The Effect of Different Extraction Conditions on the Physicochemical Properties of Novel High Methoxyl Pectin-like Polysaccharides from Green Bell Pepper (GBP)
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Experimental Design
2.3. Polysaccharide Extraction Process
2.4. Determination of Neutral Sugar and Galacturonic Contents
2.5. Degree of Methyl Esterification (DM) of GBP Polysaccharides
2.6. Determination of the Protein and Phenolic Contents of GBP Polysaccharides
2.7. Degree of Acetylation of GBP Polysaccharides
2.8. DPPH Radical Scavenging Activities of GBP Polysaccharides
2.9. Emulsifying Properties of GBP Polysaccharides
2.10. Measurement of Intrinsic Viscosity and Molecular Weight of GBP Polysaccharides
2.11. Statistical Analysis
3. Results and Discussion
3.1. Yield and Physicochemical Properties of GBP Polysaccharides
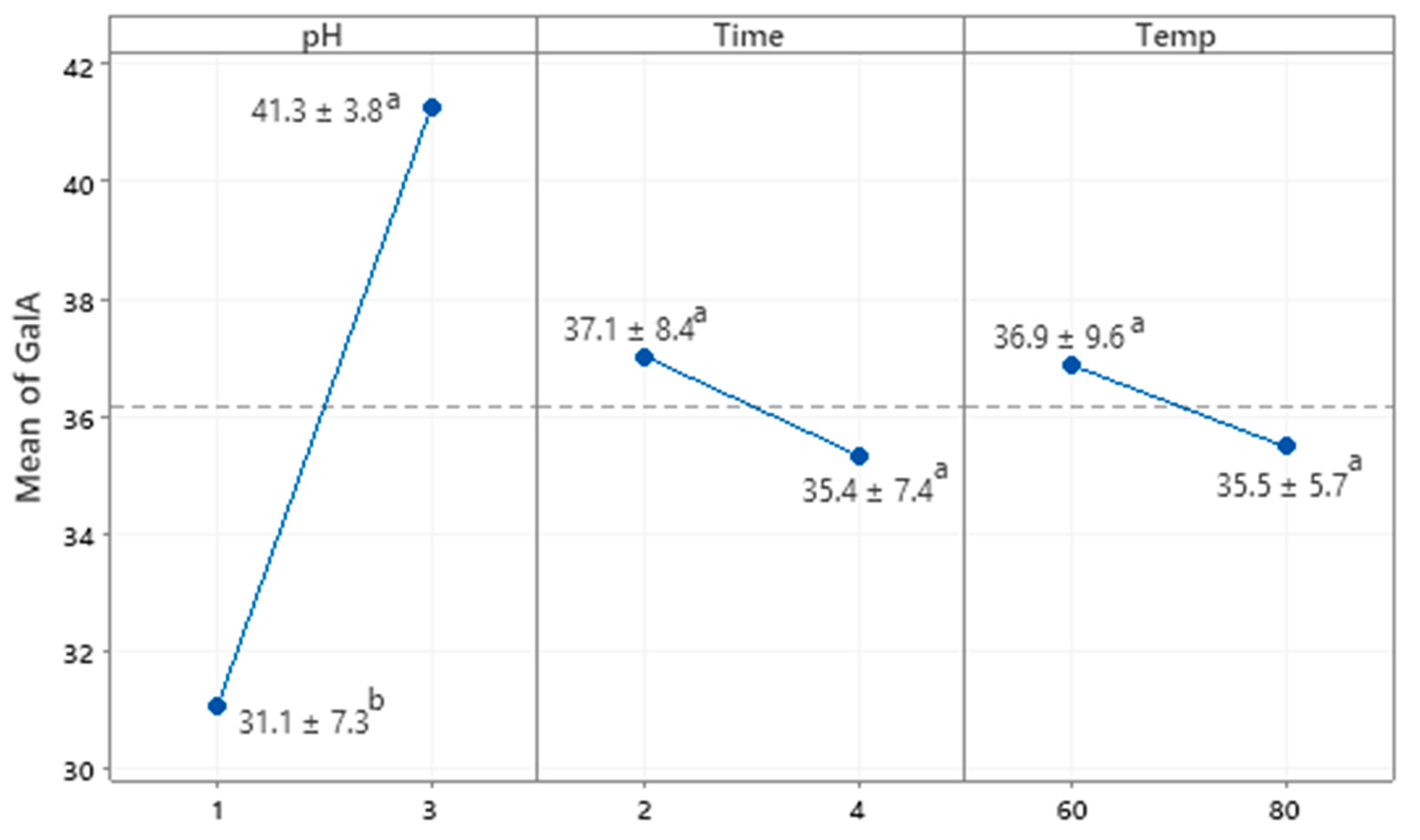
3.1.1. Neutral Sugar Ratios
3.1.2. Total Protein Content of GBP Polysaccharides
3.1.3. Total Phenolic Content of GBP Polysaccharides
3.1.4. Degree of Acetylation (DA) of GBP Polysaccharides
3.1.5. Degree of Methyl Esterification (DM) of GBP Polysaccharides
3.1.6. Intrinsic Viscosity of GBPP and the Molecular Weight of GBP Polysaccharides
3.2. Functional Properties of GBP Polysaccharides
3.2.1. Emulsifying Activity (EA) and Emulsion Stability (ES) of GBP Polysaccharides
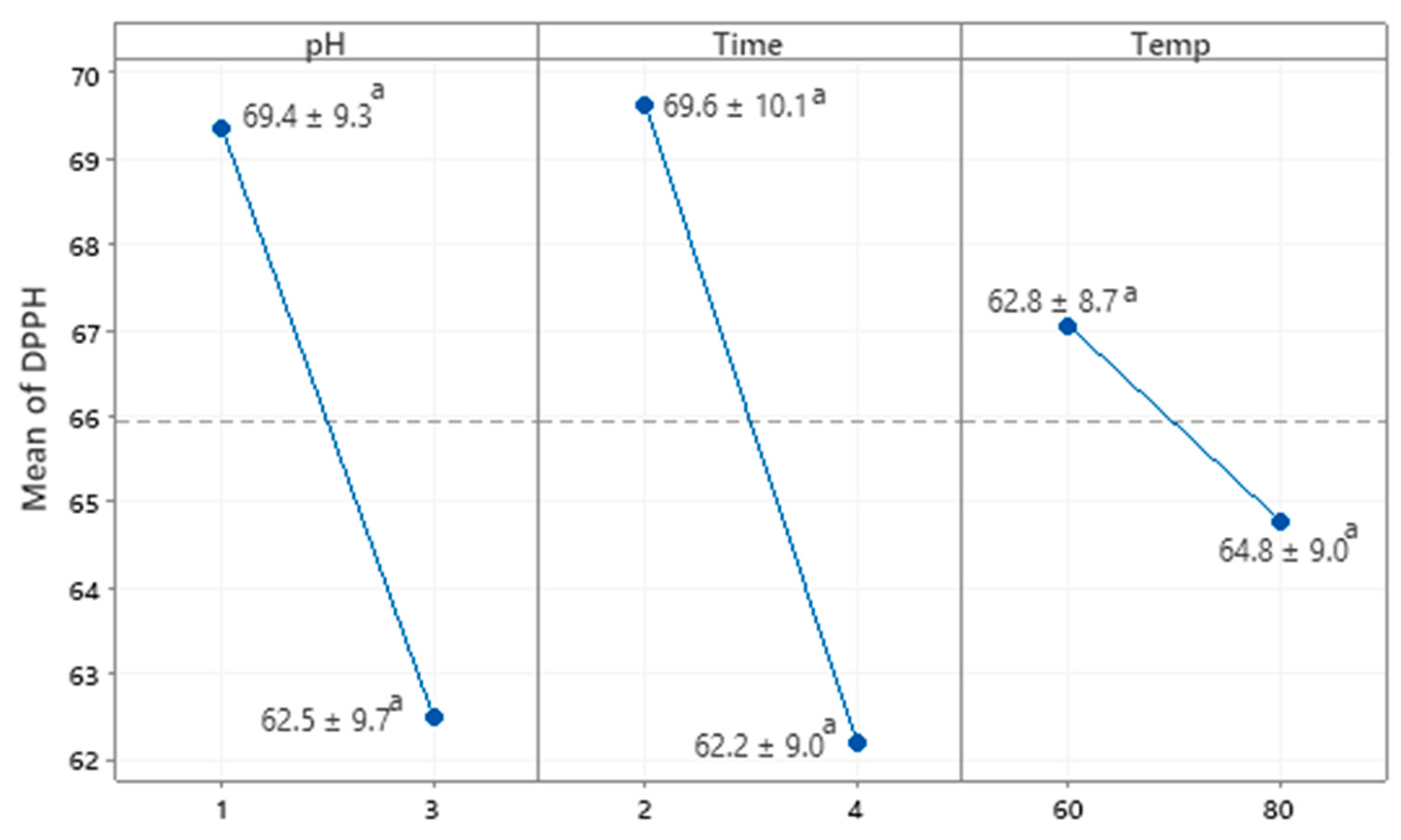
3.2.2. Antioxidant Properties of GBP Polysaccharides
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Pilnik, W.; Voragen, A.G.J. Pectin substances and other uronides. In The Biochemistry of Fruits and Their Products; Hulme, A.C., Ed.; Academic Press: New York, NY, USA, 1970; pp. 53–87. [Google Scholar]
- Rombouts, F.M.; Thibault, J.-F. Sugar beet pectins: Chemical structure and gelation through oxidative coupling. In Chemistry and Function of Pectins; Fishman, M.L., Jen, J.J., Eds.; American Chemical Society: Washington, DC, USA, 1986; pp. 49–60. [Google Scholar]
- Moelants, K.R.N.; Jolie, R.P.; Palmers, S.K.J.; Cardinaels, R.; Christiaens, S.; Van Buggenhout, S.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. The effects of process-induced pectin changes on the viscosity of carrot and tomato sera. Food Bioprocess Technol. 2013, 6, 2870–2883. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Niyigaba, T.; Diru, L.; de Dieu Habimana, J. The extraction, functionalities and applications of plant polysaccharides in fermented foods: A review. Foods 2021, 10, 3004. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Chen, X.; Zhang, J.; Zhang, Y. Dynamic changes in flavonoid, phenolic, and polysaccharide contents in leaves and fruits of sea buckthorn during the growing season in southeastern Tibet plateau. Sci. Hortic. 2023, 307, 111497. [Google Scholar] [CrossRef]
- Dong, T.T.X.; Cui, X.M.; Song, Z.H.; Zhao, K.J.; Ji, Z.N.; Lo, C.K.; Tsim, K.W.K. Chemical assessment of roots of Panax notoginseng in China: Regional and seasonal variations in its active constituents. J. Agric. Food Chem. 2003, 51, 4617–4623. [Google Scholar] [CrossRef]
- Kpodo, F.; Agbenorhevi, J.; Alba, K.; Bingham, R.; Oduro, I.; Morris, G.; Kontogiorgos, V. Pectin isolation and characterization from six okra genotypes. Food Hydrocoll. 2017, 72, 323–330. [Google Scholar] [CrossRef]
- Deng, L.-Z.; Pan, Z.; Zhang, Q.; Liu, Z.-L.; Zhang, Y.; Meng, J.-S.; Gao, Z.-J.; Xiao, H.-W. Effects of ripening stage on physicochemical properties, drying kinetics, pectin polysaccharides contents and nanostructure of apricots. Carbohydr. Polym. 2019, 222, 114980. [Google Scholar] [CrossRef]
- May, C.D. Industrial pectin: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Narasimman, P.; Sethuraman, P. An overview on the fundamentals of pectin. Int. J. Adv. Res. 2016, 4, 1855–1860. [Google Scholar] [CrossRef]
- Morris, G.A.; Binhamad, H.A. Isolation and characterisation of pectin. In Pectin: Technological and Physiological Properties; Springer: Berlin/Heidelberg, Germany, 2020; pp. 61–82. [Google Scholar]
- Alba, K.; Ritzoulis, C.; Georgiadis, N.; Kontogiorgos, V. Okra extracts as emulsifiers for acidic emulsions. Food Res. Int. 2013, 54, 1730–1737. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Schmidt, K.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Pectin of different origin and their performance in forming and stabilizing oil-in-water-emulsions. Food Hydrocoll. 2015, 46, 59–66. [Google Scholar] [CrossRef]
- Caroço, R.F.; Kim, B.; Santacoloma, P.A.; Abildskov, J.; Lee, J.H.; Huusom, J.K. Analysis and model-based optimization of a pectin extraction process. J. Food Eng. 2019, 244, 159–169. [Google Scholar] [CrossRef]
- Ghori, M.U.; Alba, K.; Smith, A.M.; Conway, B.R.; Kontogiorgos, V. Okra extracts in pharmaceutical and food applications. Food Hydrocoll. 2014, 42, 342–347. [Google Scholar] [CrossRef]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Oduro, I.N.; Morris, G.A.; Kontogiorgos, V. Structure-function relationships in pectin emulsification. Food Biophys. 2018, 13, 71–79. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, G.E.; Iacomini, M.; Cordeiro, L.M.C. New findings on green sweet pepper (Capsicum annum) pectins: Rhamnogalacturonan and type I and II arabinogalactans. Carbohydr. Polym. 2017, 171, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tai, K.; Wei, T.; Yuan, F.; Gao, Y. Physicochemical and in vitro antioxidant properties of pectin extracted from hot pepper (Capsicum annuum L. var. acuminatum (Fingerh.)) residues with hydrochloric and sulfuric acids. J. Sci. Food Agric. 2017, 97, 4953–4960. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Grønhaug, T.E.; Ghildyal, P.; Barsett, H.; Michaelsen, T.E.; Morris, G.; Diallo, D.; Inngjerdingen, M.; Paulsen, B.S. B ioactive arabinogalactans from the leaves of Opilia celtidifolia Endl. ex Walp. (Opiliaceae). Glycobiology 2010, 20, 1654–1664. [Google Scholar] [CrossRef]
- Adams, G.G.; Imran, S.; Wang, S.; Mohammad, A.; Kok, S.; Gray, D.A.; Channell, G.A.; Morris, G.A.; Harding, S.E. The hypoglycaemic effect of pumpkins as anti-diabetic and functional medicines. Food Res. Int. 2011, 44, 862–867. [Google Scholar] [CrossRef]
- Denman, L.J.; Morris, G.A. An experimental design approach to the chemical characterisation of pectin polysaccharides extracted from Cucumis melo Inodorus. Carbohydr. Polym. 2015, 117, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Jorge, N.; Veronezi, C.M.; Pereira, D.C. Extracts of red peppers: Antioxidant activity and sensory evaluation. Nutr. Food Sci. 2016, 46, 228–236. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Ferarsa, S.; Zhang, W.; Moulai-Mostefa, N.; Ding, L.; Jaffrin, M.Y.; Grimi, N. Recovery of anthocyanins and other phenolic compounds from purple eggplant peels and pulps using ultrasonic-assisted extraction. Food Bioprod. Process. 2018, 109, 19–28. [Google Scholar] [CrossRef]
- Morris, G.A.; Ralet, M.-C. The effect of neutral sugar distribution on the dilute solution conformation of sugar beet pectin. Carbohydr. Polym. 2012, 88, 1488–1491. [Google Scholar] [CrossRef]
- Morris, G.A.; Ralet, M.-C.; Bonnin, E.; Thibault, J.-F.; Harding, S.E. Physical characterisation of the rhamnogalacturonan and homogalacturonan fractions of sugar beet (Beta vulgaris) pectin. Carbohydr. Polym. 2010, 82, 1161–1167. [Google Scholar] [CrossRef]
- Morris, G.A.; Ralet, M.-C. A copolymer analysis approach to estimate the neutral sugar distribution of sugar beet pectin using size exclusion chromatography. Carbohydr. Polym. 2012, 87, 1139–1143. [Google Scholar] [CrossRef]
- Levigne, S.; Ralet, M.-C.; Thibault, J.-F. Characterisation of pectins extracted from fresh sugar beet under different conditions using an experimental design. Carbohydr. Polym. 2002, 49, 145–153. [Google Scholar] [CrossRef]
- Ngouemazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The emulsifying and emulsion-stabilizing properties of pectin: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Roman-Benn, A.; Contador, C.A.; Li, M.-W.; Lam, H.-M.; Kong, A.-H.; Ulloa, P.E.; Ravanal, M.C. Pectin: An overview of sources, extraction and applications in food products, biomedical, pharmaceutical and environmental issues. Food. Chem. Adv. 2023, 2, 100192. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.C.C.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Sayah, M.Y.; Chabir, R.; Benyahia, H.; Rodi Kandri, Y.; Ouazzani Chahdi, F.; Touzani, H.; Errachidi, F. Yield, esterification degree and molecular weight evaluation of pectins isolated from orange and grapefruit peels under different conditions. PLoS ONE 2016, 11, e0161751. [Google Scholar] [CrossRef] [PubMed]
- McCready, R.; Owens, H. Methods used at Western Regional Research Laboratory for extractions and analysis of pectin materials. Anal. Chem. 1952, 24, 54–59. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Utilization of food processing wastes of eggplant as a high potential pectin source and characterization of extracted pectin. Food Chem. 2019, 294, 339–346. [Google Scholar] [CrossRef]
- McComb, E.A.; McComb, E.A.; McCready, R.M. Determination of acetyl in pectin and in acetylated carbohydrate polymers hydroxamic acid reaction. Anal. Chem. 1957, 29, 819–821. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Ürüncüoğlu, Ş.; Alba, K.; Morris, G.A.; Kontogiorgos, V. Influence of cations, pH and dispersed phases on pectin emulsification properties. Curr. Res. Food Sci. 2021, 4, 398–404. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Eggplant peel as a high potential source of high methylated pectin: Ultrasonic extraction optimization and characterization. LWT 2019, 105, 182–189. [Google Scholar] [CrossRef]
- Evageliou, V.; Ptitchkina, N.M.; Morris, E.R. Solution viscosity and structural modification of pumpkin biopectin. Food Hydrocoll. 2005, 19, 1032–1036. [Google Scholar] [CrossRef]
- Luan, T.; Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Zhang, H. Compared molecular characterization of hyaluronan using multiple-detection techniques. Polymer 2011, 52, 5648–5658. [Google Scholar] [CrossRef]
- Solomon, O.F.; Ciutâ, I.Z. Détermination de la viscosité intrinsèque de solutions de polymères par une simple détermination de la viscosité. J. Appl. Polym. Sci. 1962, 24, 683–686. [Google Scholar] [CrossRef]
- Harding, S.E. The intrinsic viscosity of biological macromolecules. Progress in measurement, interpretation and application to structure in dilute solution. Prog. Biophys. Mol. Biol. 1997, 68, 207–262. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Azim, A.-A.A.; Atta, A.M.; Farahat, M.S.; Boutros, W.Y. Determination of intrinsic viscosity of polymeric compounds through a single specific viscosity measurement. Polymer 1998, 39, 6827–6833. [Google Scholar] [CrossRef]
- Kravtchenko, T.; Pilnik, W. A simplified method for the determination of the intrinsic viscosity of pectin solutions by classical viscosimetry. In Gums and Stabilisers for the Food Industry 5, Proceeding of the 5th International Conference, Wrexham, UK, 1989; Philips, G.O., Wedlock, D.J., Williams, P.A., Eds.; IRL Press: Oxford, UK, 1990. [Google Scholar]
- Morris, G.A.; Foster, T.J.; Harding, S.E. The effect of the degree of esterification on the hydrodynamic properties of citrus pectin. Food Hydrocoll. 2000, 14, 227–235. [Google Scholar] [CrossRef]
- Morris, G.A. Hydrodynamic Investigation of Polysaccharides and Their Interaction with Casein. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2001. [Google Scholar]
- Cui, S.W. Food Carbohydrates: Chemistry, Physical Properties, and Applications, 1st ed.; Taylor & Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Anger, H.; Berth, G. Gel permeation chromatography of sunflower pectin. Carbohydr. Polym. 1985, 5, 241–250. [Google Scholar] [CrossRef]
- Fishman, M.L.; Chau, H.K.; Hoagland, P.D.; Hotchkiss, A.T. Microwave-assisted extraction of lime pectin. Food Hydrocoll. 2006, 20, 1170–1177. [Google Scholar] [CrossRef]
- Fishman, M.L.; Chau, H.K.; Kolpak, F.; Brady, J. Solvent Effects on the Molecular Properties of Pectin. J. Agric. Food Chem. 2001, 49, 4494–4501. [Google Scholar] [CrossRef]
- Masuelli, M.A. Viscometric study of pectin. Effect of temperature on the hydrodynamic properties. Int. J. Biol. Macromol. 2011, 48, 286–291. [Google Scholar] [CrossRef]
- Morris, G.A.; Adams, G.G.; Harding, S.E. On hydrodynamic methods for the analysis of the sizes and shapes of polysaccharides in dilute solution: A short review. Food Hydrocoll. 2014, 42, 318–334. [Google Scholar] [CrossRef]
- Pasandide, B.; Khodaiyan, F.; Mousavi, Z.; Hosseini, S.S. Pectin extraction from citron peel: Optimization by Box–Behnken response surface design. Food Sci. Biotechnol. 2018, 27, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Alancay, M.M.; Lobo, M.O.; Quinzio, C.M.; Iturriaga, L.B. Extraction and physicochemical characterization of pectin from tomato processing waste. J. Food Meas. Charact. 2017, 11, 2119–2130. [Google Scholar] [CrossRef]
- Yapo, B.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chem. 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Pagan, J.; Ibarz, A. Extraction and rheological properties of pectin from fresh peach pomace. J. Food Eng. 1999, 39, 193–201. [Google Scholar] [CrossRef]
- Yamazaki, E.; Kurita, O. Extraction and characterization of the pectic substances from Japanese pepper (Zanthoxylum piperitum dc.) fruit. Int. J. Food Prop. 2007, 10, 505–513. [Google Scholar]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr. Polym. 2013, 97, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Esparza, L.M.; Mora, Z.V.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell peppers (Capsicum annum L.) losses and wastes: Source for food and pharmaceutical applications. Molecules 2021, 26, 5341. [Google Scholar] [CrossRef] [PubMed]
- Garna, H.; Mabon, N.; Robert, C.; Cornet, C.; Nott, K.; Legros, H.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield and purity of apple pomace pectin precipitated but not washed by alcohol. J. Food Sci. 2007, 72, C001–C009. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Lü, X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Houben, K.; Jolie, R.P.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Comparative study of the cell wall composition of broccoli, carrot, and tomato: Structural characterization of the extractable pectins and hemicelluloses. Carbohydr. Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Yapo, B.M.; Lerouge, P.; Thibault, J.-F.; Ralet, M.-C. Pectins from citrus peel cell walls contain homogalacturonans homogenous with respect to molar mass, rhamnogalacturonan I and rhamnogalacturonan II. Carbohydr. Polym. 2007, 69, 426–435. [Google Scholar] [CrossRef]
- William, G.W.; Paul, J.K.; Jørn, D.M. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar]
- Popov, S.V.; Ovodova, R.G.; Golovchenko, V.V.; Popova, G.Y.; Viatyasev, F.V.; Shashkov, A.S.; Ovodov, Y.S. Chemical composition and anti-inflammatory activity of a pectic polysaccharide isolated from sweet pepper using a simulated gastric medium. Food Chem. 2011, 124, 309–315. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Najari, Z. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int. J. Biol. Macromol. 2019, 125, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Kravtchenko, T.; Voragen, A.; Pilnik, W. Analytical comparison of three industrial pectin preparations. Carbohydr. Polym. 1992, 18, 17–25. [Google Scholar] [CrossRef]
- Mort, A.J.; Qiu, F.; Maness, N.O. Determination of the pattern of methyl esterification in pectin. Distribution of contiguous nonesterified residues. Carbohydr. Res. 1993, 247, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Pasandide, B.; Khodaiyan, F.; Mousavi, Z.E.; Hosseini, S.S. Optimization of aqueous pectin extraction from Citrus medica peel. Carbohydr. Polym. 2017, 178, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Application of Celluclast 1.5L in apple pectin extraction. Carbohydr. Polym. 2015, 134, 251–257. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef]
- Chen, H.-m.; Fu, X.; Luo, Z.-g. Effect of molecular structure on emulsifying properties of sugar beet pulp pectin. Food Hydrocoll. 2016, 54, 99–106. [Google Scholar] [CrossRef]
- Wusigale; Liang, L.; Luo, Y. Casein and pectin: Structures, interactions, and applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Bayar, N.; Friji, M.; Kammoun, R. Optimization of enzymatic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal. Food Chem. 2018, 241, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.C.; Denman, L.J.; Binhamad, H.A.; Morris, G.A. The effect of different extraction conditions on the physical properties, conformation and branching of pectin extracted from Cucumis melo Inodorus. Polysaccharides 2020, 1, 3–20. [Google Scholar] [CrossRef]
- Fernandes PA, R.; Coimbra, M.A. The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydr. Polym. 2023, 314, 120965. [Google Scholar] [CrossRef]
| Sample | pH | Time (h) | Temperature (°C) |
|---|---|---|---|
| 1 | 1 | 2 | 60 |
| 2 | 1 | 2 | 80 |
| 3 | 1 | 4 | 60 |
| 4 | 1 | 4 | 80 |
| 5 | 3 | 2 | 60 |
| 6 | 3 | 4 | 60 |
| 7 | 3 | 2 | 80 |
| 8 | 3 | 4 | 80 |
| Extract | Yield (%) | Phenolic Content (mg GAE/g) | Protein (%) | DA (%) | DM (%) | Glc | Gal | Ara | Rha | GalA | [η] (mL/g) | Mw (kg/mol) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15.4 ± 3.1 a | 40.2 ± 13.4 a | 2.9 ± 1.0 ab | 22.1 ± 2.3 a | 88.5 ± 0.2 a | 11.2 ± 1.0 abc | 7.7 ± 0.9 ab | 1.3 ± 0.4 a | 1.2 ± 0.2 a | 32.4 ± 2.0 bc | 20.2 ± 1.9 cd | 1533 ± 60 cd |
| 2 | 18.5 ± 7.8 a | 52.9 ± 7.7 a | 2.3 ±0.6 ab | 24.3 ± 1.8 a | 87.7 ± 5.0 a | 8.6 ± 1.3 bc | 7.9 ± 1.0 ab | 1.1 ± 0.5 a | 0.8 ± 0.1 a | 30.7 ± 1.6 bc | 36.4 ± 8.3 ab | 3434 ± 453 ab |
| 3 | 20.7 ± 1.8 a | 48.6 ± 5.2 a | 1.5 ± 0.1 b | 23.3 ± 2.8 a | 89.7 ± 1.1 a | 9.1 ± 0.4 bc | 4.4 ± 0.1 b | 0.8 ± 0.1 b | 0.3 ± 0.1 a | 26.4 ± 2.2 c | 30.8 ± 9.2 abc | 2731 ± 522 abc |
| 4 | 20.0 ± 1.7 a | 47.8 ± 1.3 a | 5.4 ± 1.1 a | 19.5 ± 2.2 a | 89.6 ± 1.8 a | 7.0 ± 1.3 bc | 7.6 ± 1.8 ab | 1.2 ± 0.4 a | 1.2 ± 0.4 a | 34.3 ± 2.7 abc | 41.4 ± 5.6 a | 4096 ± 264 a |
| 5 | 19.8 ± 2.8 a | 48.1 ± 2.8 a | 2.6 ± 0.4 ab | 15.6 ± 3.0 a | 88.9 ± 0.1 a | 9.4 ± 2.4 bc | 7.0 ± 0.9 ab | 1.4 ± 0.5 a | n. d. | 45.3 ± 8.0 a | 19.0 ± 3.3 cd | 1409 ± 128 cd |
| 6 | 19.2 ± 1.7 a | 48.3 ± 4.5 a | 4.8 ± 1.6 ab | 14.7 ± 1.7 a | 90.5 ± 0.3 a | 16.6 ± 3.9 a | 9.7 ± 2.5 a | 1.2 ± 0.8 a | n. d. | 42.8 ± 4.5 ab | 12.7 ± 2.0 d | 812 ± 65 d |
| 7 | 16.6 ± 4.8 a | 52.0 ± 13.0 a | 3.2 ± 1.1 ab | 16.9 ± 2.8 a | 89.7 ± 1.1 a | 7.4 ± 2.1 c | 5.8 ± 1.8 ab | 1.5 ± 0.8 a | 0.6 ± 0.1 a | 43.0 ± 5.2 ab | 20.2 ± 1.9 cd | 1533 ± 60 cd |
| 8 | 11.6 ± 0.6 a | 32.3 ± 13.9 a | 5.3 ± 0.8 a | 16.6 ± 3.9 a | 87.6 ± 1.8 a | 11.3 ± 0.9 abc | 5.1 ± 0.4 b | 0.8 ± 0.6 b | 2.1 ± 0.5 a | 36.6 ± 5.4 abc | 19.0 ± 0.2 cd | 1409 ± 3 cd |
| Sample | Pectin Linearity a | Pectin Quality b | c | %HG (GalA − Rha) | %RG-I (2Rha + Ara + Gal) | HG: RG-I | |
|---|---|---|---|---|---|---|---|
| 1 | 3.2 | 3.6 | 0.03 | 7.5 | 31.2 | 11.4 | 2.7 |
| 2 | 3.1 | 4.4 | 0.03 | 11.3 | 29.9 | 10.6 | 2.8 |
| 3 | 4.8 | 3.3 | 0.01 | 17.3 | 26.1 | 5.8 | 4.5 |
| 4 | 3.4 | 6.0 | 0.03 | 7.3 | 33.1 | 11.2 | 3.0 |
| 5 | 5.4 | 5.1 | - | - | 45.3 | 8.4 | 5.4 |
| 6 | 3.9 | 3.0 | - | - | 42.8 | 10.9 | 3.9 |
| 7 | 5.4 | 6.3 | 0.01 | 12.2 | 42.4 | 8.5 | 5.0 |
| 8 | 4.6 | 3.5 | 0.06 | 2.8 | 34.5 | 10.1 | 3.4 |
| Sample | EA % at 22 °C | ES at 22 °C after 1 Day (%) | ES at 22 °C after 30 Days (%) | ES at 4 °C after 1 Day (%) | ES at 4 °C after 30 Days (%) |
|---|---|---|---|---|---|
| 1 | 52.1 ± 0.1 | 96.2 ± 5.1 | 93.1 ± 1.3 | 98.0 ± 0.0 | 98.0 ± 0.0 |
| 2 | 52.1 ± 0.8 | 96.1 ± 3.1 | 93.1 ± 1.3 | 97.1 ± 1.3 | 97.0 ± 1.4 |
| 3 | 52.1 ± 0.7 | 97.5 ± 3.5 | 93.9 ± 0.2 | 97.0 ± 1.4 | 98.0 ± 0.0 |
| 4 | 51.6 ± 1.5 | 97.0 ± 4.2 | 94.0 ± 0.0 | 98.0 ± 0.1 | 98.0 ± 0.0 |
| 5 | 52.3 ± 0.4 | 95.0 ± 4.2 | 94.1 ± 0.1 | 97.0 ± 0.0 | 97.0 ± 1.4 |
| 6 | 54.1 ± 2.1 | 100 ± 0.0 | 94.0 ± 0.0 | 98.0 ± 0.0 | 98.0 ± 0.0 |
| 7 | 52.6 ± 0.0 | 100 ± 0.0 | 94.0 ± 0.0 | 97.0 ± 1.3 | 98.0 ± 0.0 |
| 8 | 52.6 ± 0.0 | 100 ± 0.0 | 94.0 ± 0.0 | 98.1 ± 0.1 | 98.0 ± 0.0 |
| Sample | Free Radical Scavenging Effect of DPPH (%) |
|---|---|
| 1 | 72.8 ± 5.1 |
| 2 | 70.4 ± 4.3 |
| 3 | 62.8 ± 17.8 |
| 4 | 61.9 ± 8.1 |
| 5 | 67.0 ± 5.1 |
| 6 | 73.4 ± 3.9 |
| 7 | 50.3 ± 5.1 |
| 8 | 66.7 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obodo-Ovie, O.; Alyassin, M.; Smith, A.M.; Morris, G.A. The Effect of Different Extraction Conditions on the Physicochemical Properties of Novel High Methoxyl Pectin-like Polysaccharides from Green Bell Pepper (GBP). Macromol 2024, 4, 420-436. https://doi.org/10.3390/macromol4020024
Obodo-Ovie O, Alyassin M, Smith AM, Morris GA. The Effect of Different Extraction Conditions on the Physicochemical Properties of Novel High Methoxyl Pectin-like Polysaccharides from Green Bell Pepper (GBP). Macromol. 2024; 4(2):420-436. https://doi.org/10.3390/macromol4020024
Chicago/Turabian StyleObodo-Ovie, Onome, Mohammad Alyassin, Alan M. Smith, and Gordon A. Morris. 2024. "The Effect of Different Extraction Conditions on the Physicochemical Properties of Novel High Methoxyl Pectin-like Polysaccharides from Green Bell Pepper (GBP)" Macromol 4, no. 2: 420-436. https://doi.org/10.3390/macromol4020024
APA StyleObodo-Ovie, O., Alyassin, M., Smith, A. M., & Morris, G. A. (2024). The Effect of Different Extraction Conditions on the Physicochemical Properties of Novel High Methoxyl Pectin-like Polysaccharides from Green Bell Pepper (GBP). Macromol, 4(2), 420-436. https://doi.org/10.3390/macromol4020024









