Gibberellin-Producing Bacteria Isolated from Coastal Soil Enhance Seed Germination of Mallow and Broccoli Plants under Saline Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Isolation of Bacteria from Rhizospheric Soil
2.2. Screening Bioassay for Growth Promotion in Waito-C Rice Seeds
2.3. Experiment Location, Method, and Design
2.4. Screening for Indole-3-Acetic Acid Production
2.5. Screening for Siderophore Production
2.6. Screening for Phosphate Solubilization Activity
2.7. Screening for Exopolysaccharides (EPS)
2.8. Quantification of Indole-3-Acetic Acid Production (IAA)
2.9. Identification and Phylogenetic Analysis
2.10. Determination of Acid Phosphatase Activity
2.11. Measurement of the Activity of Superoxide Dismutase
2.12. Measurement of the Activity of Catalase
2.13. Quantification of Gibberellins in the Culture Broth
2.14. Screening Bioassay for Growth Promotion in Mallow and Broccoli Seeds
2.15. Measurement of Germination Metrics
2.16. Statistical Analysis
3. Results
3.1. Initial Screening for PGPR Characteristics in Isolates
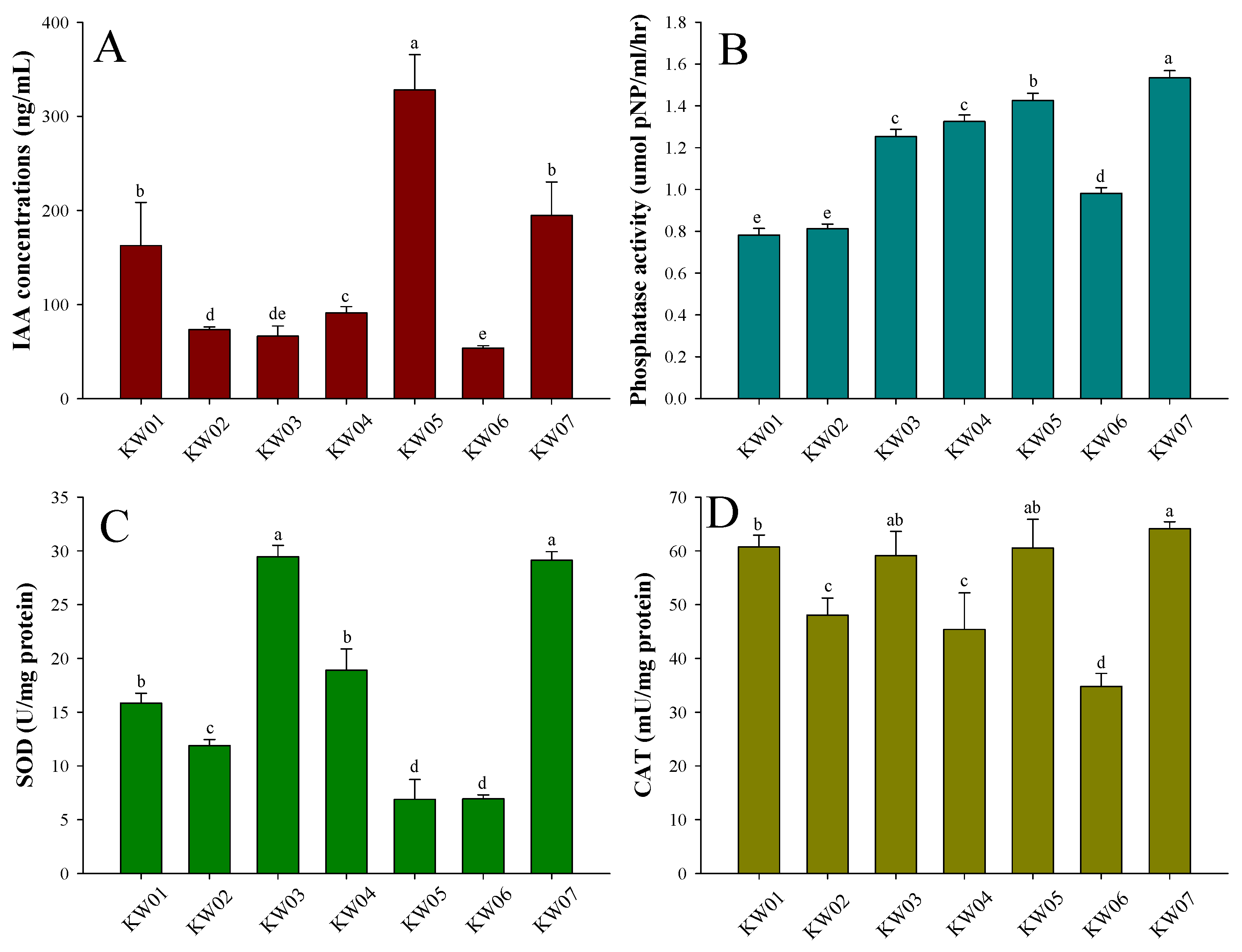
3.2. Effects of Bacterial Isolates on IAA Content, Phosphatase Activity, and Antioxidant Levels
3.3. Screening for Salt Tolerance
3.4. Effects of Bacterial Inoculation on Waito-C Rice Plants
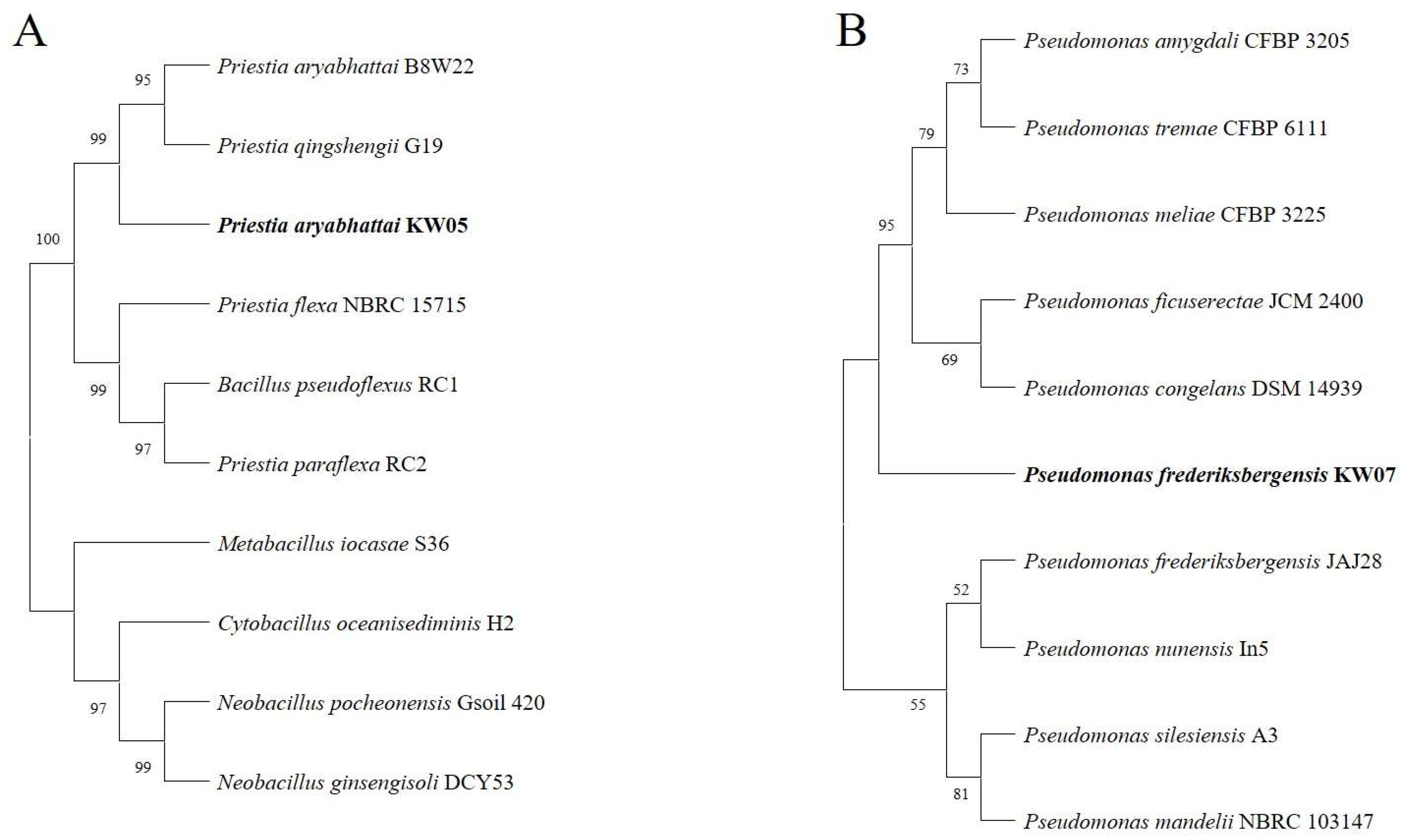
3.5. Molecular Identification of Bacterial Isolates
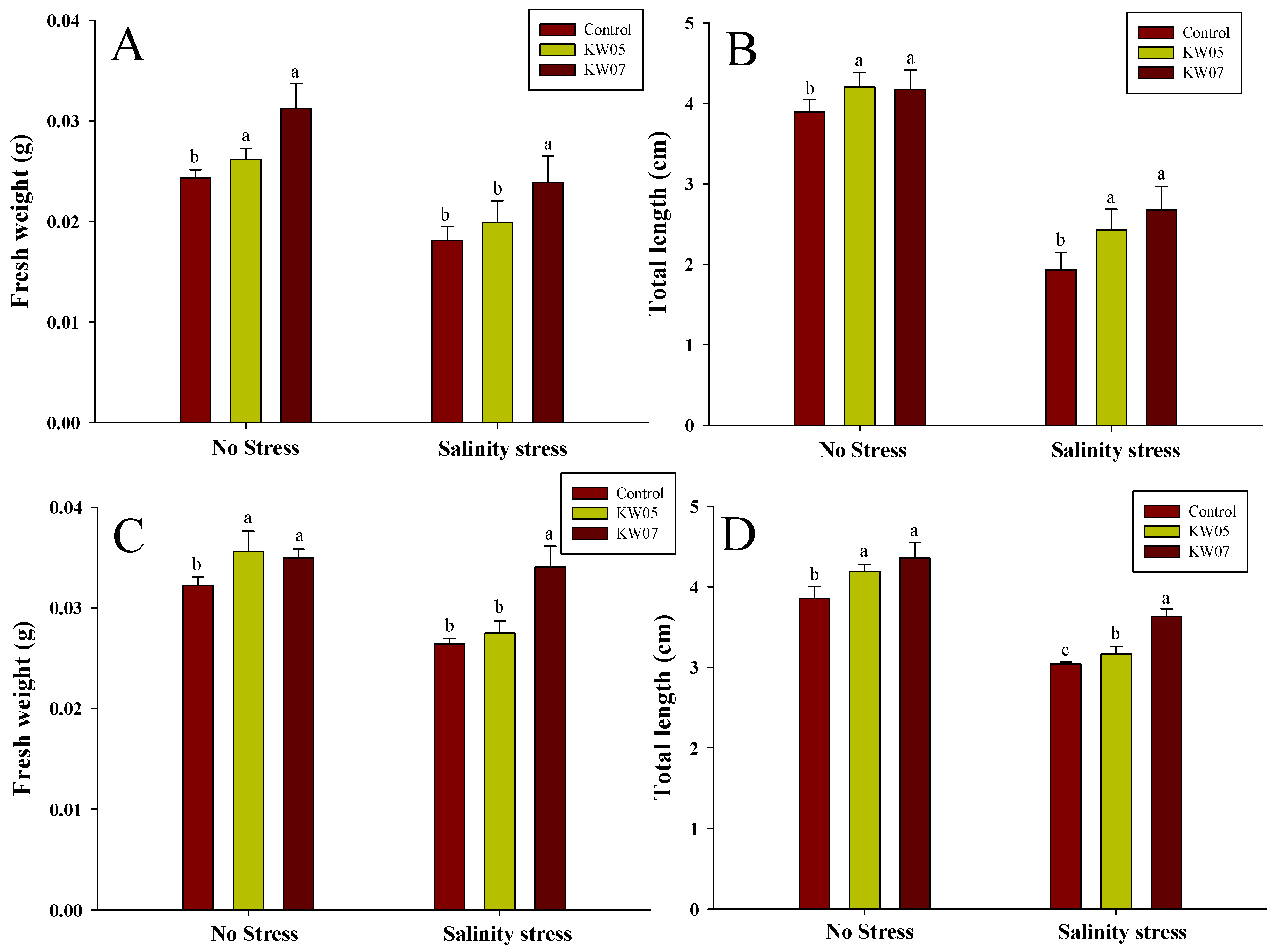
3.6. Influence of KW05 and KW07 on the Growth Characteristics of Mallow and Broccoli
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PG | Percent germination |
| MGT | Mean germination time |
| GR | Germination rate |
| GPI | Germination performance index |
| GE | Germination energy |
| MDG | Mean daily germination |
References
- Amna; Ali, B.; Azeem, M.A.; Qayyum, A.; Mustafa, G.; Ahmad, M.A.; Javed, M.T.; Chaudhary, H.J. Bio-fabricated silver nanoparticles: A sustainable approach for augmentation of plant growth and pathogen control. In Sustainable Agriculture Reviews 53: Nanoparticles: A New Tool to Enhance Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2022; pp. 345–371. [Google Scholar]
- Islam, F.; Wang, J.; Farooq, M.A.; Yang, C.; Jan, M.; Mwamba, T.M.; Hannan, F.; Xu, L.; Zhou, W. Rice responses and tolerance to salt stress: Deciphering the physiological and molecular mechanisms of salinity adaptation. In Advances in Rice Research for Abiotic Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2019; pp. 791–819. [Google Scholar]
- Abuelgasim, A.; Ammad, R. Mapping soil salinity in arid and semi-arid regions using Landsat 8 OLI satellite data. Remote Sens. Appl. Soc. Environ. 2019, 13, 415–425. [Google Scholar] [CrossRef]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Map of Salt-Affected Soils; FAO: Rome, Italy, 2021; pp. 1–20. [Google Scholar]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Hafeez, A.; Ammar Javed, M.; Ahmad, S.; Afridi, M.S.; Ullah, A.; Alwahibi, M.S.; Elsheikh, M.S.; Muresan, C.C.; Marc, R.A. Bacterial-mediated salt tolerance in maize: Insights into plant growth promotion, antioxidant defenses system, oxidative stress, and surfactant production. Front. Plant Sci. 2022, 13, 978291. [Google Scholar]
- Gupta, A.; Singh, S.K.; Singh, M.K.; Singh, V.K.; Modi, A.; Singh, P.K.; Kumar, A. Plant growth–promoting rhizobacteria and their functional role in salinity stress management. In Abatement of Environmental Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–160. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Mehmood, S.; Khatoon, Z.; Amna; Ahmad, I.; Muneer, M.A.; Kamran, M.A.; Ali, J.; Ali, B.; Chaudhary, H.J.; Munis, M.F.H. Bacillus sp. PM31 harboring various plant growth-promoting activities regulates Fusarium dry rot and wilt tolerance in potato. Arch. Agron. Soil Sci. 2023, 69, 197–211. [Google Scholar] [CrossRef]
- Wu, H.; Shabala, L.; Barry, K.; Zhou, M.; Shabala, S. Ability of leaf mesophyll to retain potassium correlates with salinity tolerance in wheat and barley. Physiol. Plant. 2013, 149, 515–527. [Google Scholar] [CrossRef]
- Faryal, S.; Ullah, R.; Khan, M.N.; Ali, B.; Hafeez, A.; Jaremko, M.; Qureshi, K.A. Thiourea-capped nanoapatites amplify osmotic stress tolerance in Zea mays L. by conserving photosynthetic pigments, osmolytes biosynthesis and antioxidant biosystems. Molecules 2022, 27, 5744. [Google Scholar] [CrossRef]
- Bibi, S.; Ullah, S.; Hafeez, A.; Khan, M.; Javed, M.; Ali, B.; Din, I.; Bangash, S.; Wahab, S.; Wahid, N. Exogenous Ca/Mg quotient reduces the inhibitory effects of PEG induced osmotic stress on Avena sativa L. Braz. J. Biol. 2022, 84. [Google Scholar] [CrossRef]
- Saleem, K.; Asghar, M.A.; Saleem, M.H.; Raza, A.; Kocsy, G.; Iqbal, N.; Ali, B.; Albeshr, M.F.; Bhat, E.A. Chrysotile-asbestos-induced damage in Panicum virgatum and Phleum pretense species and its alleviation by organic-soil amendment. Sustainability 2022, 14, 10824. [Google Scholar] [CrossRef]
- Alam, P.; Albalawi, T.H.; Altalayan, F.H.; Bakht, M.A.; Ahanger, M.A.; Raja, V.; Ashraf, M.; Ahmad, P. 24-Epibrassinolide (EBR) Confers Tolerance against NaCl Stress in Soybean Plants by Up-Regulating Antioxidant System, Ascorbate-Glutathione Cycle, and Glyoxalase System. Biomolecules 2019, 9, 640. [Google Scholar] [CrossRef]
- Ali, B.; Hafeez, A.; Ahmad, S.; Javed, M.A.; Afridi, M.S.; Dawoud, T.M.; Almaary, K.S.; Muresan, C.C.; Marc, R.A.; Alkhalifah, D.H.M. Bacillus thuringiensis PM25 ameliorates oxidative damage of salinity stress in maize via regulating growth, leaf pigments, antioxidant defense system, and stress responsive gene expression. Front. Plant Sci. 2022, 13, 921668. [Google Scholar] [CrossRef]
- Khan, I.; Raza, M.A.; Awan, S.A.; Shah, G.A.; Rizwan, M.; Ali, B.; Tariq, R.; Hassan, M.J.; Alyemeni, M.N.; Brestic, M. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): The oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol. Biochem. 2020, 156, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Muneer, M.A.; Tahir, M.; Javed, M.T.; Mahmood, T.; Afridi, M.S.; Pakar, N.P.; Abbasi, H.A.; Munis, M.F.H.; Chaudhary, H.J. Deciphering distinct biological control and growth promoting potential of multi-stress tolerant Bacillus subtilis PM32 for potato stem canker. Physiol. Mol. Biol. Plants 2021, 27, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, T.; Ali, F.; Rafique, M.; Ali, J.; Afridi, M.S.; Smith, D.; Mehmood, S.; Amna; Souleimanov, A.; Jellani, G. Biochemical Characterization and Potential of Bacillus safensis Strain SCAL1 to Mitigate Heat Stress in Solanum lycopersicum L. J. Plant Growth Regul. 2023, 42, 523–538. [Google Scholar] [CrossRef]
- Anwar, T.; Qureshi, H.; Parveen, N.; Mahmood, S.; Haider, M.Z.; Mumtaz, S.; Nawaz, H.; Khan, S.A.; Hafeez, A.; Tipu, M.I. Herbicidal effectiveness of wild poisonous plant Rhazya stricta using different media by the sandwich method. Pak. J. Bot 2023, 55, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Ngom, M.; Oshone, R.; Diagne, N.; Cissoko, M.; Svistoonoff, S.; Tisa, L.S.; Laplaze, L.; Sy, M.O.; Champion, A. Tolerance to environmental stress by the nitrogen-fixing actinobacterium Frankia and its role in actinorhizal plants adaptation. Symbiosis 2016, 70, 17–29. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Chaoxing, H. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 2011, 127, 228–233. [Google Scholar] [CrossRef]
- Safdarian, M.; Askari, H.; Nematzadeh, G.; Adriano, S. Halophile plant growth-promoting rhizobacteria induce salt tolerance traits in wheat seedlings (Triticum aestivum L.). Pedosphere 2020, 30, 684–693. [Google Scholar] [CrossRef]
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic insights of the interaction of plant growth-promoting rhizobacteria (PGPR) with plant roots toward enhancing plant productivity by alleviating salinity stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef]
- Gross, H.; Loper, J.E. Genomics of secondary metabolite production by Pseudomonas spp. Nat. Prod. Rep. 2009, 26, 1408–1446. [Google Scholar] [CrossRef] [PubMed]
- Ghequire, M.G.; De Mot, R. Ribosomally encoded antibacterial proteins and peptides from Pseudomonas. FEMS Microbiol. Rev. 2014, 38, 523–568. [Google Scholar] [CrossRef]
- Götze, S.; Stallforth, P. Structure, properties, and biological functions of nonribosomal lipopeptides from pseudomonads. Nat. Prod. Rep. 2020, 37, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Biessy, A.; Filion, M. Phloroglucinol derivatives in plant-beneficial Pseudomonas spp.: Biosynthesis, regulation, and functions. Metabolites 2021, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Dola, D.B.; Mannan, M.A.; Sarker, U.; Mamun, M.A.A.; Islam, T.; Ercisli, S.; Saleem, M.H.; Ali, B.; Pop, O.L.; Marc, R.A. Nano-iron oxide accelerates growth, yield, and quality of Glycine max seed in water deficits. Front. Plant Sci. 2022, 13, 992535. [Google Scholar] [CrossRef]
- Farooq, T.H.; Rafay, M.; Basit, H.; Shakoor, A.; Shabbir, R.; Riaz, M.U.; Ali, B.; Kumar, U.; Qureshi, K.A.; Jaremko, M. Morpho-physiological growth performance and phytoremediation capabilities of selected xerophyte grass species toward Cr and Pb stress. Front. Plant Sci. 2022, 13, 997120. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.; Salem, S.S.; Fouda, A.; Awad, M.A.; El-Gamal, M.S.; Abdo, A.M. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Radiat. Res. Appl. Sci. 2018, 11, 262–270. [Google Scholar]
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin localization and transport in plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Achard, P.; Renou, J.-P.; Berthomé, R.; Harberd, N.P.; Genschik, P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Taheri, Y.; Shaheen, S.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Brdar-Jokanović, M.; Rajkovic, J.; et al. Malva species: Insights on its chemical composition towards pharmacological applications. Phytother. Res. 2020, 34, 546–567. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Pavlović, I.; Salopek-Sondi, B. White cabbage (Brassica oleracea var. capitata f. alba): Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2017, 16, 117–135. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A.; Hosseini, H.M. Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX 2015, 2, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Kirienko, D.R.; Webster, P.; Fisher, A.L.; Kirienko, N.V. Pyoverdine, a siderophore from Pseudomonas aeruginosa, translocates into C. elegans, removes iron, and activates a distinct host response. Virulence 2018, 9, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant Biol. 2021, 21, 176. [Google Scholar] [CrossRef]
- Gandhi, A.D.; Vizhi, D.K.; Lavanya, K.; Kalpana, V.; Rajeswari, V.D.; Babujanarthanam, R. In vitro anti-biofilm and anti-bacterial activity of Sesbania grandiflora extract against Staphylococcus aureus. Biochem. Biophys. Rep. 2017, 12, 193–197. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, Y.; Jin, Y.; Bai, F.; Cheng, Z.; Wu, W. Pseudomonas aeruginosa Oligoribonuclease Controls Tolerance to Polymyxin B by Regulating Pel Exopolysaccharide Production. Antimicrob. Agents Chemother. 2022, 66, e02021–e02072. [Google Scholar] [CrossRef]
- Lubna; Asaf, S.; Hamayun, M.; Khan, A.L.; Waqas, M.; Khan, M.A.; Jan, R.; Lee, I.-J.; Hussain, A. Salt tolerance of Glycine max L. induced by endophytic fungus Aspergillus flavus CSH1, via regulating its endogenous hormones and antioxidative system. Plant Physiol. Biochem. 2018, 128, 13–23. [Google Scholar] [CrossRef]
- Chen, W.-J.; Zhang, W.; Lei, Q.; Chen, S.-F.; Huang, Y.; Bhatt, K.; Liao, L.; Zhou, X. Pseudomonas aeruginosa based concurrent degradation of beta-cypermethrin and metabolite 3-phenoxybenzaldehyde, and its bioremediation efficacy in contaminated soils. Environ. Res. 2023, 236, 116619. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.-F.; Chen, W.-J.; Zhu, X.; Mishra, S.; Bhatt, P.; Chen, S. Efficient biodegradation of multiple pyrethroid pesticides by Rhodococcus pyridinivorans strain Y6 and its degradation mechanism. Chem. Eng. J. 2023, 469, 143863. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Juma, N.G.; Tabatabai, M.A. Phosphatase activity in corn and soybean roots: Conditions for assay and effects of metals. Plant Soil 1988, 107, 39–47. [Google Scholar] [CrossRef]
- Adhikari, A.; Khan, M.A.; Lee, K.-E.; Kang, S.-M.; Dhungana, S.K.; Bhusal, N.; Lee, I.-J. The halotolerant rhizobacterium—Pseudomonas koreensis MU2 enhances inorganic silicon and phosphorus use efficiency and augments salt stress tolerance in soybean (Glycine max L.). Microorganisms 2020, 8, 1256. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Shahzad, R.; Khan, A.L.; Kang, S.-M.; Imran, Q.M.; Al-Harrasi, A.; Yun, B.-W.; Lee, I.-J. Endophytic microbial consortia of phytohormones-producing fungus Paecilomyces formosus LHL10 and bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to attenuate aluminum and zinc stresses. Front. Plant Sci. 2018, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-E.; Adhikari, A.; Kang, S.-M.; You, Y.-H.; Joo, G.-J.; Kim, J.-H.; Kim, S.-J.; Lee, I.-J. Isolation and characterization of the high silicate and phosphate solubilizing novel strain Enterobacter ludwigii GAK2 that promotes growth in rice plants. Agronomy 2019, 9, 144. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; ISTA: Bassersdorf, Switzerland, 2006. [Google Scholar]
- Scott, S.J.; Jones, R.; Williams, W. Review of data analysis methods for seed germination 1. Crop Sci. 1984, 24, 1192–1199. [Google Scholar] [CrossRef]
- Ellis, R.; Roberts, E. Improved equations for the prediction of seed longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar] [CrossRef]
- Edwards, R.; Sundstrom, F. Afterripening and harvesting effects on Tabasco pepper seed germination performance. HortScience 1987, 22, 473–475. [Google Scholar] [CrossRef]
- Uçarlı, C. Effects of salinity on seed germination and early seedling stage. Abiotic Stress Plants 2020, 211. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A. Arabidopsis seed germination under abiotic stress as a concert of action of phytohormones. OMICS A J. Integr. Biol. 2011, 15, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Khan, M. Growth, oxidative damage and antioxidant responses in greengram (Vigna radiata L.) under short-term salinity stress and its recovery. J. Agron. Crop Sci. 2009, 195, 442–454. [Google Scholar] [CrossRef]
- Sulochana, M.; Jayachandra, S.; Kumar, S.A.; Parameshwar, A.; Reddy, K.M.; Dayanand, A. Siderophore as a potential plant growth-promoting agent produced by Pseudomonas aeruginosa JAS-25. Appl. Biochem. Biotechnol. 2014, 174, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Basra, S.; Rehman, H.; Saleem, B. Seed priming enhances the performance of late sown wheat (Triticum aestivum L.) by improving chilling tolerance. J. Agron. Crop Sci. 2008, 194, 55–60. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, P.; Wang, T.; Chi, X.; Wang, M.; Chen, M.; Chen, N.; Pan, L. Role of halotolerant phosphate-solubilising bacteria on growth promotion of peanut (Arachis hypogaea) under saline soil. Ann. Appl. Biol. 2019, 174, 20–30. [Google Scholar] [CrossRef]
- Sunita, K.; Mishra, I.; Mishra, J.; Prakash, J.; Arora, N.K. Secondary metabolites from halotolerant plant growth promoting rhizobacteria for ameliorating salinity stress in plants. Front. Microbiol. 2020, 11, 567768. [Google Scholar] [CrossRef]
- Li, P.-S.; Kong, W.-L.; Wu, X.-Q. Salt tolerance mechanism of the rhizosphere bacterium JZ-GX1 and its effects on tomato seed germination and seedling growth. Front. Microbiol. 2021, 12, 657238. [Google Scholar] [CrossRef]
- Bahin, E.; Bailly, C.; Sotta, B.; Kranner, I.; Corbineau, F.; Leymarie, J. Crosstalk between reactive oxygen species and hormonal signalling pathways regulates grain dormancy in barley. Plant Cell Environ. 2011, 34, 980–993. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Bi, C.; Ma, Y.; Wu, Z.; Yu, Y.-T.; Liang, S.; Lu, K.; Wang, X.-F. Arabidopsis ABI5 plays a role in regulating ROS homeostasis by activating CATALASE 1 transcription in seed germination. Plant Mol. Biol. 2017, 94, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Deng, X.; Xiao, Q.; Han, Y.; Zhu, S.; Chen, J. IAA priming improves the germination and seedling growth in cotton (Gossypium hirsutum L.) via regulating the endogenous phytohormones and enhancing the sucrose metabolism. Ind. Crops Prod. 2020, 155, 112788. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Lee, I.-J. Gibberellins producing Bacillus methylotrophicus KE2 supports plant growth and enhances nutritional metabolites and food values of lettuce. Plant Physiol. Biochem. 2016, 109, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Khan, A.L.; You, Y.-H.; Kim, J.-G.; Kamran, M.; Lee, I.-J. Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth. J. Microbiol. Biotechnol. 2014, 24, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, P. Studies on the molecular mechanisms of seed germination. Proteomics 2015, 15, 1671–1679. [Google Scholar] [CrossRef]
- Llanes, A.; Andrade, A.; Masciarelli, O.; Alemano, S.; Luna, V. Drought and salinity alter endogenous hormonal profiles at the seed germination phase. Seed Sci. Res. 2016, 26, 1–13. [Google Scholar] [CrossRef]
- Shu, K.; Liu, X.-d.; Xie, Q.; He, Z.-h. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef]
- Nimir, N.E.A.; Zhou, G.; Guo, W.; Ma, B.; Lu, S.; Wang, Y. Effect of foliar application of GA3, kinetin, and salicylic acid on ions content, membrane permeability, and photosynthesis under salt stress of sweet sorghum [Sorghum bicolor (L.) Moench]. Can. J. Plant Sci. 2016, 97, 525–535. [Google Scholar] [CrossRef]
- Liu, X.; Hu, P.; Huang, M.; Tang, Y.; Li, Y.; Li, L.; Hou, X. The NF-YC–RGL2 module integrates GA and ABA signalling to regulate seed germination in Arabidopsis. Nat. Commun. 2016, 7, 12768. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-X.; Peng, Y.-X.; Yang, X.-Q.; Liu, J. Delayed germination of Brassica parachinensis seeds by coumarin involves decreased GA4 production and a consequent reduction of ROS accumulation. Seed Sci. Res. 2021, 31, 224–235. [Google Scholar] [CrossRef]
- Kilic, S.; Kahraman, A. The mitigation effects of exogenous hydrogen peroxide when alleviating seed germination and seedling growth inhibition on salinity-induced stress in barley. Pol. J. Environ. Stud. 2016, 25, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Jha, Y.; Yadav, K.A.; Mohamed, H.I. Plant growth-promoting bacteria and exogenous phytohormones alleviate the adverse effects of drought stress in pigeon pea plants. Plant Soil 2023, 1–21. [Google Scholar] [CrossRef]
- Kang, S.-M.; Radhakrishnan, R.; Khan, A.L.; Kim, M.-J.; Park, J.-M.; Kim, B.-R.; Shin, D.-H.; Lee, I.-J. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014, 84, 115–124. [Google Scholar] [CrossRef]
- Fatima, T.; Mishra, I.; Verma, R.; Arora, N.K. Mechanisms of halotolerant plant growth promoting Alcaligenes sp. involved in salt tolerance and enhancement of the growth of rice under salinity stress. 3 Biotech 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Wang, X.; Saleem, M.H.; Azeem, M.A.; Afridi, M.S.; Nadeem, M.; Ghazal, M.; Batool, T.; Qayyum, A.; Alatawi, A. Bacillus mycoides PM35 reinforces photosynthetic efficiency, antioxidant defense, expression of stress-responsive genes, and ameliorates the effects of salinity stress in maize. Life 2022, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Sagar, A.; Sayyed, R.; Ramteke, P.; Sharma, S.; Marraiki, N.; Elgorban, A.M.; Syed, A. ACC deaminase and antioxidant enzymes producing halophilic Enterobacter sp. PR14 promotes the growth of rice and millets under salinity stress. Physiol. Mol. Biol. Plants 2020, 26, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
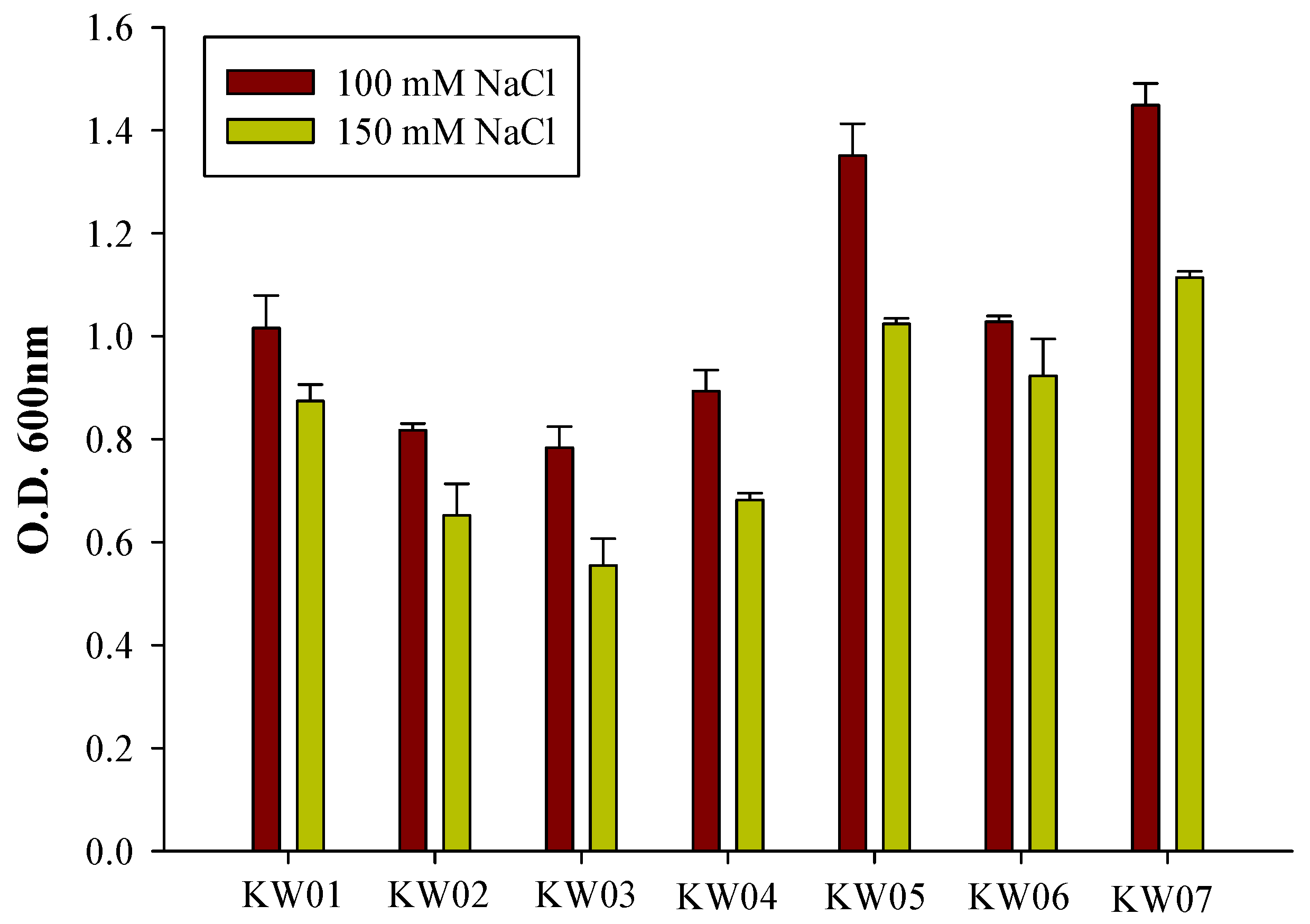


| Bacterial Isolates | Geographical Coordinates of Soil Sample | SALKOWSKI TEST | Siderophore Production | Phosphate Solubilization | EPS Production | Identification |
|---|---|---|---|---|---|---|
| KW01 | 35.784548, 129.490484 | ++ | + | + | + | Streptomyces laurentii |
| KW02 | 35.891247, 129.524323 | + | + | + | − | Priestia megaterium |
| KW03 | 35.823135, 129.507986 | + | − | ++ | − | Priestia megaterium |
| KW04 | 35.823135, 129.507986 | + | + | ++ | + | Priestia aryabhattai |
| KW05 | 35.823135, 129.507986 | +++ | ++ | +++ | + | Priestia aryabhattai |
| KW06 | 35.973071, 129.551715 | + | + | + | − | Bacillus velezensis |
| KW07 | 35.951894, 129.543674 | ++ | ++ | +++ | + | Pseudomonas frederiksbergensis |
| Crops | Treatment | PG (%) | GE (%) | GR (%/Day) | MGT (Day) | MDG | GPI |
|---|---|---|---|---|---|---|---|
| Mallow | NS | 70 ab ± 5.8 | 70 a ± 5.8 | 5.89 a ± 1.09 | 1.41 d ± 0.21 | 1.4 ab ± 0.12 | 53.5 a ± 13.4 |
| KW05 | 80 ab ± 10 | 63.3 ab ± 6.7 | 5.05 ab ± 0.73 | 2.20 c ± 0.02 | 1.6 ab ± 0.2 | 36.2 ab ± 4.3 | |
| KW07 | 83.3 a ± 3.3 | 60 ab ± 15.3 | 5.46 ab ± 0.78 | 2.31 c ± 0.44 | 1.67 a ± 0.07 | 40.2 ab ± 11.1 | |
| SS | 63.3 b ± 3.3 | 33.3 c ± 3.3 | 2.77 c ± 0.21 | 2.83 a ± 0.10 | 1.07 c ± 0.07 | 22.5 c ± 1.9 | |
| SS + KW05 | 63.3 b ± 3.3 | 46.7 b ± 3.3 | 3.35 b ± 0.14 | 2.63 b ± 0.06 | 1.27 b ± 0.07 | 26.3 b ± 1.5 | |
| SS + KW07 | 66.7 ab ± 3.3 | 53.3 b ± 3.3 | 3.64 b ± 0.19 | 2.62 ab ± 0.19 | 1.40 ab ± 0 | 25.6 b ± 1.6 | |
| Broccoli | NS | 96.7 a ± 3.3 | 96.7 ab ± 3.3 | 9.50 ab ± 0.29 | 1.03 c ± 0.03 | 1.93 a ± 0.07 | 93.6 a ± 3.2 |
| KW05 | 100 a ± 0 | 100 ab ± 0 | 9.83 a ± 0.17 | 1.03 c ± 0.03 | 2.00 a ± 0 | 97.0 a ± 3.0 | |
| KW07 | 96.7 a ± 3.3 | 96.7 ab ± 3.3 | 9.50 ab ± 0.50 | 1.04 c ± 0.04 | 1.93 a ± 0.07 | 93.7 a ± 6.3 | |
| SS | 96.7 a ± 3.3 | 90 b ± 0 | 7.33 c ± 0.14 | 1.65 a ± 0.11 | 1.93 a ± 0.07 | 58.9 c ± 1.9 | |
| SS + KW05 | 96.7 a ± 3.3 | 96.7 ab ± 3.3 | 8.72 b ± 0.39 | 1.24 b ± 0.03 | 1.93 a ± 0.07 | 78.0 b ± 2.8 | |
| SS + KW07 | 100 a ± 0 | 100 a ± 0 | 9.33 ab ± 0.33 | 1.13 bc ± 0.07 | 2.00 a ± 0 | 88.9 ab ± 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, J.-I.; Injamum-Ul-Hoque, M.; Zainurin, N.; Shaffique, S.; Kwon, E.-H.; Gam, H.-J.; Jeon, J.R.; Lee, I.-J.; Joo, G.-J.; Kang, S.-M. Gibberellin-Producing Bacteria Isolated from Coastal Soil Enhance Seed Germination of Mallow and Broccoli Plants under Saline Conditions. BioTech 2023, 12, 66. https://doi.org/10.3390/biotech12040066
Woo J-I, Injamum-Ul-Hoque M, Zainurin N, Shaffique S, Kwon E-H, Gam H-J, Jeon JR, Lee I-J, Joo G-J, Kang S-M. Gibberellin-Producing Bacteria Isolated from Coastal Soil Enhance Seed Germination of Mallow and Broccoli Plants under Saline Conditions. BioTech. 2023; 12(4):66. https://doi.org/10.3390/biotech12040066
Chicago/Turabian StyleWoo, Ji-In, Md. Injamum-Ul-Hoque, Nazree Zainurin, Shifa Shaffique, Eun-Hae Kwon, Ho-Jun Gam, Jin Ryeol Jeon, In-Jung Lee, Gil-Jae Joo, and Sang-Mo Kang. 2023. "Gibberellin-Producing Bacteria Isolated from Coastal Soil Enhance Seed Germination of Mallow and Broccoli Plants under Saline Conditions" BioTech 12, no. 4: 66. https://doi.org/10.3390/biotech12040066
APA StyleWoo, J.-I., Injamum-Ul-Hoque, M., Zainurin, N., Shaffique, S., Kwon, E.-H., Gam, H.-J., Jeon, J. R., Lee, I.-J., Joo, G.-J., & Kang, S.-M. (2023). Gibberellin-Producing Bacteria Isolated from Coastal Soil Enhance Seed Germination of Mallow and Broccoli Plants under Saline Conditions. BioTech, 12(4), 66. https://doi.org/10.3390/biotech12040066








