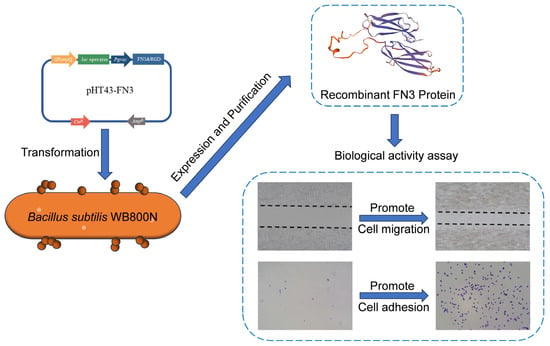
Expression and Biological Activity Analysis of Recombinant Fibronectin3 Protein in Bacillus subtilis
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Construction of the Recombinant Plasmid pHT43-FN3
2.3. Transformation and Expression of FN3 Recombinant Plasmid
2.4. Purification and Identification of the Recombinant FN3 Protein
2.5. Western Blotting
2.6. Effect of FN3 on the Proliferation of L-929 Cells
2.7. Effect of FN3 on the Migration of L-929 Cells
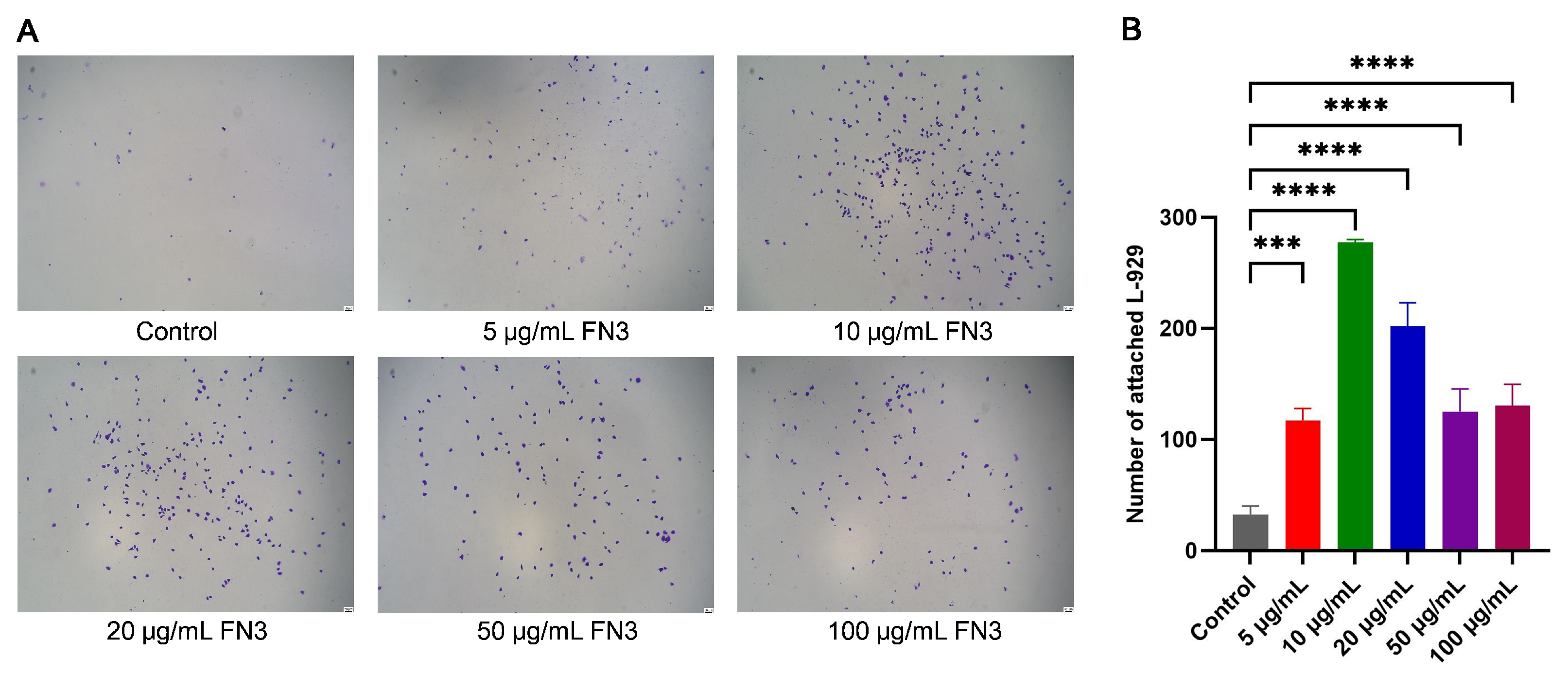
2.8. Effect of FN3 on the Adhesion of L-929 Cells
2.9. Statistical Analysis
3. Results and Discussion
3.1. Construction and Identification of the pHT43-FN3 Vector
3.2. Induced Expression of FN3 and Preliminary Optimization of Conditions
3.3. Purification and Desalination of Recombinant FN3
3.4. Identification of FN3 by Western Blot
3.5. CCK8 Assay of the Effect of FN3 on L-929 Cell Proliferation
3.6. Effect of FN3 on the Scratch Assay of L-929 Cells
3.7. Crystal Violet Assay of the Adhesion Effect of FN3 on L-929 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morrison, P.R.; Edsall, J.T.; Miller, S.G. Preparation and properties of serum and plasma proteins. XVIII. The separation of purified fibrinogen from fraction I of human plasma. J. Am. Chem. Soc. 1948, 70, 3103–3108. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug. Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Mao, Y.; Schwarzbauer, J.E. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005, 24, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Wojciechowski, K.; Gildner, C.D.; Nedelkovska, H.; Hocking, D.C. Identification of the heparin-binding determinants within fibronectin repeat III1: Role in cell spreading and growth. J. Biol. Chem. 2006, 281, 34816–34825. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Rockey, D.C.; Koteliansky, V.E.; Wang, S.S.; Bissell, D.M. Expression of variant fibronectins in wound healing: Cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J. Cell Biol. 1994, 127, 2037–2048. [Google Scholar] [CrossRef]
- Ohashi, T.; Kiehart, D.P.; Erickson, H.P. Dual labeling of the fibronectin matrix and actin cytoskeleton with green fluorescent protein variants. J. Cell Sci. 2002, 115, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhu, W.; Lei, X.; Luo, X.; Xiang, Q.; Zhu, X.; Pan, Q.; Jin, P.; Cheng, B. Treatment of Acute Wounds With Recombinant Human-Like Collagen and Recombinant Human-Like Fibronectin in C57BL/6 Mice Individually or in Combination. Front. Bioeng. Biotechnol. 2022, 10, 908585. [Google Scholar] [CrossRef]
- Cho, J.; Mosher, D.F. Enhancement of thrombogenesis by plasma fibronectin cross-linked to fibrin and assembled in platelet thrombi. Blood 2006, 107, 3555–3563. [Google Scholar] [CrossRef]
- Jara, C.P.; Wang, O.; do Prado, T.P.; Ismail, A.; Fabian, F.M.; Li, H.; Velloso, L.A.; Carlson, M.A.; Burgess, W.; Lei, Y.G.; et al. Novel fibrin-fibronectin matrix accelerates mice skin wound healing. Bioact. Mater. 2020, 5, 949–962. [Google Scholar] [CrossRef]
- Halper, J. Basic components of connective tissues and extracellular matrix: Elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv. Exp. Med. Biol. 2014, 802, 31–47. [Google Scholar] [CrossRef]
- Pankov, R.; Momchilova, A.; Stefanova, N.; Yamada, K.M. Characterization of stitch adhesions: Fibronectin-containing cell-cell contacts formed by fibroblasts. Exp. Cell Res. 2019, 384, 111616. [Google Scholar] [CrossRef]
- Forsprecher, J.; Wang, Z.; Nelea, V.; Kaartinen, M.T. Enhanced osteoblast adhesion on transglutaminase 2-crosslinked fibronectin. Amino. Acids 2009, 36, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhu, J.; Tonnesen, M.G.; Taira, B.R.; McClain, S.A.; Singer, A.J.; Clark, R.A.F. Fibronectin peptides that bind PDGF-BB enhance survival of cells and tissue under stress. J. Investig. Dermatol. 2014, 134, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.L.; Soares, D.G.; Anovazzi, G.; Anselmi, C.; Hebling, J.; de Souza Costa, C.A. Fibronectin-loaded Collagen/Gelatin Hydrogel Is a Potent Signaling Biomaterial for Dental Pulp Regeneration. J. Endod. 2021, 47, 1110–1117. [Google Scholar] [CrossRef]
- Creswell, L.; Rolnik, D.L.; Lindow, S.W.; O’Gorman, N. Preterm Birth: Screening and Prediction. Int. J. Women’s Health 2023, 15, 1981–1997. [Google Scholar] [CrossRef]
- Geng, H.; Wu, Y.; Chen, Y. C-Terminal Fibronectin Exerts Beneficial Effects in Reducing Tissue Damage and Modulating Macrophage Function in a Murine Septic Model. J. Inflamm. Res. 2023, 16, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.C.; Guimaraes, J.M.; Pereira, S.D.S.; Mariuba, L.A.M. The multifunctionality of expression systems in Bacillus subtilis: Emerging devices for the production of recombinant proteins. Exp. Biol. Med. 2021, 246, 2443–2453. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Li, J.; Du, G.; Chen, J. Synthetic biology toolbox and chassis development in Bacillus subtilis. Trends Biotechnol. 2019, 37, 548–562. [Google Scholar] [CrossRef]
- Hirooka, K.; Tamano, A. Bacillus subtilis highly efficient protein expression systems that are chromosomally integrated and controllable by glucose and rhamnose. Biosci. Biotechnol. Biochem. 2018, 82, 1942–1954. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Hao, J. A novel GH1 β-glucosidase from an Arctic bacterium: Characterization and secretory expression in Bacillus subtilis. Process Biochem. 2024, 140, 108–116. [Google Scholar] [CrossRef]
- Zweers, J.C.; Barak, I.; Becher, D.; Driessen, A.J.; Hecker, M.; Kontinen, V.P.; Saller, M.J.; Vavrova, L.; van Dijl, J.M. Towards the development of Bacillus subtilis as a cell factory for membrane proteins and protein complexes. Microb. Cell Fact. 2008, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Terpe, K. Overview of bacterial expression systems for heterologous protein production: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2006, 72, 211–222. [Google Scholar] [CrossRef]
- Phan, T.T.P.; Nguyen, H.D.; Schumann, W. Novel plasmid-based expression vectors for intra- and extracellular production of recombinant proteins in Bacillus subtilis. Protein Expr. Purif. 2006, 46, 189–195. [Google Scholar] [CrossRef]
- Calero, P.; Nikel, P.I. Chasing bacterial chassis for metabolic engineering: A perspective review from classical to non-traditional microorganisms. Microb. Biotechnol. 2019, 12, 98–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Yeung, J.C.; Duan, Y.; Ye, R.; Szarka, S.J.; Habibi, H.R.; Wong, S.L. Functional production and characterization of a fibrin-specific single-chain antibody fragment from Bacillus subtilis: Effects of molecular chaperones and a wall-bound protease on antibody fragment production. Appl. Environ. Microbiol. 2002, 68, 3261–3269. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Quyen, T.D.; Le, H.T. Cloning and enhancing production of a detergent- and organic-solvent-resistant nattokinase from Bacillus subtilis VTCC-DVN-12-01 by using an eight-protease-gene-deficient Bacillus subtilis WB800. Microb. Cell Factories 2013, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, M.J.; Smith, M.L. Multiscale relationships between fibronectin structure and functional properties. Acta Biomater. 2014, 10, 1524–1531. [Google Scholar] [CrossRef]
- Espitia Pinzon, N.; Stroo, E.; t Hart, B.A.; Bol, J.G.; Drukarch, B.; Bauer, J.; van Dam, A.M. Tissue transglutaminase in marmoset experimental multiple sclerosis: Discrepancy between white and grey matter. PLoS ONE 2014, 9, e100574. [Google Scholar] [CrossRef]
- Longstreth, J.H.; Wang, K. The role of fibronectin in mediating cell migration. Am. J. Physiol. Cell Physiol. 2024, 326, C1212–C1225. [Google Scholar] [CrossRef]
- Lim, S.S.; Chiang, C.L.; Rosli, N.; Chew, K.W. Functionalization of Chitosan-TiO2 Nanotubes Scaffolds with Fibronectin for Bone Regeneration. J. Biomim. Biomater. Biomed. Eng. 2023, 61, 51–57. [Google Scholar]
- Du, Y.; Jiang, B.; Song, S.; Pei, G.; Ni, X.; Wu, J.; Wang, S.; Wang, Z.; Yu, J. Metadherin regulates actin cytoskeletal remodeling and enhances human gastric cancer metastasis via epithelial-mesenchymal transition. Int. J. Oncol. 2017, 51, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Digiacomo, G.; Tusa, I.; Bacci, M.; Cipolleschi, M.G.; Dello Sbarba, P.; Rovida, E. Fibronectin induces macrophage migration through a SFK-FAK/CSF-1R pathway. Cell Adhes. Migr. 2017, 11, 327–337. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.Q.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Klavert, J.; van der Eerden, B.C.J. Fibronectin in fracture healing: Biological mechanisms and regenerative avenues. Front. Bioeng. Biotechnol. 2021, 9, 663357. [Google Scholar] [CrossRef]
- Parisi, L.; Toffoli, A.; Ghezzi, B.; Mozzoni, B.; Lumetti, S.; Macaluso, G.M. A glance on the role of fibronectin in controlling cell response at biomaterial interface. Jpn. Dent. Sci. Rev. 2020, 56, 50–55. [Google Scholar] [CrossRef]
- Rezvanian, P.; Alvarez-López, A.; Tabraue-Rubio, R.; Daza, R.; Colchero, L.; Elices, M.; Guinea, G.V.; González-Nieto, D.; Pérez-Rigueiro, J. Modulation of cell response through the covalent binding of fibronectin to titanium substrates. J. Funct. Biomater. 2023, 14, 342. [Google Scholar] [CrossRef]
- Siddiqui, N.; Pramanik, K. Development of fibrin conjugated chitosan/nano β-TCP composite scaffolds with improved cell supportive property for bone tissue regeneration. J. Appl. Polym. Sci. 2015, 132, 41534. [Google Scholar] [CrossRef]






| Primer Name | Sequence (5′–3′) |
|---|---|
| pHT43-FN3-BamH I-F | CAAAAACATCAGCCGTAGATATGACCCCGTCTCAGCCG |
| pHT43-FN3-BamH I-R | GGACGTCGACTCTAGAGATTAGTGGTGGTGGTGGTGATG |
| pHT43-FN3-F | GTAAAACGACGGCCAGT |
| pHT43-FN3-R | CGGGGACGTCGACTCTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Xu, G.; Tian, Y.; Yi, Z.; Tang, X. Expression and Biological Activity Analysis of Recombinant Fibronectin3 Protein in Bacillus subtilis. BioTech 2025, 14, 51. https://doi.org/10.3390/biotech14030051
Lu C, Xu G, Tian Y, Yi Z, Tang X. Expression and Biological Activity Analysis of Recombinant Fibronectin3 Protein in Bacillus subtilis. BioTech. 2025; 14(3):51. https://doi.org/10.3390/biotech14030051
Chicago/Turabian StyleLu, Chaozheng, Guangxin Xu, Yin Tian, Zhiwei Yi, and Xixiang Tang. 2025. "Expression and Biological Activity Analysis of Recombinant Fibronectin3 Protein in Bacillus subtilis" BioTech 14, no. 3: 51. https://doi.org/10.3390/biotech14030051
APA StyleLu, C., Xu, G., Tian, Y., Yi, Z., & Tang, X. (2025). Expression and Biological Activity Analysis of Recombinant Fibronectin3 Protein in Bacillus subtilis. BioTech, 14(3), 51. https://doi.org/10.3390/biotech14030051