Abstract
The present study aimed to chemically profile the hydroalcoholic extracts from the microalgae (MEs) Nannochloropsis oculata, Phaeodactylum tricornutum, Tetradesmus obliquus, and Tetraselmis tetrathele and evaluate their effects on the development of Colletotrichum lindemuthianum and anthracnose symptoms, as well as on the initial growth of bean plants. For this, MEs were analyzed using UPLC coupled with a mass spectrometer, allowing the identification of peaks and annotation of potential metabolites. Fungal mycelial growth was assessed seven days after inoculation, and conidial germination was measured 72 h after incubation, using ME concentrations of 0, 0.1, 0.5, and 1.0 mg·mL−1. Bean seeds of the IPR Uirapuru cultivar were sown and treated with 3 mL of extracts at four time points: at sowing and 72 h after each previous treatment. After 11 days of cultivation in a growth chamber, the plants were divided into two groups: one for anthracnose control assessment and the other for evaluating growth promotion by MEs. Plant length as well as fresh and dry weights of shoots and roots were determined, leaf pigments were quantified, and anthracnose severity was assessed using a diagrammatic scale. The UPLC analysis identified 32 compounds in the extracts of the four microalgae, belonging to different chemical and functional groups, with lipids being the most significant fraction. The extracts exhibited variability and diversity in chemical composition depending on the microalgal species. MEs did not affect mycelial growth yet increased the germination of C. lindemuthianum conidia, regardless of the dose or species used. Anthracnose severity was not affected by the microalgae extracts. Regarding growth promotion, the extracts showed varying effects but generally increased shoot and root length, fresh biomass, and leaf pigment content.
Key Contribution:
This study demonstrated the species-dependent variability in the chemical composition of microalgae extracts (MEs) from Nannochloropsis oculata, Phaeodactylum tricornutum, Tetradesmus obliquus, and Tetraselmis tetrathele. Despite not affecting mycelial growth or anthracnose severity, the MEs promoted the germination of Colletotrichum lindemuthianum conidia. Additionally, the extracts enhanced, to some extent, shoot and root length, fresh biomass, and leaf pigment content.
1. Introduction
Beans (Phaseolus vulgaris L.) are a staple food in the global diet and play a crucial role in smallholder farming, particularly in South America and Africa [1,2]. However, the productivity of this crop has been often threatened by both abiotic and biotic adverse factors. Among the last ones, the fungal disease anthracnose (Colletotrichum lindemuthianum) has been considered one of the most important factors being particularly difficult to control due to diversity of races and efficient dissemination of pathogen. Under optimal humidity and temperature conditions, it can cause production losses of up to 100% [3,4,5].
The intensive use of pesticides has raised concerns regarding the presence of toxic residues in the environment and potential risks to human health. In this context, microalgae have emerged as a sustainable alternative to synthetic agrochemicals, attracting increasing interest due to their broad potential applications in agriculture [6,7]. Moreover, they can be utilized both as biostimulants for plant growth and as biological control agents against pathogens [7,8].
Microalgae exhibit a wide range of bioactive compounds that can function as biostimulants and resistance inducers in plants. These compounds promote biochemical adjustments and physiological modifications, improving root system architecture and nutrient cycling [9]. Additionally, microalgae produce various secondary metabolites with antibacterial and antifungal properties, capable of inhibiting the activity of phytopathogens such as fungi, bacteria, and nematodes [10,11].
Among the most extensively studied genera, Chlorella and Arthrospira stand out due to their bioactive compounds, including polysaccharides, proteins, and lipids, which exhibit plant growth-promoting, antimicrobial, and antioxidant properties [6,8]. Studies have demonstrated that Arthrospira platensis enhances growth and biomass accumulation in various crops, such as maize (Zea mays L.), tomato (Solanum lycopersicum) [12,13], and pepper (Capsicum annuum) [13]. On the other hand, extracts of Chlorella vulgaris have shown antifungal activity against various phytopathogens, including Aspergillus niger, Cercospora beticola, and Fusarium oxysporum f. sp. melonis [6,14,15].
These findings underscore the promising role of microalgal compounds in promoting sustainable agriculture by reducing the use of chemical inputs and enhancing crop resilience. However, studies focusing on microalgae from the genera Nannochloropsis, Phaeodactylum, Tetradesmus, and Tetraselmis still remain limited concerning plant protection and growth promotion. Given this scenario, the present study aimed to characterize the compounds present in hydroalcoholic extracts of four microalgal species, evaluate their effects on common bean plant growth, and investigate their efficacy in controlling C. lindemuthianum.
2. Materials and Methods
2.1. Biological Material
The microalgae used in this study (Table 1) were maintained and cultivated in the Algae Cultivation Laboratory (LCA) of UFSC. For that, the experimental cultures of T. obliquus were developed in 2L borosilicate Schott flasks containing LCA-AD medium [16], while P. tricornutum, T. tetrathele and N. oculata were developed in LCA-AM medium [17]. The cultures were maintained at a temperature of 24 °C, with constant agitation by bubbling atmospheric air enriched with 0.2% CO2 (v/v), irradiance of 200 µmol photons·m−2s−1, and in a continuous photoperiod. After reaching the stationary phase, cultures were collected centrifuged (NT825, NovaTecnica, Piracicaba, Brazil) at 3500 RPM, −4 °C for 5 min, lyophilized (70040, Labconco, Kansas City, MO, USA), and stored at 5 °C until use.

Table 1.
Species, strain, source and abbreviation of the microalgae used in the study.
The isolate MANE 003 of race 73 of C. lindemuthianum was used in the experiments. The fungus was maintained on PDA medium, and the spores were obtained after 15 days of fungal growth on bean pods as described by De Freitas and Stadnik [4]. The conidial suspension was obtained by adding sterilized distilled water to the tubes. The tubes were vigorously agitated to dislodge the conidia, and the resulting suspension was filtered through gauze to remove mycelial fragments. The filtrate was then subjected to three successive centrifugation steps (5810R, Eppendorf, Hamburg, Germany) at 10,000 RPM and 25 °C for 10 min. Finally, the conidial concentration was measured using a Neubauer counting chamber and then diluted as required for each experiment.
The bean cultivar IPR88 Uirapuru (Agronomic Institute of Parana, IAPAR, Curitiba, Brazil), which is susceptible to race 73 of C. lindemuthianum [4], was used in the experiments. Bean seeds were sown in plastic conic tubes (75 cm3) filled with vermiculite of medium particle size, pH 6 to 9, and maximum humidity (w/w) of 8% and incubated in a growth chamber for 11 days. The chamber conditions were maintained at 22.5 °C, 80% relative humidity, and a 12 h photoperiod. The substrate was watered daily (except when treatments were applied) with 3 mL of sterile distilled water per tube.
2.2. Obtaining of Hydroalcoholic Extracts
The microalgae extracts (MEs) were obtained as previously described [18], with modifications. For that, the lyophilized biomasses were agitated in ethanol 70% (35 mg·mL−1) at 100 RPM and 25 °C for 24 h. Following this, the suspension was vacuum filtered using Qualy filter paper (No.9) in a Buchner funnel connected to a vacuum pump (121, Primastec, Itú, Brazil). The filtrate was then concentrated using a rotary evaporator (Q344B2, Quimis, Diadema, Brazil) at 40 °C. Finally, the resulting solution was collected, its volume was determined using a graduated cylinder, and it was stored at 5 °C until the assays. The crude extract was subsequently diluted with water to achieve final concentrations of 0.1, 0.5, and 1 mg·mL−1.
2.3. Chemical Characterization of MEs
For Ultra-Performance Liquid Chromatography (UPLC) analysis, stock solutions (4 mg·mL−1) of the hydroalcoholic extracts from No, Pt, So, and Ts were prepared and subsequently diluted to a final concentration of 800 µg·mL−1 using an LC-MS-grade acetonitrile/methanol (1:1) mixture. Prior to analysis, all extracts were filtered through a 0.22 µm hydrophilic PTFE membrane (Filtrilo, Colombo, Brazil).
A 2 µL aliquot of each sample was injected into a UPLC Acquity system (Waters Co., Milford, MA, USA) coupled to a Xevo G2S Q-Tof mass spectrometer (Waters Co., Milford, MA, USA), which was equipped with an electrospray ionization (ESI) source, a quadrupole, and a time-of-flight (QTof) analyzer. Chromatographic separations were performed using a Hypersil GOLD column (50 × 2.1 mm i.d., 1.9 µm particle size, Thermo Scientific, Waltham, MA, USA), maintained at 40 °C, with a mobile phase flow rate of 0.3 mL.min−1. The mobile phases consisted of ultrapure water with 0.1% formic acid (pH 3.0) (mobile phase A) and LC-MS-grade acetonitrile (mobile phase B), following the gradient program: 0 min—90% A, 10% B; 0–1 min—90% A, 10% B; 1–3 min—70% A, 30% B; 3–8 min—70% A, 30% B; 8–12 min—10% A, 90% B; 14–15 min—90% A, 10% B; and 15–20 min for column equilibration.
Data acquisition was conducted in fast data-dependent acquisition (Fast DDA) and MSE (DIA) modes, utilizing argon as the collision gas and an energy range of 20–40 eV. Spectra were recorded in centroid mode for both positive and negative ionization modes within an m/z range of 50–1500, with a scan time of 0.1 s over a 20 min runtime. Each FDDA cycle included an m/z value corresponding to MS1 above a threshold of 20,000, where peaks exceeding this threshold were selected as precursor ions for fragmentation. MS/MS spectra were acquired while the signal remained above a threshold of 10,000. Nitrogen was used as the nebulizer gas, with a cone gas flow rate of 100 L·h−1 and a desolvation gas flow rate of 800 L·h−1. The sampling cone voltage and source offset were set at 40 V and 80 V, respectively. Accurate mass measurements were ensured using a lock spray reference solution containing leucine enkephalin (Leu-Enk, m/z 554.2615, [M−H]⁻; m/z 556.2771, [M+H]⁺). Desolvation and ionization temperatures were maintained at 350 °C and 120 °C, respectively.
For metabolite annotation, raw UHPLC-MS data (.D files) obtained in FDDA mode were directly uploaded and processed using MS-DIAL software (ver. 4.7). Data collection was performed with an MS1 tolerance of 0.02 Da and an MS2 tolerance of 0.06 Da. The molecular formula finder was restricted to the elements C, H, O, P, and N. Peak detection was configured with a minimum peak height amplitude of 2000 and a mass slice width of 0.1 Da. Deconvolution parameters were set as follows: a sigma window value of 0.1 and an MS/MS abundance cutoff of 10 amplitudes.
Metabolite annotation was conducted using the MS-DIAL metabolomics MSP spectral kit, which incorporates EI-MS, MS/MS, and CCS values for ESI (+ and −)-MS/MS data from both experimental and in silico databases (16,995 unique compounds and 15,245 unique compounds) [19]. Additional annotation was performed using MS-FINDER (for molecular formula prediction based on isotopic patterns and in silico fragmentation), SIRIUS (for molecular formula and structure prediction using isotopic patterns and fragmentation trees), and relevant literature sources. Candidate metabolites were identified by querying spectral databases, including MassBank, GNPS, KNApSAcK, PubCME, COCONUT, UNPD, NANPDB, NPA, PlantCyc, FooDB, T3DB, ChEBI, STOFF, LipidMAPS, YMDB, and ECMDB. Candidates were ranked based on similarity scores, which were computed by comparing experimental MS/MS spectra against database entries [19,20].
The top-ranked candidates were assigned, but in cases where key fragment ions were not adequately explained or similarity scores were identical, manual curation and cross-referencing with available literature were performed to refine metabolite identification [21].
2.4. Assessment of Conidia Germination and Mycelial Growth
Drops (10 µL) of a suspension containing 4 × 10⁴ conidia·mL−1 of C. lindemuthianum were pipetted onto polyethylene (Con-Tact, Plavitec, São Paulo, Brazil) slides, followed by the addition of 10 µL of sterile distilled water (control) or MEs (0.1, 0.5 or 1.0 mg·mL−1). The slides were kept in a humid chamber (100% RH) and incubated at 21 °C under a 12 h photoperiod. After 72 h, the percentage of conidia germination was evaluated under a light microscope at 400× magnification after adding a 10 µL drop of Amann’s blue dye. A conidium was deemed germinated if it developed a germ tube, regardless of its length. In each drop, 100 conidia were counted and classified as germinated or non-germinated [22]. For statistical purposes, each drop was considered a replicate.
To evaluate the effect of MEs on the growth of C. lindemuthianum mycelium, 10 mL of MEs solution was added to 15 mL of potato dextrose agar (PDA) medium at 70 °C, resulting in a final volume of 25 mL at 0.1, 0.5 and 1.0 mg·mL−1 [23]. A volume of 5 mL of this compound medium was added to each Petri dish. After solidification, each plate received an 8 mm diameter disk of the fungal culture. Then, plates were incubated at 21 ± 2 °C under a 12 h photoperiod for 15 days. Control dishes contained 15 mL of PDA medium supplemented with 10 mL of sterile distilled water. Mycelial growth was assessed by measuring colony diameter along two perpendicular axes using a digital caliper (Mitutoyo, Suzano, Brazil). Each Petri dish served as an experimental unit for mycelial growth assays.
2.5. Evaluation of MEs in the Severity of Anthracnose and Plant Grownth
2.5.1. Treatments with MEs
Plants were treated by drenching on the day of and 3, 6, and 9 days after sowing with 3 mL MEs at 0.1, 0.5, or 1.0 mg·mL−1. Sterile distilled water (DW) served as control. Thereafter, one set of plants was used for infection assays and other for plant growth evaluation.
2.5.2. Inoculation and Assessment of Anthracnose Severity
Eleven days after sowing, a third set of bean plants was simultaneously inoculated with a homogeneous suspension of C. lindemuthianum at 1 × 106 conidia·mL−1 and incubated in high humidity conditions (25 °C, 99% relative humidity) for 48 h. Following the incubation period, plants were transferred back to the growth chamber for disease evaluation. The severity of anthracnose symptoms was assessed at 7 and 15 days after inoculation, with the aid of the scale proposed by Rava et al. [24], varying from 1 (no symptoms) to 9 (most plants dead).
2.5.3. Measurement of Length of Plant Shoot and Roots
Eleven days after sowing, a set of bean plants was carefully taken out of the tubes, and their roots were thoroughly rinsed under running water. Then, the shoot length, root system length, and total plant height were measured using a plastic ruler.
2.5.4. Determination of Fresh and Dry Weight
Shoots and roots were separated at the collar region with the aid of a scalpel. Then, both parts were weighed individually to determine their fresh weight. The dry weight was determined after drying seedlings at 65 °C for 72 h.
2.5.5. Quantification of Leaf Pigments
Leaf pigments were quantified as previously described [25], with modifications. For that, at eleven days after sowing, 8–10 disks (8 mm in diameter; about 100 mg) were taken from equidistant points on the primary leaf blade of a second set of plants, placed in Falcon tubes containing dimethyl sulfoxide (DMSO; 7 mL) and heated at 65 °C for 2 h. After this period, the volume in the tubes was completed to 10 mL with DMSO, and 250 µL aliquots of each sample was transferred to microplate wells. Absorbance was measured using a microplate reader (SpectraMax Paradigm, Molecular Devices, San Jose, CA, USA) at wavelengths of 480, 649, and 665 nm. Pigment concentrations were determined by the formulas described in Wellburn [26].
2.6. Experimental Design and Statistical Analysis
The experiments followed a completely randomized design with four replications. Each replication consisted of a Petri dish, a drop on a polyethylene film, and three tubes containing one plant each. These were used, respectively, to evaluate the effect of MEs on mycelial growth in solid medium, C. lindemuthianum conidial germination, and in vivo assays assessing anthracnose severity and early plant development.
After verifying homogeneity of variances, datasets were subjected to analysis of variance (ANOVA) followed by Tukey’s test at 5% significance level for mean separation or regression analysis (foliar pigments). Statistical analyses were carried out in R environment, version 4.3.0.
3. Results
3.1. Chemical Profiling of MEs
UPLC analysis of N. oculata, P. tricornutum, T. obliquus, and T. tetrathele extracts identified 32 compounds across one or more extracts, each belonging to distinct chemical and functional structural groups. This highlights the complexity and heterogeneity of the chemical composition of the extracts (Table 2). The compounds identified include monosaccharides, disaccharides, specific compound derivatives, amino acids and betaines, organic acids, terpenoids and their derivatives, lipids and their derivatives, polyphenols, and antioxidants.

Table 2.
Metabolic profile by UPLC of extracts of the microalgae Nannochloropsis oculata (No), Phaeodactylum tricornutum (Pt), Tetradesmus obliquus (To), and Tetraselmis tetrathele (Tt).
Lipids, which constituted 34% of the 32 identified compounds, were the most prevalent and specific class, with 64% of these compounds being unique to a single microalgae extract. In comparison, 38% of the compounds were common to all four microalgae extracts, including amino acids and betaines, monosaccharides, disaccharides, terpenoids, and organic acids such as linoleic acid. Of the detected compounds, 28% were present in only one extract, 25% in two extracts, and 9% in three extracts.
Regarding the different microalgae species and their respective extracts, it was observed that the P. tricornutum extract contained the highest number of characterized compounds, accounting for 75% of the total identified. The N. oculata extract followed, containing 62% of the compounds, while the T. obliquus and T. tetrathele extracts each contained 59% of the compounds (Table 2).
3.2. Effect of MEs on the Conidial Germination and Mycelial Growth of C. lindemuthianum
On control slides, the percentage of germination of C. lindemuthianum conidia was 50%. In this situation, all of the tested MEs increased significantly spore germination by 80%, 92%, 94%, and 94% at 1.0 mg·mL−1 for P. tricornutum, T. tetrathele, N. oculata, or T. obliquus, respectively (Figure 1).
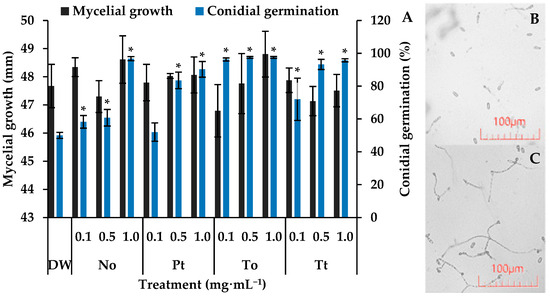
Figure 1.
Mycelial growth (mm) and germination of conidia of Colletotrichum lindemuthianum incubated for 7 days and 72 h, respectively, in distilled water (DW) and hydroalcoholic extracts of Nannochloropsis oculata (No), Phaeodactylum tricornutum (Pt), Tetradesmus obliquus (To), or Tetraselmis tetrathele (Tt) at 0.1, 0.5 or 1.0 mg·mL−1 (A). Micrograph of conidial germination in DW (B) and To at 1.0 mg·mL−1 after 72 h of incubation (C). * indicate significant differences in relation to the control (DW) (Tukey test, p ≤ 0.05, n = 4). Error bars represent the standard deviation of the means.
After seven days of incubation, C. lindemuthianum colonies in the control Petri dishes reached an average diameter of approximately 47.7 mm (Figure 1). Under these conditions, none of the four microalgae species at different concentrations affected the fugal growth, which remained around 47.8 mm.
3.3. Effect of MEs of Anthracnose Severity
The scores of anthracnose evaluated at seven and twelve days after inoculation of control bean plants were about seven and eight, respectively (Table 3). In this situation, no effect on the anthracnose severity was detected when applying the tested MEs.

Table 3.
Anthracnose severity determined at 7 and 12 days after inoculation (Dai) of 11 days-old-bean plants (Phaseolus vulgaris) drenched four times with 3 mL of different concentrations of hydroalcoholic extracts from four microalgae species.
3.4. Effect of MEs on Bean Plant Length of Plant Shoot, Roots, and Total
In control bean plants, the root, shoot, and total plant lengths were 10.5 cm, 18.1 cm, and 28.6 cm, respectively (Figure 2). Under these conditions, hydroalcoholic extract of P. tricornutum increased the total length of plants by 4% at 0.5 mg·mL−1. The extract of T. obliquus increased the length of shoots and entire bean plants by 11% at both 0.5 and 1.0 mg·mL−1. Finally, the extract of T. tetrathele increased the length of entire bean plants by 7% at all tested concentrations and the length of shoots and roots by 6%.
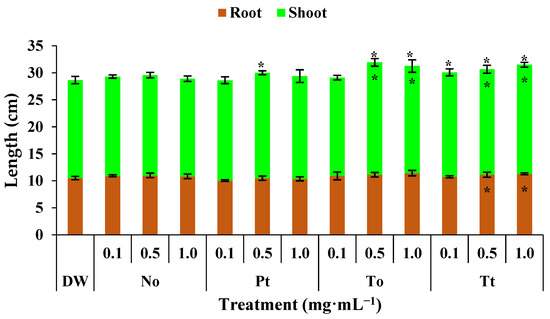
Figure 2.
Total, root, and shoot lengths (cm) of bean plants (Phaseolus vulgaris) drenched four times with varying concentrations of hydroalcoholic extracts from Nannochloropsis oculata (No), Phaeodactylum tricornutum (Pt), Tetradesmus obliquus (So), or Tetraselmis tetrathele (Tt). Plants were grown at 21 ± 2 °C, with a photoperiod of 12 h and relative humidity of 80%, for 11 days after sowing. * Within the columns indicate significant differences between treatments and the control for root or shoot length, while outside the columns indicate differences for total length (root + shoot) according to Tukey’s test (p ≤ 0.05, n = 4). Error bars represent standard deviation of means.
3.5. Effect of MEs on Fresh and Dry Weight Plant
The fresh weight of the root, aerial part, and entire bean plants treated with distilled water (DW) were 0.50 g, 1.08 g, and 1.58 g, respectively (Figure 3A). In these circumstances, the ME of N. oculata at 0.1 mg·mL−1 reduced the root fresh weight by 24%. On the other hand, at 0.5 mg·mL−1, the ME of N. oculata increased the fresh weight of entire bean plants by 11% (Figure 3A). The ME of P. tricornutum at 0.1 and 0.5 mg·mL−1 increased the fresh weight of roots by 46% and 70%, respectively, and entire bean plants by 19% and 25%, respectively. Finally, the ME of T. obliquus increased the fresh weight of roots by 26% at 1.0 mg·mL−1 (Figure 3A).
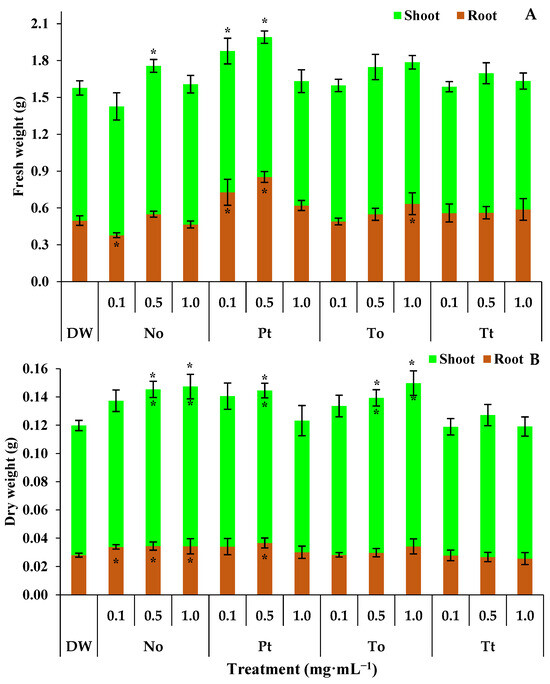
Figure 3.
Root, shoot, and total fresh weight (A) and dry weight (B) (g) of bean plants (Phaseolus vulgaris) drenched four times with varying concentrations of hydroalcoholic extracts Nannochloropsis oculata (No), Phaeodactylum tricornutum (Pt), Tetradesmus obliquus (To), or Tetraselmis tetrathele (Tt). The plants were cultivated in a growth chamber under controlled conditions: 21 ± 2 °C temperature, 12 h photoperiod, and 80% relative humidity, for 11 days post sowing. * Within the columns indicate significant differences between treatments and the control for root or shoot fresh/dry weight, while outside the columns indicate differences for total fresh/dry (root + shoot) according to Tukey Test (p ≤ 0.05, n = 4). Error bars represent standard deviation of means.
The dry weights of the shoots, roots, and whole bean plants in the water-treated control were 0.0918 g, 0.0280 g, and 0.1198 g, respectively (Figure 3B). Under these conditions, the ME of N. oculata increased the dry weight of roots by 22% at 0.1, 0.5, and 1.0 mg·mL−1 (Figure 3B). Also, the ME of N. oculata increased the dry weights of shoots and whole bean plants by 22% at both 0.5 and 1.0 mg·mL−1. On the other hand, the ME of P. tricornutum increased the dry weights of shoots, roots, and whole bean plants by 22% only at 0.5 mg·mL−1 (Figure 3B). Finally, the ME of T. obliquus increased the dry weights of shoots and whole bean plants by 22% at both 0.5 and 1.0 mg·mL−1.
3.6. Effect of MEs on Leaf Pigments
Chlorophylls a, b, and total and carotenoids determined on control bean plants were about 2.11, 0.74, 2.86, and 0.54 µg·mg of fresh weight−1 (Figure 4A–D). Under these circumstances, the MEs of N. oculata and P. tricornutum at 1.0 mg·mL−1 increased the chlorophyll and total chlorophyll and carotenoids levels by 15%, without affecting the content of chlorophyll b (Figure 4A,B). In contrast, the MEs from T. obliquus and T. tetrathele had no impact on the leaf pigment content (Figure 4C,D).
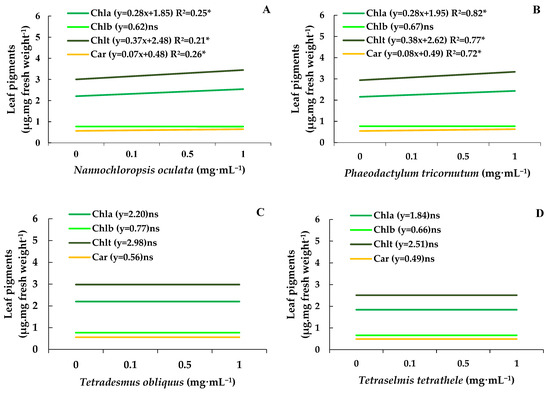
Figure 4.
Chlorophylls a and b content, total chlorophyll, and carotenoid levels (µg·mg of fresh mass−1) in leaves of bean plants (Phaseolus vulgaris) drenched with varying concentrations of the microalgae Nannochloropsis oculata (A), Phaeodactylum tricornutum (B), Tetradesmus obliquus (C), or Tetraselmis tetrathele (D). Plants were kept in a growth chamber at 21 ± 2 °C, with a 12 h photoperiod and 80% relative humidity, for 11 days after sowing. Equations marked with * indicate significant regression, while ‘ns’ means non-significant based on the F test (p ≤ 0.05; n = 4).
4. Discussion
The continuous need to increase productivity without expanding the agricultural frontiers together with the growing concerns regarding pesticide residues in both the environment and in food have driven the search for sustainable productions systems. In this plot, the use of microalgae arises as an eco-friendly strategy for both increasing yield and protecting plants against biotic and abiotic factors [27,28,29].
Most of the identified compounds present in MEs tested in the present study belong to the lipids chemical group. Indeed, microalgae can accumulate higher amounts of lipids (up to 80% of dry weight) when compared to macroalgae (up to 5%). Among accumulated lipids, fatty acids are the most common in microalgae content [30]. Curiously, the biostimulant effect observed in wheat plantlets after treatment with an Arthrospira platensis extract has been attributed to fatty acids [30].
Amino acids and betaines were also found in MEs tested in our work. Among several compounds listed in these groups, glycine betaine, a small organic molecule, can play a vital role in helping plants cope with various environmental stresses. Apparently, glycine betaine acts as an osmolyte, a molecule that helps plants maintain cell turgor (pressure) by balancing the concentration of solutes within the cell [31]. Amino acids, on the other hand, could be precursors of key phytohormones such as auxin and salicylic acid and other biomolecules including polyamines and phenolic compounds [27,28].
Sugars and their building blocks were also found in MEs used in our research. These molecules are well known inducers of plant growth and protection against both biotic and abiotic stresses [27,28]. Thus, for instance, polysaccharides extracted from P. tricornutum, Scenedesmus sp. and Porphyridium sp. increased the activity of defense-related enzymes such as phenylalanine ammonia liase, chitinase, β-1,3-glucanase, and peroxidases in tomato plants [32]. Monosaccharides and disaccharides present in microalgae’s biomass can act as biostimulants by providing energy and modulating hormonal responses [33,34], regulating osmotic balance [35,36], promoting microbial activity, and stimulating root growth [37,38].
Organic acids, terpenoids, polyphenols, and antioxidants were present in the tested MEs. Biomolecules belonging to these groups are known for possessing a wide range of biological activities [39,40]. For instance, phloroglucinol and its derivatives have potential antibacterial, antifungal, and antiviral properties [40]. Also, phloroglucinol and eckol seem to play a significant role in activating biochemical pathways essential for enhancing crop productivity [39]. Resveratrol, another polyphenol, can stimulate the growth of lettuce, probably by reducing the production of reactive oxygen species and by increasing the photosynthetic efficiency [41]. Given that citric acid can enhance plant growth and stress tolerance through improved photosynthesis, antioxidant defenses, and heavy metal chelation, it is reasonable to suppose that citric acid produced by microalgae and applied to plants can have similarly positive effects [42]. In fact, several studies have confirmed that exogenous citric acid application enhances growth, yield, and photosynthetic pigments in Gossypium barbadense (cotton) [43], boosts germination rates in Carica papaya (papaya) [44], increases growth and chlorophyll content in Phaseolus vulgaris (common bean) [43], and enhances photosynthetic pigments in Zea mays (maize) [45].
In the present work, all tested MEs increased the in vitro germination of C. lindemuthianum conidia. These results could be explained by the presence of sugars in the MEs. It is well known that sugar monomers can stimulate the growth of fungi including Colletotrichum [46]. Therefore, it would be expected that the presence of some sugars in MEs could accelerate the germination and growth of C. lindemuthianum.
The mycelial growth of C. lindemuthianum was not affected by any of the tested microalgae. In contrast, it has been demonstrated that aqueous extracts of Nannochloropsis sp., Phaeodactylum sp. and Scenedesmus sp. can reduce the mycelial growth of the plant pathogenic fungi Sclerotium rolfsii, Rhizoctonia solani, and Botrytis cinera [47]. These differences may be attributed to variations in methodology between our study and previous research. While Schmid et al. [47] spread the extracts over the semi solid PDA medium, we added them into it. Therefore, in the first, the fungus would be directly in contact with the extracts. Alternatively, our results could be explained by a different degree of sensibility of C. lindemuthianum to extracts of the tested microalgae species. For instance, the aqueous extract of S. obliquus both reduces and increases the mycelial growth of B. cinerea and Alternaria alternata, respectively [47].
None of the tested microalgae affected the anthracnose severity in bean plants. Interestingly, it has been reported that microalgae extracts can activate a wide range of defenses responses and protect plants against pathogens. For instance, the spraying of Chlorella fusca cells reduced the severity of Colletotrichum orbiculare in cucumber [48]. Two hypotheses can be raised to explain these disparities. Firstly, this could be attributed to the material applied to the leaves, while we sprayed a hydroalcoholic extract, Kim et al., [48] used live C. fusca cells probably with the growth medium. Therefore, some exopolysaccharides released by C. fusca in the growth medium would be present and could have induced defense responses in cucumber leaves. Alternatively, it is known that the conditions used during the production can directly affect the composition of microalgae [27]. Consequently, the biological effect could be entirely different. Therefore, evaluating the biological activity of microalgae grown under different conditions will be an exciting opportunity for future studies.
Extracts from tested microalgae species variably enhanced certain growth variables of bean plants, including root and shoot length, as well as their fresh and dry weights. Indeed, studies have shown that microalgae can promote the growth of various plant species, including Beta vulgaris, Solanum lycopersicum, Triticum aestivum, and Phaseolus vulgaris [30,32,49]. Despite the fact that the exact mode of action in plants is still unclear, it has been proposed that the biostimulant activity of microalgae is associated with the presence of pytohormones such as auxins, gibberelins and cytokinins, sugars, proteins, and aminoacids and antioxidant biomolecules [27,28]. These molecules could act alone or, more probably, in synergy.
The MEs of Nannochloropsis oculata and Phaeodactylum tricornutum significantly increased chlorophyll, total chlorophyll, and carotenoid levels in bean plants. Chlorophyll retention is a well-documented plant response to abiotic stress. For instance, exposure to specific chemicals, such as ethylene inhibitors, can delay senescence and chlorophyll degradation [50,51]. Interestingly, only these two microalgae species demonstrated this effect; however, no correlation with their chemical profiles was found, leaving the underlying mechanism unresolved.
5. Conclusions
In sum, our results show that hydroalcoholic extracts of the microalgae P. tricornutum, N. oculata, T. tetrathele, and T. obliquus are composed by myriad of biomolecules including lipids, sugars, aminoacids, organic acids, terpenoids, polyphenols, and antioxidants. Additionally, the tested MEs can biostimulate the initial growth of bean plants without affecting the severity of anthracnose caused by C. lindemuthianum. The microalgae tested in our work present potential applications in sustainable agriculture as natural plant growth enhancers. These findings support the exploration of microalgal extracts as eco-friendly alternatives to synthetic agrochemicals, contributing to more sustainable crop production practices.
Author Contributions
C.N. and R.B.D. cultivated and freeze-dried the different species of microalgae. A.A.d.S., C.N., M.B.d.F. and C.F.R. performed the in vitro and in vivo experiments. G.d.O.C. and L.P.S. analyzed the seaweed extracts using UPLC. A.A.d.S. wrote the main text of the manuscript, prepared figures and tables and performed statistical analyses. M.B.d.F., L.P.S., R.B.D., A.S.P. and M.J.S. corrected and revised the text, figures, and table of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The first author would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for the doctoral scholarship. This study was financially supported by grant no. 2022TR001472 from the Foundation for Research and Innovation Support of the State of Santa Catarina (FAPESC-Brazil). MJS is a fellow of CNPq.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Delgado, D.Z.; De Freitas, M.B.; Stadnik, M.J. Effectiveness of saccharin and ulvan as resistance inducers against rust and angular leaf spot in bean plants (Phaseolus vulgaris). Crop Prot. 2013, 47, 67–73. [Google Scholar] [CrossRef]
- Assefa, T.; Assibi Mahama, A.; Brown, A.V.; Canhão, E.K.S.; Rubyogo, J.C.; Rao, I.M.; Blair, M.W.; Canhão, S.B. A review of breeding objectives, genomic resources, and marker-assisted methods in common bean (Phaseolus vulgaris L.). Mol. Breed. 2019, 39, 20. [Google Scholar] [CrossRef]
- Singh, S.P.; Schwartz, H.F. Breeding common bean for resistance to diseases: A review. Crop Sci. 2010, 50, 2199–2223. [Google Scholar] [CrossRef]
- De Freitas, M.B.; Stadnik, M.J. Race-specific and ulvan-induced defense responses in bean (Phaseolus vulgaris) against Colletotrichum lindemuthianum. Physiol. Mol. Plant Pathol. 2012, 78, 8–13. [Google Scholar] [CrossRef]
- Wordell-Filho, J.A.; De Freitas, M.B.; Stadnik, M.J.; Theodoro, G.F. Manejo de doenças na cultura do feijão. In Manejo Fitossanitário na Cultura do Feijão, 1st ed.; Wordell-Filho, J.A., Chiaradia, L.A., Balbinot, A., Eds.; Epagri: Florianopolis, Brazil, 2013; pp. 9–41. ISBN 9788585014704. [Google Scholar]
- Costa, J.A.V.; Freitas, B.C.B.; Cruz, C.G.; Silveira, J.; Morais, M.G. Potential of microalgae as biopesticides to contribute to sustainable agriculture and environmental development. J. Environ. Sci. Health 2019, 54 Pt B, 366–375. [Google Scholar] [CrossRef]
- Ammar, E.E.; Aioub, A.A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; EL-Shershaby, N.A. Algae as Bio-Fertilizers: Between Current Situation and Future Prospective: The Role of Algae as a Bio-Fertilizer in Serving of Ecosystem. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef]
- Miranda, A.M.; Hernandez-Tenorio, F.; Villalta, F.; Vargas, G.J.; Sáez, A.A. Advances in the Development of Biofertilizers and Biostimulants from Microalgae. Biology 2024, 13, 199. [Google Scholar] [CrossRef]
- Boutahiri, S.; Benrkia, R.; Tembeni, B.; Idowu, O.E.; Olatunji, O.J. Effect of biostimulants on the chemical profile of food crops under normal and abiotic stress conditions. Curr. Plant Biol. 2024, 40, 100410. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as Multi-Functional Options in Modern Agriculture: Current Trends, Prospects and Challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef]
- Lee, S.M.; Ryu, C.M. Algae as new kids in the beneficial plant microbiome. Front. Plant Sci. 2021, 12, 599742. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Elarroussia, H.; Elmernissia, N.; Benhimaa, R.; Meftah, E.; Kadmiria, I.; Bendaou, N.; Smouni, A.; Wahby, I. Microalgae polysaccharides a promising plant growth biostimulant. J Algal Biomass Util. 2016, 7, 55–63. [Google Scholar]
- Vehapi, M.; Yilmaz, A.; Ozcimen, D. Antifungal Activities of Chlorella vulgaris and Chlorella minutissima Microalgae Cultivated in Bold Basal Medium, Wastewater and Tree Extract Water Against Aspergillus niger and Fusarium oxysporum. Dep. Bioeng. Rom. Biotechnol. Lett. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Ranglová, K.; Lakatos, G.E.; Câmara Manoel, J.A.; Grivalský, T.; Suárez Estrella, F.; Acién Fernández, F.G.; Molnár, Z.; Ördög, V.; Masojídek, J. Growth, biostimulant and biopesticide activity of the MACC-1 Chlorella strain cultivated outdoors in inorganic medium and wastewater. Algal Res. 2021, 53, 102136. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; D’Alessandro, E.B.; Antoniosi Filho, N.R.; Lopes, R.G.; Derner, R.B. Synergistic effect of growth conditions and organic carbon sources for improving biomass production and biodiesel quality by the microalga Choricystis minor var. minor. Sci. Total Environ. 2021, 759, 143476. [Google Scholar] [CrossRef]
- Sales, R.; Derner, R.; Tsuzuki, M. Effects of different harvesting and processing methods on Nannochloropsis oculata concentrates and their application on rotifer Brachionus sp. cultures. J. Appl. Phycol. 2019, 31, 3607–3615. [Google Scholar] [CrossRef]
- Abreu, G.F.; Talamini, V.; Stadnik, M.J. Bioprospecção de macroalgas marinhas e plantas aquáticas para o controle da antracnose do feijoeiro. Summa Phytopathol. 2008, 34, 22–26. [Google Scholar] [CrossRef]
- Tsugawa, H.; Kind, T.; Nakabayashi, R.; Yukihira, D.; Tanaka, W.; Cajka, T.; Saito, K.; Fiehn, O.; Arita, M. Hydrogen Rearrangement Rules: Computational MS/MS Fragmentation and Structure Elucidation Using MS-FINDER Software. Anal. Chem. 2016, 88, 7946–7958. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Chen, S.; Ge, Y.H.; Xiao, Y.; Zhao, M.; Wu, J.L. MS-FINDER Assisted in Understanding the Profile of Flavonoids in Temporal Dimension during the Fermentation of Pu-erh Tea. J. Agric. Food Chem. 2022, 70, 7085–7094. [Google Scholar] [CrossRef]
- Gonçalves, A.E.; Stadnik, M.J. Interferência de ulvana no desenvolvimento e melanização de apressórios de Colletotrichum gloeosporioides. Trop. Plant Pathol. 2012, 37, 431–437. [Google Scholar] [CrossRef]
- Paulert, R.; Talamini, V.; Cassolato, J.E.F.; Duarte, M.; Noseda, M.D.; Smania, J.A.; Stadnik, M.J. Effects of sulfated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). J. Plant Dis. Protect. 2009, 116, 263–270. [Google Scholar] [CrossRef]
- Rava, C.A.; Molina, J.; Kauffmann, M.; Briones, I. Determinación de razas fisiológicas de Colletotrichum lindemuthianum en Nicaragua. Fitopatol. Bras. 1992, 18, 388–391. [Google Scholar]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1978, 57, 1332–1334. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolutions. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.C.; Colla, L.M. Use of microalgae for the development of biofertilizers and biostimulants. BioEnergy Res. 2023, 16, 289–310. [Google Scholar] [CrossRef]
- Poveda, J.; Díez-Méndez, A. Use of elicitors from macroalgae and microalgae in the management of pests and diseases in agriculture. Phytoparasitica 2023, 51, 667–701. [Google Scholar] [CrossRef]
- Dmytryk, A.; Samoraj, M.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Bioactive fatty acids and compounds from Spirulina (Arthrospira) platensis: Potential as biostimulants for plant growth. Sustain. Chem. Pharm. 2022, 30, 100899. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’aversana, E.; Carrillo, P. Spatial and temporal profile of Glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef]
- Rachidi, F.; Benhima, R.; Kasmi, Y.; Sbabou, L.; Arroussi, H.E. Evaluation of microalgae polysaccharides as biostimulants of tomato plant defense using metabolomics and biochemical approaches. Sci. Rep. 2021, 11, 930. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Jiménez-Arias, D.; García-Machado, F.J.; Morales-Sierra, S.; García-García, A.L.; Herrera, A.J.; Valdés, F.; Luis, J.C.; Borges, A.A. A beginner’s guide to osmoprotection by biostimulants. Plants 2021, 10, 363. [Google Scholar] [CrossRef]
- Gómez-Ariza, J.; Campo, S.; Rufat, M.; Estopà, M.; Messeguer, J.; Segundo, B.S.; Coca, M. Sucrose-mediated priming of plant defense responses and broad-spectrum disease resistance by overexpression of the maize pathogenesis-related PRms protein in rice plants. Mol. Plant Microbe Interact. 2007, 20, 832–842. [Google Scholar] [CrossRef]
- Koyro, H.W.; Ahmad, P.; Geissler, N. Abiotic Stress Responses in Plants: An Overview. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer International Publishing: New York, NY, USA, 2012; pp. 1–28. [Google Scholar]
- Tegeder, M.; Rentsch, D. Uptake and partitioning of amino acids and peptides. Mol. Plant 2010, 3, 997–1011. [Google Scholar] [CrossRef]
- Chovanček, E.; Salazar, J.; Şirin, S.; Allahverdiyeva, Y. Microalgae from Nordic collections demonstrate biostimulant effect by enhancing plant growth and photosynthetic performance. Physiol. Plant. 2023, 175, e13911. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Kulkarni, M.G.; Stirk, W.A.; Staden, J.V. Eckol—A new plant growth stimulant from the brown seaweed Ecklonia maxima. J. Appl. Phycol. 2015, 27, 581–587. [Google Scholar] [CrossRef]
- Khan, F.; Tabassum, N.; Bamunuarachchi, N.I.; Kim, J.M. Phloroglucinol and Its Derivatives: Antimicrobial Properties toward Microbial Pathogens. J. Agric. Food Chem. 2022, 70, 4817–4838. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.L.S.; Araniti, F.; Ishii-Iwamoto, E.L.; Abenavoli, M.R. Resveratrol exerts beneficial effects on the growth and metabolism of Lactuca sativa L. Plant Pysiol. Biochem. 2022, 171, 26–37. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric acid-mediated abiotic stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef]
- El-Tohamy, W.; El-Abagy, H.; Badr, M.; Gruda, N. Drought tolerance and water status of bean plants (Phaseolus vulgaris L.) as affected by citric acid application. J. Appl. Bot. Food Qual. 2013, 86, 212–216. [Google Scholar] [CrossRef]
- Zanotti, R.F.; Lopes, J.C.; Motta, L.B.; de Freitas, A.R.; Mengarda, L.H.G. Tolerance induction to saline stress in papaya seeds treated with potassium nitrate and sildenafil citrate. Semin. Ciênc. Agrár. 2013, 34, 3669–3673. [Google Scholar] [CrossRef]
- El-Hawary, M.; Nashed, M.E. Effect of foliar application by some antioxidants on growth and productivity of maize under saline soil conditions. J. Plant Prod. 2019, 10, 93–99. [Google Scholar] [CrossRef]
- Araújo, L.; Gonçalves, A.E.; Stadnik, M.J. Ulvan effect on conidial germination and appressoria formation of Colletotrichum gloeosporioides. Phytoparasitica 2014, 42, 631–640. [Google Scholar] [CrossRef]
- Schmid, B.; Coelho, L.; Schulze, P.S.; Pereira, H.; Santos, T.; Maia, I.B.; Reis, M.; Varela, J. Antifungal properties of aqueous microalgal extracts. Bioresour. Technol. Rep. 2022, 18, 101096. [Google Scholar] [CrossRef]
- Kim, S.J.; Ko, E.J.; Hong, J.K.; Jeun, Y.C. Ultrastructures of Colletotrichum orbiculare in cucumber leaves expressing systemic acquired resistance mediated by Chlorella fusca. Plant Pathol. J. 2018, 34, 113–120. [Google Scholar] [CrossRef]
- Barone, V.; Baglieri, A.; Stevanato, P.; Broccanello, C.; Bertoldo, G.; Bertaggia, S.; Cagnin, S.; Pizzeghello, D.; Moliterni, V.M.C.; Mandolino, G.; et al. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J. Appl. Phycol. 2018, 30, 1061–1071. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, W.; Zheng, Y.; Wang, J.; Wang, Z.; Yang, Z.; Chi, X.; Dai, L.; Lu, G.; Yang, Y.; Sun, B. Ethylene-responsive SbWRKY50 suppresses leaf senescence by inhibition of chlorophyll degradation in sorghum. New Phytol. 2023, 238, 1129–1145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).