Growth Efficiency of Chlorella sorokiniana in Synthetic Media and Unsterilized Domestic Wastewater
Abstract
1. Introduction
2. Results and Discussion
2.1. Growth Characteristics of Chlorella sorokiniana in the Synthetic Media
2.2. Growth Characteristics of Chlorella sorokiniana in Domestic Wastewaters
2.3. Bacterial Community Associated with Wastewaters
3. Materials and Methods
3.1. Inoculum Preparation
3.2. Experimental Conditions in the Reactor
3.3. Cultivation Modes
3.4. Algal Culture Growth, Productivity and Nutrient Removal Analyses
3.5. Bacterial Community Structure Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The role of microalgae in the bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef]
- Khaligh, S.F.; Asoodeh, A. Recent advances in the bio-application of microalgae-derived biochemical metabolites and development trends of photobioreactor-based culture systems. 3 Biotech 2022, 12, 260. [Google Scholar] [PubMed]
- Zhang, S.; Zhang, L.; Xu, G.; Li, F.; Li, X. A review on biodiesel production from microalgae: Influencing parameters and recent advanced technologies. Front. Microbiol. 2022, 13, 970028. [Google Scholar] [PubMed]
- Ziganshina, E.E.; Bulynina, S.S.; Yureva, K.A.; Ziganshin, A.M. Growth parameters of various green microalgae species in effluent from biogas reactors: The importance of effluent concentration. Plants 2022, 11, 3583. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Kumar Pandey, A.; Ranganathan, P.; Singh, S.; Udayan, A.; Kumar Awasthi, M.; Hoang, A.T.; Chilakamarry, C.R.; Kim, S.H.; Sim, S.J. Design and applications of photobioreactors—A review. Bioresour. Technol. 2022, 349, 126858. [Google Scholar] [CrossRef]
- Pleissner, D.; Lindner, A.V.; Ambati, R.R. Techniques to control microbial contaminants in nonsterile microalgae cultivation. Appl. Biochem. Biotechnol. 2020, 192, 1376–1385. [Google Scholar] [CrossRef]
- Ganesh Saratale, R.; Ponnusamy, V.K.; Jeyakumar, R.B.; Sirohi, R.; Piechota, G.; Shobana, S.; Dharmaraja, J.; Lay, C.H.; Dattatraya Saratale, G.; Seung Shin, H.; et al. Microalgae cultivation strategies using cost-effective nutrient sources: Recent updates and progress towards biofuel production. Bioresour. Technol. 2022, 361, 127691. [Google Scholar]
- Maurya, R.; Zhu, X.; Valverde-Pérez, B.; Ravi Kiran, B.; General, T.; Sharma, S.; Kumar Sharma, A.; Thomsen, M.; Venkata Mohan, S.; Mohanty, K.; et al. Advances in microalgal research for valorization of industrial wastewater. Bioresour. Technol. 2022, 343, 126128. [Google Scholar]
- Clagnan, E.; D’Imporzano, G.; Dell’Orto, M.; Bani, A.; Dumbrell, A.J.; Parati, K.; Acién-Fernández, F.G.; Portillo-Hahnefeld, A.; Martel-Quintana, A.; Gómez-Pinchetti, J.L.; et al. Centrate as a sustainable growth medium: Impact on microalgal inocula and bacterial communities in tubular photobioreactor cultivation systems. Bioresour. Technol. 2022, 363, 127979. [Google Scholar] [CrossRef]
- Goveas, L.C.; Nayak, S.; Vinayagam, R.; Loke Show, P.; Selvaraj, R. Microalgal remediation and valorisation of polluted wastewaters for zero-carbon circular bioeconomy. Bioresour. Technol. 2022, 365, 128169. [Google Scholar] [CrossRef]
- Su, Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Mishra, V. Exploring the effects of different combinations of predictor variables for the treatment of wastewater by microalgae and biomass production. Biochem. Eng. J. 2021, 174, 108129. [Google Scholar]
- Daneshvar, E.; Wicker, R.J.; Show, P.-L.; Bhatnagar, A. Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization—A review. Chem. Eng. J. 2022, 427, 13088. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Rodrigues, C.M.; Pires, J.C.M.; Simões, M. The effect of increasing CO2 concentrations on its capture, biomass production and wastewater bioremediation by microalgae and cyanobacteria. Algal Res. 2016, 14, 127–136. [Google Scholar] [CrossRef]
- Pang, N.; Gu, X.; Chen, S.; Kirchhoff, H.; Lei, H.; Roje, S. Exploiting mixotrophy for improving productivities of biomass and co-products of microalgae. Renew. Sustain. Energy Rev. 2019, 112, 450–460. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Alam, M.A.; Mehmood, M.A. Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; A review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Comparison of the photoautotrophic growth regimens of Chlorella sorokiniana in a photobioreactor for enhanced biomass productivity. Biology 2020, 9, 169. [Google Scholar] [CrossRef]
- Ra, C.H.; Kang, C.H.; Jung, J.H.; Jeong, G.T.; Kim, S.K. Enhanced biomass production and lipid accumulation of Picochlorum atomus using light-emitting diodes (LEDs). Bioresour. Technol. 2016, 218, 1279–1283. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef]
- Sartori, R.B.; Vendruscolo, R.G.; Ribeiro, S.R.; Furlan, V.J.M.; Wagner, R.; Zepka, L.Q.; Jacob-Lopes, E. The role of photo-cycles in the modulation of growth and biochemical profile of microalgae: Part I-Food interest compounds. Life 2022, 12, 462. [Google Scholar] [CrossRef]
- Madhubalaji, C.K.; Sarat Chandra, T.; Chauhan, V.S.; Sarada, R.; Mudliar, S.N. Chlorella vulgaris cultivation in airlift photobioreactor with transparent draft tube: Effect of hydrodynamics, light and carbon dioxide on biochemical profile particularly ω-6/ω-3 fatty acid ratio. J. Food Sci. Technol. 2020, 57, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Matos, Â.P.; Cavanholi, M.G.; Moecke, E.H.S.; Sant’Anna, E.S. Effects of different photoperiod and trophic conditions on biomass, protein and lipid production by the marine alga Nannochloropsis gaditana at optimal concentration of desalination concentrate. Bioresour. Technol. 2017, 224, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Aswathnarayana Gokare, R.; Ambati, R.R. Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef]
- Poh, Z.L.; Amalina Kadir, W.N.; Lam, M.K.; Uemura, Y.; Suparmaniam, U.; Lim, J.W.; Show, P.L.; Lee, K.T. The effect of stress environment towards lipid accumulation in microalgae after harvesting. Renew. Energy 2020, 154, 1083–1091. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Y.; Xu, H.; Liu, Y.; Sun, J.; Qiao, D.; Cao, Y. Effect of nitrogen-starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochloris oleoabundans HK-129 by a two-stage process. Bioresour. Technol. 2014, 155, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Anand, J.; Arumugam, M. Enhanced lipid accumulation and biomass yield of Scenedesmus quadricauda under nitrogen starved condition. Bioresour. Technol. 2015, 188, 190–194. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Growth characteristics of Chlorella sorokiniana in a photobioreactor during the utilization of different forms of nitrogen at various temperatures. Plants 2022, 11, 1086. [Google Scholar] [CrossRef]
- Collos, Y.; Harrison, P.J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef]
- Kumbhar, A.N.; He, M.; Rajper, A.R.; Memon, K.A.; Rizwan, M.; Nagi, M.; Woldemicael, A.G.; Li, D.; Wang, C.; Wang, C. The use of urea and kelp waste extract is a promising strategy for maximizing the biomass productivity and lipid content in Chlorella sorokiniana. Plants 2020, 9, 463. [Google Scholar] [CrossRef]
- Jimenez, J.; Bott, C.; Love, N.; Bratby, J. Source separation of urine as an alternative solution to nutrient management in wastewater treatment plants: A model-based analysis. Proc. Water Environ. Fed. 2012, 10, 5308–5323. [Google Scholar] [CrossRef]
- Miao, K.; Li, X.; Guo, L.; Gao, M.; Zhao, Y.; Jin, C.; Ji, J.; She, Z. Cultivation of Chlorella pyrenoidosa with different phosphorus forms under photoautotrophic and mixotrophic modes: Biochemical component synthesis and phosphorus bioavailability appraisement. J. Clean. Prod. 2022, 359, 132058. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-derived pigments for the food industry. Mar. Drugs. 2023, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Cichoński, J.; Chrzanowski, G. Microalgae as a source of valuable phenolic compounds and carotenoids. Molecules. 2022, 27, 8852. [Google Scholar] [CrossRef]
- Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Microalgae based production of single-cell protein. Curr. Opin. Biotechnol. 2022, 75, 102705. [Google Scholar] [CrossRef]
- Nordin, N.; Yusof, N.; Md Nadzir, S.; Mohd Yusoff, M.Z.; Hassan, M.A. Effect of photo-autotrophic cultural conditions on the biomass productivity and composition of Chlorella vulgaris. Biofuels 2022, 13, 149–159. [Google Scholar] [CrossRef]
- Olguín, E.J.; Sánchez-Galván, G.; Arias-Olguín, I.I.; Melo, F.J.; González-Portela, R.E.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-based biorefineries: Challenges and future trends to produce carbohydrate enriched biomass, high-added value products and bioactive compounds. Biology 2022, 11, 1146. [Google Scholar] [CrossRef]
- Je, S.; Yamaoka, Y. Biotechnological approaches for biomass and lipid production using microalgae Chlorella and its future perspectives. J. Microbiol. Biotechnol. 2022, 32, 1357–1372. [Google Scholar] [CrossRef]
- Zurano, A.S.; Serrano, C.G.; Acién-Fernández, F.G.; Fernández-Sevilla, J.M.; Molina-Grima, E. Modelling of photosynthesis, respiration, and nutrient yield coefficients in Scenedemus almeriensis culture as a function of nitrogen and phosphorus. Appl. Microbiol. Biotechnol. 2021, 105, 7487–7503. [Google Scholar] [CrossRef]
- Arcila, J.S.; Céspedes, D.; Buitrón, G. Influence of wavelength photoperiods and N/P ratio on wastewater treatment with microalgae-bacteria. Water Sci. Technol. 2021, 84, 712–724. [Google Scholar] [CrossRef]
- Fallahi, A.; Hajinajaf, N.; Tavakoli, O.; Sarrafzadeh, M.H. Cultivation of mixed microalgae using municipal wastewater: Biomass productivity, nutrient removal, and biochemical content. Iran. J. Biotechnol. 2020, 18, e2586. [Google Scholar]
- Ramsundar, P.; Guldhe, A.; Singh, P.; Bux, F. Assessment of municipal wastewaters at various stages of treatment process as potential growth media for Chlorella sorokiniana under different modes of cultivation. Bioresour. Technol. 2017, 227, 82–92. [Google Scholar] [CrossRef]
- Gupta, P.L.; Choi, H.J.; Pawar, R.R.; Jung, S.P.; Lee, S.M. Enhanced biomass production through optimization of carbon source and utilization of wastewater as a nutrient source. J. Environ. Manag. 2016, 184, 585–595. [Google Scholar] [CrossRef]
- De Francisci, D.; Su, Y.; Iital, A.; Angelidaki, I. Evaluation of microalgae production coupled with wastewater treatment. Environ. Technol. 2018, 39, 581–592. [Google Scholar] [CrossRef]
- Arora, N.; Patel, A.; Sartaj, K.; Pruthi, P.A.; Pruthi, V. Bioremediation of domestic and industrial wastewaters integrated with enhanced biodiesel production using novel oleaginous microalgae. Environ. Sci. Pollut. Res. Int. 2016, 23, 20997–21007. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Sivaramakrishnan, R.; Incharoensakdi, A.; Cornejo, P.; Kamaraj, B.; Chi, N.T.L. Removal of nutrients from domestic wastewater by microalgae coupled to lipid augmentation for biodiesel production and influence of deoiled algal biomass as biofertilizer for Solanum lycopersicum cultivation. Chemosphere 2021, 268, 129323. [Google Scholar] [CrossRef]
- Sharma, J.; Kumar, V.; Kumar, S.S.; Malyan, S.K.; Mathimani, T.; Bishnoi, N.R.; Pugazhendhi, A. Microalgal consortia for municipal wastewater treatment—Lipid augmentation and fatty acid profiling for biodiesel production. J. Photochem. Photobiol. B 2020, 202, 111638. [Google Scholar] [CrossRef]
- Onay, M.; Aladag, E. Production and use of Scenedesmus acuminatus biomass in synthetic municipal wastewater for integrated biorefineries. Environ. Sci. Pollut. Res. Int. 2023, 30, 15808–15820. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, W.; Min, M.; Ma, X.; Chandra, C.; Doan, Y.T.; Ma, Y.; Zheng, H.; Cheng, S.; Griffith, R.; et al. Growing Chlorella sp. on meat processing wastewater for nutrient removal and biomass production. Bioresour. Technol. 2015, 198, 189–197. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Z.; Wei, D. High-yield production of biomass, protein and pigments by mixotrophic Chlorella pyrenoidosa through the bioconversion of high ammonium in wastewater. Bioresour. Technol. 2020, 313, 123499. [Google Scholar] [CrossRef]
- Lavrinovičs, A.; Mežule, L.; Cacivkins, P.; Juhna, T. Optimizing phosphorus removal for municipal wastewater post-treatment with Chlorella vulgaris. J. Environ. Manag. 2022, 324, 116313. [Google Scholar] [CrossRef]
- Arrojo, M.Á.; Regaldo, L.; Calvo Orquín, J.; Figueroa, F.L.; Abdala Díaz, R.T. Potential of the microalgae Chlorella fusca (Trebouxiophyceae, Chlorophyta) for biomass production and urban wastewater phycoremediation. AMB Express 2022, 12, 43. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Chiang, A.; Herojit, N.; Arumugam, M. Sustainable microalgal cultivation in poultry slaughterhouse wastewater for biorefinery products and pollutant removal. Bioresour. Technol. 2023, 374, 128790. [Google Scholar] [CrossRef]
- Michelon, W.; Pirolli, M.; Mezzari, M.P.; Soares, H.M.; da Silva, M.L.B. Residual sugar from microalgae biomass harvested from phycoremediation of swine wastewater digestate. Water Sci. Technol. 2019, 79, 2203–2210. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, T.Y.; Dao, G.H.; Xu, X.Q.; Wang, X.X.; Hu, H.Y. Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl. Microbiol. Biotechnol. 2017, 101, 2659–2675. [Google Scholar] [CrossRef]
- Amaro, H.M.; Salgado, E.M.; Nunes, O.C.; Pires, J.C.M.; Esteves, A.F. Microalgae systems–environmental agents for wastewater treatment and further potential biomass valorisation. J. Environ. Manag. 2023, 337, 117678. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Z.Y.; Zhao, Q.L.; Chen, D.Z.; Li, C.; Liu, M.; Yang, J.S.; Liu, J.Z.; Ge, Y.M.; Chen, J.M. Mixotrophic cultivation of microalgae coupled with anaerobic hydrolysis for sustainable treatment of municipal wastewater in a hybrid system of anaerobic membrane bioreactor and membrane photobioreactor. Bioresour. Technol. 2021, 337, 125457. [Google Scholar] [CrossRef]
- Plöhn, M.; Spain, O.; Sirin, S.; Silva, M.; Escudero-Oñate, C.; Ferrando-Climent, L.; Allahverdiyeva, Y.; Funk, C. Wastewater treatment by microalgae. Physiol. Plant. 2021, 173, 568–578. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Ruas, G.; Serejo, M.L.; Farias, S.L.; Scarcelli, P.; Boncz, M.Á. Removal of pathogens from domestic wastewater by microalgal-bacterial systems under different cultivation conditions. Int. J. Environ. Sci. Technol. 2021, 19, 10177–10188. [Google Scholar] [CrossRef]
- Newton, R.J.; McLellan, S.L.; Dila, D.K.; Vineis, J.H.; Morrison, H.G.; Murat Eren, A.; Sogin, M.L. Sewage reflects the microbiomes of human populations. MBio 2015, 6, e02574. [Google Scholar] [CrossRef]
- Alarjani, K.M.; Almutairi, A.M.; Flanet Raj, S.R.; Rajaselvam, J.; Chang, S.W.; Ravindran, B. Biofilm producing indigenous bacteria isolated from municipal sludge and their nutrient removal ability in moving bed biofilm reactor from the wastewater. Saudi J. Biol. Sci. 2021, 28, 4994–5001. [Google Scholar] [CrossRef]
- Xia, L.; Li, X.; Fan, W.; Wang, J. Heterotrophic nitrification and aerobic denitrification by a novel Acinetobacter sp. ND7 isolated from municipal activated sludge. Bioresour. Technol. 2020, 301, 122749. [Google Scholar] [CrossRef]
- Yao, S.; Ni, J.; Chen, Q.; Borthwick, A.G.L. Enrichment and characterization of a bacteria consortium capable of heterotrophic nitrification and aerobic denitrification at low temperature. Bioresour. Technol. 2013, 127, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Elufisan, T.O.; Rodríguez-Luna, I.C.; Oyedara, O.O.; Sánchez-Varela, A.; Hernández-Mendoza, A.; Dantán Gonzalez, E.; Paz-González, A.D.; Muhammad, K.; Rivera, G.; Villalobos-Lopez, M.A.; et al. The polycyclic aromatic hydrocarbon (PAH) degradation activities and genome analysis of a novel strain Stenotrophomonas sp. Pemsol isolated from Mexico. PeerJ. 2020, 8, e8102. [Google Scholar] [CrossRef] [PubMed]
- Vilchis-Carmona, J.A.; Rodríguez-Luna, I.C.; Elufisan, T.O.; Sánchez-Varela, A.; Bibbins-Martínez, M.; Rivera, G.; Paz-Gonzalez, A.D.; Villalobos-López, M.A.; Guo, X. The decolorization and degradation of azo dyes by two Stenotrophomonas strains isolated from textile effluent (Tepetitla, Mexico). Braz. J. Microbiol. 2021, 52, 1755–1767. [Google Scholar] [CrossRef]
- Maela, M.P.; Serepa-Dlamini, M.H. Draft genome sequence of Stenotrophomonas pavanii strain MHSD12, a bacterial endophyte associated with Dicoma anomala. Microbiol. Resour. Announc. 2020, 9, 28. [Google Scholar] [CrossRef]
- Chan, K.-G.; Chong, T.-M.; Adrian, T.-G.-S.; Kher, H.L.; Hong, K.-W.; Grandclément, C.; Faure, D.; Yin, W.-F.; Dessaux, Y. Whole-genome sequence of Stenotrophomonas maltophilia ZBG7B reveals its biotechnological potential. Genome Announc. 2015, 3, e01442-15. [Google Scholar] [CrossRef]
- Adegoke, A.A.; Stenström, T.A.; Okoh, A.I. Stenotrophomonas maltophilia as an emerging ubiquitous pathogen: Looking beyond contemporary antibiotic therapy. Front. Microbiol. 2017, 8, 2276. [Google Scholar]
- Lee, S.A.; Kim, M.; Kim, H.S.; Ahn, C.Y. Extra benefit of microalgae in raw piggery wastewater treatment: Pathogen reduction. Microbiome 2022, 10, 142. [Google Scholar] [CrossRef]
- Alwathnani, H.; Perveen, K. Antibacterial activity and morphological changes in human pathogenic bacteria caused by Chlorella vulgaris extracts. Biomed. Res. 2017, 28, 1610–1614. [Google Scholar]
- Fergola, P.; Cerasuolo, M.; Pollio, A.; Pinto, G.; DellaGreca, M. Allelopathy and competition between Chlorella vulgaris and Pseudokirchneriella subcapitata: Experiments and mathematical model. Ecol. Modell. 2007, 208, 205–214. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Wang, S.; Yang, G.; Zhao, L.; Pan, K. Co-culturing bacteria and microalgae in organic carbon containing medium. J. Biol. Res. 2016, 23, 8. [Google Scholar] [CrossRef]
- Guldhe, A.; Kumari, S.; Ramanna, L.; Ramsundar, P.; Singh, P.; Rawat, I.; Bux, F. Prospects, recent advancements and challenges of different wastewater streams for microalgal cultivation. J. Environ. Manag. 2017, 203, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Nichols, H.W.; Bold, H.C. Trichosarcina polymorpha Gen. et Sp. Nov. J. Phycol. 1965, 1, 34–38. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Assessment of Chlorella sorokiniana growth in anaerobic digester effluent. Plants 2021, 10, 478. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant. Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Belostotskiy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Influence of granular activated carbon on anaerobic co-digestion of sugar beet pulp and distillers grains with solubles. Processes 2020, 8, 1226. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Impact of granular activated carbon on anaerobic process and microbial community structure during mesophilic and thermophilic anaerobic digestion of chicken manure. Sustainability 2022, 14, 447. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Sagitov, I.I.; Akhmetova, R.F.; Saleeva, G.T.; Kiassov, A.P.; Gogoleva, N.E.; Shagimardanova, E.I.; Ziganshin, A.M. Comparison of the microbiota and inorganic anion content in the saliva of patients with gastroesophageal reflux disease and gastroesophageal reflux disease-free individuals. BioMed Res. Intern. 2020, 2020, 2681791. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nayak, M.; Karemore, A.; Sen, R. Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 biofixation and lipid biosynthesis for biodiesel application. Algal Res. 2016, 16, 216–223. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
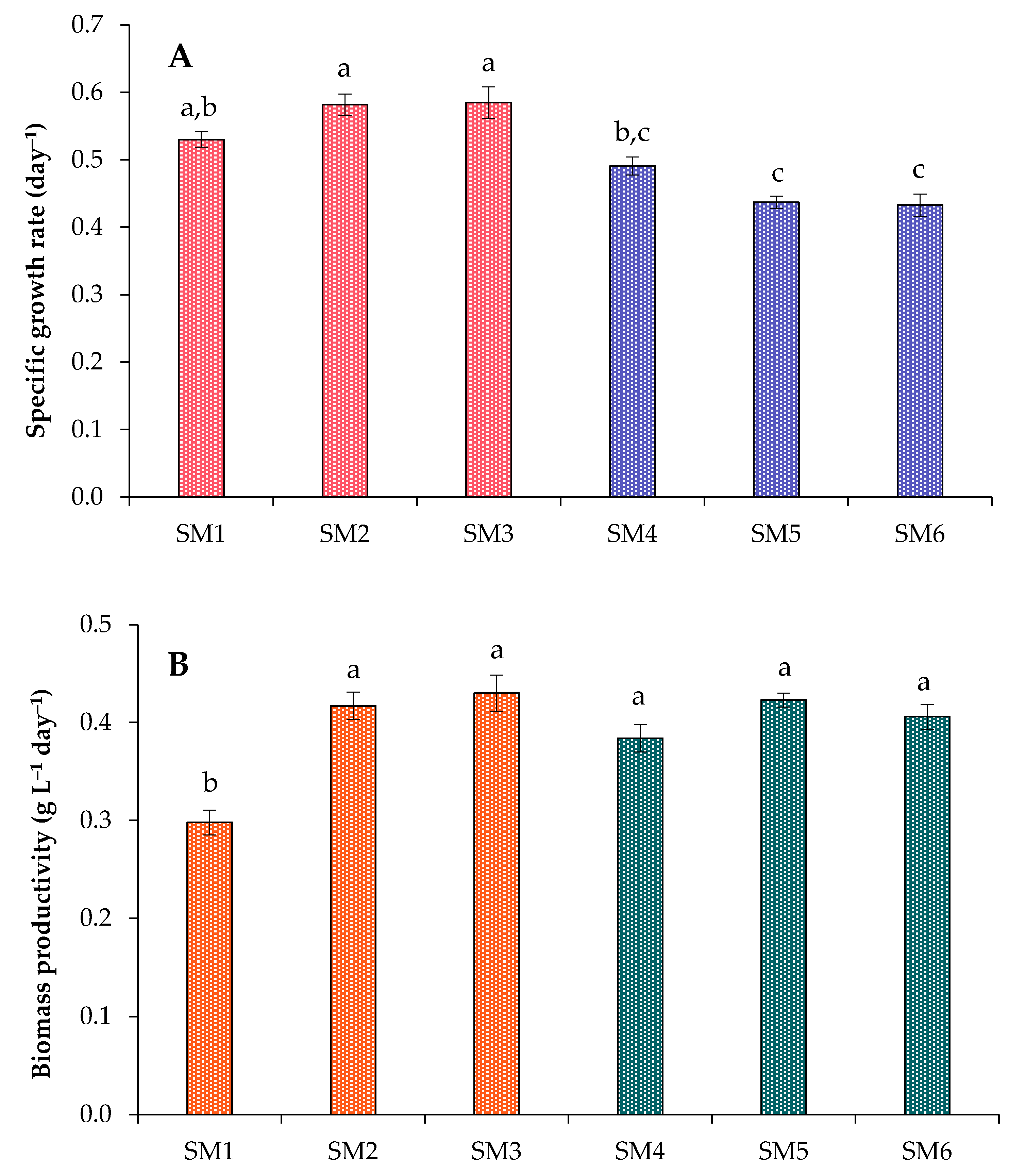

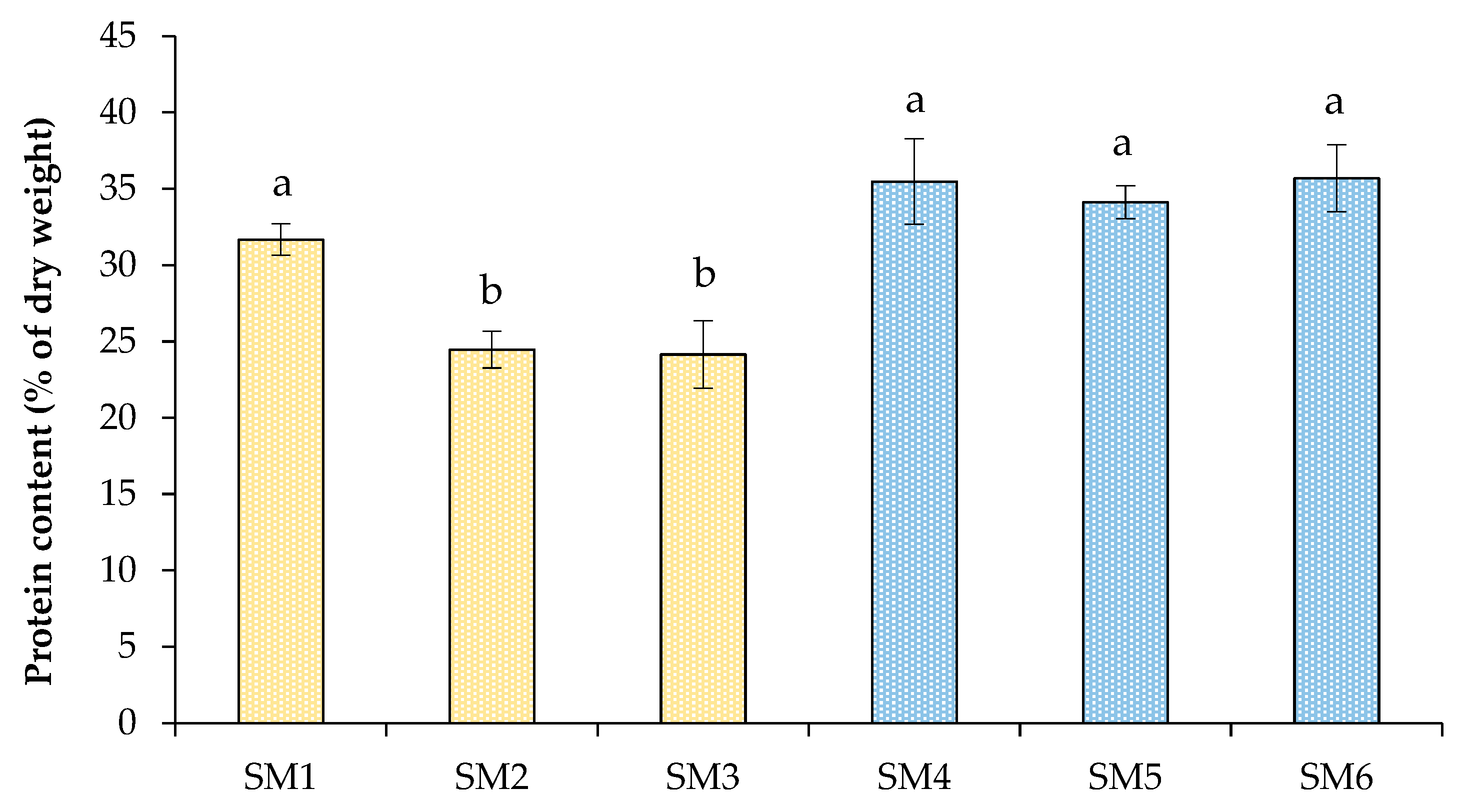
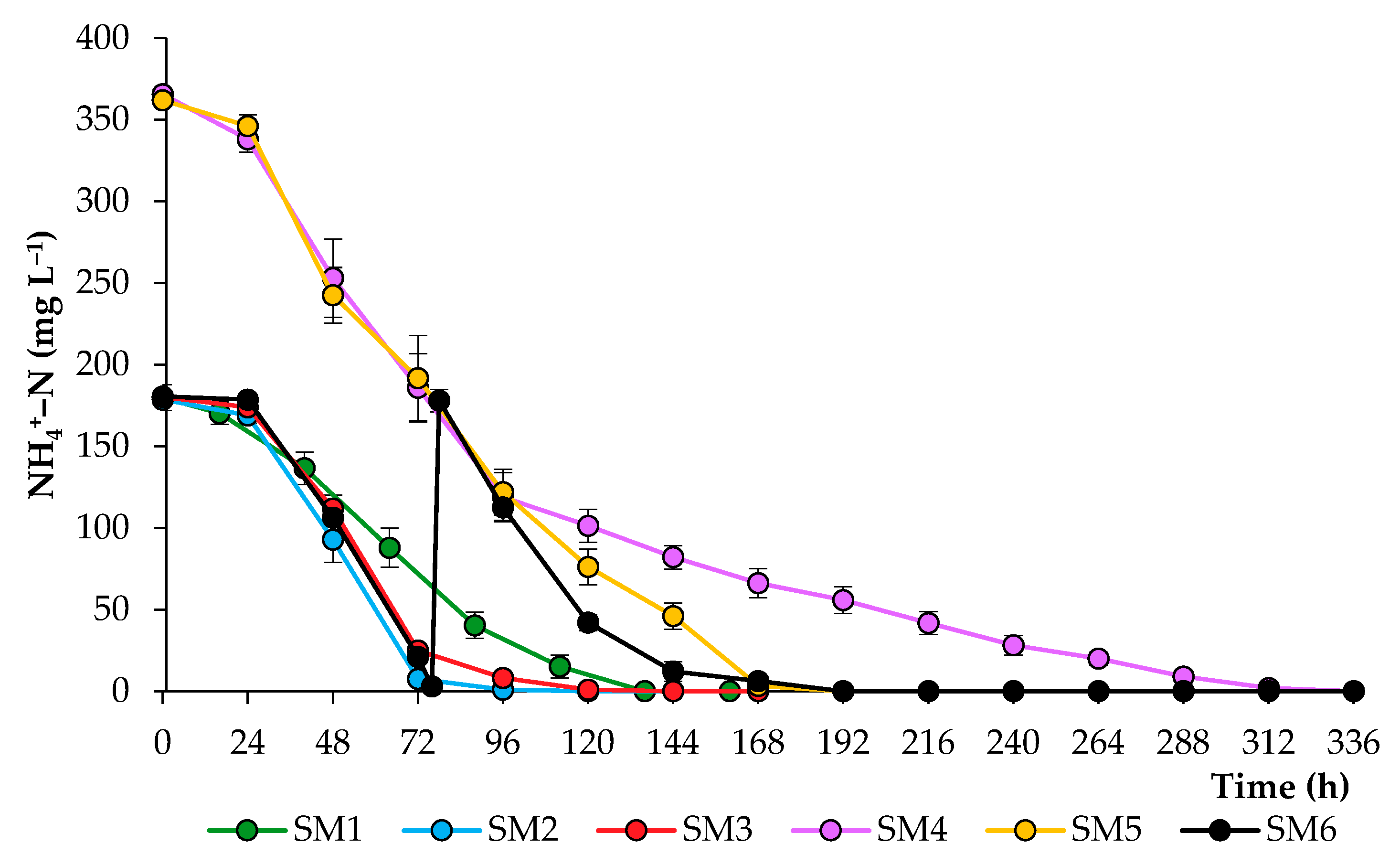



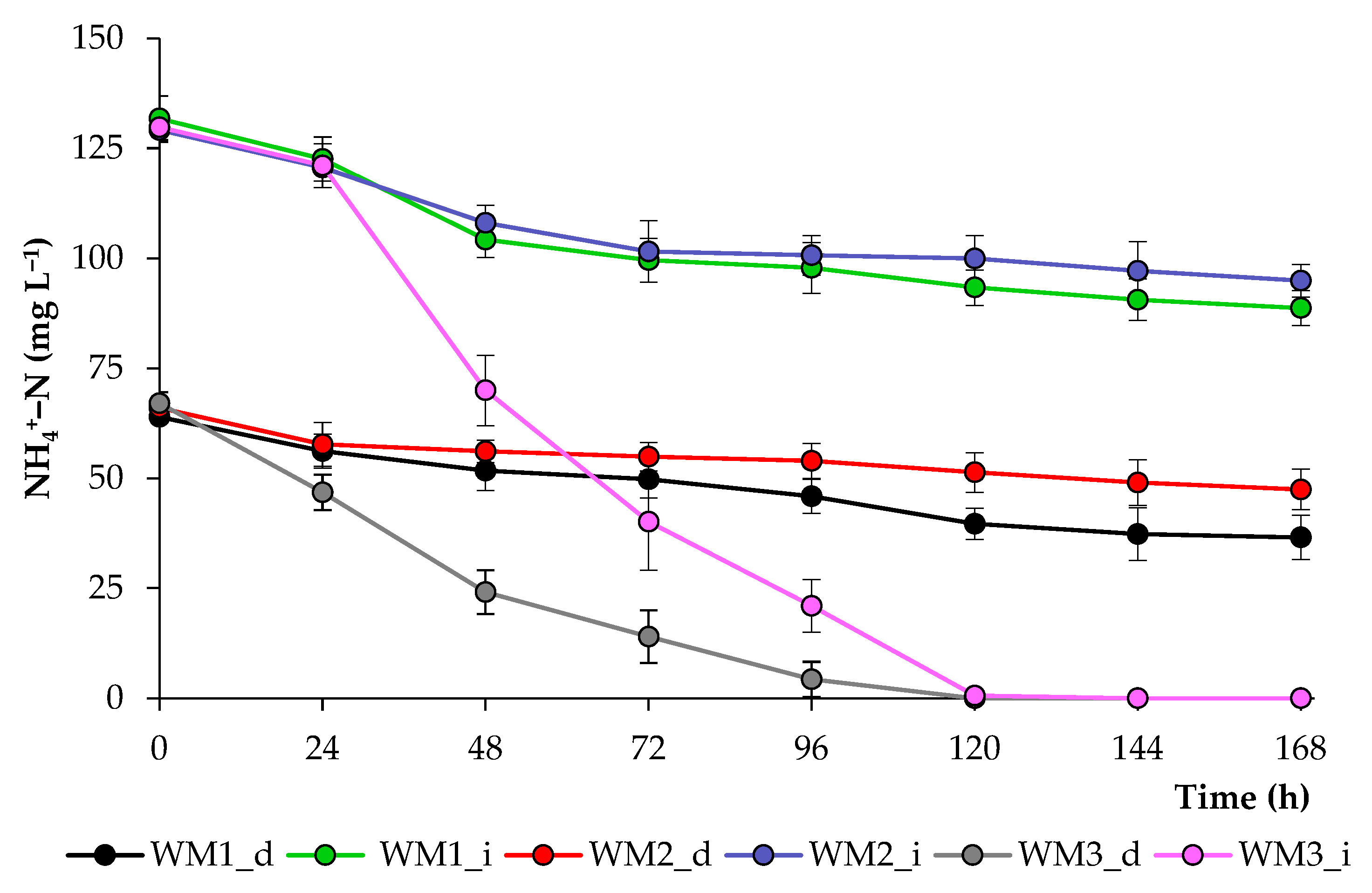
| Treatment | Light/Dark Period (h) | NH4+–N (mg L−1) | PO43−–P (mg L−1) | SO42−–S (mg L−1) | Dry Weight (g L−1) | Volatile Solids (g L−1) |
|---|---|---|---|---|---|---|
| SM1 | 16/8 | 180 | 53 | 12 | 3.17 ± 0.13 c | 2.83 ± 0.12 c |
| SM2 | 24/0 | 180 | 53 | 12 | 4.17 ± 0.14 b | 3.85 ± 0.15 b |
| SM3 | 24/0 | 180 | 80 | 36 | 4.40 ± 0.17 b | 4.10 ± 0.08 b |
| SM4 | 24/0 | 360 | 53 | 12 | 4.61 ± 0.16 b | 4.13 ± 0.09 b |
| SM5 | 24/0 | 360 | 80 | 36 | 5.93 ± 0.11 a | 5.33 ± 0.08 a |
| SM6 | 24/0 | 180 + 180 | 80 | 36 | 5.68 ± 0.18 a | 5.13 ± 0.12 a |
| Treatment | Dry Weight (g L−1) | Volatile Solids (g L−1) |
|---|---|---|
| WM1_d | 0.28 ± 0.04 c | 0.23 ± 0.02 d,e |
| WM1_i | 0.43 ± 0.05 c | 0.35 ± 0.01 c |
| WM2_d | 0.22 ± 0.05 c | 0.18 ± 0.02 e |
| WM2_i | 0.32 ± 0.04 c | 0.26 ± 0.01 d |
| WM3_d | 0.82 ± 0.06 b | 0.69 ± 0.02 b |
| WM3_i | 1.26 ± 0.07 a | 1.06 ± 0.02 a |
| Isolate (bp) | Highest BLAST Hit (Acc. No.)/ Percent Identity | Taxonomic Affiliation |
|---|---|---|
| WW1 (922) | Acinetobacter lwoffii ex19 (KF317889)/99.9% | Acinetobacter sp. |
| WW2 (932) | Pseudomonas putida B33 (KT767698)/99.9% | Pseudomonas sp. |
| WW55 (823) | Pseudomonas sp. H8 (MH885482)/100% | Pseudomonas sp. |
| WW94 (824) | Pseudomonas sp. JCM 5482 (AB685687)/99.9% | Pseudomonas sp. |
| WW8 (822) | Pseudomonas sp. CmLB7 (HM352331)/99.9% | Pseudomonas sp. |
| WW39 (857) | Pseudomonas mendocina SM5 (JX102498)/99.8% | Pseudomonas sp. |
| WW19 (876) | Pseudomonas oleovorans JCM 13980 (LC508006)/99.9% | Pseudomonas sp. |
| WW117 (802) | Pseudomonas paralactis strain DSM 29164 (NR_156987)/97.1% | Pseudomonas sp. |
| WW3 (799) | Aeromonas salmonicida HA-1 (OQ983639)/100% | Aeromonas sp. |
| WW14 (612) | Phyllobacterium myrsinacearum 608 (MT199181)/99.8% | Phyllobacterium sp. |
| WW23 (612) | Phyllobacterium myrsinacearum NBRC 100019 (NR113874)/100% | Phyllobacterium sp. |
| WW64 (684) | Stenotrophomonas maltophilia cqsm_h3 (MN826555)/99.7% | Stenotrophomonas sp. |
| WW103 (869) | Stenotrophomonas maltophilia cqsG6 (MN826545)/99.7% | Stenotrophomonas sp. |
| WW12 (838) | Stenotrophomonas maltophilia cqsG6 (MN826545)/100% | Stenotrophomonas sp. |
| WW74 (897) | Stenotrophomonas maltophilia S7-1 (MN732994)/99.8% | Stenotrophomonas sp. |
| WW11(839) | Stenotrophomonas tumulicola cqsG1 (MN826536)/99.6% | Stenotrophomonas sp. |
| WW13 (787) | Stenotrophomonas chelatiphaga P4M85 (MN421410)/99.6% | Stenotrophomonas sp. |
| WW105 (851) | Stenotrophomonas sp. V10R15 (MT165571)/100% | Stenotrophomonas sp. |
| WW18 (769) | Escherichia coli MAK15 (OP060224)/99.8% | Escherichia coli |
| WW44 (802) | Escherichia coli LCU-ID-EC1 (OL677623)/99.9% | Escherichia coli |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulynina, S.S.; Ziganshina, E.E.; Ziganshin, A.M. Growth Efficiency of Chlorella sorokiniana in Synthetic Media and Unsterilized Domestic Wastewater. BioTech 2023, 12, 53. https://doi.org/10.3390/biotech12030053
Bulynina SS, Ziganshina EE, Ziganshin AM. Growth Efficiency of Chlorella sorokiniana in Synthetic Media and Unsterilized Domestic Wastewater. BioTech. 2023; 12(3):53. https://doi.org/10.3390/biotech12030053
Chicago/Turabian StyleBulynina, Svetlana S., Elvira E. Ziganshina, and Ayrat M. Ziganshin. 2023. "Growth Efficiency of Chlorella sorokiniana in Synthetic Media and Unsterilized Domestic Wastewater" BioTech 12, no. 3: 53. https://doi.org/10.3390/biotech12030053
APA StyleBulynina, S. S., Ziganshina, E. E., & Ziganshin, A. M. (2023). Growth Efficiency of Chlorella sorokiniana in Synthetic Media and Unsterilized Domestic Wastewater. BioTech, 12(3), 53. https://doi.org/10.3390/biotech12030053






