Bacillus Pectinases as Key Biocatalysts for a Circular Bioeconomy: From Green Extraction to Process Optimization and Industrial Scale-Up
Abstract
1. Introduction
2. Review Methodology
3. Pectic Substances
3.1. Structural Characteristics and Classification
3.2. Physiological Roles and Industrial Relevance
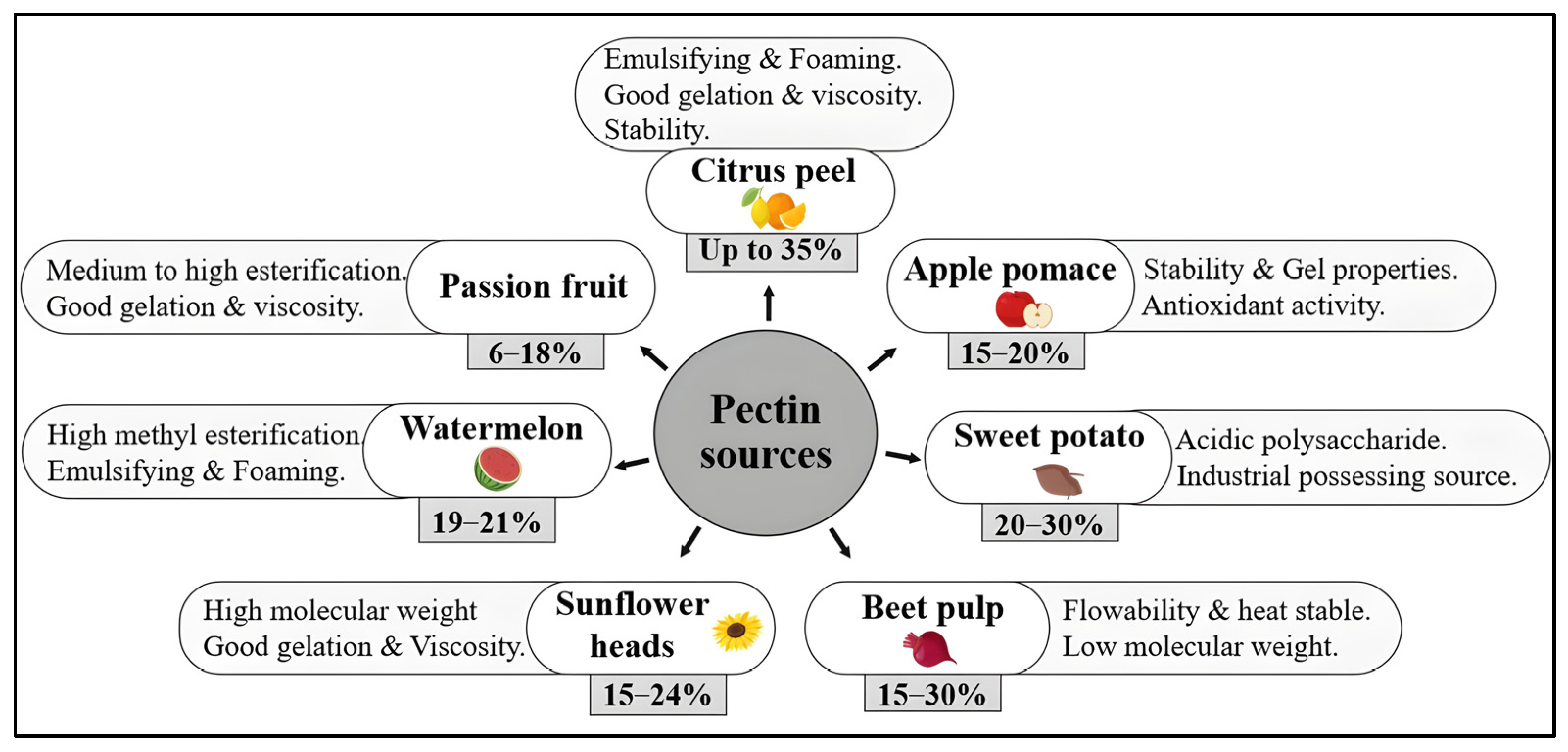
3.3. Sources and Eco-Friendly Extraction
Green Extraction Techniques
- Subcritical Water Extraction (SWE), pressurized hot water acts as a tunable solvent, allowing acid-free extraction with high yields and reduced hazardous effluents [29].
- Enzyme-Assisted Extraction (EAE), application of pectinases and complementary hydrolases under mild conditions selectively solubilizes protopectin, lowering solvent requirements and preserving native structure [30].
| Sources | Extraction Methods | DM% | DE% | Yield% | Ref. |
|---|---|---|---|---|---|
| Pomelo peels | Hot acid extraction | - | 55.67 | 15.36 | [34] |
| Microwave extraction | 55.34 | 20.43 | |||
| Ultrasound extraction | 51.42 | 17.21 | |||
| Enzyme-assisted extraction | 47.71 | 11.94 | |||
| Apple pomace | Hot acid extraction using HCl | 72.02 | 63.80 | 14 | [35] |
| Citric acid extraction | 64.05 | 63.42 | 22 | ||
| Organic acid mixture extraction | 70.25 | 64.55 | 14 | ||
| Microwave extraction | 77.0 | 64.80 | 17.6 | ||
| Ultrasound extraction | 76.75 | 64.18 | 16 | ||
| Orange Peel Waste | Extraction using HCl | - | 59.37 | 18.73 | [36] |
| Lemon peels | Extraction using HCl | - | 82.7 | 13.0 | [37] |
| Sweet lime | Hydrothermal extraction | - | 71.2 | 23.8 | [38] |
| Banana peel | Extraction using HCl | 5.84 | 27.63 | 41.84 | [39] |
| Extraction using citric acid | 11.52 | 50.27 | 59.57 | ||
| Maleic acid extraction | 10.25 | 44.88 | 56.45 | ||
| Watermelon Rind | Citric acid extraction | 24.30 | 73.3 | - | [40] |
3.4. Applications of Pectins
4. Overview of Pectinases
4.1. Classification of Pectinases
4.1.1. Optimum pH
- Acidic pectinases (pH 3–5): Predominantly fungal enzymes, endo-polygalacturonases and pectin lyases, employed for juice extraction, wine clarification, and protoplast isolation.
- Alkaline pectinases (pH 8–11): Largely bacterial enzymes (e.g., pectate lyases from Bacillus spp.), used in textile degumming, pulp bio-bleaching, and alkaline wastewater treatment.
4.1.2. Catalytic Mechanisms of Pectinases
4.1.3. Cellular Localization
4.2. Fermentation Strategies for Pectinase Production
| Microorganism | Substrate | Fermentation | Pectinase Activity | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Type | Time | T °C | pH | Agitation | ||||
| B. amyloliquefaciens TKU050 | Wheat bran | SSF | 4 days | 37 | 6.0 | 100 rpm | 0.76 U/mL | [48] |
| B. amyloliquefaciens SL9 | Pectin | SmF | 24 h | 37 | 7.0 | 150 rpm | 9.8 U/mL | [23] |
| B. subtilis NRRL B-4219 | Hazelnut shell hydrolyzate | SmF | 72 h | 30 | 7.0 | 130 rpm | 5.60 U/mL | [52] |
| B. pumilus NRRL B-212 | Pectin | SmF | 64 h | 30 | 8.0 | 150 rpm | 16.17 U/mL | [49] |
| Sugar beet pulp | SSF | 48 h | 30 | 8.0 | 150 rpm | 147.75 U/mL | ||
| B. mojavensis I4 | Carrot peels | SSF | 32 h | 37.5 | 8.0 | 150 rpm | 64.8 U/mL | [53] |
| B. tequilensis CAS-MEI-2-33 | Pectin | SmF | 40 h | 40 | 10.0 | 180 rpm | 1370 U/mL | [54] |
| B. tropicus MCCC1A01406 | Pectin | SmF | 72 h | 37 | 9.0 | - | 43 U/mL | [55] |
| B. amyloliquefaciens ADI2 | Banana peel | SSF | 48 h | 28 | 8.38 | 94 rpm | 2043.86 U/mL | [46] |
| B. subtilis strain Btk 27 | Apple pectin | SmF | 48 h | 37 | 6.5 | 120 rpm | 66.3 U/mL | [43] |
| B. subtilis MF447840.1 | Pectin | SmF | 4 days | 37 | 7.4 | 120 rpm | 345 ± 12.3 (U/mL) | [56] |
| B. subtilis PSE-8 | Cassava peel | SSF | 3 days | 45 | 9 | 100 rpm | 117.5 (U/mL) | [57] |
| B. cereus | Pectin | SmF | 24 h | 35 | 10.5 | 150 rpm | 3.37 (U/mL) | [58] |
| B. licheniformis | Orange peel | SSF | 120 h | 37 | 9.5 | - | 219 (U/mL) | [59] |
| B. subtilis ZGL14 | Pectin | SmF | 72 h | 40 | 8.0 | 200 rpm | 734.11 (U/mL) | [60] |
| Bacillus sp. Y1 | Wheat bran | SmF | 72 h | 37 | 8.2 | 100 rpm | 40 (U/mL) | [61] |
| B. safensis M35 | Citrus peel & Wheat bran | SSF | 72 h | 37 | 5.8 | 160 rpm | 411.58 (U/mL) | [62] |
| B. altitudinis J208 | Citrus peel & Wheat bran | SSF | 72 h | 37 | 6.2 | 160 rpm | 728.74 (U/mL) | [62] |
| Aspergillus niger | Apple pomace | SSF | 96 h | 25 | 4.0 | - | 6.75 U/mL | [63] |
| Aspergillus aculeatus NEJC | Mango peel | SSF | 8 days | 40 | 5.5 | - | 1360 U/mL | [64] |
| Aspergillus niger AUMC16245 | Pectin | SmF | 7 days | 40 | 7.0 | 200 rpm | 3787.04 U/mL | [65] |
| Aspergillus brasiliensis AUMC16244 | Pectin | SmF | 5 days | 45 | 7.0 | 200 rpm | 3878.35 U/mL | [65] |
| Aspergillus niveus AUMC1624 | Pectin | SmF | 7 days | 45 | 7.0 | 200 rpm | 3572.95 U/mL | [65] |
| Aspergillus foetidus | Mango peel | SSF | 96 h | 30 | 5.5 | - | 228 U/mL | [66] |
| Aspergillus spp. Gm | 0.5% Pectin | SmF | 48 h | 30 | 5.8 | 150 rpm | 112 U/mL | [44] |
| Saccharomyces cerevisiae | Corn and orange peels | SSF | 6 days | 30 | 4.0 | - | 29.57 U/mL | [67] |
| Streptomyces halstedii | Citrus pectin | SmF | 24 h | 28 | 8.0 | 200 rpm | 1.052 U/mL | [68] |
5. Bacillus vs. Fungal Pectinases
5.1. Enzymatic Properties, Stability and Robustness
5.2. Production Cycle, Substrate Flexibility, and Process Economics
5.3. Genetic Engineering and Downstream Processing
5.4. Regulatory and Safety Considerations
6. Bacillus spp. In Industrial Pectinase Production
6.1. Overview and Historical Milestones
6.2. Physiological and Molecular Advantages
6.3. Diversity of Pectinase-Bacillus Strains Producers
6.4. Ecological Sources and Isolation
| Producer Strain | Mol. Wight | Enzyme Type | Opt. pH | Opt. T °C | Specific Enzyme Activity | Stability (pH/T°C) | Kinetics | Applications | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Km | Vmax | |||||||||
| Virgibacillus salarius Strain 434 | 68 kDa | Pectinase | 9 | 40 | 104.3 U/mg | 7.0–9.5 - | 0.38 mg/mL | 120 U/mg | Pretreatment of wastewater from textile and paper industries | [83] |
| Bacillus halodurans M29 | 39 kDa | Pectinase | 10 | 80 | 142 U/mg | 9.5–10.5 | 4.1 mg/mL | 351 U/mg | - | [70] |
| Bacillus sp. DT7 | 106 kDa | Pectin lyase | 8.0 | 60 | 1433 U/mg | 7.5–8.5 40–60 °C | - | - | In textile industry, plant tissue maceration and wastewater treatments | [90] |
| B. subtilis strain BK-3 | 33 kDa | Pectinase | 5 | 50 | 143.77 U/mg | 4–10 30–60 °C | 0.4770 mg/mL | 43.46 U/mL | Clarification of fruit juice | [86] |
| Bacillus sp. strain B58-2 | - | Pectate lyase | 8.5 | 50 | 2433.26 U/mg | - | - | - | Ramie degumming | [91] |
| B. pumilus | 60 kDa | Pectinase | 8.0 | 60 | 156.5 U/mg | - | - | - | Clarification of fruit juice | [92] |
| B. tropicus P-3 | - | Alkaline pectinase | 9.0 | 37 | 65 U/mg | - | 2.2 mg/mL | 44 U/mg | Pretreatment of the fabrics | [55] |
| B. licheniformis KIBGE IB-3 | - | Polygalacturonase | 7 | 37 | 1118.12 U/mg | 5–9 - | - | - | - | [93] |
| Bacillus sp. strain BR1390 | 104 kDa | Polymethylgalacturonase | 6 | 60 | 222.6 U/mg | 5–8 - | 2.51 mg/mL | 0.066 µmol/ min | Applications in the fruit juice industry | [94] |
| B. subtilis SS | - | Pectinase | 9.5 | 70 | 5.943 U/g | 7–10 55–70 °C | - | - | Pulp and Paper Industry | [95] |
| B. subtilis 15A-B92 | 14.4 kDa | - | 4.5 | 50 | 99.6 U/mg | - | 1.72 mg/mL | 1609 U/g | Clarification of orange and apple juices | [96] |
| B. subtilis ZGL14 | 65 kDa | - | 8.6 | 50 | 52,372.52 U/mg | - | - | - | - | [60] |
| Bacillus sp. ZJ1407 | 23 kDa | - | 5.0 | 37 | 110.47 U/mg | 3–5 80–90 °C | - | - | - | [60] |
| B. subtilis PB1 | 43.1 kDa | Pectate lyase | 9.5 | 50 | 1252.82 U/mg | 5–11 - | 0.312 mg/mL | 1248 U/mL | Flue-cured tobacco leaves | [97] |
| B. pumilus DKS1 | 35 kDa | Pectate lyase | 8.5 | 75 | 6200 U/mg | - | 0.44 mg/mL | 909 U | Fibre degumming | [98] |
| B. clausii | - | Pectate lyases | 10.5 | 70 | 936.2 U/mg | - | 0.54 mg/mL | - | Ramie degumming | [99] |
7. Statistical Optimization of Bacillus spp. Pectinase Production
7.1. RSM, ANNs and DoE for Pectinase Yield Optimization
7.2. Strain-Specific Responses and Process Determinants of Pectinase Yield
7.2.1. pH and Temperature
7.2.2. Inoculum Size and Seed-Culture State
7.2.3. Agitation and Aeration
7.2.4. Substrate Composition (Carbon Source Effects and Induction)
7.2.5. Medium Composition and Non-Carbon Factors
7.2.6. Strain Robustness, Scale-Up and the Role of Statistical Optimization
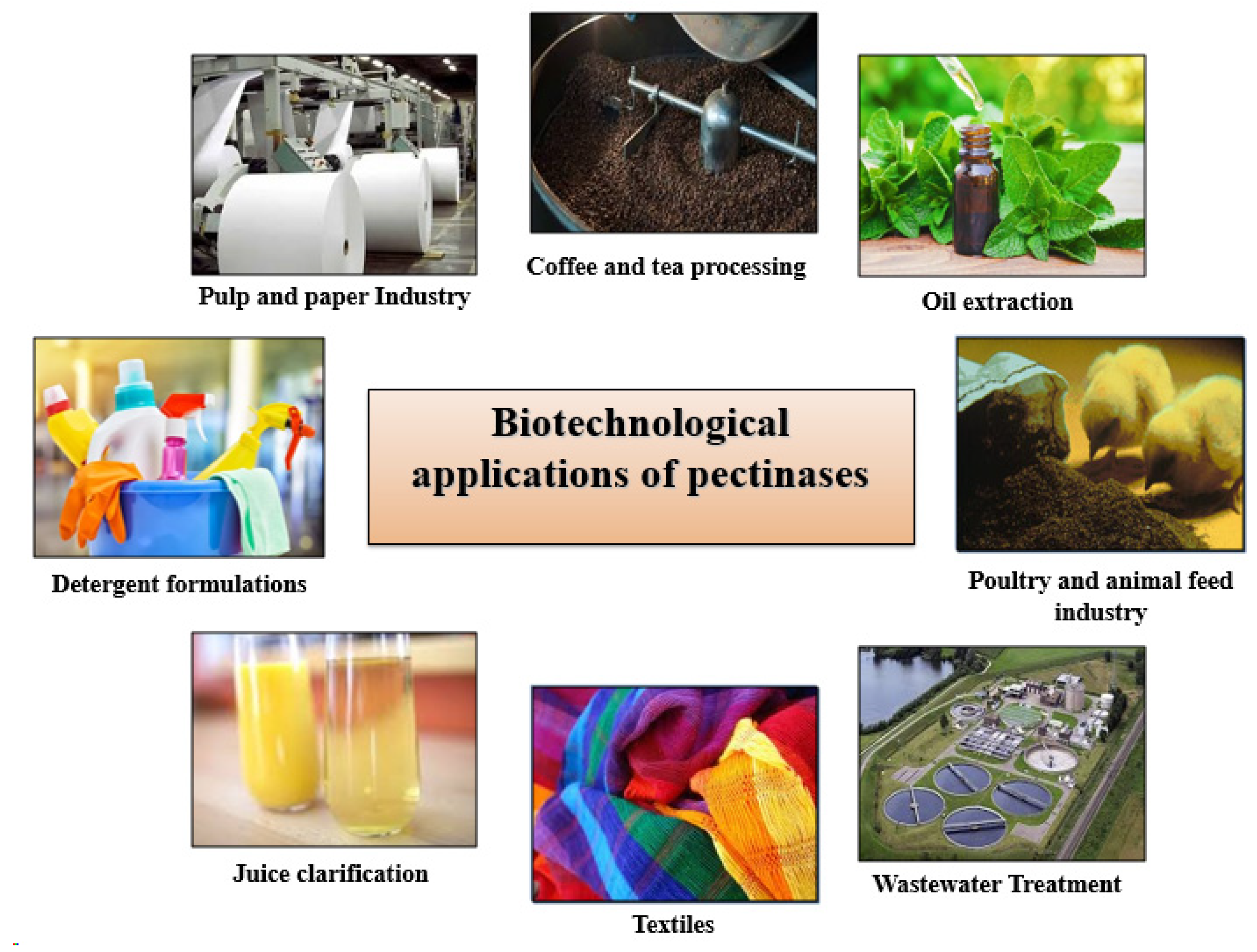
8. Industrial Significance of Bacillus Pectinases
8.1. pH-Dependent Industrial Applications of Pectinases
8.1.1. Alkaline Pectinases
- Textile Bioscouring and Degumming
- Pulp and Paper Biobleaching
- Detergent Formulations
- Coffee and Tea Processing
- Oil Extraction
- Poultry and Animal Feed Industry
- Purification of Plant Viruses
8.1.2. Acidic Pectinases and Their Industrial Applications
- Juice Clarification
- Wine Stabilization
- Food Processing: Fruit Peeling, Canning, and Product Stabilization
8.2. Bacillus Pectinases in Bioremediation and Environmental Sustainability
9. Challenges & Future Directions
9.1. Integration of Upstream and Downstream Processes
9.2. Economics of Low-Cost Production
9.3. Enzyme Engineering & Strain Diversification
9.4. Process Optimization and Scale-Up
9.5. Expanding Application Horizons
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANN | Artificial Neural Network |
| B. | Bacillus |
| CCD | Central composite design |
| CCR | Carbon catabolite repression |
| DoE | Design of Experiments |
| DM | Degree of methyl-esterification |
| DE | Degree of esterification |
| EAE | Enzyme-Assisted Extraction |
| HG | Homogalacturonans |
| HPP | High-Pressure Processing |
| MAE | Microwave-Assisted Extraction |
| OFAT | One factor at a time |
| PEF | Pulsed Electric Field |
| PG | Polygalacturonase |
| RG | Rhamnogalacturonans |
| RSM | Response surface methodology |
| SmF | Submerged fermentation |
| SSF | Solid-state fermentation |
| SWE | Subcritical water extraction |
| UAE | Ultrasound-Assisted Extraction |
References
- Morone, P.; D’Amato, D.; Befort, N.; Yilan, G. The Circular Bioeconomy: Theories and Tools for Economists and Sustainability Scientists; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar] [CrossRef]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef]
- Gallego, L.; Harvey, K.; Pevida, M.; García-Consuegra, L.; García-Suárez, O.; Meana, Á.; Alvarez-Viejo, M.; Junquera, L. From Waste to Innovation: A Circular Economy Approach for Tissue Engineering by Transforming Human Bone Waste into Novel Collagen Membranes. Biomolecules 2025, 15, 132. [Google Scholar] [CrossRef]
- Bijesh, K.; Sebastian, D. Review on bacterial production of alkaline pectinase with special emphasis on Bacillus species. Biosci. Biotechnol. Res. Commun. 2018, 11, 18–30. [Google Scholar] [CrossRef]
- Rehman, H.U. Applications of Pectinolytic Enzymes in Process Industries. In Utilization of Pectin in the Food and Drug Industries; Ahmed, M., Ed.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Kaul, K.; Rajauria, G.; Singh, R. Valorization of agro-industrial waste for pectinase production and its influence on circular economy. Food Bioprod. Process. 2024, 148, 141–153. [Google Scholar] [CrossRef]
- Shrestha, S.; Rahman, M.; Qin, W. New insights in pectinase production development and industrial applications. Appl. Microbiol. Biotechnol. 2021, 105, 9069–9087. [Google Scholar] [CrossRef]
- Vojnovic, S.; Aleksic, I.; Ilic-Tomic, T.; Stevanovic, M.; Nikodinovic-Runic, J. Bacillus and Streptomyces spp. as hosts for production of industrially relevant enzymes. Appl. Microbiol. Biotechnol. 2024, 108, 185. [Google Scholar] [CrossRef]
- Atalah, J.; Cáceres-Moreno, P.; Espina, G.; Blamey, J.M. Thermophiles and the applications of their enzymes as new biocatalysts. Bioresour. Technol. 2019, 280, 478–488. [Google Scholar] [CrossRef]
- Golgeri M, D.B.; Mulla, S.I.; Bagewadi, Z.K.; Tyagi, S.; Hu, A.; Sharma, S.; Bilal, M.; Bharagava, R.N.; Ferreira, L.F.R.; Gurumurthy, D.M.; et al. A systematic review on potential microbial carbohydrases: Current and future perspectives. Crit. Rev. Food Sci. Nutr. 2024, 64, 438–455. [Google Scholar] [CrossRef]
- El Messaoudi, N.; El Khomri, M.; El Mouden, A.; Bouich, A.; Jada, A.; Lacherai, A.; Iqbal, H.M.N.; Mulla, S.I.; Kumar, V.; Américo-Pinheiro, J.H.P. Regeneration and reusability of non-conventional low-cost adsorbents to remove dyes from wastewaters in multiple consecutive adsorption–desorption cycles: A review. Biomass Convers. Biorefin. 2024, 14, 11739–11756. [Google Scholar] [CrossRef]
- Barros, F.; Simiqueli, A.; De Andrade, C.J.; Pastore, G. Production of Enzymes from Agroindustrial Wastes by Biosurfactant-Producing Strains of Bacillus subtilis. Biotechnol. Res. Int. 2013, 2013, 103960. [Google Scholar] [CrossRef]
- Nadar, C.G.; Arora, A.; Shastri, Y. Sustainability Challenges and Opportunities in Pectin Extraction from Fruit Waste. ACS Eng. Au 2022, 2, 61–74. [Google Scholar] [CrossRef]
- Satapathy, S.; Rout, J.R.; Kerry, R.G.; Thatoi, H.; Sahoo, S.L. Biochemical Prospects of Various Microbial Pectinase and Pectin: An Approachable Concept in Pharmaceutical Bioprocessing. Front. Nutr. 2020, 7, 117. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.-J.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.d.l.L.; Lozano-Sánchez, J.; Oliver-Simancas, R.; Alañón, M.E.; Castangia, I.; Segura-Carretero, A.; Arráez-Román, D. Application of Response Surface Methodologies to Optimize High-Added Value Products Developments: Cosmetic Formulations as an Example. Antioxidants 2022, 11, 1552. [Google Scholar] [CrossRef]
- Jeilu, O. Pectinase: Substrate, Production and their Biotechnological Applications. Int. J. Environ. Agric. Biotechnol. 2017, 2, 1007–1014. [Google Scholar] [CrossRef]
- Anand, G.; Yadav, S.; Gupta, R.; Yadav, D. Pectinases: From microbes to industries. In Microorganisms for Sustainable Environment and Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 287–313. [Google Scholar]
- Hossain, M.B.; Ahmed, L. Chapter 6—Application of enzymes in juice clarification. In Value-Addition in Beverages Through Enzyme Technology; Kuddus, M., Hossain, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 97–104. [Google Scholar] [CrossRef]
- Shet, A.; Desai, S.; Achappa, S. Pectinolytic Enzymes: Classification, Production, Purification and Application. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef]
- Sakai, T.; Sakamoto, T.; Hallaert, J.; Vandamme, E.J. Pectin, Pectinase, and Protopectinase: Production, Properties, and Applications. In Advances in Applied Microbiology; Neidleman, S., Laskin, A.I., Eds.; Academic Press: Cambridge, MA, USA, 1993; Volume 39, pp. 213–294. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Li, J.; Yan, L.; Li, S.; Ye, X.; Liu, D.; Ding, T.; Linhardt, R.J.; Orfila, C.; et al. Extraction and characterization of RG-I enriched pectic polysaccharides from mandarin citrus peel. Food Hydrocoll. 2018, 79, 579–586. [Google Scholar] [CrossRef]
- Kaissar, F.Z.; Benine, M.L.; Abbouni, B.; Sid Ahmed, S.; Emiliani, G.; Barberini, S.; Lebouachera, S.E.I. Revealing of multifunctional newly isolated Bacillus strains from Algerian wastes with high pectinase activity for sustainable biotechnological application. Biologia 2025, 80, 1007–1022. [Google Scholar] [CrossRef]
- Rebello, S.; Mohandas, A.; Embalil Mathachan, A.; Sindhu, R.; Binod, P.; Pandey, A. Recent advancements in the production and applications of microbial pectinases—An overview. Reviews in Environmental Science and Biotechnology. Rev. Environ. Sci. Biotechnol. 2017, 16, 381–394. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Haque, S.M.; Kabir, A.; Ratemi, E.; Elzagheid, M.; Appu, S.; Ghani, S.; Sarief, A. Greener Pectin Extraction Techniques: Applications and Challenges. Separations 2025, 12, 65. [Google Scholar] [CrossRef]
- Öztürk, T.; Özbek, H.N.; Koçak Yanık, D. Environmentally Friendly Approach to Pectin Extraction from Grapefruit Peel: Microwave-Assisted High-Pressure CO2/H2O. Foods 2024, 13, 476. [Google Scholar] [CrossRef]
- Wani, K.M.; Uppaluri, R.V.S. Efficacy of ultrasound-assisted extraction of bioactive constituents from Psidium guajava leaves. Appl. Food Res. 2022, 2, 100096. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Faizal, A.N.M.; Che Yunus, M.A. Methods and Potential in Valorization of Banana Peels Waste by Various Extraction Processes: In Review. Sustainability 2022, 14, 10571. [Google Scholar] [CrossRef]
- Poojary, M.M.; Orlien, V.; Passamonti, P.; Olsen, K. Enzyme-assisted extraction enhancing the umami taste amino acids recovery from several cultivated mushrooms. Food Chem. 2017, 234, 236–244. [Google Scholar] [CrossRef]
- Abou-Shady, A.; El-Araby, H. A comprehensive analysis of the advantages and disadvantages of pulsed electric fields during soil electrokinetic remediation. Int. J. Environ. Sci. Technol. 2025, 22, 3895–3925. [Google Scholar] [CrossRef]
- Huang, H.-W.; Wu, S.-J.; Lu, J.-K.; Shyu, Y.-T.; Wang, C.-Y. Current status and future trends of high-pressure processing in food industry. Food Control 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Riyamol; Gada Chengaiyan, J.; Rana, S.S.; Ahmad, F.; Haque, S.; Capanoglu, E. Recent Advances in the Extraction of Pectin from Various Sources and Industrial Applications. ACS Omega 2023, 8, 46309–46324. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, P.; Yang, Y.; Ji, H.; Zhou, H.; Chen, S.; Qiu, Y.; Chen, H. Differences in physicochemical properties of pectin extracted from pomelo peel with different extraction techniques. Sci. Rep. 2024, 14, 9182. [Google Scholar] [CrossRef]
- Mahmoud, M.H.; Abu-Salem, F.M.; Azab, D.E.-S.H. A Comparative Study of Pectin Green Extraction Methods from Apple Waste: Characterization and Functional Properties. Int. J. Food Sci. 2022, 2022, 2865921. [Google Scholar] [CrossRef]
- Figueira, O.; Pereira, V.; Castilho, P.C. A Two-Step Approach to Orange Peel Waste Valorization: Consecutive Extraction of Pectin and Hesperidin. Foods 2023, 12, 3834. [Google Scholar] [CrossRef] [PubMed]
- Dambuza, A.; Rungqu, P.; Oyedeji, A.O.; Miya, G.M.; Kuria, S.K.; Hosu, S.Y.; Oyedeji, O.O. Extraction, Characterization, and Antioxidant Activity of Pectin from Lemon Peels. Molecules 2024, 29, 3878. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Arora, A. One stage hydrothermal treatment: A green strategy for simultaneous extraction of food hydrocolloid and co-products from sweet lime (Citrus limetta) peels. Food Hydrocoll. 2023, 134, 107947. [Google Scholar] [CrossRef]
- Rungraeng, N.; Kraithong, S. Effect of Acid Type and Concentration on Properties of Pectin Extracted from Unripe Cavendish Banana Peel and Its Application in Raspberry Jam. Eng. Agric. Environ. Food 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Pérez, J.; Gómez, K.; Vega, L. Optimization and Preliminary Physicochemical Characterization of Pectin Extraction from Watermelon Rind (Citrullus lanatus) with Citric Acid. Int. J. Food Sci. 2022, 2022, 3068829. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Kumar, V.; Thakur, V.K. Progress in pectin based hydrogels for water purification: Trends and challenges. J. Environ. Manag. 2019, 238, 210–223. [Google Scholar] [CrossRef]
- Ozojiofor, U.; Rasheed, Z.A. Pectinases: Structure, Functions and Biotechnological Applications. J. Appl. Nat. Sci. 2024, 5, 1448–1464. [Google Scholar]
- Oumer, O.J.; Abate, D. Comparative Studies of Pectinase Production by Bacillus subtilis strain Btk 27 in Submerged and Solid-State Fermentations. BioMed Res. Int. 2018, 2018, 1514795. [Google Scholar] [CrossRef]
- Kc, S.; Upadhyaya, J.; Joshi, D.R.; Lekhak, B.; Kumar Chaudhary, D.; Raj Pant, B.; Raj Bajgai, T.; Dhital, R.; Khanal, S.; Koirala, N.; et al. Production, Characterization, and Industrial Application of Pectinase Enzyme Isolated from Fungal Strains. Fermentation 2020, 6, 59. [Google Scholar] [CrossRef]
- Bera, S.; Rajan, E.; Shakya, S.; Perera, I.; Wijewarna, S.; Semini, M. Revisiting microbial pectinases: An understanding between structure-functional relationship in the arena of genetic engineering. J. Appl. Biol. Biotechnol. 2023, 11, 63–74. [Google Scholar] [CrossRef]
- Nawawi, M.H.; Ismail, K.I.; Sa’ad, N.; Mohamad, R.; Tahir, P.M.; Asa’ari, A.Z.; Saad, W.Z. Optimisation of Xylanase–Pectinase Cocktail Production with Bacillus amyloliquefaciens ADI2 Using a Low-Cost Substrate via Statistical Strategy. Fermentation 2022, 8, 119. [Google Scholar] [CrossRef]
- Singh, S.; Bajaj, B. Bioprocess optimization for production of thermoalkali-stable protease from Bacillus subtilis K-1 under solid state fermentation. Prep. Biochem. Biotechnol. 2016, 46, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Chen, C.-L.; Nguyen, V.B.; Tran, T.N.; Nguyen, A.D.; Wang, S.-L. Conversion of Pectin-Containing By-Products to Pectinases by Bacillus amyloliquefaciens and Its Applications on Hydrolyzing Banana Peels for Prebiotics Production. Polymers 2021, 13, 1483. [Google Scholar] [CrossRef] [PubMed]
- Tepe, Ö.; Dursun, A. Endo-pectinase Production by Bacillus pumilus NRRL B-212 and Optimization by RSM using Sugar Beet Pulp. Chem. Biochem. Eng. Q. 2022, 35, 439–453. [Google Scholar] [CrossRef]
- Jacob, N. Pectinolytic Enzymes. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Singh nee’ Nigam, P., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 383–396. [Google Scholar] [CrossRef]
- Murad, H.; Azzaz, H. Microbial Pectinases and Ruminant Nutrition. Res. J. Microbiol. 2011, 3, 246–269. [Google Scholar] [CrossRef]
- Uzuner, S.; Cekmecelioglu, D. Enhanced pectinase production by optimizing fermentation conditions of Bacillus subtilis growing on hazelnut shell hydrolyzate. J. Mol. Catal. B Enzym. 2015, 113, 62–67. [Google Scholar] [CrossRef]
- Ghazala, I.; Sayari, N.; Romdhane, M.B.; Ellouz-Chaabouni, S.; Haddar, A. Assessment of pectinase production by Bacillus mojavensis I4 using an economical substrate and its potential application in oil sesame extraction. J. Food Sci. Technol. 2015, 52, 7710–7722. [Google Scholar] [CrossRef]
- Zhang, G.; Li, S.; Xu, Y.; Wang, J.; Wang, F.; Xin, Y.; Shen, Z.; Zhang, H.; Ma, M.; Liu, H. Production of alkaline pectinase: A case study investigating the use of tobacco stalk with the newly isolated strain Bacillus tequilensis CAS-MEI-2-33. BMC Biotechnol. 2019, 19, 45. [Google Scholar] [CrossRef]
- Thakur, P.; Singh, A.; Mukherjee, G. Isolation and Characterization of Alkaline Pectinase Productive Bacillus tropicus from Fruit and Vegetable Waste Dump Soil. Braz. Arch. Biol. Technol. 2021, 64, e21200319. [Google Scholar] [CrossRef]
- Ram Balak, M.; Mukesh, Y.; Soumya, S.; Biswnath, B. Optimization of Process Parameters for Production of Pectinase using Bacillus subtilis MF447840.1. Recent Pat. Biotechnol. 2019, 13, 69–73. [Google Scholar] [CrossRef]
- Echesi, S.A.; Ire, F.S.; Odu, N.N. Optimization of Pectinase Production from Bacillus subtilis PSE-8 Using Cassava Peels as Substrate in Submerged Fermentation through Response Surface Methodology (RSM). J. Adv. Microbiol. 2022, 22, 55–66. [Google Scholar] [CrossRef]
- Kohli, P.; Sharma, N.; Gupta, R. Statistical optimization of production conditions of alkaline pectin lyase from Bacillus cereus using response surface methodology. Biocatal. Biotransform. 2017, 35, 417–426. [Google Scholar] [CrossRef]
- Bibi, N.; Ali, S.; Tabassum, R. Statistical Optimization of Pectinase Biosynthesis from Orange Peel by Bacillus licheniformis Using Submerged Fermentation. Waste Biomass Valoriz. 2016, 7, 467–481. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, Y.; Gu, D. Production optimization of a heat-tolerant alkaline pectinase from Bacillus subtilis ZGL14 and its purification and characterization. Bioengineered 2017, 8, 613–623. [Google Scholar] [CrossRef]
- Guo, F.; Li, X.; Zhao, J.; Li, G.; Gao, P.; Han, X. Optimizing Culture Conditions by Statistical Approach to Enhance Production of Pectinase from Bacillus sp. Y1. BioMed Res. Int. 2019, 2019, 8146948. [Google Scholar] [CrossRef]
- Thite, V.S.; Nerurkar, A.S.; Baxi, N.N. Optimization of concurrent production of xylanolytic and pectinolytic enzymes by Bacillus safensis M35 and Bacillus altitudinis J208 using agro-industrial biomass through Response Surface Methodology. Sci. Rep. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Joshi, V.; Parmar, M.; Rana, N. Pectin Esterase Production from Apple Pomace in Solid-State and Submerged Fermentations. Food Technol. Biotechnol. 2006, 44, 253–256. [Google Scholar]
- Omeje, K.; Nnolim, N.; Ezema, B.; Ozioko, J.; Ossai, E.; Eze, S. Valorization of agro-industrial residues for pectinase production by Aspergillus aculeatus: Application in cashew fruit juice clarification. Clean. Circ. Bioecon. 2023, 4, 100038. [Google Scholar] [CrossRef]
- Mwaheb, M.; Mohamed, B.; Abd-Elhalim, B.; El-Kassim, N.; Radwan, T. Study of Different Cultivated Plants Rhizosphere Soil Fungi-Mediated Pectinase: Insights into Production, Optimization, Purification, Biocompatibility, and Application. Microb. Ecol. 2025, 87, 165. [Google Scholar] [CrossRef]
- Kumar, Y.S.; Kumar, P.V.; Reddy, O.V.S. Pectinase Production from Mango Peel Using Aspergillus foetidus and its Application in Processing of Mango Juice. Food Biotechnol. 2012, 26, 107–123. [Google Scholar] [CrossRef]
- Ametefe, G.; Dzogbefia, V.; Apprey, C.; Kwatia, S. Optimal conditions for pectinase production by Saccharomyces cerevisiae (ATCC 52712) in solid state fermentation and its efficacy in orange juice extraction. IOSR J. Biotechnol. Biochem. 2017, 3, 78–86. [Google Scholar]
- Ramírez-Tapias, Y.; Rivero, C.; Britos, C.; Trelles, J. Alkaline and thermostable polygalacturonase from Streptomyces halstedii ATCC 10897 with applications in waste waters. Biocatal. Agric. Biotechnol. 2015, 44, 221–228. [Google Scholar] [CrossRef]
- Suhaimi, H.; Dailin, D.J.; Malek, R.A.; Hanapi, S.Z.; Ambehabati, K.K.; Keat, H.C.; Prakasham, S.; Elsayed, E.A.; Misson, M.; El Enshasy, H. Fungal Pectinases: Production and Applications in Food Industries. In Fungi in Sustainable Food Production; Dai, X., Sharma, M., Chen, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 85–115. [Google Scholar] [CrossRef]
- Mei, Y.; Chen, Y.; Zhai, R.; Liu, Y. Cloning, purification and biochemical properties of a thermostable pectinase from Bacillus halodurans M29. J. Mol. Catal. B Enzym. 2013, 94, 77–81. [Google Scholar] [CrossRef]
- Martin, N.; Souza, S.R.D.; Silva, R.D.; Gomes, E. Pectinase production by fungal strains in solid-state fermentation using agro-industrial bioproduct. Braz. Arch. Biol. Technol. 2004, 47, 813–819. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, Q.; Lv, C.; Wang, D.; Ye, X. Fermentation time-dependent pectinase activity is associated with metabolomics variation in Bacillus licheniformis DY2. Process Biochem. 2021, 101, 147–155. [Google Scholar] [CrossRef]
- Amin, F.; Bhatti, H.N.; Bilal, M. Recent advances in the production strategies of microbial pectinases—A review. Int. J. Biol. Macromol. 2019, 122, 1017–1026. [Google Scholar] [CrossRef]
- Galano, M.; van den Dungen, M.W.; van Rij, T.; Abbas, H.E. Safety evaluation of food enzymes produced by a safe strain lineage of Bacillus subtilis. Regul. Toxicol. Pharmacol. 2021, 126, 105030. [Google Scholar] [CrossRef]
- Shah, P.; Gutierrez-Sanchez, G.; Orlando, R.; Bergmann, C. A proteomic study of pectin-degrading enzymes secreted by Botrytis cinerea grown in liquid culture. Proteomics 2009, 9, 3126–3135. [Google Scholar] [CrossRef]
- Shen, Q.; Ruan, H.; Zhang, H.; Wu, T.; Zhu, K.; Han, W.; Dong, R.; Ming, T.; Qi, H.; Zhang, Y. Utilization of CRISPR-Cas genome editing technology in filamentous fungi: Function and advancement potentiality. Front. Microbiol. 2024, 15, 1375120. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Zhao, F.; Zhang, Y.; Han, S. Efficient expression of an alkaline pectin lyase from Bacillus licheniformis in Pichia pastoris. Bioresour. Bioprocess. 2024, 11, 37. [Google Scholar] [CrossRef]
- Singh, N.; Gaur, S. GRAS Fungi: A New Horizon in Safer Food Product. In Fungi in Sustainable Food Production; Dai, X., Sharma, M., Chen, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 27–37. [Google Scholar] [CrossRef]
- Danilova, I.; Sharipova, M. The Practical Potential of Bacilli and Their Enzymes for Industrial Production. Front. Microbiol. 2020, 11, 1782. [Google Scholar] [CrossRef]
- Gautam, S.; Chauhan, A.; Sharma, R.; Sehgal, R.; Shirkot, C.K. Potential of Bacillus amyloliquefaciens for biocontrol of bacterial canker of tomato incited by Clavibacter michiganensis ssp. michiganensis. Microb. Pathog. 2019, 130, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Letti, L.A.J.; Herrmann, L.W.; de Oliveira Penha, R.; de Mello, A.F.M.; Karp, S.G.; Soccol, C.R. Enzymes from Bacillus spp. for Nutraceutical Production. In Microbial Enzymes in Production of Functional Foods and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2023; pp. 65–88. [Google Scholar] [CrossRef]
- Gundala, P.B.; Chinthala, P. Extremophilic Pectinases. In Extremophilic Enzymatic Processing of Lignocellulosic Feedstocks to Bioenergy; Sani, R.K., Krishnaraj, R.N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 155–180. [Google Scholar] [CrossRef]
- Boyadzhieva, I.; Berberov, K.; Atanasova, N.; Krumov, N.; Kabaivanova, L. Extracellular Haloalkalophilic Pectinase Produced by Virgibacillus salarius Strain 434—A Useful Tool for Biotechnological Applications. Appl. Sci. 2024, 14, 9295. [Google Scholar] [CrossRef]
- Afrin, N.; Shilpi, R.Y. First report of Bacillus marisflavi as a potential pectinase producing bacteria from samples of savar, Dhaka. Bangladesh J. Bot. 2023, 52, 159–163. [Google Scholar] [CrossRef]
- Kh, A.-E.-A.S.; Attallah, A.; Abdel-Aziz, N.M.; Khalil, B.E. Isolation, screening, and molecular identification of pectinase producers from fruits, vegetables, and soil samples. Egypt. Pharm. J. 2022, 21, 302–311. [Google Scholar] [CrossRef]
- Prajapati, J.; Dudhagara, P.; Patel, K. Production of thermal and acid-stable pectinase from Bacillus subtilis strain BK-3: Optimization, characterization, and application for fruit juice clarification. Biocatal. Agric. Biotechnol. 2021, 35, 102063. [Google Scholar] [CrossRef]
- Hassan, M.K. In vitro pectate lyase activity and carbon uptake assays and whole genome sequencing of Bacillus amyloliquefaciens subsp. plantarum strains for a pectin defective pathway. bioRxiv 2021. ahead of print. [Google Scholar] [CrossRef]
- Kabir, M.S.; Tasmim, T. Isolation of pectinase producing bacteria from the rhizosphere of Andrographis paniculata nees and 16S rRNA gene sequence comparison of some potential strains. Adv. Microbiol. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Kiran Kumar, D.J.; Kavya, N.L.; Chaithra, B.S.; Poojashree, T.H.; Rama, T. Pectinase Producing Bacteria Isolation from Halophilic Soil, Water Samples and Partial Purification of the Enzyme. Int. J. Sci. Res. Sci. Eng. Technol. 2020, 7, 600–607. [Google Scholar] [CrossRef]
- Kashyap, D.; Chandra, S.; Kaul, A.; Tewari, R. Production, purification and characterization of pectinase from a Bacillus sp. DT7. World J. Microbiol. Biotechnol. 2000, 16, 277–282. [Google Scholar] [CrossRef]
- Liu, S.; Qin, Y.; Wang, Q.; Zhang, J.; Zhou, J.; He, B.; Liang, X.; Xian, L.; Wu, J. A novel pectate lyase with high specific activity from Bacillus sp. B58-2: Gene cloning, heterologous expression and use in ramie degumming. Enzym. Microb. Technol. 2024, 175, 110395. [Google Scholar] [CrossRef]
- Viayaraghavan, P.; Jeba Kumar, S.; Valan Arasu, M.; Al-Dhabi, N.A. Simultaneous production of commercial enzymes using agro industrial residues by statistical approach. J. Sci. Food Agric. 2019, 99, 2685–2696. [Google Scholar] [CrossRef]
- Jahan, N.; Shahid, F.; Aman, A.; Mujahid, T.; Ul Qader, S.A. Utilization of agro waste pectin for the production of industrially important polygalacturonase. Heliyon 2017, 3, e00330. [Google Scholar] [CrossRef]
- Rastegari, B.; Karbalaei-Heidari, H.R. Isolation and Partial Characterization of a Bacterial Thermostable Polymethyl Galacturonase from a Newly Isolated Bacillus sp. strain BR1390. Iran. J. Biotechnol. 2014, 12, 41–46. [Google Scholar] [CrossRef]
- Ahlawat, S.; Mandhan, R.P.; Dhiman, S.S.; Kumar, R.; Sharma, J. Potential Application of Alkaline Pectinase from Bacillus subtilis SS in Pulp and Paper Industry. Appl. Biochem. Biotechnol. 2008, 149, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, Y.S.; More, S.S.; Keerthana, R.; Shaikh, I.A.; Anusha, K.J.; More, V.S.; Niyonzima, F.N.; Muddapur, U.M.; Khan, A.A. Production and Purification of Pectinase from Bacillus subtilis 15A-B92 and Its Biotechnological Applications. Molecules 2022, 27, 4195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wu, J.; Wang, T.; Gao, L.; Yin, H.; Lü, X. The purification and characterization of a novel alkali-stable pectate lyase produced by Bacillus subtilis PB1. World J. Microbiol. Biotechnol. 2017, 33, 190. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Ghosh, A.; Bera, A.; Saha, M.; Chattopadhyay, D.; Chakrabarti, K. Thermodynamic characterization of a highly thermoactive extracellular pectate lyase from a new isolate Bacillus pumilus DKS1. Bioresour. Technol. 2008, 99, 8088–8094. [Google Scholar] [CrossRef]
- Zhou, C.; Xue, Y.; Ma, Y. Cloning, evaluation, and high-level expression of a thermo-alkaline pectate lyase from alkaliphilic Bacillus clausii with potential in ramie degumming. Appl. Microbiol. Biotechnol. 2017, 101, 3663–3676. [Google Scholar] [CrossRef]
- Satpathy, A.; Mukherjee, K.; Nigam, V.K. Batch cultivation and optimization of pectinase production using Bacillus sp. (BIOSMNF02) through RSM-D-optimal quadratic model. Biomass Convers. Biorefin. 2023, 14, 31979–31990. [Google Scholar] [CrossRef]
- Gammoudi, N.; Mabrouk, M.; Bouhemda, T.; Nagaz, K.; Ferchichi, A. Modeling and optimization of capsaicin extraction from Capsicum annuum L. using response surface methodology (RSM), artificial neural network (ANN), and Simulink simulation. Ind. Crops Prod. 2021, 171, 113869. [Google Scholar] [CrossRef]
- Aklilu, E.G. Artificial Neural Networks (ANNs) and Response Surface Methodology (RSM) Approach for Modeling and Optimization of Pectin Extraction from Banana Peel. Res. Sq. 2020. ahead of print. [Google Scholar] [CrossRef]
- Chakraborty, S.; Das, M.; Ghosh, T.; Dash, K.K. Application of Artificial Neural Network (ANN) in Ultrasound-Assisted Extraction of Bioactive Compounds. J. Food Process Eng. 2025, 48, e70028. [Google Scholar] [CrossRef]
- Ortiz, G.E.; Ponce-Mora, M.C.; Noseda, D.G.; Cazabat, G.; Saravalli, C.; López, M.C.; Gil, G.P.; Blasco, M.; Albertó, E.O. Pectinase production by Aspergillus giganteus in solid-state fermentation: Optimization, scale-up, biochemical characterization and its application in olive-oil extraction. J. Ind. Microbiol. Biotechnol. 2017, 44, 197–211. [Google Scholar] [CrossRef]
- Mechri, S.; Kriaa, M.; Ben Elhoul Berrouina, M.; Omrane Benmrad, M.; Zaraî Jaouadi, N.; Rekik, H.; Bouacem, K.; Bouanane-Darenfed, A.; Chebbi, A.; Sayadi, S.; et al. Optimized production and characterization of a detergent-stable protease from Lysinibacillus fusiformis C250R. Int. J. Biol. Macromol. 2017, 101, 383–397. [Google Scholar] [CrossRef]
- Sharma, D.; Satyanarayana, T. Biotechnological Potential of Agro Residues for Economical Production of Thermoalkali-Stable Pectinase by Bacillus pumilus dcsr1 by Solid-State Fermentation and Its Efficacy in the Treatment of Ramie Fibres. Enzym. Res. 2012, 2012, 281384. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, S. Exploring the Functionality of Microbes in Fermented Foods: Technological Advancements and Future Directions. Fermentation 2025, 11, 300. [Google Scholar] [CrossRef]
- Saini, P.; Mishra, P. Bioprospecting of extremophiles for industrial enzymes. In Bioprospecting of Microbial Resources for Agriculture, Environment and Bio-Chemical Industry; Springer: Cham, Switzerland, 2024; pp. 43–74. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Zhang, P.; Zhang, G.; Cai, Y.; Wang, Q.; Zhou, Z.; Ding, Y.; Zubair, M. Effect of substrate load on anaerobic fermentation of rice straw with rumen liquid as inoculum: Hydrolysis and acidogenesis efficiency, enzymatic activities and rumen bacterial community structure. Waste Manag. 2021, 124, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Haile, S.; Ayele, A. Pectinase from microorganisms and its industrial applications. Sci. World J. 2022, 2022, 1881305. [Google Scholar] [CrossRef]
- Krishna, C. Solid-state fermentation systems—An overview. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef]
- Amanullah, A.; Buckland, B.C.; Nienow, A.W. Mixing in the fermentation and cell culture industries. In Handbook of Industrial Mixing: Science and Practice; Wiley-Interscience: Hoboken, NJ, USA, 2003; pp. 1071–1170. [Google Scholar] [CrossRef]
- Oraby, A.; Weickardt, I.; Zibek, S. Foam fractionation methods in aerobic fermentation processes. Biotechnol. Bioeng. 2022, 119, 1697–1711. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Rodríguez, R.; Contreras-Esquivel, J.C.; Aguilar, C. Pectinase production from lemon peel pomace as support and carbon source in solid-state fermentation column-tray bioreactor. Biochem. Eng. J. 2012, 65, 90–95. [Google Scholar] [CrossRef]
- Singh, K.D.; Schmalisch, M.H.; Stülke, J.; Görke, B. Carbon catabolite repression in Bacillus subtilis: Quantitative analysis of repression exerted by different carbon sources. J. Bacteriol. 2008, 190, 7275–7284. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Hirooka, K. T7 expression system in Bacillus subtilis utilizing the rhiL promoter responsive to pectin and glucose. Biosci. Biotechnol. Biochem. 2025, 89, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Chotani, G.K.; Dodge, T.C.; Peres, C.M.; Moslemy, P.; Arbige, M.V. Industrial biotechnology: Discovery to delivery. In Handbook of Industrial Chemistry and Biotechnology; Springer: Cham, Switzerland, 2017; pp. 1495–1570. [Google Scholar] [CrossRef]
- Maqsood, S.; Khalid, W.; Kumar, P.; Benmebarek, I.E.; Rasool, I.F.U.; Trif, M.; Moreno, A.; Esatbeyoglu, T. Valorization of plant-based agro-industrial waste and by-products for the production of polysaccharides: Towards a more circular economy. Appl. Food Res. 2025, 5, 100954. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C. Valorization of sugar beet pulp through biotechnological approaches: Recent developments. Biotechnol. Lett. 2021, 43, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.J.; Gupta, V.K. Production of pectinolytic enzymes pectinase and pectin lyase by Bacillus subtilis SAV-21 in solid state fermentation. Ann. Microbiol. 2017, 67, 333–342. [Google Scholar] [CrossRef]
- Kavuthodi, B.; Sebastian, D. Biotechnological valorization of pineapple stem for pectinase production by Bacillus subtilis BKDS1: Media formulation and statistical optimization for submerged fermentation. Biocatal. Agric. Biotechnol. 2018, 16, 715–722. [Google Scholar] [CrossRef]
- Kashyap, D.; Vohra, P.; Chopra, S.; Tewari, R. Applications of pectinases in the commercial sector: A review. Bioresour. Technol. 2001, 77, 215–227. [Google Scholar] [CrossRef]
- Sharma, N.; Rathore, M.; Sharma, M. Microbial pectinase: Sources, characterization and applications. Rev. Environ. Sci. Bio/Technol. 2013, 12, 45–60. [Google Scholar] [CrossRef]
- Bassim Atta, M.; Ruiz-Larrea, F. Fungal Pectinases in Food Technology. In Pectins—The New-Old Polysaccharides; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Kapoor, M.; Beg, Q.; Bhushan, B.; Singh, K.; Dadhich, K.S.; Hoondal, G.S. Application of alkaline and thermostable polygalacturonase from Bacillus sp. MG-cp-2 in degumming of ramie (Boehmeria nivea) and sunn hemp (Crotolaria juncia) bast fibres. Process Biochem. 2001, 36, 803–807. [Google Scholar] [CrossRef]
- Anab-Atulomah, C.; Nwachukwu, E. Bio-Scouring of Cotton using Protease and Pectinase from Bacillus subtilis Isolated from Market Waste. Path Sci. 2021, 7, 72–73. [Google Scholar] [CrossRef]
- Khan, M.M.; Choi, Y.S.; Kim, Y.K.; Yoo, J.C. Immobilization of an alkaline endopolygalacturonase purified from Bacillus paralicheniformis exhibits bioscouring of cotton fabrics. Bioprocess Biosyst. Eng. 2018, 41, 1425–1436. [Google Scholar] [CrossRef]
- Basu, S.; Saha, M.N.; Chattopadhyay, D.; Chakrabarti, K. Degumming and characterization of ramie fibre using pectate lyase from immobilized Bacillus pumilus DKS1. Lett. Appl. Microbiol. 2009, 48, 593–597. [Google Scholar] [CrossRef]
- Colombi, B.L.; Quesli, M.; Kopsch, I.C.; Barboza, D.S.D.; Borges, V.J.A.; Jürgen, A.; Lis, A.M.J.; Valle, R.D.C.S.C. Understanding the effects of process parameters in the bioscouring of cotton and their interactions on pectate lyase activity by factorial design analysis. J. Text. Inst. 2022, 113, 857–868. [Google Scholar] [CrossRef]
- Nawawi, M.H.; Mohamad, R.; Tahir, P.M.; Asa’ari, A.Z.; Saad, W.Z. Pulp Enhancement of Oil Palm Empty Fruit Bunches (OPEFBs) via Biobleaching by Using Xylano-Pectinolytic Enzymes of Bacillus amyloliquefaciens ADI2. Molecules 2021, 26, 4279. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, S.; Battan, B.; Dhiman, S.S.; Sharma, J.; Mandhan, R.P. Production of thermostable pectinase and xylanase for their potential application in bleaching of kraft pulp. J. Ind. Microbiol. Biotechnol. 2007, 34, 763–770. [Google Scholar] [CrossRef]
- Angural, S.; Kumar, A.; Kumar, D.; Warmoota, R.; Sondhi, S.; Gupta, N. Lignolytic and hemicellulolytic enzyme cocktail production from Bacillus tequilensis LXM 55 and its application in pulp biobleaching. Bioprocess Biosyst. Eng. 2020, 43, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Kobayashi, Y. Biochemical pulping. Retting of Mitsumata bast by alkalophilic Bacillus in papermaking. Agric. Biol. Chem. 1982, 46, 109–117. [Google Scholar] [CrossRef]
- Nguyen, V.-M.-L.; Ndao, A.; Peterson, E.C.; Blais, J.-F.; Adjallé, K. Bacillus Species: Evolving Roles in Bio-Based Detergents. Processes 2025, 13, 1885. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Joseph, B.; Alhussaini, M.S.; Ramteke, P.W. Chapter 17–Current applications and future trends of extremozymes in detergent industries. In Microbial Extremozymes; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 223–230. [Google Scholar] [CrossRef]
- Bouassida, M.; Fourati, N.; Ghazala, I.; Ellouze-Chaabouni, S.; Ghribi, D. Potential application of Bacillus subtilis SPB1 biosurfactants in laundry detergent formulations: Compatibility study with detergent ingredients and washing performance. Eng. Life Sci. 2018, 18, 70–77. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Qiu, J.; Wu, D.; Qiu, M.; Xie, W.; Tan, Q. Co-expression of protease and pectinase in Bacillus subtilis using the herbal saponin extract as substrate. Int. Microbiol. 2018, 21, 223–229. [Google Scholar] [CrossRef]
- Puerta Quintero, G. Efecto de enzimas pectolíticas en la remoción del mucílago de Coffea arabica L., según el desarrollo del fruto. Cenicafé 2009, 60, 291–312. [Google Scholar]
- Silva, M.E.; Oliveira, R.L.; Moraes, M.M.; Camara, C.A.; Silva, S.P.; Porto, T.S. Application of Commercial Pectinase as a Biocatalyst During Self-Induced Anaerobic Fermentation of Coffee (Coffea arabica L. var. Typica). Fermentation 2025, 11, 361. [Google Scholar] [CrossRef]
- Umar, M.; Rehman, A.; Khan, I.; Hayat, P.; Hayat, A.; Rehman, M.; Ali Shah, T.; Dawoud, T.; Hadrach, S.; Bourhia, M. Screening and optimization of extracellular pectinase produced by Bacillus thuringiensis SH7. Open Chem. 2023, 21, 20220358. [Google Scholar] [CrossRef]
- Sivasakthi, M.; Sankar, S.S. Isolation of Pectinolytic Bacteria from Decayed Orange Peel (Citrus sinensis) in Clarification of Tea Extract. Asian J. Dairy Food Res. 2023, 1–7. [Google Scholar] [CrossRef]
- Panji, T.; Suharyanto, S.; Shabri, S.; Rohdiana, D.; Yusianto, Y. Production of fungal pectinase enzyme through solid substrate fermentation of estate waste for improvement of enzymatic oxidation and increasing the quality of CTC tea. J. Sains Teh Kina 2016, 18, 11–20. [Google Scholar] [CrossRef]
- Hoondal, G.; Tiwari, R.; Tewari, R.; Dahiya, D.; Beg, Q. Microbial alkaline pectinases and their industrial applications: A review. Appl. Microbiol. Biotechnol. 2002, 59, 409–418. [Google Scholar] [CrossRef]
- Arellano, J.; Ilich, S.; Salazar, M.; Rodríguez, I.; Torres, P.; Alarcón, W. Producción de pectinasas por Bacillus spp. a partir de cáscaras de naranja y de toronja como fuente de carbono. REBIOL 2015, 35, 62–69. [Google Scholar]
- Soares, M.M.C.N.; Da Silva, R.; Carmona, E.C.; Gomes, E. Pectinolytic enzyme production by Bacillus species and their potential application on juice extraction. World J. Microbiol. Biotechnol. 2001, 17, 79–82. [Google Scholar] [CrossRef]
- Nwafor, O.; Efeyini, I. Screening for pectinase production in solid state fermentation (ssf) of banana peels by Bacillus spp. Niger. J. Life Sci. 2014, 4, 103–111. [Google Scholar] [CrossRef]
- Latorre, J.D.; Hernandez-Velasco, X.; Wolfenden, R.E.; Vicente, J.L.; Wolfenden, A.D.; Menconi, A.; Bielke, L.R.; Hargis, B.M.; Tellez, G. Evaluation and Selection of Bacillus Species Based on Enzyme Production, Antimicrobial Activity, and Biofilm Synthesis as Direct-Fed Microbial Candidates for Poultry. Front. Vet. Sci. 2016, 3, 95. [Google Scholar] [CrossRef]
- Anadón, A.; Ares, I.; Martínez-Larrañaga, M.R.; Martínez, M.A. Enzymes in Feed and Animal Health. In Nutraceuticals in Veterinary Medicine; Gupta, R.C., Srivastava, A., Lall, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 303–313. [Google Scholar] [CrossRef]
- Singh, S.; Anjaneyulu, A.; Lapierre, H. Use of pectino-cellulolytic enzymes for improving extraction of phloem-limited plant viruses as exemplified by the rice tungro virus complex. Agronomie 1984, 4, 479–484. [Google Scholar] [CrossRef]
- Takanami, Y.; Kubo, S. Enzyme-assisted Purification of two Phloem-limited Plant Viruses: Tobacco Necrotic Dwarf and Potato Leafroll. J. Gen. Virol. 1979, 44, 153–159. [Google Scholar] [CrossRef]
- Pavlović, M.; Šokarda Slavić, M.; Kojić, M.; Margetić, A.; Ristović, M.; Drulović, N.; Vujčić, Z. Unveiling novel insights into Bacillus velezensis 16B pectin lyase for improved fruit juice processing. Food Chem. 2024, 456, 140030. [Google Scholar] [CrossRef]
- Chaudhari, A.; Dahale, I.; Pungle, R.; Jadhav, M. Optimization of Culture Conditions and Characterization of Pectinase from Isolated Bacteria and Application in Juice Clarification. J. Adv. Sci. Res. 2024, 15, 36–41. [Google Scholar] [CrossRef]
- Patrício, T.; Santos, L.; Silva, R.; Santos, R.; Ribeiro-Filho, N.; Santos, S. Pectinases production using an exotic Caatinga passion fruit waste and wheat bran as a substrate for Clarification of grape juice. Food Biosci. 2024, 61, 104163. [Google Scholar] [CrossRef]
- Zhong, C.; Dong, C.; Bo, L.; Yutuo, W.; Liang, X.; Yi, L.; Zhenzhen, L.; Ribo, H. A Novel Acid-Stable Endo-Polygalacturonase from Penicillium oxalicum CZ1028: Purification, Characterization, and Application in the Beverage Industry. J. Microbiol. Biotechnol. 2016, 26, 989–998. [Google Scholar] [CrossRef]
- Oliveira, S.C.; Dencheva, N.V.; Denchev, Z.Z. Immobilization of Enological Pectinase on Magnetic Sensitive Polyamide Microparticles for Wine Clarification. Foods 2024, 13, 420. [Google Scholar] [CrossRef]
- Rehman, H.; Baloch, A.H.; Asif Nawaz, M. Pectinase: Immobilization and Applications. A review: Immobilization and applications of pectinase. Trends Pept. Protein Sci. 2021, 6, 1–16. [Google Scholar] [CrossRef]
- Kapilavai, S.; Aluru, R.R.; Chanda, C. Purification and Production Optimization of Bacterial Pectinases Using Fruit Waste. Int. J. Pharm. Investig. 2024, 14, 1216–1220. [Google Scholar] [CrossRef]
- Gunjal, A.; Waghmode, M.; Patil, N. Role of Extremozymes in Bioremediation: A Review. Res. J. Biotechnol. 2021, 16, 240–252. [Google Scholar]
- Torimiro, N.; Raphael, O. A comparative study of pectinolytic enzyme production by Bacillus species. Afr. J. Biotechnol. 2013, 12, 6498–6503. [Google Scholar] [CrossRef]
- Thite, V.S.; Nerurkar, A.S. Physicochemical characterization of pectinase activity from Bacillus spp. and their accessory role in synergism with crude xylanase and commercial cellulase in enzyme cocktail mediated saccharification of agrowaste biomass. J. Appl. Microbiol. 2018, 124, 1147–1163. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.W.; Gu, M.B.; Tsang, Y.F.; Choi, Y.-S.; Chung, J. Application of endospore-forming Bacillus species to food waste-recycling wastewater treatment: A focus on the fate of macromolecular nutrients. J. Environ. Chem. Eng. 2022, 10, 107584. [Google Scholar] [CrossRef]
- Bhardwaj, D. Microbial Pectinases and their applications in industries: A review. Int. Res. J. Eng. Technol. (IRJET) 2017, 4, 829–836. [Google Scholar]


| Product Trade Name | Manufacturer | Country |
|---|---|---|
| Pectinex™ a | Novo Nordisk (Now Novozymes) | Denmark |
| Pectinase Enzyme a | Carolina Biological Supply Co. | United States of America |
| Pectinase a | CCM International Ltd. | Various |
| Panzym b | C.H. Boehringer Sohn, | Ingelheim, West Germany |
| Ultrazyme b | Ciba-Geigy, A.G. | Basel, Switzerland |
| Pectolase b | Grinsteelvaeket | Aarthus, Denmark |
| Sclase b | Kikkoman Shoyu, Co. | Tokyo, Japan |
| Pectinex b | Schweizerische Ferment, A.G. | Basel, Switzerland |
| Rapidase, Clarizyme b | Societe Rapidase, S.A. | Seclin, France |
| Klerzyme b | Wallerstein, Co. | Des Plaines, United States of America |
| Pectinol, Rohament b | Rohm, GmbH | Darmstadt, West Germany |
| Pectinase c | Biocatalysts Ltd. | Cardiff, United Kingdom |
| Sunson Industry Group Co. Ltd. c | Sunson® PEC-pectinase | Yinchuan, China |
| Yakult Pharmaceutical Industry Co. Ltd. c | Macerozyme, Pectinase | Tokyo, Japan |
| Esseco Group c | EnartisZym | San Martino, Italy |
| Megazyme International c | Pectate lyase, Pectinase | Wicklow, Ireland |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaissar, F.Z.; Bouacem, K.; Benine, M.L.; Mechri, S.; Sharma, S.R.; Singh, V.K.; Bakli, M.; Lebouachera, S.E.I.; Emiliani, G. Bacillus Pectinases as Key Biocatalysts for a Circular Bioeconomy: From Green Extraction to Process Optimization and Industrial Scale-Up. BioTech 2025, 14, 74. https://doi.org/10.3390/biotech14030074
Kaissar FZ, Bouacem K, Benine ML, Mechri S, Sharma SR, Singh VK, Bakli M, Lebouachera SEI, Emiliani G. Bacillus Pectinases as Key Biocatalysts for a Circular Bioeconomy: From Green Extraction to Process Optimization and Industrial Scale-Up. BioTech. 2025; 14(3):74. https://doi.org/10.3390/biotech14030074
Chicago/Turabian StyleKaissar, Fatima Zohra, Khelifa Bouacem, Mohammed Lamine Benine, Sondes Mechri, Shubha Rani Sharma, Vishal Kumar Singh, Mahfoud Bakli, Seif El Islam Lebouachera, and Giovanni Emiliani. 2025. "Bacillus Pectinases as Key Biocatalysts for a Circular Bioeconomy: From Green Extraction to Process Optimization and Industrial Scale-Up" BioTech 14, no. 3: 74. https://doi.org/10.3390/biotech14030074
APA StyleKaissar, F. Z., Bouacem, K., Benine, M. L., Mechri, S., Sharma, S. R., Singh, V. K., Bakli, M., Lebouachera, S. E. I., & Emiliani, G. (2025). Bacillus Pectinases as Key Biocatalysts for a Circular Bioeconomy: From Green Extraction to Process Optimization and Industrial Scale-Up. BioTech, 14(3), 74. https://doi.org/10.3390/biotech14030074










