Enhancing In Vitro Regeneration in Three Sweet Potato Genotypes: Interplay Between Disinfectant, Explant Age, and Genotype
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Plant Material
2.3. Preparation of the Culture Medium
2.4. Disinfection of Plant Material
2.4.1. Collection and Preparation
2.4.2. Inoculation
2.5. Evaluated Parameters
2.5.1. Assessment of the Antiseptic Properties of Disinfectants (Clean Culture Rates: CCR)
2.5.2. Assessment of Regeneration Rate (RR)
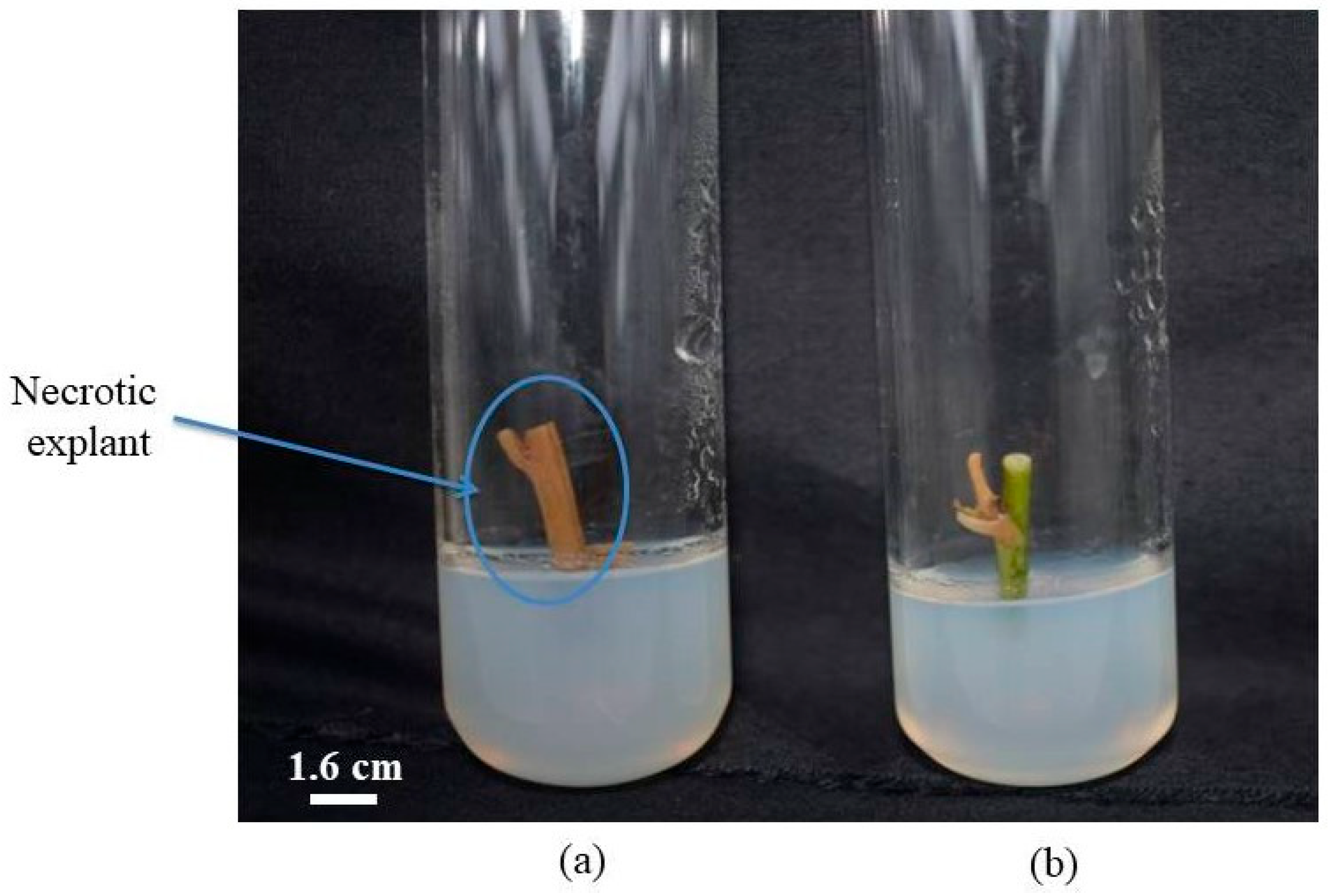
2.5.3. Evaluation of Necrosis Rate (NR)
2.6. Statistical Analysis
3. Results
3.1. Appearance and Color of Contamination Observed in Cultures After Disinfection
3.2. Influence of Sodium Hypochlorite and Mercury Chloride Concentrations on the Clean Culture and Regeneration Rates of Explants
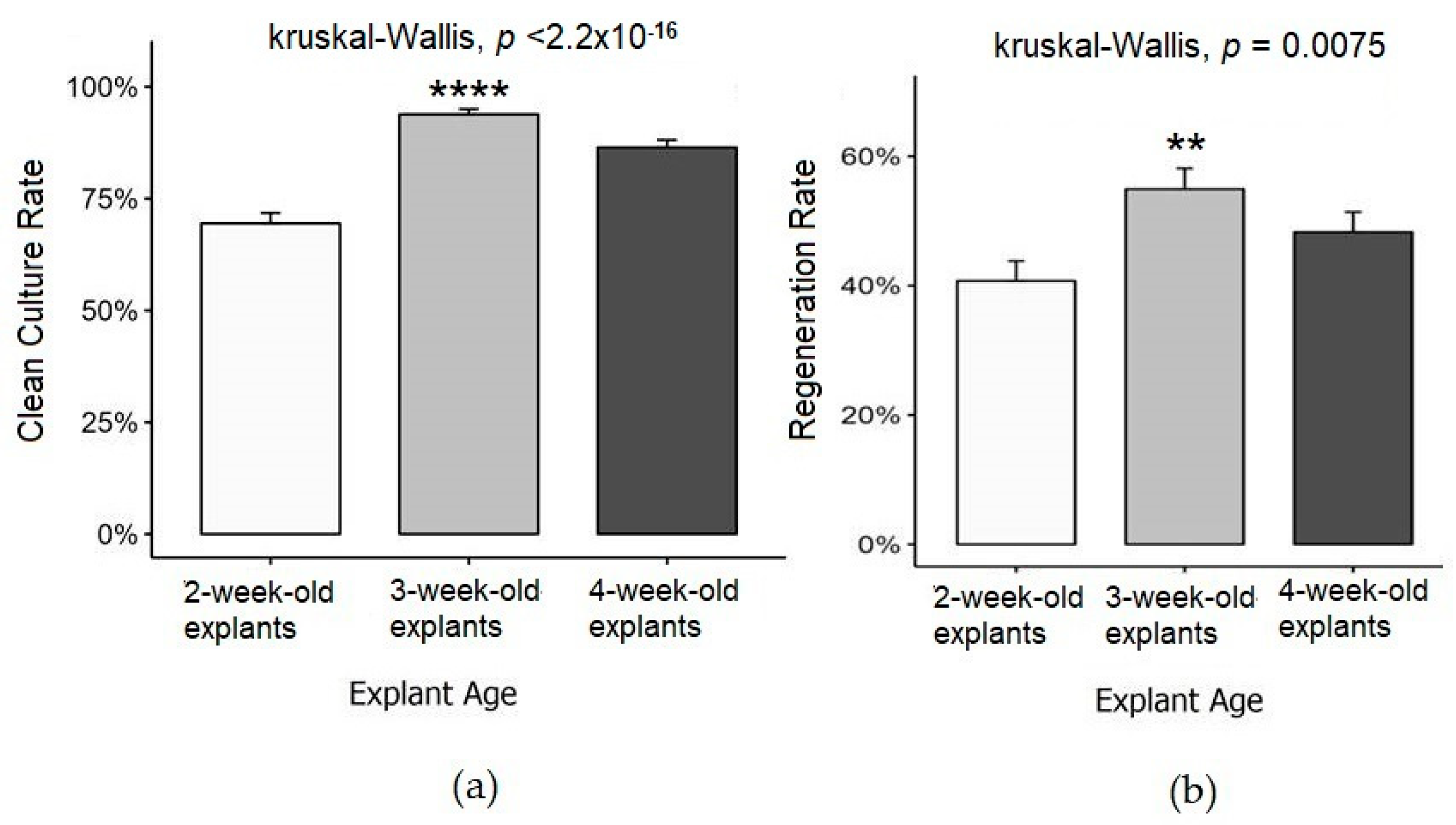
3.3. Effect of Explant Age on Clean Culture and Regeneration Rates
3.4. Effect of Genotypes on Clean Culture and Regeneration Rates
3.5. Simultaneous Influence of Genotypes and Treatments on the In Vitro Clean Culture and Regeneration Rates of Explants
3.6. Simultaneous Influence of Explant Age and Treatments on Clean Culture and Regeneration Rates In Vitro
3.7. Effect of Different Treatments on Necrosis Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monostori, T.; Szarvas, A. A Review on Sweet Potato with Special Focus on Hungarian Production: Utilization, Biology and Transplant Production. Rev. Agric. Rural. Dev. 2015, 4, 68–81. [Google Scholar]
- Drapal, M.; Rossel, G.; Heider, B.; Fraser, P.D. Metabolic Diversity in Sweet Potato (Ipomoea batatas, Lam.) Leaves and Storage Roots. Hortic. Res. 2019, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Dossou-Aminon, I.; Loko, L.Y.; Adjatin, A.; Dansi, A.; Elangovan, M.; Chaudhary, P.; Vodouhè, R.; Sanni, A. Diversity, Genetic Erosion and Farmer’s Preference of Sorghum Varieties [Sorghum bicolor (L.) Moench] in North-Eastern Benin. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 531–552. [Google Scholar]
- Ngailo, S.; Shimelis, H.; Sibiya, J.; Mtunda, K. Sweet Potato Breeding for Resistance to Sweet Potato Virus Disease and Improved Yield: Progress and Challenges. Afr. J. Agric. Res. 2013, 8, 3202–3215. [Google Scholar]
- Adikini, S.; Mukasa, S.B.; Mwanga, R.O.M.; Gibson, R.W. Effects of Sweet Potato Feathery Mottle Virus and Sweet Potato Chlorotic Stunt Virus on the Yield of SweetPotato in Uganda. J. Phytopathol. 2016, 164, 242–254. [Google Scholar] [CrossRef]
- Behera, S.; Chauhan, V.B.S.; Pati, K.; Bansode, V.; Nedunchezhiyan, M.; Verma, A.K.; Monalisa, K.; Naik, P.K.; Naik, S.K. Biology and Biotechnological Aspect of Sweet Potato (Ipomoea batatas L.): A Commercially Important Tuber Crop. Planta 2022, 256, 40. [Google Scholar] [CrossRef]
- Adu Donyina, G.; Szarvas, A.; Opoku, V.A.; Miko, E.; Tar, M.; Czóbel, S.; Monostori, T. Enhancing Sweet Potato Production: A Comprehensive Analysis of the Role of Auxins and Cytokinins in Micropropagation. Planta 2025, 261, 74. [Google Scholar] [CrossRef]
- Acedo, V.Z. Meristem Culture and Micropropagation of Cassava. J. Root Crops 2002, 28, 1–7. [Google Scholar]
- Shiji, R.; George, J.; Sunitha, S.; Muthuraj, R. Micropropagation for Rapid Multiplication of Planting Material in Cassava (Manihot Esculenta Crantz). J. Root Crops 2014, 40, 23–30. [Google Scholar]
- Sivanesan, I.; Muthu, M.; Gopal, J.; Tasneem, S.; Kim, D.-H.; Oh, J.-W. A Fumigation-Based Surface Sterilization Approach for Plant Tissue Culture. Int. J. Environ. Res. Public Health 2021, 18, 2282. [Google Scholar] [CrossRef]
- Hashim, S.N.; Ghazali, S.Z.; Sidik, N.J.; Chia-Chay, T.; Saleh, A. Surface Sterilization Method for Reducing Contamination of Clinacanthus Nutans Nodal Explants Intended for In-Vitro Culture. In E3S Web of Conferences; EDP Sciences: London, UK, 2021; Volume 306, p. 01004. [Google Scholar]
- Doussoh, A.M.; Dangou, J.S.; Cacaï, G.H.; Houedjissin, S.S.; Ahanhanzo, C. Effect of Cytokinins and Auxin on Bud Burst and Direct Organogenesis In Vitro of Some Sweet Potato Landraces (Ipomoea batatas L.) Grown in Benin. J. Appl. Biosci. 2018, 131, 13347–13358. [Google Scholar] [CrossRef]
- Pérez-Pazos, J.; Rosero, A.; Cardinale, M.; Gámez, R. Development of Control Strategies for Bacteria and Fungi Associated with a Micropropagated New Cultivar of Orange-Fleshed Sweet Potato (Ipomoea batatas Cv. Agrosavia–Aurora). Hortic. Environ. Biotechnol. 2023, 64, 859–875. [Google Scholar] [CrossRef]
- Ahanhanzo, C.; Adoukonou-Sagbadja, H.; Yehouessi, W.; Ahoton, L.; Ganglo, J.C.; Djikpo-Tchibozo, M.A.; Agbidinoukoun, A.; Agbangla, C. Étude de l’influence Du Chlorure Mercurique Sur La Survie in Vitro d’explants et de l’aptitude à La Régénération de Teck (Tectona grandis L. f., Verbenaceae). J. Appl. Biosci. 2013, 65, 4935–4944. [Google Scholar] [CrossRef]
- Yesmin, S.; Hashem, A.; Khatun, M.M.; Nasrin, S.; Tanny, T.; Islam, M.S. In Vitro Clonal Propagation of BARI Ada-1 (Zingiber officinale Rosc.). Jahangirnagar Univ. J. Biol. Sci. 2015, 4, 53–57. [Google Scholar] [CrossRef]
- Jena, R.C.; Samal, K.C. Endogenous Microbial Contamination during in Vitro Culture of Sweet Potato [Ipomoea batatas (L.) Lam]: Identification and Prevention. J. Agric. Technol. 2011, 7, 1725–1731. [Google Scholar]
- Bakhsh, A.; Hussain, T.; Caliskan, M.E. A Promising and Cost Effective Surface Sterilizing Method for Sweet Potato (Ipomoea batatas L.) Cultivated in Open Environment. Fresen. Environ. Bull. 2017, 26, 3062–3067. [Google Scholar]
- Amissah, S.; Coleman, P.A.; Sintim, H.Y.; Akromah, R. In Vitro Control of Microbial Contamination of Sweet Potatoes Cultured with Nodal Explants. Annu. Res. Rev. Biol. 2016, 9, 1. [Google Scholar] [CrossRef]
- Alula, K.; Zeleke, H.; Manikandan, M. In Vitro Propagation of Sweet Potato (Ipomoea batatas (L.) Lam.) through Apical Meristem Culture. J. Pharmacogn. Phytochem. 2018, 7, 2386–2392. [Google Scholar]
- Yadav, R.; Arora, P.; Kumar, D.; Katyal, D.; Dilbaghi, N.; Chaudhury, A. High Frequency Direct Plant Regeneration from Leaf, Internode, and Root Segments of Eastern Cottonwood (Populus deltoides). Plant Biotechnol. Rep. 2009, 3, 175–182. [Google Scholar] [CrossRef]
- Onwubiko, N.C.; Nkogho, C.S.; Anyanwu, C.P.; Onyeishi, G.C. Effect of Different Concentration of Sterilant and Exposure Time on Sweet Potato (Ipomoea batatas Lam) Explants. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 14–20. [Google Scholar]
- Doussoh, A.M.; Dangou-Sossou, J.; Houédjissin, S.S.; Cacaï, G.H.T.; Assogba, A.K.; Ahanhanzo, C. Influence of Mercuric Chloride on Survival and Suitability for In Vitro Regeneration of Three Sweet Potato Landraces (Ipomoea batatas L.) Produced in Benin. Int. J. Curr. Res. Biosci. Plant Biol. 2017, 4, 56–64. [Google Scholar] [CrossRef]
- Hammond, R.; Buah, J.N.; Asare, P.A.; Acheampong, S. Optimizing Sterilization Condition for the Initiation of Sweet Potato (Ipomoea batatas) Culture In Vitro. Asian J. Biotechnol. 2014, 6, 25–37. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Wanjala, B.W.; Srinivasulu, R.; Makokha, P.; Ssali, R.T.; McEwan, M.; Kreuze, J.F.; Low, J.W. Improving Rapid Multiplication of Sweetpotato (Ipomoea batatas L.(Lam) Pre-Basic Seed Using Sandponics Technology in East Africa. Exp. Agric. 2020, 56, 347–354. [Google Scholar] [CrossRef]
- Abrham, T.; Beshir, H.M.; Haile, A. Sweetpotato Production Practices, Constraints, and Variety Evaluation under Different Storage Types. Food Energy Secur. 2021, 10, e263. [Google Scholar] [CrossRef]
- Delgado-Paredes, G.E.; Idrogo, C.R.; Chanamé-Céspedes, J.; Floh, E.I.; Handro, W. In Vitro Direct Organogenesis in Roots of Ipomoea Batatas. Asian J. Plant Sci. Res. 2016, 6, 17–27. [Google Scholar]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A Review on the Plant Microbiome: Ecology, Functions, and Emerging Trends in Microbial Application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Santos, L.F.; Olivares, F.L. Plant Microbiome Structure and Benefits for Sustainable Agriculture. Curr. Plant Biol. 2021, 26, 100198. [Google Scholar] [CrossRef]
- Lazo-Javalera, M.F.; Troncoso-Rojas, R.; Tiznado-Hernández, M.E.; Martínez-Tellez, M.A.; Vargas-Arispuro, I.; Islas-Osuna, M.A.; Rivera-Domínguez, M. Surface Disinfection Procedure and in Vitro Regeneration of Grapevine (Vitis vinifera L.) Axillary Buds. SpringerPlus 2016, 5, 453. [Google Scholar] [CrossRef]
- Carmello, C.R.; Cardoso, J.C. Effects of Plant Extracts and Sodium Hypochlorite on Lettuce Germination and Inhibition of Cercospora Longissima In Vitro. Sci. Hortic. 2018, 234, 245–249. [Google Scholar] [CrossRef]
- Ponce de León, I.; Montesano, M. Adaptation Mechanisms in the Evolution of Moss Defenses to Microbes. Front. Plant Sci. 2017, 8, 366. [Google Scholar] [CrossRef] [PubMed]
- Aguoru, C.; Amuzie, U. Associated Microbial Contaminants in In-Vitro Micropropagation of Sweet Potato (Ipomoea batatas l). Int. J. Nat. Appl. Sci. 2009, 5. [Google Scholar] [CrossRef]
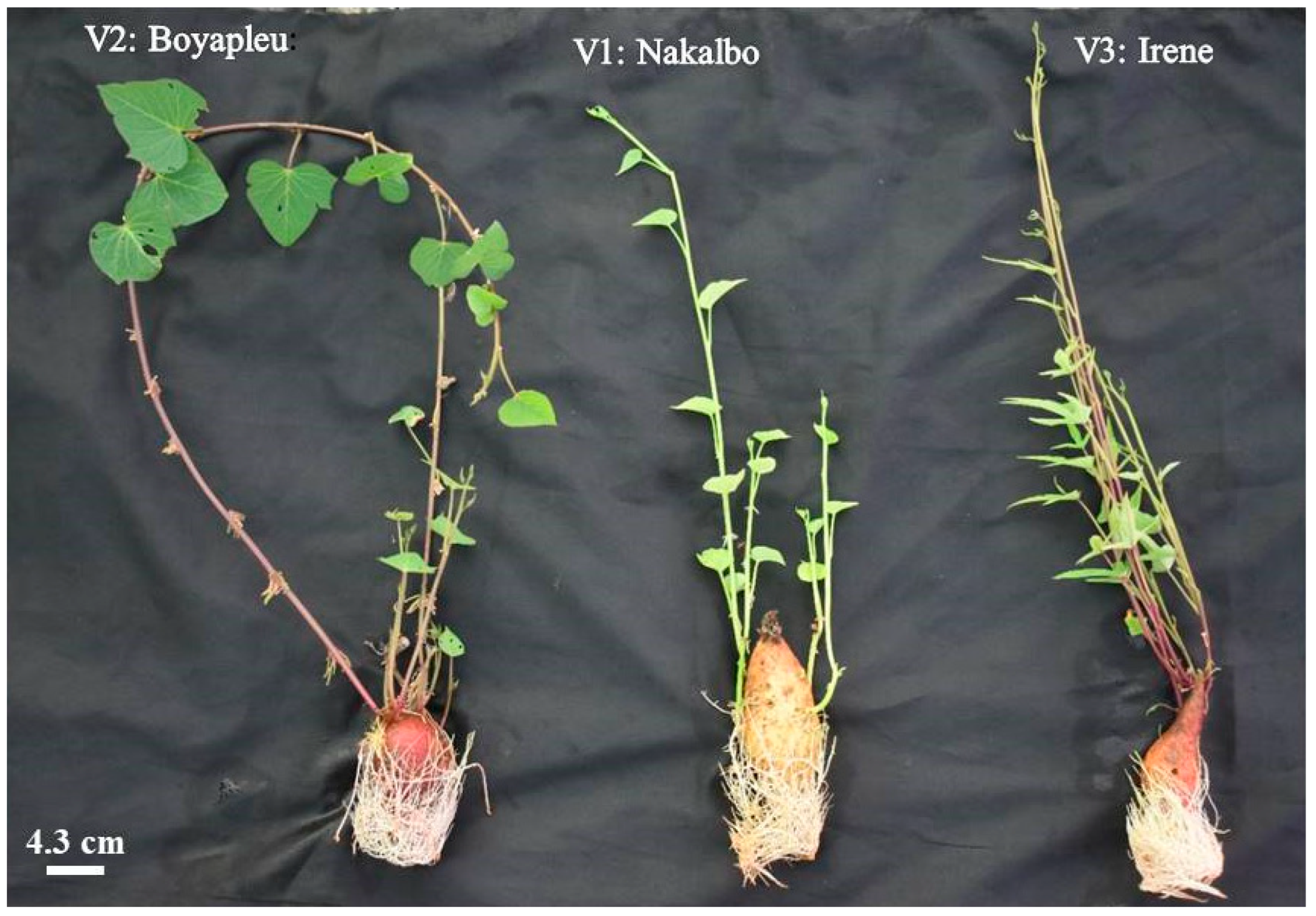





| Characters | Genotypes | ||
|---|---|---|---|
| Nakalbo | Boyapleu | Irene | |
| Origin | Côte d’Ivoire | Burkina Faso | Burkina Faso |
| Plant type | Spreading | Spreading | Erect |
| General leaf outline | Cordate | Cordate | Hastate |
| Leaf lobe number | 1 | 3 | 3 |
| Skin color | Cream | purple-red | purple-red |
| Flesh color | White | White | Orange |
| Type of Agent Disinfecting | Concentration (%) | Treatments Codes |
|---|---|---|
| Sodium hypochlorite (NaOCl) | 10.00 | SH1 |
| 20.00 | SH2 | |
| 30.00 | SH3 | |
| 40.00 | SH4 | |
| Mercuric chloride (HgCl2) | 0.05 | MC1 |
| 0.10 | MC2 | |
| 0.20 | MC3 | |
| 0.30 | MC4 |
| Treatments | Clean Culture Rates (%) | Regeneration Rates (%) |
|---|---|---|
| 10% of NaOCl | 75.72± 3.36 b | 86.83± 3.02 a |
| 20% of NaOCl | 67.48 ± 3.92 c | 78.18 ± 3.56 ab |
| 30% of NaOCl | 75.72 ± 3.31 b | 77.36 ± 4.04 b |
| 40% of NaOCl | 74.48 ±3.71 bc | 66.66 ± 4.72 c |
| 0.05% of HgCl2 | 91.58 ± 3.5 a | 62.13 ± 4.78 c |
| 0.1% of HgCl2 | 93.82 ± 1.95 a | 10.28 ± 2.90 d |
| 0.2% of HgCl2 | 94.23 ± 1.92 a | 1.23 ± 0.70 d |
| 0.3% of HgCl2 | 93.00 ± 2.16 a | 1.23 ± 0.70 d |
| p | <0.0001 | <0.0001 |
| Genotypes | Treatments | Clean Culture Rates (%) | Regeneration Rate (%) |
|---|---|---|---|
| Nakalbo | 10% of NaOCl | 64.19 ± 4.15 h | 92.59 ± 2.90 l |
| 20% of NaOCl | 51.85 ± 5.53 i | 77.78 ± 4.87 m | |
| 30% of NaOCl | 71.60 ± 3.91 g | 56.79 ± 5.87 jk | |
| 40% of NaOCl | 62.96 ± 4.59 h | 34.57 ± 5.83 l | |
| 0.05% of HgCl2 | 87.65 ± 3.59 cd | 62.96 ± 6.33 de | |
| 0.1% of HgCl2 | 86.41 ± 3.38 cde | 0.00 ± 0.00 ef | |
| 0.2% of HgCl2 | 88.88 ± 3.55 c | 0.00 ± 0.00 d | |
| 0.3% of HgCl2 | 80.24 ± 4.02 f | 0.00 ± 0.00 h | |
| Boyapleu | 10% of NaOCl | 79.01 ± 4.00 f | 81.48 ± 4.23 hi |
| 20% of NaOCl | 72.83 ± 3.98 g | 83.95 ± 4.42 j | |
| 30% of NaOCl | 85.18 ± 3.16 de | 83.95 ± 4.42 fg | |
| 40% of NaOCl | 71.60 ± 5.58 g | 86.42 ± 3.82 jk | |
| 0.05% of HgCl2 | 98.76 ± 0.87 ab | 66.67 ± 5.62 ab | |
| 0.1% of HgCl2 | 97.53 ± 1.74 ab | 18.52 ± 4.92 bc | |
| 0.2% of HgCl2 | 97.53 ± 1.21 ab | 0.00 ± 0.00 bc | |
| 0.3% of HgCl2 | 98.76 ± 0.87 ab | 0.00 ± 0.00 ab | |
| Irene | 10% of NaOCl | 83.95 ± 3.85 e | 86.42 ± 3.82 g |
| 20% of NaOCl | 77.77 ± 4.17 f | 72.84 ± 3.78 i | |
| 30% of NaOCl | 70.37 ± 4.76 g | 91.36 ± 2.97 k | |
| 40% of NaOCl | 88.88 ± 2.17 c | 79.01 ± 4.89 d | |
| 0.05% of HgCl2 | 87.65 ± 3.36 cd | 56.79 ± 5.73 de | |
| 0.1% of HgCl2 | 97.53 ± 1.21 ab | 12.35 ± 3.36 bc | |
| 0.2% of HgCl2 | 97.53 ± 1.45 ab | 3.70 ± 1.45 c | |
| 0.3% of HgCl2 | 100.00 ± 0.00 a | 3.70 ± 1.45 a | |
| p | <0.0001 | <0.0001 |
| Age of explants | Treatments | Clean Culture Rates (%) | Regeneration Rate (%) |
|---|---|---|---|
| 4-week-old explants | 10% of NaOCl | 80.24 ± 3.61 h | 80.25 ± 4.40 e |
| 20% of NaOCl | 62.96 ± 4.59 k | 66.67 ± 5.48 g | |
| 30% of NaOCl | 74.07 ± 4.04 j | 66.67 ± 5.33 g | |
| 40% of NaOCl | 85.18 ± 3.40 g | 70.37 ± 5.38 f | |
| 0.05% of HgCl2 | 100.00 ± 0.00 a | 69.14 ± 6.28 f | |
| 0.1% of HgCl2 | 100.00 ± 0.00 a | 30.86 ± 5.17 j | |
| 0.2% of HgCl2 | 98.76 ± 0.87 ab | 0.00 ± 0.00 m | |
| 0.3% of HgCl2 | 90.12 ± 2.76 ef | 2.47 ± 1.21 kl | |
| 3-week-old explants | 10% of NaOCl | 92.59 ± 2.92 d | 93.83 ± 2.52 b |
| 20% of NaOCl | 91.35 ± 2.97 de | 98.77 ± 0.87 a | |
| 30% of NaOCl | 98.76 ± 0.87 ab | 80.25 ± 5.07 e | |
| 40% of NaOCl | 86.41 ± 3.38 g | 79.01 ± 4.73 e | |
| 0.05% of HgCl2 | 97.53 ± 1.21 b | 82.72 ± 3.41 d | |
| 0.1% of HgCl2 | 86.41 ± 3.38 g | 0.00 ± 0.00 m | |
| 0.2% of HgCl2 | 97.53 ± 1.21 b | 3.70 ± 1.45 k | |
| 0.3% of HgCl2 | 100.00 ± 0.00 a | 1.23 ± 0.87 lm | |
| 2-week-old explants | 10% of NaOCl | 54.32 ± 4.38 l | 86.42 ± 3.82 c |
| 20% of NaOCl | 48.14 ± 4.76 n | 69.14 ± 3.96 f | |
| 30% of NaOCl | 54.32 ± 3.80 l | 85.19 ± 4.23 c | |
| 40% of NaOCl | 51.85 ± 5.08 m | 50.62 ± 6.56 h | |
| 0.05% of HgCl2 | 76.54 ± 4.32 i | 34.57 ± 5.55 i | |
| 0.1% of HgCl2 | 95.06 ± 2.06 c | 0.00 ± 0.00 m | |
| 0.2% of HgCl2 | 86.41 ± 3.61 g | 0.00 ± 0.00 m | |
| 0.3% of HgCl2 | 88.88 ± 3.55 f | 0.00 ± 0.00 m | |
| p | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapily, E.H.H.; Kouassi, K.M.; Kouassi, M.K.; Seka, J.S.S.; Tiendrébéogo, F.; Pita, J.S. Enhancing In Vitro Regeneration in Three Sweet Potato Genotypes: Interplay Between Disinfectant, Explant Age, and Genotype. BioTech 2025, 14, 63. https://doi.org/10.3390/biotech14030063
Tapily EHH, Kouassi KM, Kouassi MK, Seka JSS, Tiendrébéogo F, Pita JS. Enhancing In Vitro Regeneration in Three Sweet Potato Genotypes: Interplay Between Disinfectant, Explant Age, and Genotype. BioTech. 2025; 14(3):63. https://doi.org/10.3390/biotech14030063
Chicago/Turabian StyleTapily, El Hadj Hussein, Kan Modeste Kouassi, Marius Konan Kouassi, John Steven S. Seka, Fidèle Tiendrébéogo, and Justin S. Pita. 2025. "Enhancing In Vitro Regeneration in Three Sweet Potato Genotypes: Interplay Between Disinfectant, Explant Age, and Genotype" BioTech 14, no. 3: 63. https://doi.org/10.3390/biotech14030063
APA StyleTapily, E. H. H., Kouassi, K. M., Kouassi, M. K., Seka, J. S. S., Tiendrébéogo, F., & Pita, J. S. (2025). Enhancing In Vitro Regeneration in Three Sweet Potato Genotypes: Interplay Between Disinfectant, Explant Age, and Genotype. BioTech, 14(3), 63. https://doi.org/10.3390/biotech14030063






