Effects of Glycerol and Phenolics on Myceliophthora heterothallica Endoxylanase Expressed in K. phaffii
Abstract
1. Introduction
2. Materials and Methods
2.1. In Silico Analysis
2.1.1. Sequence Analysis
2.1.2. Structural Modeling
2.1.3. Molecular Docking of Phenolic Compounds
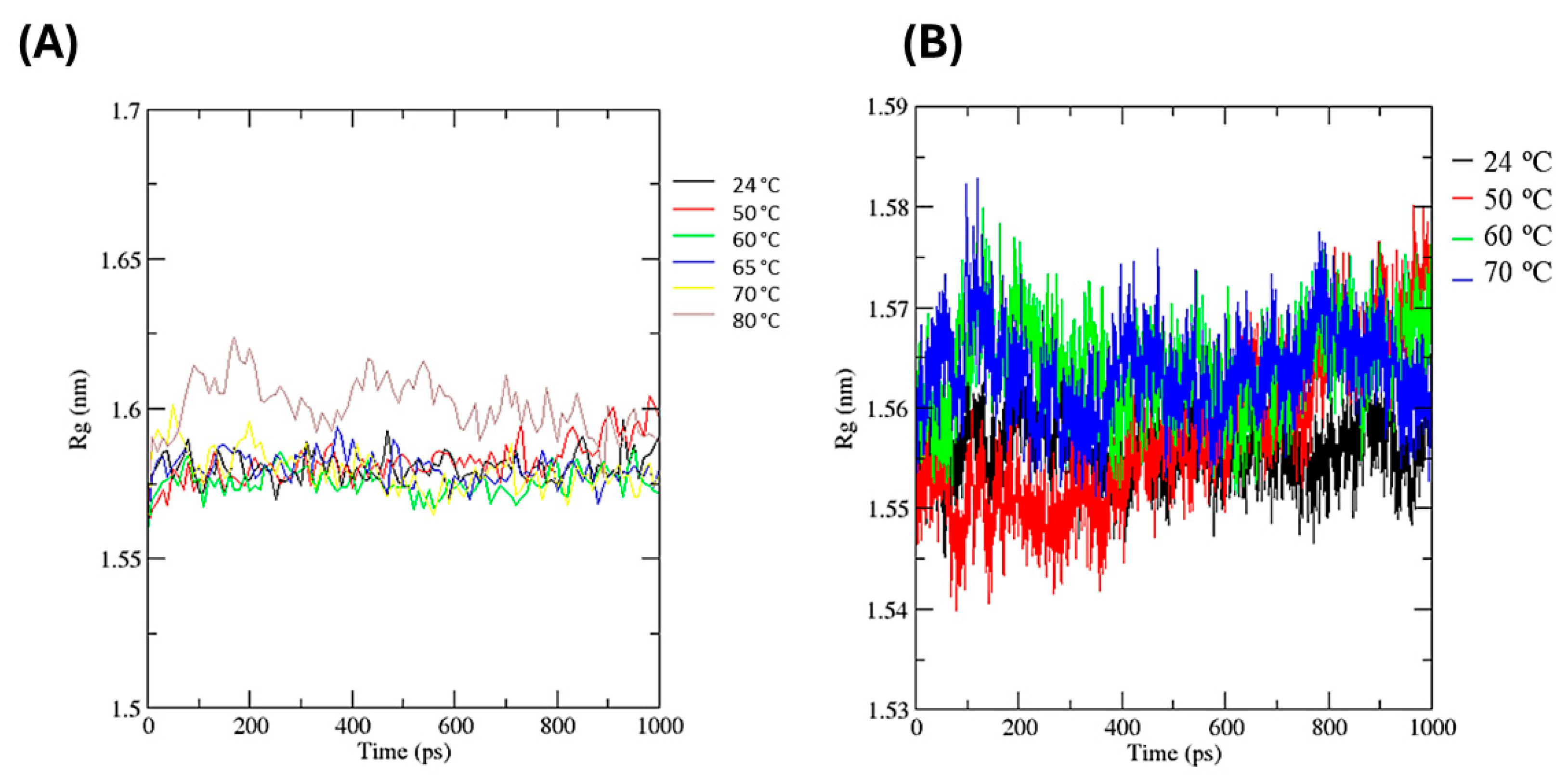
2.1.4. Investigating the Rapid Onset of Glycerol’s Stabilizing Action via Molecular Dynamics
2.2. Enzyme Characterization
2.2.1. Reagents and Materials
2.2.2. Enzyme Assays
2.2.3. Pre-Incubation of the Enzyme with Substances to Be Evaluated
2.2.4. Thermodynamic Parameters
3. Results and Discussions
3.1. Sequence and Structural Analysis in Silico
3.2. Enzyme Kinetics
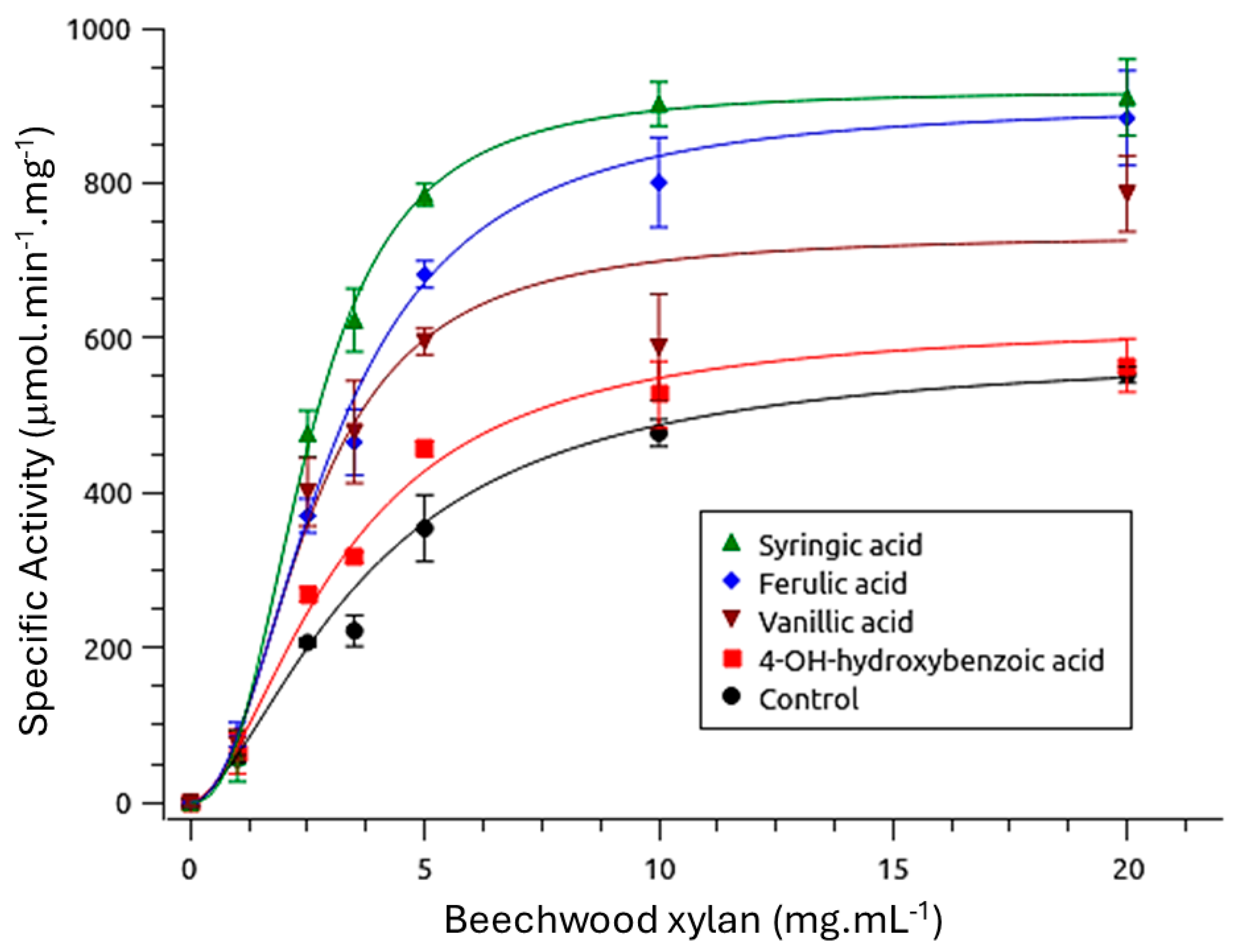
3.2.1. Effect of Phenolics on Enzymatic Kinetics
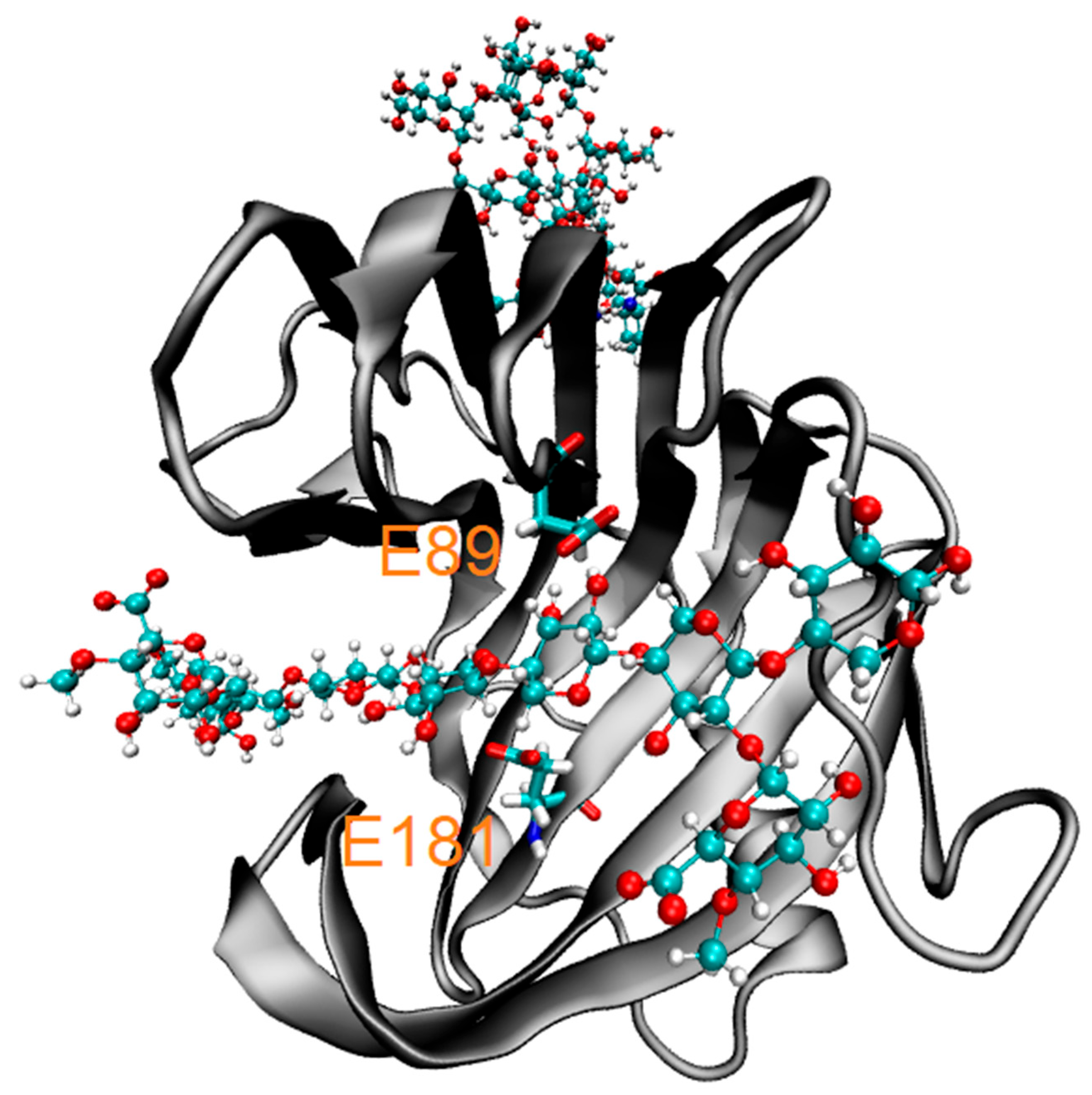
3.2.2. Molecular Docking of Phenolics
3.2.3. Effect of Glycerol on Enzyme Kinetics
3.3. Thermodynamics
3.3.1. Effect of Phenolic Compounds on Thermal Stability
3.3.2. Effect of Glycerol on Thermal Stability
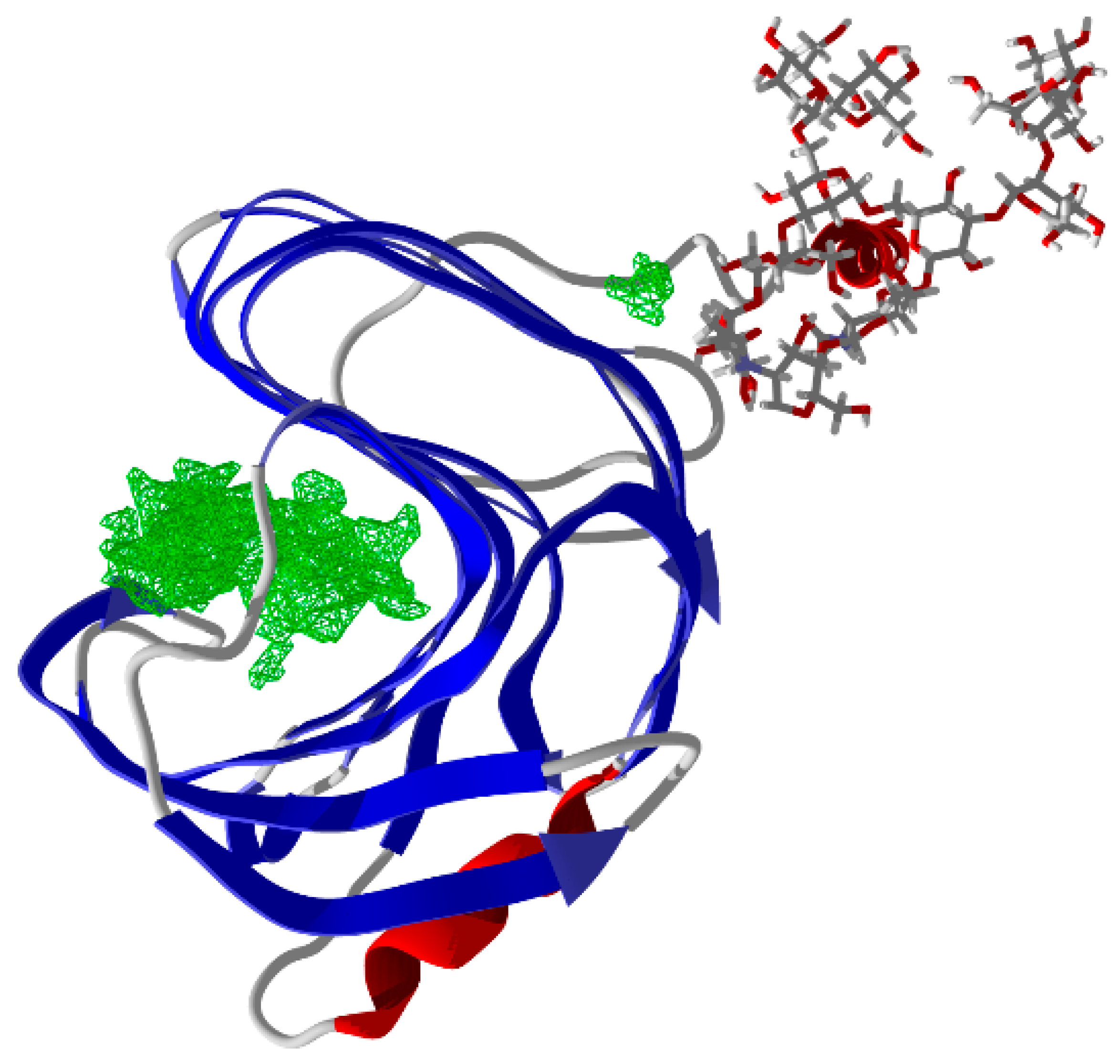
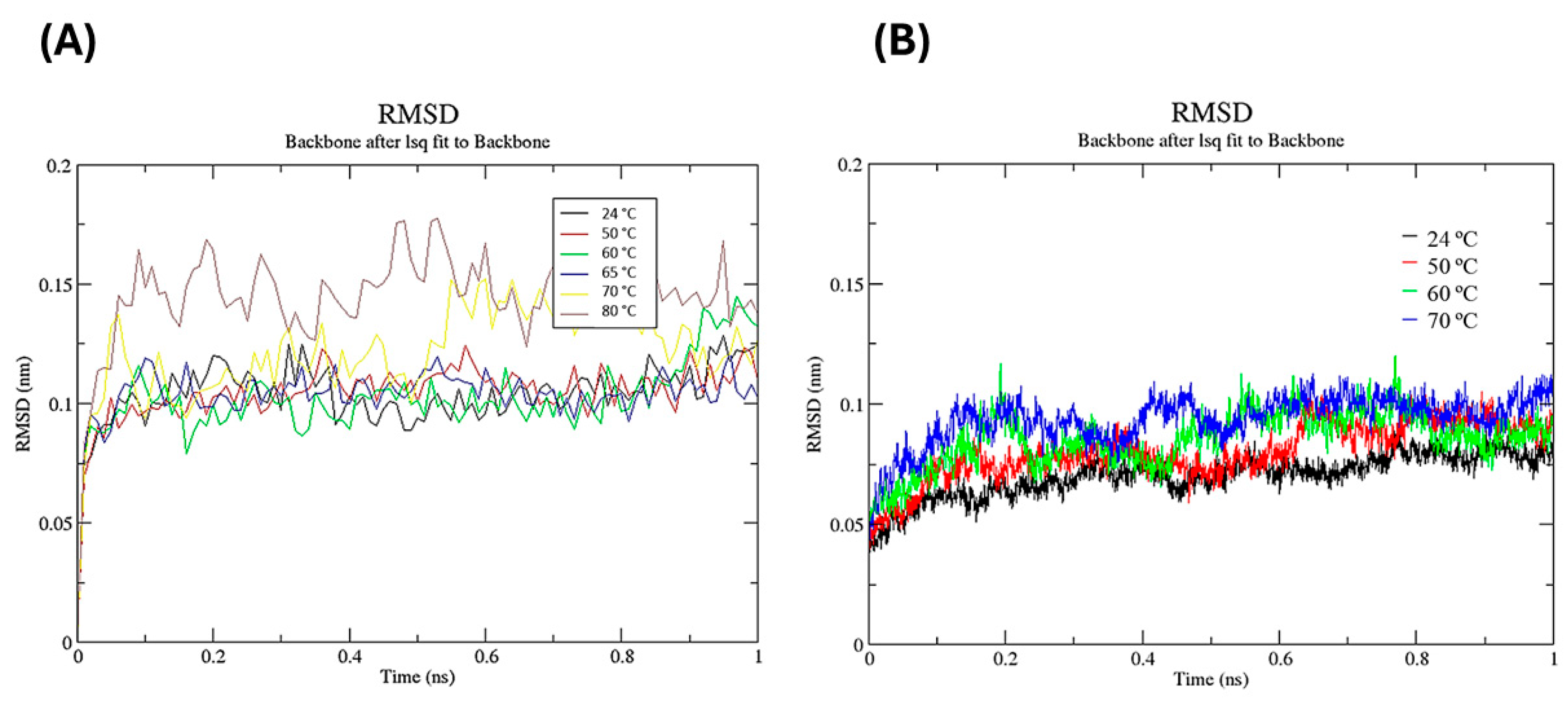
3.3.3. Molecular Dynamics and Glycerol Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| rMhXyn | Recombinant Myceliophthora heterothallica endoxylanase |
| MD | Molecular dynamics |
| XOS | Xylooligosaccharides |
| K′ | Semisaturation constant |
| Vmax | Maximum reaction rate or velocity |
| kcat | Turnover number |
| Ea | Activation energy |
| Q10 | Temperature coefficient |
| T1/2 | Half-life time |
| Ea(D) | Activation energy for thermal denaturation |
| ΔH(D) | Enthalpy of denaturation |
| ΔG(D) | Gibbs free energy of denaturation |
| ΔS(D) | Entropy of denaturation |
| ES | Enzyme–substrate complex |
References
- Bhardwaj, N.; Kumar, B.; Verma, P. A detailed overview of xylanases: An emerging biomolecule for current and future prospective. Bioresour. Bioprocess. 2019, 6, 1–36. [Google Scholar] [CrossRef]
- Bastawde, K.B. Xylan structure, microbial xylanases, and their mode of action. World J. Microbiol. Biotechnol. 1992, 8, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Dimarogona, M.; Topakas, E.; Christakopoulos, P.; Chrysina, E.D. The structure of a GH10 xylanase fromFusarium oxysporum reveals the presence of an extended loop on top of the catalytic cleft. Acta Crystallogr. Sect. D Struct. Biol. 2012, 68, 735–742. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, G.-L.; Chen, C.; Yang, Q.; Luo, X.-M.; Wang, Z.-B.; Wu, A.-M.; Feng, J.-X. A combination of mild chemical pre-treatment and enzymatic hydrolysis efficiently produces xylooligosaccharides from sugarcane bagasse. J. Clean. Prod. 2021, 291, 125972. [Google Scholar] [CrossRef]
- Palaniappan, A.; Antony, U.; Emmambux, M.N. Current status of xylooligosaccharides: Production, characterization, health benefits and food application. Trends Food Sci. Technol. 2021, 111, 506–519. [Google Scholar] [CrossRef]
- Ali, K.; Niaz, N.; Waseem, M.; Ashraf, W.; Hussain, M.; Khalid, M.U.; Bin Tahir, A.; Raza, A.; Khan, I.M. Xylooligosaccharides: A comprehensive review of production, purification, characterization, and quantification. Food Res. Int. 2025, 201, 115631. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kaur, P.; Kaur, J.; Singla, D.; Taggar, M.S. Xylanase, xylooligosaccharide and xylitol production from lignocellulosic biomass: Exploring biovalorization of xylan from a sustainable biorefinery perspective. Ind. Crops Prod. 2024, 215, 118610. [Google Scholar] [CrossRef]
- Gangwar, A.K.; Prakash, N.T.; Prakash, R. Applicability of microbial xylanases in paper pulp bleaching: A review. BioResources 2014, 9, 3733–3754. [Google Scholar] [CrossRef]
- Campestrini, E.; Silva, V.T.M.d.; Appelt, M.D. Utilização de enzimas na alimentação animal. Rev. Eletrônica Nutr. 2005, 2, 254–267. [Google Scholar]
- Yang, X.; Chen, H.; Gao, H.; Li, Z. Bioconversion of corn straw by coupling ensiling and solid-state fermentation. Bioresour. Technol. 2001, 78, 277–280. [Google Scholar] [CrossRef]
- Moretti, M.; Bocchini-Martins, D.A.; Silva, R.D.; Rodrigues, A.; Sette, L.D.; Gomes, E. Selection of thermophilic and thermotolerant fungi for the production of cellulases and xylanases under solid-state fermentation. Braz. J. Microbiol. 2012, 43, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Zha, Z.; Li, J.; Zhang, W.; Bai, Z.; Hu, Y.; Wang, X.; Chen, Y.; Chen, Z.; Wang, J.; et al. Improvement of XYL10C_∆N catalytic performance through loop engineering for lignocellulosic biomass utilization in feed and fuel industries. Biotechnol. Biofuels 2021, 14, 195. [Google Scholar] [CrossRef]
- Stamogiannou, I.; Van Camp, J.; Smagghe, G.; Van de Walle, D.; Dewettinck, K.; Raes, K. Impact of phenolic compound as activators or inhibitors on the enzymatic hydrolysis of cellulose. Int. J. Biol. Macromol. 2021, 186, 174–180. [Google Scholar] [CrossRef]
- Hirai, M.; Ajito, S.; Sugiyama, M.; Iwase, H.; Takata, S.-i.; Shimizu, N.; Igarashi, N.; Martel, A.; Porcar, L. Direct Evidence for the Effect of Glycerol on Protein Hydration and Thermal Structural Transition. Biophys. J. 2018, 115, 313–327. [Google Scholar] [CrossRef]
- Ait Braham, S.; Siar, E.H.; Arana-Peña, S.; Bavandi, H.; Carballares, D.; Morellon-Sterling, R.; de Andrades, D.; Kornecki, J.F.; Fernandez-Lafuente, R. Positive effect of glycerol on the stability of immobilized enzymes: Is it a universal fact? Process Biochem. 2021, 102, 108–121. [Google Scholar] [CrossRef]
- de Amo, G.S.; Bezerra-Bussoli, C.; da Silva, R.R.; Kishi, L.T.; Ferreira, H.; Gomes, E.; Bonilla-Rodriguez, G.O. Heterologous expression of GH11 xylanase from Myceliophthora heterothallica F.2.1.4 in Pichia pastoris. Biocatal. Agric. Biotechnol. 2024, 61, 103343. [Google Scholar] [CrossRef]
- de Amo, G.S.; Bezerra-Bussoli, C.; da Silva, R.R.; Kishi, L.T.; Ferreira, H.; Mariutti, R.B.; Arni, R.K.; Gomes, E.; Bonilla-Rodriguez, G.O. Heterologous expression, purification and biochemical characterization of a new xylanase from Myceliophthora heterothallica F.2.1.4. Int. J. Biol. Macromol. 2019, 131, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Massarente, V.S.; de Araujo Zanoni, J.; Gomes, E.; Bonilla-Rodriguez, G.O. Biochemical characterization of endoglucanases produced by Myceliophthora thermophila M. 7.7 in solid-state culture. Biocatal. Agric. Biotechnol. 2020, 27, 101684. [Google Scholar] [CrossRef]
- Vardakou, M.; Dumon, C.; Murray, J.W.; Christakopoulos, P.; Weiner, D.P.; Juge, N.; Lewis, R.J.; Gilbert, H.J.; Flint, J.E. Understanding the Structural Basis for Substrate and Inhibitor Recognition in Eukaryotic GH11 Xylanases. J. Mol. Biol. 2008, 375, 1293–1305. [Google Scholar] [CrossRef]
- Wang, J.; Cieplak, P.; Kollman, P.A. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 2000, 21, 1049–1074. [Google Scholar] [CrossRef]
- Peters, G.H.; Frimurer, T.M.; Andersen, J.N.; Olsen, O.H. Molecular Dynamics Simulations of Protein-Tyrosine Phosphatase 1B. I. Ligand-Induced Changes in the Protein Motions. Biophys. J. 1999, 77, 505–515. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Van Gunsteren, W.F.; Berendsen, H.J.C. Gromos Manual; BIOMOS, Biomolecular Software, Laboratory of Physical Chemistry, University of Groningen: Groningen, The Netherlands, 1987. [Google Scholar]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford Clarendon: Oxford, UK, 1989. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Marangoni, A.G. Enzyme Kinetics: A Modern Approach; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Saqib, A.A.N.; Farooq, A.; Iqbal, M.; Hassan, J.U.; Hayat, U.; Baig, S. A thermostable crude endoglucanase produced by Aspergillus fumigatus in a novel solid state fermentation process using isolated free water. Enzym. Res. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Saqib, A.A.; Hassan, M.; Khan, N.F.; Baig, S. Thermostability of crude endoglucanase from Aspergillus fumigatus grown under solid state fermentation (SSF) and submerged fermentation (SmF). Process. Biochem. 2010, 45, 641–646. [Google Scholar] [CrossRef]
- Bonfá, E.C.; de Souza Moretti, M.M.; Gomes, E.; Bonilla-Rodriguez, G.O. Biochemical characterization of an isolated 50 kDa beta-glucosidase from the thermophilic fungus Myceliophthora thermophila M. 7.7. Biocatal. Agric. Biotechnol. 2018, 13, 311–318. [Google Scholar] [CrossRef]
- Trindade, L.V.; Desagiacomo, C.; Polizeli, M.d.L.T.d.M.; Damasio, A.R.d.L.; Lima, A.M.F.; Gomes, E.; Bonilla-Rodriguez, G.O. Biochemical characterization, thermal stability, and partial sequence of a novel exo-polygalacturonase from the thermophilic fungus Rhizomucor pusillus A13. 36 obtained by submerged cultivation. BioMed Res. Int. 2016, 2016, 8653583. [Google Scholar] [CrossRef]
- Siddiqui, K.S.; Saqib, A.A.N.; Rashid, M.H.; Rajoka, M.I. Thermostabilization of carboxymethylcellulase from Aspergillus niger by carboxyl group modification. Biotechnol. Lett. 1997, 19, 325–330. [Google Scholar] [CrossRef]
- Vucinic, J.; Novikov, G.; Montanier, C.Y.; Dumon, C.; Schiex, T.; Barbe, S. A Comparative Study to Decipher the Structural and Dynamics Determinants Underlying the Activity and Thermal Stability of GH-11 Xylanases. Int. J. Mol. Sci. 2021, 22, 5961. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Seo, M.-H.; Kyeong, H.-H.; Kim, E.; Kim, H.-S. Molecular basis for the role of glucokinase regulatory protein as the allosteric switch for glucokinase. Proc. Natl. Acad. Sci. USA 2013, 110, 10171–10176. [Google Scholar] [CrossRef]
- Oliveira, I.C.M.; Garay, A.V.; Souza, A.A.; Valadares, N.F.; Barbosa, J.A.R.G.; Faria, F.P.; Freitas, S.M. Structural and biochemical analysis reveals how ferulic acid improves catalytic efficiency of Humicola grisea xylanase. Sci. Rep. 2022, 12, 11409. [Google Scholar] [CrossRef]
- Moreira, L.R.d.S.; Campos, M.d.C.; de Siqueira, P.H.V.M.; Silva, L.P.; Ricart, C.A.O.; Martins, P.A.; Queiroz, R.M.L.; Filho, E.X.F. Two β-xylanases from Aspergillus terreus: Characterization and influence of phenolic compounds on xylanase activity. Fungal Genet. Biol. 2013, 60, 46–52. [Google Scholar] [CrossRef]
- Mwangi, I.W.; Ngila, J.C.; Ndung’U, P.; Msagati, T.A. Removal of phenolics from aqueous media using quaternised maize tassels. J. Environ. Manag. 2014, 134, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.d.S.; Rogez, H.; Pena, R.d.S. Adsorption capacity of phenolic compounds onto cellulose and xylan. Food Sci. Technol. 2015, 35, 314–320. [Google Scholar] [CrossRef]
- Borjian, N.; Farhadian, S.; Shareghi, B.; Asgharzadeh, S.; Momeni, L.; Ghobadi, S. Binding affinity and mechanism of dicofol-lysozyme interaction: Insights from multi-spectroscopy and molecular dynamic simulations. Int. J. Biol. Macromol. 2025, 308, 142569. [Google Scholar] [CrossRef]
- Driss, D.; Bhiri, F.; Ghorbel, R.; Chaabouni, S.E. Cloning and constitutive expression of His-tagged xylanase GH 11 from Penicillium occitanis Pol6 in Pichia pastoris X33: Purification and characterization. Protein Expr. Purif. 2012, 83, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, K.; Pedroso, M.M.; Wu, B.; Gao, Z.; He, B.; Schenk, G. Sequence- and structure-guided improvement of the catalytic performance of a GH11 family xylanase from Bacillus subtilis. J. Biol. Chem. 2021, 297, 101262. [Google Scholar] [CrossRef]
- Deponte, M.; Sturm, N.; Mittler, S.; Harner, M.; Mack, H.; Becker, K. Allosteric Coupling of Two Different Functional Active Sites in Monomeric Plasmodium falciparum Glyoxalase I. J. Biol. Chem. 2007, 282, 28419–28430. [Google Scholar] [CrossRef]
- Vagenende, V.; Yap, M.G.; Trout, B.L. Mechanisms of Protein Stabilization and Prevention of Protein Aggregation by Glycerol. Biochemistry 2009, 48, 11084–11096. [Google Scholar] [CrossRef]
- Gajardo-Parra, N.F.; Akrofi-Mantey, H.O.; Ascani, M.; Cea-Klapp, E.; Garrido, J.M.; Sadowski, G.; Held, C. Osmolyte effect on enzymatic stability and reaction equilibrium of formate dehydrogenase. Phys. Chem. Chem. Phys. 2022, 24, 27930–27939. [Google Scholar] [CrossRef] [PubMed]
- Tamoliūnas, K.; Galamba, N. Protein Denaturation, Zero Entropy Temperature, and the Structure of Water around Hydrophobic and Amphiphilic Solutes. J. Phys. Chem. B 2020, 124, 10994–11006. [Google Scholar] [CrossRef] [PubMed]
- Saridakis, E.; Donta, K. Protein Thermodynamic Properties, Crystallisation, and the Hofmeister Series. Chempluschem 2024, 89, e202300733. [Google Scholar] [CrossRef] [PubMed]
- Shostka, V.; Shostka, N.; Halilov, S.; Vershitsky, V. Formation of clathrate structures in surface layers of heavily diluted aqueous solutions. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2020; Volume 1697, p. 012244. [Google Scholar]
- Sheng, Y.; Lam, S.S.; Wu, Y.; Ge, S.; Wu, J.; Cai, L.; Huang, Z.; Van Le, Q.; Sonne, C.; Xia, C. Enzymatic conversion of pretreated lignocellulosic biomass: A review on influence of structural changes of lignin. Bioresour. Technol. 2021, 324, 124631. [Google Scholar] [CrossRef]
- Manna, B.; Ghosh, A. Understanding the conformational change and inhibition of hyperthermophilic GH10 xylanase in ionic liquid. J. Mol. Liq. 2021, 332, 115875. [Google Scholar] [CrossRef]


| K′ (mg·mL−1) | Vmax (µmol·min−1·mg−1) | h | Kcat (s−1) | Kcat/K′ (M−1·s−1) | |
|---|---|---|---|---|---|
| Syringic acid | 2.50 ± 0.07 | 919.30 ± 16.29 | 2.57 ± 0.16 | 42.71 ± 0.83 | 17.08 ± 0.15 |
| Ferulic acid | 2.99 ± 0.19 | 903.44 ± 43.44 | 2.07 ± 0.15 | 41.94 ± 2.02 | 14.03 ± 0.22 |
| Vanillic acid | 2.54 ± 0.30 | 733.31 ± 52.00 | 2.20 ± 0.30 | 34.03 ± 2.40 | 13.44 ± 0.63 |
| 4-hydroxybenzoic acid | 3.18 ± 0.62 | 620.91 ± 100.07 | 1.76 ± 0.50 | 28.81 ± 4.64 | 9.10 ± 0.31 |
| Control | 3.72 ± 0.34 | 583.60 ± 25.56 | 1.64 ± 0.20 | 27.08 ± 1.19 | 7.3 ± 0.34 |
| K′ (mg·mL−1) | Vmax (µmol·min−1·mg−1) | h | Kcat (s−1) | Kcat/K′ (M−1·s−1) | |
|---|---|---|---|---|---|
| Glycerol | 2.36 ± 0.11 | 699.9 ± 19.1 | 1.85 ± 0.18 | 34.48 ± 0.89 | 13.77 ± 0.27 |
| Control | 2.41 ± 0.07 | 526.6 ± 1.20 | 2.79 ± 0.20 | 24.43 ± 0.05 | 10.15 ± 0.26 |
| Temperatura | Control | 20% Glycerol | |||||
|---|---|---|---|---|---|---|---|
| °C | K | Q10 | kd (min−1) | T ½ (min) | Q10 | kd (min−1) | T ½ (min) |
| 55 | 313.15 | 1.77 | 0.00793 | 87.39 | 1.50 | 0.00156 | 444.22 |
| 60 | 323.15 | 1.74 | 0.0184 | 37.65 | 1.48 | 0.00859 | 80.67 |
| 65 | 333.15 | 1.71 | 0.0164 | 42.27 | 1.46 | 0.00919 | 75.41 |
| 70 | 343.15 | 1.67 | 0.0203 | 34.14 | 1.45 | 0.00395 | 74.12 |
| Temperature | Control | 20% Glycerol | |||||
|---|---|---|---|---|---|---|---|
| °C | K | ∆HD (kJ·mol−1) | ∆GD (kJ·mol−1) | ∆SD (J K−1·mol−1) | ∆HD (kJ·mol−1) | ∆GD (kJ·mol−1) | ∆SD (J K−1·mol−1) |
| 55 | 328.15 | 57.13 | 93.78 | −111.72 | 116.75 | 98.21 | 56.50 |
| 60 | 333.15 | 57.09 | 92.92 | −107.59 | 116.70 | 95.03 | 65.09 |
| 65 | 338.15 | 57.05 | 94.68 | −111.34 | 116.66 | 96.31 | 60.22 |
| 70 | 343.15 | 57.01 | 95.52 | −112.26 | 116.62 | 97.73 | 55.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanoni, J.d.A.; Zilli, I.K.C.; Pretto, G.d.P.; Seixas, F.A.V.; Lima, M.M.d.F.; Lemos, E.G.d.M.; Gomes, E.; Zazeri, G.; Bonilla-Rodriguez, G.O. Effects of Glycerol and Phenolics on Myceliophthora heterothallica Endoxylanase Expressed in K. phaffii. BioTech 2025, 14, 62. https://doi.org/10.3390/biotech14030062
Zanoni JdA, Zilli IKC, Pretto GdP, Seixas FAV, Lima MMdF, Lemos EGdM, Gomes E, Zazeri G, Bonilla-Rodriguez GO. Effects of Glycerol and Phenolics on Myceliophthora heterothallica Endoxylanase Expressed in K. phaffii. BioTech. 2025; 14(3):62. https://doi.org/10.3390/biotech14030062
Chicago/Turabian StyleZanoni, Jéssica de Araujo, Izabela Karolina Costa Zilli, Guilherme de Paula Pretto, Flavio Augusto Vicente Seixas, Marcela Marques de Freitas Lima, Eliana Gertrudes de Macedo Lemos, Eleni Gomes, Gabriel Zazeri, and Gustavo Orlando Bonilla-Rodriguez. 2025. "Effects of Glycerol and Phenolics on Myceliophthora heterothallica Endoxylanase Expressed in K. phaffii" BioTech 14, no. 3: 62. https://doi.org/10.3390/biotech14030062
APA StyleZanoni, J. d. A., Zilli, I. K. C., Pretto, G. d. P., Seixas, F. A. V., Lima, M. M. d. F., Lemos, E. G. d. M., Gomes, E., Zazeri, G., & Bonilla-Rodriguez, G. O. (2025). Effects of Glycerol and Phenolics on Myceliophthora heterothallica Endoxylanase Expressed in K. phaffii. BioTech, 14(3), 62. https://doi.org/10.3390/biotech14030062








