Large-Scale Production of Isocitric Acid Using Yarrowia lipolytica Yeast with Further Down-Stream Purification
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Seed Culture
2.3. Cultivation
2.4. Specification of 10-L Lab-Scale and 500-L Pilot Scale Fermentors
2.5. Analyses
2.6. Product Isolation and Recovery
2.7. Calculations
2.8. Statistical Analysis
3. Results and Discussion
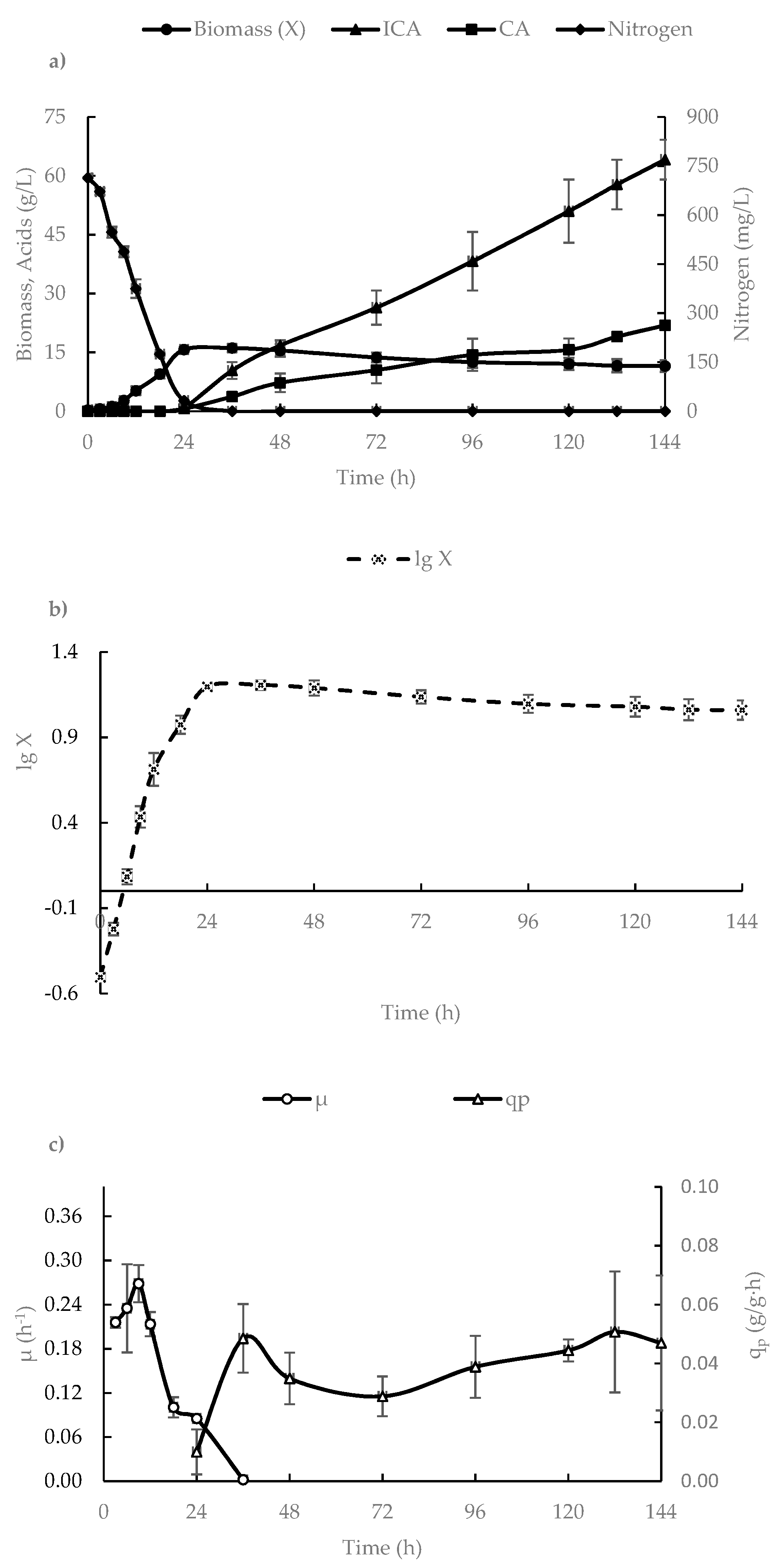
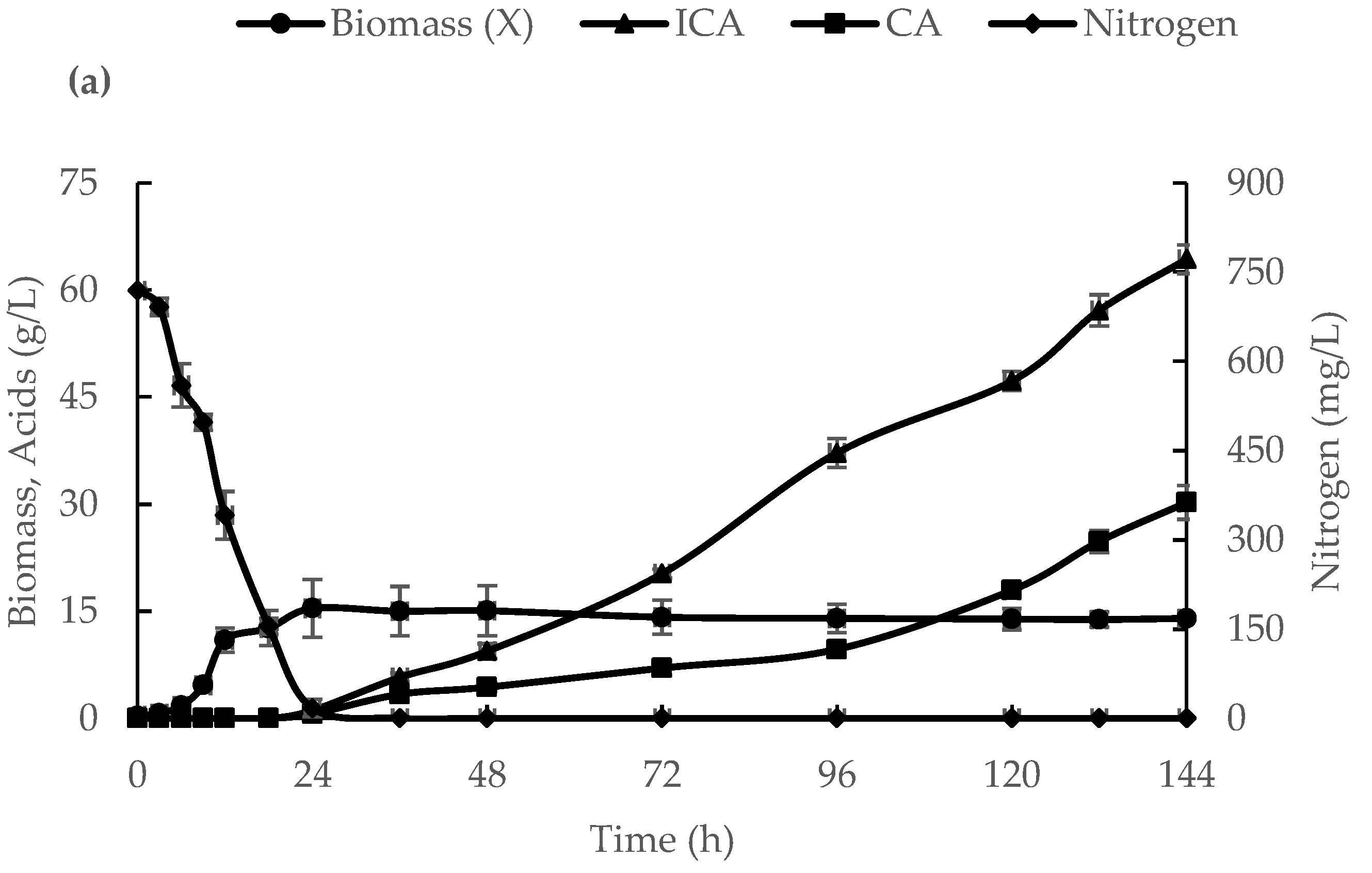
3.1. ICA Production in a 500-L Bioreactor
3.2. Isolation and Purification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finogenova, T.V.; Morgunov, I.G.; Kamzolova, S.V.; Chernyavskaya, O.G. Organic acid production by the yeast Yarrowia lipolytica: A review of prospects. Appl. Biochem. Microbiol. 2005, 41, 418–425. [Google Scholar] [CrossRef]
- Heretsch, P.; Thomas, F.; Aurich, A.; Krautscheid, H.; Sicker, D.; Giannis, A. Syntheses with a chiral building block from the citric acid cycle: (2R,3S)-isocitric acid by fermentation of sunflower oil. Angew. Chem. Int. Ed. Engl. 2008, 47, 1958–1960. [Google Scholar] [CrossRef]
- Aurich, A.; Specht, R.; Müller, R.A.; Stottmeister, U.; Yovkova, V.; Otto, C.; Holz, M.; Barth, G.; Heretsch, P.; Thomas, F.A.; et al. Microbiologically produced carboxylic acids used as building blocks in organic synthesis. In Reprogramming Microbial Metabolic Pathways. Subcellular Biochemistry; Wang, X., Chen, J., Quinn, P., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 64, pp. 391–423. [Google Scholar]
- Bullin, K.; Hennig, L.; Herold, R.; Krautscheid, H.; Richter, K.; Sicker, D. An optimized method for an (2R,3S)-isocitric acid building block. Mon. Chem. 2019, 150, 247–253. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G. Microbial production of (2 R,3 S)-isocitric acid: State of the arts and prospect. Appl. Microbiol. Biotechnol. 2019, 103, 9321–9333. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Karpukhina, O.V.; Kamzolova, S.V.; Samoilenko, V.A.; Inozemtsev, A.N. Investigation of the effect of biologically active threo-Ds-isocitric acid on oxidative stress in Paramecium caudatum. Prep. Biochem. Biotechnol. 2018, 48, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Morgunov, I.G.; Kamzolova, S.V.; Karpukhina, O.V.; Bokieva, S.V.; Inozemtsev, A.N. Biosynthesis of isocitric acid in repeated-batch culture and testing of its stress-protective activity. Appl. Microbiol. Biotechnol. 2019, 103, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Morgunov, I.G.; Kamzolova, S.V.; Karpukhina, O.V.; Bokieva, S.B.; Lunina, J.N.; Inozemtsev, A.N. Microbiological Production of Isocitric Acid from Biodiesel Waste and Its Effect on Spatial Memory. Microorganisms 2020, 8, 462. [Google Scholar] [CrossRef]
- Zinjarde, S.S. Food-related applications of Yarrowia lipolytica. Food Chem. 2014, 152, 1–10. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.V.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Finogenova, T.V.; Shishkanova, N.V.; Fausek, E.A.; Eremina, S.S. Biosynthesis of isocitric acid from ethanol by yeasts. Appl. Microbiol. Biotechnol. 1991, 36, 231–235. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Shamin, R.V.; Stepanova, N.N.; Morgunov, G.I.; Lunina, J.N.; Allayarov, R.K.; Samoilenko, V.A.; Morgunov, I.G. Fermentation conditions and media optimization for isocitric acid production from ethanol by Yarrowia lipolytica. Biomed. Res. Int. 2018, 2018, e2543210. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Dedyukhina, E.G.; Samoilenko, V.A.; Lunina, J.N.; Puntus, I.F.; Allayarov, R.K.; Chiglintseva, M.N.; Mironov, A.A.; Morgunov, I.G. Isocitric acid production from rapeseed oil by Yarrowia lipolytica yeast. Appl. Microbiol. Biotechnol. 2013, 97, 9133–9144. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Allayarov, R.K.; Lunina, J.N.; Morgunov, I.G. The effect of oxalic and itaconic acids on threo-Ds-isocitric acid production from rapeseed oil by Yarrowia lipolytica. Bioresour. Technol. 2016, 206, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Hapeta, P.; Rakicka-Pustułka, M.; Juszczyk, P.; Robak, M.; Rymowicz, W.; Lazar, Z. Overexpression of citrate synthase increases isocitric acid biosynthesis in the yeast Yarrowia lipolytica. Sustainability 2020, 12, 7364. [Google Scholar] [CrossRef]
- Aurich, A.; Hofmann, J.; Oltrogge, R.; Wecks, M.; Glaser, R.; Blömer, L.; Mauersberger, S.; Roland, A.; Müller, R.A.; Sicker, D.; et al. Improved isolation of microbiologically produced (2R,3S)-isocitric acid by adsorption on activated carbon and recovery with methanol. Org. Process Res. Dev. 2017, 21, 866–870. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Samoilenko, V.A.; Lunina, J.N.; Morgunov, I.G. Effects of medium components on isocitric acid production by Yarrowia lipolytica yeast. Fermentation 2020, 6, 112. [Google Scholar] [CrossRef]
- Förster, A.; Jacobs, K.; Juretzek, T.; Mauersberger, S.; Barth, B. Overexpression of the ICL1 gene changes the product ratio of citric acid production by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2007, 77, 861–869. [Google Scholar] [CrossRef]
- Holz, M.; Förster, A.; Mauersberger, S.; Barth, G. Aconitase overexpression changes the product ratio of citric acid production by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2009, 81, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Yuzbasheva, E.Y.; Scarcia, P.; Yuzbashev, T.V.; Messina, E.; Kosikhina, I.M.; Palmieri, L.; Shutov, A.V.; Taratynova, M.O.; Amaro, R.L.; Palmieri, F.; et al. Engineering Yarrowia lipolytica for the selective and high-level production of isocitric acid through manipulation of mitochondrial dicarboxylate-tricarboxylate carriers. Metab. Eng. 2021, 65, 156–166. [Google Scholar] [CrossRef]
- Da Silva, L.V.; Tavares, C.B.; Amaral, P.F.F.; Coehlo, M.A.Z. Production of citric acid by Yarrowia lipolytica in different crude oil concentrations and in different nitrogen sources. Chem. Eng. Trans. 2012, 27, 199–204. [Google Scholar]
- Morgunov, I.G.; Kamzolova, S.V. Physiologo-biochemical characteristics of citrate-producing yeast Yarrowia lipolytica grown on glycerol-containing waste of biodiesel industry. Appl. Microbiol. Biotechnol. 2015, 99, 6443–6450. [Google Scholar] [CrossRef]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Aseptic production of citric and isocitric acid from crude glycerol by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2019, 271, 340–344. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Samoilenko, V.A.; Lunina, J.N.; Morgunov, I.G. Isocitric Acid Production from Ethanol Industry Waste by Yarrowia lipolytica. Fermentation 2021, 7, 146. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G. Effect of Metabolic Regulators and Aeration on Isocitric Acid Synthesis by Yarrowia lipolytica Grown on Ester-Aldehyde Fraction. Fermentation 2021, 7, 283. [Google Scholar] [CrossRef]
- Rywińska, A.; Musiał, I.; Rymowicz, W.; Żarowska, B.; Boruczkowski, T. Effect of agitation and aeration on the citric acid production by Yarrowia lipolytica grown on glycerol. Prep. Biochem. Biotechnol. 2012, 42, 279–291. [Google Scholar] [CrossRef]
- Sabra, W.; Bommareddy, R.R.; Maheshwari, G.; Papanikolaou, S.; Zeng, A.P. Substrates and oxygen dependent citric acid production by Yarrowia lipolytica: Insights through transcriptome and fluxome analyses. Microb. Cell Fact. 2017, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.; Lopes, M.; Mota, M.; Belo, I. Oxygen mass transfer impact on citric acid production by Yarrowia lipolytica from crude glycerol. Biochem. Eng. J. 2016, 110, 35–42. [Google Scholar] [CrossRef]
- Fickers, P.; Ongena, M.A.R.C.; Destain, J.; Weekers, F.; Thonart, P. Production and down-stream processing of an extracellular lipase from the yeast Yarrowia lipolytica. Enzym. Microb. Technol. 2006, 38, 756–759. [Google Scholar] [CrossRef]
- Kar, T.; Destain, J.; Thonart, P.; Delvigne, F. Scale-down assessment of the sensitivity of Yarrowia lipolytica to oxygen transfer and foam management in bioreactors: Investigation of the underlying physiological mechanisms. J. Ind. Microbiol. Biotechnol. 2012, 39, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Zinjarde, S.S.; Pant, A.; Deshpande, M.V. Dimorphic transition in Yarrowia lipolytica isolated from oil-polluted sea water. Mycol. Res. 1998, 102, 553–558. [Google Scholar] [CrossRef]
- Bellou, S.; Makri, A.; Triantaphyllidou, I.-E.; Papanikolaou, S.; Aggelis, G. Morphological and metabolic shifts of Yarrowia lipolytica induced by alteration of the dissolved oxygen concentration in the growth environment. Microbiology 2014, 160, 807–817. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Diamantopoulou, P.; Blanchard, F.; Lambrinea, E.; Chevalot, I.; Stoforos, N.G.; Rondags, E. Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production. Appl. Sci. 2020, 10, 7373. [Google Scholar] [CrossRef]
- Crolla, A.; Kennedy, K.J. Fed-batch production of citric acid by Candida lipolytica grown on n-paraffins. J. Biotechnol. 2004, 110, 73–84. [Google Scholar] [CrossRef]
- Snopek, P.; Nowak, D.; Zieniuk, B.; Fabiszewska, A. Aeration and Stirring in Yarrowia lipolytica Lipase Biosynthesis during Batch Cultures with Waste Fish Oil as a Carbon Source. Fermentation 2021, 7, 88. [Google Scholar] [CrossRef]

| Indices | Volume of Bioreactor | |
|---|---|---|
| 500 L | 10 L | |
| Initial volume of cultural broth (L) | 220.00 ± 0.00 | 6.00 ± 0.00 |
| Final volume of cultural broth (L) | 266.70 ± 21.20 | 8.05 ± 0.07 |
| Total amount of biomass (g) | 3096.00 ± 639.73 | 112.35 ± 6.65 |
| Total amount of ICA (g) | 17,164.20 ± 2687.33 | 516.46 ± 22.66 |
| Total amount of CA (g) | 5847.87 ± 609.84 | 242.92 ± 21.84 |
| ICA/CA ratio | 2.9:1 | 2.1:1 |
| Rapeseed oil consumed (g) | 23,245.33 ± 2329.86 | 552.00 ± 0.00 |
| Yp (g/g) | 0.74 ± 0.05 | 0.94 ± 0.04 |
| qp (g/g·h) | 0.04 ± 0.00 | 0.03 ± 0.00 |
| Qp (g/L·h) | 0.54 ± 0.08 | 0.60 ± 0.03 |
| Stages | Indicators | Samples | |||||
|---|---|---|---|---|---|---|---|
| 1 * | 2 * | 3 * | 4 ** | 5 ** | 6 ** | ||
| Fermentation | Volume of cultural broth (L) | 286 | 244 | 270 | 7.9 | 8.1 | 8.1 |
| ICA (g/L) | 69.1 | 59.0 | 64.2 | 66.3 | 62.3 | 64.3 | |
| CA (g/L) | 22.4 | 21.30 | 22.0 | 32.7 | 28.0 | 30.0 | |
| Calculated amount of K-ICA (kg) | 23.68 | 17.25 | 20.77 | 0.620 | 0.605 | 0.620 | |
| Separation Clarification Filtration | Volume of supernatant (L) | 274 | 232 | 258 | 7.8 | 7.9 | 7.8 |
| Concentration | Volume of concentrate (L) | 54 | 35 | 47 | 1.8 | 1.5 | 1.47 |
| ICA (g/L) | 350 | 390 | 350 | 290 | 340 | 340 | |
| Acidification | pH of concentrate after acidification | 3.4 | 3.5 | 3.4 | 3.4 | 3.5 | 3.5 |
| Crystallization Washing Drying | K-ICA content in technical-grade product (%) | 92.0 | 90.9 | 92.0 | 92.0 | 92.0 | 92.0 |
| Amount of technical-grade K-ICA (kg) | 18.85 | 13.3 | 16.4 | 0.40 | 0.48 | 0.49 | |
| Yield of technical-grade salt (% of the original) | 73.2 | 69.4 | 72.6 | 58.6 | 73.0 | 72.2 | |
| Recrystallization | Purity of product (%) | 99.9 | 99.9 | 99.0 | 99.0 | 99.0 | 99.0 |
| Amount of K-ICA (kg) | 17.07 | 11.73 | 15.0 | 0.31 | 0.43 | 0.45 | |
| Yield of purified product (% of the original) | 71.0 | 67.0 | 71.0 | 49 | 71.3 | 71.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamzolova, S.V.; Samoilenko, V.A.; Lunina, J.N.; Morgunov, I.G. Large-Scale Production of Isocitric Acid Using Yarrowia lipolytica Yeast with Further Down-Stream Purification. BioTech 2023, 12, 22. https://doi.org/10.3390/biotech12010022
Kamzolova SV, Samoilenko VA, Lunina JN, Morgunov IG. Large-Scale Production of Isocitric Acid Using Yarrowia lipolytica Yeast with Further Down-Stream Purification. BioTech. 2023; 12(1):22. https://doi.org/10.3390/biotech12010022
Chicago/Turabian StyleKamzolova, Svetlana V., Vladimir A. Samoilenko, Julia N. Lunina, and Igor G. Morgunov. 2023. "Large-Scale Production of Isocitric Acid Using Yarrowia lipolytica Yeast with Further Down-Stream Purification" BioTech 12, no. 1: 22. https://doi.org/10.3390/biotech12010022
APA StyleKamzolova, S. V., Samoilenko, V. A., Lunina, J. N., & Morgunov, I. G. (2023). Large-Scale Production of Isocitric Acid Using Yarrowia lipolytica Yeast with Further Down-Stream Purification. BioTech, 12(1), 22. https://doi.org/10.3390/biotech12010022






