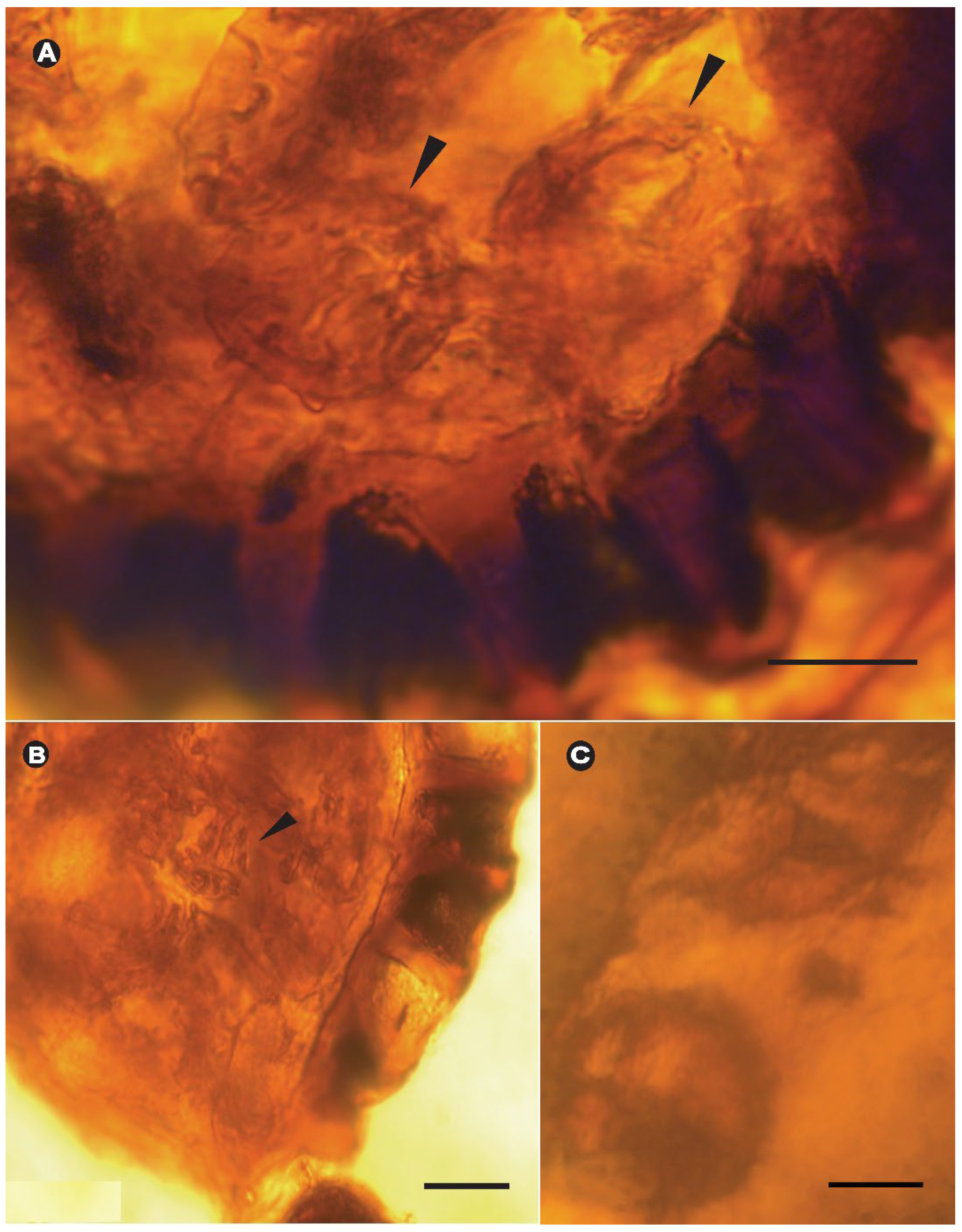
3. Results
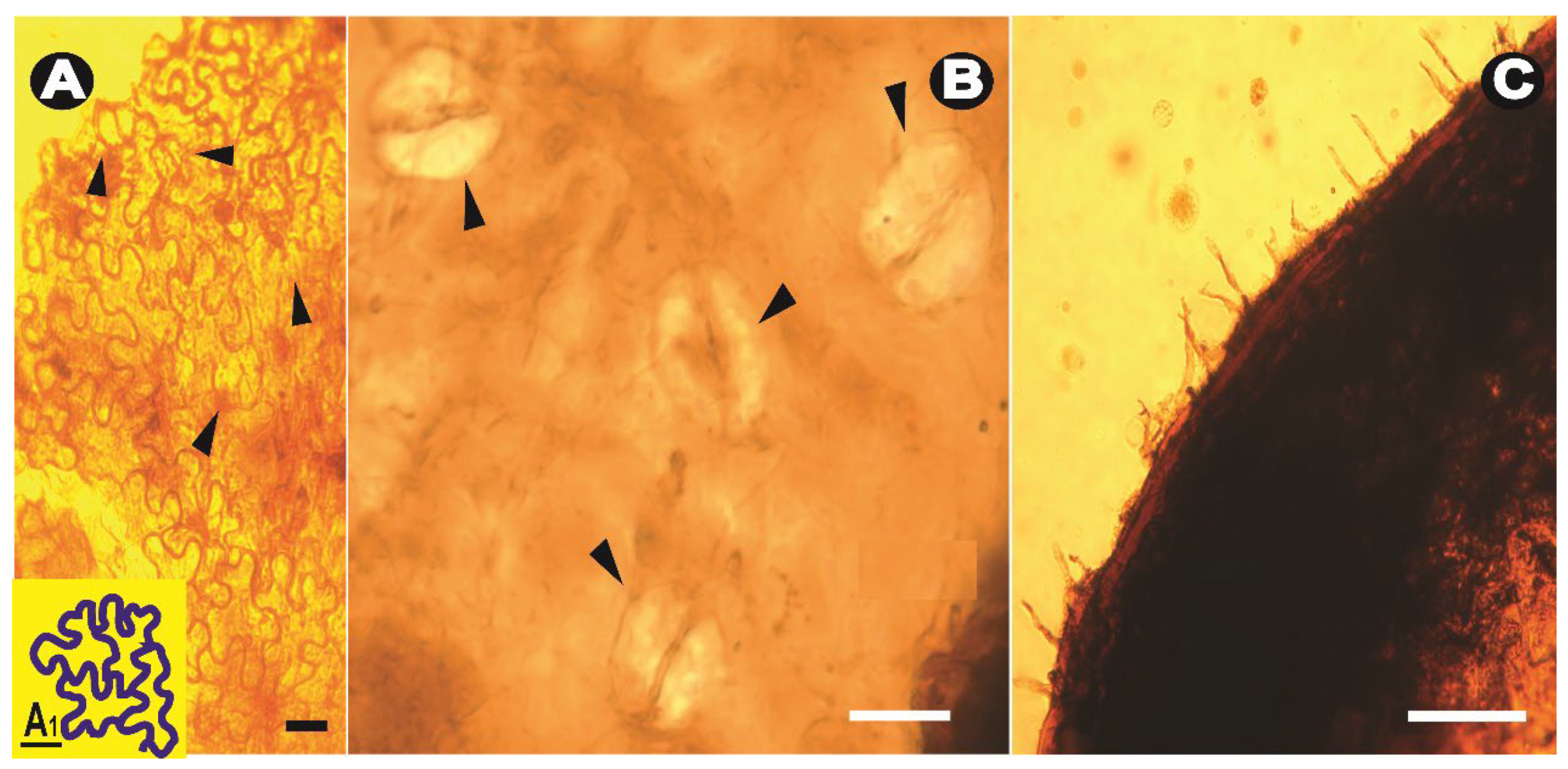
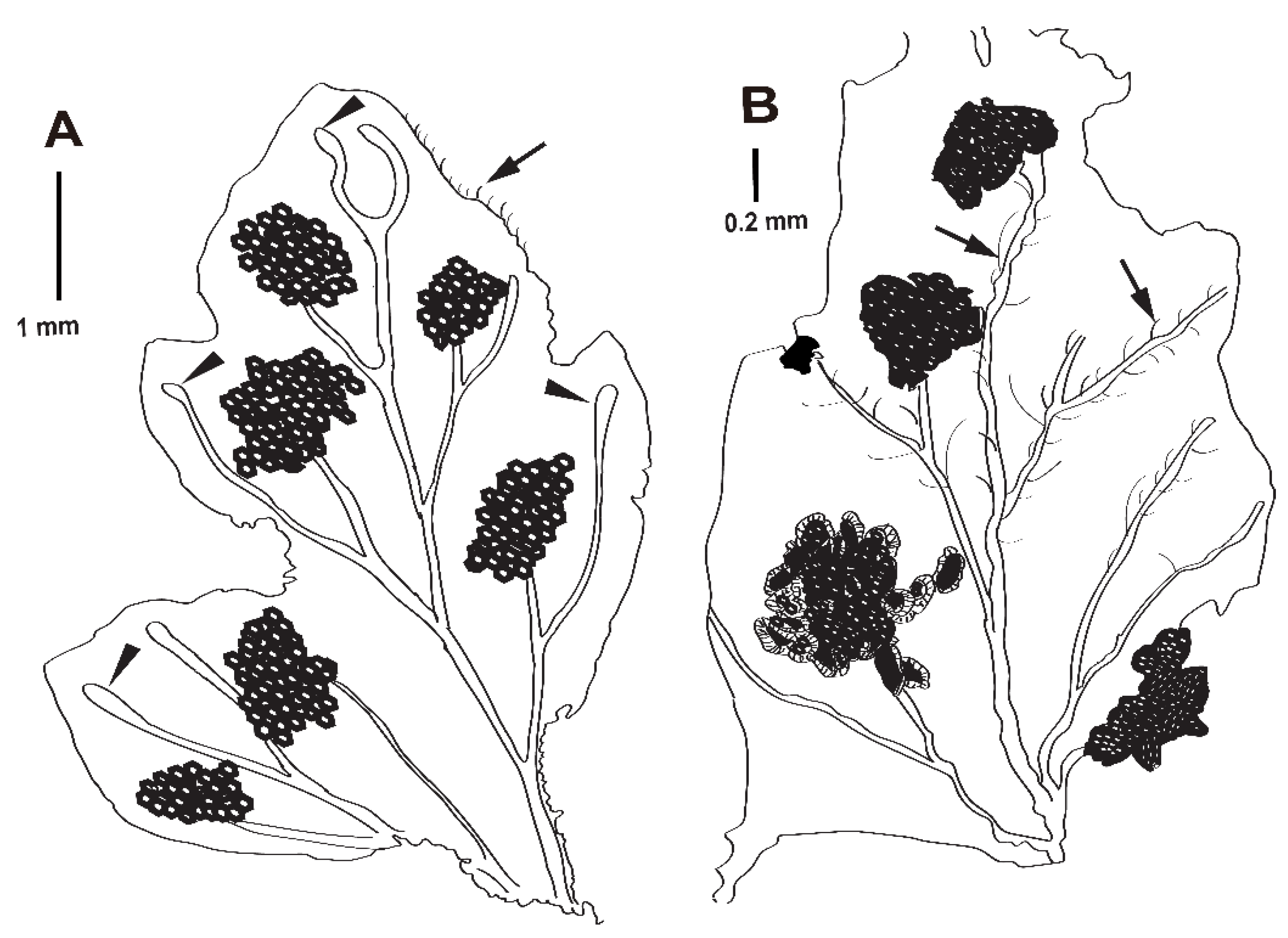
Systematic Paleontology
4. Discussion
4.1. Comparisons to Fossils from Myanmar Amber
4.2. Early Diversification of Dennstaedtiaceae and Their Potential Interactions with Arthropods
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, H.; Schuettpelz, E.; Pryer, K.M.; Cranfill, R.; Magallón, S.; Lupia, R. Ferns diversified in the shadow of angiosperms. Nature 2004, 428, 553–557. [Google Scholar] [CrossRef] [PubMed]
- The Pteridophyte Phylogeny Group. A community-derived classification for extant lycophytes and ferns. J. Syst. Evol. 2016, 54, 563–603. [Google Scholar] [CrossRef]
- Du, X.-Y.; Lu, J.-M.; Zhang, L.-B.; Wen, J.; Kuo, L.-Y.; Mynssen, C.M.; Schneider, H.; Li, D.-Z. Simultaneous diversification of Polypodiales and angiosperms in the Mesozoic. Cladistics 2021, 37, 518–539. [Google Scholar] [CrossRef] [PubMed]
- Nitta, J.H.; Schuettpelz, E.; Ramírez-Barahona, S.; Iwasaki, W. An open and continuously updated fern tree of life. Front. Plant Sci. 2022, 13, 909768. [Google Scholar] [CrossRef]
- Schneider, H.; Schmidt, A.R.; Heinrichs, J. Burmese amber fossils bridge the gap in the Cretaceous record of polypod ferns. Perspect. Plant Ecol. Evol. Syst. 2016, 18, 70–78. [Google Scholar] [CrossRef][Green Version]
- Schuettpelz, E.; Pryer, K.M. Evidence for a Cenozoic radiation of ferns in an angiosperm-dominated canopy. Proc. Natl. Acad. Sci. USA 2009, 106, 11200–11205. [Google Scholar] [CrossRef]
- Testo, W.; Sundue, M. A 4000-species dataset provides new insight into the evolution of ferns. Mol. Phylogenet. Evol. 2016, 105, 200–211. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Li, C.-X. Inferring Eupolypods divergence time using Bayesian tip-dating. Open J. Geol. 2024, 14, 247–258. [Google Scholar] [CrossRef]
- Li, C.-X.; Ma, J.-Y. Dating Suborder Polypodiineae (Eupolypods I) with Its Oldest Fossil. Open J. Geol. 2024, 14, 929–942. [Google Scholar] [CrossRef]
- Poinar, G.O., Jr.; Buckley, A.R. Cretacifilix fungiformis gen. and sp. nov., an eupolypod fern (Polypodiales) in Early Cretaceous Burmese amber. J. Bot. Res. Inst. Texas 2008, 2, 1175–1182. [Google Scholar]
- Regalado, L.; Schneider, H.; Mülle, P.; Schmidt, A.R. Character evolution of modern eupolypods supports the assignment of the fossil fern Cretacifilix fungiformis to Dryopteridaceae. Front. Ecol. Evol. 2023, 11, 1162577. [Google Scholar] [CrossRef]
- Long, X.-X.; Peng, Y.; Feng, Q.; Engel, M.S.; Shi, C.; Wang, S. A new fossil fern of the Dryopteridaceae (Polypodiales) from the mid-Cretaceous Kachin amber. Palaeobio. Palaeoenv. 2023, 103, 489–494. [Google Scholar] [CrossRef]
- Regalado, L.; Schmidt, A.R.; Krings, M.; Bechteler, J.; Schneider, H.; Heinrichs, J. Fossil evidence of eupolypod ferns in the mid-Cretaceous of Myanmar. Plant Syst. Evol. 2018, 304, 1–13. [Google Scholar] [CrossRef]
- Poinar, G., Jr. A new fern, Cladarastega burmanica gen. et sp. nov. (Dennstaedtiaceae: Polypodiales) in mid-Cretaceous Burmese amber. Palaeodiversity 2021, 14, 153–160. [Google Scholar] [CrossRef]
- Long, X.-X.; Peng, Y.; Zhang, H.-R.; Fan, Y.; Shi, C.; Wang, S. Microlepia burmasia sp. nov., a new fern species from mid-Cretaceous Kachin amber of northern Myanmar (Dennstaedtiaceae, Polypodiales). Cretac. Res. 2023, 143, 105417. [Google Scholar] [CrossRef]
- Regalado, L.; Schmidt, A.R.; Appelhans, M.S.; Ilsemann, B.; Schneider, H.; Krings, M.; Heinrichs, J. A fossil species of the enigmatic early polypod fern genus Cystodium (Cystodiaceae) in Cretaceous amber from Myanmar. Sci. Rep. 2017, 7, 14615. [Google Scholar] [CrossRef]
- Li, C.-X.; Moran, R.C.; Wang, Y.-R.; Li, Y.; Ma, J.-Y. A New Fossil of Cystodium (Cystodiaceae) from the mid-Cretaceous Myanmar amber. Cretac. Res. 2024, 160, 105882. [Google Scholar] [CrossRef]
- Regalado, L.; Schmidt, A.R.; Müller, P.; Kobbert, M.J.; Schneider, H.; Heinrichs, J. The first fossil of Lindsaeaceae (Polypodiales) from the Cretaceous amber forest of Myanmar. Cretac. Res. 2017, 72, 8–12. [Google Scholar] [CrossRef]
- Li, C.-X.; Moran, R.C.; Ma, J.-Y.; Wang, B.; Hao, J.-S. A new fossil record of Lindsaeaceae (Polypodiales) from the mid-Cretaceous amber of Myanmar. Cretac. Res. 2020, 105, 104040. [Google Scholar] [CrossRef]
- Regalado, L.; Schmidt, A.R.; Müller, P.; Niedermeier, L.; Schneider, H. Heinrichsia cheilanthoides gen. et sp. nov., a fossil fern in the family Pteridaceae (Polypodiales) from the Cretaceous amber forests of Myanmar. J. Syst. Evol. 2019, 57, 329–338. [Google Scholar] [CrossRef]
- Sokol, J. Troubled treasure. Science 2019, 364, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.S. Myanmar: Palaeontologists must stop buying conflict amber. Nature 2020, 584, 525. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, D.A.; Engel, M.S.; Nascimbene, P.C. Fossiliferous Cretaceous amber from Myanmar (Burma): Its rediscovery, biotic diversity, and paleontological significance. Am. Mus. Novit. 2002, 3361, 1–72. [Google Scholar] [CrossRef]
- Cruickshank, R.D.; Ko, K. Geology of an amber locality in the Hukawng Valley, Northern Myanmar. J. Asian Earth Sci. 2003, 21, 441–455. [Google Scholar] [CrossRef]
- Shi, G.-H.; Grimaldi, D.A.; Harlow, G.E.; Wang, J.; Wang, J.; Yang, M.-C.; Lei, W.-Y.; Li, Q.-L.; Li, X.-H. Age constraint on Burmese amber based on U–Pb dating of zircons. Cretac. Res. 2012, 37, 155–163. [Google Scholar] [CrossRef]
- Yu, T.-T.; Kelly, R.; Mu, L.; Ross, A.; Kennedy, J.; Broly, P.; Xia, F.-Y.; Zhang, H.-C.; Wang, B.; Dilcher, D. An ammonite trapped in Burmese amber. Proc. Natl. Acad. Sci. USA 2019, 116, 11345–11350. [Google Scholar] [CrossRef]
- Haug, J.T.; Azar, D.; Ross, A.; Szwedo, J.; Wang, B.; Arillo, A.; Baranov, V.; Bechteler, J.; Beutel, R.; Blagoderov, V.; et al. Comment on the letter of the Society of Vertebrate Paleontology (SVP) dated 21 April 2020 regarding “Fossils from conflict zones and reproducibility of fossil-based scientific data”: Myanmar amber. Palz 2020, 94, 431–437. [Google Scholar] [CrossRef]
- Kramer, K.U. Dennstaedtiaceae. In The Families and Genera of Vascular Plants, Volume 1 Pteridophytes and Gymnosperms; Kramer, K.U., Green, P.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 81–94. [Google Scholar]
- Kramer, K.U. Monachosoraceae. In The Families and Genera of Vascular Plants, Volume 1 Pteridophytes and Gymnosperms; Kramer, K.U., Green, P.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 187–188. [Google Scholar]
- Tryon, R.M.; Tryon, A.F. Ferns and Allied Plants with Special Reference to Tropical America; Springer-Verlag: New York, NY, USA, 1982. [Google Scholar]
- Tryon, A.F.; Lugardon, B. Spores of the Pteridophyta; Springer-Verlag: New York, NY, USA, 1991. [Google Scholar]
- Schwartsburd, P.B.; Perrie, L.R.; Brownsey, P.; Shepherd, L.D.; Sundue, M.A. New insights into the evolution of the fern family Dennstaedtiaceae from an expanded molecular phylogeny and morphological analysis. Mol. Phylogenet. Evol. 2020, 150, 106881. [Google Scholar] [CrossRef]
- Lu, J.-M.; Du, X.-Y.; Kuo, L.-Y.; Ebihara, A.; Perrie, L.R.; Zuo, Z.-Y.; Shang, H.; Chang, Y.-H.; Li, D.-Z. Plastome phylogenomic analysis reveals evolutionary divergences of Polypodiales suborder Dennstaedtiineae. BMC Plant Biol. 2022, 22, 511. [Google Scholar] [CrossRef]
- Poinar, G.O., Jr. A Naturalist’s Guide to the Hidden World of Pacific Northwest Dunes; Oregon State University Press: Corvallis, OR, USA, 2016. [Google Scholar]
- Rasnitsyn, A.P.; Quicke, D.L.J. History of Insects; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Labandeira, C.C.; Wilf, P.; Johnson, K.R.; Marsh, F. Guide to Insect (and Other) Damage Types on Compressed Plant Fossils, Version 3.0; Smithsonian Institution: Washington, DC, USA, 2007. [Google Scholar]
- Labandeira, C.C. Ecology and evolution of gall-inducing arthropods: The pattern from the terrestrial fossil record. Front. Ecol. Evol. 2021, 9, 632449. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Wang, Y.-D.; Li, L.-Q.; Lu, N.; Zhu, Y.-B.; Huang, Z.-L.; McLoughlin, S. Plant-insect interactions across the Triassic–Jurassic boundary in the Sichuan Basin, South China. Front. Ecol. Evol. 2023, 11, 1338865. [Google Scholar] [CrossRef]
- Feng, Z.; Wan, S.; Sui, Q.; Labandeira, C.; Guo, Y.; Chen, J.-B. A Triassic tritrophic triad documents an early food-web cascade. Curr. Biol. 2022, 32, 5165–5171. [Google Scholar] [CrossRef] [PubMed]
- Labandeira, C.C.; Wappler, T. Arthropod and pathogen damage on fossil and modern plants: Exploring the origins and evolution of herbivory on land. Annu. Rev. Entomol. 2023, 68, 341–361. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.R.; Jancke, S.; Lindquist, E.E.; Ragazzi, E.; Roghi, G.; Nascimbene, P.C.; Schmidt, K.; Wappler, T.; Grimaldi, D.A. Arthropods in amber from the Triassic Period. Proc. Natl. Acad. Sci. USA 2012, 109, 14796–14801. [Google Scholar] [CrossRef] [PubMed]
- Sidorchuk, E.A.; Schmidt, A.R.; Ragazzi, E.; Roghi, G.; Lindquist, E.E. Plant-feeding mite diversity in Triassic amber (Acari: Tetrapodili). J. Syst. Palaeontol. 2014, 13, 129–151. [Google Scholar] [CrossRef]
- Sidorchuk, E.A. Mites as fossils: Forever small? Int. J. Acarol. 2018, 44, 149708. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Meng, F. A New Species of Krameropteris (Dennstaedtiaceae) from Mid-Cretaceous Myanmar Amber. Taxonomy 2025, 5, 3. https://doi.org/10.3390/taxonomy5010003
Li C, Meng F. A New Species of Krameropteris (Dennstaedtiaceae) from Mid-Cretaceous Myanmar Amber. Taxonomy. 2025; 5(1):3. https://doi.org/10.3390/taxonomy5010003
Chicago/Turabian StyleLi, Chunxiang, and Fanwei Meng. 2025. "A New Species of Krameropteris (Dennstaedtiaceae) from Mid-Cretaceous Myanmar Amber" Taxonomy 5, no. 1: 3. https://doi.org/10.3390/taxonomy5010003
APA StyleLi, C., & Meng, F. (2025). A New Species of Krameropteris (Dennstaedtiaceae) from Mid-Cretaceous Myanmar Amber. Taxonomy, 5(1), 3. https://doi.org/10.3390/taxonomy5010003




