Abstract
We report a new species of shearwater, Ardenna buchananbrowni sp. nov., from the Pliocene of New Zealand. It is both the smallest and oldest known diving member of the genus, demonstrating that this now abundant form of shearwater has had a long presence in southern oceans. Ardenna buchananbrowni sp. nov. is among the few extinct shearwaters described from the Southern Hemisphere and adds to an increasingly diverse seabird assemblage in the Pliocene of the region.
Keywords:
biogeography; fossil; Piacenzian; Procellariiformes; Tangahoe Formation; Taranaki; Waipipian 1. Introduction
Shearwaters (Procellariidae) are a diverse group of procellariiforms comprising three main lineages: the relatively large Calonectris, weighing 470–1060 g; the somewhat smaller Ardenna, weighing 320–950 g; and the notably smaller Puffinus, weighing 120–575 g [1,2,3,4,5,6,7,8].
Shearwaters contribute the greatest number of species to the Neogene procellariform fossil record [9], with the oldest unambiguous records dating back to the Middle Miocene [10]. The affinities of purported older species like Puffinus raemdonckii from the early Oligocene of Belgium remain unclear [10,11,12]. Shearwater fossils are concentrated in the Northern Hemisphere [9,13], with southern occurrences previously limited to modern or unidentified taxa from the Mio-Pliocene of South Africa [14,15], Peru [16], and Chile [17].
This situation has recently begun to change with the discovery of a globally important Pliocene seabird assemblage from Taranaki, Aotearoa New Zealand [18,19,20,21,22,23]. Procellariiforms from this locality already include Ardenna davealleni, a large gliding shearwater [18]. Here, we describe a smaller diving shearwater from the same assemblage.
2. Materials and Methods
The fossils described here come from Pliocene mudstone concretions and were mechanically prepared by their collectors. Based on their advanced degree of bone fusion, both specimens are osteologically mature. We compared the new fossils to other procellariiforms, including representatives of all clades of living shearwaters, using specimens housed at the Museum of New Zealand Te Papa Tongarewa (Te Papa; NMNZ, Wellington). We limited detailed comparisons to Puffinus and ‘diving’ species of Ardenna after initial morphological comparisons showed few similarities with larger ‘gliding’ species of shearwater (see [18]). Sexual size dimorphism was not considered as shearwaters show only limited differences, with males being larger on average [24,25,26]. To visualise anatomical differences, we summarised individual bone measurements of various living and extinct shearwaters via principal component analysis (PCA) in R (v.4.3.2; [27]). All measurements were z-standardised (i.e., mean centred at 0 and standard deviation at 1) prior to analysis, and missing data were accounted for via mean value imputation. Considering the good preservation of the fossils, as well as the bone elements chosen for analysis (skull, humerus, and ulna; Table 1), we do not anticipate that damage and/or deformation will impact the results.Measurements (Table 1 and Table 2; Supplementary File S1) were taken to the nearest 0.1 mm using Vernier callipers and include the following: Skull—S-C, culmen length, taken from the tip of the beak to the suture between the lacrimal and the frontonasal process; S-CL, cranium length; S-MFF, minimum interorbital width, taken between the supraorbital glands (fossae glandulae nasalis); S-MIF, minimum interorbital width including the supraorbital glands; S-PPW, cranium width, taken at the level of the paroccipital process; S-Q, quadrate height across the mandibular and otic processes; S-TL, total length, taken from the cerebellar prominence to the tip of the beak. Coracoid—C-SmW, mid-shaft width. Humerus—H-SmD, mid-shaft depth; H-SmW, mid-shaft width. Ulna—U-PW, proximal width. Osteological terms follow [28,29].

Table 1.
Measurements (in mm) of the type specimens of Ardenna buchananbrowni sp. nov. (HOL = holotype NMNZ S.49931; PAR = paratype NMNZ S.49666), alongside mean values of measurements of specimens of Puffinus spp. and Ardenna spp. Abbreviations can be found in Section 2 of this paper; n indicates the number of specimens measured; an asterisk (*) indicates an estimated measurement from lightly damaged bones. See Supplementary File S1 for measurements of each specimen. The average weight (in grams) of living shearwaters was obtained from [25] and included for comparison.

Table 2.
Total lengths of humerus and ulna (in mm) of Ardenna buchananbrowni sp. nov., alongside those of Puffinus spp. and Ardena spp.
This published work and the nomenclatural act it contains have been registered in ZooBank, the online registration system for the International Code of Zoological Nomenclature. The ZooBank Life Science Identifier for this publication is http://zoobank.org/urn:lsid:zoobank.org:pub:7D76F702-3665-49FC-B0E1-43F2EC78CB76.
3. Systematic Palaeontology
Order Procellariiformes Fürbringer, 1888
The present fossils belong to Procellariiformes because of their straight, deeply grooved beaks, prominent dorsally opening nostrils, enlarged nasal glands above the eyes, and long, narrow wing bones.
Family Procellariidae Leach, 1820
The present fossils belong to Procellariidae because of their intermediate size (Diomedeidae are larger, Oceanitidae and Hydrobatidae smaller), gracile beaks, and dorsally projecting nostrils.
Genus Ardenna Reichenbach, 1853
Type species: Procellaria gravis O’Reilly, 1818.
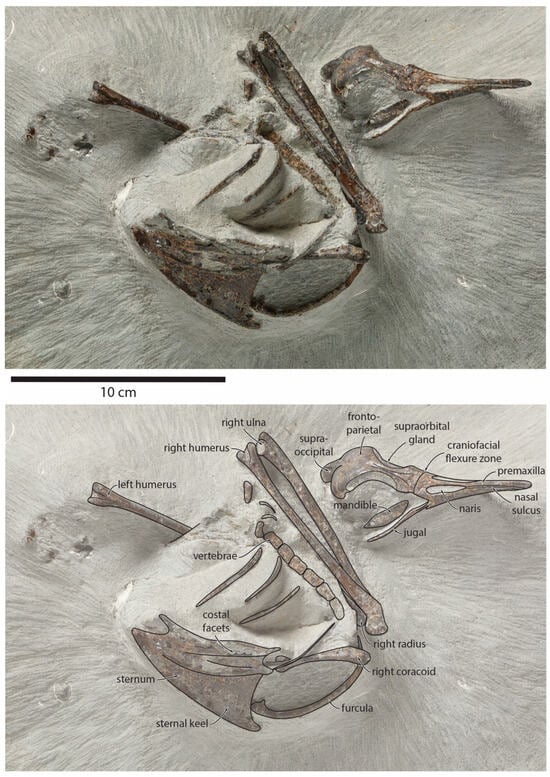
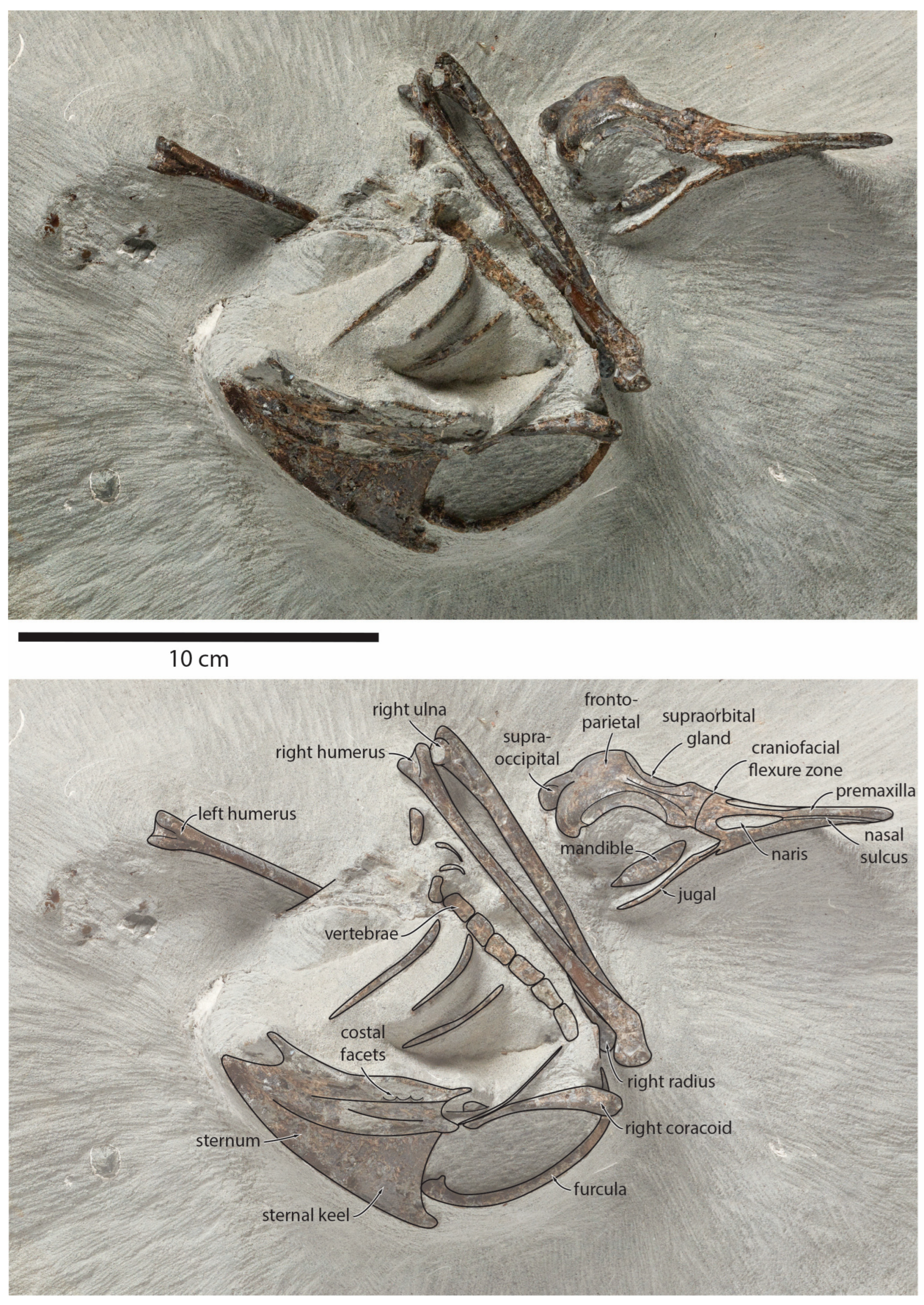
Figure 1.
Pliocene fossil shearwater Ardenna buchananbrowni sp. nov. Photograph of holotype NMNZ S.49931 (top) and explanatory line drawing (bottom).
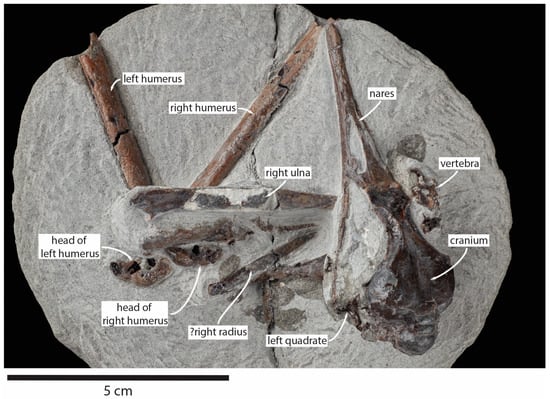
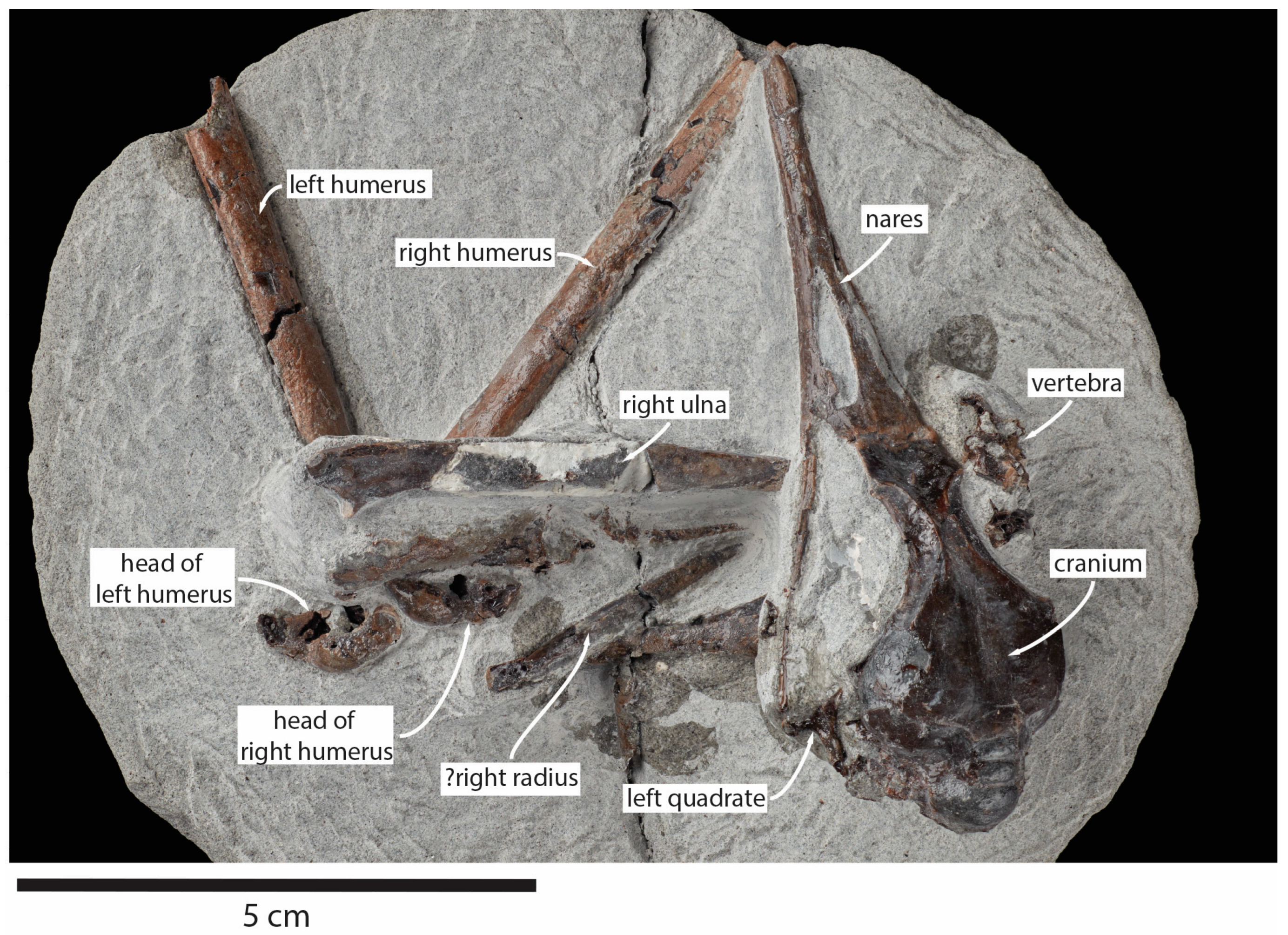
Figure 2.
Pliocene fossil shearwater Ardenna buchananbrowni sp. nov. paratype NMNZ S.49666, with elements identified.
ZooBank reg.: urn:lsid:zoobank.org:act:A24C44F5-1969-4A6E-85F7-24B468BC5BCF
Holotype: NMNZ S.49931, partial articulated skeleton collected by Karl Raubenheimer, preserving the complete skull and premaxilla, posterior right mandible, sternum, furcula, right coracoid, a row of articulated thoracic vertebrae, four ribs, both humeri, right ulna, right radius, and several small unidentified fragments (Figure 1).
Type locality and horizon: Ohawe Beach, southern Taranaki, New Zealand (39°35.55′ S 174°12.45′ E). Tangahoe Formation. (Fossil Record Electronic Database FR Number Q21/F0175).
Age: Late Pliocene, Piacenzian/Waipipian; 3.36–3.06 Ma [30].
Paratype: NMNZ S.49666, partial skeleton preserving the complete skull and premaxilla, left quadrate, right coracoid, both humeri (missing their distal ends), right ulna, probable right radius, one vertebra, and several small unidentified fragments (Figure 2). This skeleton is similar in size to the holotype and was collected by John Buchanan-Brown at Waihi Beach, South Taranaki, New Zealand (39°36.13′ S 174°14.08′ E). Formation and age as for the holotype.
Etymology: The specific epithet honours John Buchanan-Brown, the discoverer of the paratype.
Diagnosis: Small species of shearwater differing from all procellariids except Ardenna, Calonectris, and Puffinus in having a thin elongate beak and long olecranon process of the ulna. Further differs from Calonectris and all species of Ardenna except A. grisea and A. tenuirostris in having a shallow brachial fossa on the humerus, a dorsoventrally flattened humeral shaft, and a humerus/ulna length ratio ≥ 1.05 (Table 1 and Table 2). Differs from Ardenna in being smaller overall but is comparable to the largest members of Puffinus.
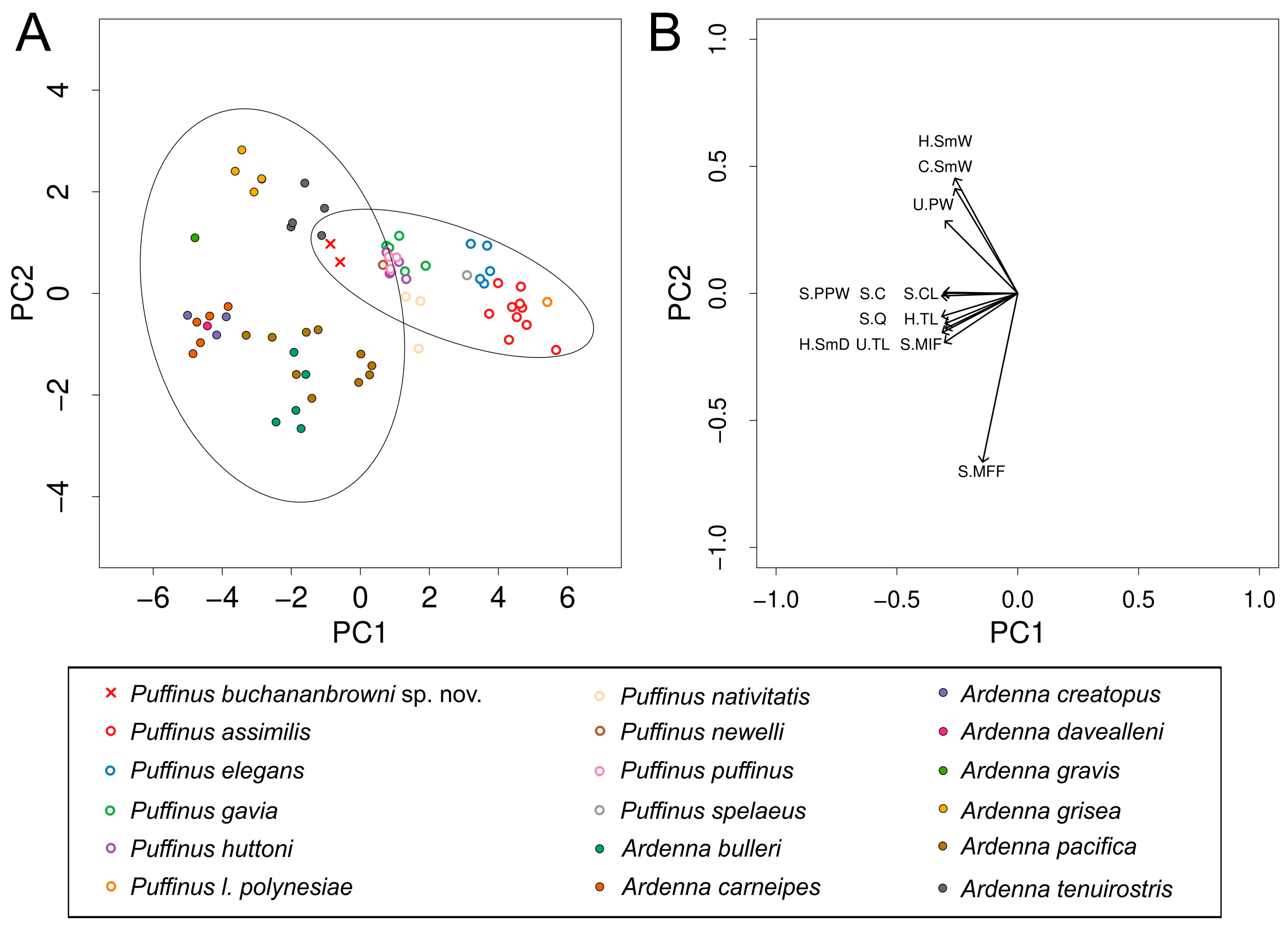
Our PCA clusters the new fossils with Ardenna but still within the 95% confidence ellipse of Puffinus, reflecting their intermediate size and morphology (Figure 3A; Supplementary File S1). PC1 accounts for 78% of the variation and largely reflects size. By contrast, PC2 accounts for 12% of the variation and broadly separates diving shearwaters (Puffinus, Ardenna grisea, and A. tenuirostris) from gliding forms (other Ardenna), with the new species clustering with the divers. Overall, it falls closest to A. tenuirostris, but differs from the latter, like from all Ardenna, in having a shorter humerus and ulna (Table 2). For a comparison of size and morphology between diving shearwaters (Puffinus, A. grisea, and A. tenuirostris) and gliding Ardenna, see Supplementary File S2.
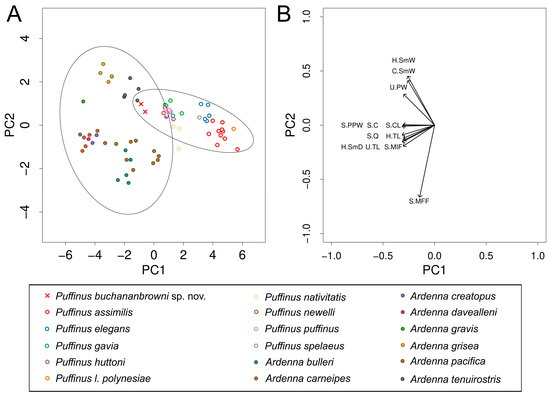
Figure 3.
Results of principal component analysis of skeletal measurements of Ardenna buchananbrowni sp. nov., Puffinus spp., and Ardena spp. (see Supplementary File S1 for specimen list and measurements). (A) Biplot of first and second principal component scores, with 95% confidence ellipses for each genus. A. buchananbrowni sp. nov. is represented by red crosses; closed circles represent Ardenna spp., and open circles represent Puffinus spp. (each colour represents one species). (B) Projection of principal component vectors (loadings) of tested parameters on to PC1–PC2 biplot.
Description: The new fossils are small to medium-sized relative to other procellariiforms. The skull is elongate with a prominent supraoccipital. The beak is long and narrow, with prominent nasal openings, deep nasal sulci, and large supraorbital glands. The wing bones are long, slender, and straight. The head of the humerus slightly overhangs the dorsal pneumotricipital fossa. The humerus is noticeably flattened and bears a capital shaft ridge extending from the proximal shaft to the head. There is no tubercle on the cranial surface of the ventral margin of the bicipital area. The dorsal supracondylar process is moderately sized and tapers to a point, but is relatively straight and not clearly angled proximally. A ridge runs along the caudal surface of the ulna to the proximal tip, which forms a prominent olecranon process. The sternum is elongate and has a large keel with a convex ventral edge. The coracoid is short and stout, with a large acrocoracoid process. Neither specimen preserves the pelvic or leg elements, but the holotype includes a row of vertebrae and ribs.
4. Discussion
In light of their morphology, supported by the PCA results, we parsimoniously interpret these fossils as a new diving species of Ardenna. Below, we provide more specific comparisons with other taxa and discuss the history of the group in New Zealand.
4.1. Convergent Evolution of Diving Adaptations
Puffinus and diving species of Ardenna convergently evolved a suite of adaptations for underwater propulsion, including flattened humeri, a shortened forewing, a stouter and more curved femur, and a more laterally compressed tarsometatarsus ([31] p. 254). Ardenna buchananbrowni sp. nov. shares many of these features and, thus, highlights the antiquity of this convergent trend.
Other potentially relevant characters in this context include the elongate sternum of the holotype, which recalls that of diving shearwaters [24,32]. Specifically, the ratio of its humerus to sternal keel lengths (84.4:69.1 = 1.22) falls within the range of Puffinus (1.17–1.28) and is only just below that of diving Ardenna (1.38–1.53), with the gliding species of Ardenna showing notably higher values (1.74–2.08) ([24] pp. 85–86). The sternal keel of the holotype also appears to protrude anteriorly just beyond the spina externa, as also only found in diving shearwaters and, especially, Puffinus ([24] pp. 108, 158); however, damage to the bone makes the exact extent of the projection hard to discern. Finally, our new fossils have a relatively thin beak and smooth nasal tubes that open more dorsally than in gliding species of Ardenna, but resemble those of Puffinus spp., A. grisea, and A. tenuirostris ([24] pp. 107, 128, 130).
4.2. Comparisons with Other Shearwater Fossils
The discovery of a third Pliocene species of Ardenna helps to elucidate the history of this genus. In the past, fossils of diving shearwaters have generally been assigned to Puffinus. Many of them are fragmentary, however, and were described when Ardenna was considered a junior synonym of Puffinus (e.g., [33]). As a result, the assignment of many Puffinus fossils should be considered tentative.
Two extinct species of shearwater have been described from New Zealand: P. spelaeus from the Holocene [34] and A. davealleni from the Pliocene [18]. Neither closely resembles A. buchananbrowni sp. nov., with P. spelaeus being notably smaller and A. davealleni being both notably larger and a gliding species (Table 1). A record of Pliocene ‘Puffinus’ from near Taihape, from similarly aged deposits as those that yielded A. buchananbrowni sp. nov., remains unnamed [35].
Some southern shearwater species migrate between hemispheres in their non-breeding season (e.g., [25]) and have even been reported from northern archaeological deposits (e.g., [36,37,38]). As a result, and despite the vast distances involved, it is crucial to compare A. buchananbrowni sp. nov. to northern shearwater fossils.
Comparisons with Puffinus raemdonckii (van Beneden, 1871) from the early Oligocene of Belgium are hampered by the loss of the type material [10,11,12]. The lectotype is a c. 10 cm long humerus missing the proximal end [11,39], which is markedly longer than the humerus of A. buchananbrowni sp. nov. (80.2 mm).
Puffinus antiquus (Milne-Edwards, 1874) from the Middle Miocene of France is based on the proximal end of a humerus [40,41]. Its original description does not clearly distinguish this species from many other shearwaters, but suggests that the humerus is slightly stouter than that of the Cape petrel Daption capense (Linnaeus, 1758), which indicates that P. antiquus was slightly larger than A. buchananbrowni sp. nov. (see [42]). The far older age of P. antiquus also argues against close affinities with A. buchananbrowni sp. nov.
Several diving shearwaters with flattened humeri and/or tarsometatarsi are known from the Mio-Pliocene of the United States [43,44,45,46]. Among the latter, Early Miocene Puffinus micraulax Brodkorb, 1963 is smaller than A. buchananbrowni sp. nov.; it is only known from the coast of the Atlantic Ocean, and considerably predates the New Zealand fossil [12,42,47]. Puffinus inceptor Wetmore, 1930 from the Middle Miocene of California differs from A. buchananbrowni sp. nov. in the distal position of its dorsal condyle on the humerus and open intercondylar furrow [44,48]. P. inceptor is probably also larger than A. buchananbrowni sp. nov. (see [42]). Puffinus calhouni Howard, 1968, P. barnesi Howard, 1978, P. priscus Miller, 1961, and P. diatomicus Miller, 1925 from the Late Miocene of California were described based on crushed or rather fragmentary material and are similar to A. buchananbrowni sp. nov. in size, but predate it [43,44,45,49,50,51,52]. Puffinus mitchelli Miller, 1961 and P. felthami Howard, 1949 from the Late Miocene and Early Pliocene of California are considerably larger than A. buchananbrowni sp. nov. [42,44,49]. Puffinus kanakoffi Howard, 1949 from the Late Pliocene of California has different humeral proportions to A. buchananbrowni sp. nov., with a humeral shaft width & depth of 5.3–5.7 & 3.3–3.7 mm versus 5.9–6.6 & 3.1–3.3 mm (Table 1; [45,49]). Puffinus tedfordi Howard, 1971 from the Early Pliocene of Mexico is only represented by partial tarsometatarsi and, thus, currently cannot be directly compared with A. buchananbrowni sp. nov. despite its potentially similar size [42,46]. P. tedfordi has, however, been noted for its unusually robust anatomy and, furthermore, still predates our material by about two million years [42]. Pending the discovery of further material, we, hence, consider these taxa to be different.
Puffinus nestori Alcover, 1989, P. holeae Walker, Wragg & Harrison, 1990, and P. olsoni McMinn, Jaune & Alcover, 1990 from the Pleistocene and Holocene of Spain all come from younger deposits than P. buchananbrowni sp. nov. [2,53,54,55]. Additionally, P. nestori and P. holeae are larger species than P. buchananbrowni sp. nov. [53,55], and P. olsoni is smaller than P. buchananbrowni sp. nov. [54]. Various other Miocene and Pliocene ‘Puffinus’ remains have been described but not assigned to species (e.g., [14,15,17,31,45,50,52]).
All other extinct shearwaters clearly differ from A. buchananbrowni sp. nov. because they resemble either Calonectris or the gliding forms of Ardenna (see [18]). These fossils include Calonectris kurodai Olson, 2009 (Middle Miocene, Chesapeake Bay, east coast of USA), C. krantzi Olson and Rasmussen, 2001 (Early Pliocene, Lee Creek Mine, NC, USA), C. wingatei Olson, 2008 (Middle Pleistocene, Bermuda), Ardenna conradi (Marsh, 1870) (Middle Miocene, MD, USA), “Puffinus” aquitanicus Milne-Edwards, 1874 (Middle Miocene, France), Ardenna gilmorei (Chandler, 1990) (Late Pliocene, San Diego, CA, USA), and Ardenna pacificoides (Olson, 1975) (Pleistocene, Saint Helena).
4.3. Shearwater Evolution in Zealandia
New Zealand today is a centre of shearwater diversity with nine of a global total of 42 species breeding there [7]. Resident species include the wedge-tailed shearwater, A. pacifica; Buller’s shearwater, A. bulleri; the sooty shearwater, A. grisea; the pale-footed shearwater, A. carneipes; the fluttering shearwater, P. gavia; Hutton’s shearwater, P. huttoni; the little shearwater, P. assimilis; and the subantarctic little shearwater, P. elegans [8].
Ardenna davealleni and A. buchananbrowni sp. nov. from the Pliocene provide a glimpse of the past diversity of shearwaters in New Zealand and evince their relatively long history in the South Pacific. More broadly, they also add to earlier undescribed records of extinct southern shearwaters from South Africa and western South America [14,15,16,17].
Ardenna buchananbrowni sp. nov. is both the earliest diving member and one of the smallest representatives of the genus Ardenna (the smallest among the diving Ardenna spp.). Modern diving Ardenna—A. grisea and A. tenuirostris—are key members of the marine community and two of the most abundant seabirds globally [56]. The new species provides the first evidence that Ardenna shearwaters have had a diving form for at least 3 million years. Details about the evolution of A. grisea and A. tenuirostris remain poorly known, but the discovery of A. buchananbrowni sp. nov. either suggests that the living species evolved from a smaller diving ancestor or that diving Ardenna species were more diverse in the past. Puffinus and Ardenna are estimated to have diverged at least 10.4 million years ago based on molecular data [5] but fossils indicate that this divergence apparently occurred by the Middle Miocene [12,18,24,48]. Therefore, any Pliocene shearwater species should already be well differentiated into their respective genera.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/taxonomy4020012/s1, File S1: Data used and results from PCA analysis; measurements in mm. Abbreviations can be found in the Materials and Methods section of this paper; File S2. Comparative image of bone elements of three recent shearwater species.
Author Contributions
Conceptualisation, investigation, A.J.D.T.; methodology, data curation, formal analysis, A.J.D.T., R.B.S. and B.M.T.; visualisation, B.M.T., F.G.M. and R.B.S.; writing—original draft preparation, A.J.D.T. and R.B.S.; writing—review and editing, all authors; funding acquisition, A.J.D.T. and F.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Te Papa Collection Development Fund (New Zealand).
Data Availability Statement
All data can be found within the article and its Supplementary Materials.
Acknowledgments
We thank Karl Raubenheimer and John Buchanan-Brown for providing their specimens to Te Papa; Jean-Claude Stahl (NMNZ) for the photos of the specimens used herein; and the two anonymous reviewers for their comments and suggestions to improve our manuscript. We also acknowledge the ongoing support provided by Ngāti Ruanui and Ngā Ruahine for Te Papa’s work in their rohe.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Austin, J.J. Molecular phylogenetics of Puffinus shearwaters: Preliminary evidence from mitochondrial cytochrome b gene sequences. Mol. Phylogenetics Evol. 1996, 6, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, P.; Amengual, J.; Wink, M. Phylogenetic relationships in Mediterranean and North Atlantic shearwaters (Aves: Procellariidae) based on nucleotide sequences of mt DNA. Biochem. Syst. Ecol. 1998, 26, 145–170. [Google Scholar] [CrossRef]
- Nunn, G.B.; Stanley, S.E. Body size effects and rates of cytochrome b evolution in tube-nosed seabirds. Mol. Biol. Evol. 1998, 15, 1360–1371. [Google Scholar] [CrossRef]
- Austin, J.J.; Bretagnolle, V.; Pasquet, E. A global molecular phylogeny of the small Puffinus shearwaters and implications for systematics of the little-Audubon’s shearwater complex. Auk 2004, 121, 847–864. [Google Scholar] [CrossRef]
- Penhallurick, J.; Wink, M. Analysis of the taxonomy and nomenclature of the Procellariiformes based on complete nucleotide sequences of the mitochondrial cytochrome b gene. Emu 2004, 104, 125–147. [Google Scholar] [CrossRef]
- Onley, D.; Scofield, P. Albatrosses, Petrels, and Shearwaters of the World; Princeton University Press: Princeton, NJ, USA, 2007; p. 240. [Google Scholar]
- Dickinson, E.C.; Remsen, J.V. The Howard and Moore Complete Checklist of the Birds of the World, 4th ed.; Aves Press: Eastbourne, UK, 2013; Volume 1, p. 461. [Google Scholar]
- Checklist Committee. Checklist of the Birds of New Zealand, 5th ed.; Ornithological Society of New Zealand: Wellington, New Zealand, 2022; p. 332. [Google Scholar]
- Warham, J. The Behaviour, Population Biology and Physiology of the Petrels; Academic Press: London, UK, 1996; p. 613. [Google Scholar]
- Mayr, G.; Smith, T. Phylogenetic affinities and taxonomy of the Oligocene Diomedeoididae, and the basal divergences amongst extant procellariiform birds. Zool. J. Linn. Soc. 2012, 166, 854–875. [Google Scholar] [CrossRef]
- Brodkorb, P. The systematic position of two Oligocene birds from Belgium. Auk 1962, 79, 706–707. [Google Scholar] [CrossRef]
- Olson, S.L. The fossil record of birds. Avian Biol. 1985, 8, 79–238. [Google Scholar]
- Warheit, K.I. The seabird fossil record and the role of paleontology in understanding seabird community structure. In Biology of Marine Birds; Schreiber, E.A., Burger, J., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 17–55. [Google Scholar]
- Olson, S.L. Early Pliocene Procellariiformes (Aves) from Langebaanweg, South-Western Cape Province, South Africa. Ann. S. Afr. Mus. 1985, 95, 123–145. [Google Scholar]
- Olson, S.L. An Early Pliocene marine avifauna from Duinefontein, Cape Province, South Africa. Ann. S. Afr. Mus. 1985, 95, 147–164. [Google Scholar]
- Stucchi, M.; Urbina, M. Nuevos restos de Procellariiformes (Aves) de la Formación Pisco, Perú. Boletín Soc. Geol. Perú 2005, 100, 67–77. [Google Scholar]
- Hoffmeister, M.C.; Carrillo, J.D.; Nielsen, S.N. The evolution of seabirds in the Humboldt Current: New clues from the Pliocene of central Chile. PLoS ONE 2014, 9, e90043. [Google Scholar] [CrossRef] [PubMed]
- Tennyson, A.J.D.; Mannering, A.A. A new species of Pliocene shearwater (Aves: Procellariidae) from New Zealand. Tuhinga 2018, 29, 1–19. [Google Scholar]
- Mayr, G.; Tennyson, A.J.D. A small, narrow-beaked albatross from the Pliocene of New Zealand demonstrates a higher past diversity in the feeding ecology of the Diomedeidae. Ibis 2020, 162, 723–734. [Google Scholar] [CrossRef]
- Thomas, D.B.; Tennyson, A.J.D.; Scofield, R.P.; Heath, T.A.; Pett, W.; Ksepka, D.T. Ancient crested penguin constrains timing of recruitment into seabird hotspot. Proc. R. Soc. B 2020, 287, 20201497. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.B.; Tennyson, A.J.D.; Marx, F.G.; Ksepka, D.T. Pliocene fossils support a New Zealand origin for the smallest extant penguins. J. Paleontol. 2023, 97, 711–721. [Google Scholar] [CrossRef]
- Tennyson, A.J.D.; Tomotani, B.M. A new fossil species of Procellaria (Aves: Procellariiformes) from the Pliocene of New Zealand. Papéis Avulsos Zool. 2021, 61, e20216116. [Google Scholar] [CrossRef]
- Tennyson, A.J.D.; Salvador, R.B. A new giant petrel (Macronectes, Aves: Procellariidae) from the Pliocene of Taranaki, New Zealand. Taxonomy 2023, 3, 57–67. [Google Scholar] [CrossRef]
- Kuroda, N. On the Classification and Phylogeny of the Order Tubinares, Particularly the Shearwaters (Puffinus), with Special Considerations on Their Osteology and Habit Differentiation; N. Kuroda: Tokyo, Japan, 1954; p. 179. [Google Scholar]
- Marchant, S.; Higgins, P.J. Handbook of Australian, New Zealand and Antarctic Birds; Ratites to Ducks. Oxford University Press: Melbourne, Australia, 1990; Volume 1, p. 1400. [Google Scholar]
- Warham, J. The Petrels: Their Ecology and Breeding Systems; Academic Press: London, UK, 1990; p. 440. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Baumel, J.J.; Witmer, L.M. Osteologia. In Handbook of Avian Anatomy: Nomina Anatomica Avium, 2nd ed.; Baumel, J.J., King, A.S., Breazile, J.E., Evans, H.E., Vanden Berge, J.C., Eds.; Publications of the Nuttall Ornithological Club 23; Nuttall Ornithological Club: Cambridge, MA, USA, 1993; pp. 45–132. [Google Scholar]
- Livezey, B.C.; Zusi, R.L. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. I. Methods and characters. Bull. Carnegie Mus. Nat. Hist. 2006, 37, 1–544. [Google Scholar] [CrossRef]
- Naish, T.R.; Wehland, F.; Wilson, G.S.; Browne, G.H.; Cook, R.A.; Morgans, H.E.G.; Rosenberg, M.; King, P.R.; Smale, D.; Nelson, C.S.; et al. An integrated sequence stratigraphic, palaeoenvironmental, and chronostratigraphic analysis of the Tangahoe Formation, southern Taranaki coast, with implications for mid-Pliocene (c. 3.4–3.0 Ma) glacio-eustatic sea-level changes. J. R. Soc. N. Z. 2005, 35, 151–196. [Google Scholar] [CrossRef]
- Olson, S.L.; Rasmussen, P.C. Miocene and Pliocene birds from the Lee Creek Mine, North Carolina. Smithson. Contrib. Paleobiol. 2001, 90, 233–365. [Google Scholar]
- Kuroda, N. On the skeletons of Puffinus nativitatis and Pagodroma nivea. Tori 1953, 13, 50–68. [Google Scholar] [CrossRef][Green Version]
- Jouanin, C.; Mougin, J.-L. Order Procellariiformes. In Check-List of Birds of the World, 2nd ed.; Mayr, E., Cottrell, G.W., Eds.; Museum of Comparative Zoology: Cambridge, MA, USA, 1979; Volume 1, pp. 48–121. [Google Scholar]
- Holdaway, R.N.; Worthy, T.H. A new fossil species of shearwater Puffinus from the Late Quaternary of the South Island, New Zealand, and notes on the biogeography and evolution of the Puffinus gavia superspecies. Emu 1994, 94, 201–215. [Google Scholar] [CrossRef]
- Henderson, N.; Gill, B.J. A mid-Pliocene shearwater skull (Aves: Procellariidae: Puffinus) from the Taihape Mudstone, central North Island, New Zealand. N. Z. J. Geol. Geophys. 2010, 53, 327–332. [Google Scholar] [CrossRef]
- Harrison, C.J.O. A re-examination of British Devensian and earlier Holocene bird bones in the British Museum (Natural History). J. Archaeol. Sci. 1980, 7, 53–68. [Google Scholar] [CrossRef]
- Bovy, K.M. Global human impacts or climate change?: Explaining the sooty shearwater decline at the Minard site, Washington State, USA. J. Archaeol. Sci. 2007, 34, 1087–1097. [Google Scholar] [CrossRef]
- Tennyson, A.J.D.; Rieth, T.M.; Cochrane, E.E. Bird remains from an early archaeological site on Tutuila Island, Sāmoa. Contrib. Cient. Mus. Argent. Cienc. Nat. Bernardino Rivadavia 2017, 7, 157–172. [Google Scholar]
- van Beneden, P.J. Les oiseaux de l’argile rupelienne. Bull. L’académie R. Sci. Belgique 1871, 32, 256–261. [Google Scholar]
- Milne-Edwards, A. Observations sur les oiseaux fossiles des Faluns de Saucats. Bibliothèque L’école Hautes Études Sect. Sci. Nat. 1874, 11, 1–12. [Google Scholar]
- Brodkorb, P. Catalogue of fossil birds—Part 1 (Archaeopterygiformes through Ardeiformes). Bull. Fla. State Mus. 1963, 7, 179–293. [Google Scholar] [CrossRef]
- Howard, H. Pliocene avian remains from Baja California. Nat. Hist. Mus. Los Angeles Co. Contrib. Sci. 1971, 217, 1–17. [Google Scholar] [CrossRef]
- Miller, L. Avian remains from the Miocene of Lompoc, California. Carnegie Inst. Wash. 1925, 349, 107–117. [Google Scholar]
- Miller, L. Birds from the Miocene of Sharktooth Hill, California. Condor 1961, 63, 399–402. [Google Scholar] [CrossRef]
- Chandler, R.M. Recent advances in the study of Neogene fossil birds. Part II. Fossil birds of the San Diego Formation, Late Pliocene, Blancan, San Diego County, California. Ornithol. Monogr. 1990, 44, 73–161. [Google Scholar] [CrossRef]
- Olson, S.L. A new diminutive species of shearwater of the genus Calonectris (Aves: Procellariidae) from the Middle Miocene Calvert Formation of Chesapeake Bay. Proc. Biol. Soc. Wash. 2009, 122, 466–470. [Google Scholar] [CrossRef]
- Brodkorb, P. Miocene birds from the Hawthorne Formation. Q. J. Fla. Acad. Sci. 1963, 2, 159–167. [Google Scholar]
- Wetmore, A. Fossil bird remains from the Temblor Formation near Bakersfield, California. Proc. Calif. Acad. Sci. 1930, 19, 85–93. [Google Scholar]
- Howard, H. New avian records for the Pliocene of California. Carnegie Inst. Wash. Publ. 1949, 584, 179–199. [Google Scholar]
- Howard, H. Tertiary birds from Laguna Hills, Orange County, California. Nat. Hist. Mus. Los Angeles Co. Contrib. Sci. 1968, 142, 1–21. [Google Scholar] [CrossRef]
- Howard, H. Late Miocene marine birds from Orange County, California. Nat. Hist. Mus. Los Angeles Co. Contrib. Sci. 1978, 290, 1–26. [Google Scholar] [CrossRef]
- Howard, H.; Barnes, L.G. Middle Miocene marine birds from the foothills of the Santa Ana Mountains, Orange County, California. Nat. Hist. Mus. Los Angeles Co. Contrib. Sci. 1987, 383, 1–9. [Google Scholar] [CrossRef]
- Alcover, J.A. Les aus fòssils de la Cova de Ca Na Reia. Endins 1989, 14–15, 95–100. [Google Scholar]
- McMinn, M.; Jaume, D.; Alcover, J.A. Puffinus olsoni n. sp.: Nova espècie de baldritja recentment extingida provinent de depòsits espeleològics de Fuerteventura i Lanzarote (Illes Canàries, Atlàntic Oriental). Endins 1990, 16, 63–71. [Google Scholar]
- Walker, C.A.; Wragg, G.M.; Harrison, C.J.O. A new shearwater from the Pleistocene of the Canary Islands and its bearing on the evolution of certain Puffinus shearwaters. Hist. Biol. 1990, 3, 203–224. [Google Scholar] [CrossRef]
- Brooke, M.L. Albatrosses and Petrels Across the World; Oxford University Press: Oxford, UK, 2004; p. 499. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).