Monitoring of the Single-Cell Behavior of an Escherichia coli Reporter Strain Producing L-phenylalanine in a Scale-Down Bioreactor by Automated Real-Time Flow Cytometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Cryopreservation
2.2. Growth Medium and Preculture Preparation
2.3. L-phenylalanine Production Process in the Stirred-Tank Bioreactor
2.4. L-phenylalanine Production Process in the Two-Compartment Bioreactor
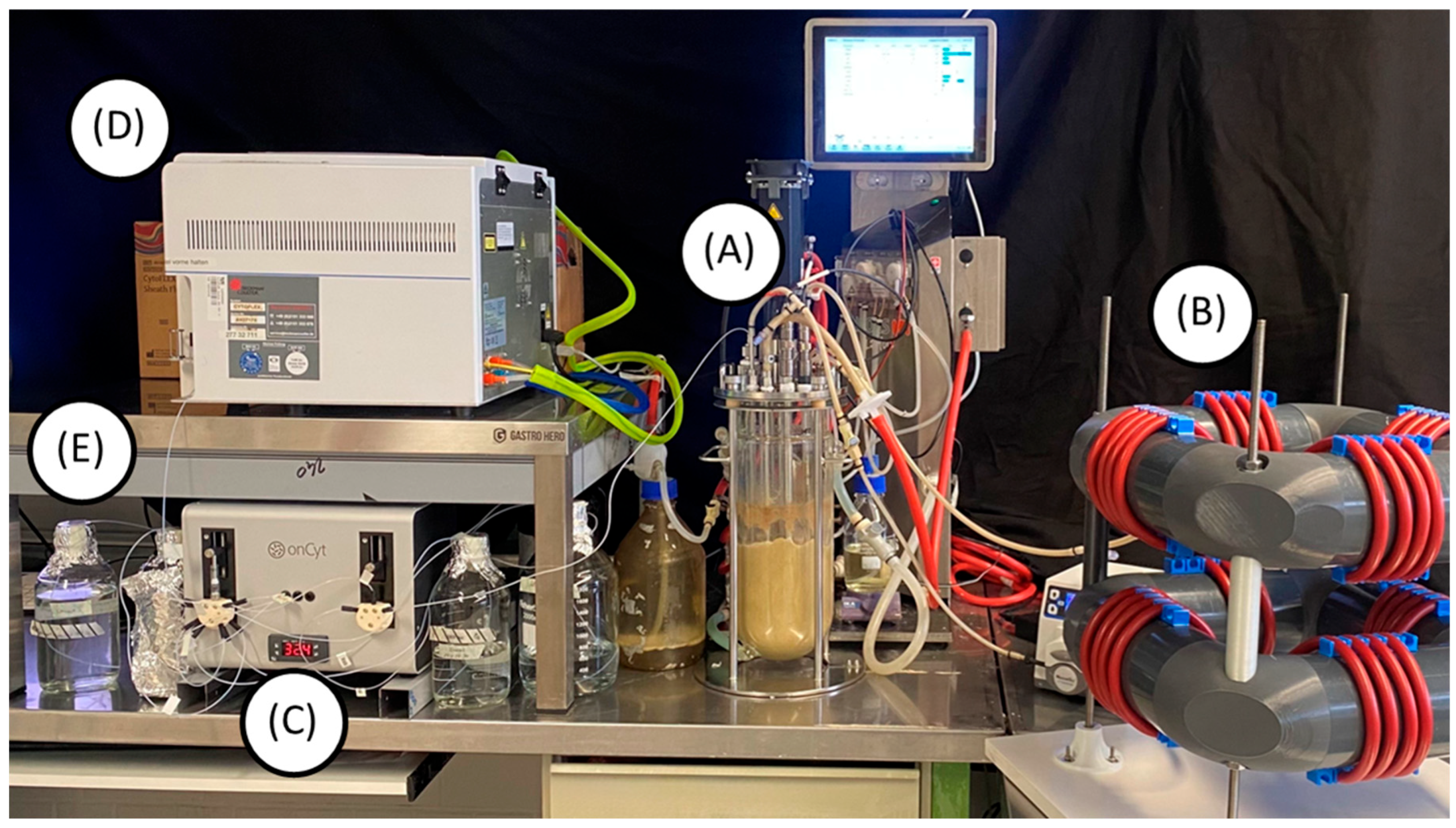
2.5. OC-300 Automation Add-On for Automated Real-Time Flow Cytometry
2.6. Analysis of Optical Density and Biomass Concentration
2.7. Quantification of Sugars, Organic Acids, and Amino Acids
2.8. Evaluation of Flow Cytometry Data
3. Results
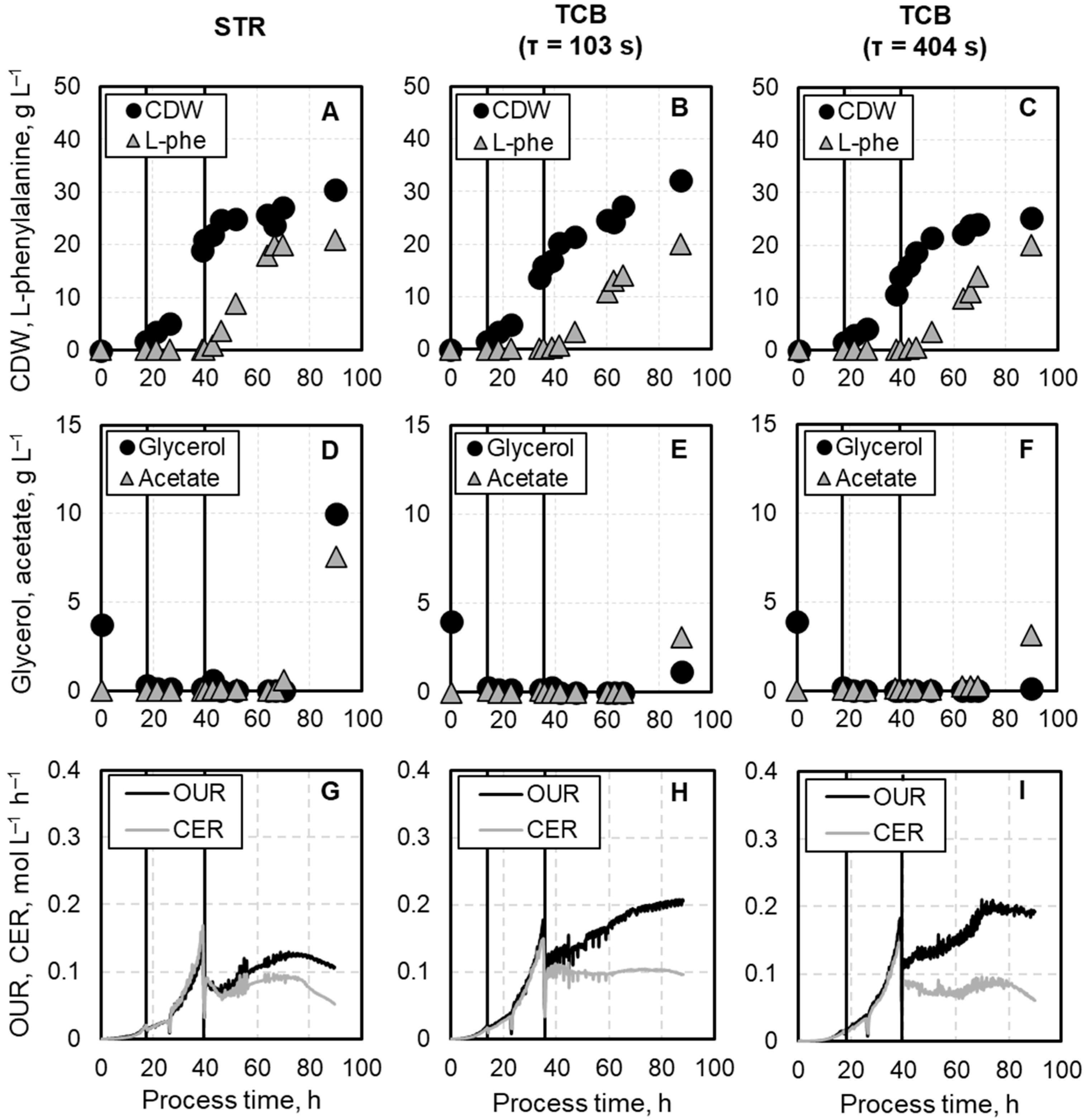
3.1. Comparison of Process Performances in STR and TCB
3.2. Evaluation of the Median Fluorescence Intensities (MFI) and Correlation to Specific Process Features
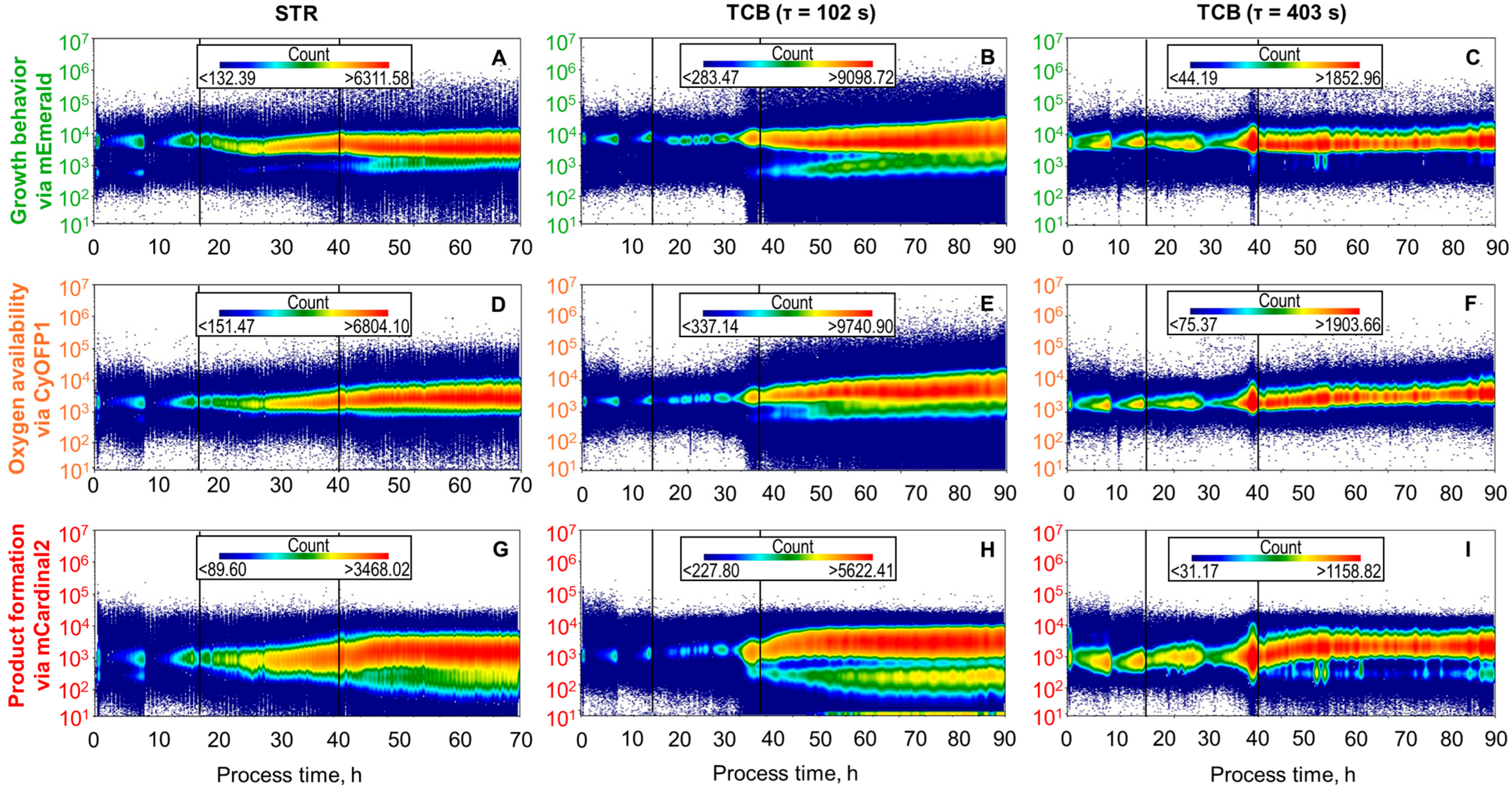
3.3. Single-Cell Fluorescence Distributions During L-phenylalanine Production in STR and TCB
3.4. Impact of Residence Time Versus Exposure Frequency on Population Heterogeneity
4. Discussion
4.1. Advantages of Automated Real-Time Flow Cytometry
4.2. Comparison of Process Performance
4.3. Comparison of the Median Fluorescence Intensities
4.4. Investigation of Population Heterogeneity Under Fluctuating Conditions
4.5. Influence of Residence Time on Population Heterogeneity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| STR | Stirred-tank bioreactor |
| CFI | Coiled flow inverter |
| ART-FCM | Automated real-time flow cytometry |
| TCB | Two-compartment bioreactor |
| DO | Dissolved oxygen |
| SDB | Scale-down bioreactor |
| OD600 | Optical density at 600 nm |
| CDW | Cell dry weight |
| PBS | Phosphate-buffered saline solution |
| HPLC | High-performance liquid chromatography |
| MMR | Mean-to-median ratio |
Appendix A
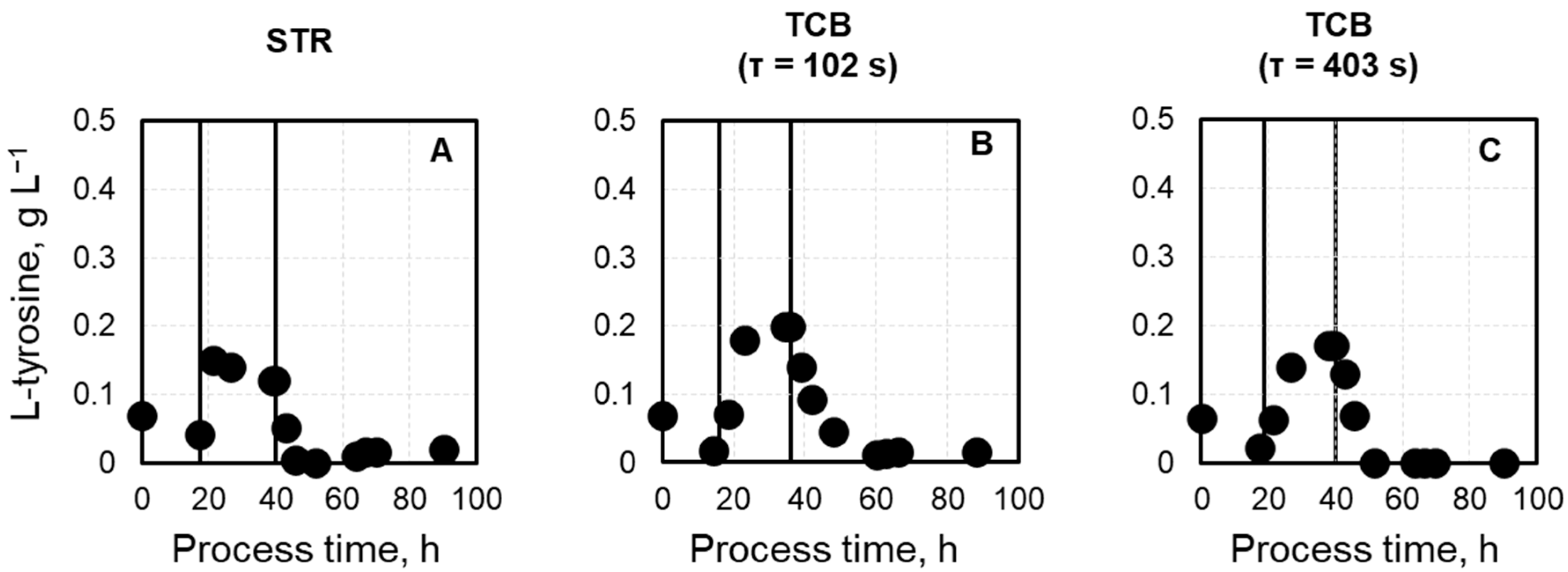
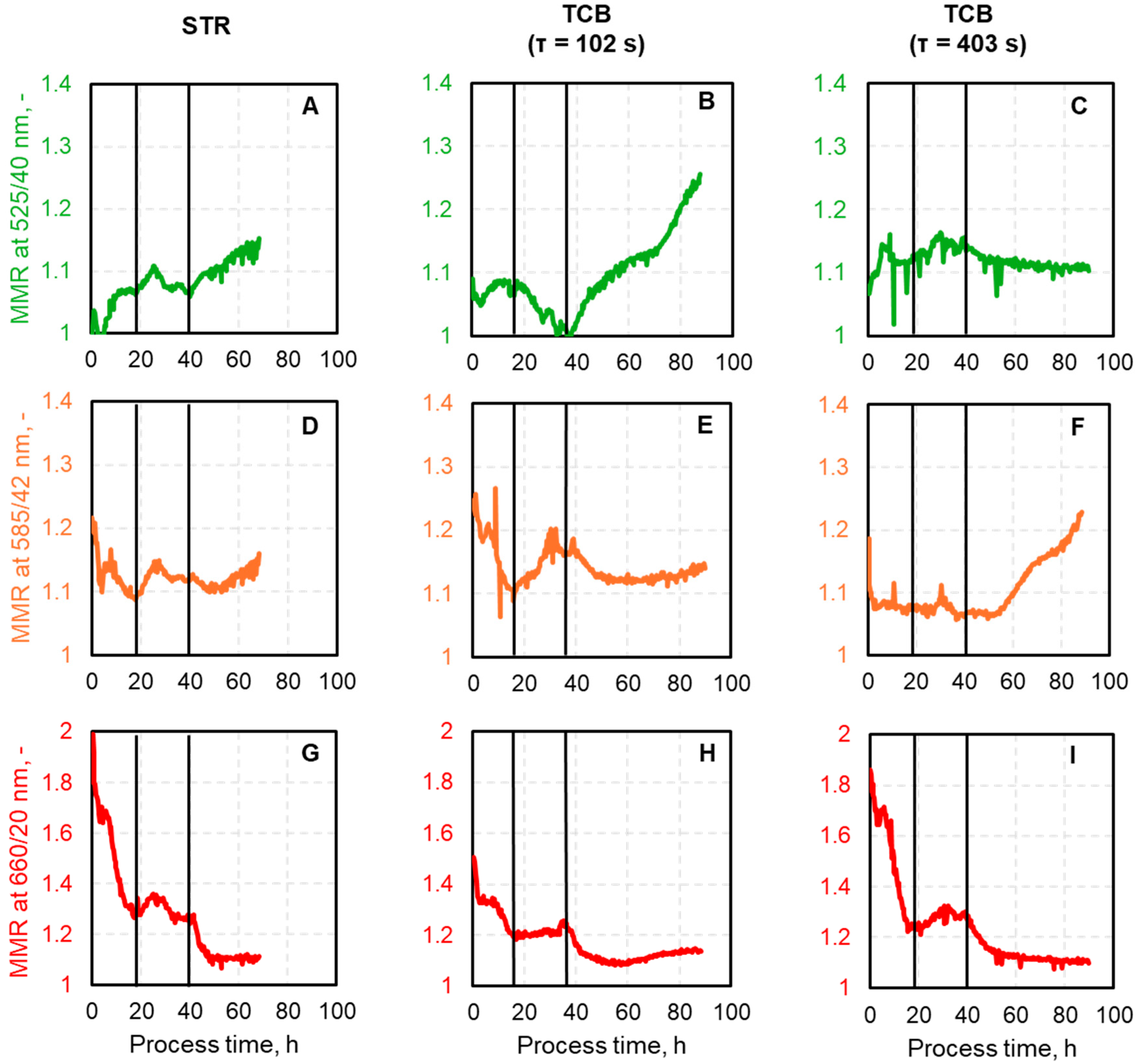
Appendix B

References
- Woodley, J.M. Towards the Sustainable Production of Bulk-Chemicals Using Biotechnology. New Biotechnol. 2020, 59, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Bajić, B.; Vučurović, D.; Vasić, Đ.; Jevtić-Mučibabić, R.; Dodić, S. Biotechnological Production of Sustainable Microbial Proteins from Agro-Industrial Residues and By-Products. Foods 2023, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Enfors, S.-O.; Jahic, M.; Rozkov, A.; Xu, B.; Hecker, M.; Jürgen, B.; Krüger, E.; Schweder, T.; Hamer, G.; O’Beirne, D.; et al. Physiological Responses to Mixing in Large Scale Bioreactors. J. Biotechnol. 2001, 85, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, P.; Junne, S. Scale-down Simulators for Metabolic Analysis of Large-Scale Bioprocesses. Curr. Opin. Biotechnol. 2010, 21, 114–121. [Google Scholar] [CrossRef]
- Heins, A.L.; Weuster-Botz, D. Population Heterogeneity in Microbial Bioprocesses: Origin, Analysis, Mechanisms, and Future Perspectives. Bioprocess Biosyst. Eng. 2018, 41, 889–916. [Google Scholar] [CrossRef]
- Heins, A.-L.; Reyelt, J.; Schmidt, M.; Kranz, H.; Weuster-Botz, D. Development and Characterization of Escherichia coli Triple Reporter Strains for Investigation of Population Heterogeneity in Bioprocesses. Microb. Cell Fact. 2020, 19, 14. [Google Scholar] [CrossRef]
- Täuber, S.; Blöbaum, L.; Steier, V.; Oldiges, M.; Grünberger, A. Microfluidic Single-Cell Scale-down Bioreactors: A Proof-of-Concept for the Growth of Corynebacterium Glutamicum at Oscillating pH Values. Biotechnol. Bioeng. 2022, 119, 3194–3209. [Google Scholar] [CrossRef]
- Arulrajah, P.; Lievonen, A.E.; Subaşı, D.; Pagal, S.; Weuster-Botz, D.; Heins, A.-L. Scale-down Bioreactors—Comparative Analysis of Configurations. Bioprocess Biosyst. Eng. 2025. [Google Scholar] [CrossRef]
- Updegrove, T.B.; Delerue, T.; Anantharaman, V.; Cho, H.; Chan, C.; Nipper, T.; Choo-Wosoba, H.; Jenkins, L.M.; Zhang, L.; Su, Y.; et al. Altruistic Feeding and Cell-Cell Signaling during Bacterial Differentiation Actively Enhance Phenotypic Heterogeneity. Sci. Adv. 2024, 10, eadq0791. [Google Scholar] [CrossRef]
- Hoang, M.D.; Polte, I.; Frantzmann, L.; von den Eichen, N.; Heins, A.-L.; Weuster-Botz, D. Impact of Mixing Insufficiencies on L-Phenylalanine Production with an Escherichia coli Reporter Strain in a Novel Two-Compartment Bioreactor. Microb. Cell Fact. 2023, 22, 153. [Google Scholar] [CrossRef]
- Gottlieb, K. Nutzung von Glycerin und Rohglycerin als C-Quelle für die Produktion von L-Phenylalanin mit rekombinanten Escherichia coli-Stämmen. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2011. [Google Scholar]
- Gottlieb, K.; Albermann, C.; Sprenger, G.A. Improvement of L-Phenylalanine Production from Glycerol by Recombinant Escherichia coli Strains: The Role of Extra Copies of glpK, glpX, and tktA Genes. Microb. Cell Factories 2014, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.; Trö Ndle, J.; Albermann, C.; Sprenger, G.A.; Weuster-Botz, D. Carbon Storage in Recombinant Escherichia coli During Growth on Glycerol and Lactic Acid. Biotechnol. Bioeng. 2014, 111, 2508–2519. [Google Scholar] [CrossRef]
- Arnoldini, M.; Heck, T.; Blanco-Fernández, A.; Hammes, F. Monitoring of Dynamic Microbiological Processes Using Real-Time Flow Cytometry. PLoS ONE 2013, 8, e80117. [Google Scholar] [CrossRef] [PubMed]
- Kuystermans, D.; Avesh, M.; Al-Rubeai, M. Online Flow Cytometry for Monitoring Apoptosis in Mammalian Cell Cultures as an Application for Process Analytical Technology. Cytotechnology 2016, 68, 399–408. [Google Scholar] [CrossRef]
- Heins, A.-L.; Hoang, M.D.; Weuster-Botz, D. Advances in Automated Real-Time Flow Cytometry for Monitoring of Bioreactor Processes. Eng. Life Sci. 2022, 22, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Natarajan, A.; Srienc, F. A Flow Injection Flow Cytometry System for On-Line Monitoring of Bioreactors. Biotechnol. Bioeng. 1999, 62, 609–617. [Google Scholar] [CrossRef]
- Sassi, H.; Nguyen, T.M.; Telek, S.; Gosset, G.; Grünberger, A.; Delvigne, F. Segregostat: A Novel Concept to Control Phenotypic Diversification Dynamics on the Example of Gram-Negative Bacteria. Microb. Biotechnol. 2019, 12, 1064–1075. [Google Scholar] [CrossRef]
- Dowdell, K.S.; Olsen, K.; Martinez Paz, E.F.; Sun, A.; Keown, J.; Lahr, R.; Steglitz, B.; Busch, A.; LiPuma, J.J.; Olson, T.; et al. Investigating the Suitability of Online Flow Cytometry for Monitoring Full-Scale Drinking Water Ozone System Disinfection Effectiveness. Water Res. 2024, 257, 121702. [Google Scholar] [CrossRef]
- Besmer, M.D.; Epting, J.; Page, R.M.; Sigrist, J.A.; Huggenberger, P.; Hammes, F. Online Flow Cytometry Reveals Microbial Dynamics Influenced by Concurrent Natural and Operational Events in Groundwater Used for Drinking Water Treatment. Sci. Rep. 2016, 6, 38462. [Google Scholar] [CrossRef]
- Besmer, M.D.; Weissbrodt, D.G.; Kratochvil, B.E.; Sigrist, J.A.; Weyland, M.S.; Hammes, F. The Feasibility of Automated Online Flow Cytometry for In-Situ Monitoring of Microbial Dynamics in Aquatic Ecosystems. Front. Microbiol. 2014, 5, 265. [Google Scholar] [CrossRef]
- Joran, A.; Schiessl, K.; Klein, G.; Besmer, M.D.; Eicher, C.; Alexandre, H. Continuous Automated Microbial Monitoring in Laboratory-Scale Winemaking Experiments. OENO One 2023, 57, 189–202. [Google Scholar] [CrossRef]
- López-Gálvez, J.; Schiessl, K.; Besmer, M.D.; Bruckmann, C.; Harms, H.; Müller, S. Development of an Automated Online Flow Cytometry Method to Quantify Cell Density and Fingerprint Bacterial Communities. Cells 2023, 12, 1559. [Google Scholar] [CrossRef] [PubMed]
- Haberkorn, I.; Off, C.L.; Besmer, M.D.; Buchmann, L.; Mathys, A. Automated Online Flow Cytometry Advances Microalgal Ecosystem Management as in Situ, High-Temporal Resolution Monitoring Tool. Front. Bioeng. Biotechnol. 2021, 9, 642671. [Google Scholar] [CrossRef]
- Weiner, M.; Trö Ndle, J.; Albermann, C.; Sprenger, G.A.; Weuster-Botz, D. Improvement of Constraint-Based Flux Estimation During L-Phenylalanine Production with Escherichia coli Using Targeted Knock-out Mutants. Biotechnol. Bioeng. 2014, 111, 1406–1416. [Google Scholar] [CrossRef]
- Hoang, M.D. L-Phenylalanine Production with Escherichia coli Reporter Strains in a Novel Two-Compartment Bioreactor. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2024. [Google Scholar]
- Vrábel, P.; van der Lans, R.G.J.M.; Luyben, K.C.A.M.; Boon, L.; Nienow, A.W. Mixing in Large-Scale Vessels Stirred with Multiple Radial or Radial and Axial Up-Pumping Impellers: Modelling and Measurements. Chem. Eng. Sci. 2000, 55, 5881–5896. [Google Scholar] [CrossRef]
- ZENTRALLABOR: M03.003. Available online: https://www.tuhh.de/zentrallabor/methoden/oc-methoden/m03003 (accessed on 4 February 2025).
- Bafna-Rührer, J.; Orth, J.V.; Sudarsan, S. Combined Oxygen and Glucose Oscillations Distinctly Change the Transcriptional and Physiological State of Escherichia coli. Microb. Biotechnol. 2024, 17, e70051. [Google Scholar] [CrossRef]
- Murray, H.D.; Appleman, J.A.; Gourse, R.L. Regulation of the Escherichia coli rrnB P2 Promoter. J. Bacteriol. 2003, 185, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.B.; Sternberg, C.; Poulsen, L.K.; Bjørn, S.P.; Givskov, M.; Molin, S. New Unstable Variants of Green Fluorescent Protein for Studies of Transient Gene Expression in Bacteria. Appl. Environ. Microbiol. 1998, 64, 2240–2246. [Google Scholar] [CrossRef]
- Sohaskey, C.D.; Wayne, L.G. Role of narK2X and narGHJI inHypoxic Upregulation of Nitrate Reduction byMycobacteriumtuberculosis. J. Bacteriol. 2003, 185, 7247–7256. [Google Scholar] [CrossRef]
- Voulgaris, I.; Arnold, S.A.; Speight, R.; Harvey, L.M.; McNeil, B. Effects of Dissolved Oxygen Availability and Culture Biomass at Induction upon the Intracellular Expression of Monoamine Oxidase by Recombinant Escherichia coli in Fed Batch Bioprocesses. Process Biochem. 2011, 46, 721–729. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Yoon, T.-S.; Kang, S.; Afzal, M. Juggling with Fluorescent Proteins: Spectrum and Structural Changes of the mCardinal2 Variants. Biochem. Biophys. Res. Commun. 2022, 593, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.; Albermann, C.; Gottlieb, K.; Sprenger, G.A.; Weuster-Botz, D. Fed-Batch Production of l-Phenylalanine from Glycerol and Ammonia with Recombinant Escherichia coli. Biochem. Eng. J. 2014, 83, 62–69. [Google Scholar] [CrossRef]
- Battesti, A.; Majdalani, N.; Gottesman, S. The RpoS-Mediated General Stress Response in Escherichia coli. Annu. Rev. Microbiol. 2011, 65, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Bouillet, S.; Bauer, T.S.; Gottesman, S. RpoS and the Bacterial General Stress Response. Microbiol. Mol. Biol. Rev. 2024, 88, e0015122. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arulrajah, P.; Riessner, S.K.; Heins, A.-L.; Weuster-Botz, D. Monitoring of the Single-Cell Behavior of an Escherichia coli Reporter Strain Producing L-phenylalanine in a Scale-Down Bioreactor by Automated Real-Time Flow Cytometry. BioTech 2025, 14, 54. https://doi.org/10.3390/biotech14030054
Arulrajah P, Riessner SK, Heins A-L, Weuster-Botz D. Monitoring of the Single-Cell Behavior of an Escherichia coli Reporter Strain Producing L-phenylalanine in a Scale-Down Bioreactor by Automated Real-Time Flow Cytometry. BioTech. 2025; 14(3):54. https://doi.org/10.3390/biotech14030054
Chicago/Turabian StyleArulrajah, Prasika, Sophi Katharina Riessner, Anna-Lena Heins, and Dirk Weuster-Botz. 2025. "Monitoring of the Single-Cell Behavior of an Escherichia coli Reporter Strain Producing L-phenylalanine in a Scale-Down Bioreactor by Automated Real-Time Flow Cytometry" BioTech 14, no. 3: 54. https://doi.org/10.3390/biotech14030054
APA StyleArulrajah, P., Riessner, S. K., Heins, A.-L., & Weuster-Botz, D. (2025). Monitoring of the Single-Cell Behavior of an Escherichia coli Reporter Strain Producing L-phenylalanine in a Scale-Down Bioreactor by Automated Real-Time Flow Cytometry. BioTech, 14(3), 54. https://doi.org/10.3390/biotech14030054





