Abstract
Background: Early identification of the stage of oral cancer development can lead to better treatment outcomes and avoid malignant transformation. Therefore, this review aims to provide a comprehensive overview that describes the development of standardized procedures for oral sample collection, characterization, and molecular risk assessment. This can help investigators to choose the appropriate sampling method and downstream analyses for different purposes. Methods: This systematic review was conducted according to the PRISMA guidelines. Using both PubMed and Web of Science databases, four independent authors conducted a literature search between 15 and 21 June 2021. We used key search terms to broaden the search for studies. Non-conforming articles were removed using an EndNote-based and manual approach. Reviewers used a designed form to extract data. Results: This review included a total of 3574 records, after eliminating duplicate articles and excluding papers that did not meet the inclusion criteria. Finally, 202 articles were included in this review. We summarized the sampling methods, biopsy samples, and downstream analysis. The biopsy techniques were classified into tissue and liquid biopsy. The common sequential analysis of tissue biopsy includes histopathological examination such as H&E or IHC to identify various pathogenic features. Meanwhile, liquid samples such as saliva, blood, and urine are analyzed for the purpose of screening to detect mutations in cancer. Commonly used technologies are PCR, RT-PCR, high-throughput sequencing, and metabolomic analysis. Conclusions: Currently, tissue biopsies provide increased diagnostic value compared to liquid biopsy. However, the minimal invasiveness and convenience of liquid biopsy make it a suitable method for mass screening and eventual clinical adoption. The analysis of samples includes histological and molecular analysis. Metabolite analysis is rising but remains scarce.
1. Introduction
Oral cancer is characterized by the presence of a malignant tumor on the lip or in the mouth [1]. Oral and pharyngeal cancers are the sixth most prevalent cancers globally [2,3] and their ranking varies regionally from the first to eleventh place as the most prevalent cancer, with the highest incidence in Southcentral Asia and Melanesia [3,4]. The incidence, mortality, and disability-adjusted life years (DALYs) of oral cancer doubled from 1990–2017 [5]. The age-standardized rates of incidence are about 2.4 per 100,000 while age-standardized disability-adjusted life years are about 64.0 per 100,000 person-years [5]. Asia, in particular, is the region with the highest prevalence and mortality of oral cancer among all continents [6].
Approximately 80% to 90% of malignant lesions in the mouth are recognized as oral squamous cell carcinoma (OSCC) and OSCC accounts for approximately 3% of all cancers globally [7]. The World Health Organization named the precancerous occurrence as oral potentially malignant disorder (OPMD), a condition that may exhibit epithelial dysplasia on histopathologic evaluation [8,9,10]. The anterior part of the tongue, the labial or buccal mucosa, the gingiva, the alveolar muscle, and the palate constitute the potential tissues of origin [11]. The heterogeneous group of conditions associated with OPMD includes leukoplakia, erythroplakia, proliferative verrucous leukoplakia, oral lichen planus, oral submucous fibrosis, palatal lesions, lupus erythematosus, epidermolysis bullosa, and dyskeratosis congenita [10]. Leukoplakia is among the most common OPMDs, with the global prevalence estimated at 1.7–2.7% and the malignant conversion rate at approximately 1.36% [12]. Meta-analysis shows that 7.9% of the OPMD cases turn into a carcinoma with moderate or severe dysplasia [13].
As with most cancer cases, early identification can result in a better treatment outcome. Research shows the 3-year survival rate for the early stage of cancer being 92.2% [14]. The identification of cancer at a later stage causes the 3-year survival rate to fall to 70.3% [14].
However, the diagnosis can be easily missed or delayed as most of the early-stage cancer cases are reported to be asymptomatic.
Due to the difficulty in identifying the site of early lesions, this leads to great difficulties in the early detection and treatment of OPMD and OSCC [15]. Therefore, it is important to confirm the margins of the tumor in order to perform better and more precise lesion removal. Biamonte et al. demonstrated the benefits of combining autofluorescence with a traditional oral examination to determine the surgical margins [16]. Moreover, the advent of precision medicine is anticipated to expedite the development of targeted therapies and screening strategies in the oncology field [17,18]. The application of near-patient diagnostics provides the opportunities to identify early precancerous lesions and intervene in the earlier stage of the disease, treating or delaying the progression of disease via screening and prevention [19]. The understanding of current sampling techniques/biopsies and how these have been used over the recent past can provide a further technical understanding regarding the development of precision medicine techniques for oral cancer. Therefore, this manuscript aims to provide a comprehensive review of oral biopsies, sample types, and detection techniques applied as sampling methods and testing techniques for clinical purposes.
2. Methods
This systematic review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [20], including all the published, international, scientific peer-reviewed original research articles.
2.1. Databases and Search Strategy
All comprehensive literature recording oral biopsy techniques were taken into account. Using two databases, PubMed and Web of Science, the literature was searched for terms associated with oral cancer (oral cancer, cancer, oral cavity), biopsy (biospecimen, specimen), and molecular assessment (molecular, risk assessment). Each keyword item was bound using the logical word “AND” in the two databases. The detailed search for terms were researched as follows: “oral cancer” AND “biopsy”; “oral cancer” AND “biospecimen”; “oral cancer” AND “specimen”; “molecular” AND “risk assessment” AND “oral cancer”; “cancer” AND “oral cavity” AND “specimen”. These studies were restricted to the human study published on the 21 June 2021. These search strategies were utilized by four authors (GY, LW, TB, and YF) to conduct independent searches in the two databases.
2.2. Eligibility Criteria and Data Extraction
The results of the databases and manual searches were exported into EndNote. Duplications were removed using EndNote-based methods as reported previously [21], and double-checked manually. Four reviewers (GY, LW, TB, and YF) independently screened by following eligibility criteria.
Studies chosen met the following eligibility criteria: (1) All literature searched was relevant to human oral cancer; (2) The samples collected were from human tissue or fluids; (3) Details of the sample collection must be described in the methods available for inclusion; (4) Original studies were eligible for inclusion. However, non-human oral cancers or samples from other species sources were not eligible for inclusion. More importantly, biopsy methods without a detailed description in the literature were not included. Articles which lacked downstream analysis of biopsies were eliminated. Non-English articles, commentaries, and secondary evidence were excluded. The difference of opinion between the reviewers was resolved by a discussion with a third party (ZK and IC). The study screening processes are shown in Figure 1.
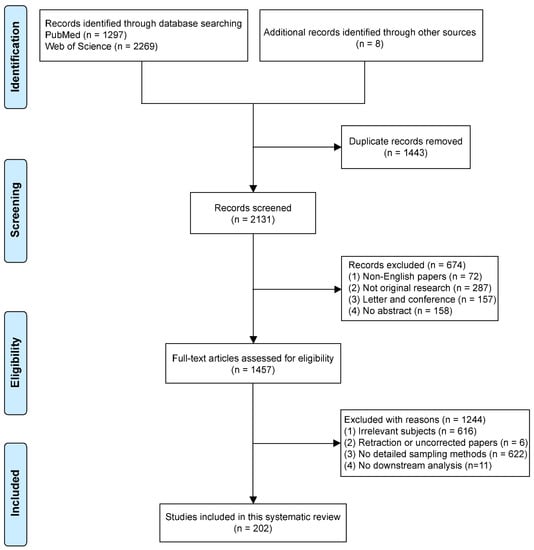
Figure 1.
Flow diagram of literature search.
The four authors (GY, LW, TB, and YF) included the publications that met the criteria in Supplementary Table S1. The main items of the article contain the first author, year of publication, patients, type of sample technology, and clinical applications, etc.
3. Results
3.1. Search Results
A total of 3574 records were included, of which 3566 were found in PubMed and Web of Science. Another eight records were obtained from related professional literature. The PRISMA flow chart for the study selection procedure is shown in Figure 1. After the elimination of duplicates, 2131 papers remained and were screened based on language, article type, and abstract. This resulted in the exclusion of 674 records, including 72 non-English articles, 287 non-original articles, 157 letters/conference reports, and 158 entries only with an abstract and an absence of a full text. The remaining 1457 articles were retrieved for further review. By reading the full text, 1244 articles were excluded because of a lack of direct relevance to the subject, for example only a single mention of oral cancer biopsy (616 papers); retraction (6 papers); manuscripts that utilized biopsies yet did not provide details regarding the sampling methods (622 papers); and no downstream analysis (11 papers). Thus, 202 articles were finally included in this review.
3.2. Characteristics of the Included Studies
Supplementary Table S1 describes the characteristics of each included study. Patients were predominantly in the early stages of oral squamous cell carcinoma and potentially malignant disorders. The advantages and limitations of tissue biopsy, liquid biopsy, and brush biopsy are summarized in Table 1. The sampling methods and the techniques used to analyze the biopsy specimens are summarized in Table 2.

Table 1.
Advantages and limitations of sampling for oral cancer.

Table 2.
Summary of downstream applications of oral cancer biopsy methods included in the literature.
The sampling types in the articles can be divided into two main categories depending on the characteristics of the sample: tissue biopsy and liquid biopsy.
3.3. Tissue Biopsy
A tissue biopsy is a method of obtaining soft tissues of the oral cavity or lymph nodes through surgery or special instruments. Traditional tissue biopsy is the most reliable basis for oral cancer diagnosis. There are several methods of tissue biopsy: surgical biopsy, punch biopsy, lymph node biopsy, brush biopsy, and needle aspiration biopsy, implemented through the utilization of various tools such as a scalpel, circular blade, hollow needle, etc. (Supplementary Table S1).
Tissue obtained from the surgical biopsy is made into frozen sections (FS) [22,23] with hematoxylin and eosin (H&E) staining [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] for pathological analysis. This forms the current gold standard for intraoperative evaluation of tumors. However, study [57] shows that FS requires an extensive waiting time and expensive FS devices. Therefore, the study utilized the multi-staining (MS) [57] method for intraoperative pathological diagnosis. They selected visual inspection acetic acid (VIA) [57] and visual inspection with Lugol’s iodine (VILI) [34,57] in cervical cancer screening due to the high diagnostic accuracy. Other researchers [58] also applied the VILI technique to distinguish epithelial carcinoma and dysplasia from other benign mucosal lesions. Additionally, Takeda et al. applied qPCR and immunofluorescence (IFC) staining to compare normal and cancerous- tissue-related genetic alterations and mitochondrial DNA amounts [59].
SLNB uses frozen section analysis with H&E during surgery to determine the presence of metastasis in cancer. The conventional method is usually paraffin blocks followed by H&E staining [60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100] to examine for possible metastasis and further immunohistochemical (IHC) [83,85,87,88,91,92,96,98,101] analysis for cytokeratin (AE1/AE3) [38,61,63,64,69,72,74,75,77,81,86] to reveal any undetected micro-metastasis if the node was free from tumors. However, the intraoperative pathology of SLNB is only moderately sensitive, and the final pathology examination might require several days. In addition, the SLN identification rate remains relatively low, and the false-negative rate is high. To improve the identification rate, researchers [102] use radioisotopes (RI) and near-infrared (NIR) fluorescence such as indocyanine green (ICG) [89,103] for lymphatic mapping. Besides H&E, these SLNs samples can be cut into 2 mm-thick sections for the preparation of multiple section imprint cytology [104]. These specimens were subsequently stained with May–Grunwald–Giemsa (MGG) and Papanicolaou (PAP) staining [105] for final histopathological diagnosis. Compared to frozen section analysis, imprint cytology requires a shorter time but a lower specificity. Therefore, the selection of techniques was recommended based on the situation. Only a small piece of tissue is required for the pathological assessment of a tissue biopsy. Hence, it is important to identify the margins of the tumor in order to obtain a suitable sample. Autofluorescence can accurately show areas of superficial squamous cell carcinoma of the oral cavity. This helps with further histochemical assessment.
A brush biopsy uses a soft-bristle brush to collect samples from the surface of oral lesions for cytological analysis. This method is usually used as a primary screening method for oral cancer or oral precancerous lesions. Oral brushing samples are then spun down onto slides and stained using the Feulgen-thionin reaction [105,106,107]. Brush biopsies are subsequently stained using PAP staining [28,36,39,43,104,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122] or argyrophilic nucleolar organizer region (AgNOR) [32,107,118,121] for conventional cytology (CC) and liquid-based cytology (LBC). For molecular analysis, mass spectrometry [123,124] and DNA-image cytometry (DNA-ICM) [42,125,126,127,128,129] are utilized. DNA-ICM analysis can detect gross alterations of cellular DNA content representing aneuploidy [130]. Due to the lower cost and accessibility of brush biopsies, they are suitable to serve as a robust non-invasive automated oral cancer screening tool for mass screening to promote early oral cancer detection and decrease the number of unnecessary invasive biopsies.
3.4. Liquid Biopsy
Liquid biopsies typically use traditional non-invasive methods to collect fluid samples such as saliva, blood, urine, and surgical drainage. When liquid biopsies are performed as a screening tool, all cells have the potential to be analyzed for markers that match the disease. In oncology, fluid samples are analyzed to find mutations in cancer.
A liquid biopsy is a fast, easy method which allows early detection and helps early diagnosis with low risk, minimal pain, and less invasion. It is easily collected and transported for various biomarker detection uses (Table 2). However, the initial histologic diagnosis is still needed for the application of a liquid biopsy. The liquid biopsy has the possibility of “over interpretation” and low sensitivity which may lead to high false results and require greater technical efforts [131].
The sequential analysis of saliva normally includes nucleic acid (PCR [132,133,134,135,136], qPCR [137,138,139,140,141,142,143], ddPCR [122,144], nucleic acid extraction [145], calculation of the DNA integrity index [137] and miRNA expression analysis [141]), protein (Western blot [122,146,147], ELISA [146,148], photometric test [149], proteomic analysis [148,150], etc.), cytological analysis (flow cytometry) [25,125], metabolite (Micro-Raman [151], FT-IR [149,151,152], ATR-FTIR [149], LC-MS/MS [148,153]), and other biochemical analyses (bacterial colony count [146]; immunoreactivity assay [154]). The saliva analysis for the oral cancer biopsy serves as a screening tool for an early diagnosis of oral and oropharyngeal cancer because it has the ability to identify hopefully potential biomarkers for oral carcinoma. In addition, it can be used for the detection of human papilloma virus (HPV) [122,125,138,145,155].
Blood is normally collected for metabolite analysis (metabolite extraction [153], high-performance liquid chromatography analysis [156], GC–MS [148,153], FTIR spectra measurement [152]), biochemical analyses (biochemical estimation [157], chemometric techniques [152], ABC-immunoperoxidase technique [53]) and exosome isolation [153]. It assists diagnosis and the clinical staging of oral carcinoma and serves as the metabolite markers for early detection or diagnosis [131].
Urine and surgical drain fluid can also be collected as a liquid biopsy. The collection of urine is for flow cytometry or high-performance liquid chromatography analysis (Table 2). The evaluation of the surgical drain fluid helps the determination of target levels of different disease outcomes.
4. Discussion
Global oral cancer incidence, mortality, and disability-adjusted life years increased by approximately 1.0-fold during 1990–2017 [5]. Obtaining tissue from the oral cavity is an essential first step towards the early identification of potentially malignant lesions and the development of targeted treatments and screening strategies. Therefore, this manuscript aims to provide a comprehensive review of oral biopsies, focusing on the detection techniques applied for different sample types, to select better sampling procedures and detection techniques for oral cancer in clinical diagnosis and precision medicine.
The initial step in these biopsy methods is to analyze the collected samples. In contrast, tissue biopsy obtains solid tissue from the tumor, lesions, or lymph nodes in an invasive manner, whereas liquid biopsy is usually performed using traditional non-invasive or micro-invasive methods to collect liquid samples such as saliva, blood, urine, and surgical drainage fluid (Table 1). Surgical biopsy is a gold standard for diagnosis. The common sequential analysis includes histopathological examination such as H&E [24,25,26,27,95,97,98,99,100] or IHC to identify various pathogenic features [158,159]. Autofluorescence can accurately show areas of superficial squamous cell carcinoma of the oral cavity. This helps with further histochemical assessment [16]. However, it is associated with an increased risk of infection and requires highly skilled professionals.
Meanwhile, the purpose of liquid biopsy is to improve the ability to identify oral cancer and detect prognostic markers of the oral cancer at an early stage. In practice, liquid samples such as saliva, blood, and urine are analyzed for the purpose of screening to detect mutations in cancer. Commonly used technologies are polymerase chain reaction (PCR), real-time polymerase chain reaction (RT-PCR), high throughput sequencing [156], and metabolomic analysis [160].
Liquid biopsy has many advantages over conventional tissue biopsy. In early diagnosis, surgical biopsy is still considered the gold standard, but it causes significant discomfort in the patient. Comparatively, liquid biopsy is a better option for screening and identification of mutations in metastatic cancers and for dynamically following changes that occur during treatment [161,162]. Importantly, in addition to blood, there are other body fluids such as urine, saliva, and surgical drainage fluid samples that can be used for liquid biopsy in oral cancer [131,163,164].
The downstream analyses of liquid biopsies commonly examine circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), circulating tumor RNA (ctRNA), proteins, and exosomes [153,165,166]. CTCs are released from the primary tumor or distant metastatic areas of the tumor cells into the bloodstream and share most of the mutational profile with the primary tumor [167]. Therefore, they could be used as a predictor for the recurrence of oral cancer [168,169]. Circulating cell-free DNA (cfDNA) also has the potential to detect malignancies [170,171], as do exosomes [172,173]. However, such techniques still require overcoming the low detection rates in the early stages of oral cancer, and further standardization is needed prior to any clinical application [174,175].
As liquid biopsy analyzes the cfDNA of cancer patients whose tumor cells are excreted into the bloodstream, it can be expected to provide a complete picture of all the mutations found in all tumors [176]. However, this means that not all mutations are equally well captured by cfDNA [177,178]. Liquid biopsy may not detect a tumor mutation in situ. There is also a lack of competence in the qualitative assessment of tumors. Therefore, performing tumor biopsy at the time of cancer diagnosis can provide important pathological information and the ability to assess biomarkers that do not involve DNA alterations [179].
Similarly, salivary biomarkers have the potential to be utilized as adjunct diagnostic methods. In the case of oral cancer, salivary mitochondrial DNA has been proved to be a prognostic marker [180,181]. In addition, the development of high-throughput sequencing technology is very promising for the application of liquid biopsy to a large-scale clinical screening [131,161]. Liquid biopsy is an innovative, promising approach for cancer detection and therapy. Although liquid biopsies revolutionized oncology, they are not ready to completely replace tissue biopsy [182].
In contrast to liquid biopsy methodologies, the brush biopsy has been used as a common screening technique for oral cancer and does not require strict pre-procedure preparation. It collects cells from the deeper layers of the oral mucosa with minimal pain and bleeding [183]. A brush biopsy is a painless non-invasive method that involves the application of a brush to collect oral cancer specimens [184].
The biopsy can easily rule out the atypical hyperplasia and cancer under limited conditions [185]. The results are reported as negative, atypical, and positive. This is a viable chair-side method for mass screening and initial judgment (decision making) before performing a painful biopsy. The test requires basal cells, thus is likely to result in minor, localized bleeding. However, the keratinization or deep lesions might underrepresent the samples and result in a false-negative outcome [186].
Currently, traditional exfoliative cytology techniques are gradually transforming into computerized cell morphometry through DNA index measurements, micronucleus analysis, and the evaluation of nucleated tissue zones. The addition of molecular methods, such as immunohistochemistry [34,187], cytological analysis [28,36,39,109,125,188], and fluorescence-activated cell scanning [189] can significantly improve efficiency and revolutionize the technique.
Liquid and tissue biopsy techniques are used as two different types of examination methods and functions. More research and advances are necessary to make a decisive choice of superior biopsy. More research is needed on the effectiveness of liquid biopsy as a promising technique for the clinical diagnosis of oral cancer and for guiding treatment.
5. Limitations
Non-English articles were excluded from this review, which may have led to the absence of some research in this area. In addition, none of the pre-print depositions were considered, and that might provide a reduced ability to detect any latest developments in the field. A large number of articles were excluded as those articles provided insufficient or unspecific information on the type of biopsy methods used and downstream analysis. Beyond the need for emphasizing methodological accuracy and completeness, this may have resulted in a lack of comprehensive evaluation of trends in the clinical application of biopsy methods.
6. Conclusions
The main modalities for oral cancer biopsy remain the invasive ones as they form the current clinical gold standard (such as surgical biopsy, lymph node biopsy, puncture biopsy, etc.). However, increasingly non-invasive methods are applied (such as liquid biopsy and brush biopsy) and the anticipation is that the clinical adoption of the latter will continue to increase. Furthermore, the relationship between oral cancer biopsy samples and downstream analyses remains complex, with the need for harmonization and standardization for a number of downstream molecular methods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biotech11010005/s1, Table S1: Summary characteristics of included studies [190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251].
Author Contributions
Conceptualization: X.L. (Xiaoguang Li) and H.W.; Methodology: G.Y., L.W., B.K.S.T. and Y.F.; Software: G.Y., L.W. and B.K.S.T.; Validation: G.Y., L.W., B.K.S.T., Z.K. and I.H.C.; Writing—Original draft preparation: G.Y., L.W. and B.K.S.T.; Writing—Review and Editing: G.Y., L.W., B.K.S.T., Y.F., Z.K. and I.H.C.; Supervision: Z.K., I.H.C., X.L. (Xue Li), H.W. and X.L. (Xiaoguang Li); Project administration: H.W. and X.L. (Xiaoguang Li). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/WHO.
References
- Mishra, R. Biomarkers of oral premalignant epithelial lesions for clinical application. Oral Oncol. 2012, 48, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- García-Martín, J.M. Epidemiology of Oral Cancer. In Oral Cancer Detection: Novel Strategies and Clinical Impact; Panta, P., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 81–93. [Google Scholar]
- Fitzmaurice, C.; Dicker, D.; Pain, A.; Hamavid, H.; Moradi-Lakeh, M.; MacIntyre, M.F.; Allen, C.; Hansen, G.; Woodbrook, R.; Wolfe, C.; et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.H.; Hu, C.Y.; He, H.R.; Li, Y.J.; Lyu, J. Global and regional burdens of oral cancer from 1990 to 2017: Results from the global burden of disease study. Cancer Commun. 2020, 40, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Sarode, G.; Maniyar, N.; Sarode, S.C.; Jafer, M.; Patil, S.; Awan, K.H. Epidemiologic aspects of oral cancer. Dis. Mon. 2020, 66, 100988. [Google Scholar] [CrossRef]
- Johnson, N.W.; Jayasekara, P.; Amarasinghe, A.A. Squamous cell carcinoma and precursor lesions of the oral cavity: Epidemiology and aetiology. Periodontology 2000 2011, 57, 19–37. [Google Scholar] [CrossRef]
- Rethman, M.P.; Carpenter, W.; Cohen, E.E.; Epstein, J.; Evans, C.A.; Flaitz, C.M.; Graham, F.J.; Hujoel, P.P.; Kalmar, J.R.; Koch, W.M.; et al. Evidence-based clinical recommendations regarding screening for oral squamous cell carcinomas. J. Am. Dent. Assoc. 2010, 141, 509–520. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Johnson, N.W.; Van der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Dhanuthai, K.; Rojanawatsirivej, S.; Thosaporn, W.; Kintarak, S.; Subarnbhesaj, A.; Darling, M.; Kryshtalskyj, E.; Chiang, C.P.; Shin, H.I.; Choi, S.Y.; et al. Oral cancer: A multicenter study. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e23–e29. [Google Scholar] [CrossRef]
- Petti, S. Pooled estimate of world leukoplakia prevalence: A systematic review. Oral Oncol. 2003, 39, 770–780. [Google Scholar] [CrossRef]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardinas Lopez, S.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Cheraghlou, S.; Schettino, A.; Zogg, C.K.; Judson, B.L. Changing prognosis of oral cancer: An analysis of survival and treatment between 1973 and 2014. Laryngoscope 2018, 128, 2762–2769. [Google Scholar] [CrossRef]
- Farah, C.S. Narrow Band Imaging-guided resection of oral cavity cancer decreases local recurrence and increases survival. Oral Dis. 2018, 24, 89–97. [Google Scholar] [CrossRef]
- Biamonte, F.; Buffone, C.; Santamaria, G.; Battaglia, A.M.; Mignogna, C.; Fortunato, L.; Costanzo, F.S.; Giudice, A. Gene expression analysis of autofluorescence margins in leukoplakia and oral carcinoma: A pilot study. Oral Dis. 2021, 27, 193–203. [Google Scholar] [CrossRef]
- Bashraheel, S.S.; Domling, A.; Goda, S.K. Update on targeted cancer therapies, single or in combination, and their fine tuning for precision medicine. Biomed. Pharmacother. 2020, 125, 110009. [Google Scholar] [CrossRef]
- Loomans-Kropp, H.A.; Umar, A. Cancer prevention and screening: The next step in the era of precision medicine. NPJ Precis. Oncol. 2019, 3, 3. [Google Scholar] [CrossRef]
- Price, C.P. Point of care testing. BMJ 2001, 322, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Giustini, D.; de Jonge, G.B. Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243. [Google Scholar] [CrossRef]
- Buchakjian, M.R.; Tasche, K.K.; Robinson, R.A.; Pagedar, N.A.; Sperry, S.M. Association of Main Specimen and Tumor Bed Margin Status With Local Recurrence and Survival in Oral Cancer Surgery. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Bahr, W.; Stoll, P. Intraoperative histological evaluation of tumor resection borders without prolonging surgery. Int. J. Oral Maxillofac. Surg. 1992, 21, 90–91. [Google Scholar] [CrossRef]
- Obade, A.Y.; Pandarathodiyil, A.K.; Oo, A.L.; Warnakulasuriya, S.; Ramanathan, A. Application of optical coherence tomography to study the structural features of oral mucosa in biopsy tissues of oral dysplasia and carcinomas. Clin. Oral Investig. 2021, 25, 5411–5419. [Google Scholar] [CrossRef] [PubMed]
- Mada, L.N.; Kumarguru, B.N.; Gaur, U. A Clinical Study on Detection of Dysplastic Cells Through Saliva. Indian J. Otolaryngol. Head Neck Surg. 2021, 73, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Giovannacci, I.; Magnoni, C.; Pedrazzi, G.; Vescovi, P.; Meleti, M. Clinicopathological Features Associated with Fluorescence Alteration: Analysis of 108 Oral Malignant and Potentially Malignant Lesions. Photobiomodulation Photomed. Laser Surg. 2021, 39, 53–61. [Google Scholar] [CrossRef]
- De Koning, K.J.; Koppes, S.A.; de Bree, R.; Dankbaar, J.W.; Willems, S.M.; van Es, R.J.; Noorlag, R. Feasibility study of ultrasound-guided resection of tongue cancer with immediate specimen examination to improve margin control—Comparison with conventional treatment. Oral Oncol. 2021, 116, 105249. [Google Scholar] [CrossRef]
- Bhatia, P.V.; Dudhia, B.B.; Patel, T.S.; Jani, R.K.; Shah, E.M.; Patel, R.A. Don’t rush, first brush: A comparative study between Modified Brush Biopsy (MBB) and Liquid-Based Cytology (LBC). J. Indian Acad. Oral Med. Radiol. 2020, 32, 134–139. [Google Scholar]
- Muraki, Y.; Hasegawa, T.; Takeda, D.; Ueha, T.; Iwata, E.; Saito, I.; Amano, R.; Sakakibara, A.; Akashi, M.; Komori, T. Induced Pluripotent Stem Cell-related Genes Correlate With Poor Prognoses of Oral Squamous Cell Carcinoma. Anticancer Res. 2019, 39, 1205–1216. [Google Scholar] [CrossRef]
- Quang, T.; Tran, E.Q.; Schwarz, R.A.; Williams, M.D.; Vigneswaran, N.; Gillenwater, A.M.; Richards-Kortum, R. Prospective Evaluation of Multimodal Optical Imaging with Automated Image Analysis to Detect Oral Neoplasia In Vivo. Cancer Prev. Res. 2017, 10, 563–570. [Google Scholar] [CrossRef]
- Liese, J.; Winter, K.; Glass, Ä.; Bertolini, J.; Kämmerer, P.W.; Frerich, B.; Schiefke, I.; Remmerbach, T.W. Advances toward fully automated in vivo assessment of oral epithelial dysplasia by nuclear endomicroscopy—A pilot study. J. Oral Pathol. Med. 2017, 46, 911–920. [Google Scholar] [CrossRef]
- Jajodia, E.; Raphael, V.; Shunyu, N.B.; Ralte, S.; Pala, S.; Jitani, A.K. Brush Cytology and AgNOR in the Diagnosis of Oral Squamous Cell Carcinoma. Acta Cytol. 2017, 61, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Grillone, G.A.; Wang, Z.; Krisciunas, G.P.; Tsai, A.C.; Kannabiran, V.R.; Pistey, R.W.; Zhao, Q.; Rodriguez-Diaz, E.; A’Amar, O.M.; Bigio, I.J. The color of cancer: Margin guidance for oral cancer resection using elastic scattering spectroscopy. Laryngoscope 2017, 127, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.D.D.; Maraschin, B.J.; Laureano, N.K.; Daroit, N.; Brochier, F.; Bündrich, L.; Visioli, F.; Rados, P.V. Expression of E-cadherin and involucrin in leukoplakia and oral cancer: An immunocytochemical and immunohistochemical study. Braz. Oral Res. 2017, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Angelelli, G.; Moschetta, M.; Limongelli, L.; Albergo, A.; Lacalendola, E.; Brindicci, F.; Favia, G.; Maiorano, E. Endocavitary sonography of early oral cavity malignant tumors. Head Neck-J. Sci. Spec. Head Neck 2017, 39, 1349–1356. [Google Scholar] [CrossRef]
- Nanayakkara, P.G.C.L.; Dissanayaka, W.L.; Nanayakkara, B.G.; Amaratunga, E.A.P.D.; Tilakaratne, W.M. Comparison of spatula and cytobrush cytological techniques in early detection of oral malignant and premalignant lesions: A prospective and blinded study. J. Oral Pathol. Med. 2016, 45, 268–274. [Google Scholar] [CrossRef]
- Hande, A.H.; Mohite, D.P.; Chaudhary, M.S.; Patel, M.; Agarwal, P.; Bohra, S. Evidence based demonstration of the concept of ’field cancerization’ by p53 expression in mirror image biopsies of patients with oral squamous cell carcinoma-an immunohistochemical study. Rom. J. Morphol. Embryol. 2015, 56, 1027–1033. [Google Scholar]
- De Bree, R.; Pouw, B.; Heuveling, D.A.; Castelijns, J.A. Fusion of Freehand SPECT and Ultrasound to Perform Ultrasound-Guided Fine-Needle Aspiration Cytology of Sentinel Nodes in Head and Neck Cancer. Am. J. Neuroradiol. 2015, 36, 2153–2158. [Google Scholar] [CrossRef]
- Gupta, S.; Shah, J.S.; Parikh, S.; Limbdiwala, P.; Goel, S. Clinical correlative study on early detection of oral cancer and precancerous lesions by modified oral brush biopsy and cytology followed by histopathology. J. Cancer Res. Ther. 2014, 10, 232–238. [Google Scholar] [CrossRef]
- Graveland, A.P.; Bremmer, J.F.; De Maaker, M.; Brink, A.; Cobussen, P.; Zwart, M.; Braakhuis, B.J.; Bloemena, E.; Van Der Waal, I.; Leemans, C.R.; et al. Molecular screening of oral precancer. Oral Oncol. 2013, 49, 1129–1135. [Google Scholar] [CrossRef]
- Costa Fontes, K.B.F.D.; Cunha, K.S.G.; Rodrigues, F.R.; Silva, L.E.D.; Dias, E.P. Concordance between cytopathology and incisional biopsy in the diagnosis of oral squamous cell carcinoma. Braz. Oral Res. 2013, 27, 122–127. [Google Scholar] [CrossRef]
- Saini, R.; Tang, T.H.; Zain, R.B.; Cheong, S.C.; Musa, K.I.; Saini, D.; Ismail, A.R.; Abraham, M.T.; Mustafa, W.M.W.; Santhanam, J. Significant association of high-risk human papillomavirus (HPV) but not of p53 polymorphisms with oral squamous cell carcinomas in Malaysia. J. Cancer Res. Clin. Oncol. 2011, 137, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, M.; Ibrahim, R.; Mehrotra, R. Utility of toluidine blue staining and brush biopsy in precancerous and cancerous oral lesions. Acta Cytol. 2007, 51, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Siar, C.H. Chemiluminescence as a diagnostic aid in the detection of oral cancer and potentially malignant epithelial lesions. Int. J. Oral Maxillofac. Surg. 2005, 34, 521–527. [Google Scholar] [CrossRef]
- Sokolov, K.; Nieman, L.T.; Myakov, A.; Gillenwater, A. Polarized reflectance spectroscopy for pre-cancer detection. Technol. Cancer Res. Treat. 2004, 3, 1–14. [Google Scholar] [CrossRef]
- Seoane, J.; Varela-Centelles, P.I.; Ramírez, J.R.; Cameselle-Teijeiro, J.; Romero, M.A. Artefacts in oral incisional biopsies in general dental practice: A pathology audit. Oral Dis. 2004, 10, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Beevi, S.S.S.; Rasheed, A.M.H.; Geetha, A. Evaluation of oxidative stress and nitric oxide levels in patients with oral cavity cancer. Jpn. J. Clin. Oncol. 2004, 34, 379–385. [Google Scholar] [CrossRef]
- McCullough, M.; Jaber, M.; Barrett, A.W.; Bain, L.; Speight, P.M.; Porter, S.R. Oral yeast carriage correlates with presence of oral epithelial dysplasia. Oral Oncol. 2002, 38, 391–393. [Google Scholar] [CrossRef]
- Da Costa Filho, L.C.; Da Costa, C.C.; Sória, M.L.; Taga, R. Effect of home bleaching and smoking on marginal gingival epithelium proliferation: A histologic study in women. J. Oral Pathol. Med. 2002, 31, 473–480. [Google Scholar] [CrossRef]
- Sciubba, J.J.; US Collaborative OralCDx Study Group. Collaborative Oral, Improving detection of precancerous and cancerous oral lesions-Computer-assisted analysis of the oral brush biopsy. J. Am. Dent. Assoc. 1999, 130, 1445–1457. [Google Scholar] [CrossRef]
- Erenmemisoglu, A.; Ustun, H.; Kartal, M. Carcinoma of buccal mucosa in smokeless tobacco users: A preliminary study of the use of cytology for early detection. Cytopathology 1995, 6, 403–438. [Google Scholar] [CrossRef]
- Wood, M.W.; Medina, J.E.; Thompson, G.C.; Houck, J.R.; Min, K.W. Accumulation of the p53 tumor-suppressor gene product in oral leukoplakia. Otolaryngol. Head Neck Surg. 1994, 111, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Migliorati, C.A.; Migliorati, E.K.J.; Silverman, S., Jr.; Greenspan, D.; Greenspan, J.S. Phenotypic identification of mononuclear cells in oral premalignant lesions and cancer by monoclonal antibodies. J. Oral Pathol. 1986, 15, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Pindborg, J.J.; Mehta, F.S.; Gupta, P.C.; Daftary, D.K.; Smith, C.J. Reverse smoking in Andhra Pradesh, India: A study of palatal lesions among 10,169 villagers. Br. J. Cancer 1971, 25, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Pindborg, J.J.; Poulsen, H.E.; Zachariah, J. Oral epithelial changes in thirty Indians with oral cancer and submucous fibrosis. Cancer 1967, 20, 1141–1146. [Google Scholar] [CrossRef]
- Glucksmann, A.; Walter, L.; Cherry, C.P. The role of serial biopsies in the treatment of oral cancer. Clin. Radiol. 1967, 18, 310–312. [Google Scholar] [CrossRef]
- Putri, M.I.A.; Panigoro, S.S.; Harahap, A.S.; Pakasi, T.A.; Brahma, B. Acetic Acid and Iodine Staining for Determining Malignancy in Solid Tumors. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 463–469. [Google Scholar] [CrossRef]
- Elimairi, I.; Altay, M.A.; Abdoun, O.; Elimairi, A.; Tozoglu, S.; Baur, D.A.; Quereshy, F. Clinical relevance of the utilization of vital Lugol’s iodine staining in detection and diagnosis of oral cancer and dysplasia. Clin. Oral Investig. 2017, 21, 589–595. [Google Scholar] [CrossRef]
- Takeda, D.; Hasegawa, T.; Ueha, T.; Sakakibara, A.; Kawamoto, T.; Minamikawa, T.; Sakai, Y.; Komori, T. Decreased mitochondrial copy numbers in oral squamous cell carcinoma. Head Neck 2016, 38, 1170–1175. [Google Scholar] [CrossRef]
- Chikamatsu, K.; Kamada, H.; Ninomiya, H.; Takahashi, K.; Sakurai, T.; Oriuchi, N.; Furuya, N. A preliminary study on sentinel lymph node biopsy: Feasibility and predictive ability in oral cavity cancer. Ann. Nuclear Med. 2004, 18, 257–262. [Google Scholar] [CrossRef]
- Kovács, A.F.; Döbert, N.; Walendzik, H.; Zaplatnikov, K.; Landes, C.A. The diagnostic role of radioactivity in sentinel nodes in oral and oropharyngeal cancer. Cancer Biother. Radiopharm. 2006, 21, 535–543. [Google Scholar] [CrossRef]
- Civantos, F.J.; Moffat, F.L.; Goodwin, W.J. Lymphatic mapping and sentinel lymphadenectomy for 106 head and neck lesions: Contrasts between oral cavity and cutaneous malignancy. Laryngoscope 2006, 116, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bilde, A.; Von Buchwald, C.; Mortensen, J.; Marving, J.; Hamilton Therkildsen, M.; Kirkegaard, J.; Charabi, B.; Specht, L. The role of SPECT-CT in the lymphoscintigraphic identification of sentinel nodes in patients with oral cancer. Acta Oto-Laryngol. 2006, 126, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.B.; Sorensen, J.A.; Krogdahl, A. Sentinel lymph nodes in cancer of the oral cavity-isolated tumour cells. J. Oral Pathol. Med. 2005, 34, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.B.; Sørensen, J.A.; Grupe, P.; Krogdahl, A. Sentinel lymph node biopsy in oral cancer: Validation of technique and clinical implications of added oblique planar lymphoscintigraphy and/or tomography. Acta Radiol. 2005, 46, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.B.; Sørensen, J.A.; Grupe, P.; Karstoft, J.; Krogdahl, A. Staging N0 oral cancer: Lymphoscintigraphy and conventional imaging. Acta Radiol. 2005, 46, 492–496. [Google Scholar] [CrossRef]
- Terada, A.; Hasegawa, Y.; Goto, M.; Sato, E.; Hyodo, I.; Ogawa, T.; Nakashima, T.; Yatabe, Y. Sentinel lymph node radiolocalization in clinically negative neck oral cancer. Head Neck 2006, 28, 114–120. [Google Scholar] [CrossRef]
- Stoeckli, S.J. Sentinel node biopsy for oral and oropharyngeal squamous cell carcinoma of the head and neck. Laryngoscope 2007, 117, 1539–1551. [Google Scholar] [CrossRef]
- Vigili, M.G.; Tartaglione, G.; Rahimi, S.; Mafera, B.; Pagan, M. Lymphoscintigraphy and radioguided sentinel node biopsy in oral cavity squamous cell carcinoma: Same day protocol. Eur. Arch. Oto-Rhino-Laryngol. 2007, 264, 163–167. [Google Scholar] [CrossRef]
- Thomsen, J.B.; Christensen, R.K.; Sørensen, J.A.; Krogdahl, A. Sentinel lymph nodes in cancer of the oral cavity: Is central step-sectioning enough? J. Oral Pathol. Med. 2007, 36, 425–429. [Google Scholar] [CrossRef]
- Matsuzuka, T.; Kano, M.; Ogawa, H.; Miura, T.; Tada, Y.; Matsui, T.; Yokoyma, S.; Suzuki, Y.; Suzuki, M.; Omori, K. Sentinel Node Mapping for Node Positive Oral Cancer: Potential to Predict Multiple Metastasis. Laryngoscope 2008, 118, 646–649. [Google Scholar] [CrossRef]
- Keski-säntti, H.; Kontio, R.; Leivo, I.; Törnwall, J.; Mätzke, S.; Mäkitie, A.A.; Atula, T. Sentinel lymph node biopsy as an alternative to wait and see policy in patients with small T1 oral cavity squamous cell carcinoma. Acta Oto-Laryngol. 2008, 128, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Chone, C.T.; Magalhães, R.S.; Etchehebere, E.; Camargo, E.; Altemani, A.; Crespo, A.N. Predictive value of sentinel node biopsy in head and neck cancer. Acta Oto-Laryngol. 2008, 128, 920–924. [Google Scholar] [CrossRef]
- Bilde, A.; von Buchwald, C.; Therkildsen, M.H.; Mortensen, J.; Kirkegaard, J.; Charabi, B.; Specht, L. Need for Intensive Histopathologic Analysis to Determine Lymph Node Metastases When Using Sentinel Node Biopsy in Oral Cancer. Laryngoscope 2008, 118, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Santaolalla, F.; Sanchez, J.M.; Ereño, C.; Gonzalez, A.; Rodriguez, M.L.; Sanchez, A.; Martinez, A. Non-sentinel node tumor invasion in oropharyngeal and oral cancer: Risk of misdiagnosis of metastasis. Acta Oto-Laryngol. 2008, 128, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.; Foster, A.; Walshe, P.; O’Dwyer, T. Sentinel lymph node biopsy in node-negative squamous cell carcinoma of the oral cavity and oropharynx. J. Laryngol. Otol. 2009, 123, 439–443. [Google Scholar] [CrossRef]
- Atula, T.; Hunter, K.D.; Cooper, L.A.; Shoaib, T.; Ross, G.L.; Soutar, D.S. Micrometastases and isolated tumour cells in sentinel lymph nodes in oral and oropharyngeal squamous cell carcinoma. Eur. J. Surg. Oncol. 2009, 35, 532–538. [Google Scholar] [CrossRef]
- Civantos, F.J.; Zitsch, R.P.; Schuller, D.E.; Agrawal, A.; Smith, R.B.; Nason, R.; Petruzelli, G.; Gourin, C.G.; Wong, R.J.; Ferris, R.L.; et al. Sentinel Lymph Node Biopsy Accurately Stages the Regional Lymph Nodes for T1-T2 Oral Squamous Cell Carcinomas: Results of a Prospective Multi-Institutional Trial. J. Clin. Oncol. 2010, 28, 1395–1400. [Google Scholar] [CrossRef]
- Terada, A.; Hasegawa, Y.; Yatabe, Y.; Hanai, N.; Ozawa, T.; Hirakawa, H.; Maruo, T.; Kawakita, D.; Mikami, S.; Suzuki, A.; et al. Follow-up after intraoperative sentinel node biopsy of N0 neck oral cancer patients. Eur. Arch. Oto-Rhino-Laryngol. 2011, 268, 429–435. [Google Scholar] [CrossRef]
- Melkane, A.E.; Mamelle, G.; Wycisk, G.; Temam, S.; Janot, F.; Casiraghi, O.; Lumbroso, J. Sentinel node biopsy in early oral squamous cell carcinomas: A 10-year experience. Laryngoscope 2012, 122, 1782–1788. [Google Scholar] [CrossRef]
- Den Toom, I.J.; Heuveling, D.A.; Flach, G.B.; Van Weert, S.; Karagozoglu, K.H.; Van Schie, A.; Bloemena, E.; Leemans, C.R.; De Bree, R. Sentinel node biopsy for early-stage oral cavity cancer: The VU University Medical Center experience. Head Neck J. Sci. Spec. Head Neck 2015, 37, 573–578. [Google Scholar] [CrossRef]
- Schilling, C.; Stoeckli, S.J.; Haerle, S.K.; Broglie, M.A.; Huber, G.F.; Sorensen, J.A.; Bakholdt, V.; Krogdahl, A.; Von Buchwald, C.; Bilde, A.; et al. Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur. J. Cancer 2015, 51, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, G.; Stoeckli, S.J.; De Bree, R.; Schilling, C.; Flach, G.B.; Bakholdt, V.; Sorensen, J.A.; Bilde, A.; Von Buchwald, C.; Lawson, G.; et al. Sentinel Node in Oral Cancer The Nuclear Medicine Aspects. A Survey from the Sentinel European Node Trial. Clin. Nuclear Med. 2016, 41, 534–542. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hiraki, A.; Fukuma, D.; Nagata, M.; Shiraishi, S.; Kawahara, K.; Matsuoka, Y.; Nakagawa, Y.; Yoshida, R.; Tanaka, T.; Yoshitake, Y.; et al. Sentinel lymph node biopsy reduces the incidence of secondary neck metastasis in patients with oral squamous cell carcinoma. Mol. Clin. Oncol. 2016, 5, 57–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boeve, K.; Schepman, K.P.; van der Vegt, B.; Schuuring, E.; Roodenburg, J.L.; Brouwers, A.H.; Witjes, M.J. Lymphatic drainage patterns of oral maxillary tumors: Approachable locations of sentinel lymph nodes mainly at the cervical neck level. Head Neck 2017, 39, 486–491. [Google Scholar] [CrossRef]
- Miura, K.; Hirakawa, H.; Uemura, H.; Yoshimoto, S.; Shiotani, A.; Sugasawa, M.; Homma, A.; Yokoyama, J.; Tsukahara, K.; Yoshizaki, T.; et al. Sentinel node biopsy for oral cancer: A prospective multicenter Phase II trial. Auris Nasus Larynx 2017, 44, 319–326. [Google Scholar] [CrossRef]
- Riese, C.G.; Karstadt, J.A.; Schramm, A.; Gueleryuez, S.; Dressel, G.; Lorenz, K.J.; Klemenz, B.; Sailer, A.; Seitz, S.; Wilde, F. Validity of sentinel node biopsy in early oral and oropharyngeal carcinoma. J. Cranio-Maxillofac. Surg. 2018, 46, 1748–1752. [Google Scholar] [CrossRef]
- Vishnoi, J.R.; Kumar, V.; Gupta, S.; Chaturvedi, A.; Misra, S.; Akhtar, N.; Agarwal, P.; Jamal, N.; Pareek, P. Outcome of sentinel lymph node biopsy in early-stages quamous cell carcinoma of the oral cavity with methylene blue dye alone: A prospective validation study. Br. J. Oral Maxillofac. Surg. 2019, 57, 755–759. [Google Scholar] [CrossRef]
- Kim, J.H.; Byeon, H.K.; Kim, D.H.; Kim, S.H.; Choi, E.C.; Koh, Y.W. ICG-Guided Sentinel Lymph Node Sampling during Robotic Retroauricular Neck Dissection in cN0 Oral Cancer. Otolaryngology-Head Neck Surg. 2020, 162, 410–413. [Google Scholar] [CrossRef]
- Ishiguro, K.; Iwai, T.; Izumi, T.; Sugiyama, S.; Baba, J.; Oguri, S.; Hirota, M.; Mitsudo, K. Sentinel lymph node biopsy with preoperative CT lymphography and intraoperative indocyanine green fluorescence imaging for N0 early tongue cancer: A long-term follow-up study. J. Cranio-Maxillofac. Surg. 2020, 48, 217–222. [Google Scholar] [CrossRef]
- Hernando, J.; Aguirre, P.; Aguilar-Salvatierra, A.; Leizaola-Cardesa, I.O.; Bidaguren, A.; Gómez-Moreno, G. Magnetic detection of sentinel nodes in oral squamous cell carcinoma by means of superparamagnetic iron oxide contrast. J. Surg. Oncol. 2020, 121, 244–248. [Google Scholar] [CrossRef]
- Rathod, R.; Bakshi, J.; Panda, N.K.; Verma, R.; Bhattacharya, A.; Bal, A. Can Sentinel Lymph Node Biopsy Predict Various Levels of Echelon Nodes in Oral Cancers? Int. Arch. Otorhinolaryngol. 2020, 24, E51–E57. [Google Scholar] [CrossRef]
- Vigili, M.G.; Rahimi, S.; Marani, C.; Natale, M.E.; Tartaglione, G. Radioguided sentinel node biopsy to avoid unnecessary neck dissection in T1-T2N0 oral cavity squamous cell carcinoma: Personal experience with same day protocol. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 3479–3487. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, J.; Hasegawa, Y.; Sugasawa, M.; Shiotani, A.; Murakami, Y.; Ohba, S.; Kohno, N. Long term-follow-up multicenter feasibility study of ICG fluorescence-navigated sentinel node biopsy in oral cancer. Mol. Clin. Oncol. 2020, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- De Kerangal, Q.; Kapso, R.; Morinière, S.; Laure, B.; Bonastre, J.; Moya-Plana, A. Sentinel lymph node biopsy versus selective neck dissection in patients with early oral squamous cell carcinoma: A cost analysis. J. Stomatol. Oral Maxillofac. Surg. 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Boeve, K.; Mastik, M.F.; Slagter-Menkema, L.; van Dijk, B.A.; Roodenburg, J.L.; van der Laan, B.F.; Witjes, M.J.; van der Vegt, B.; Schuuring, E. Cortactin expression assessment improves patient selection for a watchful waiting strategy in pT1cN0-staged oral squamous cell carcinomas with a tumor infiltration depth below 4 mm. Head Neck J. Sci. Spec. Head Neck 2021, 43, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Den Toom, I.J.; Mahieu, R.; van Rooij, R.; van Es, R.J.; Hobbelink, M.G.; Krijger, G.C.; Tijink, B.M.; de Keizer, B.; de Bree, R. Sentinel lymph node detection in oral cancer: A within-patient comparison between Tc-99m Tc-tilmanocept and Tc-99m Tc-nanocolloid. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 851–858. [Google Scholar] [CrossRef]
- Mahieu, R.; Den Toom, I.J.; Boeve, K.; Lobeek, D.; Bloemena, E.; Donswijk, M.L.; De Keizer, B.; Klop, W.M.C.; Leemans, C.R.; Willems, S.M.; et al. Contralateral Regional Recurrence in Lateralized or Paramedian Early-Stage Oral Cancer Undergoing Sentinel Lymph Node Biopsy-Comparison to a Historic Elective Neck Dissection Cohort. Front. Oncol. 2021, 11, 644306. [Google Scholar] [CrossRef]
- Sugiyama, S.; Iwai, T.; Izumi, T.; Baba, J.; Oguri, S.; Hirota, M.; Mitsudo, K. Sentinel lymph node mapping of clinically N0 early oral cancer: A diagnostic pitfall on CT lymphography. Oral Radiol. 2021, 37, 251–255. [Google Scholar] [CrossRef]
- Park, W.; Jin, H.; Heo, Y.; Jeong, H.S.; Son, Y.I.; Chung, M.K.; Baek, C.H. Sentinel lymph node biopsy versus elective neck dissection: Long-term oncologic outcomes in clinically node-negative tongue cancer. Clin. Exp. Otorhinolaryngol. 2021, 15, 107–114. [Google Scholar] [CrossRef]
- Mahieu, R. Diagnostic accuracy of Tc-99m Tc-tilmanocept compared to Tc-99m Tc-nanocolloid for sentinel lymph node identification in early-stage oral cancer. Clin. Otolaryngol. 2021, 46, 1383–1388. [Google Scholar] [CrossRef]
- Nakamura, T.; Kogashiwa, Y.; Nagafuji, H.; Yamauchi, K.; Kohno, N. Validity of Sentinel Lymph Node Biopsy by ICG Fluorescence for Early Head and Neck Cancer. Anticancer Res. 2015, 35, 1669–1674. [Google Scholar]
- Peng, H.; Wang, S.J.; Niu, X.; Yang, X.; Chi, C.; Zhang, G. Sentinel node biopsy using indocyanine green in oral/oropharyngeal cancer. World J. Surg. Oncol. 2015, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Terada, A.; Hasegawa, Y.; Yatabe, Y.; Hyodo, I.; Ogawa, T.; Hanai, N.; Ikeda, A.; Nagashima, Y.; Masui, T.; Hirakawa, H.; et al. Intraoperative diagnosis of cancer metastasis in sentinel lymph node of oral cancer patients. Oral Oncol. 2008, 44, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.M.; Zhou, T.J.; Wang, R.; Shan, J.; Wu, Y.N.; Song, X.L.; Gu, N.; Fan, Y. Brush biopsy with DNA-image cytometry: A useful and noninvasive method for monitoring malignant transformation of potentially malignant oral disorders. Eur. Arch. Otorhinolaryngol. 2014, 271, 3291–3295. [Google Scholar] [CrossRef] [PubMed]
- Maraki, D.; Yalcinkaya, S.; Pomjanski, N.; Megahed, M.; Boecking, A.; Becker, J. Cytologic and DNA-cytometric examination of oral lesions in lichen planus. J. Oral Pathol. Med. 2006, 35, 227–232. [Google Scholar] [CrossRef]
- Remmerbach, T.W.; Meyer-Ebrecht, D.; Aach, T.; Würflinger, T.; Bell, A.A.; Schneider, T.E.; Nietzke, N.; Frerich, B.; Böcking, A. Toward a Multimodal Cell Analysis of Brush Biopsies for the Early Detection of Oral Cancer. Cancer Cytopathol. 2009, 117, 228–235. [Google Scholar] [CrossRef]
- Gaida, K.; Deuerling, L.; Neumann, H.; Remmerbach, T.W. Comparison between two cell collecting methods for liquid-based brush biopsies: A consecutive and retrospective study. BMC Oral Health 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Velleuer, E.; Dietrich, R.; Pomjanski, N.; de Santana Almeida Araujo, I.K.; Silva de Araujo, B.E.; Sroka, I.; Biesterfeld, S.; Böcking, A.; Schramm, M. Diagnostic accuracy of brush biopsy-based cytology for the early detection of oral cancer and precursors in Fanconi anemia. Cancer Cytopathol. 2020, 128, 403–413. [Google Scholar] [CrossRef]
- Raman, R.K.; Kamboj, M.; Narwal, A. The Diagnostic Role of Methyl Green-Pyronin Y Staining in Oral Leukoplakia and Oral Squamous Cell Carcinoma: An Exfoliative Cytology-Based Cytomorphometric Analysis. Acta Cytol. 2019, 63, 401–410. [Google Scholar] [CrossRef]
- Deuerling, L.; Gaida, K.; Neumann, H.; Remmerbach, T.W. Evaluation of the Accuracy of Liquid-Based Oral Brush Cytology in Screening for Oral Squamous Cell Carcinoma. Cancers 2019, 11, 1813. [Google Scholar] [CrossRef]
- Pereira, T.; Kesarkar, K.; Tamgadge, A.; Bhalerao, S.; Shetty, S. Comparative analysis of oral rinse-based cytology and conventional exfoliative cytology: A pilot study. J. Cancer Res. Ther. 2018, 14, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Kiran, K.; Agarwal, P.; Kumar, S.; Jain, K. Micronuclei as a Predictor for Oral Carcinogenesis. J. Cytol. 2018, 35, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Alsarraf, A.; Kujan, O.; Farah, C.S. Liquid-based oral brush cytology in the diagnosis of oral leukoplakia using a modified Bethesda Cytology system. J. Oral Pathol. Med. 2018, 47, 887–894. [Google Scholar] [CrossRef]
- Remmerbach, T.W.; Pomjanski, N.; Bauer, U.; Neumann, H. Liquid-based versus conventional cytology of oral brush biopsies: A split-sample pilot study. Clin. Oral Investig. 2017, 21, 2493–2498. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Handa, U.; Mohan, H.; Dass, A. Evaluation of Brush Cytology and DNA Image Cytometry for the Detection of Cancer of the Oral Cavity. Diagn. Cytopathol. 2016, 44, 201–205. [Google Scholar] [CrossRef]
- Mulki, S.; Shetty, P.; Pai, P. Oral rinse-based cytology and conventional exfoliative cytology: A comparative study. J. Cancer Res. Ther. 2015, 11, 129–135. [Google Scholar] [CrossRef]
- Rajput, D.V.; Tupkari, J.V. Early detection of oral cancer: PAP and AgNOR staining in brush biopsies. J. Oral Maxillofac. Pathol. JOMFP 2010, 14, 52–58. [Google Scholar]
- Delavarian, Z.; Mohtasham, N.; Mosannen-Mozaffari, P.; Pakfetrat, A.; Shakeri, M.T.; Ghafoorian Maddah, R. Evaluation of the diagnostic value of a Modified Liquid-Based Cytology using OralCDx (R) Brush in early detection of oral potentially malignant lesions and oral cancer. Med. Oral Patol. Oral Cir. Bucal 2010, 15, E671–E676. [Google Scholar] [CrossRef]
- Maraki, D.; Becker, J.; Boecking, A. Cytologic and DNA-cytometric very early diagnosis of oral cancer. J. Oral Pathol. Med. 2004, 33, 398–404. [Google Scholar] [CrossRef]
- Remmerbach, T.W.; Weidenbach, H.; Müller, C.; Hemprich, A.; Pomjanski, N. Buckstegge, B.; Böcking, A. Diagnostic value of nucleolar organizer regions (AgNORs) in brush biopsies of suspicious lesions of the oral cavity. Anal. Cell. Pathol. 2003, 25, 139–146. [Google Scholar] [CrossRef]
- Wang, Z.; Li, F.; Rufo, J.; Chen, C.; Yang, S.; Li, L.; Zhang, J.; Cheng, J.; Kim, Y.; Wu, M.; et al. Acoustofluidic Salivary Exosome Isolation A Liquid Biopsy Compatible Approach for Human Papillomavirus-Associated Oropharyngeal Cancer Detection. J. Mol. Diagn. 2020, 22, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Remmerbach, T.W.; Maurer, K.; Janke, S.; Schellenberger, W.; Eschrich, K.; Bertolini, J.; Hofmann, H.; Rupf, S. Oral brush biopsy analysis by matrix assisted laser desorption/ionisation-time of flight mass spectrometry profiling—A pilot study. Oral Oncol. 2011, 47, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.; Eschrich, K.; Schellenberger, W.; Bertolini, J.; Rupf, S.; Remmerbach, T.W. Oral brush biopsy analysis by MALDI-ToF Mass Spectrometry for early cancer diagnosis. Oral Oncol. 2013, 49, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, M.I.; Morelatto, R.A.; Belardinelli, P.A.; Mosmann, J.M.; Cuffini, C.; de Blanc, S.A.L. Oral Human Papillomavirus: A multisite infection. Med. Oral Patol. Oral Cir. Bucal 2020, 25, E425–E430. [Google Scholar] [CrossRef]
- Zarate, A.M.; Don, J.; Secchi, D.; Carrica, A.; Galindez Costa, F.; Panico, R.; Brusa, M.; Barra, J.L.; Brunotto, M. Study of the TP53 codon 72 polymorphism in oral cancer and oral potentially malignant disorders in Argentine patients. Tumor Biol. 2017, 39, 1010428317699113. [Google Scholar] [CrossRef]
- Jalouli, J.; Ibrahim, S.O.; Sapkota, D.; Jalouli, M.M.; Vasstrand, E.N.; Hirsch, J.M.; Larsson, P.A. Presence of human papilloma virus, herpes simplex virus and Epstein-Barr virus DNA in oral biopsies from Sudanese patients with regard to toombak use. J. Oral Pathol. Med. 2010, 39, 599–604. [Google Scholar] [CrossRef]
- Nunes, D.N.; Kowalski, L.P.; Simpson, A.J.G. Detection of oral and oropharyngeal cancer by microsatellite analysis in mouth washes and lesion brushings. Oral Oncol. 2000, 36, 525–528. [Google Scholar] [CrossRef]
- Harty, L.C.; Shields, P.G.; Winn, D.M.; Caporaso, N.E.; Hayes, R.B. Self-collection of oral epithelial cell DNA under instruction from epidemiologic interviewers. Am. J. Epidemiol. 2000, 151, 199–205. [Google Scholar] [CrossRef][Green Version]
- Parfenova, E.; Liu, K.Y.; Harrison, A.; MacAulay, C.; Guillaud, M.; Poh, C.F. An improved algorithm using a Health Canada-approved DNA-image cytometry system for non-invasive screening of high-grade oral lesions. J. Oral Pathol. Med. 2021, 50, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Lousada-Fernandez, F.; Rapado-Gonzalez, O.; Lopez-Cedrun, J.L.; Lopez-Lopez, R.; Muinelo-Romay, L.; Suarez-Cunqueiro, M.M. Liquid Biopsy in Oral Cancer. Int. J. Mol. Sci. 2018, 19, 1704. [Google Scholar] [CrossRef]
- Hettmann, A.; Demcsák, A.; Bach, Á.; Decsi, G.; Dencs, Á.; Pálinkó, D.; Rovó, L.; Nagy, K.; Minarovits, J.; Takács, M. Detection and Phylogenetic Analysis of Torque Teno Virus in Salivary and Tumor Biopsy Samples from Head and Neck Carcinoma Patients. Intervirology 2016, 59, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Schussel, J.; Zhou, X.C.; Zhang, Z.; Pattani, K.; Bermudez, F.; Jean-Charles, G.; McCaffrey, T.; Padhya, T.; Phelan, J.; Spivakovsky, S.; et al. EDNRB and DCC Salivary Rinse Hypermethylation Has a Similar Performance as Expert Clinical Examination in Discrimination of Oral Cancer/Dysplasia versus Benign Lesions. Clin. Cancer Res. 2013, 19, 3268–3275. [Google Scholar] [CrossRef] [PubMed]
- Kugimoto, T.; Morita, K.I.; Omura, K. Development of oral cancer screening test by detection of squamous cell carcinoma among exfoliated oral mucosal cells. Oral Oncol. 2012, 48, 794–798. [Google Scholar] [CrossRef]
- Majumder, M.; Sikdar, N.; Ghosh, S.; Roy, B. Polymorphisms at XPD and XRCC1 DNA repair loci and increased risk of oral leukoplakia and cancer among NAT2 slow acetylators. Int. J. Cancer 2007, 120, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.J.; Gridley, G.; Harty, L.C.; Diehl, S.R.; Brown, L.M.; Winn, D.M.; Bravo-Otero, E.; Hayes, R.B. Folate intake, serum homocysteine and methylenetetrahydrofolate reductase (MTHFR) C677T genotype are not associated with oral cancer risk in Puerto Rico. J. Nutr. 2002, 132, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Azab, N.A.; Fat’heya, M.Z.; Amin, A.A.W.; Rady, N.H. DNA integrity in diagnosis of premalignant lesions. Med. Oral Patol. Oral Cir. Bucal 2020, 26, e445. [Google Scholar] [CrossRef]
- Tang, K.D.; Baeten, K.; Kenny, L.; Frazer, I.H.; Scheper, G.; Punyadeera, C. Unlocking the Potential of Saliva-Based Test to Detect HPV-16-Driven Oropharyngeal Cancer. Cancers 2019, 11, 473. [Google Scholar] [CrossRef]
- Tang, K.D.; Kenny, L.; Perry, C.; Frazer, I.; Punyadeera, C. The overexpression of salivary cytokeratins as potential diagnostic biomarkers in head and neck squamous cell carcinomas. Oncotarget 2017, 8, 72272–72280. [Google Scholar] [CrossRef]
- Martin, J.L. Validation of Reference Genes for Oral Cancer Detection Panels in a Prospective Blinded Cohort. PLoS ONE 2016, 11, e0158462. [Google Scholar] [CrossRef]
- Zahran, F.; Ghalwash, D.; Shaker, O.; Al-Johani, K.; Scully, C. Salivary microRNAs in oral cancer. Oral Dis. 2015, 21, 739–747. [Google Scholar] [CrossRef]
- Matthews, A.M.; Kaur, H.; Dodd, M.; D’Souza, J.; Liloglou, T.; Shaw, R.J.; Risk, J.M. Saliva collection methods for DNA biomarker analysis in oral cancer patients. Br. J. Oral Maxillofac. Surg. 2013, 51, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Kusukawa, J.; Suefuji, Y.; Ryu, F.; Noguchi, R. Iwamoto, O.; Kameyama, T. Dissemination of cancer cells into circulation occurs by incisional biopsy of oral squamous cell carcinoma. J. Oral Pathol. Med. 2000, 29, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Crimi, S.; Falzone, L.; Gattuso, G.; Grillo, C.M.; Candido, S.; Bianchi, A.; Libra, M. Droplet Digital PCR Analysis of Liquid Biopsy Samples Unveils the Diagnostic Role of hsa-miR-133a-3p and hsa-miR-375-3p in Oral Cancer. Biology 2020, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Conway, D.I.; Robertson, C.; Gray, H.; Young, L.; McDaid, L.M.; Winter, A.J.; Campbell, C.; Pan, J.; Kavanagh, K.; Kean, S.; et al. Human Papilloma Virus (HPV) Oral Prevalence in Scotland (HOPSCOTCH): A Feasibility Study in Dental Settings. PLoS ONE 2016, 11, e0165847. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, C.; Jiang, S.; Kong, X.; Teng, Y.; Niu, B.; Zhu, C.; Xin, W.; Chen, X.; Wen, L.; et al. Ultra-Sensitive and Selective Electrochemical Bio-Fluid Biopsy for Oral Cancer Screening. Small Methods 2021, 5, 2001205. [Google Scholar] [CrossRef]
- Yang, Y.; Rhodus, N.L.; Ondrey, F.G.; Wuertz, B.R.; Chen, X.; Zhu, Y.; Griffin, T.J. Quantitative Proteomic Analysis of Oral Brush Biopsies Identifies Secretory Leukocyte Protease Inhibitor as a Promising, Mechanism-Based Oral Cancer Biomarker. PLoS ONE 2014, 9, e95389. [Google Scholar] [CrossRef]
- Sivadasan, P.; Gupta, M.K.; Sathe, G.; Sudheendra, H.V.; Sunny, S.P.; Renu, D.; Hari, P.S.; Gowda, H.; Suresh, A.; Kuriakose, M.A.; et al. Salivary proteins from dysplastic leukoplakia and oral squamous cell carcinoma and their potential for early detection. J. Proteom. 2020, 212, 103574. [Google Scholar] [CrossRef]
- Shaikh, S.; Yadav, D.K.; Rawal, R. Saliva based non invasive screening of Oral Submucous Fibrosis using ATR-FTIR spectroscopy. J. Pharm. Biomed. Anal. 2021, 203, 114202. [Google Scholar] [CrossRef]
- Sivadasan, P.; Gupta, M.K.; Sathe, G.J.; Balakrishnan, L.; Palit, P.; Gowda, H.; Suresh, A.; Kuriakose, M.A.; Sirdeshmukh, R. Human salivary proteome--a resource of potential biomarkers for oral cancer. J. Proteom. 2015, 127, 89–95. [Google Scholar] [CrossRef]
- Falamas, A.; Faur, C.I.; Ciupe, S.; Chirila, M.; Rotaru, H.; Hedesiu, M.; Pinzaru, S.C. Rapid and noninvasive diagnosis of oral and oropharyngeal cancer based on micro-Raman and FT-IR spectra of saliva. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119477. [Google Scholar] [CrossRef]
- Rai, V.; Mukherjee, R.; Routray, A.; Ghosh, A.K.; Roy, S.; Ghosh, B.P.; Mandal, P.B.; Bose, S.; Chakraborty, C. Serum-based diagnostic prediction of oral submucous fibrosis using FTIR spectrometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Wojakowska, A.; Zebrowska, A.; Skowronek, A.; Rutkowski, T.; Polanski, K.; Widlak, P.; Marczak, L.; Pietrowska, M. Metabolic Profiles of Whole Serum and Serum-Derived Exosomes Are Different in Head and Neck Cancer Patients Treated by Radiotherapy. J. Pers. Med. 2020, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Peisker, A.; Raschke, G.F.; Fahmy, M.D.; Guentsch, A.; Roshanghias, K.; Hennings, J.; Schultze-Mosgau, S. Salivary MMP-9 in the detection of oral squamous cell carcinoma. Med. Oral Patol. Oral Cir. Bucal 2017, 22, E270–E275. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.C.; Campredon, L.P.; Brouwer, A.F.; Walline, H.M.; Marinelli, B.M.; Lau, Y.K.; Thomas, T.B.; Delinger, R.L.; Sullivan, T.S.; Yost, M.L.; et al. Dynamics and Determinants of HPV Infection: The Michigan HPV and Oropharyngeal Cancer (M-HOC) Study. BMJ Open 2018, 8, e021618. [Google Scholar] [CrossRef]
- Gabriel, H.E.; Liu, Z.; Crott, J.W.; Choi, S.W.; Song, B.C.; Mason, J.B.; Johnson, E.J. A comparison of carotenoids, retinoids, and tocopherols in the serum and buccal mucosa of chronic cigarette smokers versus nonsmokers. Cancer Epidemiol. Biomark. Prev. 2006, 15, 993–999. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chinnannavar, S.N.; Ashok, L.; Vidya, K.C.; Setty, S.M.K.; Narasimha, G.E.; Garg, R. Evaluation of serum sialic acid, fucose levels and their ratio in oral squamous cell carcinoma. J. Int. Soc. Prev. Community Dent. 2015, 5, 446–450. [Google Scholar] [CrossRef]
- Ding, Y.; Bu, J.; Tian, J. Significance of Sentinel Lymph Node Biopsy and Immunohistochemistry in Diagnosis and Staging of Stage-cN_0 Oral Squamous Cell Carcinoma. Chin. J. Clin. Oncol. 2007, 34, 640–643. [Google Scholar]
- Chone, C.T.; Aniteli, M.B.; Magalhães, R.S.; Freitas, L.L.; Altemani, A.; Ramos, C.D.; Etchebehere, E.; Crespo, A.N. Impact of immunohistochemistry in sentinel lymph node biopsy in head and neck cancer. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 313–317. [Google Scholar] [CrossRef]
- Mazumder, S.; Datta, S.; Ray, J.G.; Chaudhuri, K.; Chatterjee, R. Liquid biopsy: miRNA as a potential biomarker in oral cancer. Cancer Epidemiol. 2019, 58, 137–145. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B. Updates of liquid biopsy in oral cancer and multiomics analysis. Oral Dis. 2021, 1–11. [Google Scholar] [CrossRef]
- Baby, N.T.; Abdullah, A.; Kannan, S. The scope of liquid biopsy in the clinical management of oral cancer. Int. J. Oral Maxillofac. Surg. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arantes, L.M.R.B.; De Carvalho, A.C.; Melendez, M.E.; Lopes Carvalho, A. Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev. Mol. Diagn. 2018, 18, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Lassig, A.A.D.; Joseph, A.M.; Lindgren, B.R.; Yueh, B. Association of Oral Cavity and Oropharyngeal Cancer Biomarkers in Surgical Drain Fluid With Patient Outcomes. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Zhang, R.; Li, Z.; Li, J. Clinical and biological significance of circulating tumor cells, circulating tumor DNA, and exosomes as biomarkers in colorectal cancer. Oncotarget 2017, 8, 55632–55645. [Google Scholar] [CrossRef]
- Ma, M.; Zhu, H.; Zhang, C.; Sun, X.; Gao, X.; Chen, G. “Liquid biopsy”-ctDNA detection with great potential and challenges. Ann. Transl. Med. 2015, 3, 235. [Google Scholar]
- Patel, S.; Shah, K.; Mirza, S.; Shah, K.; Rawal, R. Circulating tumor stem like cells in oral squamous cell carcinoma: An unresolved paradox. Oral Oncol. 2016, 62, 139–146. [Google Scholar] [CrossRef]
- Gröbe, A.; Blessmann, M.; Hanken, H.; Friedrich, R.E.; Schön, G.; Wikner, J.; Effenberger, K.E.; Kluwe, L.; Heiland, M.; Pantel, K.; et al. Prognostic relevance of circulating tumor cells in blood and disseminated tumor cells in bone marrow of patients with squamous cell carcinoma of the oral cavity. Clin. Cancer Res. 2014, 20, 425–433. [Google Scholar] [CrossRef]
- Inhestern, J.; Oertel, K.; Stemmann, V.; Schmalenberg, H.; Dietz, A.; Rotter, N.; Veit, J.; Görner, M.; Sudhoff, H.; Junghanß, C. Prognostic Role of Circulating Tumor Cells during Induction Chemotherapy Followed by Curative Surgery Combined with Postoperative Radiotherapy in Patients with Locally Advanced Oral and Oropharyngeal Squamous Cell Cancer. PLoS ONE 2015, 10, e0132901. [Google Scholar] [CrossRef]
- Salvi, S.; Gurioli, G.; De Giorgi, U.; Conteduca, V.; Tedaldi, G.; Calistri, D.; Casadio, V. Cell-free DNA as a diagnostic marker for cancer: Current insights. Oncotargets Ther. 2016, 9, 6549–6559. [Google Scholar] [CrossRef]
- Van Ginkel, J.H.; Slieker, F.J.; de Bree, R.; van Es, R.J.; Van Cann, E.M.; Willems, S.M. Cell-free nucleic acids in body fluids as biomarkers for the prediction and early detection of recurrent head and neck cancer: A systematic review of the literature. Oral Oncol. 2017, 75, 8–15. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xia, W.; Lv, Z.; Xin, Y.; Ni, C.; Yang, L. Liquid Biopsy for Cancer: Circulating Tumor Cells, Circulating Free DNA or Exosomes? Cell. Physiol. Biochem. 2017, 41, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Lee, M.; Jeffrey, S.S. Circulating Tumor Cells and Circulating Tumor DNA: Challenges and Opportunities on the Path to Clinical Utility. Clin. Cancer Res. 2015, 21, 4786–4800. [Google Scholar] [CrossRef] [PubMed]
- Rodda, A.E.; Parker, B.J.; Spencer, A.; Corrie, S.R. Extending Circulating Tumor DNA Analysis to Ultralow Abundance Mutations: Techniques and Challenges. ACS Sens. 2018, 3, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.R.; Leshchiner, I.; Elagina, L.; Goyal, L.; Levovitz, C.; Siravegna, G.; Livitz, D.; Rhrissorrakrai, K.; Martin, E.E.; Van Seventer, E.E.; et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 2019, 25, 1415–1421. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Burton, K.A.; Blau, S.; Rose, A.L.; Parker, S.; Lyman, G.H.; Blau, C.A. Comparison of 2 Commercially Available Next-Generation Sequencing Platforms in Oncology. JAMA Oncol. 2017, 3, 996–998. [Google Scholar] [CrossRef]
- Chae, Y.K.; Davis, A.A.; Carneiro, B.A.; Chandra, S.; Mohindra, N.; Kalyan, A.; Kaplan, J.; Matsangou, M.; Pai, S.; Costa, R.; et al. Concordance between genomic alterations assessed by next-generation sequencing in tumor tissue or circulating cell-free DNA. Oncotarget 2016, 7, 65364–65373. [Google Scholar] [CrossRef]
- Upile, T.; Fisher, C.; Jerjes, W.; El Maaytah, M.; Singh, S.; Sudhoff, H.; Searle, A.; Archer, D.; Michaels, L.; Hopper, C.; et al. Recent technological developments: In Situ histopathological interrogation of surgical tissues and resection margins. Head Face Med. 2007, 3, 1–12. [Google Scholar] [CrossRef][Green Version]
- Momen-Heravi, F.; Trachtenberg, A.J.; Kuo, W.P.; Cheng, Y.S. Genomewide Study of Salivary MicroRNAs for Detection of Oral Cancer. J. Dent. Res. 2014, 93, 86S–93S. [Google Scholar] [CrossRef]
- Park, N.J.; Zhou, H.; Elashoff, D.; Henson, B.S.; Kastratovic, D.A.; Abemayor, E.; Wong, D.T. Salivary microRNA: Discovery, characterization, and clinical utility for oral cancer detection. Clin. Cancer Res. 2009, 15, 5473–5477. [Google Scholar] [CrossRef]
- Ilié, M.; Hofman, P. Pros: Can tissue biopsy be replaced by liquid biopsy? Transl. Lung Cancer Res. 2016, 5, 420–423. [Google Scholar] [CrossRef]
- Naugler, C. Practice tips. Brush biopsy sampling of oral lesions. Can. Fam. Physician 2008, 54, 194. [Google Scholar]
- Mehrotra, R.; Singh, M.K.; Pandya, S.; Singh, M. The use of an oral brush biopsy without computer-assisted analysis in the evaluation of oral lesions: A study of 94 patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Fedele, S. Diagnostic aids in the screening of oral cancer. Head Neck Oncol. 2009, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Drinnan, A.J. Screening for oral cancer and precancer--a valuable new technique. Gen. Dent. 2000, 48, 656–660. [Google Scholar]
- Hartmann, S.; Kipke, R.U.; Rauthe, S.; Mutzbauer, G.; Brands, R.C.; Ebhardt, H.; Kübler, A.C.; Müller-Richter, U.D. Oral brush biopsy and melanoma-associated antigens A (MAGE-A) staining in clinically suspicious lesions. J. Cranio-Maxillofac. Surg. 2015, 43, 2214–2218. [Google Scholar] [CrossRef] [PubMed]
- Kujan, O.; Huang, G.; Ravindran, A.; Vijayan, M.; Farah, C.S. CDK4, CDK6, cyclin D1 and Notch1 immunocytochemical expression of oral brush liquid-based cytology for the diagnosis of oral leukoplakia and oral cancer. J. Oral Pathol. Med. 2019, 48, 566–573. [Google Scholar] [CrossRef]
- Hall, W.; Ogden, G.R.; Saleh, A.H.; Hopwood, D.; Ross, P.E. Fluid phase endocytosis in oral epithelia: Variation with site and effect of cancer. J. Oral Pathol. Med. 2000, 29, 220–225. [Google Scholar] [CrossRef]
- Shah, S.N.; Patel, F.H.; Patil, N.; Patel, U. Evaluation of Diagnostic Value of Sediment Cytology in Oral Malignant and Oral Potentially Malignant Disorders. J. Clin. Diagn. Res. 2021, 15, 24–26. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Deng, Y.; Shen, X.; Shi, L.; Liu, W. Development and validation of a risk model for noninvasive detection of cancer in oral potentially malignant disorders using DNA image cytometry. Cancer Biol. Med. 2021, 18, 763–771. [Google Scholar] [CrossRef]
- Galíndez, M.F.; Carrica, A.; Zarate, A.M.; Secchi, D.; Don, J.; Barra, J.L.; Brunotto, M. DNA repair, NFKβ, and TP53 polymorphisms associated with potentially malignant disorders and oral squamous cell carcinoma in Argentine patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Aaboubout, Y.; Barroso, E.M.; Algoe, M.; Ewing-Graham, P.C.; Hove, I.T.; Mast, H.; Hardillo, J.A.; Sewnaik, A.; Monserez, D.A.; Keereweer, S.; et al. Intraoperative Assessment of Resection Margins in Oral Cavity Cancer: This is the Way. J. Vis. Exp. 2021, 171, e62446. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Lu, M.; Wang, L.; Jiang, Z.; Wu, M.; Li, J.; Hu, Z.; Cheng, X.; Li, T.; Zhang, Z.; et al. Contrast-Enhanced Ultrasound Guided Transoral Core Needle Biopsy: A Novel, Safe and Well-Tolerated Procedure for Obtaining High-Quality Tissue in Patients with Oral Cancer. Ultrasound Med. Biol. 2020, 46, 3210–3217. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.W.H.; Van Lanschot, C.G.F.; Aaboubout, Y.; De Ridder, M.; Hegt, V.N.; Barroso, E.M.; Meeuwis, C.A.; Sewnaik, A.; Hardillo, J.A.; Monserez, D.; et al. Intraoperative Assessment of the Resection Specimen Facilitates Achievement of Adequate Margins in Oral Carcinoma. Front. Oncol. 2020, 10, 14593. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, K.; Girija, K.; Venugopal, M.; Thomas, V.; Ramachandran, S.; Asish, R. Cervical lymph node evaluation in oral squamous cell carcinoma patients using ultrasound-guided fine-needle aspiration cytology—A descriptive diagnostic evaluation study in a tertiary care center. Contemp. Clin. Dent. 2020, 11, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, L.; Deng, Y.; Shen, X.; Liu, W.; Shi, L. DNA aneuploidy with image cytometry for detecting dysplasia and carcinoma in oral potentially malignant disorders: A prospective diagnostic study. Cancer Med. 2020, 9, 6411–6420. [Google Scholar] [CrossRef]
- Hasegawa, H.; Kaneko, T.; Kanno, C.; Endo, M.; Yamazaki, M.; Kitabatake, T.; Monma, T.; Takeishi, E.; Sato, E.; Kano, M. Preoperative intra-arterial chemotherapy with docetaxel, cisplatin, and peplomycin combined with intravenous chemotherapy using 5-fluorouracil for oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2020, 49, 984–992. [Google Scholar] [CrossRef]
- Durham, J.S.; Brasher, P.; Anderson, D.W.; Yoo, J.; Hart, R.; Dort, J.C.; Seikaly, H.; Kerr, P.; Rosin, M.P.; Poh, C.F. Effect of Fluorescence Visualization-Guided Surgery on Local Recurrence of Oral Squamous Cell Carcinoma A Randomized Clinical Trial. Jama Otolaryngol. Head Neck Surg. 2020, 146, 1149–1155. [Google Scholar] [CrossRef]
- Aggarwal, N.; Panja, T.; Dutta, S.; Sinha, R.; Mittal, A. Evaluation of the Role of Toluidine Blue Paint as an Adjunctive Method to Biopsy in Suspicious Oral Lesion: A Hospital Based Study. Indian J. Otolaryngol. Head Neck Surg. 2020, 1–8. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, L.; Wang, C.; Qian, L.; Yang, J.; Zhao, Z.; Fan, Y.; Peng, Z. Assessment of the cancerization risk for oral potentially malignant disorders by clinical risk model combined with autofluorescence and brush biopsy with DNA-image cytometry. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 2549–2557. [Google Scholar] [CrossRef]
- Tandon, N.; Mishra, N.; Fatima, N.; Srivastava, A.; Lal, N.; Kumar, V. Immunohistochemical expression of human telomerase reverse transcriptase in oral cancer and precancer: A case–control study. J. Oral Maxillofac. Pathol. 2019, 23, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Gnanatheepam, E.; Kanniyappan, U.; Dornadula, K.; Prakasarao, A.; Singaravelu, G. Synchronous Luminescence Spectroscopy as a Tool in the Discrimination and Characterization of Oral Cancer Tissue. J. Fluoresc. 2019, 29, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Al-Dam, A.; Precht, C.; Barbe, A.; Kohlmeier, C.; Hanken, H.; Wikner, J.; Schön, G.; Heiland, M.; Assaf, A.T. Sensitivity and specificity of sentinel lymph node biopsy in patients with oral squamous cell carcinomas using indocyanine green fluorescence imaging. J. Cranio-Maxillofac. Surg. 2018, 46, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, F.; Ebrahimi, R.; Andisheh-Tadbir, A.; Ashraf, M.J.; Khademi, B. Evaluation of the Ki-67 and MCM3 Expression in Cytologic Smear of Oral Squamous Cell Carcinoma. J. Dent. 2017, 18, 207–211. [Google Scholar]
- Perera, M.; Al-Hebshi, N.N.; Perera, I.; Ipe, D.; Ulett, G.C.; Speicher, D.J.; Chen, T.; Johnson, N.W. A dysbiotic mycobiome dominated by Candida albicans is identified within oral squamous-cell carcinomas. J. Oral Microbiol. 2017, 9, 1385369. [Google Scholar] [CrossRef]
- Parakh, M.K.; Reddy, R.C.J.; Subramani, P. Toluidine blue staining in identification of a biopsy site in potentially malignant lesions: A case–control study. Asia-Pac. J. Oncol. Nurs. 2017, 4, 356–360. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kawaguchi, K.; Fujihara, H.; Hasebe, M.; Kishi, Y.; Yasukawa, M.; Kumagai, K.; Hamada, Y. Detection accuracy for epithelial dysplasia using an objective autofluorescence visualization method based on the luminance ratio. Int. J. Oral Sci. 2017, 9, 200–209. [Google Scholar] [CrossRef]
- Nair, K.K. Estimation of Serum Butyryl Cholinesterase in Patients with Oral Squamous Cell Carcinoma: A Cross-Sectional Study. J. Clin. Diagn. Res. 2017, 11, ZC59–ZC62. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sugimoto, M.; Kitabatake, K.; Tu, M.; Sugano, A.; Yamamori, I.; Iba, A.; Yusa, K.; Kaneko, M.; Ota, S.; et al. Effect of timing of collection of salivary metabolomic biomarkers on oral cancer detection. Amino Acids 2017, 49, 761–770. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Arora, S.K.; Kumar, G.; Sarin, D. Isolated Perifacial Lymph Node Metastasis in Oral Squamous Cell Carcinoma With Clinically Node-Negative Neck. Laryngoscope 2016, 126, 2252–2256. [Google Scholar] [CrossRef]
- Suominen, S.; Acarturk, T.O.; Bäck, L.; Lassus, P.; Vuola, J.; Mäkitie, A.; Husso, A. Submental Artery Flap with Sentinel Lymph Node Biopsy in the Reconstruction of Oral Cancer. J. Reconstr. Microsurg. 2015, 32, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Lejoy, A.; Arpita, R.; Krishna, B.; Venkatesh, N.; Abraham, L.; Rai, A.; Burde, K.; Naikmasur, V. Methylene Blue as a Diagnostic Aid in the Early Detection of Potentially Malignant and Malignant Lesions of Oral Mucosa. Ethiop. J. Heal. Sci. 2016, 26, 201–208. [Google Scholar] [CrossRef]
- Sagheb, K.; Sagheb, K.; Rahimi-Nedjat, R.; Taylor, K.; Al-Nawas, B.; Walter, C. Sentinel lymph node biopsy in T1/T2 squamous cell carcinomas of the tongue: A prospective study. Oncol. Lett. 2016, 11, 600–604. [Google Scholar] [CrossRef]
- Graham, K.A.; Mulhall, H.J.; Labeed, F.H.; Lewis, M.P.; Hoettges, K.F.; Kalavrezos, N.; McCaul, J.; Liew, C.; Porter, S.; Fedele, S.; et al. A dielectrophoretic method of discrimination between normal oral epithelium, and oral and oropharyngeal cancer in a clinical setting. Analyst 2015, 140, 5198–5204. [Google Scholar] [CrossRef]
- Chianeh, Y.R.; Manjunath, R.; Prabhu, K.; Fernandes, N.; Vidyasagar, M.; Kamath, A. Protein Thiols and Butryrylcholinestrase in Saliva of Oral Cancer Patients. Indian J. Clin. Biochem. 2013, 29, 238–241. [Google Scholar] [CrossRef][Green Version]
- Riaz, A.; Shreedhar, B.; Kamboj, M.; Natarajan, S. Methylene blue as an early diagnostic marker for oral precancer and cancer. SpringerPlus 2013, 2, 95. [Google Scholar] [CrossRef]
- Mori, K.; Sato, S.; Kodama, M.; Habu, M.; Takahashi, O.; Nishihara, T.; Tominaga, K.; Takenaka, S. Oral Cancer Diagnosis via a Ferrocenylnaphthalene Diimide–Based Electrochemical Telomerase Assay. Clin. Chem. 2013, 59, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kämmerer, P.W.; Koch, F.P.; Santoro, M.; Babaryka, G.; Biesterfeld, S.; Brieger, J.; Kunkel, M. Prospective, blinded comparison of cytology and DNA-image cytometry of brush biopsies for early detection of oral malignancy. Oral Oncol. 2013, 49, 420–426. [Google Scholar] [CrossRef]
- Gallè, F.; Colella, G.; Di Onofrio, V.; Rossiello, R.; Angelillo, I.F.; Liguori, G. Candida spp. in oral cancer and oral precancerous lesions. New Microbiol. 2013, 36, 283–288. [Google Scholar]
- Flach, G.B.; Tenhagen, M.; de Bree, R.; Brakenhoff, R.H.; van der Waal, I.; Bloemena, E.; Kuik, D.J.; Castelijns, J.A.; Leemans, C.R. Outcome of patients with early stage oral cancer managed by an observation strategy towards the N0 neck using ultrasound guided fine needle aspiration cytology: No survival difference as compared to elective neck dissection. Oral Oncol. 2013, 49, 157–164. [Google Scholar] [CrossRef]
- Cankovic, M.; Ilic, M.P.; Vuckovic, N.; Bokor-Bratic, M. The histological characteristics of clinically normal mucosa adjacent to oral cancer. J. Cancer Res. Ther. 2013, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Hasegawa, Y.; Matsuzuka, T.; Shiotani, A.; Takahashi, K.; Kohno, N.; Yoshida, T.; Kitano, H. Sentinel node biopsy for oral and laryngopharyngeal squamous cell carcinoma: A retrospective study of 177 patients in Japan. Auris Nasus Larynx 2012, 39, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Meric, A.; Özücer, B.; Gedikli, O.; Yildirim, Y.; Korkut, A.Y.; Kahya, V.; Eren, S.B.; Somay, A. Impact of Smoking on p65 Nuclear Factor κB, p38 Mitogen-Activated Protein Kinase, and Inducible Nitric Oxide Synthase Expression Levels in Oral Mucosa. J. Craniofac. Surg. 2012, 23, 970–973. [Google Scholar] [CrossRef] [PubMed]
- MacAulay, C.; Poh, C.F.; Guillaud, M.; Williams, P.M.; Laronde, D.M.; Zhang, L.; Rosin, M.P. High throughput image cytometry for detection of suspicious lesions in the oral cavity. J. Biomed. Opt. 2012, 17, 0860041–08600411. [Google Scholar] [CrossRef]
- Lohavanichbutr, P.; Houck, J.; Doody, D.R.; Wang, P.; Méndez, E.; Futran, N.; Upton, M.P.; Holsinger, F.C.; Schwartz, S.M.; Chen, C. Gene Expression in Uninvolved Oral Mucosa of OSCC Patients Facilitates Identification of Markers Predictive of OSCC Outcomes. PLoS ONE 2012, 7, e46575. [Google Scholar] [CrossRef]
- Paderni, C.; Compilato, D.; Carinci, F.; Nardi, G.; Rodolico, V.; Muzio, L.L.; Spinelli, G.; Mazzotta, M.; Campisi, G. Direct Visualization of Oral-Cavity Tissue Fluorescence as Novel Aid for Early Oral Cancer Diagnosis and Potentially Malignant Disorders Monitoring. Int. J. Immunopathol. Pharmacol. 2011, 24, 121–128. [Google Scholar] [CrossRef]
- Kolokythas, A.; Schwartz, J.L.; Pytynia, K.B.; Panda, S.; Yao, M.; Homann, B.; Sroussi, H.Y.; Epstein, J.B.; Gordon, S.C.; Adami, G.R. Analysis of RNA from brush cytology detects changes in B2M, CYP1B1 and KRT17 levels with OSCC in tobacco users. Oral Oncol. 2011, 47, 532–536. [Google Scholar] [CrossRef]
- Weigum, S.; Floriano, P.N.; Redding, S.W.; Yeh, C.-K.; Westbrook, S.D.; McGuff, H.S.; Lin, A.; Miller, F.R.; Villarreal, F.; Rowan, S.D.; et al. Nano-Bio-Chip Sensor Platform for Examination of Oral Exfoliative Cytology. Cancer Prev. Res. 2010, 3, 518–528. [Google Scholar] [CrossRef]
- Hohlweg-Majert, B.; Deppe, H.; Metzger, M.C.; Schumm, S.; Hoefler, H.; Kesting, M.R.; Hölzle, F.; Wolff, K.-D. Sensitivity and Specificity of Oral Brush Biopsy. Cancer Investig. 2009, 27, 293–297. [Google Scholar] [CrossRef]
- Sanjay, P.R.; Hallikeri, K.; Shivashankara, A. Evaluation of salivary sialic acid, total protein, and total sugar in oral cancer: A preliminary report. Indian J. Dent. Res. 2008, 19, 288–291. [Google Scholar]
- Navone, R.; Pentenero, M.; Rostan, I.; Burlo, P.; Marsico, A.; Broccoletti, R.; Scully, C.; Gandolfo, S. Oral potentially malignant lesions: First-level micro-histological diagnosis from tissue fragments sampled in liquid-based diagnostic cytology. J. Oral Pathol. Med. 2008, 37, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Hirshberg, A.; Yarom, N.; Amariglio, N.; Yahalom, R.; Adam, I.; Stanchescu, R.; Ben-Dov, I.; Taicher, S.; Rechavi, G.; Trakhtenbrot, L. Detection of non-diploid cells in premalignant and malignant oral lesions using combined morphological and FISH analysis—A new method for early detection of suspicious oral lesions. Cancer Lett. 2007, 253, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Lin, J.-S.; Wu, C.-H.; Lui, M.-T.; Kao, S.-Y.; Fong, Y. Application of In Vivo Stain of Methylene Blue as a Diagnostic Aid in the Early Detection and Screening of Oral Squamous Cell Carcinoma and Precancer Lesions. J. Chin. Med. Assoc. 2007, 70, 497–503. [Google Scholar] [CrossRef]
- Songra, A.; Ng, S.; Farthing, P.; Hutchison, I.; Bradley, P. Observation of tumour thickness and resection margin at surgical excision of primary oral squamous cell carcinoma—Assessment by ultrasound. Int. J. Oral Maxillofac. Surg. 2006, 35, 324–331. [Google Scholar] [CrossRef]
- Poh, C.F.; Zhang, L.; Anderson, D.W.; Durham, J.S.; Williams, P.M.; Priddy, R.W.; Berean, K.W.; Ng, S.; Tseng, O.L.; MacAulay, C.; et al. Fluorescence Visualization Detection of Field Alterations in Tumor Margins of Oral Cancer Patients. Clin. Cancer Res. 2006, 12, 6716–6722. [Google Scholar] [CrossRef]
- Kujan, O.; Desai, M.; Sargent, A.; Bailey, A.; Turner, A.; Sloan, P. Potential applications of oral brush cytology with liquid-based technology: Results from a cohort of normal oral mucosa. Oral Oncol. 2006, 42, 810–818. [Google Scholar] [CrossRef]
- Khafif, A.; Schneebaum, S.; Fliss, D.M.; Lerman, H.; Metser, U.; Ben-Yosef, R.; Gil, Z.; Reider-Trejo, L.; Genadi, L.; Even-Sapir, E. Lymphoscintigraphy for sentinel node mapping using a hybrid single photon emission CT (SPECT)/CT system in oral cavity squamous cell carcinoma. Head Neck-J. Sci. Spec. Head Neck 2006, 28, 874–879. [Google Scholar] [CrossRef]
- Myo, K.; Uzawa, N.; Miyamoto, R.; Sonoda, I.; Yuki, Y.; Amagasa, T. Cyclin D1 gene numerical aberration is a predictive marker for occult cervical lymph node metastasis in TNM Stage I and II squamous cell carcinoma of the oral cavity. Cancer 2005, 104, 2709–2716. [Google Scholar] [CrossRef]
- Minamikawa, T.; Umeda, M.; Komori, T. Reliability of sentinel lymph node biopsy with squamous cell carcinoma of the oral cavity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 532–538. [Google Scholar] [CrossRef]
- Hsu, E.R.; Gillenwater, A.M.; Richards-Kortum, R.R. Detection of the Molecular Changes Associated with Oral Cancer Using a Molecular-Specific Fluorescent Contrast Agent and Single-Wavelength Spectroscopy. Appl. Spectrosc. 2005, 59, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.J.; Epstein, J.B.; Morton, T.H.; Schwartz, S.M. Reliability of histologic diagnosis of clinically normal intraoral tissue adjacent to clinically suspicious lesions in former upper aerodigestive tract cancer patients. Oral Oncol. 2005, 41, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Hamakawa, H.; Onishi, A.; Sumida, T.; Terakado, N.; Hino, S.; Nakashiro, K.-I.; Shintani, S. Intraoperative real-time genetic diagnosis for sentinel node navigation surgery. Int. J. Oral Maxillofac. Surg. 2004, 33, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Feldman, R.; Dolor, R.; Porter, S.R. The utility of tolonium chloride rinse in the diagnosis of recurrent or second primary cancers in patients with prior upper aerodigestive tract cancer. Head Neck 2003, 25, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.A.; Dünne, A.A.; Ramaswamy, A.; Folz, B.J.; Brandt, D.; Külkens, C.; Moll, R.; Lippert, B.M. Number and location of radiolabeled, intraoperatively identified sentinel nodes in 48 head and neck cancer patients with clinically staged N0 and N1 neck. Eur. Arch. Oto-Rhino-Laryngol. 2002, 259, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Betz, C.S.; Stepp, H.; Janda, P.; Arbogast, S.; Grevers, G.; Baumgartner, R.; Leunig, A. A comparative study of normal inspection, autofluorescence and 5-ALA-induced PPIX fluorescence for oral cancer diagnosis. Int. J. Cancer 2002, 97, 245–252. [Google Scholar] [CrossRef]
- Epstein, J.B.; Oakley, C.; Millner, A.; Emerton, S.; van der Meij, E.; Le, N. The utility of toluidine blue application as a diagnostic aid in patients previously treated for upper oropharyngeal carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1997, 83, 537–547. [Google Scholar] [CrossRef]
- Cox, M.; Maitlan, N.; Scully, C. Human herpes simplex-1 and papillomavirus type 16 homologous DNA sequences in normal, potentially malignant and malignant oral mucosa. Oral Oncol. 1993, 29, 215–219. [Google Scholar] [CrossRef]
- Abdulkader, M.; Ravindran, A.; Sudha, L.; Vijayakumar, T.; Vasudevan, D.M. Demonstration of a tumour associated antigen in human oral cancers. Indian J. Med. Res. 1981, 74, 428–432. [Google Scholar]
- Benson, J.W. Combined chemotherapy and cryosurgery for oral cancer. Am. J. Surg. 1975, 130, 596–600. [Google Scholar] [CrossRef]
- Dunn, R.J.; Devine, K.D. Tetracycline-induced fluorescence of laryngeal, pharyngeal, and oral cancer. Laryngoscope 1972, 82, 189–198. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).