A Simple and Safe Protocol for Intra-Testicular Gene Delivery in Neonatal Mice Using a Convenient Isoflurane-Based Anesthesia System
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
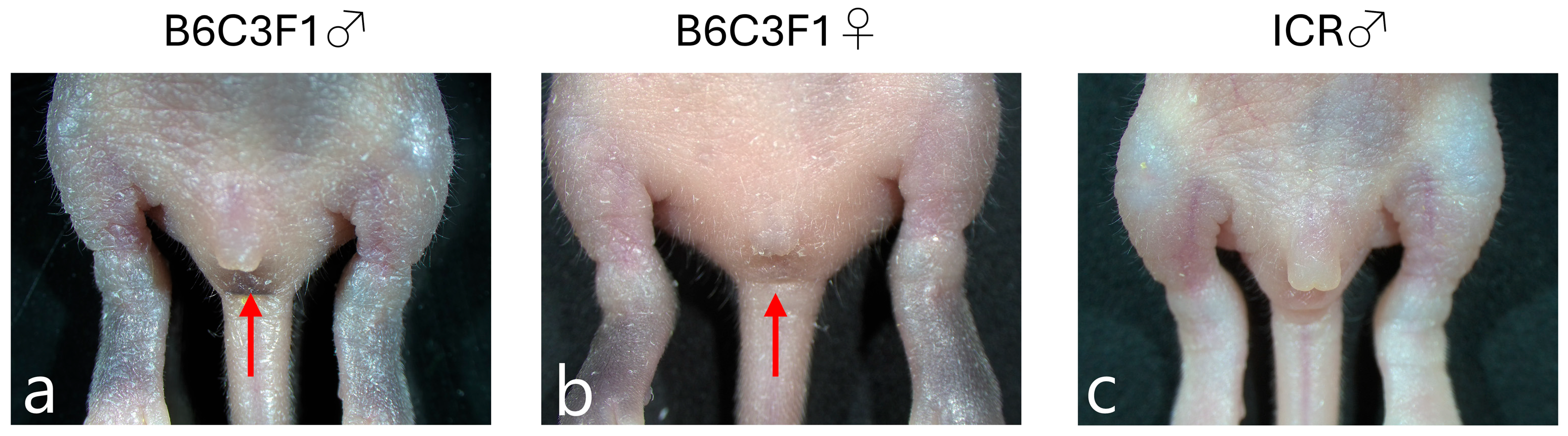
2.2. Sex Determination of Neonatal Mice
2.3. Plasmid DNA
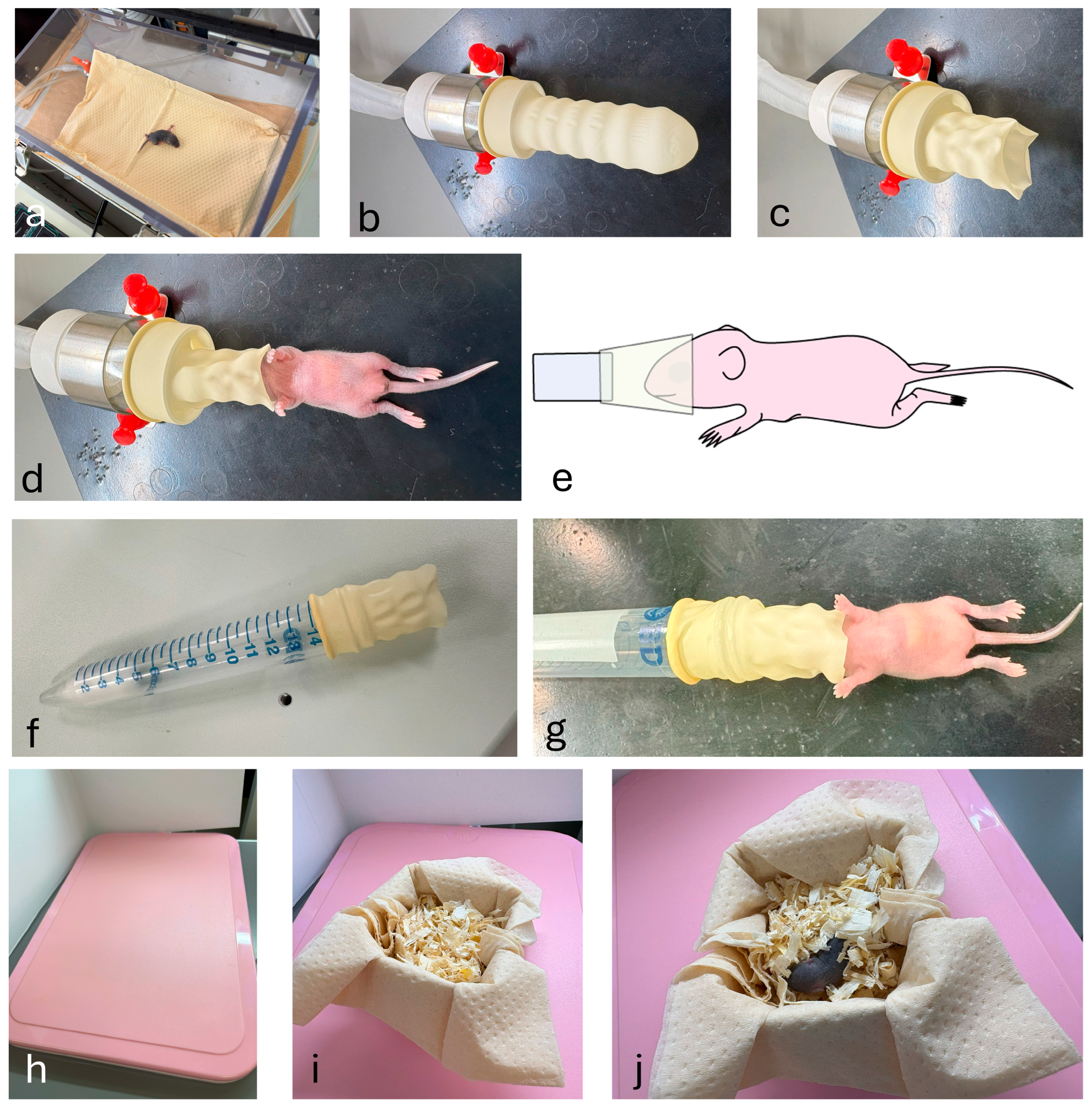
2.4. Convenient Inhalation Anesthesia System for Neonatal Mice
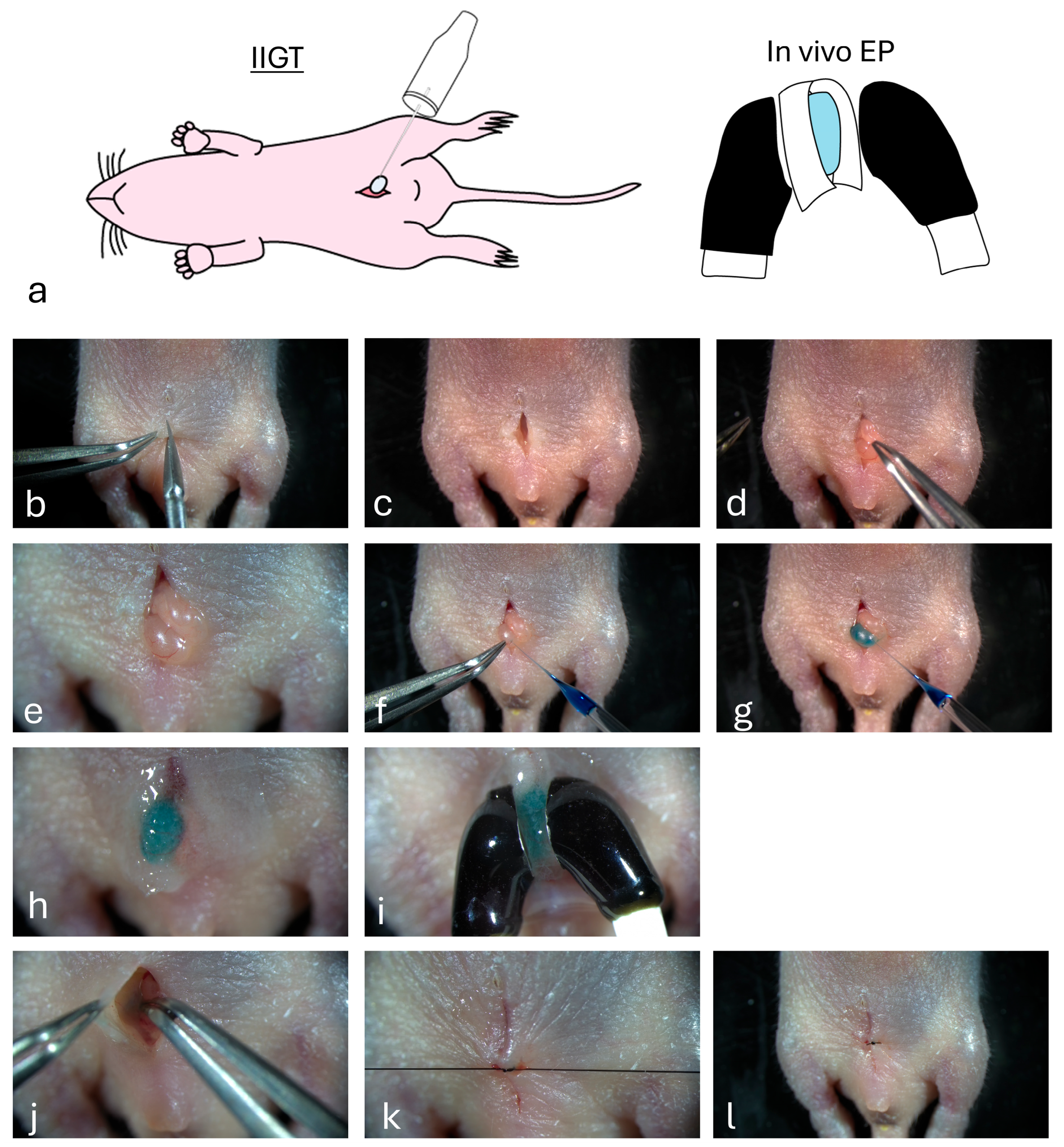
2.5. Intratesticular Injection-Based Gene Transfer (IIGT)
2.6. Recovery
2.7. Fluorescence Microscopy
2.8. Statistical Analysis
3. Results
3.1. Establishment of a Stable Inhalation Anesthesia System for Neonatal Mice and a High Survival Rate After Surgery
3.2. Gene Transfer in the Neonatal Testis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishizono, H.; Yasuda, R.; Laviv, T. Methodologies and Challenges for CRISPR/Cas9 Mediated Genome Editing of the Mammalian Brain. Front. Genome Ed. 2020, 2, 602970. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Shivalila, C.S.; Cheng, A.W.; Shi, L.; Jaenisch, R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell 2013, 154, 1370–1379. [Google Scholar] [CrossRef]
- Mashiko, D.; Fujihara, Y.; Satouh, Y.; Miyata, H.; Isotani, A.; Ikawa, M. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci. Rep. 2013, 3, 3355. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Sakuma, T.; Yamamoto, T.; Mashimo, T. Simple knockout by electroporation of engineered endonucleases into intact rat embryos. Sci. Rep. 2014, 4, 6382. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, M.; Sato, M.; Miura, H.; Takabayashi, S.; Matsuyama, M.; Koyano, T.; Arifin, N.; Nakamura, S.; Wada, K.; Gurumurthy, C.B. i-GONAD: A robust method for in situ germline genome engineering using CRISPR nucleases. Genome Biol. 2018, 19, 25. [Google Scholar] [CrossRef]
- Takabayashi, S.; Aoshima, T.; Kabashima, K.; Aoto, K.; Ohtsuka, M.; Sato, M. i-GONAD (improved genome-editing via oviductal nucleic acids delivery), a convenient in vivo tool to produce genome-edited rats. Sci. Rep. 2018, 8, 12059. [Google Scholar] [CrossRef]
- Sato, T.; Sakuma, T.; Yokonishi, T.; Katagiri, K.; Kamimura, S.; Ogonuki, N.; Ogura, A.; Yamamoto, T.; Ogawa, T. Genome Editing in Mouse Spermatogonial Stem Cell Lines Using TALEN and Double-Nicking CRISPR/Cas9. Stem Cell Rep. 2015, 5, 75–82. [Google Scholar] [CrossRef]
- Moradbeigi, P.; Hosseini, S.; Salehi, M.; Mogheiseh, A. Methyl β-Cyclodextrin-sperm-mediated gene editing (MBCD-SMGE): A simple and efficient method for targeted mutant mouse production. Biol. Proced. Online 2024, 26, 3. [Google Scholar]
- Nakamura, S.; Morohoshi, K.; Inada, E.; Sato, Y.; Watanabe, S.; Saitoh, I.; Sato, M. Recent Advances in In Vivo Somatic Cell Gene Modification in Newborn Pups. Int. J. Mol. Sci. 2023, 24, 15301. [Google Scholar] [CrossRef]
- Herrmann, K.; Flecknell, P. Retrospective review of anesthetic and analgesic regimens used in animal research proposals. Altex 2019, 36, 65–80. [Google Scholar] [CrossRef]
- Frei, C.; Darocha, T.; Debaty, G.; Dami, F.; Blancher, M.; Carron, P.N.; Oddo, M.; Pasquier, M. Clinical characteristics and outcomes of witnessed hypothermic cardiac arrest: A systematic review on rescue collapse. Resuscitation 2019, 137, 41–48. [Google Scholar] [CrossRef]
- Ho, H.; Fowle, A.; Coetzee, M.; Greger, I.H.; Watson, J.F. An inhalation anaesthesia approach for neonatal mice allowing streamlined stereotactic injection in the brain. J. Neurosci. Methods 2020, 342, 108824. [Google Scholar] [CrossRef]
- Maloney, S.E.; Yuede, C.M.; Creeley, C.E.; Williams, S.L.; Huffman, J.N.; Taylor, G.T.; Noguchi, K.N.; Wozniak, D.F. Repeated neonatal isoflurane exposures in the mouse induce apoptotic degenerative changes in the brain and relatively mild long-term behavioral deficits. Sci. Rep. 2019, 9, 2779. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Fan, Z.; Hu, J.; Zhu, Y.; Lin, C.; Shen, T.; Li, Z.; Li, K.; Liu, Z.; Chen, Y.; et al. The differential effects of isoflurane and sevoflurane on neonatal mice. Sci. Rep. 2020, 10, 19345. [Google Scholar] [CrossRef]
- Xu, D.; Liu, J.; Meng, S.; Sun, M.; Chen, Y.; Hong, Y. Isoflurane-induced neuroinflammation and NKCC1/KCC2 dysregulation result in long-term cognitive disorder in neonatal mice. BMC Anesthesiol. 2024, 24, 200. [Google Scholar] [CrossRef]
- Niwa, H.; Yamamura, K.; Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991, 108, 193–199. [Google Scholar] [CrossRef]
- Nakamura, S.; Sato, M. Possible Production of Genome-Edited Animals Using Gene-Engineered Sperm. In Gene Editing-Technologies and Applications; Chen, Y.-C., Chen, S.-J., Eds.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Gotoh, H.; Matsumoto, Y.; Imamura, K. General anesthesia of infant mice by isoflurane inhalation for medium-duration surgery. Exp. Anim. 2004, 53, 63–65. [Google Scholar] [CrossRef][Green Version]
- Ju, H.-m.; Bai, L.-j.; Ren, H.-y.; Mu, Y.-l.; Yang, S.-l.; Li, K. Production of Transgenic Mice by Type-A Spermatogonia-Mediated Gene Transfer. Agric. Sci. China 2011, 10, 431–437. [Google Scholar] [CrossRef]
- Iketani, M.; Hatomi, M.; Fujita, Y.; Watanabe, N.; Ito, M.; Kawaguchi, H.; Ohsawa, I. Inhalation of hydrogen gas mitigates sevoflurane-induced neuronal apoptosis in the neonatal cortex and is associated with changes in protein phosphorylation. J. Neurochem. 2024, 168, 2775–2790. [Google Scholar] [CrossRef] [PubMed]
- Wali, B.; Sayeed, I.; Stein, D.G.; Raper, J. Prophylactic progesterone prevents adverse behavioural and neurocognitive effects of neonatal anaesthesia exposure in rat. Br. J. Anaesth. 2022, 128, 301–310. [Google Scholar] [CrossRef]
- Oatley, J.M.; Brinster, R.L. Regulation of spermatogonial stem cell self-renewal in mammals. Annu. Rev. Cell Dev. Biol. 2008, 24, 263–286. [Google Scholar] [CrossRef] [PubMed]
- Dhup, S.; Majumdar, S.S. Transgenesis via permanent integration of genes in repopulating spermatogonial cells in vivo. Nat. Methods 2008, 5, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Usmani, A.; Ganguli, N.; Sarkar, H.; Dhup, S.; Batta, S.R.; Vimal, M.; Ganguli, N.; Basu, S.; Nagarajan, P.; Majumdar, S.S. A non-surgical approach for male germ cell mediated gene transmission through transgenesis. Sci. Rep. 2013, 3, 3430. [Google Scholar] [CrossRef]
- Pramod, R.K.; Mitra, A. Intratesticular injection followed by electroporation allows gene transfer in caprine spermatogenic cells. Sci. Rep. 2018, 8, 3169. [Google Scholar] [CrossRef]
- Takehashi, M.; Kanatsu-Shinohara, M.; Inoue, K.; Ogonuki, N.; Miki, H.; Toyokuni, S.; Ogura, A.; Shinohara, T. Adenovirus-mediated gene delivery into mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 2596–2601. [Google Scholar] [CrossRef]
- Michaelis, M.; Sobczak, A.; Weitzel, J.M. In vivo microinjection and electroporation of mouse testis. J. Vis. Exp. 2014, 90, 51802. [Google Scholar]
- Wang, S.; Duan, Y.; Chen, B.; Qiu, S.; Huang, T.; Si, W. Generation of Transgenic Sperm Expressing GFP by Lentivirus Transduction of Spermatogonial Stem Cells In Vivo in Cynomolgus Monkeys. Vet. Sci. 2023, 10, 104. [Google Scholar] [CrossRef] [PubMed]

| No. of Experiment | Total No. of Newborns Subjected to IIGT | No. Pups Died or Killed By a Mother | No. Pups Survived 1 |
|---|---|---|---|
| 1 | 9 | 1 2 | 8 |
| 2 | 13 | 1 2 | 12 |
| 3 | 10 | 0 | 10 |
| 4 | 7 | 0 | 7 |
| 5 | 6 | 1 | 5 |
| total | 45 | 3 1 | 42 |
| success rate | 93% (95% CI: 83.0–98.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morohoshi, K.; Ohba, M.; Sato, M.; Nakamura, S. A Simple and Safe Protocol for Intra-Testicular Gene Delivery in Neonatal Mice Using a Convenient Isoflurane-Based Anesthesia System. BioTech 2025, 14, 81. https://doi.org/10.3390/biotech14040081
Morohoshi K, Ohba M, Sato M, Nakamura S. A Simple and Safe Protocol for Intra-Testicular Gene Delivery in Neonatal Mice Using a Convenient Isoflurane-Based Anesthesia System. BioTech. 2025; 14(4):81. https://doi.org/10.3390/biotech14040081
Chicago/Turabian StyleMorohoshi, Kazunori, Miho Ohba, Masahiro Sato, and Shingo Nakamura. 2025. "A Simple and Safe Protocol for Intra-Testicular Gene Delivery in Neonatal Mice Using a Convenient Isoflurane-Based Anesthesia System" BioTech 14, no. 4: 81. https://doi.org/10.3390/biotech14040081
APA StyleMorohoshi, K., Ohba, M., Sato, M., & Nakamura, S. (2025). A Simple and Safe Protocol for Intra-Testicular Gene Delivery in Neonatal Mice Using a Convenient Isoflurane-Based Anesthesia System. BioTech, 14(4), 81. https://doi.org/10.3390/biotech14040081






