Spatial and Temporal Aspects of Fungicide Resistance in Venturia inaequalis (Apple Scab) Populations in Northern Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Area
2.2. Reagents
2.3. Characterisation of Isolates for Fungicide Resistance
2.4. Fungicide Resistance Screening
2.5. Data Analysis
3. Results
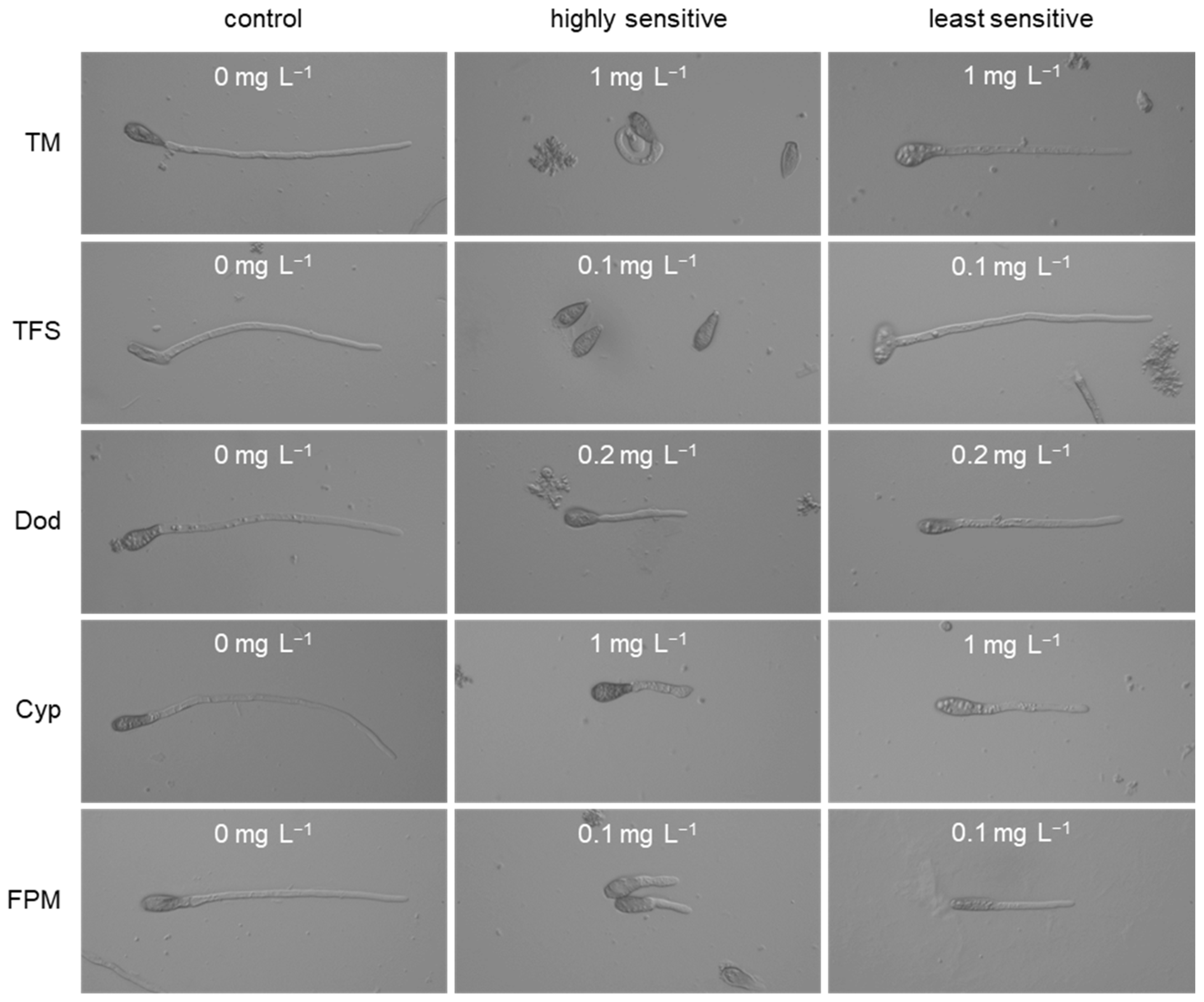
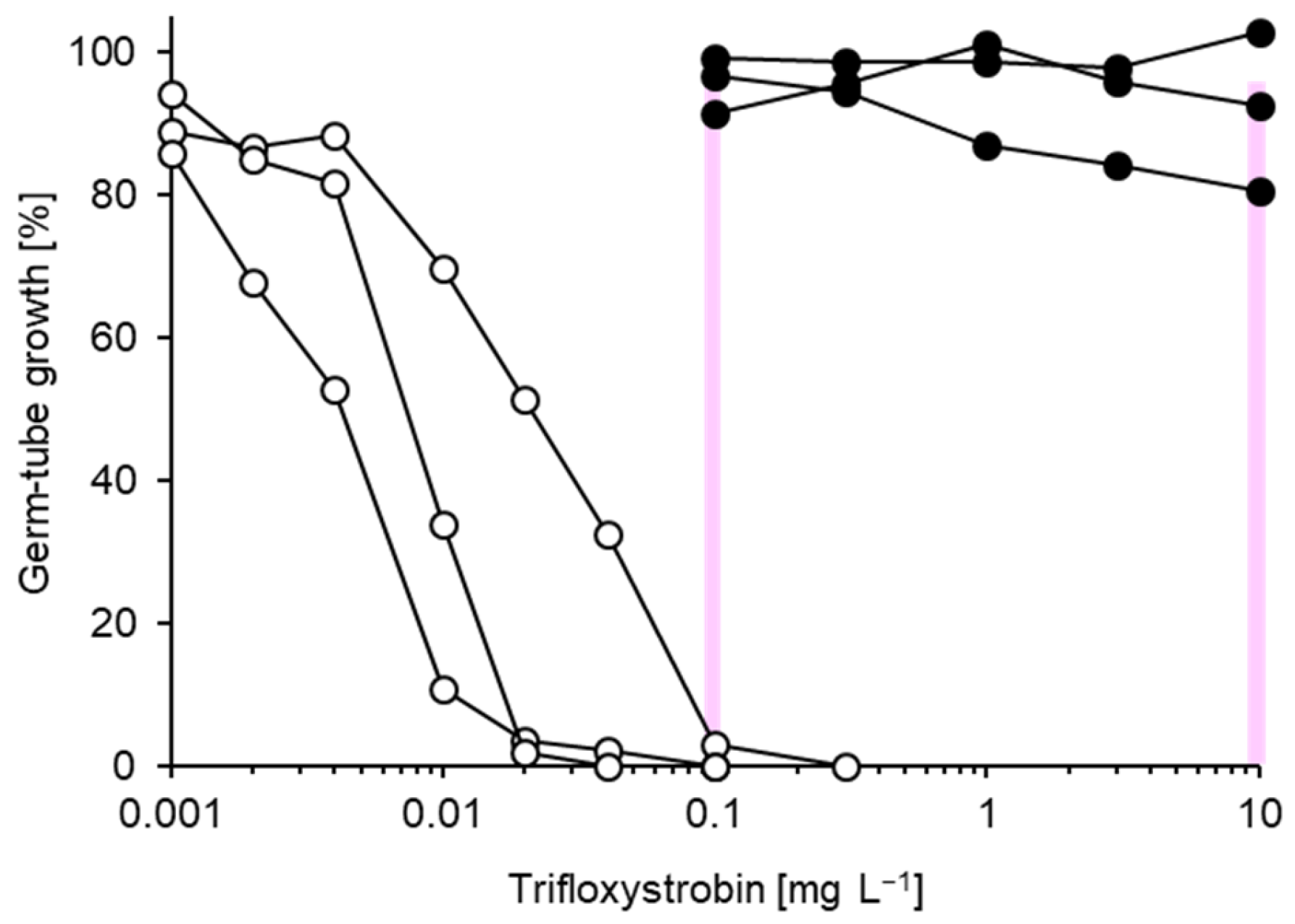
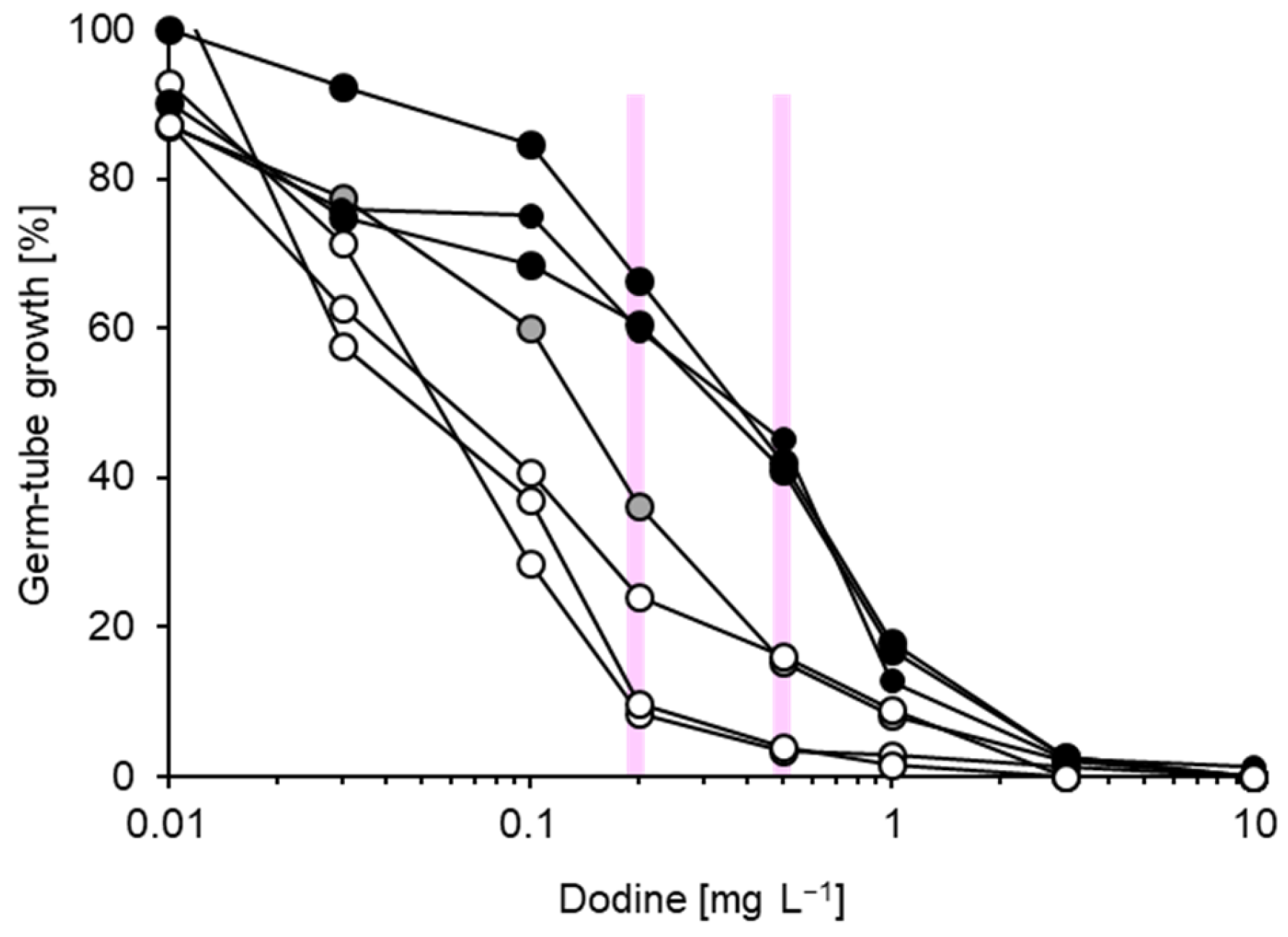
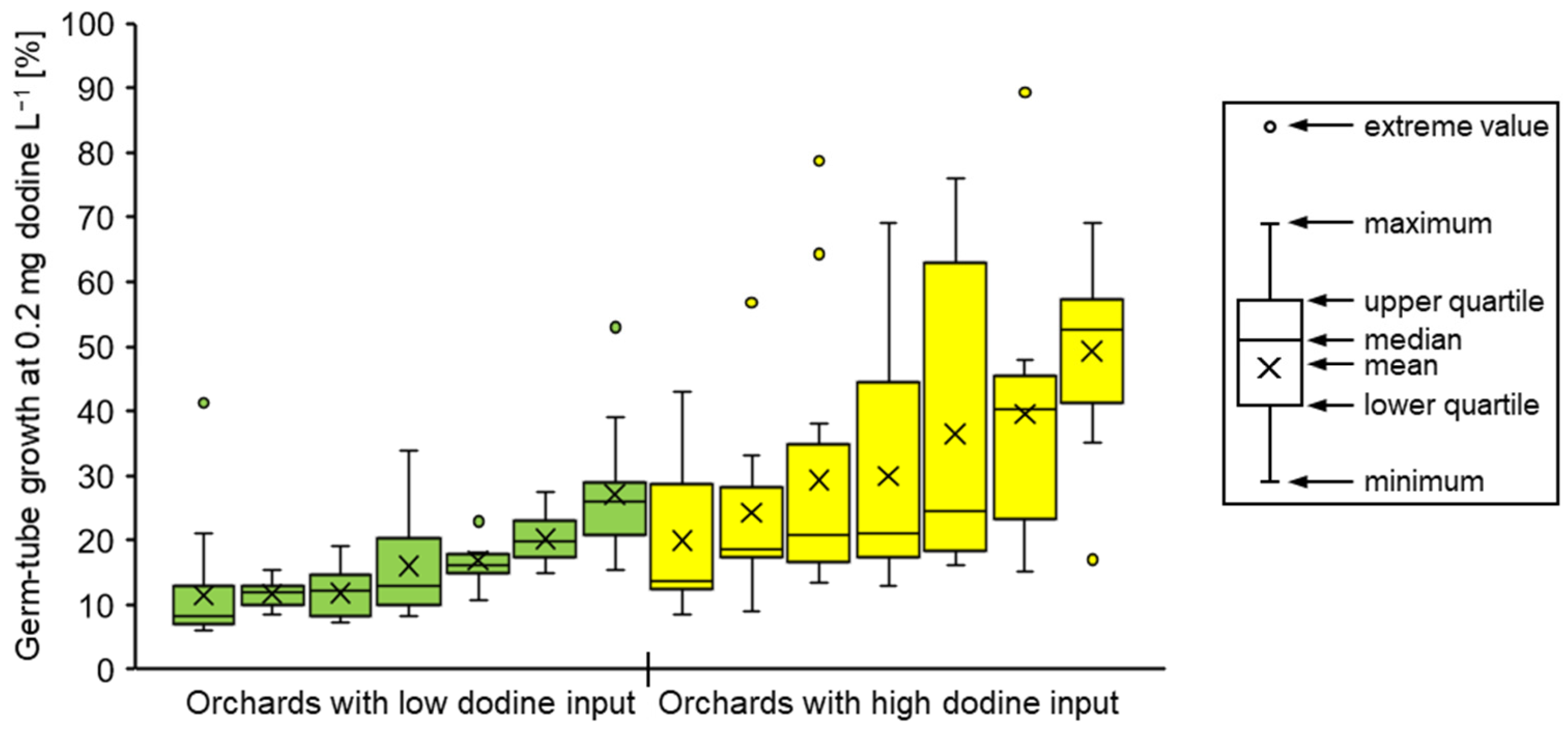
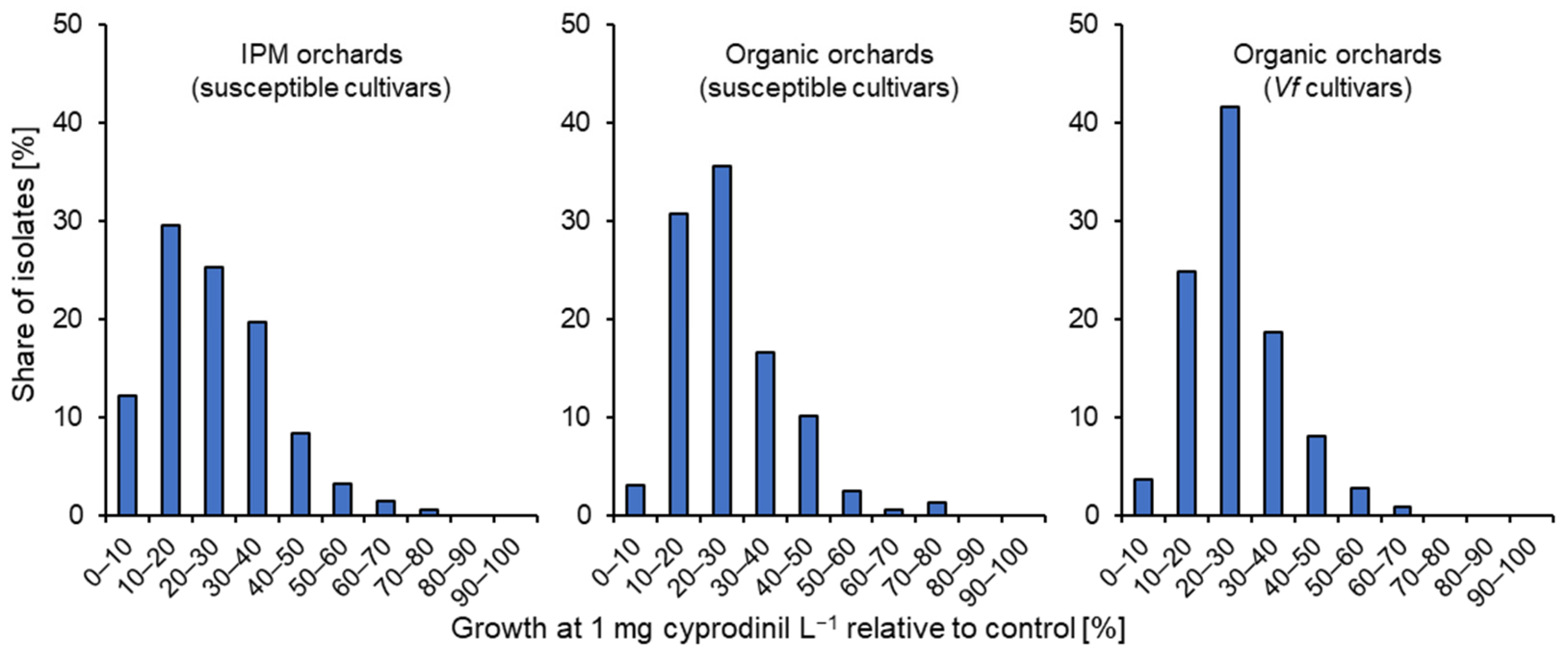
3.1. Characterisation of Fungicide Resistance
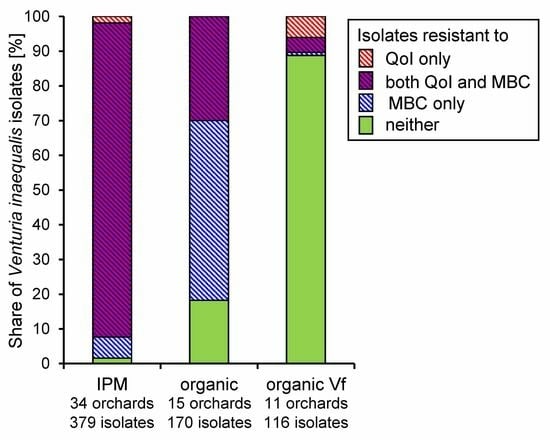
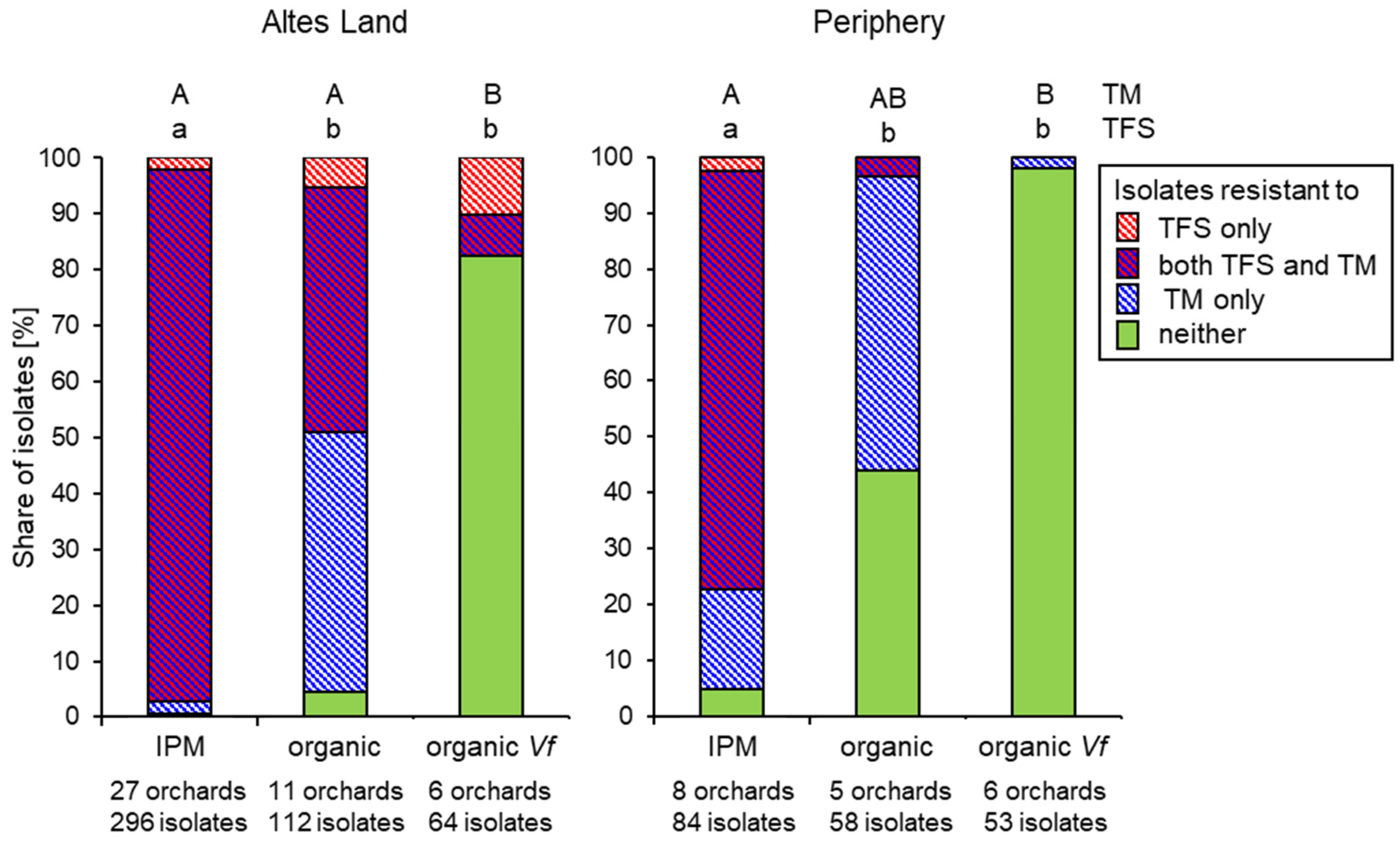
3.2. Fungicide Resistance in Commercial Orchards Within the Lower Elbe Region
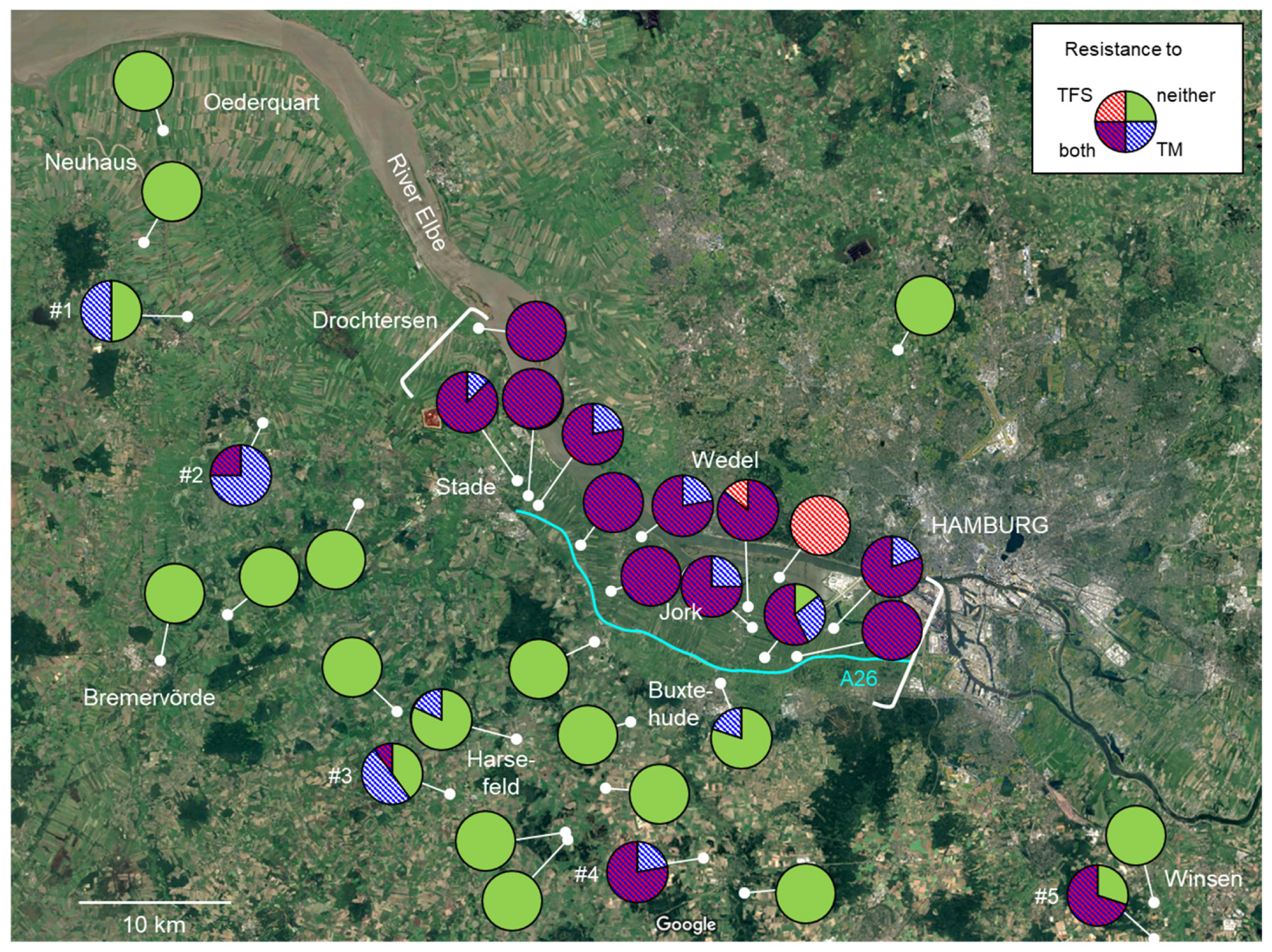
3.3. Regional Distribution of Fungicide Resistance in Abandoned Orchards
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Anilinopyrimidine |
| EC50 | Effective fungicide concentration causing a 50% growth inhibition |
| MBC | Methyl benzimidazole carbamate |
| MEA | Malt extract agar |
| PDA | Potato dextrose agar |
| QoI | Quinone-outside inhibitor |
| SDHI | Succinate dehydrogenase inhibitor |
References
- MacHardy, W.E. Apple Scab: Biology, Epidemiology, and Management; APS Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Biggs, A.R.; Stensvand, A. Apple scab. In Compendium of Apple and Pear Diseases, 2nd ed.; Sutton, T.B., Aldwinckle, H.S., Agnello, A.M., Walgenbach, J.F., Eds.; APS Press: St. Paul, MN, USA, 2014; pp. 8–11. [Google Scholar]
- Görgens, M. Betriebsvergleich Niederelbe 2022/2023. Mitt. Obstbauversuchsr. Alt. Land. 2024, 79, 424–434. [Google Scholar]
- Weber, R.W.S.; Späth, S.; Buchleither, S.; Mayr, U. A review of sooty blotch and flyspeck disease in German organic apple production. Erwerbs-Obstbau 2016, 58, 63–79. [Google Scholar] [CrossRef]
- Gessler, C.; Pertot, I. Vf scab resistance of Malus. Trees 2012, 26, 95–108. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Kruse, P. Analyse der Apfelschorfsaison 2019. Mitt. Obstbauversuchsr. Alt. Land. 2022, 77, 407–412. [Google Scholar]
- Weber, R.W.S.; Kruse, P. Spät- und Lagerschorf an Äpfeln an der Niederelbe 2015. Mitt. Obstbauversuchsr. Alt. Land. 2016, 71, 126–131. [Google Scholar]
- Fillinger, S.; Walker, A.-S. Chemical control and resistance management of Botrytis diseases. In Botrytis—The Fungus, the Pathogen and its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 165–187. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Petridis, A. Fungicide resistance in Botrytis spp. and regional strategies for its management in Northern European strawberry production. BioTech 2023, 12, 64. [Google Scholar] [CrossRef]
- Cox, K.D. Fungicide resistance in Venturia inaequalis, the causal agent of apple scab, in the United States. In Fungicide Resistance in Plant Pathogens; Ishii, H., Holloman, D.W., Eds.; Springer: Tokyo, Japan, 2015; pp. 433–447. [Google Scholar] [CrossRef]
- Chatzidimopoulos, M.; Zambounis, A.; Lioliopoulou, F.; Vellios, E. Detection of Venturia inaequalis isolates with multiple resistance in Greece. Microorganisms 2022, 10, 2354. [Google Scholar] [CrossRef]
- Vagt, W. Die Schorfsituation 1974 und unsere Spritzempfehlungen für 1975. Mitt. Obstbauversuchsr. Alt. Land. 1975, 30, 76–80. [Google Scholar]
- Palm, G. Sensitivitätsverlust des Apfelschorfes gegenüber Ergosterolbiosynthesehemmern—notwendige Antiresistenzstrategie in der Schorfbekämpfung. Mitt. Obstbauversuchsr. Alt. Land. 1999, 54, 98–107. [Google Scholar]
- Palm, G. Wie sicher ist noch die Wirkung von Discus in der Bekämpfung des Apfelschorfes? Mitt. Obstbauversuchsr. Alt. Land. 1999, 54, 389–392. [Google Scholar]
- Palm, G. Anilinopyrimidin-Resistenz beim Apfelschorf. Mitt. Obstbauversuchsr. Alt. Land. 2006, 61, 55–58. [Google Scholar]
- Köller, W.; Wilcox, W.F. Evidence for the predisposition of fungicide-resistant phenotypes of Venturia inaequalis to a preferential selection for resistance to other fungicides. Phytopathology 2001, 91, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.S.; Sundin, G.W.; Beckerman, J.L. Identification of resistance to multiple fungicides in field populations of Venturia inaequalis. Plant Dis. 2011, 95, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Polat, Z.; Bayraktar, H. Resistance of Venturia inaequalis to multiple fungicides in Turkish apple orchards. J. Phytopathol. 2021, 169, 360–368. [Google Scholar] [CrossRef]
- Szkolnik, M.; Gilpatrick, J.D. Apparent resistance of Venturia inaequalis to dodine in New York apple orchards. Plant Dis. Rep. 1969, 53, 861–864. [Google Scholar]
- Palm, G.; Kruse, P. Syllit-Versuche zur kurativen Bekämpfung des Apfelschorfes und Fruchtverträglichkeit. Mitt. Obstbauversuchsr. Alt. Land. 2010, 65, 104–107. [Google Scholar]
- Weber, R.W.S.; Kruse, P. Die Schorfjahre 2013 und 2014 an der Niederelbe. Mitt. Obstbauversuchsr. Alt. Land. 2015, 70, 110–123. [Google Scholar]
- Weber, R.W.S.; Kruse, P. Fungizide gegen Apfelschorf im Kontext aktueller Behandlungsempfehlungen. Mitt. Obstbauversuchsr. Alt. Land. 2018, 73, 127–134. [Google Scholar]
- Özkılınç, H.; Fidanoğlu, B.T.; Öncel, S.; Kurtuluş, E.; Kadıoğlu, İ.E. Resistance evolution and local adaptation of Venturia inaequalis to old and new generation SDHI fungicides. Fungal Biol. 2025, 129, 101543. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Palm, G. Resistance of storage rot fungi Neofabraea perennans, N. alba, Glomerella acutata and Neonectria galligena against thiophanate-methyl in Northern German apple production. J. Plant Dis. Protect. 2010, 117, 185–191. [Google Scholar] [CrossRef]
- Görgens, M. Baumobsterhebung 2022—Ergebnisse für das Niederelbegebiet (Niedersachsen und Hamburg). Mitt. Obstbauversuchsr. Alt. Land. 2022, 77, 360–365. [Google Scholar]
- Tiemann, K.-H. Der Erwerbsobstbau an der Niederelbe mit dem Zentrum Altes Land; Obstbauversuchsring des Alten Landes: Jork, Germany, 2012. [Google Scholar]
- Kienzle, J.; Oeser, N. Gesunderhaltung der Kulturpflanzen im Ökologischen Tafelapfelanbau; FÖKO: Weinsberg, Germany, 2023. [Google Scholar]
- Smith, D.; Onions, A.H.S. The Preservation and Maintenance of Living Fungi; Commonwealth Mycological Institute: Kew, UK, 1983. [Google Scholar]
- Weber, R.W.S.; Hahn, M. A rapid and simple method for determining fungicide resistance in Botrytis. J. Plant Dis. Protect. 2011, 118, 17–25. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Entrop, A.-P.; Goertz, A.; Mehl, A. Status of sensitivity of Northern German Botrytis populations to the new SDHI fungicide fluopyram prior to its release as a commercial fungicide. J. Plant Dis. Protect. 2015, 122, 81–90. [Google Scholar] [CrossRef]
- Wise, K.A.; Bradley, C.A.; Pasche, J.S.; Gudmestad, N.C.; Dugan, F.M.; Chen, W. Baseline sensitivity of Ascochyta rabiei to azoxystrobin, pyraclostrobin, and boscalid. Plant Dis. 2008, 92, 295–300. [Google Scholar] [CrossRef]
- Köller, W.; Wilcox, W.F.; Jones, A.L. Quantification, persistence, and status of dodine resistance in New York and Michigan orchard populations of Venturia inaequalis. Plant Dis. 1999, 83, 66–70. [Google Scholar] [CrossRef]
- Palm, G.; Kuck, K.-H.; Mehl, A.; Marr, J. Aktueller Stand der Strobilurin-Apfelschorf-Resistenz an der Niederelbe. Mitt. Obstbauversuchsr. Alt. Land. 2004, 59, 291–295. [Google Scholar]
- Köller, W.; Parker, D.M.; Turechek, W.W.; Avila-Adame, C.; Cronshaw, K. A two-phase resistance response of Venturia inaequalis populations to the QoI fungicides kresoxim-methyl and trifloxystrobin. Plant Dis. 2004, 88, 537–544. [Google Scholar] [CrossRef]
- Koenraadt, H.; Somerville, S.C.; Jones, A.L. Characterization of mutations in the beta-tubulin gene of benomyl-resistant field strains of Venturia inaequalis and other plant pathogenic fungi. Phytopathology 1992, 82, 1348–1354. [Google Scholar] [CrossRef]
- McGee, D.C.; Zuck, M.G. Competition between benomyl-resistant and sensitive strains of Venturia inaequalis on apple seedlings. Phytopathology 1981, 71, 529–532. [Google Scholar] [CrossRef]
- Frederick, Z.A.; Villani, S.M.; Cooley, D.R.; Biggs, A.R.; Raes, J.J.; Cox, K.D. Prevalence and stability of qualitative QoI resistance in populations of Venturia inaequalis in the northeastern United States. Plant Dis. 2014, 98, 1122–1230. [Google Scholar] [CrossRef]
- Fiaccadori, R. Persistence of Venturia inaequalis populations resistant to strobilurins in the field and in the glasshouse. Amer. J. Plant Sci. 2018, 9, 552–560. [Google Scholar] [CrossRef]
- Hauschildt, M.; Steinkellner, S.; Weber, R.W.S. Grey mould populations in Northern German sweet cherry and plum orchards: Selection of fungicide-resistant Botrytis cinerea strains over sensitive B. pseudocinerea by fungicide treatments. Eur. J. Plant Pathol. 2020, 157, 615–623. [Google Scholar] [CrossRef]
- Hu, M.-J.; Fernández-Ortuño, D.; Schnabel, G. Monitoring resistance to SDHI fungicides in Botrytis cinerea from strawberry fields. Plant Dis. 2016, 100, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ortuño, D.; Pérez-García, A.; Chamorro, M.; de la Peña, E.; de Vicente, A.; Torés, J.A. Resistance to the SDHI fungicides boscalid, fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from commercial strawberry fields in Spain. Plant Dis. 2017, 101, 1306–1313. [Google Scholar] [CrossRef]
- Fiaccadori, R.; Battistini, G. Biological methodologies on SDHI fungicides to assess reductions of sensitivity and activity on Venturia inaequalis and cross-resistance tests. Am. J. Plant Sci. 2021, 12, 1124–1134. [Google Scholar] [CrossRef]
- Villani, S.M.; Ayer, K.; Cox, K.D. Molecular characterization of the sdhB gene and baseline sensitivity to penthiopyrad, fluopyram, and benzovindiflupyr in Venturia inaequalis. Plant Dis. 2016, 100, 1709–1716. [Google Scholar] [CrossRef]
- Palm, G. Schorfbekämpfung 1997 unter Berücksichtigung neuer Fungizide. Mitt. Obstbauversuchsr. Alt. Land. 1997, 52, 139–152. [Google Scholar]
- Köller, W.; Wilcox, W.F.; Parker, D.M. Sensitivity of Venturia inaequalis populations to anilinopyrimidine fungicides and their contribution to scab management in New York. Plant Dis. 2005, 89, 357–365. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Kruse, P.; Holthusen, H.H.F. Neubewertung von Faban als Schorffungizid an der Niederelbe. Mitt. Obstbauversuchsr. Alt. Land. 2022, 77, 103–108. [Google Scholar]
- Bakker, G.R. Sensitivity of Venturia inaequalis and V. pirina to dodine in New Zealand. Proc. 52nd N. Z. Plant Prot. Conf. 1999, 52, 167–170. [Google Scholar] [CrossRef]
- Beresford, R.M.; Wright, P.J.; Wood, P.N.; Park, N.M.; Larsen, N.J.; Fisher, B.M. Resistance of Venturia inaequalis to demethylation inhibitor and dodine fungicides in four New Zealand apple-growing regions. N. Z. Plant Prot. 2013, 66, 274–283. [Google Scholar] [CrossRef]
- Gilpatrick, J.D.; Blowers, D.R. Ascospore tolerance to dodine in relation to orchard control of apple scab. Phytopathology 1974, 64, 649–652. [Google Scholar] [CrossRef]
- McKay, M.C.R.; MacNeill, B.H. Spectrum of sensitivity to dodine in field populations of Venturia inaequalis. Can. J. Plant Pathol. 1979, 1, 76–78. [Google Scholar] [CrossRef]
- Lesniak, K.E.; Proffer, T.J.; Beckerman, J.L.; Sundin, G.W. Occurrence of QoI resistance and detection of the G143A mutation in Michigan populations of Venturia inaequalis. Plant Dis. 2011, 95, 927–934. [Google Scholar] [CrossRef]
- Quello, K.L.; Chapman, K.S.; Beckerman, J.L. In situ detection of benzimidazole resistance in field isolates of Venturia inaequalis in Indiana. Plant Dis. 2010, 94, 744–750. [Google Scholar] [CrossRef]
- Guérin, F.; Le Cam, B. Breakdown of the scab resistance gene Vf in apple leads to a founder effect in populations of the fungal pathogen Venturia inaequalis. Phytopathology 2004, 94, 364–369. [Google Scholar] [CrossRef]
- Guérin, F.; Gladieux, P.; Le Cam, B. Origin and colonization history of newly virulent strains of the phytopathogenic fungus Venturia inaequalis. Fungal Genet. Biol. 2007, 44, 284–292. [Google Scholar] [CrossRef]






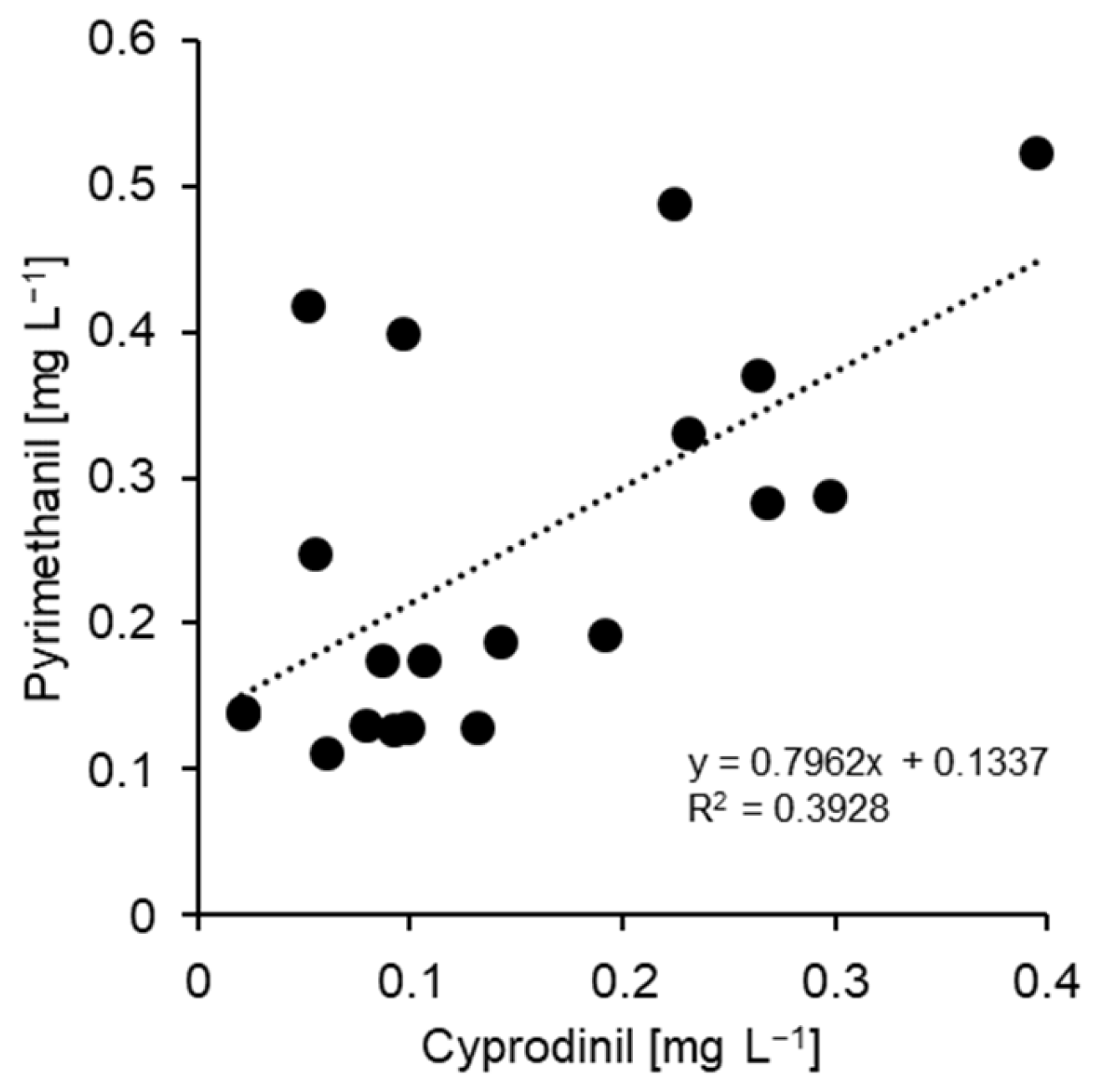
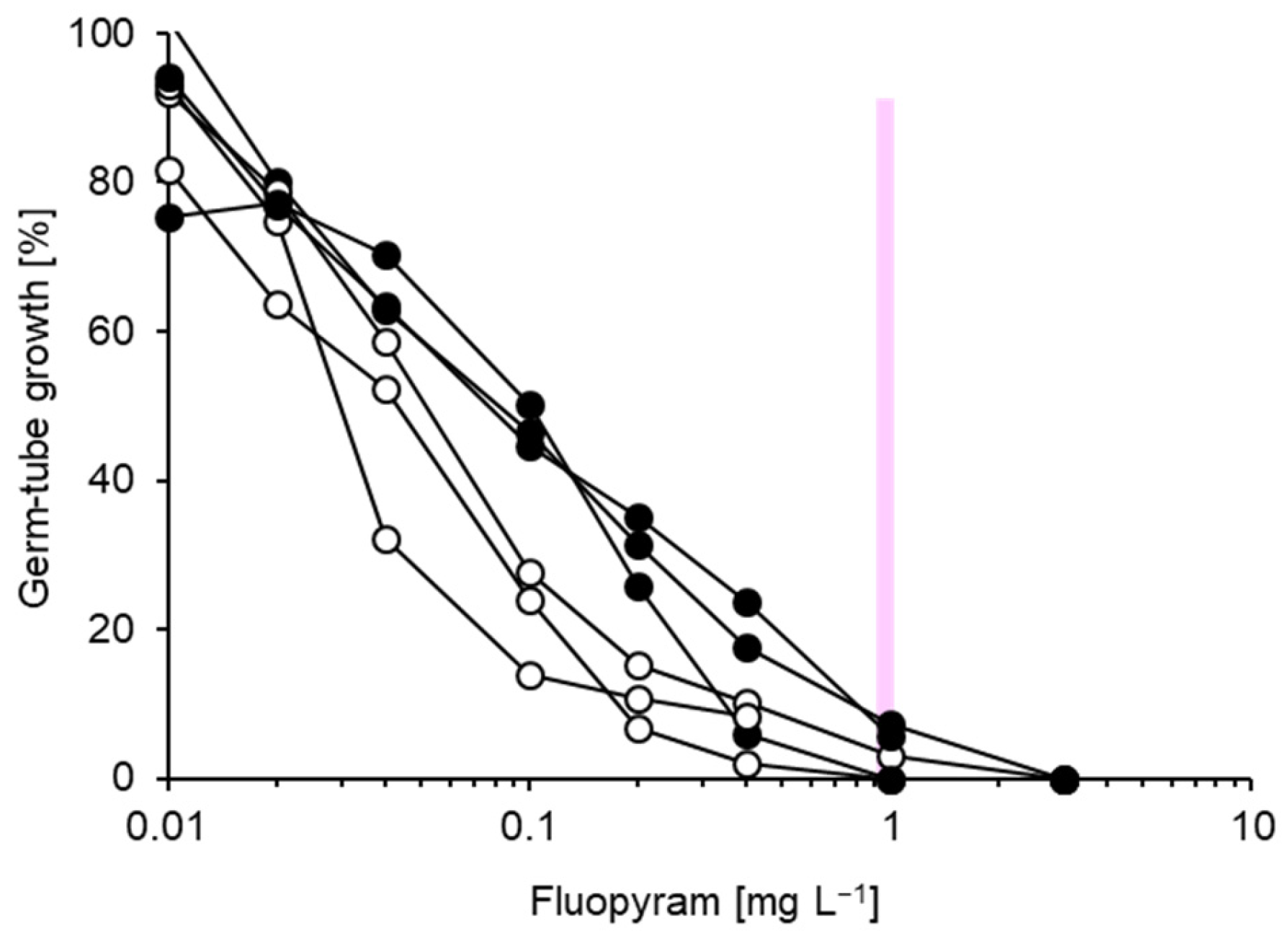
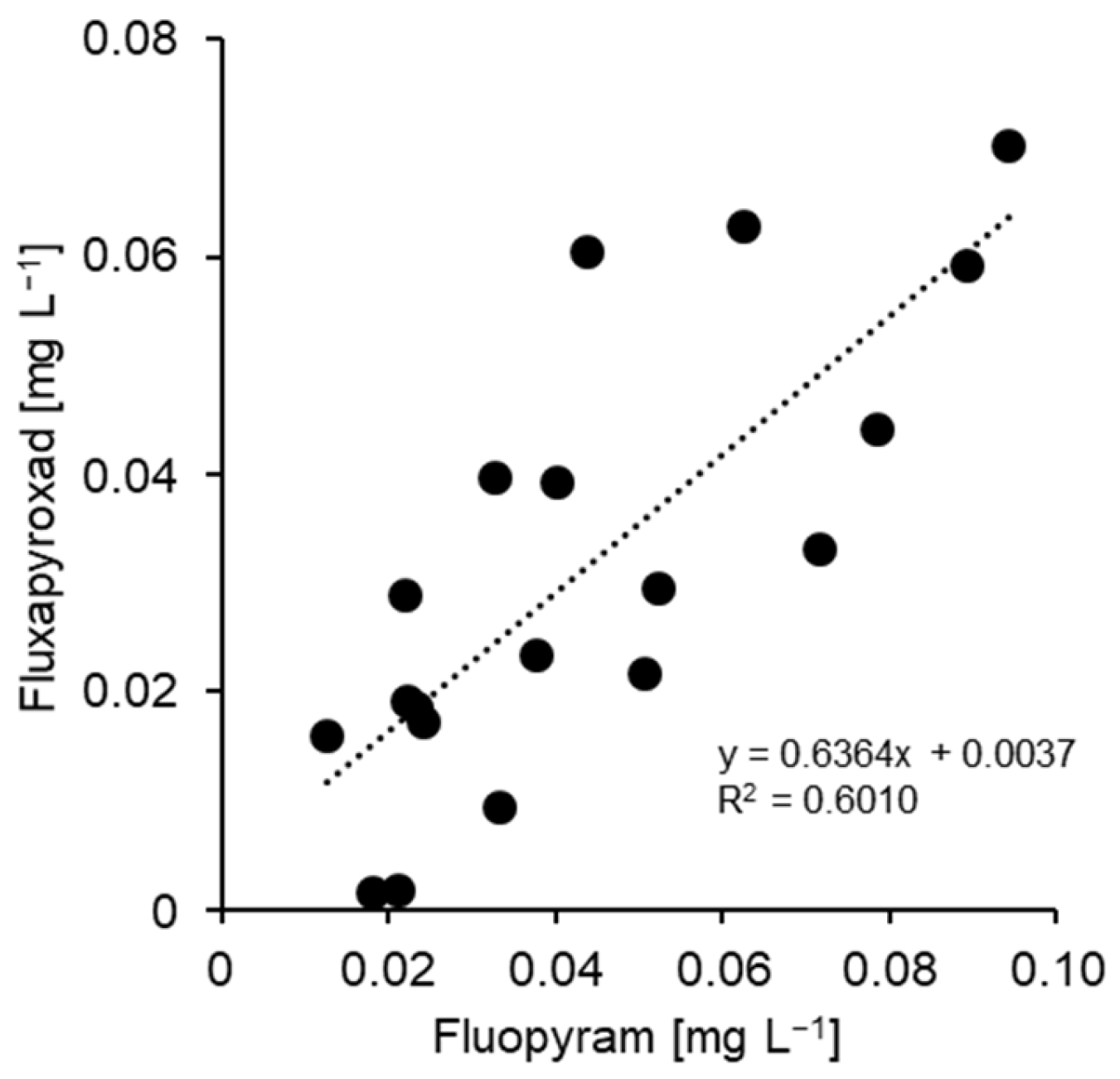


| Isolate | Cultivar | Production (Location) | TM | TFS | Dod | Cyp | Pyr | FPM | FPX |
|---|---|---|---|---|---|---|---|---|---|
| 22-006 | Red Jonaprince | abandoned (Borstel) | 1.6846 | >10 | 0.1133 | 0.0986 | 0.1298 | 0.0400 | 0.0394 |
| 28-26 | ZIN17 (Vf res.) | organic (Moorende) | 0.0777 | >10 | 0.2651 | 0.0967 | 0.3997 | 0.0241 | 0.0173 |
| 28-27 | ZIN17 (Vf res.) | organic (Moorende) | 0.1126 | 0.0025 | 0.0905 | 0.2309 | 0.3318 | 0.0222 | 0.0192 |
| 28-28 | ZIN17 (Vf res.) | organic (Moorende) | 4.3362 | >10 | 0.0732 | 0.0790 | 0.1303 | 0.0506 | 0.0218 |
| 28-29 | ZIN17 (Vf res.) | organic (Moorende) | 0.0936 | 0.0141 | 0.0778 | 0.0548 | 0.2487 | 0.0210 | 0.0019 |
| 29-25 | ZIN17 (Vf res.) | organic (Königreich) | 0.0727 | 0.0050 | 0.0451 | 0.0517 | 0.4185 | 0.0181 | 0.0017 |
| 30-25 | Red Jonaprince | IPM (Rübke) | 3.0845 | >10 | 0.3521 | 0.2637 | 0.3711 | 0.0715 | 0.0333 |
| 30-27 | Red Jonaprince | IPM (Rübke) | 2.4043 | >10 | 0.1644 | 0.3948 | 0.5229 | 0.0326 | 0.0399 |
| 35-18 | Braeburn | IPM (Neuenkirchen) | 0.0940 | 0.0039 | 0.0699 | 0.0608 | 0.1123 | 0.0376 | 0.0234 |
| 35-21 | Braeburn | IPM (Neuenkirchen) | 13.9703 | >10 | 0.1376 | 0.1313 | 0.1292 | 0.0623 | 0.0628 |
| 37-03 | Braeburn | IPM (Mittelnkirchen) | 4.0155 | >10 | 0.2298 | 0.0216 | 0.1411 | 0.0891 | 0.0592 |
| 37-19 | Braeburn | IPM (Neuenfelde) | 16.6829 | >10 | 0.1617 | 0.0869 | 0.1749 | 0.0522 | 0.0296 |
| 38-15 | Red Jonaprince | IPM (Hedendorf) | 5.8886 | >10 | 0.2584 | 0.1912 | 0.1921 | 0.0942 | 0.0702 |
| 39-21 | Braeburn | IPM (Winsen) | 5.3119 | >10 | 0.0874 | 0.1067 | 0.1759 | 0.0490 | 0.0412 |
| 40-20 | Jonagold | IPM (Sommerland) | 0.0929 | 0.0065 | 0.0676 | 0.2239 | 0.4880 | 0.0331 | 0.0095 |
| 42-14 | Topaz | organic (Osten) | 0.0880 | 0.0055 | 0.0528 | 0.0211 | 0.1390 | 0.0234 | 0.0187 |
| 42-23 | Topaz | organic (Osten) | 0.0672 | 0.0064 | 0.0942 | 0.2971 | 0.2884 | 0.0126 | 0.0161 |
| 45-11 | Red Jonaprince | IPM (Rübke) | 0.1015 | 0.0024 | 0.0562 | 0.2681 | 0.2835 | 0.0218 | 0.0289 |
| 45-29 | Elstar | IPM (Neuenfelde) | 25.0823 | >10 | 0.1331 | 0.0917 | 0.1278 | 0.0785 | 0.0443 |
| 45-30 | Elstar | IPM (Neuenfelde) | 6.9531 | >10 | 0.0717 | 0.1421 | 0.1887 | 0.0438 | 0.0606 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, R.W.S.; Busch, R.; Wesche, J. Spatial and Temporal Aspects of Fungicide Resistance in Venturia inaequalis (Apple Scab) Populations in Northern Germany. BioTech 2025, 14, 44. https://doi.org/10.3390/biotech14020044
Weber RWS, Busch R, Wesche J. Spatial and Temporal Aspects of Fungicide Resistance in Venturia inaequalis (Apple Scab) Populations in Northern Germany. BioTech. 2025; 14(2):44. https://doi.org/10.3390/biotech14020044
Chicago/Turabian StyleWeber, Roland W. S., Rebekka Busch, and Johanna Wesche. 2025. "Spatial and Temporal Aspects of Fungicide Resistance in Venturia inaequalis (Apple Scab) Populations in Northern Germany" BioTech 14, no. 2: 44. https://doi.org/10.3390/biotech14020044
APA StyleWeber, R. W. S., Busch, R., & Wesche, J. (2025). Spatial and Temporal Aspects of Fungicide Resistance in Venturia inaequalis (Apple Scab) Populations in Northern Germany. BioTech, 14(2), 44. https://doi.org/10.3390/biotech14020044