Extracts of Argemone mexicana L. Contain Antifungal Compounds for the In Vitro Control of Monilinia fructicola, Colletotrichum gloeosporioides, Fusarium oxysporum, and Sclerotinia sclerotiorum: Preliminary Evidence for Field Application
Abstract
1. Introduction
2. Materials and Methods
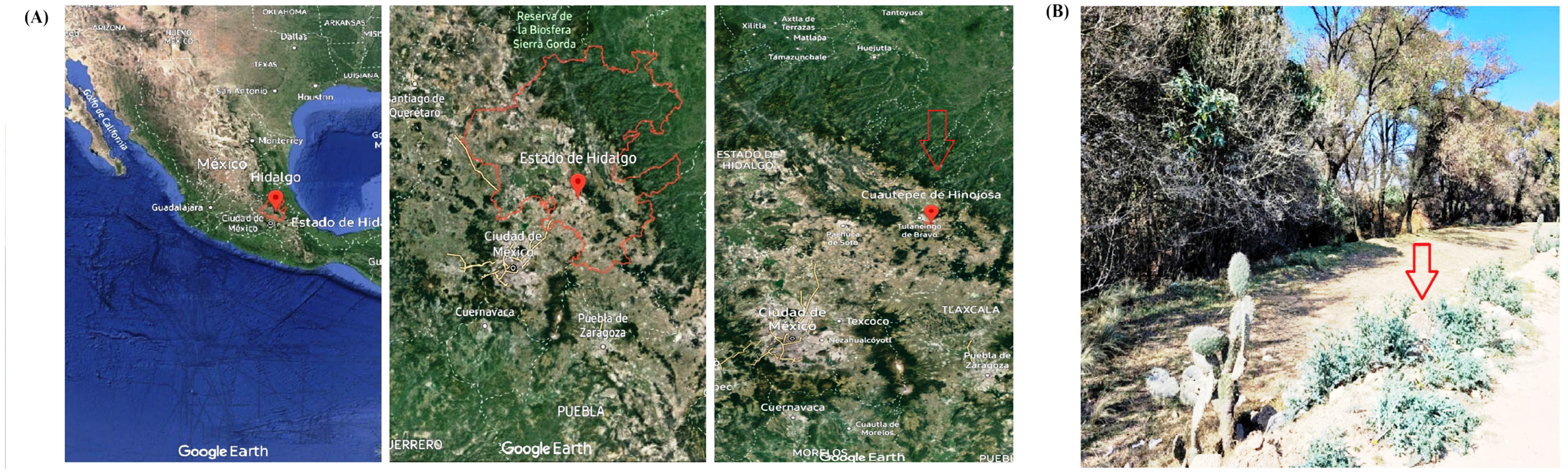
2.1. Plant Material
2.2. Extraction of the Phytochemical Compounds
2.3. Gas Chromatography-Mass Spectrometry Analysis (GC-MS)
2.4. In Vitro Evaluation of the Extracts Against Phytopathogenic Fungi
2.5. Statistical Analysis
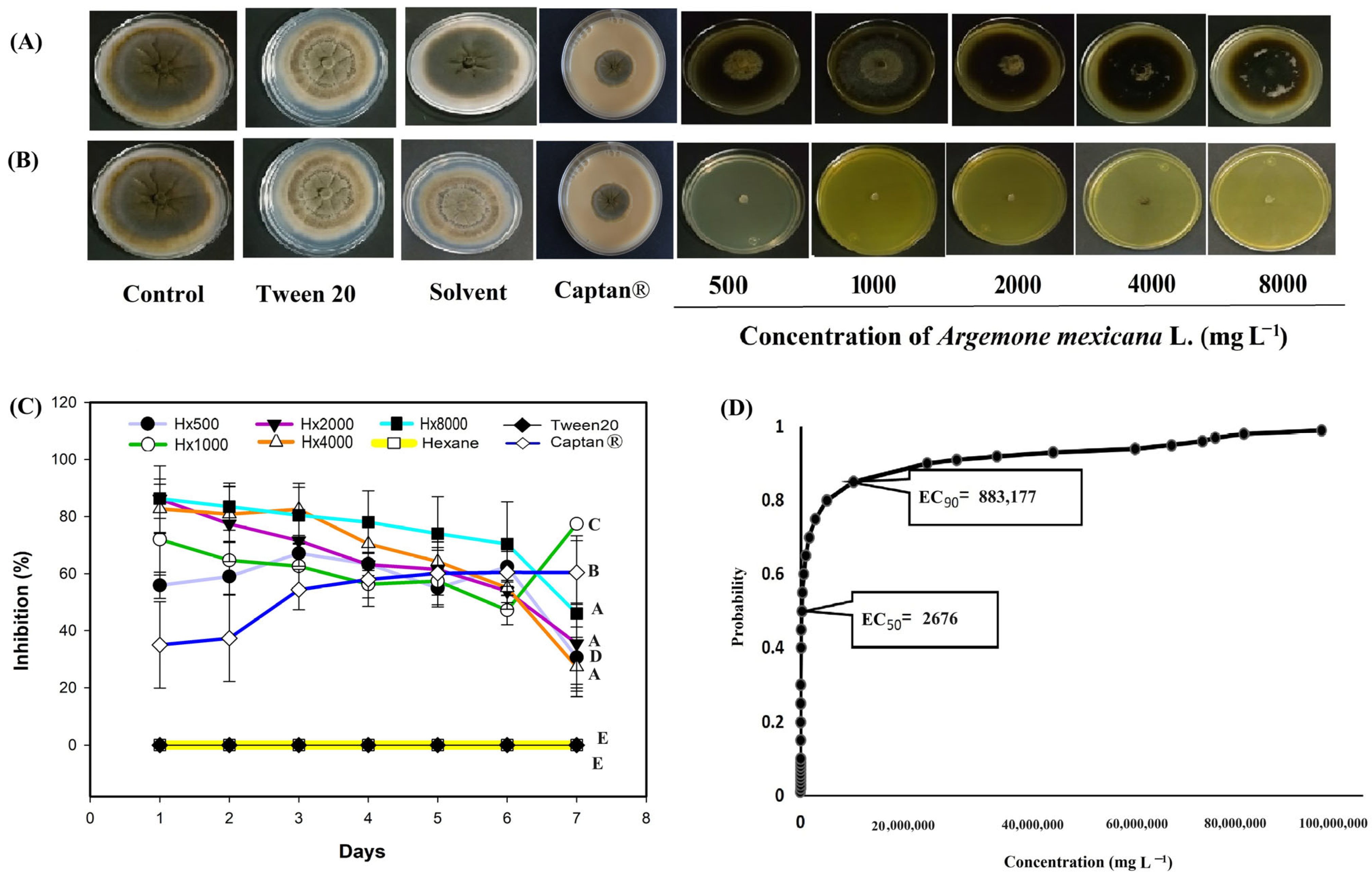
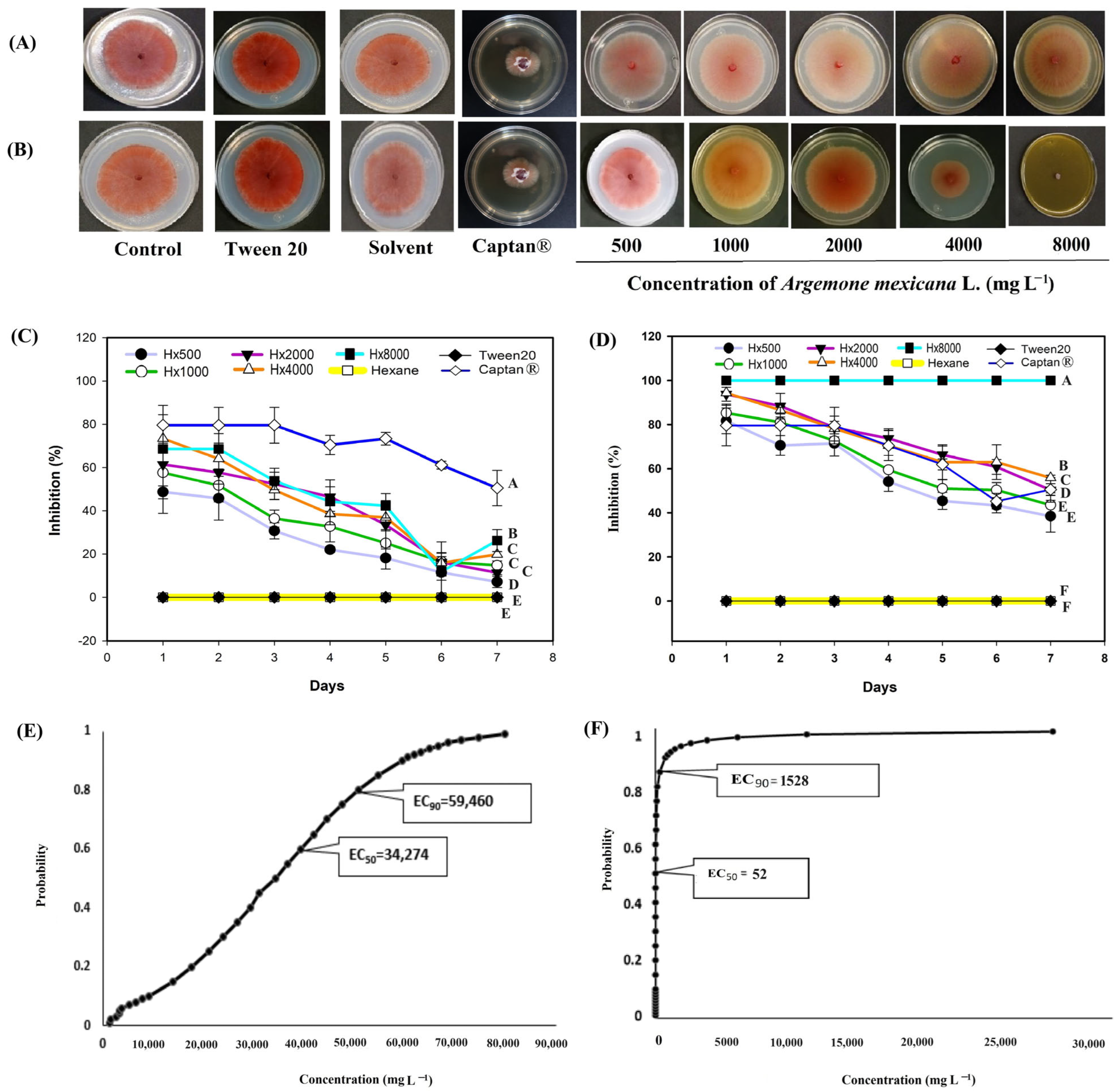
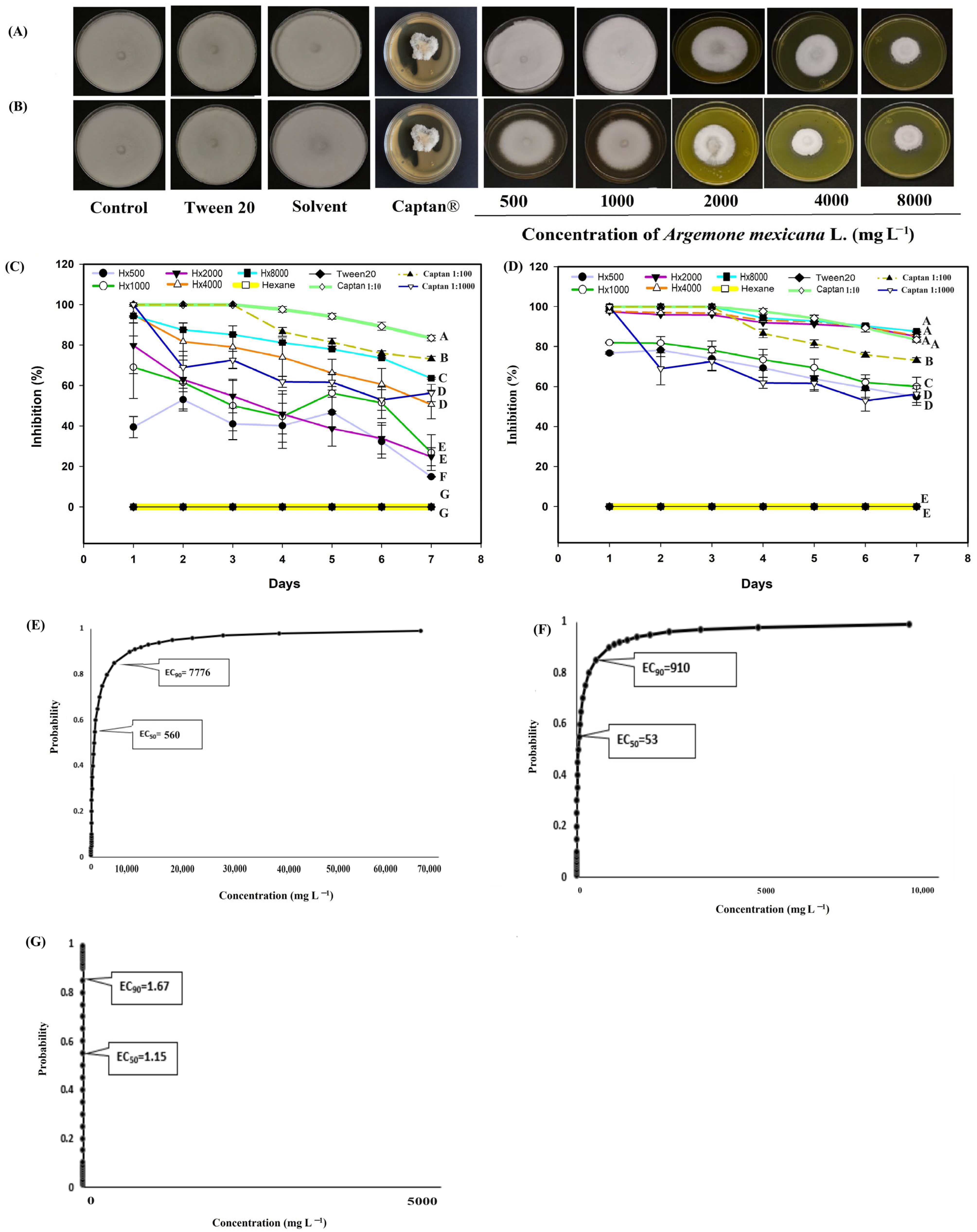
3. Results
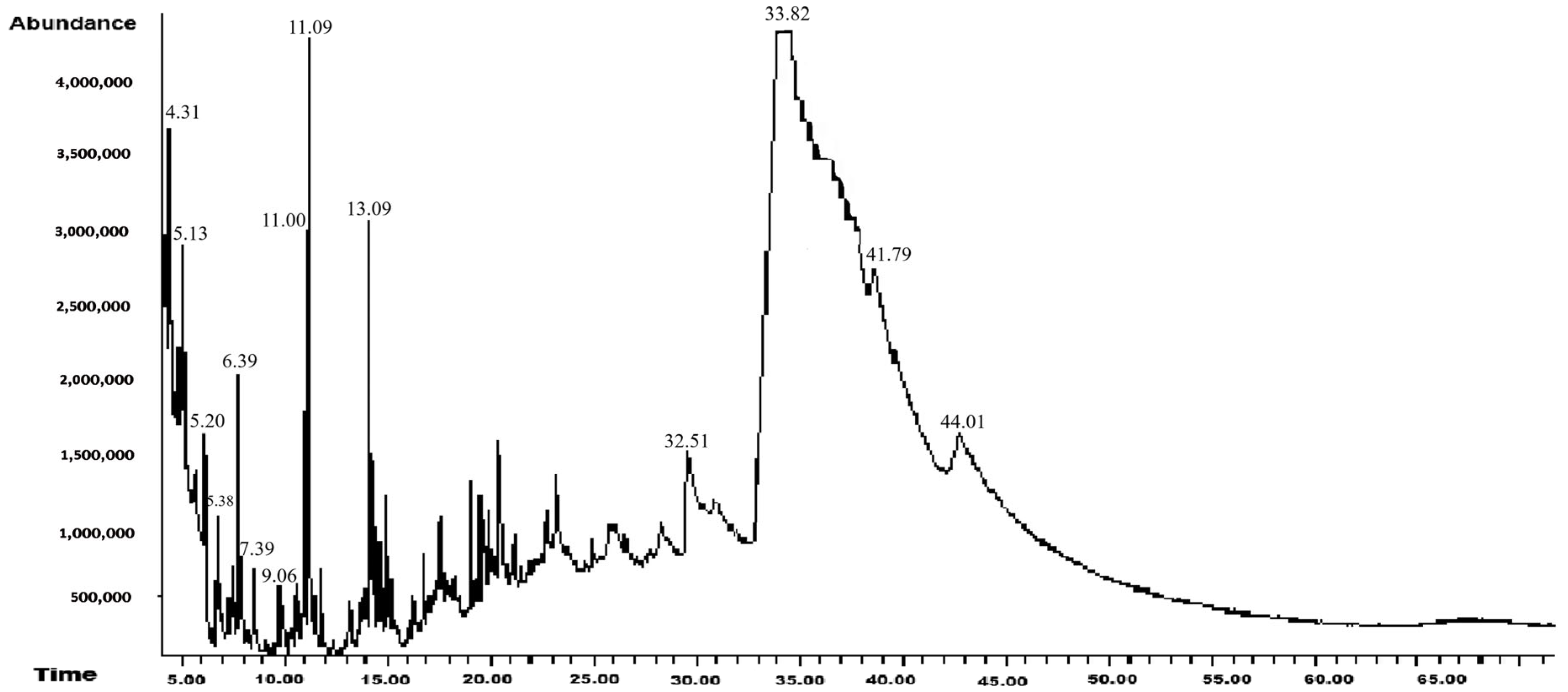
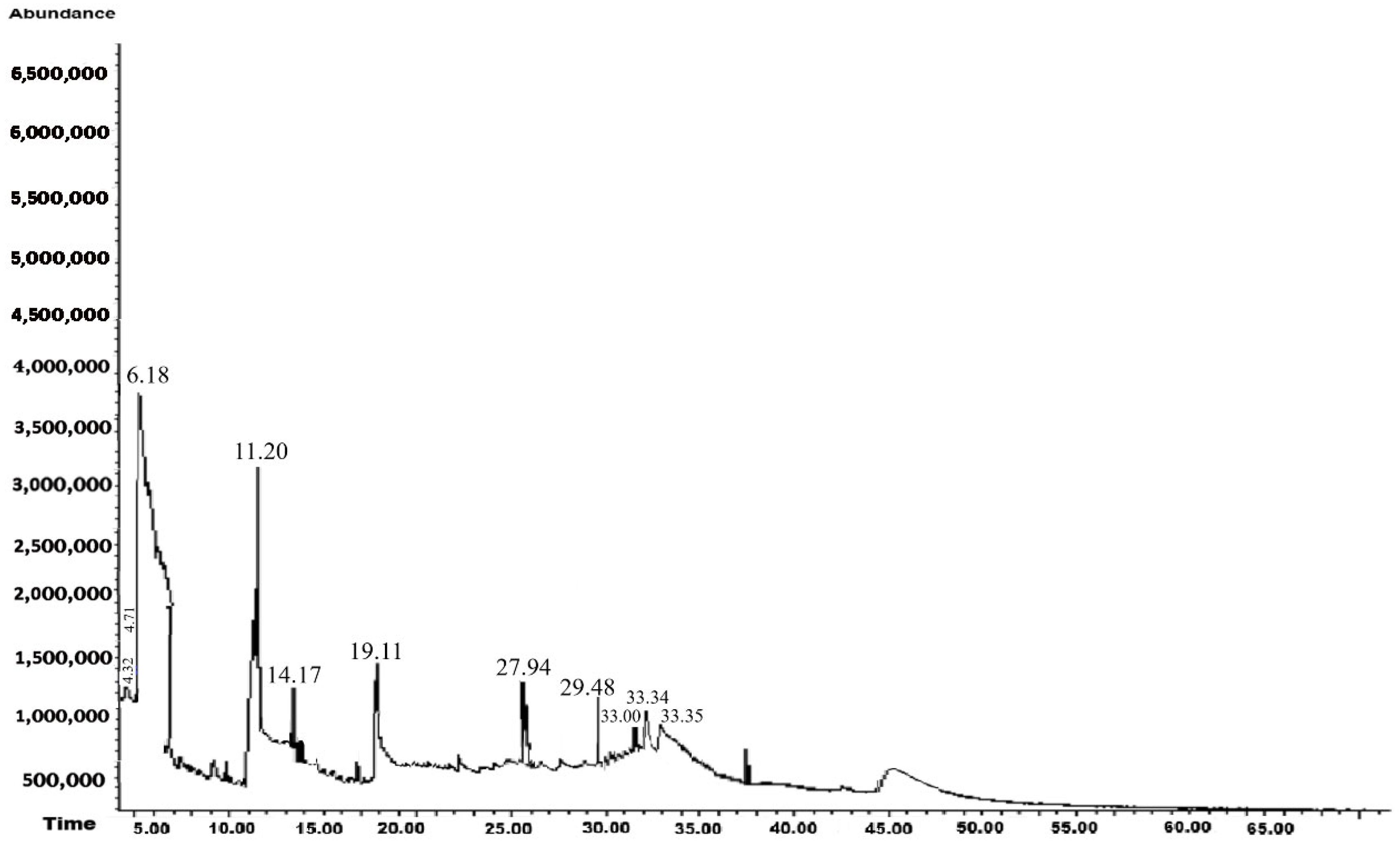
3.1. GC-MS Analysis
3.1.1. Hexanic Extract
3.1.2. Methanolic Extract
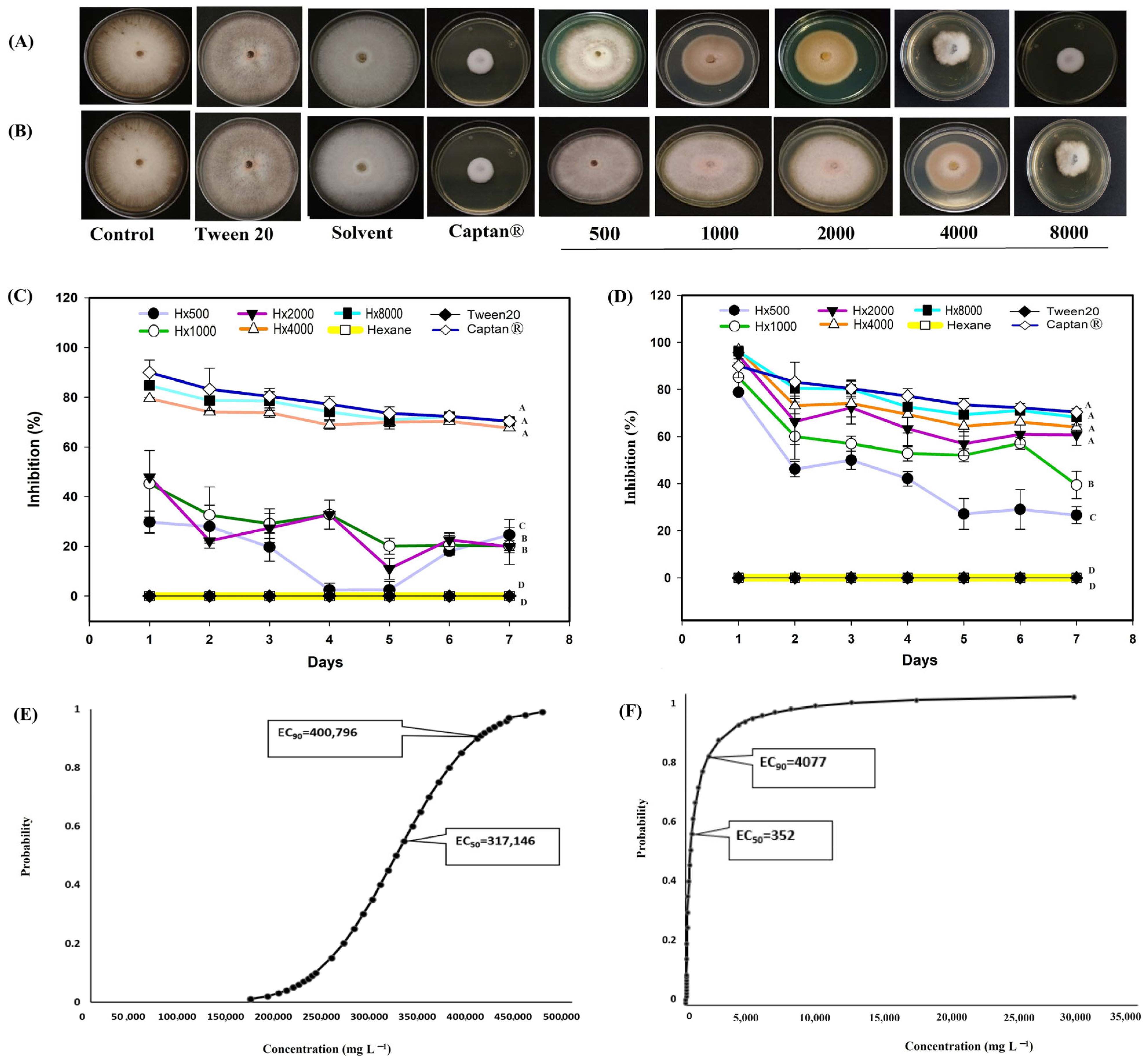
3.2. In Vitro Evaluation of the Extracts Against Phytopathogenic Fungi
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, I. Phytopathogenic fungi and their biocontrol applications. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Academic Press: Cambridge, MA, USA, 2021; Volume 1, pp. 155–188. [Google Scholar] [CrossRef]
- Di Liberto, M.G.; Stegmayer, M.I.; Fernández, L.N.; Quiroga, A.D.; Svetaz, L.A.; Derita, M.G. Control of Brown Rot Produced by Monilinia fructicola in Peaches Using a Full-Spectrum Extract of Zuccagnia punctata Cav. Horticulturae 2023, 9, 1141. [Google Scholar] [CrossRef]
- Kolytaitė, A.; Vaitiekūnaitė, D.; Antanynienė, R.; Baniulis, D.; Frercks, B. Monilinia fructigena suppressing and plant growth promoting endophytic Pseudomonas spp. bacteria isolated from plum. Microorganisms 2022, 10, 2402. [Google Scholar] [CrossRef]
- Peralta-Ruiz, Y.; Rossi, C.; Grande-Tovar, C.D.; Chaves-López, C. Green management of postharvest anthracnose caused by Colletotrichum gloeosporioides. J. Fungi 2023, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Gomdola, D.; Jeewon, R.; McKenzie, E.H.C.; Jayawardena, R.S.; Al-Otibi, F.; Tang, X.; Wang, Y.; Hyde, K.D.; Fu, L. Phylogenetic diversity of Colletotrichum species (Sordariomycetes, Glomerellales, Glomerellaceae) associated with plant diseases in Thailand. MycoKeys 2025, 119, 137–195. [Google Scholar] [CrossRef] [PubMed]
- Bhunjun, C.S.; Jayawardena, R.S.; Wei, D.P.; Huanraluek, N.; Abeywickrama, P.D.; Jeewon, R.; Monkai, J.; Hyde, K.D. Multigene phylogenetic characterisation of Colletotrichum artocarpicola sp. nov. from Artocarpus heterophyllus in northern Thailand. Phytotaxa 2019, 418, 273–286. [Google Scholar] [CrossRef]
- Rodríguez-Velázquez, N.D.; la Cruz, I.G.-D.; López-Guillen, G.; Chávez-Ramírez, B.; Santos, P.E.-D.L. Isolation and biological control of Colletotrichum sp. causing anthracnosis in Theobroma cacao L. in Chiapas, Mexico. J. Fungi 2025, 11, 312. [Google Scholar] [CrossRef]
- Crous, P.; Sandoval-Denis, M.; Costa, M.; Groenewald, J.; van Iperen, A.; Starink-Willemse, M.; Hernández-Restrepo, M.; Kandemir, H.; Ulaszewski, B.; de Boer, W.; et al. Fusarium and allied fusarioid taxa (FUSA). 1. Fungal Syst. Evol. 2022, 9, 161–200. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, C.; Akbar, S.; Wu, S.; Yue, Y.; Wang, G.; Zhou, Y.; Powell, C.A.; Yao, W.; Xu, J.; et al. Characterization and genome analysis of Fusarium oxysporum provides insights into the pathogenic mechanisms of the Pokkah Boeng disease in China. Microorganisms 2025, 13, 573. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Li, W.; Tran, L.S.P.; Mostofa, M.G. Sclerotinia sclerotiorum (Lib.) de Bary: Insights into the pathogenomic features of a global pathogen. Cells 2023, 12, 1063. [Google Scholar] [CrossRef]
- Macioszek, V.K.; Marciniak, P.; Kononowicz, A.K. Impact of Sclerotinia sclerotiorum infection on lettuce (Lactuca sativa L.) survival and phenolics content—A case study in a horticulture farm in Poland. Pathogens 2023, 12, 1416. [Google Scholar] [CrossRef]
- Pandit, M.A.; Kumar, J.; Gulati, S.; Bhandari, N.; Mehta, P.; Katyal, R.; Rawat, C.D.; Mishra, V.; Kaur, J. Major biological control strategies for plant pathogens. Pathogens 2022, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Seepe, H.A.; Nxumalo, W.; Amoo, S.O. Natural products from medicinal plants against phytopathogenic Fusarium species: Current research endeavours, challenges and prospects. Molecules 2021, 26, 6539. [Google Scholar] [CrossRef]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi, 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Soto, I.H.; Méndez, J.P.; Navarrete, A.M.; Montiel, R.G.C.; Alvarado, R.J.; Fuentes, A.D.H. Actividad biológica in vitro del extracto acuoso de Argemone mexicana L. en un hongo fitopatógeno: Sclerotinia sclerotiorum. Boletín Cienc. Agropecu. Del ICAP 2020, 6, 12–14. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Beier, R.C. Natural pesticides and bioactive components in foods. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1990; Volume 113. [Google Scholar] [CrossRef]
- Cenobio-Galindo, A.D.J.; Hernández-Fuentes, A.D.; González-Lemus, U.; Zaldívar-Ortega, A.K.; González-Montiel, L.; Madariaga-Navarrete, A.; Hernández-Soto, I. Biofungicides based on plant extracts: On the road to organic farming. Int. J. Mol. Sci. 2024, 25, 6879. [Google Scholar] [CrossRef]
- Soto, I.H.; Maldonado, A.J.; Montiel, R.G.C.; Álvarez, G.A.; Hernández-Fuentes, A.D. Argemone mexicana contiene metabolitos secundarios que controlan hongos fitopatógenos. Boletín Cienc. Agropecu. Del ICAP 2022, 8, 6–10. [Google Scholar] [CrossRef]
- Singh, S.; Singh, T.D.; Singh, V.P.; Pandey, V.B. Quaternary alkaloids of Argemone mexicana. Pharm. Biol. 2010, 48, 158–160. [Google Scholar] [CrossRef]
- Yaulema, C.S.C.; Molina, W.E.M.; Vilema, W.P.T.; Astudillo, L.J.P.; D’Armas, H. Composición química y actividad antifúngica del látex de Argemone mexicana (Cardo Santo). FACSALUD-UNEMI 2023, 7, 19–36. [Google Scholar] [CrossRef]
- Duan, W.-Y.; Zhu, X.-M.; Zhang, S.-B.; Lv, Y.-Y.; Zhai, H.-C.; Wei, S.; Ma, P.-A.; Hu, Y.-S. Antifungal effects of carvacrol, the main volatile compound in Origanum vulgare L. essential oil, against Aspergillus flavus in postharvest wheat. Int. J. Food Microbiol. 2024, 410, 110514. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Ghanem, A.W.; AbuMadi, S.; Thaher, D.; Jaghama, W.; Karaman, R.; Scrano, L.; Bufo, S.A. Antibacterial activity and antifungal activity of monomeric alkaloids. Toxins 2024, 16, 489. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Ao, C.; Yu, J.; Zhao, Y.; Huang, H. GC-MS combined with proteomic analysis of volatile compounds and formation mechanisms in green teas with different aroma types. Foods 2024, 13, 848. [Google Scholar] [CrossRef]
- De Rodríguez, D.J.; García, R.R.; Castillo, F.H.; González, C.A.; Galindo, A.S.; Quintanilla, J.V.; Zuccolotto, L.M. In vitro antifungal activity of extracts of Mexican Chihuahuan Desert plants against postharvest fruit fungi. Ind. Crops Prod. 2011, 34, 960–966. [Google Scholar] [CrossRef]
- Hernández-Soto, I.; González-García, Y.; Juárez-Maldonado, A.; Hernández-Fuentes, A.D. Impact of Argemone mexicana L. on tomato plants infected with Phytophthora infestans. PeerJ 2024, 12, e16666. [Google Scholar] [CrossRef]
- Ullah, R.; Bakht, J.; Shafi, M.; Shah, M.R. GC-MS profile of bioactive compounds from medicinally important Periploca hydaspidis. Pak. J. Pharm. Sci. 2018, 31, 1967–1973. [Google Scholar]
- Rajeswari, N.R.; RamaLakshmi, S.; Muthuchelian, K. GC-MS analysis of bioactive components from the ethanolic leaf extract of Canthium dicoccum (Gaertn.) Teijsm & Binn. J. Chem. Pharm. Res. 2011, 3, 792–798. [Google Scholar]
- Vásquez-Covarrubias, D.A.; Belmont, R.M.; Pérez, A.J.; Flores Moctezuma, H.E. Essential oils and aqueous extracts for the in vitro management of Fusarium oxysporum f. sp. lycopersici and F. solani. Rev. Mex. Fitopatol. 2013, 31, 170–180. [Google Scholar]
- Hernández-Soto, I.H.; Prieto-Méndez, J.; Aquino-Torres, E.; Pacheco-Trejo, J. Evaluation of the effect of the methanolic extract of Argemone ochroleuca for environmentally friendly control of Colletotrichum gloeosporioides, Fusarium oxysporum and Rhizoctonia solani. Ciência Técnica Vitivinícola 2018, 33, 65–74. [Google Scholar]
- Kobylina, T.; Novikov, A.; Sadyrova, G.; Kyrbassova, E.; Nazarbekova, S.; Imanova, E.; Parmanbekova, M.; Tynybekov, B. The volatile compounds composition of different parts of wild Kazakhstan Sedum ewersii Ledeb. Separations 2024, 11, 208. [Google Scholar] [CrossRef]
- Ilya, E.; Kulikova, L.; Van der Eycken, E.V.; Voskressensky, L. Recent advances in phthalan and coumaran chemistry. ChemistryOpen 2018, 7, 914–929. [Google Scholar] [CrossRef]
- Buttery, R.G.; Takeoka, G.R.; Krammer, G.E.; Ling, L.C. Identification of 2,5-dimethyl-4-hydroxy-3(2H)-furanone (furaneol) and 5-methyl-4-hydroxy-3(2H)-furanone in fresh and processed tomato. LWT Food Sci. Technol. 1994, 27, 592–594. [Google Scholar] [CrossRef]
- Bindu, T.K.; Udayan, P.S. GC-MS analysis of bioactive compounds in methanolic extract of tubers of Pueraria tuberosa. Int. J. Environ. Agric. Biotechnol. 2018, 3, 1493–1498. [Google Scholar] [CrossRef][Green Version]
- Rizvi, S.N.R.; Afzal, S.; Khan, K.-U.; Aati, H.Y.; Rao, H.; Ghalloo, B.A.; Shahzad, M.N.; Khan, D.A.; Esatbeyoglu, T.; Korma, S.A. Chemical characterisation, antidiabetic, antibacterial, and in silico studies for different extracts of Haloxylon stocksii (Boiss.) Benth: A promising halophyte. Molecules 2023, 28, 3847. [Google Scholar] [CrossRef] [PubMed]
- Naga Lakshmi, M.; Pavithra, S.; Ponmozhi, V.; Jaganathan, J.; Karthik, L.; Shree Devi, M.S. Anti-bacterial activity of selected medicinal plants. Malaya J. Biosci. 2015, 2, 153–159. [Google Scholar]
- Ahmad, S.; Zafar, R.; Khan, I.H.; Javaid, A.; Iqbal, M. Control of khapra beetle by leaf extract of Melia azedarach and identification of possible insecticidal compounds through GC-MS analysis. Pak. J. Weed Sci. Res. 2022, 28, 419–426. [Google Scholar]
- Mansour, S.A.A.; Roff, M.; Noor, M.R.M.; Saad, K.A.; Abuzid, I.; Kermani, N. Identification of semiochemicals released by brinjal, tomato, okra and chilli plants infested with whitefly, B. tabaci. Libyan J. Basic Sci. 2015, 2, 25–40. [Google Scholar]
- Akachukwu, D.; Uchegbu, R. Phytochemical, antimicrobial and free radical scavenging activity of Ficus capensis Thunb leaves. J. Complement. Altern. Med. Res. 2016, 1, 29851. [Google Scholar] [CrossRef]
- Minh, T.N.; Xuan, T.D.; Van, T.M.; Andriana, Y.; Viet, T.D.; Khanh, T.D.; Tran, H.D. Phytochemical analysis and potential biological activities of essential oil from rice leaf. Molecules 2019, 24, 546. [Google Scholar] [CrossRef]
- Marzouk, T.; Chaouachi, M.; Sharma, A.; Jallouli, S.; Mhamdi, R.; Kaushik, N.; Djébali, N. Biocontrol of Rhizoctonia solani using volatile organic compounds of solanaceae seed-borne endophytic bacteria. Postharvest Biol. Technol. 2021, 181, 111655. [Google Scholar] [CrossRef]
- Marengo, A.; Maciel, L.S.; Cagliero, C.; Rubiolo, P.; Herodes, K. Free amino acids and biogenic amines profiling and variation in wild and sub-endemic Cardueae species from Sardinia and Corse. Plants 2023, 12, 319. [Google Scholar] [CrossRef]
- Shettima, A.Y.; Karumi, Y.; Sodipo, O.A.; Usman, H.; Tijjani, M.A. Gas chromatography–mass spectrometry (GC-MS) analysis of bioactive components of ethyl acetate root extract of Guiera senegalensis J.F. Gmel. J. Appl. Pharm. Sci. 2013, 3, 146–150. [Google Scholar]
- Gayathri, M.; Ramasamy, M.; Santhiya, N. GC-MS analysis of bioactive compounds and antipathogenic activity in freshwater ampullariidae Pila virens. J. Environ. Life Sci. 2017, 2, 80–84. [Google Scholar]
- Jayashree, G.V.; Rachitha, P.; Krupashree, K.; Kumar, K.H.; Khanum, F. Chemical composition of Asparagus racemosus root by GC–MS analysis. Science, Technol. Arts Res. J. 2015, 4, 124–126. [Google Scholar] [CrossRef]
- Prabhu, V.; Devi, K.V.; Priya, M.K. GC-MS analysis of bioactive compounds present in the petroleum ether, chloroform and methanol extract of Ixora coccinea’s flower and in vitro cytotoxic activity of. Int. J. Res. Anal. Rev. 2018, 5, 801–807. [Google Scholar]
- Mohan, T.; Pandiyan, J.; Chandru, G.; Jayakumar, S.; Elumalai, K.; Vijayakumar, S.; Subasri, K.S.; Krishnappa, K.; Annathurai, M.; Paramanandham, J.P. Phyto-constituents of Manihot esculenta Crantz. (Euphorbiaceae): A novel bio-weapon against human threats ecto-parasitic vectors and lesser environmental risk. Indian J. Sci. Technol. 2023, 16, 4401–4409. [Google Scholar] [CrossRef]
- Khan, A.; More, K.C.; Mali, M.H.; Deore, S.V.; Patil, M.B. Phytochemical screening and gas chromatography–mass spectrometry analysis on Ischaemum pilosum (Klein ex Willd.). Plant Sci. Today 2023, 10, 88–96. [Google Scholar] [CrossRef]
- Omoregie, G.O.; Ovuakporie-Uvo, O.; Idu, M. Phyto-composition and antimicrobial activities of the ethanol seed extracts of Buchholzia coriacea. Afr. J. Pharmacol. Ther. 2018, 7, 1–6. [Google Scholar]
- Kale, M.V. GC-MS analysis of phytocomponents on whole plant extracts Adiantum capillus-veneris L.—A potential folklore medicinal plant. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2015, 2, 116–121. [Google Scholar] [CrossRef]
- Bhardwaj, R. GC-MS analysis and antimicrobial activity of alkaloids of Tecomella undulata. J. Med. Plant Stud. 2018, 6, 68–72. [Google Scholar]
- Shahla, F.; Muthuswami, R. GC-MS analysis of ethanolic leaf extract of Achyranthes aspera Linn. J. Pharmacogn. Phytochem. 2020, 9, 1207–1210. [Google Scholar]
- Afrin, N.S.; Hossain, M.A.; Saha, K. Phytochemical screening of plant extracts and GC-MS analysis of n-hexane soluble part of crude chloroform extract of Cuscuta reflexa (Roxb.). J. Pharmacogn. Phytochem. 2019, 8, 560–564. [Google Scholar]
- Chetia, J.; Saikia, L.R. Phytochemical analysis of Leucas aspera (Willd.) Link. from Dibrugarh. J. Sci. Res. 2020, 64, 96–98. [Google Scholar] [CrossRef]
- Nyaberi, M.O.; Onyango, C.A.; Mathooko, F.M.; Maina, J.M.; Makobe, M. Profiling active phytochemical compounds of Ziziphus abyssinica herb responsible for antioxidant and antimicrobial activity. J. Anim. Plant Sci. 2017, 34, 5413–5424. [Google Scholar]
- Shakiba, Y.; Rezatofighi, S.E.; Nejad, S.M.S.; Ardakani, M.R. Inhibition of foot-and-mouth disease virus replication by hydro-alcoholic and aqueous-acetic acid extracts of Alhagi maurorum: Antiviral activity of Alhagi maurorum against FMDV. Iran. J. Pharm. Sci. 2018, 14, 85–96. [Google Scholar] [CrossRef]
- Lu, J.; Li, N.; Li, S.; Liu, W.; Li, M.; Zhang, M.; Chen, H. Biochemical composition, antioxidant activity and antiproliferative effects of different processed garlic products. Molecules 2023, 28, 804. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Gupta, P.; Pruthi, P.A.; Pruthi, V. Role of exopolysaccharides in biofilm formation. In Introduction to Biofilm Engineering; American Chemical Society: Washington, DC, USA, 2019; pp. 17–57. [Google Scholar] [CrossRef]
- Marei, G.I.K.; Abdel Rasoul, M.A.; Abdelgaleil, S.A.M. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic. Biochem. Physiol. 2012, 103, 56–61. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 1998, 33, 339–347. [Google Scholar] [CrossRef]
- Campos, E.V.; Proença, P.L.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Xu, S.; Gaquerel, E. Evolution of plant specialized metabolites: Beyond ecological drivers. Trends Plant Sci. 2025, 30, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Mendoza, H.; Perlin, M.H.; Schirawski, J. Mitochondrial inheritance in phytopathogenic fungi—Everything is known, or is it? Int. J. Mol. Sci. 2020, 21, 3883. [Google Scholar] [CrossRef]
- Navarro-Espíndola, R.; Suaste-Olmos, F.; Peraza-Reyes, L. Dynamic regulation of peroxisomes and mitochondria during fungal development. J. Fungi 2020, 6, 302. [Google Scholar] [CrossRef]
- Agus, H.H. Terpene toxicity and oxidative stress. In Toxicology; Academic Press: Cambridge, MA, USA, 2021; Volume 6, pp. 33–42. [Google Scholar] [CrossRef]
- Chen, Q.; Ruan, D.; Shi, J.; Du, D.; Bian, C. The multifaceted roles of natural products in mitochondrial dysfunction. Front. Pharmacol. 2023, 14, 1093038. [Google Scholar] [CrossRef]
- Gbadebo, O.S.; Oke, E.D.; Ajibuwa, F.A. Anticancer properties of beta-caryophyllene and d-limonene terpenes: A review. Asian Pac. J. Trop. Biomed. 2025, 15, 129–140. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, X.; Zhao, T.; Zhou, L. Effects of Origanum vulgare essential oil and its two main components, carvacrol and thymol, on the plant pathogen Botrytis cinerea. PeerJ 2020, 8, e9626. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhang, J.; Sun, C.; Chen, X.; Wang, Y.; Lu, H.; Chen, J.; Shi, Z.; Zhang, L.; Yang, L.; et al. Thymol induces cell death of Fusarium oxysporum f. sp. niveum. Agronomy 2023, 13, 189. [Google Scholar] [CrossRef]
- Lu, X.; Hu, K.; Ou, M.; Zhang, X.; Zhan, X.; Liao, X.; Li, M.; Li, R. Synergistic antifungal activity and mechanism of carvacrol/citral against Fusarium oxysporum. Pestic. Biochem. Physiol. 2025, in press. [Google Scholar] [CrossRef]
- Castaño-Duque, L.; Lebar, M.D.; Mack, B.M.; Lohmar, J.M.; Carter-Wientjes, C. Investigating the impact of flavonoids on Aspergillus flavus: Insights into cell wall damage and biofilms. J. Fungi 2024, 10, 665. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future antimicrobials: Natural and functionalized phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Ribera, A.E.; Zuñiga, G. Induced plant secondary metabolites for phytopathogenic fungi control: A review. J. Soil Sci. Plant Nutr. 2012, 12, 893–911. [Google Scholar] [CrossRef]
- Hollomon, D.W. Fungicide resistance: 40 years on and still a major problem. In Fungicide Resistance in Plant Pathogens; Ishii, H., Hollomon, D., Eds.; Springer: Tokyo, Japan, 2015. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, T.; Liang, Z.; Hao, J.; Liu, P.; Liu, X. Dynamic changes in plant secondary metabolites induced by Botrytis cinerea infection. Metabolites 2023, 13, 654. [Google Scholar] [CrossRef]
- Xu, D.; Xue, M.; Shen, Z.; Jia, X.; Hou, X.; Lai, D.; Zhou, L. Phytotoxic secondary metabolites from fungi. Toxins 2021, 13, 261. [Google Scholar] [CrossRef]
- Priyashantha, A.K.H.; Dai, D.-Q.; Bhat, D.J.; Stephenson, S.L.; Promputtha, I.; Kaushik, P.; Tibpromma, S.; Karunarathna, S.C. Plant–fungi interactions: Where it goes? Biology 2023, 12, 809. [Google Scholar] [CrossRef]
- Spitzer, M.; Robbins, N.; Wright, G.D. Combinatorial strategies for combating invasive fungal infections. Virulence 2017, 8, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Ojima, Y.; Yokota, N.; Tanibata, Y.; Nerome, S.; Azuma, M. Concentrative nucleoside transporter, CNT, results in selective toxicity of toyocamycin against Candida albicans. Microbiol. Spectr. 2022, 10, e0113822. [Google Scholar] [CrossRef]
- Schneider-Poetsch, T.; Ju, J.; E Eyler, D.; Dang, Y.; Bhat, S.; Merrick, W.C.; Green, R.; Shen, B.; Liu, J.O. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat. Chem. Biol. 2010, 6, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Garreau de Loubresse, N.; Prokhorova, I.; Holtkamp, W.; Rodnina, M.V.; Yusupova, G.; Yusupov, M. Structural basis for the inhibition of the eukaryotic ribosome. Nature 2014, 513, 517–522. [Google Scholar] [CrossRef]
- Burgess-Beusse, B.; Farrell, C.; Gaszner, M.; Litt, M.; Mutskov, V.; Recillas-Targa, F.; Simpson, M.; West, A.; Felsenfeld, G. The insulation of genes from external enhancers and silencing chromatin. Proc. Natl. Acad. Sci. 2002, 99, 16433–16437. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Lu, A.; Xue, W. Synthesis and biological activity of novel oxazinyl flavonoids as antiviral and anti-phytopathogenic fungus agents. Molecules 2022, 27, 6875. [Google Scholar] [CrossRef]
- Mitidieri, M.S.; Barbieri, M.O.; Brambilla, M.V.; Piris, E. In vitro effect of lemon essential oil and garlic extract on Monilinia fructicola growth. Agrociencia Urug. 2021, 26, e403. [Google Scholar] [CrossRef]
- Álvarez-García, S.; Moumni, M.; Romanazzi, G. Antifungal activity of volatile organic compounds from essential oils against the postharvest pathogens Botrytis cinerea, Monilinia fructicola, Monilinia fructigena, and Monilinia laxa. Front. Plant Sci. 2023, 14, 1274770. [Google Scholar] [CrossRef]
- FeFeng, G.; Zhang, X.-S.; Zhang, Z.-K.; Ye, H.-C.; Liu, Y.-Q.; Yang, G.-Z.; Chen, C.; Chen, M.; Yan, C.; Wang, L.-Y.; et al. Fungicidal activities of camptothecin semisynthetic derivatives against Colletotrichum gloeosporioides in vitro and in mango fruit. Postharvest Biol. Technol. 2019, 147, 139–147. [Google Scholar] [CrossRef]
- Jeewon, R.; Pudaruth, S.B.; Bhoyroo, V.; Aullybux, A.A.; Rajeshkumar, K.C.; Alrefaei, A.F. Antioxidant and antifungal properties of cinnamon, cloves, Melia azedarach L. and Ocimum gratissimum L. extracts against Fusarium oxysporum isolated from Infected Vegetables in Mauritius. Pathogens 2024, 13, 436. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Cakir, A.; Akcin, T.A.; Mete, E.; Akcin, A.; Aydin, T.; Kilic, H. Antifungal and herbicidal properties of essential oils and n-hexane extracts of Achillea gypsicola Hub-Mor. and Achillea biebersteinii Afan. (Asteraceae). Ind. Crops Prod. 2009, 29, 562–570. [Google Scholar] [CrossRef]
- Saha, S.; Walia, S.; Kundu, A.; Kumar, B.; Joshi, D. Antifungal acetylenic thiophenes from Tagetes minuta: Potential biopesticide. J. Appl. Bot. Food Qual. 2013, 85, 207. [Google Scholar]
- Pansera, M.R.; Pauletti, M.; Fedrigo, C.P.; Sartori, V.C.; Ribeiro, R.T.d.S. Utilization of essential oil and vegetable extracts of Salvia officinalis L. in the control of rot sclerotinia in lettuce. Appl. Res. Agrotechnol. 2013, 6, 83–88. [Google Scholar]
- Tortelli, B.; Cappellaro, S.; Nava, F.F.d.M.; Tonial, F.; Huzar-Novakowiski, J.; Milanesi, P.M.; Chiomento, J.L.T. Bioactivity of an extract from the endophyte Diaporthe infecunda on soybean seeds inoculated with Colletotrichum truncatum and Sclerotinia sclerotiorum. Eur. J. Plant Pathol. 2023, 166, 509–520. [Google Scholar] [CrossRef]
- Onaran, A.; Yanar, Y. In vivo and in vitro antifungal activities of five plant extracts against various plant pathogens. Egypt. J. Biol. Pest Control. 2016, 26, 405. [Google Scholar]
- Mihajlović, M.; Rekanović, E.; Hrustić, J.; Grahovac, M.; Tanović, B. In vitro and in vivo toxicity of fungicides and biofungicides. Pestic. Fitomedicina 2021, 36, 23–33. [Google Scholar] [CrossRef]
- Chandini, A.; Kumar, R.; Sinha, B. Testing the efficacy of different fungicides against Colletotrichum capsici under in vitro. Asian J. Agric. Biol. Res. 2022, 2, 101–107. [Google Scholar]
- Zhang, Y.Z.; Li, Y.; Pi, Y.; Cheng, Y. FgCWM1 encodes a cell wall mannoprotein contributing to cell wall integrity and pathogenicity in Fusarium graminearum. Toxins 2019, 11, 628. [Google Scholar] [CrossRef]
- Yoshimi, A.; Miyazawa, K.; Kawauchi, M.; Abe, K. Cell wall integrity and its industrial applications in filamentous fungi. J. Fungi 2022, 8, 435. [Google Scholar] [CrossRef]
- Jiao, W.; Li, M.; Lei, T.; Liu, X.; Zhang, J.; Hu, J.; Zhang, X.; Liu, J.; Shi, S.; Pan, H.; et al. The APSES transcription factor SsStuA regulating cell wall integrity is essential for sclerotia formation and pathogenicity in Sclerotinia sclerotiorum. J. Fungi 2024, 10, 238. [Google Scholar] [CrossRef] [PubMed]
| RT (min) | Name of the Compound | Molecular Formula | MW | Area (%) | Previous Reports | Reference |
|---|---|---|---|---|---|---|
| 4.31 | Butanoic acid, 4-hydroxy- | C4H8O3 | 104 | 4.6 | Suppressor of the central nervous system | [31] |
| 5.13 | Phthalan | C8H8O | 120 | 6.8 | Antidepressant, antioxidant, antifungal, antibacterial, antitumor, and anti-inflammatory properties | [32] |
| 5.20 | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone | C6H8O3 | 128 | 5.8 | Aromatic compound that contributes to the flavor of tomatoes | [33] |
| 5.38 | 2-Hydroxy-gamma-butyrolactone | C4H6O3 | 102 | 5.5 | Antithyroid and antiviral activity | [34] |
| 6.39 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144 | 11.6 | Antioxidant, antibacterial, and antidiabetic activity | [35] |
| 7.39 | 2-Propanamine, N-methyl-N-nitroso- | C4H10N2O | 102 | 2.2 | Antibacterial activity against E. coli | [36] |
| 9.06 | 1-(3,6,6-Trimethyl-1,6,7,7a-tetrahydrocyclopenta[c]pyran-1-yl)ethanone | C13H18O2 | 206 | 2.0 | Insecticidal activity | [37] |
| 11.00 | 2,4,6-Tris(1,1-dimethylethyl)-4-methylcyclohexa-2,5-dien-1-one | C19H32O | 276 | 4.7 | Insecticidal activity | [38] |
| 11.09 | 4-(2,6,6-Trimethylcyclohexa-1,3-dienyl)but-3-en-2-one | C13H18O | 190 | 13.1 | Antifungal, antibacterial, antioxidant, anticancer, antispasmodic, hypotensive, and vasorelaxant activity | [39] |
| 13.09 | Megastigmatrienone | C13H18O | 190 | 11.2 | Herbicidal activity against Echinochloa crus-galli and Bidens pilosa and is a biostimulant in rice (Oryza sativa L.) cultivation | [40] |
| 32.51 | N-Methyl-2-isopropoxycarbonylazetidine | C8H15NO2 | 157 | 1.2 | ||
| 33.82 | 1,2,4-Triazol-4-amine, N-(2-thienylmethyl)- | C7H8N4S | 180 | 29.3 | Antifungal activity against Rhizoctonia solani | [41] |
| 41.79 | Methanesulfinyl fluoride, trifluoro- | CF4OS | 136 | 0.6 | Reported for the first time | |
| 44.01 | dl-Methionine, N-[(4-methylphenyl)sulfonyl]- | C12H17NO4S2 | 303 | 1.5 | Reported for the first time |
| RT (min) | Name of the Compound | Molecular Formula | MW | Area (%) | Previous Reports | Reference |
|---|---|---|---|---|---|---|
| 4.32 | Methylamine | CH5N | 31 | 3.37 | Detected in Mediterranean plant species | [42] |
| 4.71 | Decane, 6-ethyl-2-methyl- | C13H28 | 184 | 1.13 | Industrial use as a solvent for varnishes, paints, and inks | [43] |
| 6.18 | Benzene, 1,3-bis(1,1-dimethylethyl)- | C14H22 | 190 | 31.35 | Antibacterial and antifungal activity and reports of lipid oxidation activity has been detected in plant extracts of species such as Asparagus racemosus Willd and Ixora coccinea L. | [44,45,46] |
| 11.20 | Pentanoic acid, 5-hydroxy-, 2,4-di-t-butylphenyl esters | C19H30O3 | 306 | 17.15 | Carboxylic acid with larvicidal, antifungal, flavoring, immunostimulant, antidepressant, and hypocholesterolemic properties | [47,48,49] |
| 14.17 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 296 | 10.94 | Antibacterial, antifungal and pharmacological activity | [50,51,52] |
| 19.11 | 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | C17H24O3 | 276 | 13.43 | Medicinal, antifungal, and antibacterial activity | [53,54] |
| 27.94 | Phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-ethyl- | C25H36O2 | 368 | 10.51 | Antioxidant and antiviral activity | [55,56] |
| 29.48 | 10-Nonadecanol | C19H40O | 284 | 7.55 | Antioxidant activity and is present in the hexanic extract of garlic (Allium sativum L.) | [57] |
| 33.00 | 2-Octen-4-one, 2-methyl- | C9H16O | 140 | 1.55 | Reported for the first time | |
| 33.34 | 2-Benzyl-8-methyl-1H-piperidino[4,3-b]indole | C19H20N2 | 276 | 1.88 | Reported for the first time | |
| 33.45 | Isoxazolo[4,3-a]phenazine, 1-phenyl- | C19H11N3O | 297 | 1.14 | Reported for the first time |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Soto, I.; Juárez-Maldonado, A.; Madariaga-Navarrete, A.; Sharma, A.; Cenobio-Galindo, A.d.J.; Pinedo-Espinoza, J.M.; Hernández-Pérez, A.; Hernández-Fuentes, A.D. Extracts of Argemone mexicana L. Contain Antifungal Compounds for the In Vitro Control of Monilinia fructicola, Colletotrichum gloeosporioides, Fusarium oxysporum, and Sclerotinia sclerotiorum: Preliminary Evidence for Field Application. BioTech 2025, 14, 82. https://doi.org/10.3390/biotech14040082
Hernández-Soto I, Juárez-Maldonado A, Madariaga-Navarrete A, Sharma A, Cenobio-Galindo AdJ, Pinedo-Espinoza JM, Hernández-Pérez A, Hernández-Fuentes AD. Extracts of Argemone mexicana L. Contain Antifungal Compounds for the In Vitro Control of Monilinia fructicola, Colletotrichum gloeosporioides, Fusarium oxysporum, and Sclerotinia sclerotiorum: Preliminary Evidence for Field Application. BioTech. 2025; 14(4):82. https://doi.org/10.3390/biotech14040082
Chicago/Turabian StyleHernández-Soto, Iridiam, Antonio Juárez-Maldonado, Alfredo Madariaga-Navarrete, Ashutosh Sharma, Antonio de Jesus Cenobio-Galindo, Jose Manuel Pinedo-Espinoza, Aracely Hernández-Pérez, and Alma Delia Hernández-Fuentes. 2025. "Extracts of Argemone mexicana L. Contain Antifungal Compounds for the In Vitro Control of Monilinia fructicola, Colletotrichum gloeosporioides, Fusarium oxysporum, and Sclerotinia sclerotiorum: Preliminary Evidence for Field Application" BioTech 14, no. 4: 82. https://doi.org/10.3390/biotech14040082
APA StyleHernández-Soto, I., Juárez-Maldonado, A., Madariaga-Navarrete, A., Sharma, A., Cenobio-Galindo, A. d. J., Pinedo-Espinoza, J. M., Hernández-Pérez, A., & Hernández-Fuentes, A. D. (2025). Extracts of Argemone mexicana L. Contain Antifungal Compounds for the In Vitro Control of Monilinia fructicola, Colletotrichum gloeosporioides, Fusarium oxysporum, and Sclerotinia sclerotiorum: Preliminary Evidence for Field Application. BioTech, 14(4), 82. https://doi.org/10.3390/biotech14040082








