Engineered Human Dental Pulp Stem Cells with Promising Potential for Regenerative Medicine
Abstract
1. Introduction
2. Isolation and Culture of DPSCs
2.1. DPSCs from Enzymatic Digestion of DP Tissue (DPSC-ED) vs. DPSCs Obtained Through Outgrowth from Tissue Explants (DPSC-OG)
2.2. Optimizing Conditions for DPSCs in Cell Culture
2.3. Requirement for Xeno-Free Cell Culture System of DPSCs for Human Cell Therapy
2.4. Single-Cell Cloning and Single-Cell-Based RNA-Seq (scRNA-Seq)
2.5. Usefulness of mRNA Analysis of a Small Number of DPSCs for Creating Gene Expression Profiles
2.6. Markers for DPSCs
2.7. Multilineage Differentiation Potential of DPSCs
2.7.1. Osteogenic (Osteoblastic) Differentiation
2.7.2. Odontoblastic Differentiation
2.7.3. Differentiation into Adipogenic Cell Lineages
2.7.4. Differentiation into Neurogenic Cell Lineage
2.7.5. Differentiation into Hepatocytes
2.7.6. Differentiation into Endothelial Lineage
2.7.7. Differentiation into Cardiomyocytes
2.7.8. Differentiation into Pancreatic Lineage
2.7.9. Differentiation into Smooth Muscle Cells (SMCs)
3. Gene Engineering of DPSCs
3.1. Development of Methods for Gene Introduction into DPSCs
3.2. Immortalization
3.3. Alteration of Cell Behavior
3.3.1. Enhanced Differentiation into Odontoblast/Mineralization
3.3.2. Enhanced Differentiation into Osteogenic Lineage
3.3.3. Enhanced Cell Proliferation
3.3.4. Enhanced Angiogenic Commitment
3.3.5. Enhanced Neurogenic Differentiation and Neuroprotective Effects
3.3.6. Enhanced Adipogenic Commitment
3.3.7. Reduced Inflammatory Responses
3.3.8. Enhanced Cell Migration
3.3.9. Generation of IPCs and Oligodendrocyte Progenitors (OPs) from DPSCs by Forced Expression of Exogenous Genes
3.3.10. Apoptosis
3.3.11. Skeletal Myogenic Differentiation
3.3.12. Enhanced Pluripotency and Multilineage Differentiation Capability
3.4. Critical Evaluation of Gene-Engineering Strategies
4. Generation of Immature Cells by Forced Expression of Exogenous Genes in DPSCs
4.1. Generation of iPSCs
4.2. Generation of iTSCs from iPSCs
5. Usefulness of Feeder Cells to Maintain the Integrity of DPSCs
6. Genome Editing
6.1. CRISPR/Cas9-Based Manipulation of Bacterial Genes in S. aureus
6.2. CRISPR/Cas9-Based Manipulation of Genes Associated with Dental Health
7. Effect of DPSCs on Cancer Development
8. Exosomes and Cytokines Secreted from DPSCs
9. Immunomodulatory Effects of DPSCs
10. Application of Engineered DPSCs in Regenerative Medicine
10.1. Cell-Based Therapy Using Intact or Engineered DPSCs
10.2. Cell-Based Dental Stem Cell Therapy Using Intact DPSCs
10.3. Cell-Based Dental Stem Cell Therapy Using iPSC-Derived Dental Cells for Dentin–Pulp Complex Regeneration
11. Conclusions and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABEs | Adenine base editors |
| ADGRA2 | Adhesion G protein-coupled receptor A2 |
| ADM | Adrenomedullin |
| AFP | Alpha-fetoprotein |
| AI | Amelogenesis imperfecta |
| AKT | Protein kinase B |
| ALCAM | Activated leucocyte cell adhesion molecule |
| ALP | Alkaline phosphatase |
| AMPKα1 | Catalytic alpha 1 subunit of AMP-activated protein kinase (AMPK) |
| AMY2A | Amylase-2a |
| ANTXR1 | Anthrax toxin receptor 1 |
| AO | Ameloblast organoid |
| α-SMA | Alpha smooth muscle actin |
| ATP8B1 | ATPase phospholipid transporting 8B1 |
| BAX | BCL2-associated X |
| BCL-2 | B cell lymphoma-2 |
| BDNF | Brain-derived neurotrophic factor |
| BET | Betaine |
| bFGF | Basic fibroblast growth factor |
| BMP4 | Bone morphogenetic protein 4 |
| BMSCs | Bone marrow stem cells |
| CASP3 | Caspase-3 |
| CASP9 | Caspase-9 |
| CGFe | Concentrated growth factor exudate |
| CM | Conditioned medium |
| CNN | Calponin |
| CNS | Central nervous system |
| COL1 | Collagen type I |
| COL1A2 | Collagen type I alpha 2 chain |
| COL3A1 | Collagen type III alpha 1 chain |
| Con A | Concanavalin A |
| CPS | Carbamoyl phosphate synthetase |
| CRC | Colorectal cancer |
| Cx43 | Connexin 43 |
| DDIT3 | DNA damage-inducible transcript 3 |
| DECs | Dental epithelial cells |
| DES | Desmin |
| DFSCs | Dental follicle stem cells |
| DLL1 | Notch ligand Delta1 |
| DMCs | Dental mesenchymal cells |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMP-1 | Dentin matrix protein-1 |
| DPCs | Dental pulp cells |
| DPSCs | Dental pulp stem cells |
| DPSC-ED | DPSC from the enzymatic digestion of DP tissue |
| DPSC-OG | DPSC obtained through the outgrowth of tissue explants |
| DSP | Desmoplakin |
| DSPP | Dentinal sialophosphoprotein |
| E8 | Serum-free essential 8 medium |
| EBs | Embryoid bodies |
| ECM | Extracellular matrix |
| EFNB2 | EphrinB2 |
| EGF | Epidermal growth factor |
| Epfn | Epiprofin |
| EpiSCs | Epi-stem cells |
| ERK | Extracellular signal-regulated kinase |
| ESCs | Embryonic stem cells |
| ET-1 | Endothelin-1 |
| ETV2 | Ets variant transcription factor 2 |
| FABP4 | Fatty acid-binding protein 4 |
| FACS | Fluorescence-activated cell sorting |
| Fam83h | Family with sequence similarity 83 members H |
| FBS | Fetal bovine serum |
| Flk-1 | Functioning legal knowledge 1 |
| GALC | Galactosyl–ceramidase |
| GAP43 | Growth-associated protein 43 |
| GCG | Glucagon |
| Gdf11 | Growth/differentiation factor 11 |
| GDF15 | Growth differentiation factor 15 |
| GDNF | Glial cell-derived neurotrophic factor |
| GelMA | Gelatin methacryloyl |
| GFAP | Glial fibrillary acid protein |
| GING SCs | Gingival stem cells |
| GLUT4 | Glucose transporter type 4 |
| GM | Genetically modified |
| GMP | Good manufacturing practice |
| gtfs | Glucosyltransferases |
| GOI | Gene of interest |
| gRNA | Guide RNA |
| HDDPC | Human deciduous teeth-derived DPCs |
| HDR | Homology-directed repair |
| HERS/ERM | Hertwig’s epithelial root sheath/epithelial rests of Malassez |
| HGF | Hepatocyte growth factor |
| HIF-1 | Hypoxia-inducible factor-1 |
| HIF-1α | Hypoxia-inducible factor-1α |
| H/R | Hypoxia and reoxygenation |
| HA/TCP | Hydroxyapatite/tricalcium phosphate |
| HHEX | Hematopoietically expressed homeobox |
| HNF4α | Hepatic nuclear factor-4 alpha |
| HPV16 | E6/E7 proteins from human papillomavirus 16 |
| HS | Human serum |
| hTERT | Human telomerase reverse transcriptase protein |
| HUVECs | Human umbilical vein endothelial cells |
| IBMX | 3-isobutyl-1-methylxanthine |
| ICAM1 | Intercellular adhesion molecule-1 |
| ICCs | Islet-like cell clusters |
| ID1 | Inhibitor of DNA binding 1 |
| IFN-γ | Interferon-gamma |
| IGF-1 | Insulin-like growth factor-1 |
| IGFBP3 | Insulin-like growth factor binding protein-3 |
| IKK | κB kinase |
| IL | Interleukin |
| IL-8 | Interleukin-8 |
| IMDM | Iscove’s modified Dulbecco’s medium |
| IP | Intrapancreatic |
| IPC | Insulin-producing cell |
| iPSCs | Induced pluripotent stem cells |
| i-TSCs | Induced tissue-specific stem cells |
| IV | Intravenous |
| JAG-1 | Notch ligand, Jagged-1 |
| KDR | Kinase insert domain-containing receptor |
| KI | Knockin |
| KLF4 | Krüppel-like factor 4 |
| KO | Knockout |
| LIF | Leukemia inhibitory factor |
| LncRNA H19 | Long noncoding RNA H19 |
| LPL | Lipoprotein lipase |
| LPS | Lipopolysaccharide |
| Mafa | MAF bZIP transcription factor A |
| MAP2 | Microtubule-associated protein 2 |
| MCP-1 | Monocyte chemotactic protein-1 |
| MEF2C | Myocyte enhancer factor 2C |
| MEFs | Mouse embryonic fibroblasts |
| MEK1 | Mitogen-activated protein kinase kinase 1 |
| MEPE | Matrix extracellular phosphoglycoprotein |
| MESP | Mesoderm posterior BHLH transcription factor 1 |
| miR | MicroRNA |
| MMC | Mitomycin-C |
| MNX1 | Motor neuron and pancreas homeobox 1 |
| MSI1 | Musashi1 |
| MYH6 | Myosin heavy chain 6 |
| MyHC | Myosin heavy chain |
| MyoD | Myogenic differentiation 1 |
| MyoG | Myogenin |
| nCas9 | Cas9 nickase |
| MCAM | Melanoma cell adhesion molecule |
| NCAM | Neural cell adhesion molecule |
| NEFH | Neurofilament heavy chain |
| NES | Nestin |
| NeuN | Neuronal nuclei |
| NF-κB | Nuclear factor kappa B |
| NGF | Nerve growth factor |
| NGFR | Nerve growth factor receptor |
| Ngn3 | Neurogenin 3 |
| Nkx2.5 | NK-2 transcription factor-related, locus 5 |
| NKX6-1 | NK6 homeobox 1 |
| NOTCH1 | Notch1 |
| NSCs | Neuronal stem cells |
| OCN | Osteocalcin |
| OCT3/4 | Octamer-binding transcription factor 3/4 |
| Olig2 | Oligodendrocyte transcription factor 2 |
| ON | Osteonectin |
| OP | Oligodendrocyte progenitors |
| OPN | Osteopontin |
| OSX | Osterix |
| OT | Oxytocin |
| OXTR | Oxytocin receptor |
| PA-CM | Preameloblast-conditioned medium |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PAX4 | Paired box 4 |
| PAX6 | Paired box 6 |
| PAX9 | Paired box 9 |
| PDGF-BB | Platelet-derived growth factor BB |
| PDLSCs | Periodontal ligament stem cells |
| PDX1 | Pancreatic and duodenal homeobox 1 |
| PECAM-1 | Platelet/endothelial cell adhesion molecule-1 |
| PHA | Phytohemagglutinin |
| PI3K | Phosphatidylinositol 3 kinase |
| PIN1 | Peptidylprolyl cis/trans isomerase, NIMA-interacting 1 |
| PL | Platelet lysate |
| PLGA | Poly(lactic-co-glycolic acid) |
| PPAR-γ | Peroxisome proliferator-activated receptor γ |
| PPY | Pancreatic polypeptide |
| RA | Retinoic acid |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| RNAi | RNA interference |
| RNA-seq | RNA sequencing |
| RNA-snMIFxC | RNA analysis based on a small number of manually isolated fixed cells |
| ROS | Reactive oxygen species |
| RUNX2 | Runt-related transcription factor 2 |
| S18 | N-oleoyl serinol |
| S100 | S100 calcium-binding proteins |
| S100B | S100 calcium-binding protein B |
| SCAP | Stem cell apical papilla |
| SCI | Spinal cord injury |
| SCIP | Scratch-based isolation of primary cells from human dental pulps |
| scRNA-seq | Single-cell-based RNA-sequencing |
| SHED | Stem cells from human exfoliated deciduous teeth |
| SDF-1α | Stromal cell-derived factor-1α |
| SEMA3A | Semaphorin 3A |
| SERPINE1 | Serpin family E member 1 |
| SMCs | Smooth muscle cells |
| SORT1 | Sortilin 1 |
| SOX2 | SRY-related HMG-box 2 |
| SRGN | Serglycin |
| SSEA-1 | Stage-specific embryonic antigen-1 |
| SST | Somatostatin |
| STZ | Streptozotocin |
| SV40T | Simian virus 40 large T antigen |
| SYN1 | Synapsin I |
| SYP | Synaptophysin |
| T1D | Type 1 diabetes |
| TET2 | Ten-eleven-translocation 2 |
| TGFβ | Transforming growth factor beta |
| TGF-β1 | Transforming growth factor beta 1 |
| TIMP-1 | Tissue inhibitor of metalloproteinase-1 |
| TNF-α | Tumor necrosis factor-alpha |
| TUBB3 | β-III tubulin |
| uPA | Urokinase-type plasminogen activator |
| VEGF | Vascular endothelial growth factor |
| VEGFR2 | VEGF receptor 2 |
| VIM | Vimentin |
| VWF | von-Willebrand factor |
| WNT4 | Wnt family member 4 |
| WTA | Whole transcriptome amplification |
| XFM | Xenogeneic serum-free culture medium |
| XPC | Xeroderma pigmentosum complementation group C protein |
| ZBTB20 | Zinc finger and BTB domain-containing 20 |
| Zfp521 | Zinc finger protein 521 |
References
- Kim, K.; Evans, G. Tissue engineering: The future of stem cells. Top. Tissue Eng. 2005, 2, 1–21. [Google Scholar]
- Herberts, C.A.; Kwa, M.S.; Hermsen, H.P. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011, 9, 29. [Google Scholar] [CrossRef]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry--part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, I.; Sato, M.; Kiyokawa, Y.; Inada, E.; Iwase, Y.; Ibano, N.; Noguchi, H. Induced tissue-specific stem cells (iTSCs): Their generation and possible use in regenerative medicine. Pharmaceutics 2021, 13, 780. [Google Scholar] [CrossRef] [PubMed]
- Via, A.G.; Frizziero, A.; Oliva, F. Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J. 2012, 2, 154–162. [Google Scholar] [PubMed]
- Chu, D.T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, D.K.; Thanh, V.V.; Quang, T.L.; Truong, D.T.; Pham, V.H.; Ngoc, V.T.N.; Chu-Dinh, T.; et al. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int. J. Mol. Sci. 2020, 21, 708. [Google Scholar] [CrossRef]
- Fujii, S.; Fujimoto, K.; Goto, N.; Abiko, Y.; Imaoka, A.; Shao, J.; Kitayama, K.; Kanawa, M.; Sosiawan, A.; Suardita, K.; et al. Characterization of human dental pulp cells grown in chemically defined serum-free medium. Biomed. Rep. 2018, 8, 350–358. [Google Scholar] [CrossRef]
- Aurrekoetxea, M.; Garcia-Gallastegui, P.; Irastorza, I.; Luzuriaga, J.; Uribe-Etxebarria, V.; Unda, F.; Ibarretxe, G. Dental pulp stem cells as a multifaceted tool for bioengineering and the regeneration of craniomaxillofacial tissues. Front. Physiol. 2015, 6, 289. [Google Scholar] [CrossRef]
- Nuti, N.; Corallo, C.; Chan, B.M.; Ferrari, M.; Gerami-Naini, B. Multipotent differentiation of human dental pulp stem cells: A literature review. Stem Cell Rev. Rep. 2016, 12, 511–523. [Google Scholar] [CrossRef]
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Mesenchymal stem cells derived from dental pulp: A review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Mayo, V.; Sawatari, Y.; Huang, C.Y.; Garcia-Godoy, F. Neural crest-derived dental stem cells--where we are and where we are going. J. Dent. 2014, 42, 1043–1051. [Google Scholar] [CrossRef]
- Sonoda, S.; Tomoda, E.; Tanaka, Y.; Yamaza, T. Properties and possibilities of human dental pulp-derived stem cells. Arch. Stem Cell Res. 2015, 2, 1012. [Google Scholar] [CrossRef]
- Gimble, J.M.; Guilak, F.; Nuttall, M.E.; Sathishkumar, S.; Vidal, M.; Bunnell, B.A. In vitro differentiation potential of mesenchymal stem cells. Transfus. Med. Hemother. 2008, 35, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Ponnaiyan, D.; Jegadeesan, V. Comparison of phenotype and differentiation marker gene expression profiles in human dental pulp and bone marrow mesenchymal stem cells. Eur. J. Dent. 2014, 8, 307–313. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.S.; Lee, S.Y.; Kim, K.H.; Lee, Y.M.; Kim, W.K.; Lee, Y.K. Gene expression profile in mesenchymal stem cells derived from dental tissues and bone marrow. J. Periodontal Implant. Sci. 2011, 41, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, V.; Rattan, V.; Jha, V.; Bhattacharyya, S. Secretome cues modulate the neurogenic potential of bone marrow and dental stem cells. Mol. Neurobiol. 2017, 54, 4672–4682. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Tamaki, Y.; Nakahara, T.; Ishikawa, H.; Sato, S. In vitro analysis of mesenchymal stem cells derived from human teeth and bone marrow. Odontology 2013, 101, 121–132. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Deng, Z.; Tang, L.; Li, Y.; Shi, J.; Jin, Y. Odontogenic capability: Bone marrow stromal stem cells versus dental pulp stem cells. Biol. Cell 2007, 99, 465–474. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z. CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy. Comput. Struct. Biotechnol. J. 2020, 18, 2401–2415. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Wang, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef]
- Inada, E.; Saitoh, I.; Watanabe, S.; Aoki, R.; Miura, H.; Ohtsuka, M.; Murakami, T.; Sawami, T.; Yamasaki, Y.; Sato, M. PiggyBac transposon-mediated gene delivery efficiently generates stable transfectants derived from cultured primary human deciduous tooth dental pulp cells (HDDPCs) and HDDPC-derived iPS cells. Int. J. Oral Sci. 2015, 7, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Mitrano, T.I.; Grob, M.S.; Carrión, F.; Nova-Lamperti, E.; Luz, P.A.; Fierro, F.S.; Quintero, A.; Chaparro, A.; Sanz, A. Culture and characterization of mesenchymal stem cells from human gingival tissue. J. Periodontol. 2010, 81, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Megat Abdul Wahab, R.; Mohamed Rozali, N.A.; Senafi, S.; Zainol Abidin, I.Z.; Zainal Ariffin, Z.; Zainal Ariffin, S.H. Impact of isolation method on doubling time and the quality of chondrocyte and osteoblast differentiated from murine dental pulp stem cells. PeerJ 2017, 5, e3180. [Google Scholar] [CrossRef] [PubMed]
- Rodas-Junco, B.A.; Villicaña, C. Dental pulp stem cells: Current advances in isolation, expansion and preservation. Tissue Eng. Regen. Med. 2017, 14, 333–347. [Google Scholar] [CrossRef]
- Hilkens, P.; Gervois, P.; Fanton, Y.; Vanormelingen, J.; Martens, W.; Struys, T.; Politis, C.; Lambrichts, I.; Bronckaers, A. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 2013, 353, 65–78. [Google Scholar] [CrossRef]
- Kiyokawa, Y.; Terajima, M.; Sato, M.; Inada, E.; Hori, Y.; Bando, R.; Iwase, Y.; Kubota, N.; Murakami, T.; Tsugane, H.; et al. Scratch-based isolation of primary cells (SCIP): A novel method to obtain a large number of human dental pulp cells through one-step cultivation. J. Clin. Med. 2024, 13, 7058. [Google Scholar] [CrossRef] [PubMed]
- La Noce, M.; Paino, F.; Spina, A.; Naddeo, P.; Montella, R.; Desiderio, V.; De Rosa, A.; Papaccio, G.; Tirino, V.; Laino, L. Dental pulp stem cells: State of the art and suggestions for a true translation of research into therapy. J. Dent. 2014, 42, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Khanna-Jain, R. Growth and differentiation of human dental pulp stem cells maintained in fetal bovine serum, human serum and serum-free/xeno-free culture media. J. Stem Cell Res. Ther. 2012, 2, 126. [Google Scholar] [CrossRef]
- Brindley, D.A.; Davie, N.L.; Culme-Seymour, E.J.; Mason, C.; Smith, D.W.; Rowley, J.A. Peak serum: Implications of serum supply for cell therapy manufacturing. Regen. Med. 2012, 7, 7–13. [Google Scholar] [CrossRef]
- Mochizuki, M.; Nakahara, T. Establishment of xenogeneic serum-free culture methods for handling human dental pulp stem cells using clinically oriented in-vitro and in-vivo conditions. Stem Cell Res. Ther. 2018, 9, 25. [Google Scholar] [CrossRef]
- Xiao, J.; Yang, D.; Li, Q.; Tian, W.; Guo, W. The establishment of a chemically defined serum-free culture system for human dental pulp stem cells. Stem Cell Res. Ther. 2018, 9, 191. [Google Scholar] [CrossRef]
- Marrazzo, P.; Paduano, F.; Palmieri, F.; Marrelli, M.; Tatullo, M. Highly efficient in vitro reparative behaviour of dental pulp stem cells cultured with standardised platelet lysate supplementation. Stem Cells Int. 2016, 2016, 7230987. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Stark, R.; Grzelak, M.; Hadfield, J. RNA sequencing: The teenage years. Nat. Rev. Genet. 2019, 20, 631–656. [Google Scholar] [CrossRef]
- Kobayashi, T.; Torii, D.; Iwata, T.; Izumi, Y.; Nasu, M.; Tsutsui, T.W. Characterization of proliferation, differentiation potential, and gene expression among clonal cultures of human dental pulp cells. Hum. Cell 2020, 33, 490–501. [Google Scholar] [CrossRef]
- Inada, E.; Saitoh, I.; Kubota, N.; Iwase, Y.; Kiyokawa, Y.; Noguchi, H.; Yamasaki, Y.; Sato, M. RNA analysis based on a small number of manually isolated fixed cells (RNA-snMIFxC) to profile stem cells from human deciduous tooth-derived dental pulp cells. Biol. Proced. Online 2021, 23, 12. [Google Scholar] [CrossRef]
- Inada, E.; Saitoh, I.; Kubota, N.; Soda, M.; Matsueda, K.; Murakami, T.; Sawami, T.; Kagoshima, A.; Yamasaki, Y.; Sato, M. Alkaline phosphatase and OCT-3/4 as useful markers for predicting susceptibility of human deciduous teeth-derived dental pulp cells to reprogramming factor-induced iPS cells. J. Investig. Clin. Dent. 2017, 8, e12236. [Google Scholar] [CrossRef] [PubMed]
- Mutisheva, I.; Robatel, S.; Bäriswyl, L.; Schenk, M. An innovative approach to tissue processing and cell sorting of fixed cells for subsequent single-cell RNA sequencing. Int. J. Mol. Sci. 2022, 23, 10233. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wen, Q.; Zhao, Q.; Wang, N.; Zhao, Y. Atlas of human dental pulp cells at multiple spatial and temporal levels based on single-cell sequencing analysis. Front. Physiol. 2022, 13, 993478. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N. Characterisation of dental pulp stem cells: A new horizon for tissue regeneration? Arch. Oral Biol. 2012, 57, 1439–1458. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura-Yamada, S.; Kusano, K.; Baba, S. Clinical potential and current progress of dental pulp stem cells for various systemic diseases in regenerative medicine: A concise review. Int. J. Mol. Sci. 2019, 20, 1132. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef]
- Luke, A.M.; Patnaik, R.; Kuriadom, S.; Abu-Fanas, S.; Mathew, S.; Shetty, K.P. Human dental pulp stem cells differentiation to neural cells, osteocytes and adipocytes-An in vitro study. Heliyon 2020, 6, e03054. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhan, X.; Zhang, C.; Hargreaves, K.M.; Jin, L.; Tong, E.H. Coculture of dental pulp stem cells with endothelial cells enhances osteo-/odontogenic and angiogenic potential in vitro. J. Endod. 2012, 38, 454–463. [Google Scholar] [CrossRef]
- Pisciotta, A.; Riccio, M.; Carnevale, G.; Beretti, F.; Gibellini, L.; Maraldi, T.; Cavallini, G.M.; Ferrari, A.; Bruzzesi, G.; De Pol, A. Human serum promotes osteogenic differentiation of human dental pulp stem cells in vitro and in vivo. PLoS ONE 2012, 7, e50542. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Zhang, Y.; Du, X.; Yan, Z.; Li, J.; Wu, B. CGFe and TGF-β1 enhance viability and osteogenic differentiation of human dental pulp stem cells through the MAPK pathway. Exp. Ther. Med. 2021, 22, 1048. [Google Scholar] [CrossRef] [PubMed]
- Kornsuthisopon, C.; Nantanapiboon, D.; Rochanavibhata, S.; Nowwarote, N.; Namangkalakul, W.; Osathanon, T. Betaine promotes osteogenic differentiation in immortalized human dental pulp-derived cells. BDJ Open 2022, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.N.; Sun, J.Q.; Li, Q.H.; Jiao, K.; Shen, L.J.; Wu, D.; Tay, F.; Chen, J.H. Intrafibrillar-silicified collagen scaffolds enhance the osteogenic capacity of human dental pulp stem cells. J. Dent. 2014, 42, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Bar, J.K.; Lis-Nawara, A.; Kowalczyk, T.; Grelewski, P.G.; Stamnitz, S.; Gerber, H.; Klimczak, A. Osteogenic potential of human dental pulp stem cells (hDPSCs) growing on poly L-lactide-co-caprolactone and hyaluronic acid (HYAFF-11TM) scaffolds. Int. J. Mol. Sci. 2023, 24, 16747. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, J.; Wei, C.; Jin, C.; Zhu, M.; Zhao, S.; Xie, H. Enhancing osteogenic differentiation of dental pulp stem cells with covalently bonded all-carbon scaffolds. Adv. Funct. Mater. 2024, 34, 2400766. [Google Scholar] [CrossRef]
- Luzuriaga, J.; García-Gallastegui, P.; García-Urkia, N.; Pineda, J.R.; Irastorza, I.; Fernandez-San-Argimiro, F.J.; Briz, N.; Olalde, B.; Unda, F.; Madarieta, I.; et al. Osteogenic differentiation of human dental pulp stem cells in decellularised adipose tissue solid foams. Eur. Cell Mater. 2022, 43, 112–129. [Google Scholar] [CrossRef]
- Alipour, M.; Firouzi, N.; Aghazadeh, Z.; Samiei, M.; Montazersaheb, S.; Khoshfetrat, A.B.; Aghazadeh, M. The osteogenic differentiation of human dental pulp stem cells in alginate-gelatin/Nano-hydroxyapatite microcapsules. BMC Biotechnol. 2021, 21, 6. [Google Scholar] [CrossRef]
- Ajlan, S.A.; Ashri, N.Y.; Aldahmash, A.M.; Alnbaheen, M.S. Osteogenic differentiation of dental pulp stem cells under the influence of three different materials. BMC Oral Health 2015, 15, 132. [Google Scholar] [CrossRef]
- Soares, D.G.; Rosseto, H.L.; Scheffel, D.S.; Basso, F.G.; Huck, C.; Hebling, J.; de Souza Costa, C.A. Odontogenic differentiation potential of human dental pulp cells cultured on a calcium-aluminate enriched chitosan-collagen scaffold. Clin. Oral Investig. 2017, 21, 2827–2839. [Google Scholar] [CrossRef]
- Baldión, P.A.; Velandia-Romero, M.L.; Castellanos, J.E. Odontoblast-like cells differentiated from dental pulp stem cells retain their phenotype after subcultivation. Int. J. Cell Biol. 2018, 2018, 6853189. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, D.S.; Choung, H.W.; Shon, W.J.; Seo, B.M.; Lee, E.H.; Cho, J.Y.; Park, J.C. Odontogenic differentiation of human dental pulp stem cells induced by preameloblast-derived factors. Biomaterials 2011, 32, 9696–9706. [Google Scholar] [CrossRef]
- Liu, C.H.; Hung, C.J.; Huang, T.H.; Lin, C.C.; Kao, C.T.; Shie, M.Y. Odontogenic differentiation of human dental pulp cells by calcium silicate materials stimulating via FGFR/ERK signaling pathway. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 359–366. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Odontogenic differentiation of human dental pulp stem cells on hydrogel scaffolds derived from decellularized bone extracellular matrix and collagen type I. PLoS ONE 2016, 11, e0148225. [Google Scholar] [CrossRef]
- Huang, G.T.; Shagramanova, K.; Chan, S.W. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J. Endod. 2006, 32, 1066–1073. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Hu, J.; Wang, L.; Li, N.; Wu, D.; Shi, X.; Yuan, M.; Hu, W.; Wang, X. Endothelial cells and endothelin-1 promote the odontogenic differentiation of dental pulp stem cells. Mol. Med. Rep. 2018, 18, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Kawashima, N.; Noda, S.; Murano, H.; Han, P.; Hashimoto, K.; Kaneko, T.; Okiji, T. VEGFA promotes odonto/osteoblastic differentiation in dental pulp stem cells via ERK/p38 signaling. J. Dent. Sci. 2025, 20, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Moseti, D.; Regassa, A.; Kim, W.-K. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Zalduendo, M. Review Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy 2018, 20, 479–498. [Google Scholar] [CrossRef]
- Garcia-Urkia, N.; Luzuriaga, J.; Uribe-Etxebarria, V.; Irastorza, I.; Fernandez-San-Argimiro, F.J.; Olalde, B.; Briz, N.; Unda, F.; Ibarretxe, G.; Madarieta, I.; et al. Enhanced adipogenic differentiation of human dental pulp stem cells in enzymatically decellularized adipose tissue solid foams. Biology 2022, 11, 1099. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chang, K.-C.; Tsai, S.-J.; Chang, H.-H.; Lin, C.-P. Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. J. Formos. Med. Assoc. 2014, 113, 956–965. [Google Scholar] [CrossRef]
- Osathanon, T.; Sawangmake, C.; Nowwarote, N.; Pavasant, P. Neurogenic differentiation of human dental pulp stem cells using different induction protocols. Oral Dis. 2014, 20, 352–358. [Google Scholar] [CrossRef]
- Lee, J.-H.; Um, S.; Song, I.-S.; Kim, H.Y.; Seo, B.M. Neurogenic differentiation of human dental stem cells in vitro. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Haratizadeh, S.; Bojnordi, M.N.; Darabi, S.; Karimi, N.; Naghikhani, M.; Hamidabadi, H.G.; Seifi, M. Condition medium of cerebrospinal fluid and retinoic acid induces the transdifferentiation of human dental pulp stem cells into neuroglia and neural like cells. Anat. Cell Biol. 2017, 50, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Seonwoo, H.; Jang, K.-J.; Lee, D.; Park, S.; Lee, M.; Park, S.; Lim, K.-T.; Kim, J.; Chung, J.H. Neurogenic differentiation of human dental pulp stem cells on graphene-polycaprolactone hybrid nanofibers. Nanomaterials 2018, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Darabi, S.; Tiraihi, T.; Bojnordi, M.N.; Hamidabadi, H.G.; Rezaei, N.; Zahiri, M.; Alizadeh, R. Trans-differentiation of human dental pulp stem cells into cholinergic-like neurons via nerve growth factor. Basic Clin. Neurosci. 2019, 10, 609–617. [Google Scholar] [CrossRef]
- Kogo, Y.; Seto, C.; Totani, Y.; Mochizuki, M.; Nakahara, T.; Oka, K.; Yoshioka, T.; Ito, E. Rapid differentiation of human dental pulp stem cells to neuron-like cells by high K+ stimulation. Biophys. Physicobiol. 2020, 17, 132–139. [Google Scholar] [CrossRef]
- Al-Maswary, A.A.; O’Reilly, M.; Holmes, A.P.; Walmsley, A.D.; Cooper, P.R.; Scheven, B.A. Exploring the neurogenic differentiation of human dental pulp stem cells. PLoS ONE 2022, 17, e0277134. [Google Scholar] [CrossRef]
- Ishkitiev, N.; Yaegaki, K.; Imai, T.; Tanaka, T.; Nakahara, T.; Ishikawa, H.; Mitev, V.; Haapasalo, M. High-purity hepatic lineage differentiated from dental pulp stem cells in serum-free medium. J. Endod. 2012, 38, 475–480. [Google Scholar] [CrossRef]
- Bento, L.W.; Zhang, Z.; Imai, A.; Nör, F.; Dong, Z.; Shi, S.; Araujo, F.B.; Nör, J.E. Endothelial differentiation of SHED requires MEK1/ERK signaling. J. Dent. Res. 2013, 92, 51–57. [Google Scholar] [CrossRef]
- Bronckaers, A.; Hilkens, P.; Fanton, Y.; Struys, T.; Gervois, P.; Politis, C.; Martens, W.; Lambrichts, I. Angiogenic properties of human dental pulp stem cells. PLoS ONE 2013, 8, e71104. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Hargreaves, K.M.; Jin, L.; Samaranayake, L.P.; Zhang, C. The interplay of dental pulp stem cells and endothelial cells in an Injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng. Part A 2014, 21, 550–563. [Google Scholar] [CrossRef]
- Luzuriaga, J.; Irurzun, J.; Irastorza, I.; Unda, F.; Ibarretxe, G.; Pineda, J.R. Vasculogenesis from human dental pulp stem cells grown in Matrigel with fully defined serum-free culture media. Biomedicines 2020, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Pizzicannella, J.; Merciaro, I.; Antonucci, I.; Guarnieri, S.; Ballerini, P.; Stuppia, L.; Trubiani, O. In vitro cardiomyogenic differentiation of human dental pulp stem cells. The role of 5-azacytidine. Ital. J. Anat. Embryol. 2014, 119 (Suppl. S1), 71. [Google Scholar] [CrossRef]
- Carnevalea, G.; Riccioa, M.; Pisciottaa, A.; Berettia, F.; Maraldia, T.; Zavattia, M.; Cavallinia, G.M.; La Salab, G.B.; Ferraric, A.; De Pola, A. In vitro differentiation into insulin-producing β-cells of stem cells isolated from human amniotic fluid and dental pulp. Dig. Liver Dis. 2013, 45, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Ishkitiev, N.; Yaegaki, K.; Kozhuharova, A.; Tanaka, T.; Okada, M.; Mitev, V.; Fukuda, M.; Imai, T. Pancreatic differentiation of human dental pulp CD117+ stem cells. Regen. Med. 2013, 8, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Kanafi, M.M.; Rajeshwari, Y.B.; Gupta, S.; Dadheech, N.; Nair, P.D.; Gupta, P.K.; Bhonde, R.R. Transplantation of islet-like cell clusters derived from human dental pulp stem cells restores normoglycemia in diabetic mice. Cytotherapy 2013, 15, 1228–1236. [Google Scholar] [CrossRef]
- Mendoza, H.Y.; Yokoyama, T.; Tanaka, T.; Ii, H.; Yaegaki, K. Regeneration of insulin-producing islets from dental pulp stem cells using a 3D culture system. Regen. Med. 2018, 13, 673–687. [Google Scholar] [CrossRef]
- Shivakumar, S.B.; Lee, H.-J.; Son, Y.-B.; Bharti, D.; Ock, S.A.; Lee, S.-L.; Kang, Y.-H.; Park, B.-W.; Rho, G.-J. In vitro differentiation of single donor derived human dental mesenchymal stem cells into pancreatic β cell-like cells. Biosci. Rep. 2019, 39, BSR20182051. [Google Scholar] [CrossRef]
- Kuncorojakti, S.; Rodprasert, W.; Le, Q.D.; Osathanon, T.; Pavasant, P.; Sawangmake, C. In vitro induction of human dental pulp stem cells toward pancreatic lineages. J. Vis. Exp. 2021, 175, e62497. [Google Scholar] [CrossRef]
- Aly, R.M.; Aglan, H.A.; Eldeen, G.N.; Ahmed, H.H. Optimization of differentiation protocols of dental tissues stem cells to pancreatic β-cells. BMC Mol. Cell Biol. 2022, 23, 41. [Google Scholar] [CrossRef]
- Abuarqoub, D.; Adwan, S.; Zaza, R.; Wehaibi, S.; Aslam, N.; Jafar, H.; Qinna, N.A.; Awidi, A. Effective generation of functional pancreatic β cells from human-derived dental stem cells of apical papilla and bone-marrow-derived stem cells: A comparative study. Pharmaceuticals 2023, 16, 649. [Google Scholar] [CrossRef]
- Song, B.; Jiang, W.; Alraies, A.; Liu, Q.; Gudla, V.; Oni, J.; Wei, X.; Sloan, A.; Ni, L.; Agarwal, M. Bladder smooth muscle cells differentiation from dental pulp stem cells: Future potential for bladder tissue engineering. Stem Cells Int. 2016, 2016, 6979368. [Google Scholar] [CrossRef]
- Xu, J.G.; Zhu, S.Y.; Heng, B.C.; Dissanayaka, W.L.; Zhang, C.F. TGF-β1-induced differentiation of SHED into functional smooth muscle cells. Stem Cell Res. Ther. 2017, 8, 10. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, J.; Lin, S.; Hu, M.; Liu, J.; Kang, J.; Qi, Y.; Basabrain, M.S.; Zou, T.; Zhang, C. Direct contact with endothelial cells drives dental pulp stem cells toward smooth muscle cells differentiation via TGF-β1 secretion. Int. Endod. J. 2023, 56, 1092–1107. [Google Scholar] [CrossRef]
- Ghanavatinejad, F.; Tabrizi, Z.P.F.; Omidghaemi, S.; Sharifi, E.; Møller, S.G.; Jami, M.-S. Protein biomarkers of neural system. J. Otol. 2019, 14, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, J.; Nie, S.; Wang, X.; Li, B.; Sun, D.; Refukati, D.; Liu, Y. Transfection of stem cells derived from rat dental pulp with green fluorescent protein infection by lentiviral vector. Chin. J. Tissue Eng. Res. 2014, 53, 7299–7305. [Google Scholar] [CrossRef]
- Zhan, Y.; Li, X.; Gou, X.; Yuan, G.; Fan, M.; Yang, G. DLX3 inhibits the proliferation of human dental pulp cells through inactivation of canonical Wnt/β-catenin signaling pathway. Front. Physiol. 2018, 9, 1637. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, F.; Zhang, X.; Wang, S.; Jin, Y.; Zhang, W.; Jiang, X. The effects of platelet-derived growth factor-BB on human dental pulp stem cells mediated dentin-pulp complex regeneration. Stem Cells Transl. Med. 2017, 6, 2126–2134. [Google Scholar] [CrossRef]
- Guirado, E.; Zhang, Y.; George, A. Establishment of stable cell lines from primary human dental pulp stem cells. Methods Mol. Biol. 2019, 1922, 21–27. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, C.; Geng, T.; Liu, Y.; Zhu, S.; Zhang, C.; Liu, Z.; Wang, P. EphrinB2 overexpression enhances osteogenic differentiation of dental pulp stem cells partially through ephrinB2-mediated reverse signaling. Stem Cell Res. Ther. 2020, 11, 40. [Google Scholar] [CrossRef]
- Janebodin, K.; Chavanachat, R.; Hays, A.; Gil, M.R. Silencing VEGFR-2 hampers odontoblastic differentiation of dental pulp stem cells. Front. Cell Dev. Biol. 2021, 9, 665886. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Z.; Xie, Y.; Hu, J.; Wang, H.; Fan, Z.; Zhang, C.; Wang, J.; Wu, C.-T.; Wang, S. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res. Ther. 2015, 6, 249. [Google Scholar] [CrossRef]
- Orimoto, A.; Kyakumoto, S.; Eitsuka, T.; Nakagawa, K.; Kiyono, T.; Fukuda, T. Efficient immortalization of human dental pulp stem cells with expression of cell cycle regulators with the intact chromosomal condition. PLoS ONE 2020, 15, e0229996. [Google Scholar] [CrossRef]
- Nakashima, M.; Mizunuma, K.; Murakami, T.; Akamine, A. Induction of dental pulp stem cell differentiation into odontoblasts by electroporation-mediated gene delivery of growth/differentiation factor 11 (Gdf11). Gene Ther. 2002, 9, 814–818. [Google Scholar] [CrossRef]
- Rizk, A.; Rabie, B.M. Electroporation for transfection and differentiation of dental pulp stem cells. Biores. Open Access 2013, 2, 155–162. [Google Scholar] [CrossRef]
- Inada, E.; Saitoh, I.; Kubota, N.; Iwase, Y.; Kiyokawa, Y.; Shibasaki, S.; Noguchi, H.; Yamasaki, Y.; Sato, M. piggyBac transposon-based immortalization of human deciduous tooth dental pulp cells with multipotency and non-tumorigenic potential. Int. J. Mol. Sci. 2019, 20, 4904. [Google Scholar] [CrossRef]
- Murakami, T.; Saitoh, I.; Sato, M.; Inada, E.; Soda, M.; Oda, M.; Domon, H.; Iwase, Y.; Sawami, T.; Matsueda, K.; et al. Isolation and characterization of lymphoid enhancer factor-1-positive deciduous dental pulp stem-like cells after transfection with a piggyBac vector containing LEF1 promoter-driven selection markers. Arch. Oral Biol. 2017, 81, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Irfan, M.; Sreekumar, S.; Phimon, A.; Kim, S.; Chung, S. CRISPR-edited DPSCs constitutively expressing BDNF enhance dentin regeneration in injured teeth. eLife 2025, 14, RP105153. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Seonwoo, H.; Jang, K.J.; Pandey, S.; Lim, J.; Park, S.; Kim, J.E.; Choung, Y.-H.; Garg, P.; Chung, J.H. Development of novel gene carrier using modified nano hydroxyapatite derived from equine bone for osteogenic differentiation of dental pulp stem cells. Bioact. Mater. 2021, 6, 2742–2751. [Google Scholar] [CrossRef] [PubMed]
- Salkın, H.; Gönen, Z.B.; Ergen, E.; Bahar, D.; Çetin, M. Effects of TGF-β1 overexpression on biological characteristics of human dental pulp-derived mesenchymal stromal cells. Int. J. Stem Cells. 2019, 12, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.-L.; Zhang, Y.-J.; Wang, J.-W.; Tian, F.; Wang, C.-F. Studies on microRNA regulation of multidirectional differentiation of dental pulp stem cells: A narrative review. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lin, H.; Zhang, L.; Sun, Q.; Yuan, G.; Zhang, L.; Chen, S.; Chen, Z. miR-145 and miR-143 regulate odontoblast differentiation through targeting Klf4 and Osx genes in a feedback Loop. J. Biol. Chem. 2013, 288, 9261–9271. [Google Scholar] [CrossRef] [PubMed]
- Stacey, G.; MacDonald, C. Immortalisation of primary cells. Cell Biol. Toxicol. 2001, 17, 231–246. [Google Scholar] [CrossRef]
- Sato, M.; Saitoh, I.; Inada, E.; Nakamura, S.; Watanabe, S. Potential for isolation of immortalized hepatocyte cell lines by liver-directed in vivo gene delivery of transposons in mice. Stem Cells Int. 2019, 2019, 5129526. [Google Scholar] [CrossRef]
- Kitagawa, M.; Ueda, H.; Iizuka, S.; Sakamoto, K.; Oka, H.; Kudo, Y.; Ogawa, I.; Miyauchi, M.; Tahara, H.; Takata, T. Immortalization and characterization of human dental pulp cells with odontoblastic differentiation. Arch. Oral Biol. 2007, 52, 727–731. [Google Scholar] [CrossRef]
- Zanini, M.; Sautier, J.M.; Berdal, A.; Simon, S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J. Endod. 2012, 38, 1220–1226. [Google Scholar] [CrossRef]
- Villa, I.; Senesi, P.; Montesano, A.; Ferraretto, A.; Vacante, F.; Spinello, A.; Bottani, M.; Bolamperti, S.; Rubinacci, A.; Luzi, L.; et al. Betaine promotes cell differentiation of human osteoblasts in primary culture. J. Transl. Med. 2017, 15, 132. [Google Scholar] [CrossRef]
- Zhang, C.; Chang, J.; Sonoyama, W.; Shi, S.; Wang, C.-Y. Inhibition of human dental pulp stem cell differentiation by Notch signaling. J. Dent. Res. 2008, 87, 250–255. [Google Scholar] [CrossRef]
- Lin, H.; Xu, L.; Liu, H.; Chen, Z.; Yuan, G.; Chen, Z. KLF4 promotes the odontoblastic differentiation of human dental pulp cells. J. Endod. 2011, 37, 948–954. [Google Scholar] [CrossRef]
- Wang, X.; He, F.; Tan, Y.; Tian, W.; Qiu, S. Inhibition of Delta1 promotes differentiation of odontoblasts and inhibits proliferation of human dental pulp stem cell in vitro. Arch. Oral Biol. 2011, 56, 837–845. [Google Scholar] [CrossRef]
- Hara, E.S.; Ono, M.; Eguchi, T.; Kubota, S.; Pham, H.T.; Sonoyama, W.; Tajima, S.; Takigawa, M.; Calderwood, S.K.; Kuboki, T. miRNA-720 controls stem cell phenotype, proliferation and differentiation of human dental pulp cells. PLoS ONE 2013, 8, e83545. [Google Scholar] [CrossRef]
- Chen, K.-L.; Huang, Y.-Y.; Lung, J.; Yeh, Y.-Y.; Yuan, K. CD44 is involved in mineralization of dental pulp cells. J. Endod. 2013, 39, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, H.; Song, F.; Fu, D.; Wang, J. DDIT3 overexpression increases odontoblastic potential of human dental pulp cells. Cell Prolif. 2014, 47, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, I.; Sakowicz-Burkiewicz, M.; Pawelczyk, T. Id1 expression level determines the differentiation of human dental pulp stem cells. J. Dent. Res. 2014, 93, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Ran, S.; Qin, F.; Cao, D.; Wang, J.; Liu, B.; Liang, J. Human dental pulp stem cells via the NF-κB pathway. Cell Physiol. Biochem. 2015, 36, 1725–1734. [Google Scholar] [CrossRef]
- Heng, B.C.; Ye, X.; Liu, Y.; Dissanayaka, W.L.; Shun, G.; Cheung, P.; Zhang, C. Effects of recombinant overexpression of Bcl2 on the proliferation, apoptosis, and osteogenic/odontogenic differentiation potential of dental pulp stem cells. J. Endod. 2016, 42, 575–583. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Liu, X.; Chen, Y.; Liu, P.; Zhao, L. Effect of SOX2 on odontoblast differentiation of dental pulp stem cells. Mol. Med. Rep. 2017, 16, 9659–9663. [Google Scholar] [CrossRef]
- Ou, Y.; Zhou, Y.; Liang, S.; Wang, Y. Sclerostin promotes human dental pulp cells senescence. PeerJ 2018, 6, e5808. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, J.; Wang, X.-X. Effects of Btbd7 knockdown on the proliferation of human dental pulp cells and expression of Dspp. Int. J. Clin. Exp. Pathol. 2018, 11, 1460–1465. [Google Scholar]
- Zeng, L.; Zhao, N.; Li, F.; Han, D.; Liu, Y.; Liu, H.; Sun, S.; Wang, Y.; Feng, H. miR-675 promotes odontogenic differentiation of human dental pulp cells by epigenetic regulation of DLX3. Exp. Cell Res. 2018, 367, 104–111. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Yang, Y.; Hu, R.; Wang, W.; Wang, Y. The suppressive effects of miR-508-5p on the odontogenic differentiation of human dental pulp stem cells by targeting glycoprotein non-metastatic melanomal protein B. Stem Cell Res. Ther. 2019, 10, 35. [Google Scholar] [CrossRef]
- Huang, X.; Liu, F.; Hou, J.; Chen, K. Inflammation-induced overexpression of microRNA-223-3p regulates odontoblastic differentiation of human dental pulp stem cells by targeting SMAD3. Int. Endod. J. 2019, 52, 491–503. [Google Scholar] [CrossRef]
- Guirado, E.; Chen, Y.; Ross, R.D.; Zhang, Y.; Chaussain, C.; George, A. Disrupted protein expression and altered proteolytic events in hypophosphatemic dentin can be rescued by dentin matrix protein 1. Front. Physiol. 2020, 11, 82. [Google Scholar] [CrossRef]
- Zhong, J.; Tu, X.; Kong, Y.; Guo, L.; Li, B.; Zhong, W.; Cheng, Y.; Jiang, Y.; Jiang, Q. LncRNA H19 promotes odontoblastic differentiation of human dental pulp stem cells by regulating miR-140-5p and BMP-2/FGF9. Stem Cell Res. Ther. 2020, 11, 202. [Google Scholar] [CrossRef]
- Guirado, E.; Villani, C.; Petho, A.; Chen, Y.; Maienschein-Cline, M.; Lei, Z.; Los, N.; George, A. Wnt pathway inhibitors are upregulated in XLH dental pulp cells in response to odontogenic differentiation. Int. J. Oral Sci. 2023, 15, 13. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Sun, Z.; Zhao, S.; Deng, W. MicroRNA-15b-5p modulates the differentiation of human dental pulp stem cells into odontoblasts via targeting IGF1. J. Biol. Regul. Homeost. Agents. 2023, 37, 7191. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, X.; Chen, Y.; Zhao, Y.; Liu, P.; Zhao, L.; Han, W. Effect of SOX2 on osteogenic differentiation of dental pulp stem cells. Cell Mol. Biol. 2017, 63, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Gao, Y.; Qiao, H.; Zhou, H.; Liu, Y. Elevated osteogenic potential of stem cells from inflammatory dental pulp tissues by Wnt4 overexpression for treating bone defect in rats. Ann. Palliat. Med. 2020, 9, 2962–2969. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Zhu, L.; Pan, J.; Shen, Z.; Yang, Z.; Wang, J.; Bai, X.; Lin, Y.; Tao, J. hsa_circ_0026827 promotes osteoblast differentiation of human dental pulp stem cells through the Beclin1 and RUNX1 signaling pathways by sponging miR-188-3p. Front. Cell Dev. Biol. 2020, 8, 470. [Google Scholar] [CrossRef]
- Kim, I.H.; Jeon, M.; Lee, Y.H.; Shin, J.S.; Lee, T.; Lee, S.K.; Cheon, K.; Song, J.S. HIF-1α overexpression using a protein transduction domain to increase the osteogenic potential of SHED. J. Dent. Oral Biol. 2023, 8, 1215. [Google Scholar]
- Wen, W.; Zheng, P.; Meng, H.; Liu, H.; Yuan, C. Overexpression of Sema3A promotes osteogenic differentiation of dental pulp stem cells and MC3T3-E1. Chin. J. Tissue Eng. Res. 2024, 28, 993–999. [Google Scholar] [CrossRef]
- Deng, P.; Yang, B.; Huang, C.; Li, Y.; Mei, Z.; Li, Y.; Li, J. GDF15 promotes osteogenic differentiation of human dental pulp stem cells by activating the TGF-β/SMAD signaling pathway. J. Tissue Eng. 2025, 16, 20417314251357752. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, Y.; Ohta, K.; Okoshi, R.; Suehara, M.; Kizaki, H.; Nakagawa, K. Hypoxia induces expression and activation of AMPK in rat dental pulp cells. J. Dent. Res. 2007, 86, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cai, J.; Dong, D.; Chen, Y.; Liu, X.; Wang, Y.; Zhou, Y. Effects of SOX2 on proliferation, migration and adhesion of human dental pulp stem cells. PLoS ONE 2015, 10, e0141346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, C.; Li, S.; Zhang, L.; Wu, X.; Zhang, H.; Wang, F.; Tan, Y. MiR-633 promotes cell proliferation and differentiation by targeting MEPE in human adult dental pulp stem cells. Int. J. Clin. Exp. Pathol. 2016, 9, 8066–8074. [Google Scholar]
- Yang, X.; He, L.; Pan, S.; Li, Y.; Niu, Y. Effect of Satb2 overexpression on proliferation and migration of human dental pulp stem cells. J. Oral Sci. Res. 2017, 33, 597–600. [Google Scholar] [CrossRef]
- Zhu, L.; Dissanayaka, W.L.; Zhang, C. Dental pulp stem cells overexpressing stromal-derived factor-1α and vascular endothelial growth factor in dental pulp regeneration. Clin. Oral Investig. 2019, 23, 2497–2509. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, N.; Miao, J.; Li, C.; Wang, X.; Ruan, J. Lin28 promotes dental pulp cell proliferation via upregulation of cyclin-dependent proteins and interaction with let-7a/IGF2BP2 pathways. Biomed. Pharmacother. 2019, 113, 108742. [Google Scholar] [CrossRef]
- Yuan, X.; Huan, Z.; Wang, H. Effects of microRNA-210-3p on proliferation and differentiation of human dental pulp cells under hypoxic condition. J. Oral Sci. Res. 2019, 35, 970–974. [Google Scholar] [CrossRef]
- Ok, C.Y.; Park, S.; Jang, H.-O.; Takata, T.; Bae, M.-K.; Kim, Y.-D.; Ryu, M.H.; Bae, S.-K. Visfatin induces senescence of human dental pulp cells. Cells 2020, 9, 193. [Google Scholar] [CrossRef]
- Dong, X.; Kong, F.; Liu, C.; Dai, S.; Zhang, Y.; Xiao, F.; Zhang, H.; Wu, C.-T.; Wang, H. Pulp stem cells with hepatocyte growth factor overexpression exhibit dual effects in rheumatoid arthritis. Stem Cell Res. Ther. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sun, C.; Peng, R.; Liu, Z. The effect of LncRNA H19 on human dental pulp cells through tumor growth factor-β1 (Tgf-Β1)/Smad signaling pathway. J. Biomat. Tissue Eng. 2022, 12, 1247–1251. [Google Scholar] [CrossRef]
- Dou, W.; Xie, J.; Chen, J.; Zhou, J.; Xu, Z.; Wang, Z.; Zhu, Q. Overexpression of adrenomedullin (ADM) alleviates the senescence of human dental pulp stem cells by regulating the miR-152/CCNA2 pathway. Cell Cycle 2023, 22, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gong, Q.; Ling, J.; Zhang, W.; Liu, Z.; Quan, J. Role of MiR-424 on angiogenic potential in human dental pulp cells. J. Endod. 2014, 40, 76–82. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Han, Y.; Zhang, L.; Zou, T.; Zhang, C. Bcl-2 Overexpression and hypoxia synergistically enhance angiogenic properties of dental pulp stem cells. Int. J. Mol. Sci. 2020, 21, 6159. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Li, N.; Wu, T.; Zheng, X.; Heng, B.C.; Zou, D.; Xu, J. Upregulation of ETV2 expression promotes endothelial differentiation of human dental pulp stem cells. Cell Transplant. 2021, 30, 963689720978739. [Google Scholar] [CrossRef]
- Jiang, W.; Duan, S.; Li, W.; Yan, H.; Si, C.; Xu, N.; Li, Y.; Zhang, W.; Gu, S. PDGF-BB overexpressing dental pulp stem cells improve angiogenesis in dental pulp regeneration. Front. Bioeng. Biotechnol. 2025, 13, 1578410. [Google Scholar] [CrossRef]
- Behrouznezhad, F.; Ejeian, F.; Emadi-Baygi, M.; Nikpour, P.; Nasr-Esfahani, M.H. Hypothesis: A challenge of overexpression Zfp521 in neural tendency of derived dental pulp stem cells. Cell J. 2019, 21, 99–102. [Google Scholar] [CrossRef]
- Li, J.; Diao, S.; Yang, H.; Cao, Y.; Du, J.; Yang, D. IGFBP5 promotes angiogenic and neurogenic differentiation potential of dental pulp stem cells. Dev. Growth Differ. 2019, 61, 457–465. [Google Scholar] [CrossRef]
- Gancheva, M.R.; Kremer, K.; Breen, J.; Arthur, A.; Hamilton-Bruce, A.; Thomas, P.; Gronthos, S.; Koblar, S. Effect of octamer-binding transcription factor 4 overexpression on the neural induction of human dental pulp stem cells. Stem Cell Rev. Rep. 2024, 20, 797–815. [Google Scholar] [CrossRef]
- Balam-Lara, J.A.; Carrillo-Cocom, L.M.; Rodas-Junco, B.; Villanueva-Lizama, L.; Nic-Can, G. TEN ELEVEN TRANSLOCATION 2 (TET2) improves the adipogenic potential of dental pulp stem cells. J. Mex. Che. Soc. 2023, 67, 2023. [Google Scholar] [CrossRef]
- Tancharoen, S.; Tengrungsun, T.; Suddhasthira, T.; Kikuchi, K.; Vechvongvan, N.; Tokuda, M.; Maruyama, I. Overexpression of receptor for advanced glycation end products and high-mobility group box 1 in human dental pulp inflammation. Mediat. Inflamm. 2014, 2014, 754069. [Google Scholar] [CrossRef]
- Meng, H.; Wei, F.; Zhou, Y.; Hu, L.; Ge, Z.; Jin, J.; Wang, H.; Wu, C.-T. Overexpression of hepatocyte growth factor in dental pulp stem cells ameliorates the severity of psoriasis by reducing inflammatory responses. Stem Cells Dev. 2021, 30, 876–889. [Google Scholar] [CrossRef]
- Ni, C.; Wu, G.; Miao, T.; Xu, J. Wnt4 prevents apoptosis and inflammation of dental pulp cells induced by LPS by inhibiting the IKK/NF-κB pathway. Exp. Thera. Med. 2023, 25, 75. [Google Scholar] [CrossRef]
- Kim, S.-A.; Choi, H.S.; Ahn, S.-G. Pin1 induces the ADP-induced migration of human dental pulp cells through P2Y1 stabilization. Oncotarget 2016, 7, 85381–85392. [Google Scholar] [CrossRef]
- Shi, J.-F.; Zhu, C.-H.; Liu, J.; Sun, J.-Y.; Rao, G.-Z.; Li, A. Recombinant hFOXA2 and hPDX1 lentivirus induced dental pulp stem cells from deciduous teeth reprogramming for insulin-producing cells. Shanghai J. Stomatal. 2013, 22, 634–642. [Google Scholar]
- Nozaki, T.; Ohura, K. Inhibition of miR-183 induces insulin in dental pulp cells. J. Hard Tissue Biol. 2017, 26, 319–322. [Google Scholar] [CrossRef]
- Nozaki, T.; Ohura, K. Regulation of miRNA during direct reprogramming of dental pulp cells to insulin-producing cells. Biochem. Biophys. Res. Commun. 2014, 444, 195–198. [Google Scholar] [CrossRef]
- Askari, N.; Yaghoobi, M.M.; Shamsara, M.; Esmaeili-Mahani, S. Human dental pulp stem cells differentiate into oligodendrocyte progenitors using the expression of Olig2 transcription factor. Cells Tissues Organs 2014, 200, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Huang, Q.; Chen, Y.-X.; Liu, Q.; Fang, J.-X.; Ye, M.-W. Caspase-9 was involved in cell apoptosis in human dental pulp stem cells from deciduous teeth. Mol. Med. Rep. 2018, 18, 1067–1073. [Google Scholar] [CrossRef]
- Qiao, W.; Li, D.; Shi, Q.; Wang, H.; Wang, H.; Guo, J. miR-224-5p protects dental pulp stem cells from apoptosis by targeting Rac1. Exp. Ther. Med. 2020, 19, 9–18. [Google Scholar] [CrossRef]
- Li, D.; Deng, T.; Li, H.; Li, Y. MiR-143 and miR-135 inhibitors treatment induces skeletal myogenic differentiation of human adult dental pulp stem cells. Arch. Oral Biol. 2015, 60, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shen, G. MicroRNA-139-5p elevates skeletal myogenic differentiation of human adult dental pulp stem cells through Wnt/β-catenin signaling pathway. Exp. Ther. Med. 2018, 16, 2835–2842. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, L.; Wei, X.; Ling, J. Induced overexpression of Oct4A in human dental pulp cells enhances pluripotency and multilineage differentiation capability. Stem Cells Dev. 2015, 24, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Peng, Z.; Xu, Z.; Wei, X. XPC promotes pluripotency of human dental pulp cells through regulation of Oct-4/Sox2/c-Myc. Stem Cells Int. 2016, 2016, 3454876. [Google Scholar] [CrossRef]
- Abuarqoub, D.; Theeb, L.S.; Omari, M.B.; Hamadneh, Y.I.; Alrawabdeh, J.A.; Aslam, N.; Jafar, H.; Awidi, A. The osteogenic role of biomaterials combined with human-derived dental stem cells in bone tissue regeneration. Tissue Eng. Regen. Med. 2023, 20, 251–270. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, W.; Wang, G.; Li, Y. miR-143 suppresses the osteogenic differentiation of dental pulp stem cells by inactivation of NF-κB signaling pathway via targeting TNF-α. Arch. Oral Biol. 2018, 87, 172–179. [Google Scholar] [CrossRef]
- Lim, J.; Heo, Y.; Choi, S.S. Investigation of changes in DNA methylation associated with alterations in gene expression resulting in differences between lean and obese adipogenesis. Genomics 2023, 115, 110623. [Google Scholar] [CrossRef]
- Argaez-Sosa, A.A.; Rodas-Junco, B.A.; Carrillo-Cocom, L.M.; Rojas-Herrera, R.A.; Coral-Sosa, A.; Aguilar-Ayala, F.J.; Aguilar-Pérez, D.; Nic-Can, G. Higher expression of DNA (de)methylation-related genes reduces adipogenicity in dental pulp stem cells. Front. Cell Dev. Biol. 2022, 10, 791667. [Google Scholar] [CrossRef]
- Shimizu, K.; Taniyama, Y.; Sanada, F.; Iwabayashi, M.; Azuma, J.; Iekushi, K.; Katsuragi, N.; Otsu, R.; Shibata, K.; Ishikawa, Y.; et al. Novel mechanism of hepatocyte growth factor against prevention of inflammation and oxidative stress. Inflamm. Regen. 2013, 33, 136–142. [Google Scholar] [CrossRef]
- Zhou, Q.; Brown, J.; Kanarek, A.; Rajagopal, J.; Melton, D.A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008, 455, 627–632. [Google Scholar] [CrossRef]
- Mauda-Havakuk, M.; Litichever, N.; Chernichovski, E.; Nakar, O.; Winkler, E.; Mazkereth, R.; Orenstein, A.; Bar-Meir, E.; Ravassard, P.; Meivar-Levy, I.; et al. Ectopic PDX-1 expression directly reprograms human keratinocytes along pancreatic insulin-producing cells fate. PLoS ONE 2011, 6, e26298. [Google Scholar] [CrossRef] [PubMed]
- Dambal, S.; Shah, M.; Mihelich, B.; Nonn, L. The microRNA-183 cluster: The family that plays together stays together. Nucleic Acids Res. 2015, 43, 7173–7188. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ruan, H.; Lu, F.; Peng, H.; Luan, W. miR-224-5p acts as a tumour suppressor and reverses the resistance to BRAF inhibitor in melanoma through directly targeting PAK4 to block the MAPK pathway. Pathol. Res. Pract. 2023, 249, 154772. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Li, Y.; Wang, Y.; Teng, Y.; Hao, Y. MicroRNA-194-5p attenuates hypoxia/reoxygenation-induced apoptosis in H9C2 cardiomyocytes by inhibiting the over-activation of RAC1 protein. Mol. Med. Rep. 2022, 27, 33. [Google Scholar] [CrossRef]
- Kapplingattu, S.V.; Bhattacharya, S.; Adlakha, Y.K. MiRNAs as major players in brain health and disease: Current knowledge and future perspectives. Cell Death Discov. 2025, 11, 7. [Google Scholar] [CrossRef]
- Kent, O.A.; McCall, M.N.; Cornish, T.C.; Halushka, M.K. Lessons from miR-143/145: The importance of cell-type localization of miRNAs. Nucleic Acids Res. 2014, 42, 7528–7538. [Google Scholar] [CrossRef]
- You, Y.-H.; Wang, X.-G.; Xu, M.; Zhao, J.-P. Expression and clinical significance of miR-139-5p in non-small cell lung cancer. J. Int. Med. Res. 2019, 47, 867–874. [Google Scholar] [CrossRef]
- Zeineddine, D.; Hammoud, A.A.; Mortada, M.; Boeuf, H. The Oct4 protein: More than a magic stemness marker. Am. J. Stem Cells 2014, 3, 74–82. [Google Scholar]
- Sterneckert, J.; Hoing, S.; Scholer, H.R. Concise review: Oct4 and more: The reprogramming expressway. Stem Cells 2012, 30, 15–21. [Google Scholar] [CrossRef]
- Mitchell, R.; Szabo, E.; Shapovalova, Z.; Aslostovar, L.; Makondo, K.; Bhatia, M. Molecular evidence for OCT4-induced plasticity in adult human fibroblasts required for direct cell fate conversion to lineage specific progenitors. Stem Cells 2014, 32, 2178–2187. [Google Scholar] [CrossRef]
- Edwards, P.C.; Mason, J.M. Gene-enhanced tissue engineering for dental hard tissue regeneration: (2) dentin-pulp and periodontal regeneration. Head Face Med. 2006, 2, 16. [Google Scholar] [CrossRef]
- Movahed, A.Y.; Bagheri, R.; Savatier, P.; Šarić, T.; Moradi, S. Elimination of tumorigenic pluripotent stem cells from their differentiated cell therapy products: An important step toward ensuring safe cell therapy. Stem Cell Rep. 2025, 20, 102543. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-O.; Moon, S.H.; Jeong, H.-C.; Yi, J.-Y.; Lee, T.-H.; Shim, S.H.; Rhee, Y.-H.; Lee, S.-H.; Oh, S.-J.; Lee, M.-Y.; et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc. Natl. Acad. Sci. USA 2013, 110, E3281–E3290. [Google Scholar] [CrossRef] [PubMed]
- Nakao, H.; Yamaguchi, T.; Kawabata, K.; Higashi, K.; Nonaka, M.; Tuiji, M.; Nagai, Y.; Toyoda, H.; Yamaguchi, Y.; Kawasaki, N.; et al. Characterization of novel antibodies that recognize sialylated keratan sulfate and lacto-N-fucopentaose I on human induced pluripotent cells: Comparison with existing antibodies. Glycobiology 2023, 33, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Miyoshi, H.; Nagoshi, N.; Kohyama, J.; Itakura, G.; Kawabata, S.; Ozaki, M.; Iida, T.; Sugai, K.; Ito, S.; et al. Selective ablation of tumorigenic cells following human induced pluripotent stem cell-derived neural stem/progenitor cell transplantation in spinal cord injury. Stem Cells Transl. Med. 2018, 8, 260–270. [Google Scholar] [CrossRef]
- Tamaoki, N.; Takahashi, K.; Tanaka, T.; Ichisaka, T.; Aoki, H.; Takeda-Kawaguchi, T.; Iida, K.; Kunisada, T.; Shibata, T.; Yamanaka, S.; et al. Dental pulp cells for induced pluripotent stem cell banking. J. Dent. Res. 2010, 89, 773–778. [Google Scholar] [CrossRef]
- Yan, X.; Qin, H.; Qu, C.; Tuan, R.S.; Shi, S.; Huang, G.T.-J. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue, origin. Stem Cells Dev. 2010, 19, 469–480. [Google Scholar] [CrossRef]
- Lizier, N.F.; Kerkis, I.; Wenceslau, C.V. Generation of induced pluripotent stem cells from dental pulp somatic cells. In Pluripotent Stem Cells; Bhartiya, D., Lenka, N., Eds.; InTechOpen: Rijeka, Croatia, 2013; pp. 131–149. [Google Scholar] [CrossRef]
- Iida, K.; Takeda-Kawaguchi, T.; Hada, M.; Yuriguchi, M.; Aoki, H.; Tamaoki, N.; Hatakeyama, D.; Kunisada, T.; Shibata, T.; Tezuka, K. Hypoxia-enhanced derivation of iPSCs from human dental pulp cells. J. Dent. Res. 2013, 92, 905–910. [Google Scholar] [CrossRef]
- Takeda-Kawaguchi, T.; Sugiyama, K.; Chikusa, S.; Iida, K.; Aoki, H.; Tamaoki, N.; Hatakeyama, D.; Kunisada, T.; Shibata, T.; Fusaki, N.; et al. Derivation of iPSCs after culture of human dental pulp cells under defined conditions. PLoS ONE 2014, 9, e115392. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N. Induced pluripotent stem (iPS) cells in dentistry: A review. Int. J. Stem Cells. 2016, 9, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Hamano, S.; Sugiura, R.; Yamashita, D.; Tomokiyo, A.; Hasegawa, D.; Maeda, H. Current application of iPS cells in the dental tissue regeneration. Biomedicines 2022, 10, 3269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, Z.; Shelat, H.; Geng, Y.-J. Reprogramming somatic cells to pluripotency: A fresh look at Yamanaka’s model. Cell Cycle 2013, 12, 3594–3598. [Google Scholar] [CrossRef]
- Singh, V.K.; Kalsan, M.; Kumar, N.; Saini, A.; Chandra, R. Induced pluripotent stem cells: Applications in regenerative medicine, disease modeling, and drug discovery. Front. Cell Dev. Biol. 2015, 3, 2. [Google Scholar] [CrossRef]
- Soda, M.; Saitoh, I.; Murakami, T.; Inada, E.; Iwase, Y.; Noguchi, H.; Shibasaki, S.; Kurosawa, M.; Sawami, T.; Terunuma, M.; et al. Repeated human deciduous tooth-derived dental pulp cell reprogramming factor transfection yields multipotent intermediate cells with enhanced iPS cell formation capability. Sci. Rep. 2019, 9, 1490. [Google Scholar] [CrossRef]
- Chin, M.H.; Mason, M.J.; Xie, W.; Volinia, S.; Singer, M.; Peterson, C.; Ambartsumyan, G.; Aimiuwu, O.; Richter, L.; Zhang, J.; et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 2009, 5, 111–123. [Google Scholar] [CrossRef]
- Chandrabose, S.T.; Sriram, S.; Subramanian, S.; Cheng, S.; Ong, W.K.; Rozen, S.; Kasim, N.H.A.; Sugii, S. Amenable epigenetic traits of dental pulp stem cells underlie high capability of xeno-free episomal reprogramming. Stem Cell Res. Ther. 2018, 9, 68. [Google Scholar] [CrossRef]
- Andrews, P.W.; Gokhale, P.J. A short history of pluripotent stem cells markers. Stem Cell Rep. 2024, 19, 1–10. [Google Scholar] [CrossRef]
- Russell, A.J.; Silpa, L. Chemical-induced naive pluripotency. Cell Chem. Biol. 2016, 23, 532–534. [Google Scholar] [CrossRef]
- Inada, E.; Saitoh, I.; Kubota, N.; Iwase, Y.; Murakami, T.; Sawami, T.; Yamasaki, Y.; Sato, M. Increased expression of cell surface SSEA-1 is closely associated with naïve-like conversion from human deciduous teeth dental pulp cells-derived iPS cells. Int. J. Mol. Sci. 2019, 20, 1651. [Google Scholar] [CrossRef]
- Nakashima, Y.; Miyagi-Shiohira, C.; Saitoh, I.; Watanabe, M.; Matsushita, M.; Tsukahara, M.; Noguchi, H. Induced hepatic stem cells are suitable for human hepatocyte production. iScience 2022, 25, 105052. [Google Scholar] [CrossRef]
- Saitoh, I.; Sato, M.; Soda, M.; Inada, E.; Iwase, Y.; Murakami, T.; Ohshima, H.; Hayasaki, H.; Noguchi, H. Tissue-specific stem cells obtained by reprogramming of non-obese diabetic (NOD) mouse-derived pancreatic cells confer insulin production in response to glucose. PLoS ONE 2016, 11, e0163580. [Google Scholar] [CrossRef]
- Noguchi, H.; Miyagi-Shiohira, C.; Nakashima, Y.; Onishi, Y.; Saitoh, I.; Watanabe, M. Establishment of induced pancreatic stem cells by Yes-associated protein 1. Cell Transplant. 2024, 33, 09636897241248942. [Google Scholar] [CrossRef]
- Kiyokawa, Y.; Sato, M.; Noguchi, H.; Inada, E.; Iwase, Y.; Kubota, N.; Sawami, T.; Terunuma, M.; Maeda, T.; Hayasaki, H.; et al. Drug-induced naïve iPS cells exhibit better performance than primed iPS cells with respect to the ability to differentiate into pancreatic β-cell lineage. J. Clin. Med. 2020, 9, 2838. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lee, H.J.; Sung, H.H.; Ko, K.; Han, D.W.; Ko, K. Optimization of Matrigel-based culture for expansion of neural stem cells. Anim. Cells Syst. 2015, 19, 175–180. [Google Scholar] [CrossRef]
- Li, L.; Bennett, S.A.L.; Wang, L. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adh. Migr. 2012, 6, 59–70. [Google Scholar] [CrossRef]
- Ibano, N.; Inada, E.; Otake, S.; Kiyokawa, Y.; Sakata, K.; Sato, M.; Kubota, N.; Noguchi, H.; Iwase, Y.; Murakami, T.; et al. The role of genetically modified human feeder cells in maintaining the integrity of primary cultured human deciduous dental pulp cells. J. Clin. Med. 2022, 11, 6087. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, I.; Inada, E.; Iwase, Y.; Noguchi, H.; Murakami, T.; Soda, M.; Kubota, N.; Hasegawa, H.; Akasaka, E.; Matsumoto, Y.; et al. Choice of feeders is important when first establishing iPSCs derived from primarily cultured human deciduous tooth dental pulp cells. Cell Med. 2015, 8, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Javaid, D.; Ganie, S.Y.; Hajam, Y.A.; Reshi, M.S. CRISPR/Cas9 system: A reliable and facile genome editing tool in modern biology. Mol. Biol. Rep. 2022, 49, 12133–12150. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Yang, J.; Mishina, Y.; Giannobile, W.V. Genome editing: A new horizon for oral and craniofacial research. J. Dent. Res. 2019, 98, 36–45. [Google Scholar] [CrossRef]
- Bales, L.E. CRISPR-Cas in the Field of Dentistry: A Comprehensive Collection of the Potential Uses of CRISPR-Cas9 in Dental Health Care. Chancellor’s Honors Program Projects. 2022. Available online: https://trace.tennessee.edu/utk_chanhonoproj/2499 (accessed on 15 January 2025).
- Bikard, D.; Euler, C.W.; Jiang, W.; Nussenzweig, P.M.; Goldberg, G.W.; Duportet, X.; Fischetti, V.A. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol. 2014, 32, 1146–1150. [Google Scholar] [CrossRef]
- Gong, T.; Tang, B.; Zhou, X.; Zeng, J.; Lu, M.; Guo, X.; Peng, X.; Lei, L.; Gong, B.; Li, Y. Genome editing in Streptococcus mutans through self-targeting CRISPR arrays. Mol. Oral Microbiol. 2018, 33, 440–449. [Google Scholar] [CrossRef]
- Stern, A.; Keren, L.; Wurtzel, O.; Amitai, G.; Sorek, R. Self-targeting by CRISPR: Gene regulation or autoimmunity? Trends Genet. 2010, 26, 335–340. [Google Scholar] [CrossRef]
- Kato, Y.; Yokose, S. Oxytocin facilitates dentinogenesis of rat dental pulp cells. J. Endod. 2021, 47, 592–599. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Yao, H.; Zhang, Z.; Song, Y. CRISPR/Cas9-mediated deletion of Fam83h induces defective tooth mineralization and hair development in rabbits. J. Cell. Mol. Med. 2022, 26, 5670–5679. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, C.; Zhao, H.; Gao, J.; Hu, J. Chitosan hydrogel-delivered ABE8e corrects PAX9 mutant in dental pulp stem cells. Gels 2023, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Molla, K.A.; Yang, Y. CRISPR/Cas-mediated base editing: Technical considerations and practical applications. Trends Biotechnol. 2019, 37, 1121–1142. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, A.; Guri, I.; Zebrowska, P.; Llopis-Hernandez, V.; Brooks, I.R.; Tekkela, S.; Subramaniam, K.; Gebrezgabher, R.; Naso, G.; Petrova, A.; et al. ABE8e adenine base editor precisely and efficiently corrects a recurrent COL7A1 nonsense mutation. Sci. Rep. 2022, 12, 19643. [Google Scholar] [CrossRef]
- Rehman, A.; Panda, S.K.; Torsiello, M.; Marigliano, M.; Tufano, C.C.; Nigam, A.; Parveen, Z.; Papaccio, G.; La Noce, M. The crosstalk between primary MSCs and cancer cells in 2D and 3D cultures: Potential therapeutic strategies and impact on drug resistance. Stem Cells Transl. Med. 2024, 13, 1178–1185. [Google Scholar] [CrossRef]
- Nikkhah, E.; Kalalinia, F.; Rezaee, M.A.; Tayarani-Najaran, Z. Suppressive effects of dental pulp stem cells and its conditioned medium on development and migration of colorectal cancer cells through MAPKinase pathways. Iran. J. Basic Med. Sci. 2021, 24, 1292–1300. [Google Scholar] [CrossRef]
- He, Y.; Li, R.; She, W.; Ai, Y.; Li, K.; Kumeria, T.; Jiang, Z.; Shao, Q.; Zou, C.; Albashari, A.A.; et al. Inhibitory effects of the nanoscale lysate derived from xenogenic dental pulp stem cells in lung cancer models. J. Nanobiotechnol. 2023, 21, 488. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Yang, H.; Wu, Y.; Li, K.; Tang, Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: A comprehensive review. Stem Cell Res. Ther. 2019, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y.; Tan, T.; Pang, A.S.R.; Srinivasan, D.K. The role of cytokines in wound healing: From mechanistic insights to therapeutic applications. Explor. Immunol. 2025, 5, 1003183. [Google Scholar] [CrossRef]
- Yi, J.; Tang, Q.; Sun, S.; Xie, H.; Wang, L.; Yin, X. Exosomes in diabetic wound healing: Mechanisms, applications, and perspectives. Diabetes Metab. Syndr. Obes. 2025, 18, 2955–2976. [Google Scholar] [CrossRef] [PubMed]
- Roszkowski, S. Therapeutic potential of mesenchymal stem cell-derived exosomes for regenerative medicine applications. Clin. Exp. Med. 2024, 24, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Deng, R.; Zhang, G.; He, X.; Chen, H.; Chen, B.; Wan, L.; Kang, X. Therapeutic effect of exosomes derived from stem cells in spinal cord injury: A systematic review based on animal studies. Front. Neurol. 2022, 13, 847444. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal stem cell immunomodulation: Mechanisms and therapeutic potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Haddad, R.; Saldanha-Araujo, F. Mechanisms of T-Cell immunosuppression by mesenchymal stromal cells: What do we know so far? Biomed. Res. Int. 2014, 2014, 216806. [Google Scholar] [CrossRef]
- Yoshida, S.; Tomokiyo, A.; Hasegawa, D.; Hamano, S.; Sugii, H.; Maeda, H. Insight into the role of dental pulp stem cells in regenerative therapy. Biology 2020, 9, 160. [Google Scholar] [CrossRef]
- Pierdomenico, L.; Bonsi, L.; Calvitti, M.; Rondelli, D.; Arpinati, M.; Chirumbolo, G.; Becchetti, E.; Marchionni, C.; Alviano, F.; Fossati, V.; et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 2005, 80, 836–842. [Google Scholar] [CrossRef]
- Demircan, P.C.; Sariboyaci, A.E.; Unal, Z.S.; Gacar, G.; Subasi, C.; Karaoz, E. Immunoregulatory effects of human dental pulp-derived stem cells on T cells: Comparison of transwell co-culture and mixed lymphocyte reaction systems. Cytotherapy 2011, 13, 1205–1220. [Google Scholar] [CrossRef]
- Wada, N.; Menicanin, D.; Shi, S.; Bartold, P.M.; Gronthos, S. Immunomodulatory properties of human periodontal ligament stem cells. J. Cell. Physiol. 2009, 219, 667–676. [Google Scholar] [CrossRef]
- Hata, M.; Omi, M.; Kobayashi, Y.; Nakamura, N.; Miyabe, M.; Ito, M.; Makino, E.; Kanada, S.; Saiki, T.; Ohno, T.; et al. Transplantation of human dental pulp stem cells ameliorates diabetic polyneuropathy in streptozotocin-induced diabetic nude mice: The role of angiogenic and neurotrophic factors. Stem Cell Res. Ther. 2020, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- El-Kersh, A.O.F.O.; El-Akabawy, G.; Al-Serwi, R.H. Transplantation of human dental pulp stem cells in streptozotocin-induced diabetic rats. Anat. Sci. Int. 2020, 95, 523–539. [Google Scholar] [CrossRef]
- Inada, R.; Mendoza, H.Y.; Tanaka, T.; Horie, T.; Satomi, T. Preclinical study for the treatment of diabetes mellitus using β-like cells derived from human dental pulp stem cells. Regen. Med. 2022, 17, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7544–7556. [Google Scholar] [CrossRef]
- Sowa, K.; Nito, C.; Nakajima, M.; Ueda, M.; Kimura, K.; Okada, T. Impact of dental pulp stem cells overexpressing hepatocyte growth factor after cerebral ischemia/reperfusion in rats. Mol. Ther. Methods Clin. Dev. 2018, 10, 281–290. [Google Scholar] [CrossRef]
- Hilkens, P.; Bronckaers, A.; Ratajczak, J.; Gervois, P.; Wolfs, E.; Lambrichts, I. The angiogenic potential of DPSCs and SCAPs in an in vivo model of dental pulp regeneration. Stem Cells Int. 2017, 2017, 2582080. [Google Scholar] [CrossRef]
- Samiei, M.; Harmsen, M.C.; Abdolahinia, E.D.; Barar, J.; Petridis, X. Scaffold-free strategies in dental pulp/dentine tissue engineering: Current status and implications for regenerative biological processes. Bioengineering 2025, 12, 198. [Google Scholar] [CrossRef]
- Mohd, N.; Razali, M.; Fauzi, M.B.; Kasim, N.H.A. In vitro and in vivo biological assessments of 3D-bioprinted scaffolds for dental applications. Int. J. Mol. Sci. 2023, 24, 12881. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental enamel formation and implications for oral health and disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Mai, H.N.; Lee, D.J.; Kim, K.H.; Lee, S.J.; Jung, H.S. Strategies for differentiation of hiPSCs into dental epithelial cell lineage. Cell Tissue Res. 2021, 386, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Kim, K.-H.; Kim, H.-Y.; Lee, D.-J.; Li, S.; Han, M.N.; Jung, H.-S. Harnessing the dental cells derived from human induced pluripotent stem cells for hard tissue engineering. J. Adv. Res. 2023, 61, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Yoon, K.-S.; Arakaki, M.; Otsu, K.; Fukumoto, S.; Harada, H.; Green, D.W.; Lee, J.-M.; Jung, H.-S. Effective differentiation of induced pluripotent stem cells into dental cells. Dev. Dyn. 2019, 248, 129–139. [Google Scholar] [CrossRef]
- Kim, G.-H.; Yang, J.; Jeon, D.-H.; Kim, J.-H.; Chae, G.Y.; Jang, M.; Lee, G. Differentiation and establishment of dental epithelial-like stem cells derived from human ESCs and iPSCs. Int. J. Mol. Sci. 2020, 21, 4384. [Google Scholar] [CrossRef]
- Miao, X.; Niibe, K.; Fu, Y.; Zhang, M.; Nattasit, P.; Ohori-Morita, Y.; Nakamura, T.; Jiang, X.; Egusa, H. Epiprofin transcriptional activation promotes ameloblast induction from mouse induced pluripotent stem cells via the BMP-Smad signaling axis. Front. Bioeng. Biotechnol. 2022, 10, 890882. [Google Scholar] [CrossRef]
- Hemeryck, L.; Hermans, F.; Chappell, J.; Kobayashi, H.; Lambrechts, D.; Lambrichts, I.; Bronckaers, A.; Vankelecom, H. Organoids from human tooth showing epithelial stemness phenotype and differentiation potential. Cell. Mol. Life Sci. 2022, 79, 153. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kim, E.-J.; Kim, H.-Y.; Li, S.; Jung, H.-S. Fabrication of functional ameloblasts from hiPSCs for dental application. Front. Cell Dev. Biol. 2023, 11, 1164811. [Google Scholar] [CrossRef]
- Zielińska, A.; Karczewski, J.; Eder, P.; Kolanowski, T.; Szalata, M.; Wielgus, K.; Szalata, M.; Kim, D.; Shin, S.R.; Słomski, R.; et al. Scaffolds for drug delivery and tissue engineering: The role of genetics. J. Control Release 2023, 359, 207–223. [Google Scholar] [CrossRef]
- dos Santos, L.R.K.; Pelegrine, A.A.; da Silveira Bueno, C.E.; Ferreira, J.R.M.; Aloise, A.C.; Stringheta, C.P.; Martinez, E.F.; Pelegrine, R.A. Pulp–dentin complex regeneration with cell transplantation technique using stem cells derived from human deciduous teeth: Histological and immunohistochemical study in immunosuppressed rats. Bioengineering 2023, 10, 610. [Google Scholar] [CrossRef]
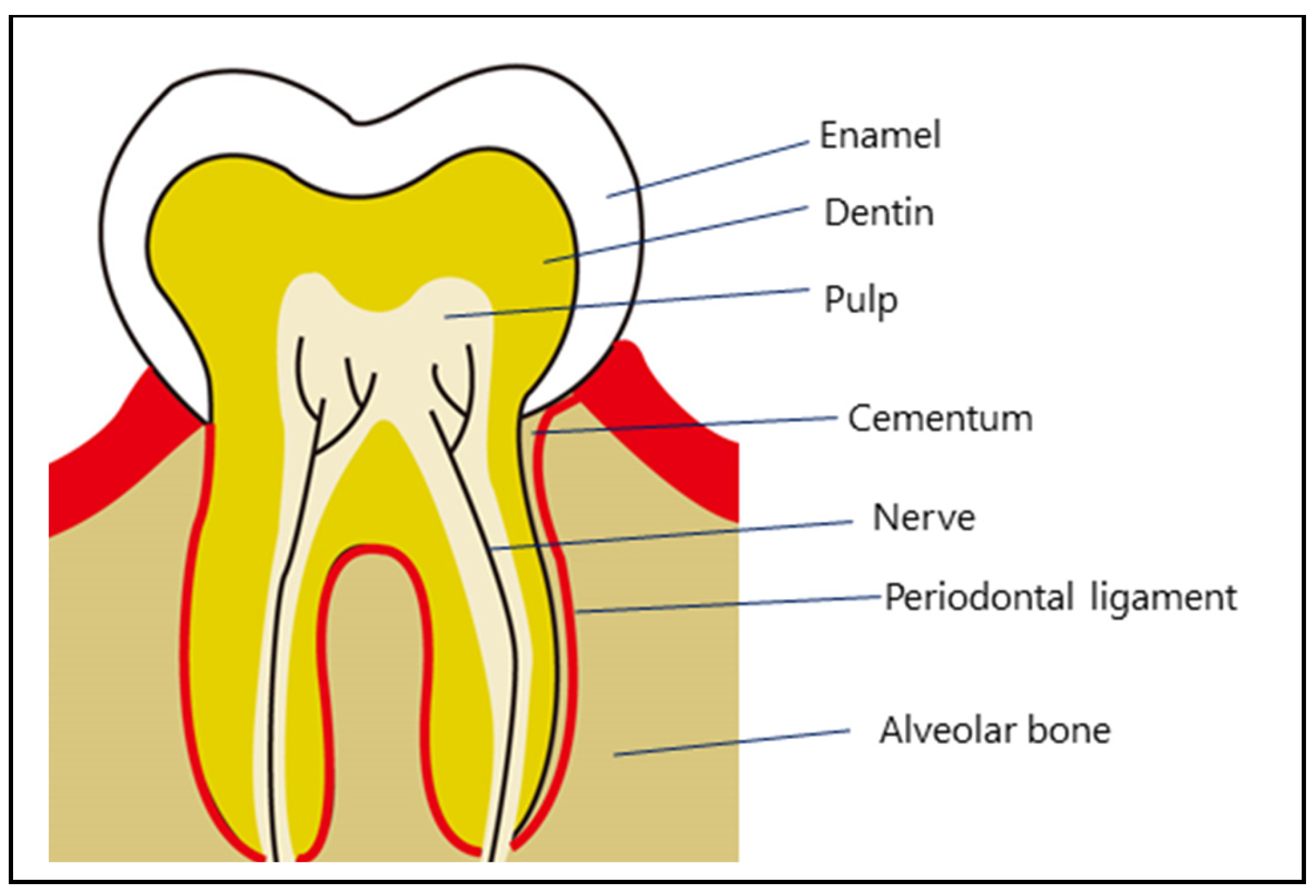

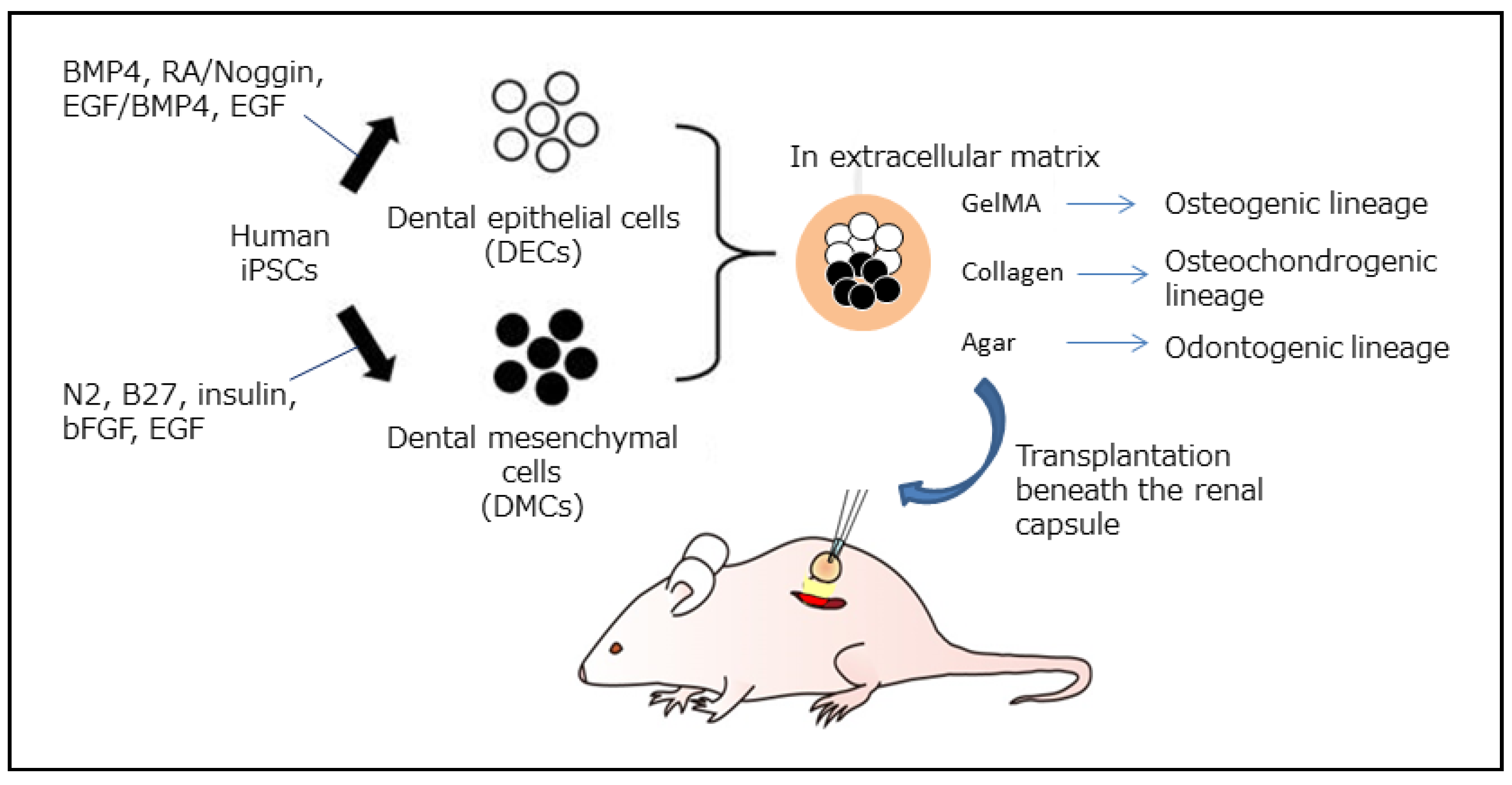
| Feature | DPSC (Dental Pulp Stem Cell) | BMSC (Bone Marrow Stem Cells) |
|---|---|---|
| Origin | Neural crest originates from the dental pulp tissue. | Mesodermal origin from the bone marrow. |
| Multilineage potential | Can differentiate into multiple cell neural lineages (osteogenic, adipogenic, chondrogenic) plus specific odontogenic and neurogenic differentiation. | Can differentiate into multiple cell neural lineages (osteogenic, adipogenic, chondrogenic), with a strong predisposition for osteogenic differentiation. |
| Surface markers | Typically positive for STRO-1, CD29, CD44, CD73, CD90, CD105, CD59, and CD106. | Typically positive for CD29, CD44, CD73, CD90, CD105, CD166, and CD146; lower STRO-1 expression than DPSCs. |
| Proliferation rate | High proliferation rate and clonogenic potential. | Slower proliferation rate compared to DPSCs. |
| Type of Differentiated Cells, Originating from DPSCs | Methods for Differentiation Induction | Markers or Staining Method | References |
|---|---|---|---|
| Osteogenic differentiation | Cultivation in medium containing dexamethasone, L-ascorbic acid, and β-glycerol phosphate; co-cultivation with endothelium; cultivation in the presence of human serum, concentrated growth factor exudate (CGFe), and TGF-β1 or betaine; cultivation with scaffolds, decellularised adipose tissue solid foams, or microcapsules | Alkaline phosphatase (ALP) Collagen type I (COL I) Osteocalcin (OCN) Osteonectin (ON) Osteopontin (OPN) Osterix (OSX) Runt-related transcription factor 2 (RUNX2) | [47,48,49,50,51,52,53,54,55,56,57,58] |
| Odontogenic differentiation | Cultivation in medium containing TGF-β1, dexamethasone, β-glycerophosphate, and L-ascorbic acid; cultivating on dentin, calcium silicate materials, or scaffolds; cultivation in preameloblast-conditioned medium or medium containing endothelin-1 (ET-1) or vascular endothelial growth factor A (VEGFA) | ALP Dentin sialoprotein (DSP) Dentin sialophosphoprotein (DSPP), Dentin matrix protein 1 (DMP-1) Matrix extracellular phosphoglycoprotein (MEPE) Alizarin red S staining | [59,60,61,62,63,64,65,66] |
| Adipogenic differentiation | Cultivation in medium containing insulin, dexamethasone, indomethacin, and 3-isobutyl-1-methylxanthine (IBMX); cultivation in the presence of enzymatically decellularized adipose tissue solid foams | Peroxisome proliferator-activated receptor γ (PPAR-γ) Glucose transporter type 4 (GLUT4) Fatty acid binding protein 4 (FABP4) Lipoprotein lipase (LPL) Oil red O staining | [52,67,68,69,70] |
| Neurogenic differentiation | Cultivation in neuroinduction medium containing B27, L-glutamine, basic FGF, and EGF for 7 days, and subsequently in neuroinduction medium supplemented with retinoic acid for another 7 days; cultivation in commercially available neurogenic differentiation medium, conditioned medium of cerebrospinal fluid and retinoic acid, or medium containing nerve growth factor (NGF); culturing in the presence of graphene–polycaprolactone hybrid nanofibers or highly concentrated K+ (50 mM KCl) for K+ stimulation | Neuronal nuclei (NeuN) Microtubule-associated protein 2 (MAP2) Neural cell adhesion molecule (NCAM) Growth-associated protein 43 (GAP43) Glial fibrillary acid protein (GFAP) Synapsin I (SYN1) Neuron-specific class III beta-tubulin (TUBB3) Gamma-aminobutyric acid (GABA receptors) Enolase 2/neuron-specific enolase (ENO2/NSE) Nestin (NES) Peripherin (PRPH) | [71,72,73,74,75,76,77,78] |
| Hepatogenic differentiation | Cultivation in hepatoinduction medium containing hepatic growth factor, insulin-transferrin-selenium-x, dexamethasone, and oncostatin M | Alpha fetoprotein (AFP) Albumin (ALB) Hepatic nuclear factor-4 alpha (HNF4α) Insulin-like growth factor-1 (IGF-1) Carbamoyl phosphate synthetase (CPS) | [79] |
| Angiogenic differentiation | Cultivation in a medium supplemented with a mixture of B27, heparin, and growth factors, including vascular endothelial growth factor (VEGF)-A165 or VEGF alone; co-cultivation with human umbilical vein endothelial cells (HUVECs) after encapsulation by a scaffold system, called self-assembling peptide nanofibers | CD54/intercellular adhesion molecule-1 (ICAM-1) CD146/melanoma cell adhesion molecule (MCAM) Monocyte chemotactic protein-1 (MCP-1) von-Willebrand factor (VWF) (domains 1 and 2) CD31/platelet/endothelial cell adhesion molecule-1 (PECAM-1) Vascular endothelial growth factor (VEGF) CD34 Functioning legal knowledge 1 (Flk-1) Vascular endothelial growth factor receptor 2 (VEGFR-2) Fibroblast growth factor 2 (FGF-2) Insulin-like growth factor binding protein-3 (IGFBP3) Interleukin-8 (IL-8) Plasminogen activator inhibitor-1 (PAI-1) Tissue inhibitors of metalloproteinase-1 (TIMP-1) Urokinase-type plasminogen activator (uPA) | [80,81,82,83] |
| Cardiogenic differentiation | DPSCs are first incubated in the presence of 5-azacytidine for 2 days; then, the resulting embryoid bodies (EBs) are plated onto gelatin-coated tissue culture dishes, through which functional cardiomyocytes with cardiac markers are developed | Myosin heavy chain 6 (MYH6) Mesoderm posterior BHLH transcription factor 1 (MESP) NK-2 transcription factor related, locus 5 (Nkx2.5) Connexin 43 (Cx43) | [84] |
| Differentiation into pancreatic lineage | CD117+ DPCs have the ability to differentiate into pancreatic lineage, following the 3-step induction protocol used for pancreatic cell lineage induction; cultivation of DPSCs using a stepwise protocol to generate islet-like cell clusters; cultivation of DPSCs in a 3D culture system using a stepwise protocol to generate organoid-like 3D structures that are similar to pancreatic islets | Glucose transporter 2 (GLUT2) Pancreatic forkhead box protein A2 (Foxa2) SRY-box transcription factor 17 (Sox17) Insulin-like growth factor I (IGF-1) Fibroblast growth factor 10 (FGF 10) Pancreatic and duodenal homeobox 1 (PDX1) Hematopoietically expressed homeobox (HHEX) Motor neuron and pancreas homeobox 1 (MNX1) Neurogenin 3 (NGN3) Paired box 4 (PAX4) Paired box 6 (PAX6) NK6 homeobox 1 (NKX6-1) Blood glucose Serum insulin (INS) c-peptide (CP) Visfatin (VF) Pancreatic glucagon (GC) Somatostatin (SS) Pancreatic polypeptide, Amylase-2a (AMY2A) | [85,86,87,88,89,90,91,92] |
| Differentiation into bladder smooth muscle | Incubation of DPSCs in bladder smooth muscle cell-conditioned medium with transforming growth factor beta 1 (TGF-β1) for 2 weeks; incubation of DPSCs with direct contact with endothelial cells in the presence of TGF-β1 | Alpha smooth muscle actin (α-SMA) Smooth muscle protein 22α (SM22α) Smooth muscle myosin heavy chain (SM-MHC) Desmin (DES) Calponin (CNN1) | [93,94,95] |
| Biological Systems | Type of Gene Expression | Target Gene or Transgene (Method for Gene Delivery) | Cell Species | Outcome | References |
|---|---|---|---|---|---|
| Differentiation into odontoblast/mineralization | Gain-of-function (overexpression) | Growth/differentiation factor 11 (Gdf11) (electroporation) | Mouse | In vivo transfer of Gdf11 stimulated the reparative dentin formation during pulpal wound healing in canine teeth | [105] |
| Differentiation into odontoblast/mineralization | Gain-of-function (overexpression) | Jagged canonical Notch ligand 1 (Jagged-1; JAG-1) (retrovirus) | Human | Overexpression of JAG-1 caused activation of the Notch signaling pathway, inhibition of odontoblastic differentiation, and the formation of mineralized tissues | [119] |
| Differentiation into odontoblast/mineralization | Gain-of-function (overexpression) | Krüppel-like factor 4 (KLF4) (plasmid, FuGENE) | Human | Overexpression of KLF4 caused increased alkaline phosphatase (ALP) activity and expression of odontoblastic differentiation markers | [120] |
| Differentiation into odontoblast/mineralization | Small interfering RNA (siRNA)-based knockdown | Notch ligand Delta1 (lentivirus) | Human | Suppression of Notch ligand Delta1 resulted in inhibition of proliferation but increased differentiation into odontoblasts | [121] |
| Differentiation into odontoblast/mineralization | miR-based knockdown | miR-720 (lipofectamine) | Human | miR-720, which targets NANOG, promoted odontogenic differentiation through suppression of NANOG | [122] |
| Differentiation into odontoblast/mineralization | siRNA-based knockdown | CD44 [HCAM (homing cell adhesion molecule)] (lentivirus) | Human | RNAi for CD44, which is expressed in odontogenic cells, resulted in suppression of mineralization activities | [123] |
| Differentiation into odontoblast/mineralization | Gain-of-function (overexpression) | DNA damage-inducible transcript 3 (DDIT3) (lentivirus) | Human | Overexpression of DDIT3, an apoptotic transcription factor, increased calcium nodule formation related to odontoblastic differentiation | [124] |
| Differentiation into odontoblast/mineralization | Gain-of-function (overexpression) siRNA-based knockdown | Inhibitor of DNA binding 1 (ID1) (lentivirus) | Human | Overexpression of ID1 resulted in an enhanced odontogenic differentiation; ID1 silencing produced an opposite effect | [125] |
| Differentiation into odontoblast/mineralization | Gain-of-function (overexpression) siRNA-based knockdown | Zinc finger and BTB domain-containing 20 (ZBTB20) (lentivirus) | Human | Inhibition of ZBTB20 reduced odontogenic differentiation, while overexpression of ZBTB20 enhanced odontogenic differentiation along with increased ALP activity | [126] |
| Differentiation into odontoblast/mineralization | Gain-of-function (overexpression) | B cell lymphoma 2 gene (BCL2) (lentivirus) | Human | Overexpression of BCL2 caused decreased osteogenic/odontogenic potential | [127] |
| Differentiation into odontoblast/mineralization | Gain-of-function (overexpression) | SRY-box 2 (SOX2) (retrovirus) | Human | Overexpression of SOX2 resulted in enhanced odontoblast differentiation, which was also associated with the activation of the Wnt signaling pathway | [128] |
| Differentiation into odontoblast/mineralization | Gain-of-function (overexpression) | Platelet-derived growth factor-BB (PDGF-BB) (lentivirus) | Human | PDGF-BB enhanced odontoblastic differentiation; subcutaneous grafting of PDGF-BB-expressing DPSCs generated dentin-like mineralized tissue | [99] |
| Differentiation into odontoblast/mineralization | Gain-of-function (overexpression) siRNA-based knockdown | Sclerostin (SOST) (lentivirus) | Human | Overexpression of SOST, a protein that acts as a negative regulator of bone formation, resulted in promoted senescence-related decline of odontoblastic differentiation potential; knockdown of SOST enhances odontoblastic differentiation | [129] |
| Differentiation into odontoblast/mineralization | siRNA-based knockdown | BTB/POZ domain-containing protein 7 (BTBD7) (lentivirus) | Human | Knockdown of BTBD7, a regulatory gene that promotes epithelial tissue remodeling and branching morphogenesis, resulted in reduced expression of odontoblast markers | [130] |
| Differentiation into odontoblast/mineralization | miR-based knockdown | miR-675 (lentivirus) | Human | Overexpression of miR-675, which is involved in odontogenic differentiation, resulted in enhancement of odontogenic differentiation | [131] |
| Differentiation into odontoblast/mineralization | miR-based knockdown | miR-508-5p (lipofectamine) | Human | Overexpression of miR-508-5p, which targets the glycoprotein non-metastatic melanoma protein B (GPNMB) gene, resulted in suppression of odontogenesis; ectopic expression of GPNMB (lacking 3′-UTR) rescued the effects of miR-508-5p on odontogenic differentiation | [132] |
| Differentiation into odontoblast/mineralization | miR-based knockdown | miR-223-3p (lentivirus) | Human | Overexpression of miR-223-3p, one of the inflammation-induced miRs, resulted in increased production of odontogenic markers but decreased the production of SMAD family member 3 (SMAD3), a potential target of miR-223-3p | [133] |
| Differentiation into odontoblast/mineralization | Gain-of-function (overexpression) | Dentin matrix protein 1 (DMP-1) (lentivirus) | Human | Overexpression of DMP-1 in the DPSCs derived from X-linked hypophosphatemia (XLH), associated with deficient dentin formation and mineralization, restored the irregular protein processing patterns to near-physiological levels | [134] |
| Differentiation into odontoblast/mineralization | Gain-of-function (overexpression) siRNA-based knockdown | Long noncoding RNA H19 (LncRNA H19) (plasmid; lentivirus) | Human | Overexpression of LncRNA H19 promoted enhanced ALP activity and increased expression of odontogenic markers via regulating the TGF-β1/Smad signaling pathway | [135] |
| Differentiation into odontoblast/mineralization | Gain-of-function (overexpression) | DMP-1 (lentivirus) | Human | Expression of DMP-1, a factor stimulating canonical Wnt signaling, in the XLH-DPSCs resulted in downregulation of Wnt inhibitors and improved mineralization | [136] |
| Differentiation into odontoblast/mineralization | miR-based knockdown | miR-15b-5p (lipofectamine) | Human | MiR-15b-5p suppressed the differentiation into odontoblasts by targeting insulin-like growth factor 1 (IGF1), while the miR-15b-5p inhibitor enhanced the differentiation into odontoblasts | [137] |
| Osteogenic differentiation | Gain-of-function (overexpression) | BCL2 (lentivirus) | Human | Overexpression of BCL2 resulted in enhanced cell survivability because of the inhibition of apoptosis by BCL2; it also caused decreased osteogenic/odontogenic potential | [127] |
| Osteogenic differentiation | Gain-of-function (overexpression) | SOX2 (retrovirus) | Human | Overexpression of SOX2 resulted in enhanced osteogenic differentiation, together with the activation of the Hippo signal pathway | [138] |
| Osteogenic differentiation | Gain-of-function (overexpression) | Transforming growth factor beta 1 (TGF-β1) (plasmid; electroporation) | Human | Overexpression of TGF-β1 resulted in increased osteogenic and chondrogenic differentiation; it also resulted in increased proliferation rate and decreased apoptosis | [111] |
| Osteogenic differentiation | Gain-of-function (overexpression) | Wnt family member 4 (WNT4) (lentivirus) | Human | Overexpression of WNT4 exhibited effective repair of rat bone defects, suggesting that WNT4-expressing DPSCs may be a feasible resource of seed cells for bone regeneration | [139] |
| Osteogenic differentiation | Gain-of-function (overexpression) | EphrinB2 (EFNB2) (lentivirus) | Human canine | Overexpression of EFNB2 resulted in enhanced osteogenic differentiation capacity | [101] |
| Osteogenic differentiation | Gain-of-function (overexpression) miR-based knockdown | hsa_circ_0026827 miR-188-3p (plasmid; lipofectamine) | Human | Overexpression of hsa_circ_0026827, one of the circular RNAs (circRNAs), resulted in increased osteoblast differentiation, while knockdown of hsa_circ_0026827 suppressed osteoblast differentiation and promoted miR-188-3p expression | [140] |
| Osteogenic differentiation | Gain-of-function (overexpression) | Hypoxia-inducible factor 1α (HIF-1α) (protein transduction domains (PTDs)) (lentivirus) | Human | Overexpression of HIF-1α, a protein known to be expressed in hypoxia and affect stemness and bone differentiation, resulted in enhanced osteogenic differentiation | [141] |
| Osteogenic differentiation | Gain-of-function (overexpression) | Semaphorin 3A (SEMA3A) (lentivirus) | Unknown | Overexpression of Sema3A, a secretory osteoprotective factor, exhibited enhanced the osteogenic differentiation | [142] |
| Osteogenic differentiation | Gain-of-function (overexpression) siRNA-based knockdown | Growth differentiation factor 15 (GDF15) (lentivirus; lipofectamine) | Human | Overexpression of GDF15 caused enhanced osteogenic differentiation through activation of the TGF-β/SMAD signaling pathway, while knockdown of GDF15 produced the opposite effect | [143] |
| Enhanced cell proliferation | siRNA-based knockdown | Catalytic alpha1 of AMP-activated protein kinase (AMPK alpha1) (siRNA; lipofectamine) | Rat | Knockdown of AMPKalpha1, a stress-responsive enzyme that is activated by hypoxia, resulted in decreased cell proliferation under both normoxia and hypoxia | [144] |
| Enhanced cell proliferation | siRNA-based knockdown | Notch ligand Delta1 (lentivirus) | Human | Knockdown of Notch ligand Delta1 resulted in inhibition of proliferation but increased differentiation into odontoblasts | [121] |
| Enhanced cell proliferation | miRNA-based knockdown | miR-720 (lipofectamine) | Human | miR-720, targeting NANOG, caused decreased proliferation and promoted odontogenic differentiation through suppression of NANOG | [122] |
| Enhanced cell proliferation | Gain-of-function (overexpression) | DDIT3 (lentivirus) | Human | Overexpression of DDIT3, an apoptotic transcription factor, resulted in reduced cell proliferation but increased calcium nodule formation related to odontoblastic differentiation | [124] |
| Enhanced cell proliferation | Gain-of-function (overexpression) | SOX2 (retrovirus) | Human | SOX2 overexpression resulted in the enhancement of cell proliferation, migration, and adhesion | [145] |
| Enhanced cell proliferation | miR-based knockdown | MiR-633 (lentivirus) | Human | miR-633 overexpression increased cell proliferation and differentiation through direct interaction with the 3′-UTR of matrix extracellular phosphoglycoprotein (MEPE) gene | [146] |
| Enhanced cell proliferation | Gain-of-function (overexpression) | Special AT-rich binding protein 2 (SATB2) (lentivirus) | Human | Overexpression of SATB2 resulted in accelerated proliferation and cell migration | [147] |
| Enhanced cell proliferation | Gain-of-function (overexpression) | PDGF-BB (lentivirus) | Human | PDGF-BB caused enhanced proliferation, odontoblastic differentiation, and cell migration via the activation of the phosphatidylinositol 3 kinase (PI3K)/Akt signaling pathway | [99] |
| Enhanced cell proliferation | Gain-of-function (overexpression) | Vascular endothelial growth factor (VEGF)/ stromal cell-derived factor-1α (SDF-1α) (lentivirus) | Human | Overexpression of SDF-1α or VEGF resulted in enhanced cell proliferation, endothelial cell migration, and vascular tube formation on Matrigel in vitro; expression of both VEGF and SDF-1α resulted in enhanced vascularized dental pulp regeneration in vivo | [148] |
| Enhanced cell proliferation | Gain-of-function (overexpression) | Lin28 (lentivirus) | Human | Overexpression of Lin28, a conserved RNA-binding protein in eukaryotes, caused increased proliferation through interaction with let-7a/IGF2BP2 pathways | [149] |
| Enhanced cell proliferation | miR-based knockdown | miR-210-3p (lentivirus) | Human | Overexpression of miR-633, targeting the 3′ UTR of the MEPE gene, caused increased cell proliferation and differentiation | [150] |
| Enhanced cell proliferation | Gain-of-function (overexpression) | TGF-β1 (plasmid; electroporation) | Human | Overexpression of TGF-β1 resulted in increased osteogenic and chondrogenic differentiation but decreased adipogenic differentiation; it also resulted in increased proliferation rate and decreased apoptosis | [111] |
| Enhanced cell proliferation | siRNA-based knockdown | Visfatin (VIS) (unknown) | Human | Knockdown of VIS, a novel adipokine associated with cellular senescence, resulted in reduced senescence | [151] |
| Enhanced cell proliferation | Gain-of-function (overexpression) | Hepatocyte growth factor (HGF) (adenovirus) | Human | Overexpression of HGF inhibited rheumatoid arthritis progression by its immunosuppressive effects, while in the late phase, HGF promoted synovitis by activating fibroblast-like synoviocytes and exhibited accelerated cell proliferation and apoptosis resistance, suggesting a dual role of HGF in RA | [152] |
| Enhanced cell proliferation | Gain-of-function (overexpression) | Long noncoding RNA H19 (LncRNA H19) (plasmid; lentivirus) | Human | Overexpression of LncRNA H19 caused increased cell proliferation, enhanced ALP activity, and increased odontoblast markers via regulating the TGF-β1/Smad signaling pathway | [135] |
| Enhanced cell proliferation | Gain-of-function (overexpression) | LncRNA H19 (plasmid; lentivirus) | Human | Overexpression of LncRNA H19 promoted cell proliferation, enhanced ALP activity, and increased odontoblast markers via regulating the TGF-β1/Smad signaling pathway | [153] |
| Enhanced cell proliferation | Gain-of-function (overexpression) | Adrenomedullin (ADM) (lentivirus) | Human | Overexpression of ADM resulted in promotion of cell cycle progression, inhibition of p53 expression, decreased reactive oxygen species (ROS) accumulation, and resistance against cellular senescence | [154] |
| Angio-/vasculogenic commitment | miR-based knockdown | MiR-424 (lentivirus) | Human | Overexpression of miR-424 caused decreased levels of VEGF and kinase insert domain-containing receptor (KDR) protein | [155] |
| Angio-/vasculogenic commitment | Gain-of-function (overexpression) | PDGF-BB (lentivirus) | Human | PDGF-BB caused enhanced proliferation, odontoblastic differentiation, and cell migration via the activation of the PI3K/Akt signaling pathway | [99] |
| Angio-/vasculogenic commitment | Gain-of-function (overexpression) | VEGF SDF-1α (lentivirus) | Human | Overexpression of SDF-1α or VEGF resulted in enhanced cell proliferation, endothelial cell migration, and vascular tube formation on Matrigel in vitro; expression of both VEGF and SDF-1α resulted in enhanced vascularized dental pulp regeneration in vivo | [148] |
| Angio-/vasculogenic commitment | Gain-of-function (overexpression) | BCL-2/ green fluorescent protein (GFP) (lentivirus) | Human | Overexpression of BCL-2 resulted in enhanced endothelial cell proliferation, migration, and vascular tube formation on Matrigel, suggesting that BCL-2 overexpression enhances angio-/vasculogenic properties of DPSCs | [156] |
| Angio-/vasculogenic commitment | Gain-of-function (overexpression) | Ets variant transcription factor 2 (ETV2) (lentivirus) | Human | Overexpression of ETV2 resulted in the appearance of endothelial-like morphology and increased expression of endothelial-specific genes, which also correlated with enhanced capillary-like tube formation on Matrigel in vitro | [157] |
| Angio-/vasculogenic commitment | Gain-of-function (overexpression) | PDGF-BB (lentivirus) | Human | Co-cultivation of PDGF-BB-overexpressing cells with human umbilical vein endothelial cells (HUVECs) exhibited increased formation of vascular tubes, suggesting that PDGF-BB engineering is an effective strategy to amplify DPSCs’ angiogenic potential | [158] |
| Neurogenic commitment | Gain-of-function (overexpression) | zinc finger protein 521 (Zfp521) (unknown) | unknown | Overexpression of Zfp521, a transcription factor, can facilitate differentiation into neural cells through chromatin modification | [159] |
| Neurogenic commitment | Gain-of-function (overexpression) | Insulin-like growth factor-binding protein 5 (IGFBP5) (retrovirus) | Human | Overexpression of IGFBP5 prompted neurogenic differentiation potential of DPSCs, suggesting that IGFBP5 is a potential target for dental pulp–dentin functional regeneration | [160] |
| Neurogenic commitment | Gain-of-function (overexpression) | OCT3/4 (lentivirus) | Human | Overexpression of OCT3/4 under neural inductive conditions caused reprogramming into the neural lineage | [161] |
| Adipogenic commitment | Gain-of-function (overexpression) | Ten-eleven-translocation 2 (TET2) (plasmid; lipofectamine) | Human | Overexpression of TET2, a key regulator of DNA methylation during adipogenic induction, resulted in increased expression of adipogenic marker genes and enhancement of the transition of DPSCs toward adipogenic commitment | [162] |
| Inflammation commitment | siRNA-mediated knockdown | Receptor for advanced glycation end products (RAGE) (lipofectamine) | Human | Binding of RAGE to high-mobility group box 1 (HMGB1), a nonhistone DNA-binding protein that promotes inflammation, is directly related to eliciting inflammation | [163] |
| Inflammation commitment | miR-based knockdown | miR-223-3p (lentivirus) | Human | Overexpression of miR-223-3p, one of the inflammation-induced miRNAs, resulted in increased production of odontoblast marker genes; knockdown of Smad3 increased the level of ALP, thereby promoting odontoblast differentiation | [133] |
| Inflammation commitment | Gain-of-function (overexpression) | HGF (adenovirus) | Human | Overexpression of HGF resulted in enhanced downregulation of inflammation-related factors | [164] |
| Inflammation commitment | Gain-of-function (overexpression) | WNT4 (plasmid; lipofectamine) | Human | Overexpression of WNT4 resulted in amelioration of cell inflammatory response, enhanced BCL-2 expression, and decreased apoptosis rate in the DPCs inflamed by lipopolysaccharide (LPS) | [165] |
| Cell migration | siRNA-based knockdown | Peptidylprolyl cis/trans isomerase, NIMA-interacting 1 (PIN1) (plasmid; lipofectamine) | Human | siRNA-based silencing of PIN1 (which specifically binds to phosphorylated Ser/Thr-pro motifs to catalytically regulate the post-phosphorylation conformation of its substrates) decreased the cell migration of DPSC | [166] |
| Cell migration | Gain-of-function (overexpression) | PDGF-BB (lentivirus) | Human | PDGF-BB enhanced odontoblastic differentiation and cell migration | [99] |
| Enhanced induction into insulin-producing cells (IPCs) | Gain-of-function (overexpression) | Forkhead box A2 (FOXA2) Pancreatic and duodenal homeobox 1 (PDX1) (lentivirus) | Human | DPSCs can be reprogrammed to generate insulin-producing cells (IPCs) by transducing them with the transcription factor genes FOXA2 and PDX1 | [167] |
| Enhanced induction into IPCs | miR-based knockdown using miR inhibitor | miR-183 inhibitor (FuGENE) | Rat | Downregulation of miR-183 through transfection with miR-183 inhibitor resulted in the generation of cells expressing insulin 72 h after transfection | [168] |
| Enhanced induction into IPCs | Gain-of-function (overexpression) | Pdx1 Neurogenin 3 (Neurog3) (plasmid; FuGENE) | Rat | Transfection with vectors carrying transcription factors Pdx1 and Neurog3 caused direct conversion into IPCs | [169] |
| Enhanced induction into oligodendrocyte progenitors | Gain-of-function (overexpression) | Oligodendrocyte transcription factor 2 (Olig2) (plasmid; X-tremeGENE) | Human | Transfection of DPSCs with the Oligo2 gene and subsequent cultivation in the differentiation-inducing medium resulted in the generation of cells showing oligodendrogenic markers | [170] |
| Cell apoptosis | RNAi (siRNA oligo)- mediated knockdown | Caspase-8 and caspase-9 (unknown) | Human | Knockdown of caspase-9 exhibited a reduction in apoptosis, caspase-3 expression, and its activity | [171] |
| Cell apoptosis | miR-based knockdown | miR-224-5p (lipofectamine) | Human | Inhibition of miR-224-5p, which targets the 3′-untranslated region (3′-UTR) of the Rac family small GTPase 1 (Rac1) gene, caused increased cell apoptosis | [172] |
| Skeletal myogenic differentiation | miR-based knockdown | miR-143 and miR-135 inhibitors (lipofectamine) | Human | miR-135 and miR-143 inhibitors induce myogenic differentiation of DPSCs | [173] |
| Skeletal myogenic differentiation | miR-based knockdown | miRNA-139-5p (lipofectamine) | Human | Overexpression of miR-139-5p induced skeletal myogenic differentiation via the Wnt/β-catenin signaling pathway; downregulation of miR-139-5p inhibited cell growth and reduced skeletal myogenic differentiation | [174] |
| Pluripotency and multilineage differentiation capability | Gain-of-function (overexpression) | Octamer-binding transcription factor 4A (OCT4A) (lentivirus) | Human | Overexpression of OCT4A resulted in upregulation of expression of stemness factors, as well as increased cell proliferation, pluripotency, and multilineage differentiation potential | [175] |
| Pluripotency and multilineage differentiation capability | Gain-of-function (overexpression) | Xeroderma pigmentosum complementation group C protein (XPC) (lentivirus) | Human | XPC, a component of the DNA repair pathway, interacts with OCT3/4 to modulate pluripotency; overexpression of XPC enhances proliferation rate, reduces apoptosis, and improves DPSC’s multilineage differentiation capabilities | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inada, E.; Saitoh, I.; Terajima, M.; Kiyokawa, Y.; Kubota, N.; Yamaza, H.; Morohoshi, K.; Nakamura, S.; Sato, M. Engineered Human Dental Pulp Stem Cells with Promising Potential for Regenerative Medicine. BioTech 2025, 14, 88. https://doi.org/10.3390/biotech14040088
Inada E, Saitoh I, Terajima M, Kiyokawa Y, Kubota N, Yamaza H, Morohoshi K, Nakamura S, Sato M. Engineered Human Dental Pulp Stem Cells with Promising Potential for Regenerative Medicine. BioTech. 2025; 14(4):88. https://doi.org/10.3390/biotech14040088
Chicago/Turabian StyleInada, Emi, Issei Saitoh, Masahiko Terajima, Yuki Kiyokawa, Naoko Kubota, Haruyoshi Yamaza, Kazunori Morohoshi, Shingo Nakamura, and Masahiro Sato. 2025. "Engineered Human Dental Pulp Stem Cells with Promising Potential for Regenerative Medicine" BioTech 14, no. 4: 88. https://doi.org/10.3390/biotech14040088
APA StyleInada, E., Saitoh, I., Terajima, M., Kiyokawa, Y., Kubota, N., Yamaza, H., Morohoshi, K., Nakamura, S., & Sato, M. (2025). Engineered Human Dental Pulp Stem Cells with Promising Potential for Regenerative Medicine. BioTech, 14(4), 88. https://doi.org/10.3390/biotech14040088







