Enhanced Bioactive Compounds and Antioxidant Activity in Germinated Seeds of the New Peanut Variety
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growth Conditions
2.3. Instruments and Reagents
2.4. Preparation of SPK Extracts
2.5. Sample Preparation for Antioxidant Activities
2.6. ABTS+ Radical Scavenging Activity of SPK
2.7. DPPH Radical Scavenging Activity of SPK
2.8. Total Polyphenol Content (TPC) of SPK
2.9. Total Flavonoid Content (TFC) of SPK
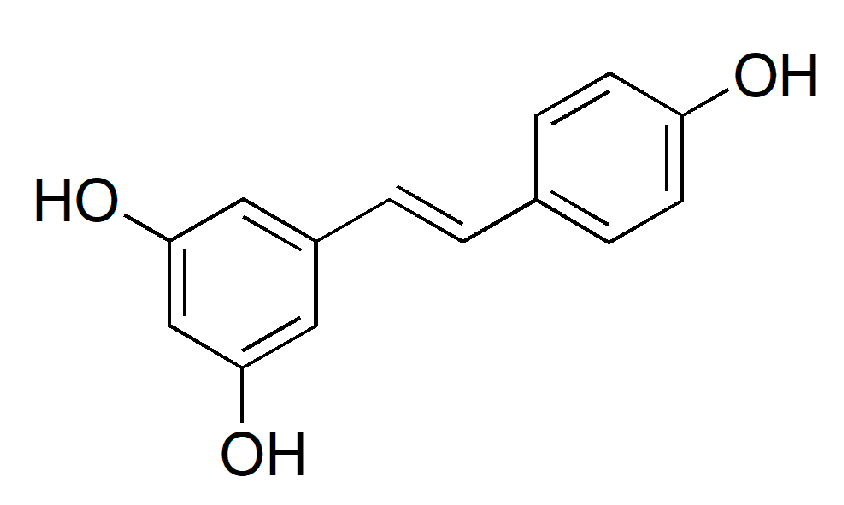
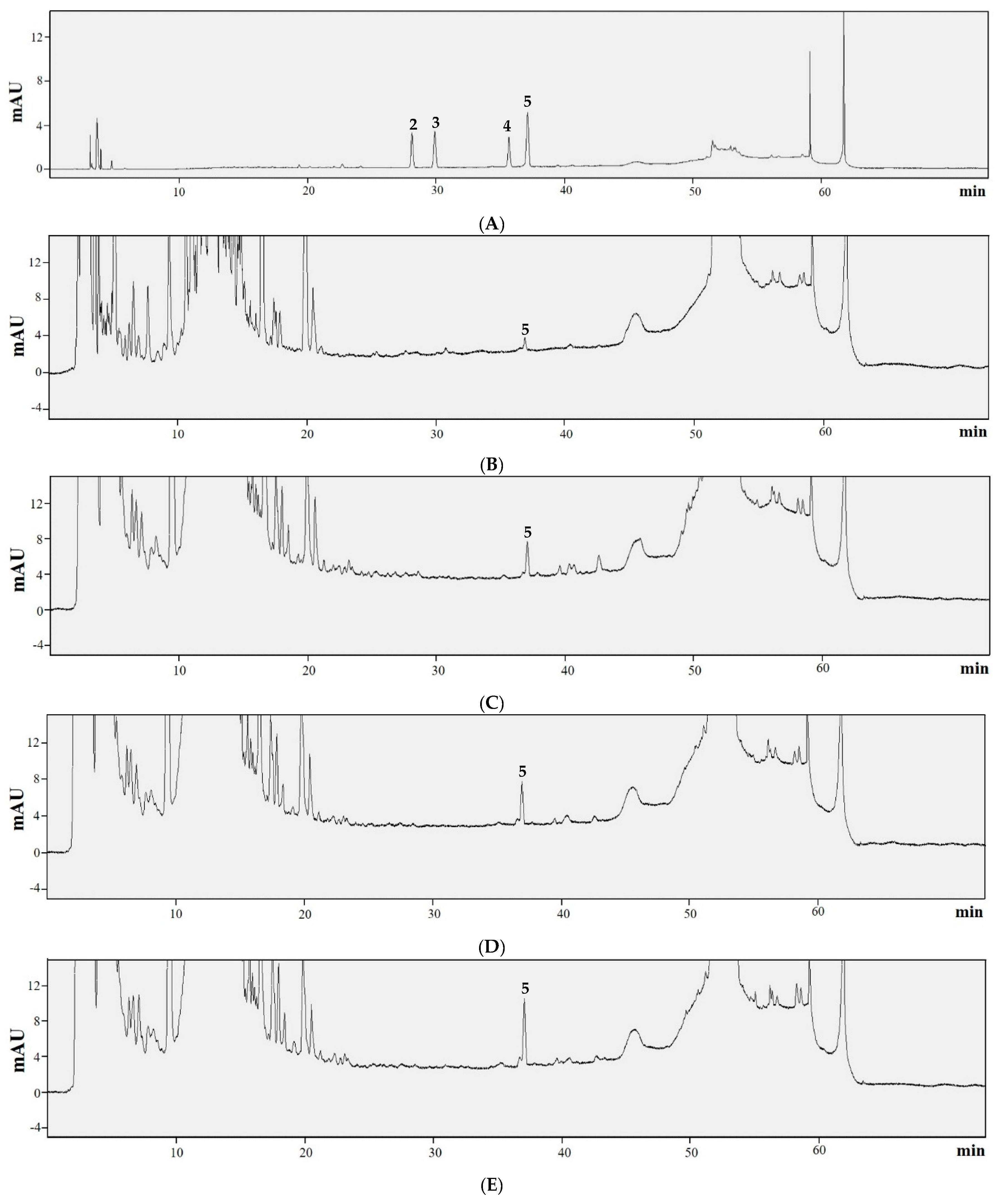
2.10. HPLC Analysis of Trans-Resveratrol
2.11. HPLC Analysis of Soyasaponins Aa, Ab, Ba, and Bb
2.12. Calibration Curves
2.13. Statistical Analysis
3. Results and Discussion
3.1. SPK Extracts
3.2. ABTS+ and DPPH Radical Scavenging Activity of SPK
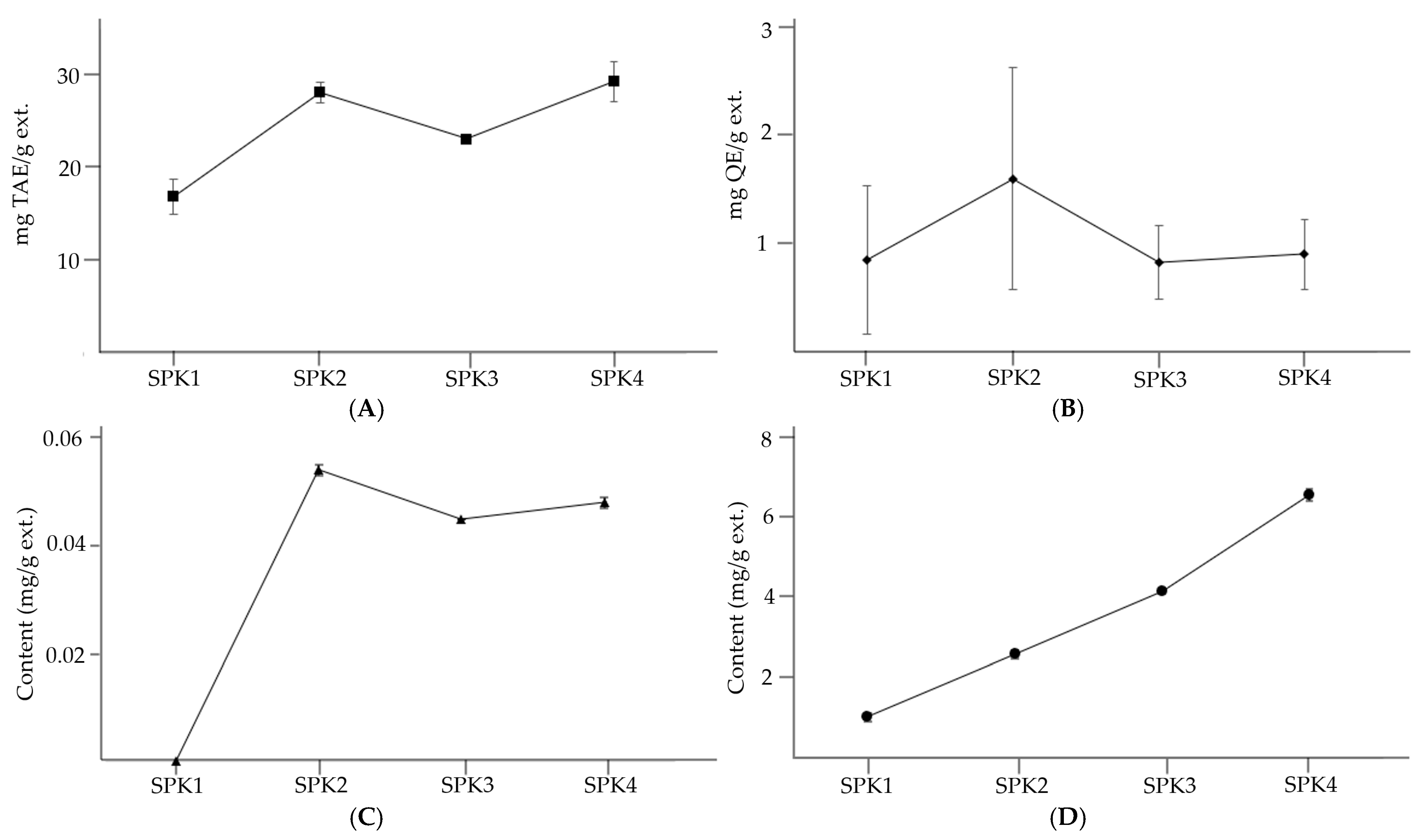
3.3. TPC and TFC of SPK
3.4. Content of Trans-Resveratrol
3.5. Content of Soyasponins Aa, Ab, Ba, and Bb
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, M.; Kim, J.; Ahn, S.M. Factors associated with frequency of peanut consumption in Korea: A national population-based study. Nutrients 2020, 12, 1207. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Blumberg, J.; Ingwersen, L.; Jenab, M.; Tucker, K.L. Tree nuts and peanuts as components of a healthy diet. J. Nutr. 2008, 138, 1736S–1740S. [Google Scholar] [CrossRef]
- Kim, J.; Choi, C.H.; Lee, A.Y.; Lee, S. Antioxidant, anticancer, and neuroprotective activities and phytochemical analysis of germinated shoots. J. Food Biochem. 2023, 2023, 1–9. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, F.; Luo, H.; He, W.; Li, D.; Bao, Y.; Zhang, Z.; Zhou, C. Changes in phytochemical profiles, relevant enzyme activity and antioxidant capacity of different germinated maize varieties. Food Biosci. 2023, 56, 103410. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Jung, Y.-S.; Cho, E.-C.; Kim, D.-H.; Shin, K.-O. Physiological Activity and Nutritional Properties of Peanut Sprout Extracts. J. Korean Soc. Food Sci. Nutr. 2024, 53, 599–607. [Google Scholar] [CrossRef]
- Gonçalves, J.P.; Gasparini, K.; Picoli, E.A.T.; Costa, M.D.L.; Araujo, W.L.; Zsögön, A.; Ribeiro, D.M. Metabolic control of seed germination in legumes. J. Plant Physiol. 2024, 295, 154206. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.-H.; Lee, Y.-J.; Kim, J.-H.; Kim, Y.-H.; Kim, D.-B.; Lee, J.S.; Lim, J.-H.; Lee, O.-H. Antioxidant activities of commonly used Brassica spp. sprout vegetables in Korea. J. Korean Soc. Food Preserv. 2014, 21, 587–592. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Jung, Y.-S.; Cho, E.-C.; Kim, D.-H.; Shin, K.-O. Quality Characteristics of Madeleines Prepared with the Addition of Peanut Sprout Powder. J. East Asian Soc. Diet. Life 2024, 34, 298–309. [Google Scholar] [CrossRef]
- Pae, S.B.; Hwang, C.D.; Kim, S.U.; Lee, M.-H.; Shim, K.B.; Park, C.; Lee, C.-K.; Baek, I.Y.; Lee, J.-K. A new large grain and high-yielding Virginia type peanut cultivar ‘Sinpalkwang’. Korean J. Breed. Sci. 2016, 48, 66–71. [Google Scholar] [CrossRef]
- Das, M.; Das, D.K. Resveratrol and cardiovascular health. Mol. Asp. Med. 2010, 31, 503–512. [Google Scholar] [CrossRef]
- Uggioni, M.L.R.; Ronsani, L.; Motta, S.; Júnior, J.C.D.; Marçal, F.; Ferraz, S.D.; Rosa, M.I.D.; Colonetti, T. Effects of resveratrol on the lipid profile of post-menopause women: Systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2024, 103827. [Google Scholar] [CrossRef] [PubMed]
- Lindner, G.R.; Khalil, N.M.; Mainardes, R.M. Resveratrol-loaded polymeric nanoparticles: Validation of an HPLC-PDA method to determine the drug entrapment and evaluation of its antioxidant activity. Sci. World J. 2013. [Google Scholar] [CrossRef]
- Rigon, R.B.; Fachinetti, N.; Severino, P.; Durazzo, A.; Lucarini, M.; Atanasov, A.G.; El Mamouni, S.; Chorilli, M.; Santini, A.; Souto, E.B. Quantification of trans-resveratrol-loaded solid lipid nanoparticles by a validated reverse-phase HPLC photodiode array. Appl. Sci. 2019, 9, 4961. [Google Scholar] [CrossRef]
- Langley, B.; Sauve, A. Sirtuin deacetylases as therapeutic targets in the nervous system. Neurotherapeutics 2013, 10, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Chen, Y.; Tang, X.; Wang, S.; Yin, K. PAD2: A potential target for tumor therapy. Biochim. Et Biophys. Acta 2023, 1878, 188931. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Et Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar] [CrossRef]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef] [PubMed]
- Penantian, R.M.; Antarianto, R.D.; Hardiany, N.S. Effect of calorie restriction on the expression of sirtuin1 as an antiaging biomarker. Makara J. Sci. 2023, 27, 179–185. [Google Scholar]
- Perinelli, D.R.; Mustafa, A.M.; Angeloni, S.; Sagratini, G.; Caprioli, G.; Cespi, M.; Bonacucina, G. Surface and emulsifying properties of raw ethanolic extracts from legumes containing soyasaponins and proteins. Sustain. Chem. Pharm. 2022, 30, 100872. [Google Scholar] [CrossRef]
- Tsuno, Y.; Fujimatsu, T.; Endo, K.; Sugiyama, A.; Yazaki, K. Soyasaponins: A new class of root exudates in soybean (Glycine max). Plant Cell Physiol. 2018, 59, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Chitisankul, W.T.; Itabashi, M.; Son, H.; Takahashi, Y.; Ito, A.; Varanyanond, W.; Tsukamoto, C. Soyasaponin composition complexities in soyfoods relating nutraceutical properties and undesirable taste characteristics. LWT 2021, 146, 111337. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, C.; Huang, B. Hepatoprotective action mechanism and quantification of soyasaponin Bb in Abri Herba by HPLC and network pharmacology. J. Ethnopharmacol. 2025, 337, 118850. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Hemamalini, R.; Khare, S. Effect of processing conditions on saponin content and antioxidant activity of Indian varieties of soybean (Glycine max Linn.). Ann. Phytomed. 2012, 1, 62–68. [Google Scholar]
- Jyothi, T.C.; Sindhu Kanya, T.C.; Appu Rao, A.G. Influence of germination on saponins in soybean and recovery of soy sapogenol I. J. Food Biochem. 2007, 31, 1–13. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Chen, Y.-H.; Chien, Y.-W.; Huang, W.-H.; Lin, S.-H. Effect of soy saponin on the growth of human colon cancer cells. World J. Gastroenterol. 2010, 16, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Gu, X.; Chen, J.; Liu, M.; Xiong, F.; Wu, X.; Zhang, Y.; Chen, F.; Chen, H.; Li, M.; et al. Soyasaponins reduce inflammation and improve serum lipid profiles and glucose homeostasis in high fat diet-induced obese mice. Mol. Nutr. Food Res. 2018, 62, e1800205. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-Z.; Liu, Z.-Q. Free-radical-scavenging effect of carbazole derivatives on DPPH and ABTS radicals. J. Am. Oil Chem. Soc. 2007, 84, 1095–1100. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I. Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chem. 2005, 90, 199–206. [Google Scholar] [CrossRef]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Zhou, Z.; Fan, Z.; Meenu, M.; Xu, B. Impact of germination time on resveratrol, phenolic acids, and antioxidant capacities of different varieties of peanut (Arachis hypogaea Linn.) from China. Antioxidants 2021, 10, 1714. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.; Lee, H.-D.; Cho, H.; Paje, L.A.; Shin, H.; Lee, S. Development of an analytical approach for the utilization of edible tree sprouts. Nat. Prod. Sci. 2022, 28, 27–32. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Tran, G.-H.; Uy, N.-P.; Kang, S.-H.; Heo, W.; Lee, E.-S.; Roh, S.-S.; Lee, S. HPLC/DAD analysis and antioxidant activity of adlay sprouts and seeds. Separations 2024, 11, 32. [Google Scholar] [CrossRef]
- Lee, S.S.; Lee, S.M.; Kim, M.; Chun, J.; Cheong, Y.K.; Lee, J. Analysis of trans-resveratrol in peanuts and peanut butters consumed in Korea. Food Res. Int. 2004, 37, 247–251. [Google Scholar] [CrossRef]
- Pae, S.-B.; Ha, T.-J.; Lee, M.-H.; Hwang, C.-D.; Shim, K.-B.; Park, C.-H.; Park, K.-Y.; Baek, I.-Y. Evaluation of characteristics of peanut sprout using Korean cultivars. Korean J. Crop Sci. 2011, 56, 394–399. [Google Scholar] [CrossRef]
- Silva, C.G.; Monteiro, J.; Marques, R.R.N.; Silva, A.M.T.; Martínez, C.; Canle, L.M.; Faria, J.L. Photochemical and photocatalytic degradation of trans-resveratrol. Photochem. Photobiol. Sci. 2013, 12, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, M.; Ye, J.H.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R. Photo-induced chemical reaction of trans-resveratrol. Food Chem. 2015, 171, 137–143. [Google Scholar] [CrossRef]
- Santos Wagner, A.L.; Araniti, F.; Ishii-Iwamoto, E.L.; Abenavoli, M.R. Resveratrol exerts beneficial effects on the growth and metabolism of Lactuca sativa L. Plant Physiol. Biochem. 2022, 171, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yuk, H.J.; Ryu, H.W.; Oh, S.R.; Song, D.Y.; Lee, K.S.; Park, K.I.; Choi, S.W.; Seo, W.D. Biofunctional soyasaponin Bb in peanut (Arachis hypogaea L.) sprouts enhances bone morphogenetic protein-2-dependent osteogenic differentiation via activation of runt-related transcription factor 2 in C2C12 cells. Phytother. Res. 2019, 33, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Lee, H.L.; Kang, J.Y.; Kim, J.M.; Heo, H.J. Peanut (Arachis hypogaea) sprout prevents high-fat diet-induced cognitive impairment by improving mitochondrial function. Sci. Rep. 2022, 12, 6213. [Google Scholar] [CrossRef]
- Kim, M.Y.; Jang, G.Y.; Lee, Y.J.; Kim, K.M.; Kim, D.J.; Lee, J.; Jeong, H.S. Application of high hydrostatic pressure for production of bioactive soyasaponin from black soybean [Glycine max (L.)] sprout. J. Food Nutr. Res. 2019, 7, 270–278. [Google Scholar]
- Shimoyamada, M.; Okubo, K. Variation in saponin contents in germinating soybean seeds and effect of light irradiation. Agric. Biol. Chem. 1991, 55, 577–579. [Google Scholar]
- Yoshiki, Y.; Kudou, S.; Okubo, K. Relationship between chemical structure and biological activities of triterpenoid saponins from soybean. Biosci. Biotechnol. Biochem. 1998, 62, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
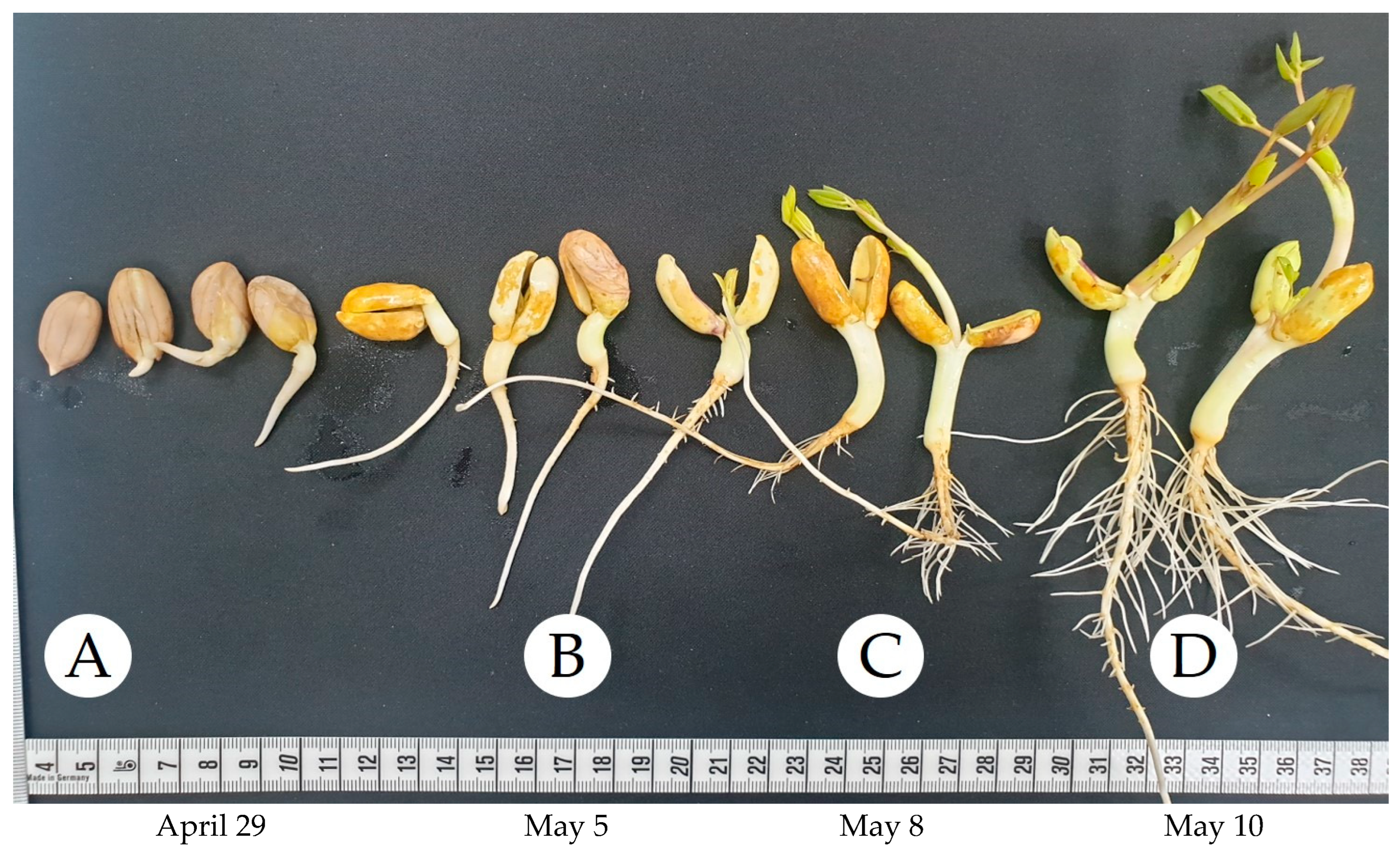

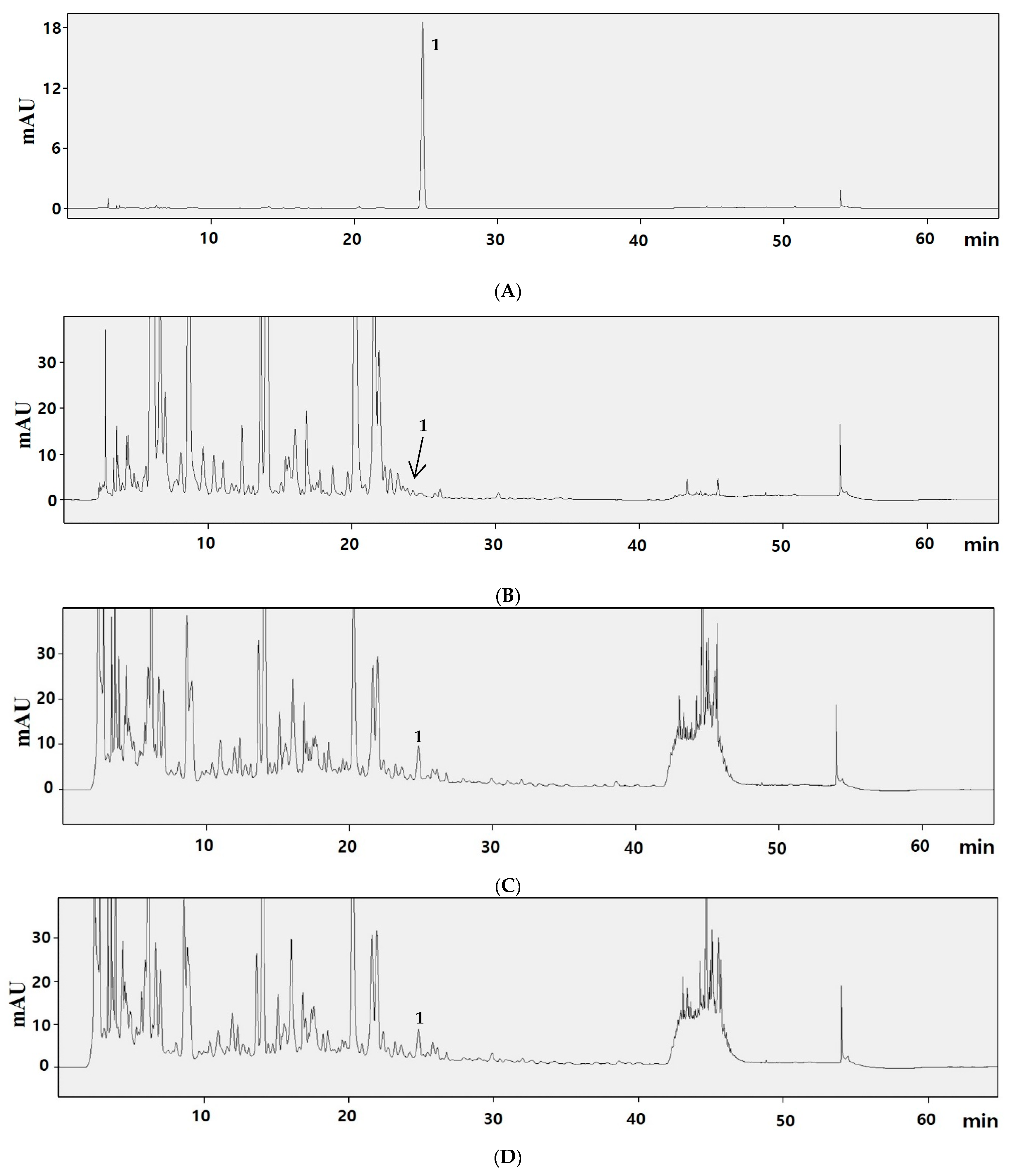
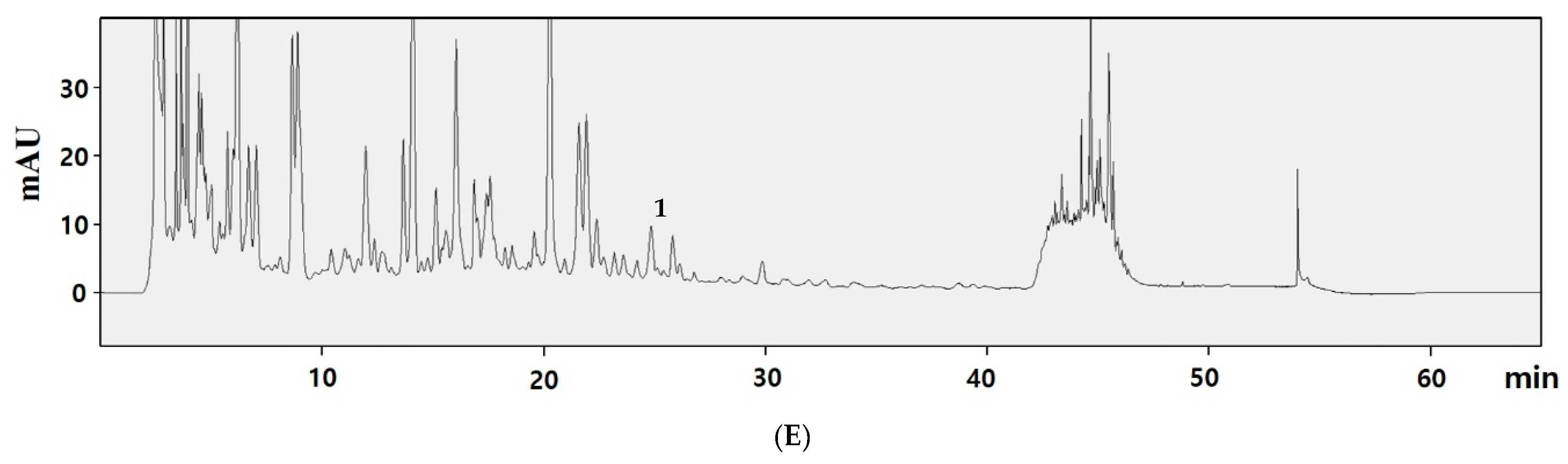

| Sample | DW (g) | Ext. Weight (g) | Yield (%) |
|---|---|---|---|
| SPK 1 | 6 | 3.0 | 50.0 |
| SPK 2 | 6 | 3.1 | 51.7 |
| SPK 3 | 6 | 3.3 | 55.0 |
| SPK 4 | 6 | 3.2 | 53.3 |
| Sample | IC50 (mg/mL) | |
|---|---|---|
| ABTS+ | DPPH | |
| SPK 1 | 23.32 ± 0.30 a | 44.24 ± 0.66 a |
| SPK 2 | 6.20 ± 0.08 d | 17.61 ± 0.80 b |
| SPK 3 | 11.75 ± 0.02 b | 14.48 ± 0.87 c |
| SPK 4 | 7.63 ± 0.08 c | 15.05 ± 0.71 c |
| Ascorbic acid | 0.11 ± 0.00 | 0.13 ± 0.00 |
| Sample | TPC (mg TAE/g ext.) | TFC (mg QE/g ext.) |
|---|---|---|
| SPK 1 | 16.92 ± 1.93 c | 0.86 ± 0.69 a |
| SPK 2 | 28.22 ± 1.11 a | 1.61 ± 1.03 a |
| SPK 3 | 23.16 ± 0.04 b | 0.84 ± 0.34 a |
| SPK 4 | 29.38 ± 2.18 a | 0.91 ± 0.32 a |
| Sample | Content (mg/g ext.) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Total | |
| SPK 1 | Trace | ND | ND | ND | 1.025 ± 0.102 d | 1.025 |
| SPK 2 | 0.054 ± 0.001 a | ND | ND | ND | 2.587 ± 0.102 c | 2.641 |
| SPK 3 | 0.045 ± 0.000 c | ND | ND | ND | 4.149 ± 0.045 b | 4.194 |
| SPK 4 | 0.048 ± 0.001 b | ND | ND | ND | 6.543 ± 0.146 a | 6.591 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-H.; Park, J.-S.; Lee, S. Enhanced Bioactive Compounds and Antioxidant Activity in Germinated Seeds of the New Peanut Variety. BioTech 2025, 14, 12. https://doi.org/10.3390/biotech14010012
Yu H-H, Park J-S, Lee S. Enhanced Bioactive Compounds and Antioxidant Activity in Germinated Seeds of the New Peanut Variety. BioTech. 2025; 14(1):12. https://doi.org/10.3390/biotech14010012
Chicago/Turabian StyleYu, Hwan-Hee, Jong-Suk Park, and Sanghyun Lee. 2025. "Enhanced Bioactive Compounds and Antioxidant Activity in Germinated Seeds of the New Peanut Variety" BioTech 14, no. 1: 12. https://doi.org/10.3390/biotech14010012
APA StyleYu, H.-H., Park, J.-S., & Lee, S. (2025). Enhanced Bioactive Compounds and Antioxidant Activity in Germinated Seeds of the New Peanut Variety. BioTech, 14(1), 12. https://doi.org/10.3390/biotech14010012








