Influence of Thermal and Chemical Stresses on Thermal Properties, Crystal Morphology, and Mechanical Strength Development of a Sulfur Polymer Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation and Calculations
2.2. Synthesis and Exposure of Samples
3. Results and Discussion
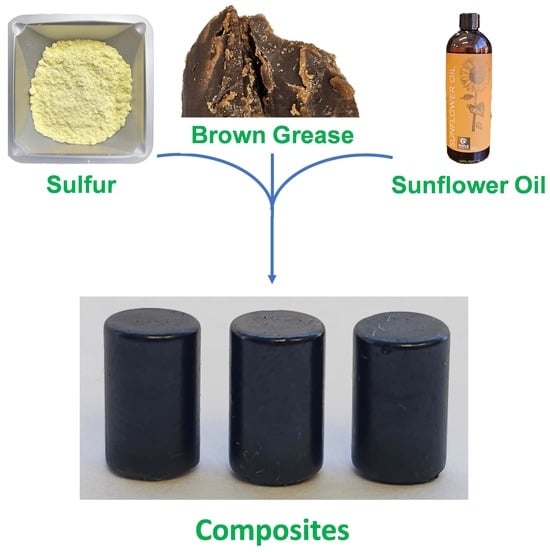
3.1. Design and Rationale
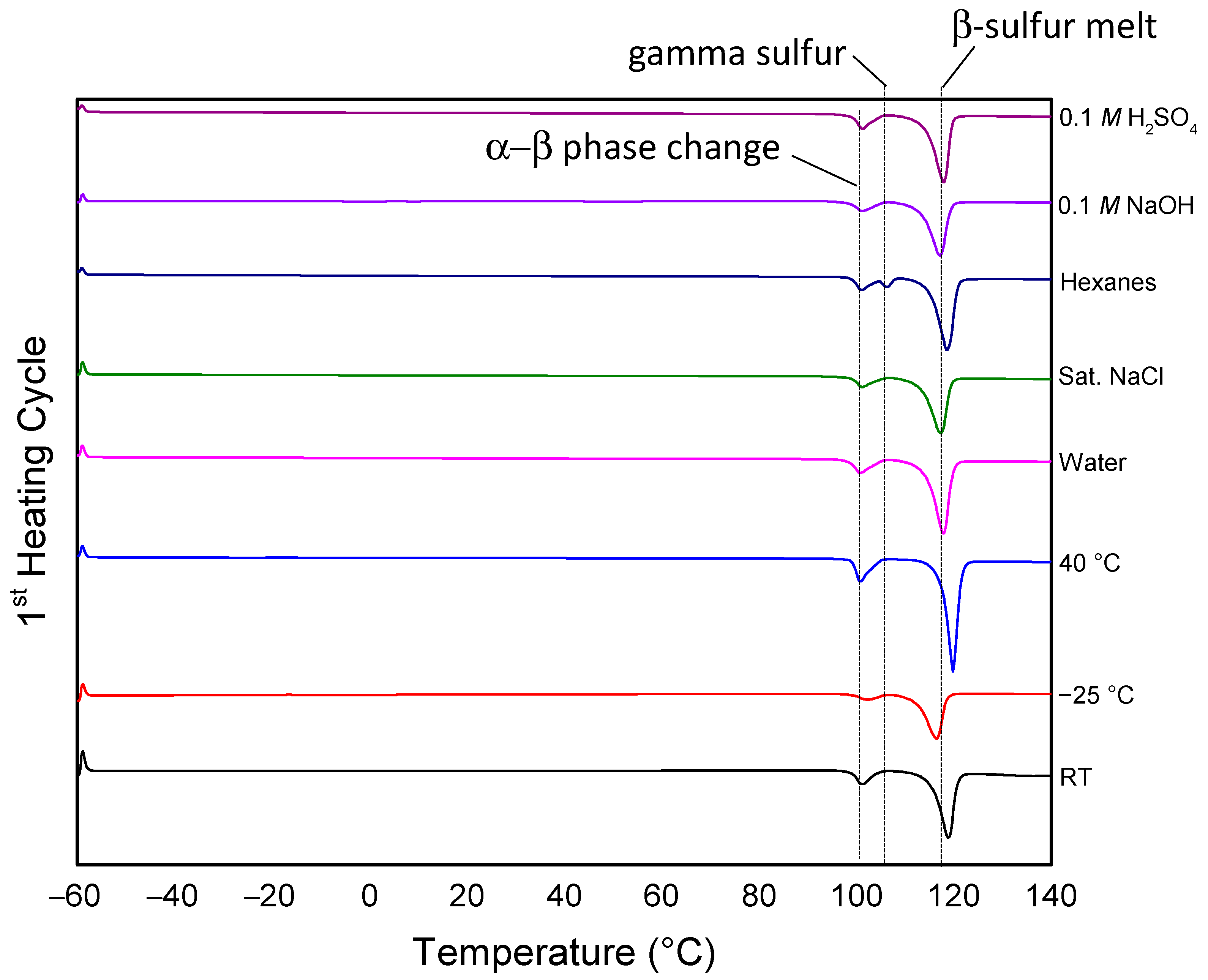
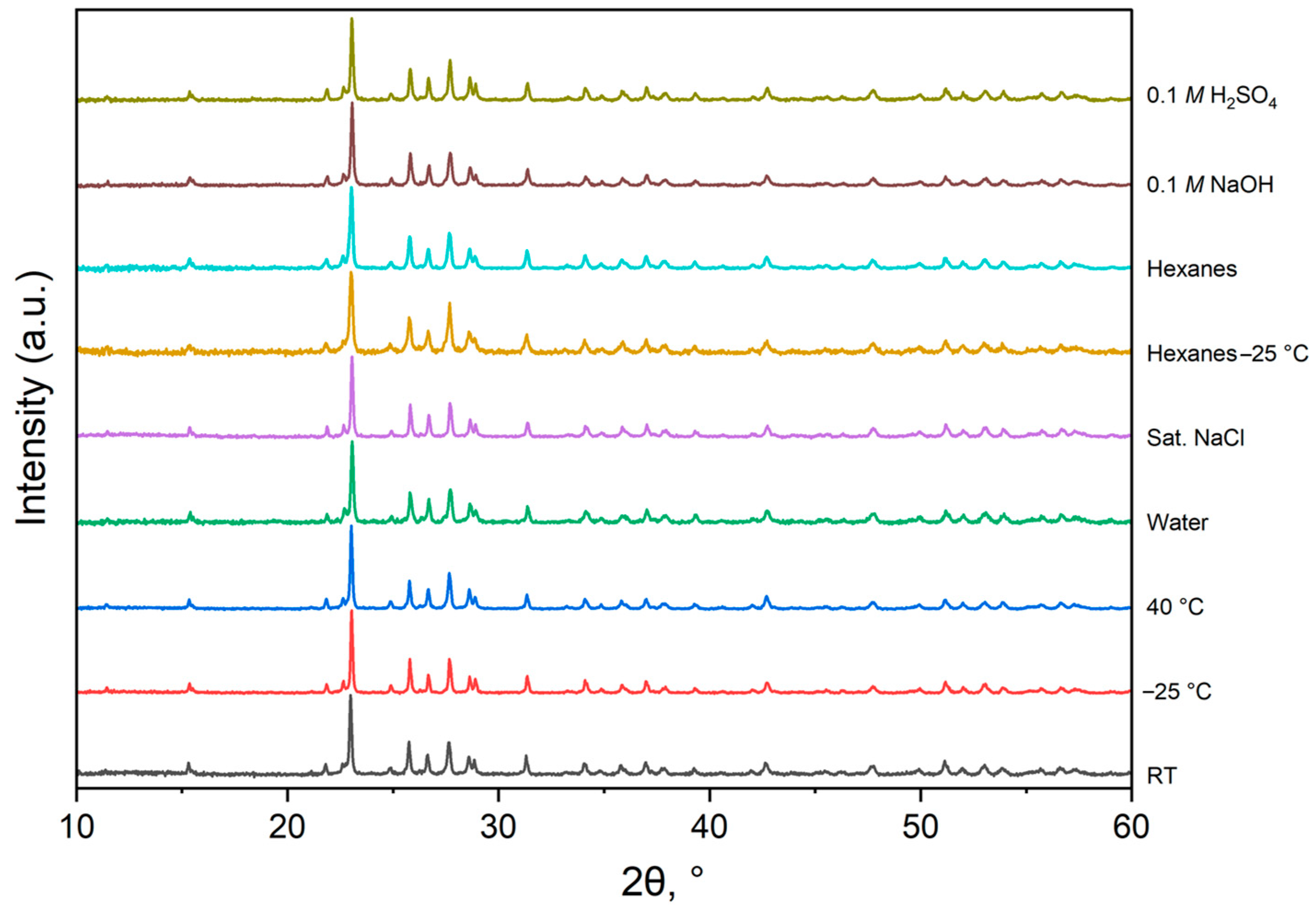
3.2. Effects of Environmental Factors on Physical and Thermo-Morphological Properties
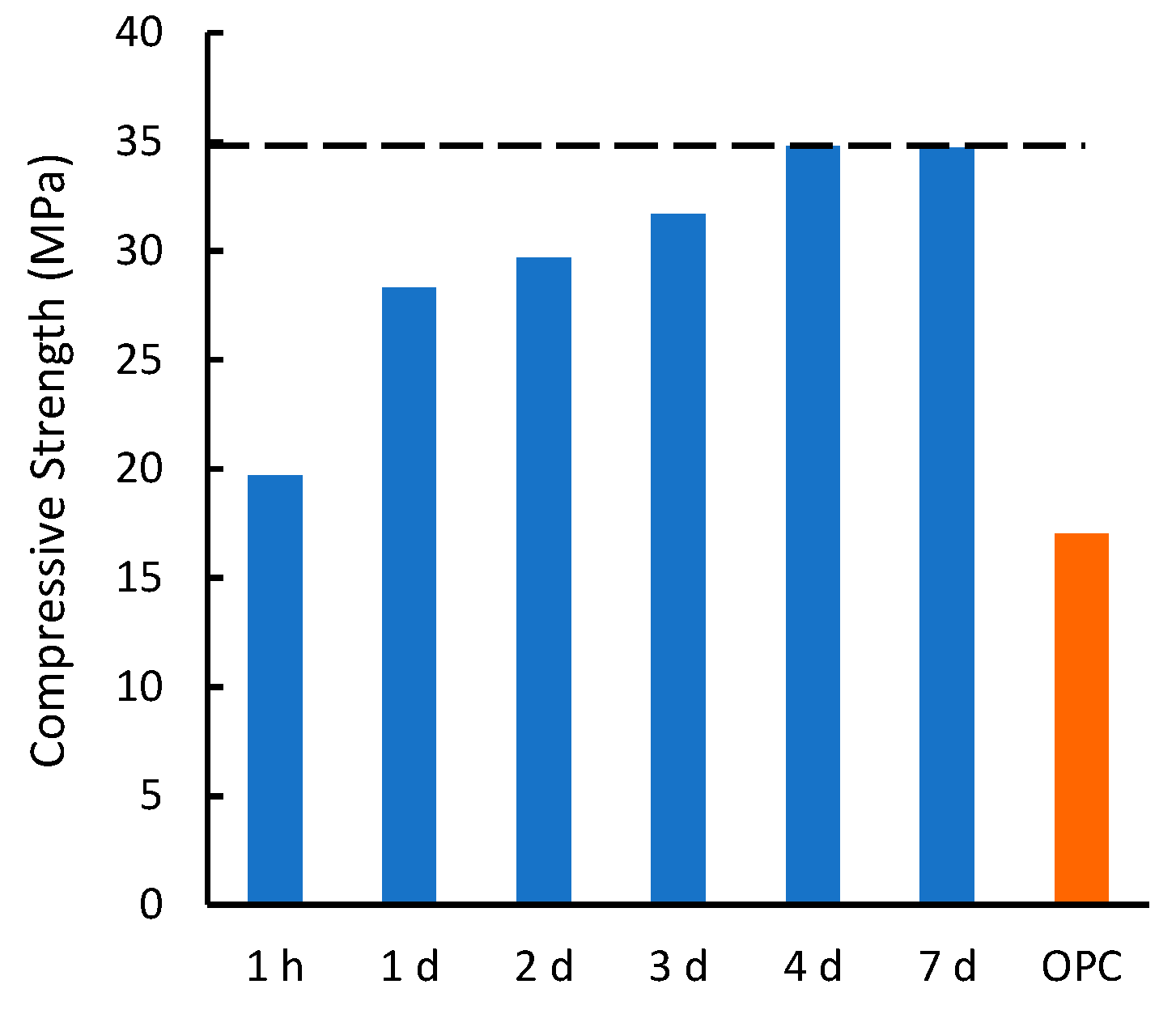
3.3. Effects of Environmental Stresses on Compressional Strength Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gutarowska, B.; Piotrowska, M.; Kozirog, A.; Berlowska, J.; Dziugan, P.; Kotynia, R.; Bielinski, D.; Anyszka, R.; Wreczycki, J. New Sulfur Organic Polymer-Concrete Composites Containing Waste Materials: Mechanical Characteristics and Resistance to Biocorrosion. Materials 2019, 12, 2602. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, M.; Kotynia, R. Assessment of sulfur concrete properties for use in civil engineering. MATEC Web Conf. 2018, 219, 3006. [Google Scholar] [CrossRef]
- Gwon, S.; Jeong, Y.; Oh, J.E.; Shin, M. Sustainable sulfur composites with enhanced strength and lightweightness using waste rubber and fly ash. Constr. Build. Mater. 2017, 135, 650–664. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, R.C.; Tennyson, A.G. Polymer cements by copolymerization of waste sulfur, oleic acid, and pozzolan cements. Sust. Chem. Pharm. 2020, 16, 100249. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Furtos, G.; Molnar, L.; Silaghi-Dumitrescu, L.; Pascuta, P.; Korniejenko, K. Mechanical and thermal properties of wood fiber reinforced geopolymer composites. J. Nat. Fibers 2022, 19, 6676–6691. [Google Scholar] [CrossRef]
- Furtos, G.; Silaghi-Dumitrescu, L.; Pascuta, P.; Sarosi, C.; Korniejenko, K. Mechanical Properties of Wood Fiber Reinforced Geopolymer Composites with Sand Addition. J. Nat. Fibers 2021, 18, 285–296. [Google Scholar] [CrossRef]
- Weil, E.D. Recent industrial organosulfur chemistry. Phosphorus Sulfur Silicon Relat. Elem. 1991, 59, 325–340. [Google Scholar] [CrossRef]
- Thiounn, T.; Karunarathna, M.S.; Slann, L.M.; Lauer, M.K.; Smith, R.C. Sequential Crosslinking for Mechanical Property Development in High Sulfur Content Composites. J. Poly. Sci. 2020, 58, 2943–2950. [Google Scholar] [CrossRef]
- Thiounn, T.; Tennyson, A.G.; Smith, R.C. Durable, acid-resistant copolymers from industrial by-product sulfur and microbially-produced tyrosine. RSC Adv. 2019, 9, 31460–31465. [Google Scholar] [CrossRef]
- Gregor, R.; Hackl, A. A new approach to sulfur concrete. Adv. Chem. Ser. 1978, 165, 54–78. [Google Scholar]
- Shrive, N.G.; Gillott, J.E.; Jordaan, I.J.; Loov, R.E. The potential and properties of sulfur concretes. ACS Symp. Ser. 1982, 183, 137–154. [Google Scholar] [CrossRef]
- Okumura, H.A. Early sulfur concrete installations. Concr. Int. 1998, 20, 72–75. [Google Scholar]
- Davis, A.E.; Sayer, K.B.; Jenkins, C.L. A comparison of adhesive polysulfides initiated by garlic essential oil and elemental sulfur to create recyclable adhesives. Polym. Chem. 2022, 13, 4634–4640. [Google Scholar] [CrossRef]
- Eder, M.L.; Call, C.B.; Jenkins, C.L. Utilizing Reclaimed Petroleum Waste to Synthesize Water-Soluble Polysulfides for Selective Heavy Metal Binding and Detection. ACS Appl. Polym. Mater. 2022, 4, 1110–1116. [Google Scholar] [CrossRef]
- Eder, M.L.; Jenkins, C. Inverse vulcanization of sulfur and charged monomers to enhance solubility and create inexpensive metal binding materials. Abstr. Pap. Am. Chem. Soc. 2019, 257, POLY-0336. [Google Scholar]
- Herrera, C.; Ysinga, K.J.; Jenkins, C.L. Polysulfides Synthesized from Renewable Garlic Components and Repurposed Sulfur Form Environmentally Friendly Adhesives. ACS Appl. Mater. Interfaces 2019, 11, 35312–35318. [Google Scholar] [CrossRef]
- Orme, K.; Fistrovich, A.H.; Jenkins, C.L. Tailoring Polysulfide Properties through Variations of Inverse Vulcanization. Macromolecules 2020, 53, 9353–9361. [Google Scholar] [CrossRef]
- Westerman, C.R.; Jenkins, C.L. Dynamic Sulfur Bonds Initiate Polymerization of Vinyl and Allyl Ethers at Mild Temperatures. Macromolecules 2018, 51, 7233–7238. [Google Scholar] [CrossRef]
- Duarte, M.E.; Huber, B.; Theato, P.; Mutlu, H. The unrevealed potential of elemental sulfur for the synthesis of high sulfur content bio-based aliphatic polyesters. Polym. Chem. 2020, 11, 241–248. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Hoefling, A.; Yee, M.; Nguyen, G.T.H.; Theato, P.; Lee, Y.J.; Song, S.-W. Enabling High-Rate and Safe Lithium Ion-Sulfur Batteries by Effective Combination of Sulfur-Copolymer Cathode and Hard-Carbon Anode. ChemSusChem 2019, 12, 480–486. [Google Scholar] [CrossRef]
- Mutlu, H.; Theato, P.; Ceper Ezgi, B.; Ozmen Mehmet, M.; Li, X.; Yang, J.; Dong, W.; Theato, P.; Yang, J. Sulfur Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2019, 40, e1800650. [Google Scholar] [CrossRef]
- Dale, J.J.; Stanley, J.; Dop, R.A.; Chronowska-Bojczuk, G.; Fielding, A.J.; Neill, D.R.; Hasell, T. Exploring Inverse Vulcanisation Mechanisms from the Perspective of Dark Sulfur. Eur. Polym. J. 2023, 195, 112198. [Google Scholar] [CrossRef]
- Jia, J.; Liu, J.; Wang, Z.-Q.; Liu, T.; Yan, P.; Gong, X.-Q.; Zhao, C.; Chen, L.; Miao, C.; Zhao, W.; et al. Photoinduced inverse vulcanization. Nat. Chem. 2022, 14, 1249–1257. [Google Scholar] [CrossRef]
- Dale, J.J.; Petcher, S.; Hasell, T. Dark Sulfur: Quantifying Unpolymerized Sulfur in Inverse Vulcanized Polymers. ACS Appl. Polym. Mater. 2022, 4, 3169–3173. [Google Scholar] [CrossRef]
- Dodd, L.J.; Omar, O.; Wu, X.; Hasell, T. Investigating the Role and Scope of Catalysts in Inverse Vulcanization. ACS Catal. 2021, 11, 4441–4455. [Google Scholar] [CrossRef]
- Tonkin, S.J.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Karton, A.; Hasell, T.; Chalker, J.M. Chemically induced repair, adhesion, and recycling of polymers made by inverse vulcanization. Chem. Sci. 2020, 11, 5537–5546. [Google Scholar] [CrossRef] [PubMed]
- Tikoalu, A.D.; Lundquist, N.A.; Chalker, J.M. Mercury Sorbents Made By Inverse Vulcanization of Sustainable Triglycerides: The Plant Oil Structure Influences the Rate of Mercury Removal from Water. Adv. Sustain. Syst. 2020, 4, 1900111. [Google Scholar] [CrossRef]
- Mann, M.; Kruger, J.E.; Andari, F.; McErlean, J.; Gascooke, J.R.; Smith, J.A.; Worthington, M.J.H.; McKinley, C.C.C.; Campbell, J.A.; Lewis, D.A.; et al. Sulfur polymer composites as controlled-release fertilizers. Org. Biomol. Chem. 2019, 17, 1929–1936. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Chalker, J.M. Green chemistry and polymers made from sulfur. Green Chem. 2017, 19, 2748–2761. [Google Scholar] [CrossRef]
- Bao, J.; Martin, K.P.; Cho, E.; Kang, K.-S.; Glass, R.S.; Coropceanu, V.; Bredas, J.-L.; Parker, W.O.N., Jr.; Njardarson, J.T.; Pyun, J. On the Mechanism of the Inverse Vulcanization of Elemental Sulfur: Structural Characterization of Poly(sulfur-random-(1,3-diisopropenylbenzene)). J. Am. Chem. Soc. 2023, 145, 12386–12397. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Dirlam, P.T.; Njardarson, J.T.; Glass, R.S.; Pyun, J. Polymerizations with Elemental Sulfur: From Petroleum Refining to Polymeric Materials. J. Am. Chem. Soc. 2022, 144, 5–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Glass, R.S.; Char, K.; Pyun, J. Recent advances in the polymerization of elemental sulphur, inverse vulcanization and methods to obtain functional Chalcogenide Hybrid Inorganic/Organic Polymers (CHIPs). Polym. Chem. 2019, 10, 4078–4105. [Google Scholar] [CrossRef]
- Kolet, M.; Zerbib, D.; Nakonechny, F.; Nisnevitch, M. Production of Biodiesel from Brown Grease. Catalysts 2020, 10, 1189. [Google Scholar] [CrossRef]
- Sim, Y.-L.; Meyappan, N.; Yen, N.S.; Kamala a/p Subramaniam, S.; Khoo, C.H.; Cheah, W.L.; St. Hilaire, D.; Pinnock, T.; Bacolod, B.; Cai, Z.B.; et al. Chemical reactions in the pyrolysis of brown grease. Fuel 2017, 207, 274–282. [Google Scholar] [CrossRef]
- Lopez, C.V.; Smith, A.D.; Smith, R.C. High strength composites from low-value animal coproducts and industrial waste sulfur. RSC Adv. 2022, 12, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Sauceda-Olono, P.Y.; Borbon-Almada, A.C.; Gaxiola, M.; Smith, A.D.; Tennyson, A.G.; Smith, R.C. Thermal and Mechanical Properties of Recyclable Composites Prepared from Bio-Olefins and Industrial Waste. J. Compos. Sci. 2023, 7, 248. [Google Scholar] [CrossRef]
- Ruedrich, J.; Knell, C.; Enseleit, J.; Rieffel, Y.; Siegesmund, S. Stability assessment of marble statuaries of the Schlossbrücke (Berlin, Germany) based on rock strength measurements and ultrasonic wave velocities. Environ. Earth Sci. 2013, 69, 1451–1469. [Google Scholar] [CrossRef]
- Ozcelik, Y.; Ozguven, A. Water absorption and drying features of different natural building stones. Constr. Build. Mater. 2014, 63, 257–270. [Google Scholar] [CrossRef]
- Medvedev, V.Y.; Stepanov, P. Density characteristics of ancient rocks of the western tien-shan. Int. Geol. Rev. 1962, 4, 67–78. [Google Scholar] [CrossRef]
- Choudhary, R. An Experimental Investigation to Evaluate Abrasion Wear Characteristics and Study the Effect of Micro Cracks on the Mechanical Properties of Various Marbles Types of Rajasthan Region. Doctoral Dissertation, MNIT, Jaipur, India, 2015. [Google Scholar]
- Broz, M.E.; Cook, R.F.; Whitney, D.L. Microhardness, toughness, and modulus of Mohs scale minerals. Am. Mineral. 2006, 91, 135–142. [Google Scholar] [CrossRef]
- Marble Institute of America. Report 2016-VIII. In Stone Testing; Marble Institute of America: Oberlin, OH, USA, 2016. [Google Scholar]
- Lopez, C.V.; Smith, A.D.; Smith, R.C. Evaluation of Animal Fats and Vegetable Oils as Comonomers in Polymer Composite Synthesis: Effects of Plant/Animal Sources and Comonomer Composition on Composite Properties. Macromol. Chem. Phys. 2023, 9, 2300233. [Google Scholar] [CrossRef]
- Lopez, C.V.; Karunarathna, M.S.; Lauer, M.K.; Maladeniya, C.P.; Thiounn, T.; Ackley, E.D.; Smith, R.C. High Strength, Acid-Resistant Composites from Canola, Sunflower, or Linseed Oils: Influence of Triglyceride Unsaturation on Material Properties. J. Poly. Sci. 2020, 58, 2259–2266. [Google Scholar] [CrossRef]
- ACI Committee 332. Guide to Residential Concrete Construction; ACI 332.1R-06, American Concrete Institute: Farmington Hills, MI, USA, 2006. [Google Scholar]
- Liu, L.; Shen, D.; Chen, H.; Sun, W.; Qian, Z.; Zhao, H.; Jiang, J. Analysis of damage development in cement paste due to ice nucleation at different temperatures. Cem. Concr. Compos. 2014, 53, 1–9. [Google Scholar] [CrossRef]
- Wu, M.; Fridh, K.; Johannesson, B.; Geiker, M. Influence of frost damage and sample preconditioning on the porosity characterization of cement based materials using low temperature calorimetry. Thermochim. Acta 2015, 607, 30–38. [Google Scholar] [CrossRef]
- Fu, Y.-F.; Wong, Y.-L.; Poon, C.-S.; Tang, C.-A.; Lin, P. Experimental study of micro/macro crack development and stress–strain relations of cement-based composite materials at elevated temperatures. Cem. Concr. Res. 2004, 34, 789–797. [Google Scholar] [CrossRef]
- Fabbri, A.; Corvisier, J.; Schubnel, A.; Brunet, F.; Goffé, B.; Rimmele, G.; Barlet-Gouédard, V. Effect of carbonation on the hydro-mechanical properties of Portland cements. Cem. Concr. Res. 2009, 39, 1156–1163. [Google Scholar] [CrossRef]
- Sanchez, L. Contribution to the Assessment of Damage in Aging Concrete Infrastructures Affected by Alkali-Aggregate Reaction. Doctoral Dissertation, Université Laval, Québec, QC, Canada, 2014. [Google Scholar]
- Qiao, C.; Suraneni, P.; Weiss, J. Damage in cement pastes exposed to NaCl solutions. Constr. Build. Mater. 2018, 171, 120–127. [Google Scholar] [CrossRef]
- Qiao, C.; Suraneni, P.; Tsui Chang, M.; Weiss, J. Damage in cement pastes exposed to MgCl2 solutions. Mater. Struct. 2018, 51, 1–15. [Google Scholar] [CrossRef]
- Hosseinzadeh, N.; Montanari, L.; Qiao, C.; Suraneni, P. Damage in cement pastes and mortars exposed to CaCl2 and low-temperature cycles. Mater. Struct. 2022, 55, 105. [Google Scholar] [CrossRef]
- Zivica, V.r.; Bajza, A. Acidic attack of cement based materials—A review: Part 1. Principle of acidic attack. Constr. Build. Mater. 2001, 15, 331–340. [Google Scholar] [CrossRef]
- Lauer, M.K.; Estrada-Mendoza, T.A.; McMillen, C.D.; Chumanov, G.; Tennyson, A.G.; Smith, R.C. Durable Cellulose-Sulfur Composites Derived from Agricultural and Petrochemical Waste. Adv. Sustain. Syst. 2019, 3, 1900062. [Google Scholar] [CrossRef]
- Tisdale, K.A.; Maladeniya, C.P.; Lopez, C.V.; Tennyson, A.G.; Smith, R.C. Sustainable Composites from Waste Sulfur, Terpenoids, and Pozzolan Cements. J. Compos. Sci. 2023, 7, 35. [Google Scholar] [CrossRef]
- Graham, M.J.; Lopez, C.V.; Maladeniya, C.P.; Tennyson, A.G.; Smith, R.C. Influence of pozzolans on plant oil-sulfur polymer cements: More sustainable and chemically-resistant alternatives to Portland cement. J. Appl. Polym. Sci. 2023, 140, e53684. [Google Scholar] [CrossRef]
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar] [CrossRef]
- Muthmann, W. XXIV. Untersuchungen über den Schwefel und das Selen. Z. Für Krist. Cryst. Mater. 1890, 17, 336–367. [Google Scholar] [CrossRef][Green Version]
- Pai, R.; Singh, A.; Tang, M.H.; Kalra, V. Stabilization of gamma sulfur at room temperature to enable the use of carbonate electrolyte in Li-S batteries. Commun. Chem. 2022, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Park, M.J. Thirty-minute synthesis of hierarchically ordered sulfur particles enables high-energy, flexible lithium-sulfur batteries. Nano Energy 2021, 89, 106459. [Google Scholar] [CrossRef]
- Douglas, S.; Yang, H. Mineral biosignatures in evaporites: Presence of rosickyite in an endoevaporitic microbial community from Death Valley, California. Geology 2002, 30, 1075–1078. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Khawaja, S.Z.; Kumar, S.V.; Jena, K.K.; Alhassan, S.M. Flexible sulfur film from inverse vulcanization technique. Mater. Lett. 2017, 203, 58–61. [Google Scholar] [CrossRef]
- Lian, Q.; Li, Y.; Li, K.; Cheng, J.; Zhang, J. Insights into the vulcanization mechanism through a simple and facile approach to the sulfur cleavage behavior. Macromolecules 2017, 50, 803–810. [Google Scholar] [CrossRef]
- Meyer, B. Solid allotropes of sulfur. Chem. Rev. 1964, 64, 429–451. [Google Scholar] [CrossRef]
- Meyer, B. Elemental sulfur. Chem. Rev. 1976, 76, 367–388. [Google Scholar] [CrossRef]
- Shankarayya Wadi, V.K.; Jena, K.K.; Khawaja, S.Z.; Yannakopoulou, K.; Fardis, M.; Mitrikas, G.; Karagianni, M.; Papavassiliou, G.; Alhassan, S.M. NMR and EPR structural analysis and stability study of inverse vulcanized sulfur copolymers. ACS Omega 2018, 3, 3330–3339. [Google Scholar] [CrossRef] [PubMed]
- Tobolsky, A.; MacKnight, W.; Beevers, R.; Gupta, V. The glass transition temperature of polymeric sulphur. Polymer 1963, 4, 423–427. [Google Scholar] [CrossRef]
- Tobolsky, A.V. Polymeric sulfur and related polymers. In Proceedings of the Journal of Polymer Science Part C: Polymer Symposia; Wiley Subscription Services, Inc.: Hoboken, NJ, USA, 1966; pp. 71–78. [Google Scholar]
- Dawood, A.O.; Mussa, F.I.; Al Khazraji, H.; Abd Ulsada, H.A.; Yasser, M.M. Investigation of compressive strength of straw reinforced unfired clay bricks for sustainable building construction. Civ. Environ. Eng. 2021, 17, 150–163. [Google Scholar] [CrossRef]
- Coppens, P.; Yang, Y.; Blessing, R.; Copper, W.; Larsen, F. The experimental charge distribution in sulfur containing molecules. Analysis of cyclic octasulfur at 300 and 100 K. J. Am. Chem. Soc. 1977, 99, 760–766. [Google Scholar] [CrossRef]
- Gallacher, A.; Pinkerton, A. A redetermination of monclinic γ-sulfur. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1993, 49, 125–126. [Google Scholar] [CrossRef]
- Rettig, S.; Trotter, J. Refinement of the structure of orthorhombic sulfur, α-S8. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 2260–2262. [Google Scholar] [CrossRef]
- Templeton, L.K.; Templeton, D.H.; Zalkin, A. Crystal structure of monoclinic sulfur. Inorg. Chem. 1976, 15, 1999–2001. [Google Scholar] [CrossRef]
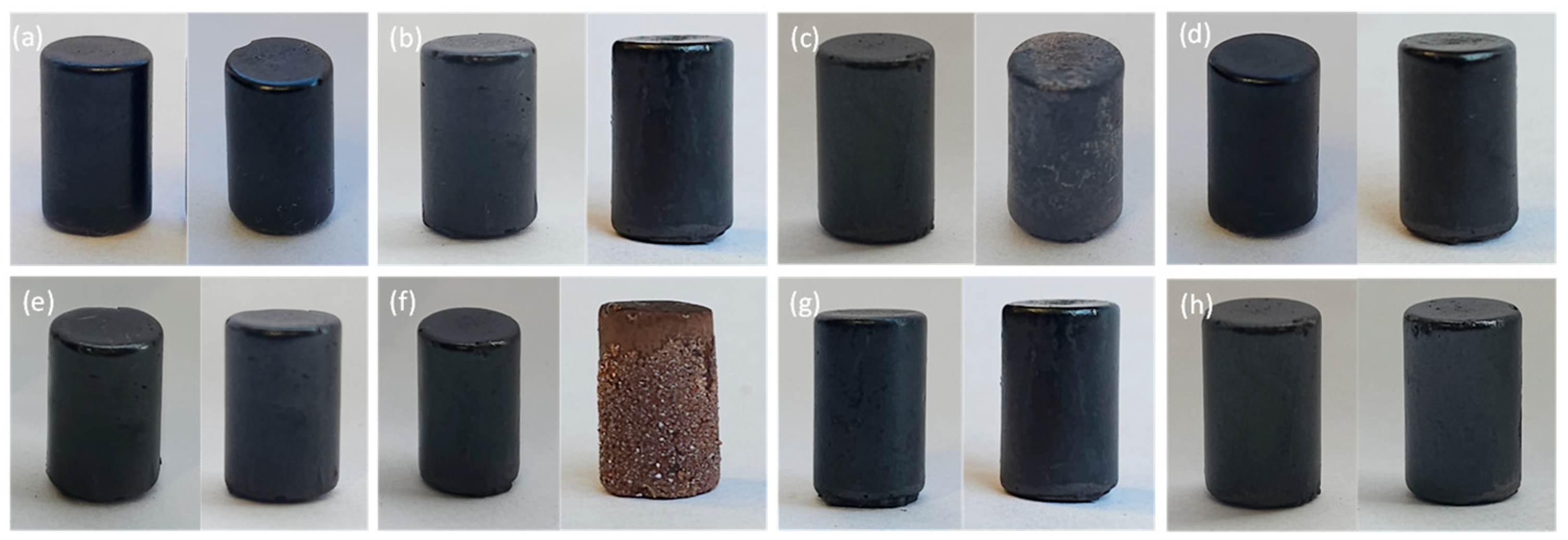
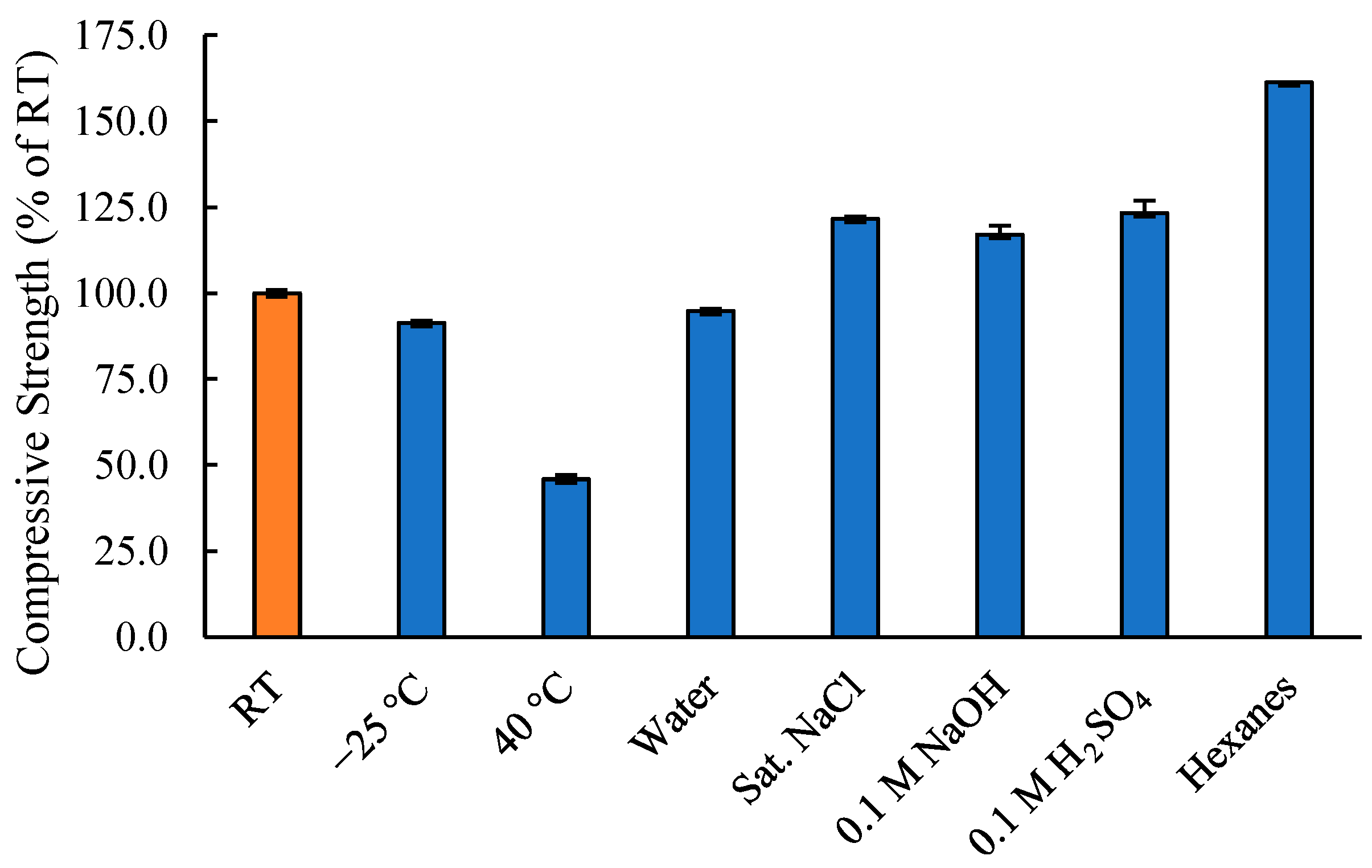
| Test/Metric | Test Name/Number | Value of Metric | |
|---|---|---|---|
| SunBG90 | Marble | ||
| Compressive strength | Compressive strength | 35.9 MPa | 12.45 MPa |
| Flexural strength | Flexural strength | 7.7 MPa | 3.4 MPa |
| Water absorption | ASTM C140 | 0.83% | 0.12% |
| Density | ASTM C140 | 106.5 lb./ft3 | 164.8 lb./ft3 |
| Abrasion resistance (Iw) | ASTM C1353 | 16 (unitless) | 10 (unitless) |
| Thermal conductivity | ISO 8302 | 0.126 W/(m·K) | 2.95 W/(m·K) |
| Moh’s Hardness | Moh’s hardness test | 2.5 | 3 |
| Condition | Tm[a]/°C | Tg,DSC [b]/°C | Cold Crystallization Peaks/°C | ∆Hm J/g | ∆Hcc J/g | Percent Crystallinity [c] |
|---|---|---|---|---|---|---|
| RT | 119 | −36.0 | NA | 29.3 | −2.2 | 30 |
| −25 °C | 118 | −36.2 | 34 | 28.2 | −1.5 | 46 |
| 40 °C | 119 | −36.2 | NA | 39.4 | −1.1 | 10 |
| Water | 118 | −36.2 | NA | 33.8 | −1.1 | 22 |
| Sat. NaCl | 117 | −36.2 | 6.1, 44 | 28.2 | −1.5 | 34 |
| Hexanes | 118 | −36.5 | NA | 34.6 | −0.3 | 22 |
| 0.1 M NaOH | 117 | −36.2 | 47.5 | 28.2 | −0.8 | 35 |
| 0.1 M H2SO4 | 117 | −36.3 | −0.50, 42 | 32.1 | −0.4 | 27 |
| Composite/Material | Compressive Strength (MPa) | |
|---|---|---|
| SunBG90 | RT | 35.9 |
| −25 °C | 32.8 | |
| 40 °C | 16.5 | |
| Water | 34.0 | |
| Sat. NaCl | 43.7 | |
| Hexanes | 42.0 | |
| 0.1 M NaOH | 44.3 | |
| 0.1 M H2SO4 | 57.9 | |
| CanS | 9.3 | |
| SunS | 17.9 | |
| CFS90 | 24.7 | |
| Clay bricks | 10.0 | |
| OPC | 17.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauceda-Oloño, P.Y.; Lopez, C.V.; Patel, B.K.; Smith, A.D.; Smith, R.C. Influence of Thermal and Chemical Stresses on Thermal Properties, Crystal Morphology, and Mechanical Strength Development of a Sulfur Polymer Composite. Macromol 2024, 4, 240-252. https://doi.org/10.3390/macromol4020013
Sauceda-Oloño PY, Lopez CV, Patel BK, Smith AD, Smith RC. Influence of Thermal and Chemical Stresses on Thermal Properties, Crystal Morphology, and Mechanical Strength Development of a Sulfur Polymer Composite. Macromol. 2024; 4(2):240-252. https://doi.org/10.3390/macromol4020013
Chicago/Turabian StyleSauceda-Oloño, Perla Y., Claudia V. Lopez, Bhakti K. Patel, Ashlyn D. Smith, and Rhett C. Smith. 2024. "Influence of Thermal and Chemical Stresses on Thermal Properties, Crystal Morphology, and Mechanical Strength Development of a Sulfur Polymer Composite" Macromol 4, no. 2: 240-252. https://doi.org/10.3390/macromol4020013
APA StyleSauceda-Oloño, P. Y., Lopez, C. V., Patel, B. K., Smith, A. D., & Smith, R. C. (2024). Influence of Thermal and Chemical Stresses on Thermal Properties, Crystal Morphology, and Mechanical Strength Development of a Sulfur Polymer Composite. Macromol, 4(2), 240-252. https://doi.org/10.3390/macromol4020013