Electrospun Scaffolds for Tissue Engineering: A Review
Abstract
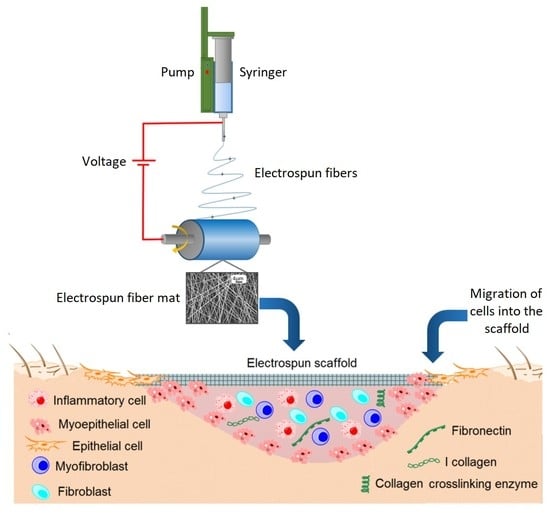
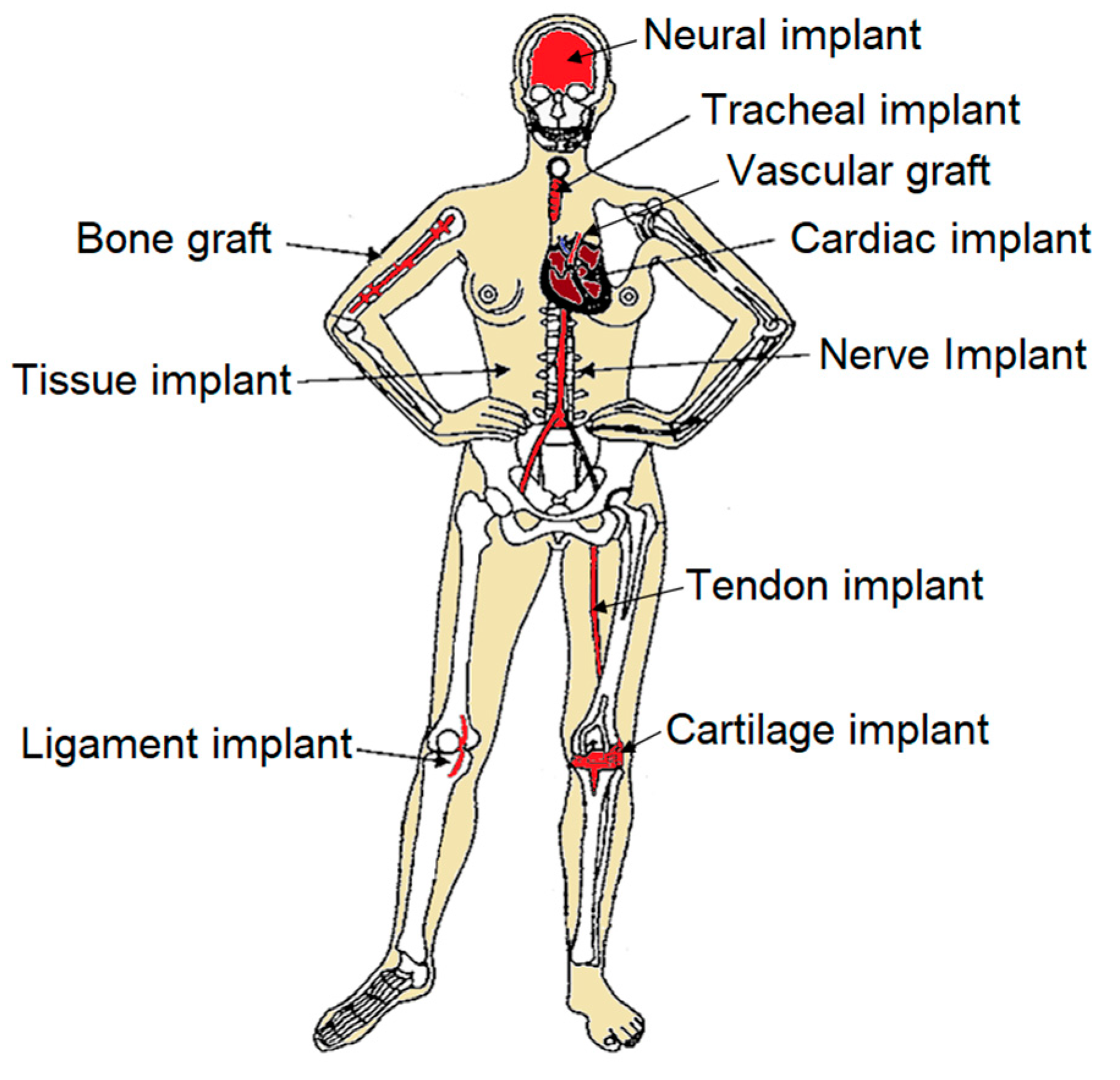
1. Introduction
2. Ideal Scaffold System
- The scaffold should feature an interconnected porous structure with controlled pore geometry and size. This structure should maintain mechanical stability over a period of time, facilitating adequate tissue regeneration within the scaffold. The interconnected porous structure is crucial for cell clearance, nutrient transport, and removal of cellular waste, all of which are vital for cell formation and tissue growth. The optimal size and morphology of the pores can vary depending on the cell type, but they should be large enough to support three-dimensional tissue formation through multilayered cell growth. Additionally, a highly porous scaffold not only maximizes tissue growth but also minimizes material usage [24,25,26].
3. Scaffold Materials
4. Electrospinning
- (a)
- Spinning time; electrospinning is particularly suitable for fabricating 2D structures, such as membranes with random or aligned orientations [92]. However, conventional electrospinning leads to changes in membrane thickness over time, resulting in 3D structures with thicknesses ranging from tens to hundreds of microns. Moreover, multilayered 3D macrostructures comprising different materials can be achieved through sequential electrospinning [15,93] or co-electrospinning [94], involving the exchange and electrospinning of polymer solutions under different conditions [95].
- (b)
- (c)
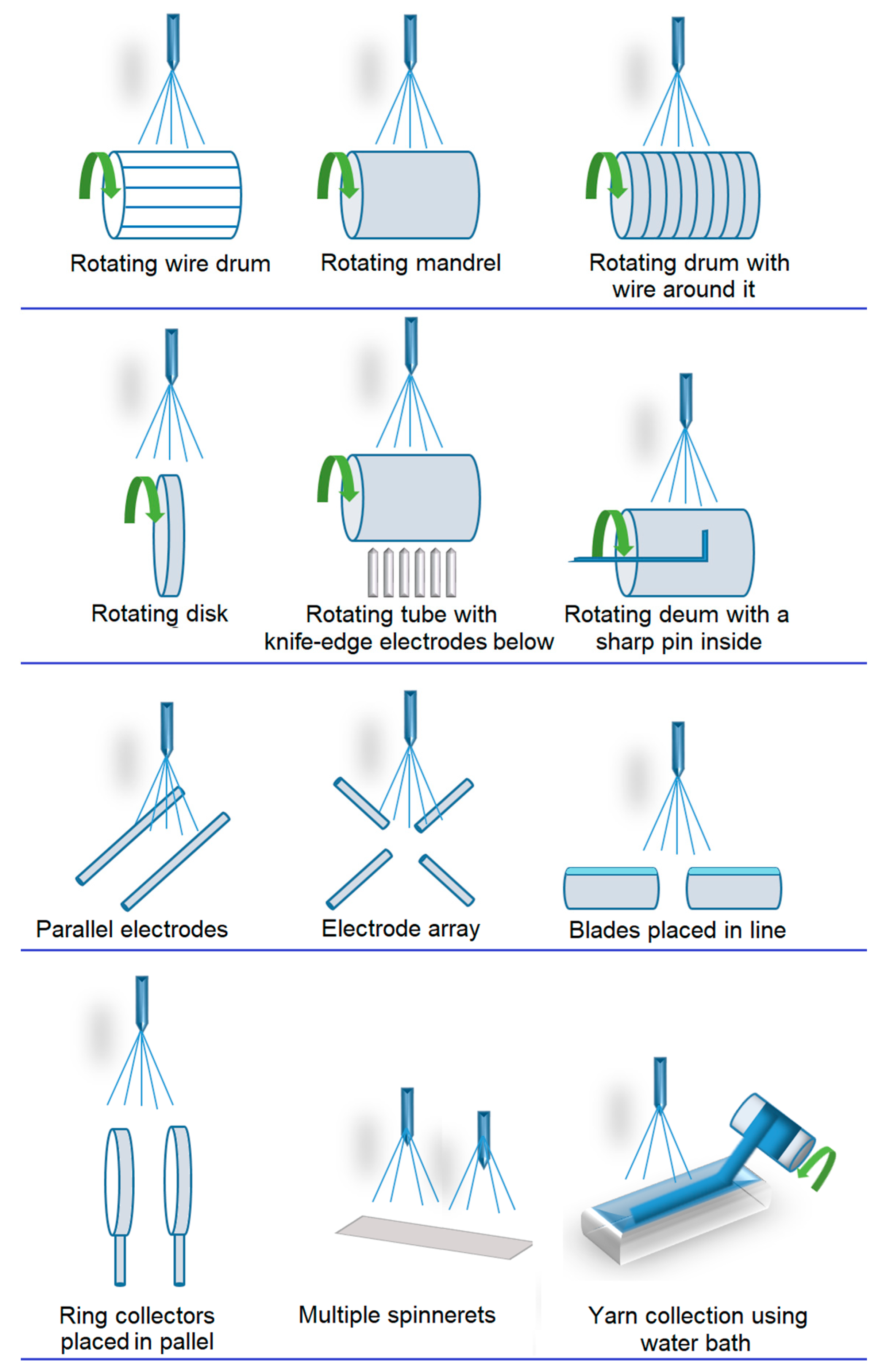
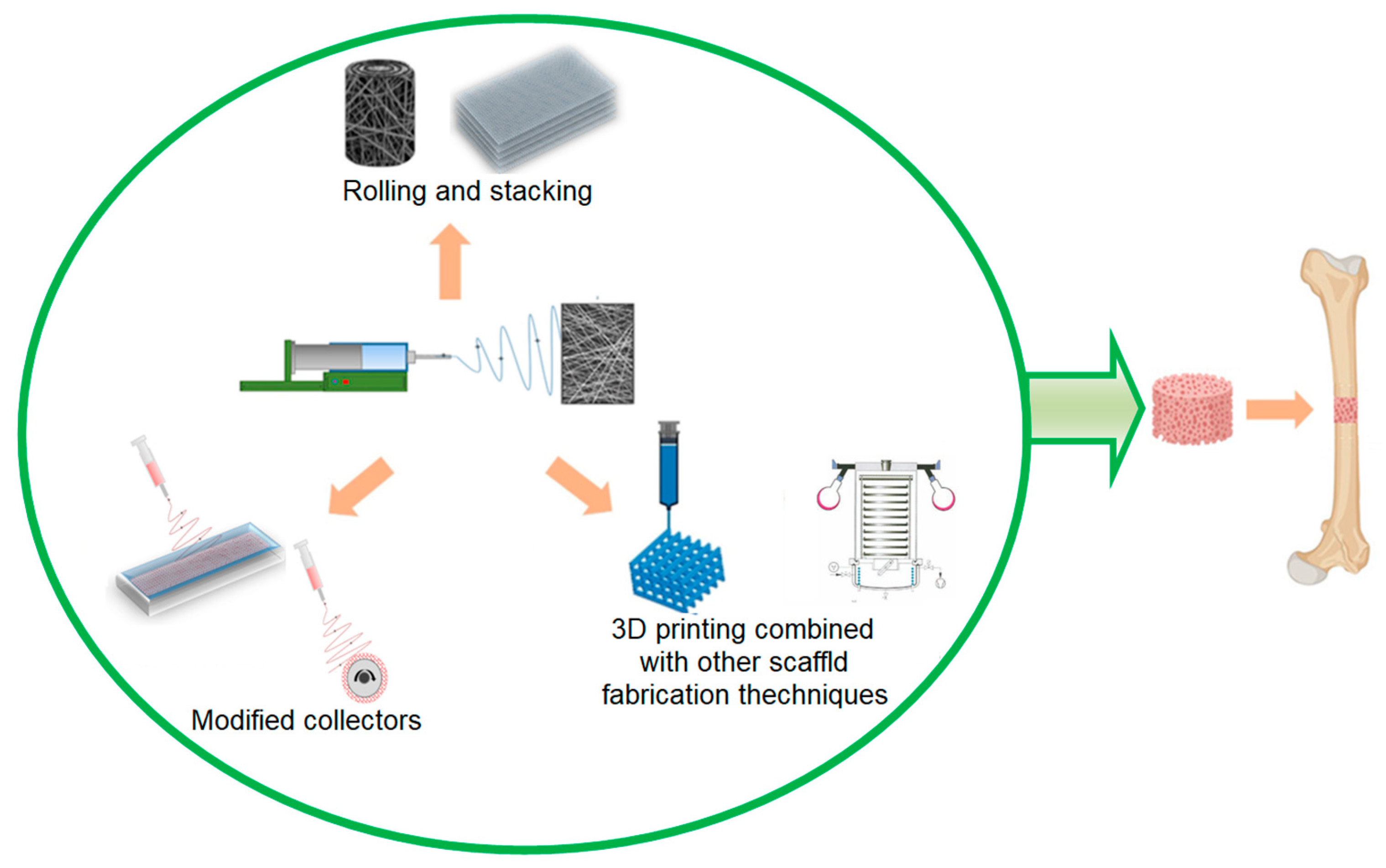
- Direct assembly using auxiliary factors, such as 3D templates [99], liquid collectors, or porogenic agents. Templates are commonly in the form of mechanical collectors with desired shapes (e.g., rotating collectors or static collectors) or other fibrous structures (e.g., microfibers), which serve as matrix templates [100,101].
- -
- Rotating collectors enable the fabrication of single or interconnected micro- and macro-tubes with multiple micropatterns [102].
- -
- -
- Liquid collectors are effective for manufacturing 3D fibrous structures by utilizing liquid deposition or vortex formation to solidify the fibers, resulting in a 3D fibrous structure [106].
- -
- The addition of porogenic agents, such as ice crystals [107,108], salt particles [109] or even certain polymers (e.g., PEO), is ideal for fabricating highly porous 3D fibrous structures [110,111]. These materials, acting as porogens, are typically mixed with the precursor during the electrospinning process and later washed away after reaching the desired thickness [109].
- (d)
- Self-assembly is a strategy for creating nest-like fibrous structures through the utilization of electrostatic forces between already collected fibers, where flying fibers are directed to settle on nearby conductive regions to dissipate their charges [112].
5. Applications
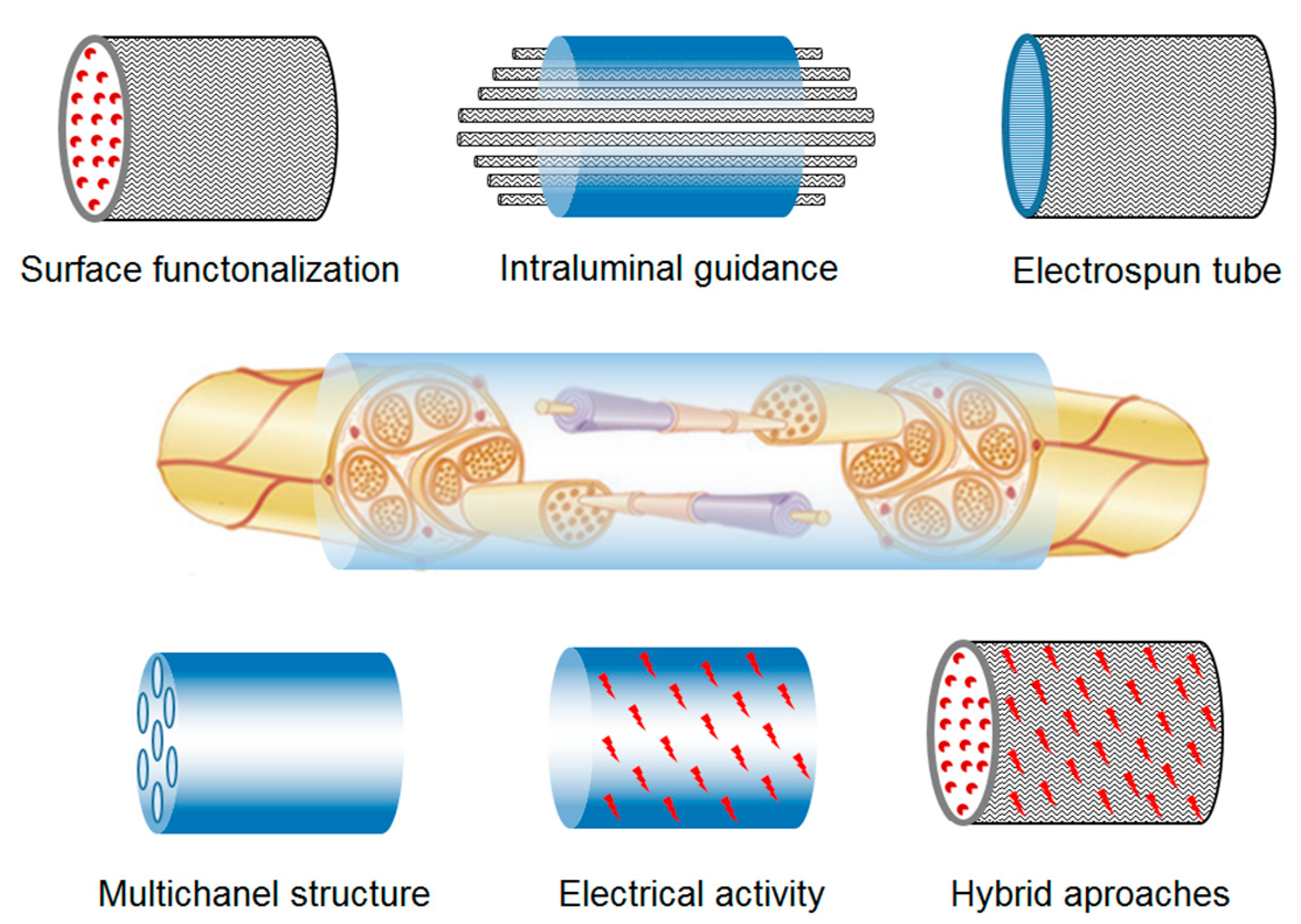
5.1. Nerve and Neural Regeneration
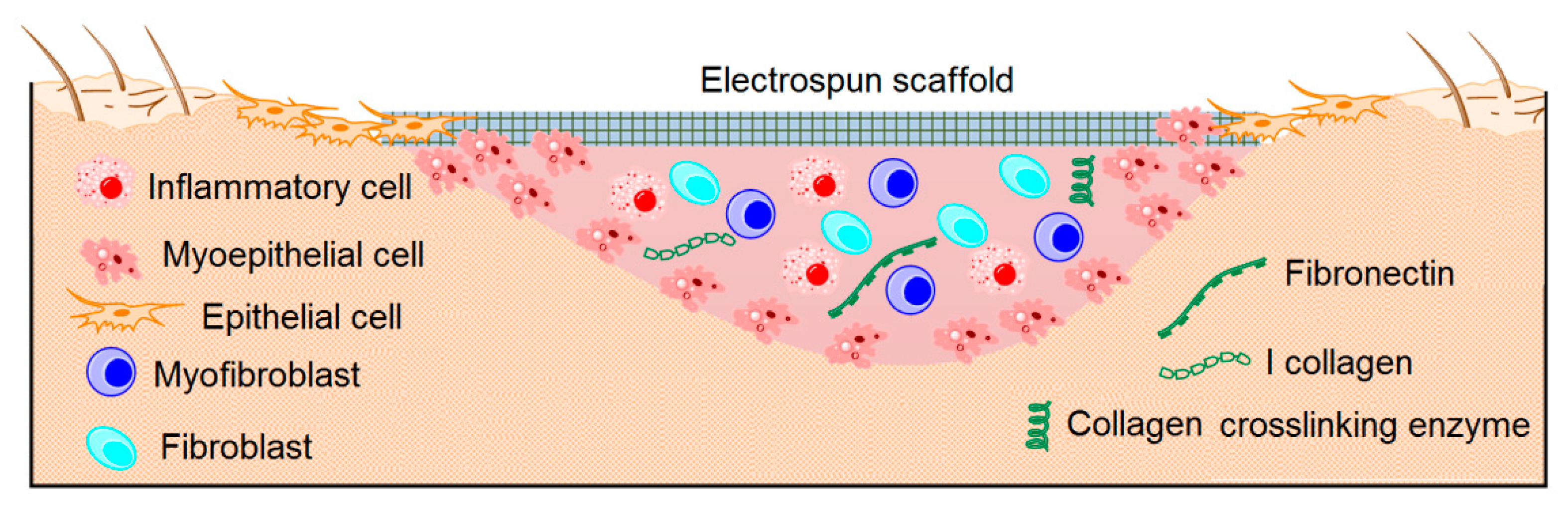
5.2. Skin Regeneration
5.3. Bone Regeneration
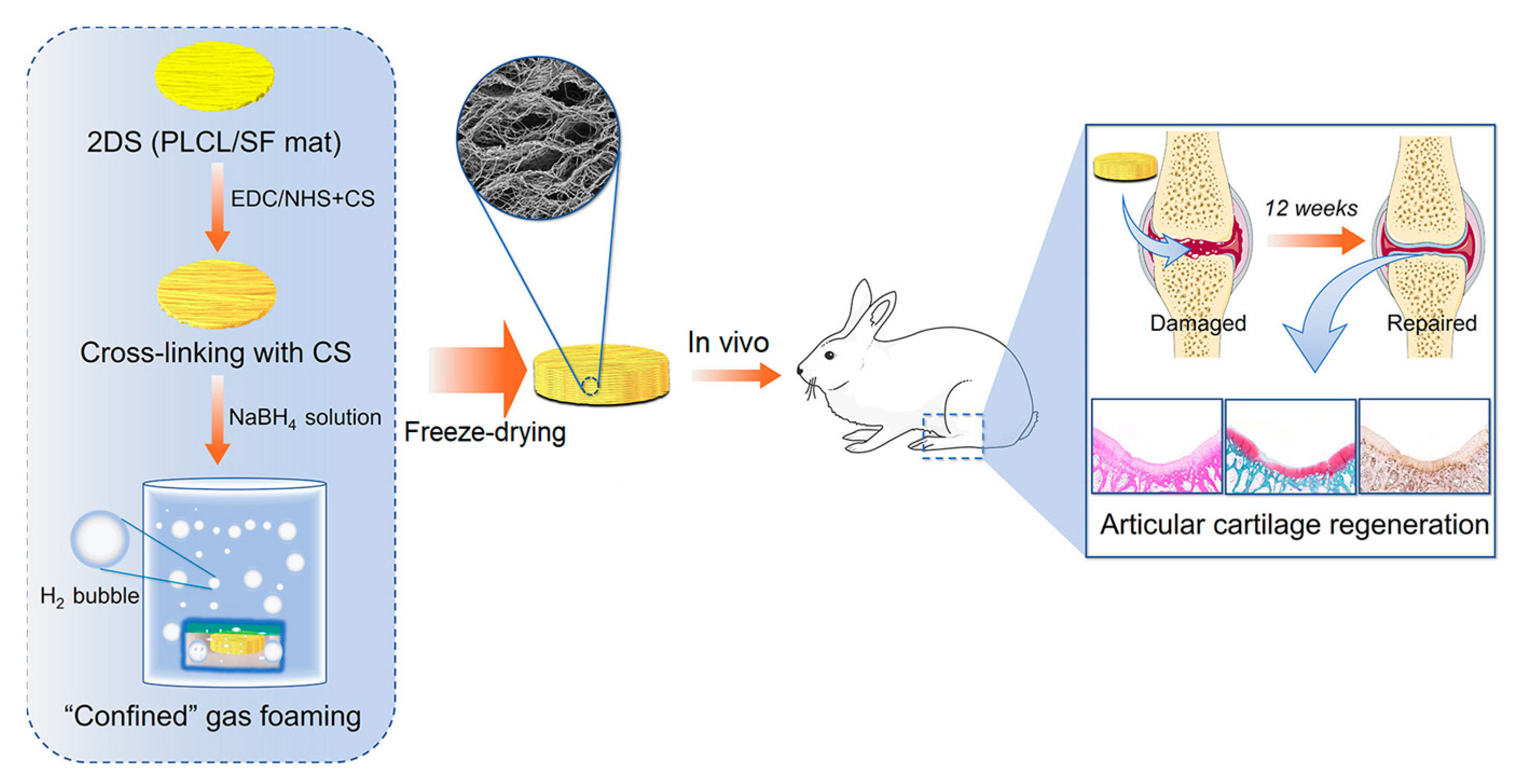
5.4. Cartilage Regeneration
5.5. Tendon and Ligament Regeneration
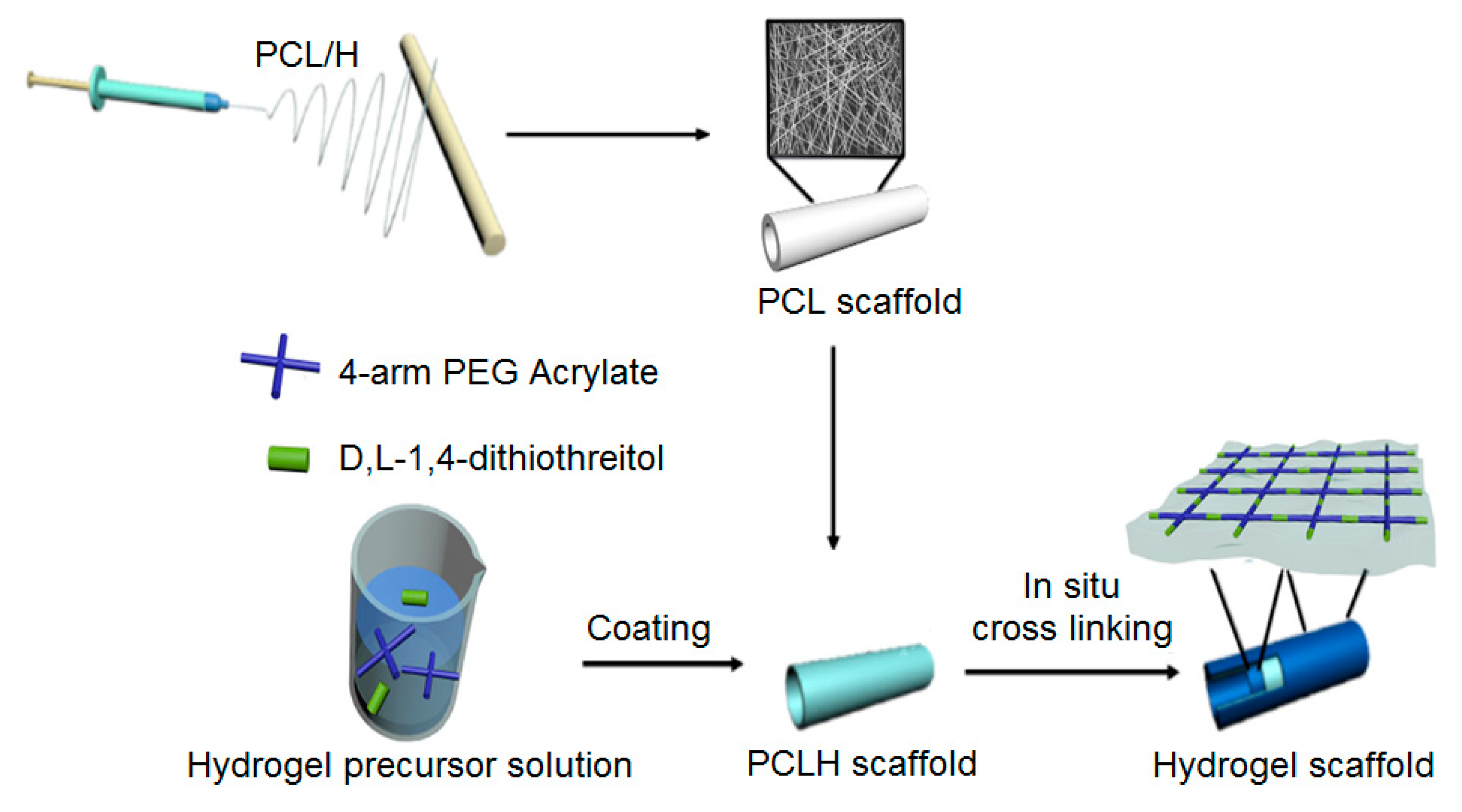
5.6. Vascular Regeneration
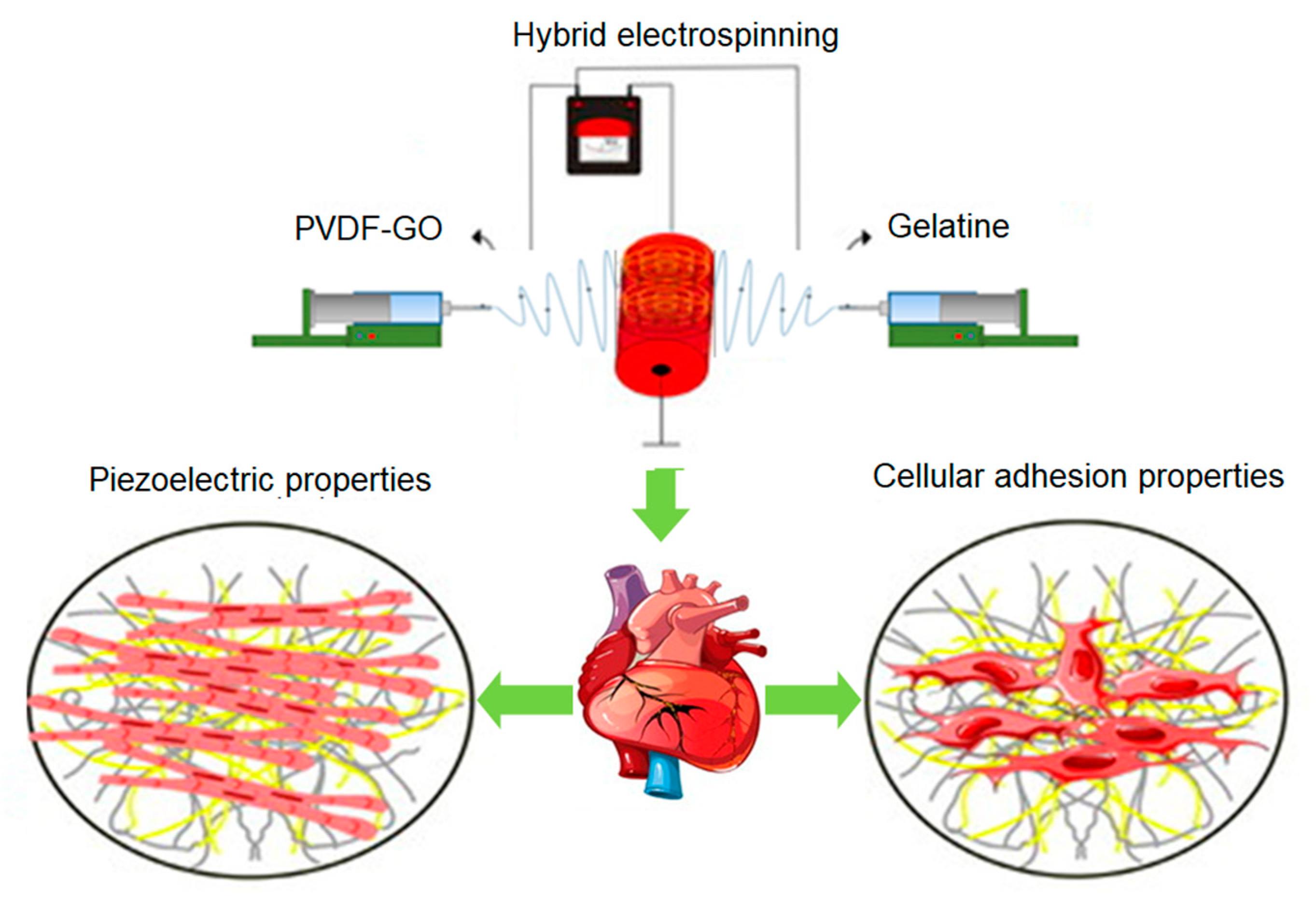
5.7. Cardiac Regeneration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tabata, Y. Tissue regeneration based on tissue engineering technology. Congenit. Anom. 2004, 44, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, E.; Goldhaber, J.I.; Marbán, E. Next-generation pacemakers: From small devices to biological pacemakers. Nat. Rev. Cardiol. 2018, 15, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Lacour, S.P.; Courtine, G.; Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016, 1, 16063. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- Guan, J.; Wang, F.; Li, Z.; Chen, J.; Guo, X.; Liao, J.; Moldovan, N.I. The stimulation of the cardiac differentiation of mesenchymal stem cells in tissue constructs that mimic myocardium structure and biomechanics. Biomaterials 2011, 32, 5568–5580. [Google Scholar] [CrossRef]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef]
- Kishan, A.P.; Cosgriff-Hernandez, E.M. Recent advancements in electrospinning design for tissue engineering applications: A review. J. Biomed. Mater. Res. Part A 2017, 105, 2892–2905. [Google Scholar] [CrossRef]
- Townsend-Nicholson, A.; Jayasinghe, S.N. Cell electrospinning: A unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules 2006, 7, 3364–3369. [Google Scholar] [CrossRef] [PubMed]
- Zussman, E.; Yarin, A.L.; Bazilevsky, A.V.; Avrahami, R.; Feldman, M. Electrospun polyacrylonitrile/poly (methyl methacrylate)-derived turbostratic carbon micro-/nanotubes. Adv. Mater. 2006, 18, 348–353. [Google Scholar] [CrossRef]
- Qiao, Y.; Shi, C.; Wang, X.; Wang, P.; Zhang, Y.; Wang, D.; Qiao, R.; Wang, X.; Zhong, J. Electrospun Nanobelt-Shaped Polymer Membranes for Fast and High-Sensitivity Detection of Metal Ions. ACS Appl. Mater. Interfaces 2019, 11, 5401–5413. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Jin, M.; Wang, F.; Greiner, A.; Agarwal, S.; Jiang, S. Core effect on mechanical properties of one dimensional electrospun core-sheath composite fibers. Compos. Commun. 2021, 25, 100773. [Google Scholar] [CrossRef]
- Trinca, R.B.; Westin, C.B.; da Silva, J.A.F.; Moraes, Â.M. Electrospun multilayer chitosan scaffolds as potential wound dressings for skin lesions. Eur. Polym. J. 2017, 88, 161–170. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, F.; Hou, L.; Li, S.; Gao, Y.; Xin, Z.; Li, Q.; Xie, S.; Wang, N.; Zhao, Y. Synergistic engineering of 1D electrospun nanofibers and 2D nanosheets for sustainable applications. Sustain. Mater. Technol. 2020, 26, e00214. [Google Scholar] [CrossRef]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: A review. WIREs Rev. Nanomed. Nanobiotechnol. 2020, 12, e1626. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Kong, Y.P.; Tu, C.H.; Donovan, P.J.; Yee, A.F. Expression of Oct4 in human embryonic stem cells is dependent on nanotopographical configuration. Acta Biomater. 2013, 9, 6369–6380. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Zhong, H.; Huang, J.; Wu, J.; Du, J. Electrospinning nanofibers to 1D, 2D, and 3D scaffolds and their biomedical applications. Nano Res. 2022, 15, 787–804. [Google Scholar] [CrossRef]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Dave, K.; Gomes, V.G. Interactions at scaffold interfaces: Effect of surface chemistry, structural attributes and bioaffinity. Mater. Sci. Eng. C 2019, 105, 110078. [Google Scholar] [CrossRef]
- Chernozem, R.V.; Guselnikova, O.; Surmeneva, M.A.; Postnikov, P.S.; Abalymov, A.A.; Parakhonskiy, B.V.; De Roo, N.; Depla, D.; Skirtach, A.G.; Surmenev, R.A. Diazonium chemistry surface treatment of piezoelectric polyhydroxybutyrate scaffolds for enhanced osteoblastic cell growth. Appl. Mater. Today 2020, 20, 100758. [Google Scholar] [CrossRef]
- Grafahrend, D.; Heffels, K.H.; Beer, M.V.; Gasteier, P.; Möller, M.; Boehm, G.; Dalton, P.D.; Groll, J. Degradable polyester scaffolds with controlled surface chemistry combining minimal protein adsorption with specific bioactivation. Nat. Mater. 2011, 10, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.S.P.; Valainis, D.; Bhattacharya, K.; van Griensven, M.; Dondl, P. Optimization of Bone Scaffold Porosity Distributions. Sci. Rep. 2019, 9, 9170. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Annabi, N. Engineering porous scaffolds using gas-based techniques. Curr. Opin. Biotechnol. 2011, 22, 661–666. [Google Scholar] [CrossRef]
- Tran, T.T.; Hamid, Z.A.; Cheong, K.Y. A Review of Mechanical Properties of Scaffold in Tissue Engineering: Aloe Vera Composites. J. Phys. Conf. Ser. 2018, 1082, 012080. [Google Scholar] [CrossRef]
- Fang, Z.; Starly, B.; Sun, W. Computer-aided characterization for effective mechanical properties of porous tissue scaffolds. CAD Comput. Aided Des. 2005, 37, 65–72. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Frenkel, S.R.; Toolan, B.; Menche, D.; Pitman, M.I.; Pachence, J.M. Chondrocyte Transplantation Using a Collagen Bilayer Matrix for Cartilage Repair. J. Bone Jt. Surg. Br. 1997, 79, 831–836. [Google Scholar] [CrossRef]
- Yap, J.X.; Leo, C.P.; Mohd Yasin, N.H.; Show, P.L.; Chu, D.T.; Singh, V.; Derek, C.J.C. Recent advances of natural biopolymeric culture scaffold: Synthesis and modification. Bioengineered 2022, 13, 2226–2247. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.C.; Yaszemski, M.J.; Powers, J.M.; Mikos, A.G. Fabrication of biodegradable polymer scaffolds to engineer trabecular bone. J. Biomater. Sci. Polym. Ed. 1996, 7, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Vert, M.; Christel, P.; Chabot, F.; Leray, J. Bioresorbable plastic materials for bone surgery. In Macromolecular Materials; CRC Press: Boca Raton, FL, USA, 2018; pp. 119–142. [Google Scholar] [CrossRef]
- Pulapura, S.; Li, C.; Kohn, J. Structure-property relationships for the design of polyiminocarbonates. Biomaterials 1990, 11, 666–678. [Google Scholar] [CrossRef]
- Daniels, A.U.; Chang, M.K.O.; Andriano, K.P.; Heller, J. Mechanical properties of biodegradable polymers and composites proposed for internal fixation of bone. J. Appl. Biomater. 1990, 1, 57–78. [Google Scholar] [CrossRef]
- Kai, D.; Liow, S.S.; Loh, X.J. Biodegradable polymers for electrospinning: Towards biomedical applications. Mater. Sci. Eng. C 2015, 45, 659–670. [Google Scholar] [CrossRef]
- Kaihara, S.; Borenstein, J.; Koka, R.; Lalan, S.; Ochoa, E.R.; Ravens, M.; Pien, H.; Cunningham, B.; Vacanti, J.P. Silicon Micromachining to Tissue Engineer Branched Vascular Channels for Liver Fabrication. Tissue Eng. 2000, 6, 105–117. [Google Scholar] [CrossRef]
- Atala, A. Tissue Engineering in the Genitourinary System. In Synthetic Biodegradable Polymer Scaffolds; Atala, A., Mooney, D.J., Eds.; Birkhäuser Boston: Boston, MA, USA, 1997; pp. 149–164. ISBN 978-1-4612-4154-6. [Google Scholar]
- Nerem, R.M.; Braddon, L.G.; Seliktar, D.; Ziegler, T. Tissue Engineering and the Vascular System. In Synthetic Biodegradable Polymer Scaffolds; Atala, A., Mooney, D.J., Eds.; Birkhäuser Boston: Boston, MA, USA, 1997; pp. 165–185. ISBN 978-1-4612-4154-6. [Google Scholar]
- Yu, N.Y.C.; Schindeler, A.; Little, D.G.; Ruys, A.J. Biodegradable poly(α-hydroxy acid) polymer scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 285–295. [Google Scholar] [CrossRef]
- Ginjupalli, K.; Shavi, G.V.; Averineni, R.K.; Bhat, M.; Udupa, N.; Nagaraja Upadhya, P. Poly(α-hydroxy acid) based polymers: A review on material and degradation aspects. Polym. Degrad. Stab. 2017, 144, 520–535. [Google Scholar] [CrossRef]
- Hertz, A.; Bruce, I.J. Inorganic materials for bone repair or replacement applications. Nanomedicine 2007, 2, 899–918. [Google Scholar] [CrossRef]
- Ninan, N.; Muthiah, M.; Park, I.K.; Wong, T.W.; Thomas, S.; Grohens, Y. Natural polymer/inorganic material based hybrid scaffolds for skin wound healing. Polym. Rev. 2015, 55, 453–490. [Google Scholar] [CrossRef]
- Terzaki, K.; Kissamitaki, M.; Skarmoutsou, A.; Fotakis, C.; Charitidis, C.A.; Farsari, M.; Vamvakaki, M.; Chatzinikolaidou, M. Pre-osteoblastic cell response on three-dimensional, organic-inorganic hybrid material scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef]
- Colombo, A.; Chieffo, A.; Frasheri, A.; Garbo, R.; Masotti-Centol, M.; Salvatella, N.; Dominguez, J.F.O.; Steffanon, L.; Tarantini, G.; Presbitero, P.; et al. Second-generation drug-eluting stent implantation followed by 6- Versus 12-month dual antiplatelet therapy. J. Am. Coll. Cardiol. 2014, 64, 2086–2097. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.T.L.; Serruys, P.W. Technology Insight: An overview of research in drug-eluting stents. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hwang, G.; Kim, T.H.; Kwon, S.J.; Kim, J.U.; Koh, K.; Park, B.; Hong, H.; Yu, K.J.; Chae, H.; et al. On-Demand Drug Release from Gold Nanoturf for a Thermo- and Chemotherapeutic Esophageal Stent. ACS Nano 2018, 12, 6756–6766. [Google Scholar] [CrossRef]
- Chen, H.; Ni, J.; Chen, J.; Xue, W.; Wang, J.; Na, H.; Zhu, J. Activation of corn cellulose with alcohols to improve its dissolvability in fabricating ultrafine fibers via electrospinning. Carbohydr. Polym. 2015, 123, 174–179. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Cheong, J.Y.; KP, A.; Makvandi, P.; Zare, E.N.; Torres-Mendieta, R.; Wacławek, S.; Černík, M.; Kim, I.-D.; Varma, R.S. Electrospun fibers based on carbohydrate gum polymers and their multifaceted applications. Carbohydr. Polym. 2020, 247, 116705. [Google Scholar] [CrossRef]
- Sanhueza, C.; Acevedo, F.; Rocha, S.; Villegas, P.; Seeger, M.; Navia, R. Polyhydroxyalkanoates as biomaterial for electrospun scaffolds. Int. J. Biol. Macromol. 2019, 124, 102–110. [Google Scholar] [CrossRef]
- Palanisamy, C.P.; Cui, B.; Zhang, H.; Gunasekaran, V.P.; Ariyo, A.L.; Jayaraman, S.; Rajagopal, P.; Long, Q. A critical review on starch-based electrospun nanofibrous scaffolds for wound healing application. Int. J. Biol. Macromol. 2022, 222, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Blackford, A.C.; Wegst, U.G.K.; Schauer, C.L. Carbon black immobilized in electrospun chitosan membranes. Carbohydr. Polym. 2011, 84, 1252–1257. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Stijnman, A.C.; Bodnar, I.; Hans Tromp, R. Electrospinning of food-grade polysaccharides. Food Hydrocoll. 2011, 25, 1393–1398. [Google Scholar] [CrossRef]
- Vicini, S.; Castellano, M.; Mauri, M.; Marsano, E. Gelling process for sodium alginate: New technical approach by using calcium rich micro-spheres. Carbohydr. Polym. 2015, 134, 767–774. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, C.; Lv, S.; Ren, F.; Wang, J. Study on electrospinning of wheat gluten: A review. Food Res. Int. 2023, 169, 112851. [Google Scholar] [CrossRef]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Hernández-Rangel, A.; Martin-Martinez, E.S. Collagen based electrospun materials for skin wounds treatment. J. Biomed. Mater. Res. Part A 2021, 109, 1751–1764. [Google Scholar] [CrossRef]
- Rodríguez-Zamora, P.; Peña-Juárez, M.C.; Cedillo-Servín, G.; Paloalto-Landon, A.; Ortega-García, I.; Maaza, M.; Vera-Graziano, R. Characterization of mechanically reinforced electrospun dextrin-polyethylene oxide sub-microfiber mats. Polym. Eng. Sci. 2019, 59, 1778–1786. [Google Scholar] [CrossRef]
- Perumcherry, S.R.; Chennazhi, K.P.; Nair, S.V.; Menon, D.; Afeesh, R. A novel method for the fabrication of fibrin-based electrospun nanofibrous scaffold for tissue-engineering applications. Tissue Eng. Part C Methods 2011, 17, 1121–1130. [Google Scholar] [CrossRef]
- Jiang, Q.; Reddy, N.; Yang, Y. Cytocompatible cross-linking of electrospun zein fibers for the development of water-stable tissue engineering scaffolds. Acta Biomater. 2010, 6, 4042–4051. [Google Scholar] [CrossRef] [PubMed]
- Arampatzis, A.S.; Giannakoula, K.; Kontogiannopoulos, K.N.; Theodoridis, K.; Aggelidou, E.; Rat, A.; Kampasakali, E.; Willems, A.; Christofilos, D.; Kritis, A.; et al. Novel electrospun poly-hydroxybutyrate scaffolds as carriers for the wound healing agents alkannins and shikonins. Regen. Biomater. 2021, 8, rbab011. [Google Scholar] [CrossRef]
- Zhuravleva, M.; Gilazieva, Z.; Grigoriev, T.E.; Shepelev, A.D.; Tenchurin, T.K.; Kamyshinsky, R.; Krasheninnikov, S.V.; Orlov, S.; Caralogli, G.; Archipova, S.; et al. In vitro assessment of electrospun polyamide-6 scaffolds for esophageal tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Janmohammadi, M.; Nourbakhsh, M.S. Electrospun polycaprolactone scaffolds for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 527–539. [Google Scholar] [CrossRef]
- Ciarfaglia, N.; Laezza, A.; Lods, L.; Lonjon, A.; Pepe, A.; Bochicchio, B.; Ciarfaglia, N.; Laezza, A.; Lods, L.; Lonjon, A.; et al. Thermal and dynamic mechanical behavior of poly(lactic acid) (PLA)-based electrospun scaffolds for tissue engineering. J. Appl. Polym. Sci. 2021, 138, 51313. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J. Electrospun poly(lactic-co-glycolic acid)/wool keratin fibrous composite scaffolds potential for bone tissue engineering applications. J. Bioact. Compat. Polym. 2013, 28, 141–153. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2020, 12, 7. [Google Scholar] [CrossRef]
- Boland, E.D.; Wnek, G.E.; Simpson, D.G.; Pawlowski, K.J.; Bowlin, G.L. Tailoring tissue engineering scaffolds using electrostatic processing techniques: A study of poly(glycolic acid) electrospinning. J. Macromol. Sci. Pure Appl. Chem. 2001, 38, 1231–1243. [Google Scholar] [CrossRef]
- Huang, A.; Peng, X.; Geng, L.; Zhang, L.; Huang, K.; Chen, B.; Gu, Z.; Kuang, T. Electrospun poly (butylene succinate)/cellulose nanocrystals bio-nanocomposite scaffolds for tissue engineering: Preparation, characterization and in vitro evaluation. Polym. Test. 2018, 71, 101–109. [Google Scholar] [CrossRef]
- Griffin, J.; Delgado-Rivera, R.; Meiners, S.; Uhrich, K.E. Salicylic acid-derived poly(anhydride-ester) electrospun fibers designed for regenerating the peripheral nervous system. J. Biomed. Mater. Res. Part A 2011, 97, 230–242. [Google Scholar] [CrossRef]
- Herwig, G.; Perez-Madrigal, M.M.; Dove, A.P. Customized Fading Scaffolds: Strong Polyorthoester Networks via Thiol-Ene Cross-linking for Cytocompatible Surface-Eroding Materials in 3D Printing. Biomacromolecules 2021, 22, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Choueka, J.; Charvet, J.L.; Koval, K.J.; Alexander, H.; James, K.S.; Hooper, K.A.; Kohn, J. Canine bone response to tyrosine-derived polycarbonates and poly (L-lactic acid). J. Biomed. Mater. Res. 1996, 31, 35–41. [Google Scholar] [CrossRef]
- Ibim, S.E.M.; Uhrich, K.E.; Attawia, M.; Shastri, V.R.; El-Amin, S.F.; Bronson, R.; Langer, R.; Laurencin, C.T. Preliminary in vivo report on the osteocompatibility of poly(anhydride- co-imides) evaluated in a tibial model. J. Biomed. Mater. Res. 1998, 43, 374–379. [Google Scholar] [CrossRef]
- Peter, S.J.; Lu, L.; Kim, D.J.; Mikos, A.G. Marrow stromal osteoblast function on a poly(propylene fumarate)/β-tricalcium phosphate biodegradable orthopaedic composite. Biomaterials 2000, 21, 1207–1213. [Google Scholar] [CrossRef]
- Yousefzade, O.; Katsarava, R.; Puiggalí, J. Biomimetic hybrid systems for tissue engineering. Biomimetics 2020, 5, 49. [Google Scholar] [CrossRef]
- Carampin, P.; Conconi, M.T.; Lora, S.; Menti, A.M.; Baiguera, S.; Bellini, S.; Grandi, C.; Parnigotto, P.P. Electrospun polyphosphazene nanofibers for in vitro rat endothelial cells proliferation. J. Biomed. Mater. Res. Part A 2007, 80, 661–668. [Google Scholar] [CrossRef]
- Caracciolo, P.C.; Thomas, V.; Vohra, Y.K.; Buffa, F.; Abraham, G.A. Electrospinning of novel biodegradable poly(ester urethane)s and poly(ester urethane urea)s for soft tissue-engineering applications. J. Mater. Sci. Mater. Med. 2009, 20, 2129–2137. [Google Scholar] [CrossRef]
- Gao, X.; Wen, M.; Liu, Y.; Hou, T.; An, M. Mechanical performance and cyocompatibility of PU/PLCL nanofibrous electrospun scaffolds for skin regeneration. Eng. Regen. 2022, 3, 53–58. [Google Scholar] [CrossRef]
- Dorkhani, E.; Noorafkan, Y.; Salehi, Z.; Ghiass, M.A.; Tafti, S.H.A.; Heirani-Tabasi, A.; Tavafoghi, M. Design and fabrication of polyvinylidene fluoride-graphene oxide/gelatine nanofibrous scaffold for cardiac tissue engineering. J. Biomater. Sci. Polym. Ed. 2023, 34, 1195–1216. [Google Scholar] [CrossRef]
- SalehHudin, H.S.; Mohamad, E.N.; Mahadi, W.N.L.; Muhammad Afifi, A. Multiple-jet electrospinning methods for nanofiber processing: A review. Mater. Manuf. Process. 2018, 33, 479–498. [Google Scholar] [CrossRef]
- Bera, B. Literature Review on Electrospinning Process (A Fascinating Fiber Fabrication Technique). Imp. J. Interdiscip. Res. 2016, 2, 972–984. [Google Scholar]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Park, S.; Park, K.; Yoon, H.; Son, J.; Min, T.; Kim, G. Apparatus for preparing electrospun nanofibers: Designing an electrospinning process for nanofiber fabrication. Polym. Int. 2006, 55, 961–969. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, X.; Zhang, L.; Liu, H.; Wang, X.; Li, Q. Polymer scaffolds for vascular tissue engineering fabricated by combined electrospinning and hot embossing. Biomed. Mater. 2018, 13, 015003. [Google Scholar] [CrossRef] [PubMed]
- Borojeni, I.A.; Gajewski, G.; Riahi, R.A. Application of Electrospun Nonwoven Fibers in Air Filters. Fibers 2022, 10, 15. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, X.; Shafiq, M.; Myles, G.; Radacsi, N.; Mo, X. Recent Advancements on Three-Dimensional Electrospun Nanofiber Scaffolds for Tissue Engineering. Adv. Fiber Mater. 2022, 4, 959–986. [Google Scholar] [CrossRef]
- Jiang, S.; Helfricht, N.; Papastavrou, G.; Greiner, A.; Agarwal, S. Low-Density Self-Assembled Poly(N-Isopropyl Acrylamide) Sponges with Ultrahigh and Extremely Fast Water Uptake and Release. Macromol. Rapid Commun. 2018, 39, 1700838. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Agarwal, S.; Greiner, A. Low-Density Open Cellular Sponges as Functional Materials. Angew. Chemie Int. Ed. 2017, 56, 15520–15538. [Google Scholar] [CrossRef]
- Robinson, A.J.; Pérez-Nava, A.; Ali, S.C.; González-Campos, J.B.; Holloway, J.L.; Cosgriff-Hernandez, E.M. Comparative analysis of fiber alignment methods in electrospinning. Matter 2021, 4, 821–844. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly (ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef]
- Thomas, V.; Dean, D.R.; Jose, M.V.; Mathew, B.; Chowdhury, S.; Vohra, Y.K. Nanostructured biocomposite scaffolds based on collagen coelectrospun with nanohydroxyapatite. Biomacromolecules 2007, 8, 631–637. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Zussman, E.; Xu, H. Electrospinning of Nanofibers from Polymer Solutions and Melts. Adv. Appl. Mech. 2007, 41, 43–195, 345–346. [Google Scholar] [CrossRef]
- Shim, I.K.; Suh, W.H.; Lee, S.Y.; Lee, S.H.; Heo, S.J.; Lee, M.C.; Lee, S.J. Chitosan nano-/microfibrous double-layered membrane with rolled-up three-dimensional structures for chondrocyte cultivation. J. Biomed. Mater. Res. Part A 2009, 90, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, S.; Zhao, G.; Fu, Q. Constructing fibrillated skeleton with highly aligned boron nitride nanosheets confined in alumina fiber via electrospinning and sintering for thermally conductive composite. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106282. [Google Scholar] [CrossRef]
- Shim, I.K.; Jung, M.R.; Kim, K.H.; Seol, Y.J.; Park, Y.J.; Park, W.H.; Lee, S.J. Novel three-dimensional scaffolds of poly (L-lactic acid) microfibers using electrospinning and mechanical expansion: Fabrication and bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 150–160. [Google Scholar]
- Navaneethan, B.; Chou, C.F. Self-Searching Writing of Human-Organ-Scale Three-Dimensional Topographic Scaffolds with Shape Memory by Silkworm-like Electrospun Autopilot Jet. ACS Appl. Mater. Interfaces 2022, 14, 42841–42851. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, S. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720. [Google Scholar] [CrossRef]
- Lee, J.K.Y.; Chen, N.; Peng, S.; Li, L.; Tian, L.; Thakor, N.; Ramakrishna, S. Polymer-based composites by electrospinning: Preparation & functionalization with nanocarbons. Prog. Polym. Sci. 2018, 86, 40–84. [Google Scholar] [CrossRef]
- Daming, Z.; Jiang, C. Electrospinning of three-dimensional nanofibrous tubes with controllable architectures. Nano Lett. 2008, 8, 3283–3287. [Google Scholar] [CrossRef]
- Pensa, N.W.; Curry, A.S.; Bonvallet, P.P.; Bellis, N.F.; Rettig, K.M.; Reddy, M.S.; Eberhardt, A.W.; Bellis, S.L. 3D printed mesh reinforcements enhance the mechanical properties of electrospun scaffolds. Biomater. Res. 2019, 23, 22. [Google Scholar] [CrossRef]
- Mayoral, I.; Bevilacqua, E.; Gómez, G.; Hmadcha, A.; González-Loscertales, I.; Reina, E.; Sotelo, J.; Domínguez, A.; Pérez-Alcántara, P.; Smani, Y.; et al. Tissue engineered in-vitro vascular patch fabrication using hybrid 3D printing and electrospinning. Mater. Today Bio 2022, 14, 100252. [Google Scholar] [CrossRef] [PubMed]
- Centola, M.; Rainer, A.; Spadaccio, C.; De Porcellinis, S.; Genovese, J.A.; Trombetta, M. Combining electrospinning and fused deposition modeling for the fabrication of a hybrid vascular graft. Biofabrication 2010, 2, 014102. [Google Scholar] [CrossRef]
- Ki, C.S.; Kim, J.W.; Hyun, J.H.; Lee, K.H.; Hattori, M.; Rah, D.K.; Park, Y.H. Electrospun three-dimensional silk fibroin nanofibrous scaffold. J. Appl. Polym. Sci. 2007, 106, 3922–3928. [Google Scholar] [CrossRef]
- Leong, M.F.; Rasheed, M.Z.; Lim, T.C.; Chian, K.S. In vitro cell infiltration and in vivo cell infiltration and vascularization in a fibrous, highly porous poly(D,L-lactide) scaffold fabricated by cryogenic electrospinning technique. J. Biomed. Mater. Res. Part A 2009, 91, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Oliaro-Bosso, S.; Zonari, D.; Zattoni, A.; Ugazio, E. Mesoporous silica nanoparticles as a promising skin delivery system for methotrexate. Int. J. Pharm. 2017, 530, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Chung, H.J.; Park, T.G. Macroporous and nanofibrous hyaluronic acid/collagen hybrid scaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. Acta Biomater. 2008, 4, 1611–1619. [Google Scholar] [CrossRef]
- Phipps, M.C.; Clem, W.C.; Grunda, J.M.; Clines, G.A.; Bellis, S.L. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials 2012, 33, 524–534. [Google Scholar] [CrossRef]
- Klumpp, D.; Rudisile, M.; Kühnle, R.I.; Hess, A.; Bitto, F.F.; Arkudas, A.; Bleiziffer, O.; Boos, A.M.; Kneser, U.; Horch, R.E.; et al. Three-dimensional vascularization of electrospun PCL/collagen-blend nanofibrous scaffolds in vivo. J. Biomed. Mater. Res. Part A 2012, 100, 2302–2311. [Google Scholar] [CrossRef]
- Sun, B.; Long, Y.Z.; Yu, F.; Li, M.M.; Zhang, H.D.; Li, W.J.; Xu, T.X. Self-assembly of a three-dimensional fibrous polymer sponge by electrospinning. Nanoscale 2012, 4, 2134–2137. [Google Scholar] [CrossRef]
- Han, D.; Gouma, P.I. Electrospun bioscaffolds that mimic the topology of extracellular matrix. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 37–41. [Google Scholar] [CrossRef]
- Xu, Y.; Patnaik, S.; Guo, X.; Li, Z.; Lo, W.; Butler, R.; Claude, A.; Liu, Z.; Zhang, G.; Liao, J.; et al. Cardiac differentiation of cardiosphere-derived cells in scaffolds mimicking morphology of the cardiac extracellular matrix. Acta Biomater. 2014, 10, 3449–3462. [Google Scholar] [CrossRef]
- Weiss, D.J.; Bates, J.H.T.; Gilbert, T.; Liles, W.C.; Lutzko, C.; Rajagopal, J.; Prockop, D. Stem cells and cell therapies in lung biology and diseases: Conference report. Ann. Am. Thorac. Soc. 2013, 10, S25–S44. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Hwang, T.I.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Controlled Design of Aligned and Random Nanofibers for 3D Bi-functionalized Nerve Conduits Fabricated via a Novel Electrospinning Set-up. Sci. Rep. 2016, 6, 23761. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Kim, G.H. Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater. 2020, 107, 102–114. [Google Scholar] [CrossRef]
- Mejía Suaza, M.L.; Moncada, M.E.; Ossa-Orozco, C.P. Characterization of Electrospun Silk Fibroin Scaffolds for Bone Tissue Engineering: A Review. TecnoLógicas 2020, 23, 33–51. [Google Scholar] [CrossRef]
- Cui, W.; Zhou, Y.; Chang, J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci. Technol. Adv. Mater. 2010, 11, 014108. [Google Scholar] [CrossRef]
- Liu, Z.; Ramakrishna, S.; Liu, X. Electrospinning and emerging healthcare and medicine possibilities. APL Bioeng. 2020, 4, 030901. [Google Scholar] [CrossRef]
- Tanaka, E.M.; Ferretti, P. Considering the evolution of regeneration in the central nervous system. Nat. Rev. Neurosci. 2009, 10, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Bard, P. Anatomical organization of the central nervous system in relation to control of the heart and blood vessels. Physiol. Rev. Suppl. 1960, 4, 3–26. [Google Scholar]
- Popović, D.B.; Sinkjær, T. Diseases and Injuries of the Central Nervous System Leading to Sensory–Motor Impairment. In Introduction to Neural Engineering for Motor Rehabilitation; IEEE Press: Piscataway, NJ, USA, 2013; pp. 3–20. ISBN 9781118628522. [Google Scholar]
- Gaudin, R.; Knipfer, C.; Henningsen, A.; Smeets, R.; Heiland, M.; Hadlock, T. Approaches to peripheral nerve repair: Generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. BioMed Res. Int. 2016, 2016, 3856262. [Google Scholar] [CrossRef]
- Muheremu, A.; Ao, Q. Past, Present, and Future of Nerve Conduits in the Treatment of Peripheral Nerve Injury. BioMed Res. Int. 2015, 2015, 237507. [Google Scholar] [CrossRef]
- Manktelow, R.T. Functioning Muscle Transplantation to the Upper Limb. Clin. Plast. Surg. 1984, 11, 65–71. [Google Scholar] [CrossRef]
- Quan, Q.; Chang, B.; Meng, H.Y.; Liu, R.X.; Wang, Y.; Lu, S.B.; Peng, J.; Zhao, Q. Use of electrospinning to construct biomaterials for peripheral nerve regeneration. Rev. Neurosci. 2016, 27, 761–768. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; Lowery, J.; Del Carro, U.; Taraballi, F.; Amadio, S.; Vescovi, A.; Gelain, F. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 2008, 8, 39. [Google Scholar] [CrossRef]
- Li, Y.F.; Gregersen, H.; Nygaard, J.V.; Cheng, W.; Yu, Y.; Huang, Y.; Dong, M.; Besenbacher, F.; Chen, M. Ultraporous nanofeatured PCL-PEO microfibrous scaffolds enhance cell infiltration, colonization and myofibroblastic differentiation. Nanoscale 2015, 7, 14989–14995. [Google Scholar] [CrossRef]
- Gregory, H.; Phillips, J.B. Materials for peripheral nerve repair constructs: Natural proteins or synthetic polymers? Neurochem. Int. 2021, 143, 104953. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Hild, M.; Toskas, G.; Aibibu, D.; Wittenburg, G.; Meissner, H.; Cherif, C.; Hund, R.D. Chitosan/gelatin micro/nanofiber 3D composite scaffolds for regenerative medicine. Compos. Interfaces 2014, 21, 301–308. [Google Scholar] [CrossRef]
- Jiang, T.; Carbone, E.J.; Lo, K.W.H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24. [Google Scholar] [CrossRef]
- Entekhabi, E.; Haghbin Nazarpak, M.; Shafieian, M.; Mohammadi, H.; Firouzi, M.; Hassannejad, Z. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2021, 109, 300–312. [Google Scholar] [CrossRef]
- Hurtado, A.; Cregg, J.M.; Wang, H.B.; Wendell, D.F.; Oudega, M.; Gilbert, R.J.; McDonald, J.W. Robust CNS regeneration after complete spinal cord transection using aligned poly-l-lactic acid microfibers. Biomaterials 2011, 32, 6068–6079. [Google Scholar] [CrossRef]
- Taskin, M.B.; Xu, R.; Gregersen, H.; Nygaard, J.V.; Besenbacher, F.; Chen, M. Three-Dimensional Polydopamine Functionalized Coiled Microfibrous Scaffolds Enhance Human Mesenchymal Stem Cells Colonization and Mild Myofibroblastic Differentiation. ACS Appl. Mater. Interfaces 2016, 8, 15864–15873. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Haftel, V.K.; Kumar, S.; Bellamkonda, R.V. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials 2008, 29, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.B.; Kolodkin, A.L.; Ginty, D.D.; Cloutier, J.F. Signaling at the growth cone: Ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 2003, 26, 509–563. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.; Sun, J.; Zhuang, Y.; Shi, J.; Guan, D.; Chen, Y.; Dai, J. Radially Aligned Electrospun Fibers with Continuous Gradient of SDF1α for the Guidance of Neural Stem Cells. Small 2016, 12, 5009–5018. [Google Scholar] [CrossRef]
- Lee, D.J.; Fontaine, A.; Meng, X.; Park, D. Biomimetic Nerve Guidance Conduit Containing Intraluminal Microchannels with Aligned Nanofibers Markedly Facilitates in Nerve Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1403–1410. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Y.; Ding, S.; Zhang, K.; Mao, H.Q.; Yang, Y. Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering. Biomaterials 2020, 255, 120164. [Google Scholar] [CrossRef]
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic scaffolds for tissue engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Roldán, S.; Vargas, C.; Mejía, M.; Zapata, J.; Moncada, M. Tipos de Biomateriales Empleaos en la Ingenieria de Tejidos. In Ingeniería de Tejidos y Aplicaciones; Fondo Editorial ITM: Medellín, Colombia, 2016; ISBN 9789588743844. [Google Scholar]
- Heumann, R.; Lindholm, D.; Bandtlow, C.; Meyer, M.; Radeke, M.J.; Misko, T.P.; Shooter, E.; Thoenen, H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: Role of macrophages. Proc. Natl. Acad. Sci. USA 1987, 84, 8735–8739. [Google Scholar] [CrossRef]
- Liu, H.M.; Yang, L.H.; Yang, Y.J. Schwann Cell Properties: 3. C-fos Expression, bFGF Production, Phagocytosis and Proliferation During Wallerian Degeneration. J. Neuropathol. Exp. Neurol. 1995, 54, 487–496. [Google Scholar] [CrossRef]
- Wittmer, C.R.; Claudepierre, T.; Reber, M.; Wiedemann, P.; Garlick, J.A.; Kaplan, D.; Egles, C. Multifunctionalized electrospun silk fibers promote axon regeneration in the central nervous system. Adv. Funct. Mater. 2011, 21, 4232–4242. [Google Scholar] [CrossRef]
- Dinis, T.M.; Elia, R.; Vidal, G.; Auffret, A.; Kaplan, D.L.; Egles, C. Method to form a fiber/growth factor dual-gradient along electrospun silk for nerve regeneration. ACS Appl. Mater. Interfaces 2014, 6, 16817–16826. [Google Scholar] [CrossRef] [PubMed]
- Roman, J.A.; Reucroft, I.; Martin, R.A.; Hurtado, A.; Mao, H.Q. Local Release of Paclitaxel from Aligned, Electrospun Microfibers Promotes Axonal Extension. Adv. Healthc. Mater. 2016, 5, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Guo, B.; Shao, Y.; Ma, P.X. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials 2016, 87, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Maurel, P.; Einheber, S.; Galinska, J.; Thaker, P.; Lam, I.; Rubin, M.B.; Scherer, S.S.; Murakami, Y.; Gutmann, D.H.; Salzer, J.L. Nectin-like proteins mediate axon-Schwann cell interactions along the internode and are essential for myelination. J. Cell Biol. 2007, 178, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar] [CrossRef]
- Wang, W.; Itoh, S.; Konno, K.; Kikkawa, T.; Ichinose, S.; Sakai, K.; Ohkuma, T.; Watabe, K. Effects of Schwann cell alignment along the oriented electrospun chitosan nanofibers on nerve regeneration. J. Biomed. Mater. Res. Part A 2009, 91, 994–1005. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Fabrication of uniaxially aligned 3D electrospun scaffolds for neural regeneration. Biomed. Mater. 2011, 6, 025004. [Google Scholar] [CrossRef]
- Wang, T.Y.; Forsythe, J.S.; Nisbet, D.R.; Parish, C.L. Promoting engraftment of transplanted neural stem cells/progenitors using biofunctionalised electrospun scaffolds. Biomaterials 2012, 33, 9188–9197. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Losquadro, W.D. Anatomy of the Skin and the Pathogenesis of Nonmelanoma Skin Cancer. Facial Plast. Surg. Clin. N. Am. 2017, 25, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.E.; He, W.; Ramakrishna, S. Electrospun scaffold tailored for tissue-specific extracellular matrix. Biotechnol. J. Healthc. Nutr. Technol. 2006, 1, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chen, H.H.; Chang, S.H.; Ni, T.S. Pectin-chitosan-PVA nanofibrous scaffold made by electrospinning and its potential use as a skin tissue scaffold. J. Biomater. Sci. Polym. Ed. 2013, 24, 470–484. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef]
- Sun, L.; Gao, W.; Fu, X.; Shi, M.; Xie, W.; Zhang, W.; Zhao, F.; Chen, X. Enhanced wound healing in diabetic rats by nanofibrous scaffolds mimicking the basketweave pattern of collagen fibrils in native skin. Biomater. Sci. 2018, 6, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cheng, L.; Zhu, W.; Hu, C.; Jin, R.; Sun, B.; Shi, Y.; Zhang, Y.; Cui, W. Use of ginsenoside Rg3-loaded electrospun PLGA fibrous membranes as wound cover induces healing and inhibits hypertrophic scar formation of the skin. Colloids Surf. B Biointerfaces 2014, 115, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cheng, L.; Zhao, J.; Jin, R.; Sun, B.; Shi, Y.; Zhang, L.; Zhang, Y.; Cui, W. BFGF-grafted electrospun fibrous scaffolds via poly(dopamine) for skin wound healing. J. Mater. Chem. B 2014, 2, 3636–3645. [Google Scholar] [CrossRef]
- Larjava, H.; Häkkinen, L.; Koivisto, L. Re-epithelialization of wounds. In Oral Wound Healing; Larjava, H., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 81–123. [Google Scholar]
- Chen, J.; Zhang, G.; Zhao, Y.; Zhou, M.; Zhong, A.; Sun, J. Promotion of skin regeneration through co-axial electrospun fibers loaded with basic fibroblast growth factor. Adv. Compos. Hybrid Mater. 2022, 5, 1111–1125. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Ran, B.; Yang, X.; Zheng, W.; Long, Y.; Jiang, X. Small Molecular TGF-β1-Inhibitor-Loaded Electrospun Fibrous Scaffolds for Preventing Hypertrophic Scars. ACS Appl. Mater. Interfaces 2017, 9, 32545–32553. [Google Scholar] [CrossRef]
- Shin, S.H.; Purevdorj, O.; Castano, O.; Planell, J.A.; Kim, H.W. A short review: Recent advances in electrospinning for bone tissue regeneration. J. Tissue Eng. 2012, 3, 2041731412443530. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Castano, O.; Kim, H.W. Electrospun materials as potential platforms for bone tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.W.; Weiss, L.E.; Sundine, M.J. New frontiers in bone tissue engineering. Clin. Plast. Surg. 2003, 30, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Cornell, C.N. Osteoconductive materials and their role as substitutes for autogenous bone grafts. Orthop. Clin. N. Am. 1999, 30, 591–598. [Google Scholar] [CrossRef]
- Di Martino, A.; Liverani, L.; Rainer, A.; Salvatore, G.; Trombetta, M.; Denaro, V. Electrospun scaffolds for bone tissue engineering. Musculoskelet. Surg. 2011, 95, 69–80. [Google Scholar] [CrossRef]
- Pramanik, S.; Pingguan-Murphy, B.; Abu Osman, N.A. Progress of key strategies in development of electrospun scaffolds: Bone tissue. Sci. Technol. Adv. Mater. 2012, 13, 043002. [Google Scholar] [CrossRef]
- Yu, Y.; Hua, S.; Yang, M.; Fu, Z.; Teng, S.; Niu, K.; Zhao, Q.; Yi, C. Fabrication and characterization of electrospinning/3D printing bone tissue engineering scaffold. RSC Adv. 2016, 6, 110557–110565. [Google Scholar] [CrossRef]
- Jain, S.; Krishna Meka, S.R.; Chatterjee, K. Curcumin eluting nanofibers augment osteogenesis toward phytochemical based bone tissue engineering. Biomed. Mater. 2016, 11, 055007. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Allo, B.A.; Rizkalla, A.S.; Mequanint, K. Synthesis and electrospinning of ε-polycaprolactone-bioactive glass hybrid biomaterials via a sol-gel process. Langmuir 2010, 26, 18340–18348. [Google Scholar] [CrossRef]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore Size of Porous Hydroxyapatite as the Cell-Substratum Controls BMP-Induced Osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef]
- Kim, H.-W.; Lee, H.-H.; Knowles, J.C. Electrospinning biomedical nanocomposite fibers of hydroxyapatite/poly(lactic acid) for bone regeneration. J. Biomed. Mater. Res. Part A 2006, 79A, 643–649. [Google Scholar] [CrossRef]
- Barbon, S.; Contran, M.; Stocco, E.; Todros, S.; Macchi, V.; De Caro, R.; Porzionato, A. Enhanced biomechanical properties of polyvinyl alcohol-based hybrid scaffolds for cartilage tissue engineering. Processes 2021, 9, 730. [Google Scholar] [CrossRef]
- Morejón, L.; Delgado, J.A.; Ribeiro, A.A.; de Oliveira, M.V.; Mendizábal, E.; García, I.; Alfonso, A.; Poh, P.; van Griensven, M.; Balmayor, E.R. Development, characterization and in vitro biological properties of scaffolds fabricated from calcium phosphate nanoparticles. Int. J. Mol. Sci. 2019, 20, 1790. [Google Scholar] [CrossRef]
- Song, L.; Xie, X.; Lv, C.; ur Rehman Khan, A.; Sun, Y.; Li, R.; Yao, J.; El-Newehy, M.; El-Hamshary, H.; Morsi, Y.; et al. Electrospun biodegradable nanofibers loaded with epigallocatechin gallate for guided bone regeneration. Compos. Part B Eng. 2022, 238, 109920. [Google Scholar] [CrossRef]
- Guo, B.; Glavas, L.; Albertsson, A.C. Biodegradable and electrically conducting polymers for biomedical applications. Prog. Polym. Sci. 2013, 38, 1263–1286. [Google Scholar] [CrossRef]
- Alonso, L.M.; Torres, I.F.; Tamayo, Á.M.Z.; Lozano, O.E.L.; Ramos, I.D.; García-Menocal, J.Á.D.; Rios-Donato, N.; Mendizábal, E. Antibacterial effect of acrylic bone cements loaded with drugs of different action’s mechanism. J. Infect. Dev. Ctries 2019, 13, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.R.; Low, S.; Choon, A.T.; Kumar, A.B.; Ramakrishna, S. Nanobioengineered electrospun composite nanofibers and osteoblasts for bone regeneration. Artif. Organs 2008, 32, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Morejón, L.; Mendizábal, A.E.; García-Menocal, J.A.D.; Ginebra, M.P.; Aparicio, C.; Mur, F.J.G.; Marsal, M.; Davidenko, N.; Ballesteros, M.E.; Planell, J.A. Static mechanical properties of hydroxyapatite (HA) powder-filled acrylic bone cements: Effect of type of HA powder. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72, 345–352. [Google Scholar] [CrossRef]
- Caeiro, J.R.; González, P.; Guede, D. Biomechanics and bone (& II): Trials in different hierarchical levels of bone and alternative tools for the determination of bone strength. Rev. Osteoporos. Metab. Miner. 2013, 5, 99–108. [Google Scholar]
- Madhurakkat Perikamana, S.K.; Lee, J.; Ahmad, T.; Jeong, Y.; Kim, D.G.; Kim, K.; Shin, H. Effects of immobilized BMP-2 and nanofiber morphology on in vitro osteogenic differentiation of hMSCs and in vivo collagen assembly of regenerated bone. ACS Appl. Mater. Interfaces 2015, 7, 8798–8808. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, W.; Müller, T.; Schubert, D.W.; Boccaccini, A.R.; Yao, Q.; Roether, J.A. Electrospun Polyhydroxybutyrate/Poly(ϵ-caprolactone)/58S Sol-Gel Bioactive Glass Hybrid Scaffolds with Highly Improved Osteogenic Potential for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17098–17108. [Google Scholar] [CrossRef] [PubMed]
- Banimohamad-Shotorbani, B.; Rahmani Del Bakhshayesh, A.; Mehdipour, A.; Jarolmasjed, S.; Shafaei, H. The efficiency of PCL/HAp electrospun nanofibers in bone regeneration: A review. J. Med. Eng. Technol. 2021, 45, 511–531. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Song, J.; Ji, P.; Zhang, X.; Li, X.; Xu, X.; Wang, M.; Zhang, S.; Deng, Y.; Deng, F.; et al. Polydopamine-templated hydroxyapatite reinforced polycaprolactone composite nanofibers with enhanced cytocompatibility and osteogenesis for bone tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 3499–3515. [Google Scholar] [CrossRef]
- Dhinasekaran, D.; Vimalraj, S.; Rajendran, A.R.; Saravanan, S.; Purushothaman, B.; Subramaniam, B. Bio-inspired multifunctional collagen/electrospun bioactive glass membranes for bone tissue engineering applications. Mater. Sci. Eng. C 2021, 126, 111856. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, Y.; Lv, H.; Si, Y.; Liu, L.; Zhang, Q.; Cao, J.; Yu, J.; Li, X.; Ding, B. 3D Superelastic Scaffolds Constructed from Flexible Inorganic Nanofibers with Self-Fitting Capability and Tailorable Gradient for Bone Regeneration. Adv. Funct. Mater. 2019, 29, 1901407. [Google Scholar] [CrossRef]
- Sofi, H.S.; Ashraf, R.; Beigh, M.A.; Sheikh, F.A. Scaffolds Fabricated from Natural Polymers/Composites by Electrospinning for Bone Tissue Regeneration. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Chun, H.J., Park, C.H., Kwon, I.K., Khang, G., Eds.; Springer: Singapore, 2018; pp. 49–78. ISBN 978-981-13-0950-2. [Google Scholar]
- Castilla-Casadiego, D.A.; Maldonado, M.; Sundaram, P.; Almodovar, J. “Green” electrospinning of a collagen/hydroxyapatite composite nanofibrous scaffold. MRS Commun. 2016, 6, 402–407. [Google Scholar] [CrossRef]
- Schröder, H.C.; Wang, X.; Neufurth, M.; Wang, S.; Tan, R.; Müller, W.E.G. Inorganic Polymeric Materials for Injured Tissue Repair: Biocatalytic Formation and Exploitation. Biomedicines 2022, 10, 658. [Google Scholar] [CrossRef]
- Wren, A.W. 45S5 Bioglass Based Scaffolds for Skeletal Repair. In Biocompatible Glasses: From Bone Regeneration to Cancer Treatment; Marchi, J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 183–201. ISBN 978-3-319-44249-5. [Google Scholar]
- Müller, A.; Schleid, T.; Doser, M.; Müschenborn, N.; Tautzenberger, A.; Ignatius, A.; Clauß, B.; Buchmeiser, M.R. Calcium Cl/OH-apatite, Cl/OH-apatite/Al2O3 and Ca3(PO4)2 fibre nonwovens: Potential ceramic components for osteosynthesis. J. Eur. Ceram. Soc. 2014, 34, 3993–4000. [Google Scholar] [CrossRef]
- Dong, S.; Sun, J.; Li, Y.; Li, J.; Cui, W.; Li, B. Electrospun nanofibrous scaffolds of poly (l-lactic acid)-dicalcium silicate composite via ultrasonic-aging technique for bone regeneration. Mater. Sci. Eng. C 2014, 35, 426–433. [Google Scholar] [CrossRef]
- Sanaei-rad, P.; Jafarzadeh Kashi, T.S.; Seyedjafari, E.; Soleimani, M. Enhancement of stem cell differentiation to osteogenic lineage on hydroxyapatite-coated hybrid PLGA/gelatin nanofiber scaffolds. Biologicals 2016, 44, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Che, L.; Ha, Y.; Ryu, W. Mechanically-reinforced electrospun composite silk fibroin nanofibers containing hydroxyapatite nanoparticles. Mater. Sci. Eng. C 2014, 40, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yao, H.; Wang, J.; Wang, D.; Liu, Q.; Li, Z. Greener synthesis of electrospun collagen/ hydroxyapatite composite fibers with an excellent microstructure for bone tissue engineering. Int. J. Nanomed. 2015, 10, 3203–3215. [Google Scholar] [CrossRef]
- Zhang, Y.; Venugopal, J.R.; El-Turki, A.; Ramakrishna, S.; Su, B.; Lim, C.T. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials 2008, 29, 4314–4322. [Google Scholar] [CrossRef]
- Saveleva, M.S.; Ivanov, A.N.; Kurtukova, M.O.; Atkin, V.S.; Ivanova, A.G.; Lyubun, G.P.; Martyukova, A.V.; Cherevko, E.I.; Sargsyan, A.K.; Fedonnikov, A.S.; et al. Hybrid PCL/CaCO3 scaffolds with capabilities of carrying biologically active molecules: Synthesis, loading and in vivo applications. Mater. Sci. Eng. C 2018, 85, 57–67. [Google Scholar] [CrossRef]
- Coverdale, B. Incorporation of Surfactants into Electrospun Scaffolds for Improved Bone Tissue Engineering Applications. Ph.D. Thesis, University of Manchester, Manchester, UK, 2016. [Google Scholar]
- Gualandi, C.; Celli, A.; Zucchelli, A.; Focarete, M.L. Nanohybrid Materials by Electrospinning. In Organic-Inorganic Hybrid Nanomaterials; Kalia, S., Haldorai, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 87–142. ISBN 978-3-319-13593-9. [Google Scholar]
- Jeong, S.I.; Ko, E.K.; Yum, J.; Jung, C.H.; Lee, Y.M.; Shin, H. Nanofibrous poly(lactic acid)/hydroxyapatite composite scaffolds for guided tissue regeneration. Macromol. Biosci. 2008, 8, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1253–1263. [Google Scholar] [CrossRef]
- McKeon-Fischer, K.D.; Freeman, J.W. Characterization of electrospun poly (L-lactide) and gold nanoparticle composite scaffolds for skeletal muscle tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 560–568. [Google Scholar] [CrossRef]
- Bahremandi-Toloue, E.; Mohammadalizadeh, Z.; Mukherjee, S.; Karbasi, S. Incorporation of inorganic bioceramics into electrospun scaffolds for tissue engineering applications: A review. Ceram. Int. 2022, 48, 8803–8837. [Google Scholar] [CrossRef]
- Martins, A.; Chung, S.; Pedro, A.J.; Sousa, R.A.; Marques, A.P.; Reis, R.L.; Neves, N.M. Hierarchical starch-based fibrous scaffold for bone tissue engineering applications. J. Tissue Eng. Regen. Med. 2009, 3, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Z.; Zhang, G.R.; Wang, L.L.; Jiang, Y.Z.; Ouyang, H.W.; Zou, X.H. Novel biodegradable three-dimensional macroporous scaffold using aligned electrospun nanofibrous yarns for bone tissue engineering. J. Biomed. Mater. Res. Part A 2012, 100, 1187–1194. [Google Scholar] [CrossRef]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef]
- Stockwell, R.A. The cell density of human articular and costal cartilage. J. Anat. 1967, 101, 753–763. [Google Scholar] [PubMed]
- Caplan, A.I. Cartilage. Sci. Am. 1984, 251, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Myers, E.R.; Lai, W.M.; Mow, V.C. A Continuum Theory and an Experiment for the Ion-Induced Swelling Behavior of Articular Cartilage. J. Biomech. Eng. 1984, 106, 151–158. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Dong, C.; Jiang, H.; Xue, B.; Gao, X.; Qin, M.; Wang, W.; Chen, B.; Cao, Y. Rationally designed synthetic protein hydrogels with predictable mechanical properties. Nat. Commun. 2018, 9, 620. [Google Scholar] [CrossRef]
- Mahmood, H.; Eckold, D.; Stead, I.; Shepherd, D.E.T.; Espino, D.M.; Dearn, K.D. A method for the assessment of the coefficient of friction of articular cartilage and a replacement biomaterial. J. Mech. Behav. Biomed. Mater. 2020, 103, 103580. [Google Scholar] [CrossRef]
- Liu, S.H.; Yang, R.S.; Al-Shaikh, R.; Lane, J.M. Collagen in tendon, ligament, and bone healing: A current review. Clin. Orthop. Relat. Res. 1995, 318, 265–278. [Google Scholar]
- Knutsen, G.; Drogset, J.O.; Engebretsen, L.; Grøntvedt, T.; Isaksen, V.; Ludvigsen, T.C.; Roberts, S.; Solheim, E.; Strand, T.; Johansen, O. A randomized trial comparing autologous chondrocyte implantation with microfracture: Findings at five years. J. Bone Jt. Surg. 2007, 89, 2105–2112. [Google Scholar] [CrossRef]
- Vilela, C.A.; Correia, C.; Oliveira, J.M.; Sousa, R.A.; Espregueira-Mendes, J.; Reis, R.L. Cartilage Repair Using Hydrogels: A Critical Review of in Vivo Experimental Designs. ACS Biomater. Sci. Eng. 2015, 1, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Katti, A.; Shimpi, N.; Roy, S.; Lu, H.; Fabrizio, E.F.; Dass, A.; Capadona, L.A.; Leventis, N. Chemical, physical, and mechanical characterization of isocyanate cross-Linked amine-modified silica aerogels. Chem. Mater. 2006, 18, 285–296. [Google Scholar] [CrossRef]
- Li, Y.; Guo, J.; Li, M.; Tang, Y.; Murugadoss, V.; Seok, I.; Yu, J.; Sun, L.; Sun, C.; Luo, Y. Recent Application of Cellulose Gel in Flexible Sensing-A Review. ES Food Agrofor. 2021, 4, 9–27. [Google Scholar] [CrossRef]
- Bosworth, L.A.; Turner, L.A.; Cartmell, S.H. State of the art composites comprising electrospun fibres coupled with hydrogels: A review. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 322–335. [Google Scholar] [CrossRef]
- Moroni, L.; Schotel, R.; Hamann, D.; de Wijn, J.R.; van Blitterswijk, C.A. 3D fiber-deposited electrospun integrated scaffolds enhance cartilage tissue formation. Adv. Funct. Mater. 2008, 18, 53–60. [Google Scholar] [CrossRef]
- Brunelle, A.R.; Horner, C.B.; Low, K.; Ico, G.; Nam, J. Electrospun thermosensitive hydrogel scaffold for enhanced chondrogenesis of human mesenchymal stem cells. Acta Biomater. 2018, 66, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.T.; Everett, D.; Budhwani, K.I.; Dickinson, B.; Thomas, V. Wet-laid soy fiber reinforced hydrogel scaffold: Fabrication, mechano-morphological and cell studies. Mater. Sci. Eng. C 2016, 63, 308–316. [Google Scholar] [CrossRef]
- Butcher, A.L.; Offeddu, G.S.; Oyen, M.L. Nanofibrous hydrogel composites as mechanically robust tissue engineering scaffolds. Trends Biotechnol. 2014, 32, 564–570. [Google Scholar] [CrossRef]
- Wise, J.K.; Yarin, A.L.; Megaridis, C.M.; Cho, M. Chondrogenic Differentiation of Human Mesenchymal Stem Cells on Oriented Nanofibrous Scaffolds: Engineering the Superficial Zone of Articular Cartilage. Tissue Eng. Part A 2008, 15, 913–921. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Liu, Y.; Wang, Z.; Li, Y.; Jiang, G.; Mo, X.; Zhou, G. Three-dimensional printed electrospun fiber-based scaffold for cartilage regeneration. Mater. Des. 2019, 179, 107886. [Google Scholar] [CrossRef]
- Zheng, R.; Duan, H.; Xue, J.; Liu, Y.; Feng, B.; Zhao, S.; Zhu, Y.; Liu, Y.; He, A.; Zhang, W.; et al. The influence of Gelatin/PCL ratio and 3-D construct shape of electrospun membranes on cartilage regeneration. Biomaterials 2014, 35, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Lee, C.H.; Cho, I.H.; Kim, Y.J.; Lee, Y.J.; Kim, I.A.; Park, K.D.; Yui, N.; Shin, J.W. Electrospun PLGA nanofiber scaffolds for articular cartilage reconstruction: Mechanical stability, degradation and cellular responses under mechanical stimulation in vitro. J. Biomater. Sci. Polym. Ed. 2006, 17, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Su, C.H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011, 7, 234–243. [Google Scholar] [CrossRef]
- Martin, A.R.; Patel, J.M.; Locke, R.C.; Eby, M.R.; Saleh, K.S.; Davidson, M.D.; Sennett, M.L.; Zlotnick, H.M.; Chang, A.H.; Carey, J.L.; et al. Nanofibrous hyaluronic acid scaffolds delivering TGF-β3 and SDF-1α for articular cartilage repair in a large animal model. Acta Biomater. 2021, 126, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Davoudi, Z.; Xing, X.; Yu, X.; Cheng, X.; Li, Z.; Deng, H.; Wang, Q. Advanced Silk Fibroin Biomaterials for Cartilage Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 2704–2715. [Google Scholar] [CrossRef]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104, 1003–1011. [Google Scholar] [CrossRef]
- Mohabatpour, F.; Karkhaneh, A.; Sharifi, A.M. A hydrogel/fiber composite scaffold for chondrocyte encapsulation in cartilage tissue regeneration. RSC Adv. 2016, 6, 83135–83145. [Google Scholar] [CrossRef]
- De Mori, A.; Fernández, M.P.; Blunn, G.; Tozzi, G.; Roldo, M. 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef]
- Baker, B.M.; Gee, A.O.; Metter, R.B.; Nathan, A.S.; Marklein, R.A.; Burdick, J.A.; Mauck, R.L. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 2008, 29, 2348–2358. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, W.; Shafiq, M.; Song, D.; Xie, X.; Yuan, Z.; EL-Newehy, M.; EL-Hamshary, H.; Morsi, Y.; Liu, Y.; et al. Chondroitin sulfate cross-linked three-dimensional tailored electrospun scaffolds for cartilage regeneration. Biomater. Adv. 2022, 134, 112643. [Google Scholar] [CrossRef] [PubMed]
- Leong, N.L.; Kator, J.L.; Clemens, T.L.; James, A.; Enamoto-Iwamoto, M.; Jiang, J. Tendon and Ligament Healing and Current Approaches to Tendon and Ligament Regeneration. J. Orthop. Res. 2020, 38, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Murakami, G.; Minoura, N. Crocodilian bone-tendon an bone-ligament interfaces. Ann. Anat. 2003, 185, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Zhang, T.; Ju, W.; Chen, X.; Heng, B.C.; Shen, W.; Yin, Z. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact. Mater. 2021, 6, 2491–2510. [Google Scholar] [CrossRef]
- Hasslund, S.; Jacobson, J.A.; Dadali, T.; Basile, P.; Ulrich-Vinther, M.; Søballe, K.; Schwarz, E.M.; O’Keefe, R.J.; Mitten, D.J.; Awad, H.A. Adhesions in a murine flexor tendon graft model: Autograft versus allograft reconstruction. J. Orthop. Res. 2008, 26, 824–833. [Google Scholar] [CrossRef]
- Moshiri, A. Tendon and Ligament Tissue Engineering, Healing and Regenerative Medicine. J. Sports Med. Doping Stud. 2013, 3, 1000126. [Google Scholar] [CrossRef]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef]
- Sensini, A.; Massafra, G.; Gotti, C.; Zucchelli, A.; Cristofolini, L. Tissue Engineering for the Insertions of Tendons and Ligaments: An Overview of Electrospun Biomaterials and Structures. Front. Bioeng. Biotechnol. 2021, 9, 645544. [Google Scholar] [CrossRef]
- Sensini, A.; Gualandi, C.; Focarete, M.L.; Belcari, J.; Zucchelli, A.; Boyle, L.; Reilly, G.C.; Kao, A.P.; Tozzi, G.; Cristofolini, L. Multiscale hierarchical bioresorbable scaffolds for the regeneration of tendons and ligaments. Biofabrication 2019, 11, 035026. [Google Scholar] [CrossRef]
- Czaplewski, S.K.; Tsai, T.L.; Duenwald-Kuehl, S.E.; Vanderby, R.; Li, W.J. Tenogenic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells dictated by properties of braided submicron fibrous scaffolds. Biomaterials 2014, 35, 6907–6917. [Google Scholar] [CrossRef]
- Deepthi, S.; Jeevitha, K.; Nivedhitha Sundaram, M.; Chennazhi, K.P.; Jayakumar, R. Chitosan-hyaluronic acid hydrogel coated poly(caprolactone) multiscale bilayer scaffold for ligament regeneration. Chem. Eng. J. 2015, 260, 478–485. [Google Scholar] [CrossRef]
- Yang, G.; Lin, H.; Rothrauff, B.B.; Yu, S.; Tuan, R.S. Multilayered polycaprolactone/gelatin fiber-hydrogel composite for tendon tissue engineering. Acta Biomater. 2016, 35, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Zhang, E.; Yang, L.; Yuan, H.; Tu, W.; Zhang, H.; Yin, Z.; Shen, W.; Chen, X.; et al. An epigenetic bioactive composite scaffold with well-aligned nanofibers for functional tendon tissue engineering. Acta Biomater. 2018, 66, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Lei, D.; Li, S.; Huang, P.; Qi, Q.; Sun, Y.; Zhang, Y.; Wang, Z.; You, Z.; Ye, X.; et al. Hybrid small-diameter vascular grafts: Anti-expansion effect of electrospun poly ε-caprolactone on heparin-coated decellularized matrices. Biomaterials 2016, 76, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Handa, R.; Sharma, S. Vascular Graft Failure Of Leg Arterial Bypasses—A Review. J. Hypertens. Cardiol. 2014, 1, 17–21. [Google Scholar] [CrossRef]
- Niu, Y.; Galluzzi, M.; Fu, M.; Hu, J.; Xia, H. In vivo performance of electrospun tubular hyaluronic acid/collagen nanofibrous scaffolds for vascular reconstruction in the rabbit model. J. Nanobiotechnology 2021, 19, 349. [Google Scholar] [CrossRef]
- Emechebe, G.A.; Obiweluozor, F.O.; Jeong, I.S.; Park, J.K.; Park, C.H.; Kim, C.S. Merging 3D printing with electrospun biodegradable small-caliber vascular grafts immobilized with VEGF. Nanomed. Nanotechnol. Biol. Med. 2020, 30, 102306. [Google Scholar] [CrossRef]
- Spadaccio, C.; Nappi, F.; De Marco, F.; Sedati, P.; Sutherland, F.W.H.; Chello, M.; Trombetta, M.; Rainer, A. Preliminary in vivo evaluation of a hybrid armored vascular graft combining electrospinning and additive manufacturing techniques. Drug Target Insights 2016, 10, DTI.S35202. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, M.E.; Nah, H.; Seok, J.M.; Jeong, M.H.; Park, K.; Kwon, I.K.; Lee, J.S.; Park, S.A. Vascular endothelial growth factor immobilized on mussel-inspired three-dimensional bilayered scaffold for artificial vascular graft application: In vitro and in vivo evaluations. J. Colloid Interface Sci. 2019, 537, 333–344. [Google Scholar] [CrossRef]
- Zhou, F.; Jia, X.; Yang, Y.; Yang, Q.; Gao, C.; Hu, S.; Zhao, Y.; Fan, Y.; Yuan, X. Nanofiber-mediated microRNA-126 delivery to vascular endothelial cells for blood vessel regeneration. Acta Biomater. 2016, 43, 303–313. [Google Scholar] [CrossRef]
- Braghirolli, D.I.; Helfer, V.E.; Chagastelles, P.C.; Dalberto, T.P.; Gamba, D.; Pranke, P. Electrospun scaffolds functionalized with heparin and vascular endothelial growth factor increase the proliferation of endothelial progenitor cells. Biomed. Mater. 2017, 12, 025003. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Wang, H.; Yin, G.; Dong, Z. Fabrication and properties of the electrospun polylactide/silk fibroin-gelatin composite tubular scaffold. Biomacromolecules 2009, 10, 2240–2244. [Google Scholar] [CrossRef]
- Yuan, H.; Qin, J.; Xie, J.; Li, B.; Yu, Z.; Peng, Z.; Yi, B.; Lou, X.; Lu, X.; Zhang, Y. Highly aligned core-shell structured nanofibers for promoting phenotypic expression of vSMCs for vascular regeneration. Nanoscale 2016, 8, 16307–16322. [Google Scholar] [CrossRef]
- Geng, X.; Xu, Z.Q.; Tu, C.Z.; Peng, J.; Jin, X.; Ye, L.; Zhang, A.Y.; Gu, Y.Q.; Feng, Z.G. Hydrogel Complex Electrospun Scaffolds and Their Multiple Functions in in Situ Vascular Tissue Engineering. ACS Appl. Bio Mater. 2021, 4, 2373–2384. [Google Scholar] [CrossRef]
- Ren, J.; Xu, Q.; Chen, X.; Li, W.; Guo, K.; Zhao, Y.; Wang, Q.; Zhang, Z.; Peng, H.; Li, Y.G. Superaligned Carbon Nanotubes Guide Oriented Cell Growth and Promote Electrophysiological Homogeneity for Synthetic Cardiac Tissues. Adv. Mater. 2017, 29, 1702713. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; He, R.; Sha, B.; Li, W.; Qing, H.; Teng, R.; Xu, F. Electrospun three-dimensional aligned nanofibrous scaffolds for tissue engineering. Mater. Sci. Eng. C 2018, 92, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, X.; Lu, T.J.; Xu, F. Recent Advances in Electrospun Nanofibrous Scaffolds for Cardiac Tissue Engineering. Adv. Funct. Mater. 2015, 25, 5726–5738. [Google Scholar] [CrossRef]
- Parrag, I.C.; Zandstra, P.W.; Woodhouse, K.A. Fiber alignment and coculture with fibroblasts improves the differentiated phenotype of murine embryonic stem cell-derived cardiomyocytes for cardiac tissue engineering. Biotechnol. Bioeng. 2012, 109, 813–822. [Google Scholar] [CrossRef]
- Sirivisoot, S.; Harrison, B.S. Skeletal myotube formation enhanced by electrospun polyurethane carbon nanotube scaffolds. Int. J. Nanomed. 2011, 6, 2483–2497. [Google Scholar] [CrossRef]
- Carlberg, B.; Axell, M.Z.; Nannmark, U.; Liu, J.; Kuhn, H.G. Electrospun polyurethane scaffolds for proliferation and neuronal differentiation of human embryonic stem cells. Biomed. Mater. 2009, 4, 045004. [Google Scholar] [CrossRef]
- Genchi, G.G.; Sinibaldi, E.; Ceseracciu, L.; Labardi, M.; Marino, A.; Marras, S.; De Simoni, G.; Mattoli, V.; Ciofani, G. Ultrasound-activated piezoelectric P(VDF-TrFE)/boron nitride nanotube composite films promote differentiation of human SaOS-2 osteoblast-like cells. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2421–2432. [Google Scholar] [CrossRef] [PubMed]


| Category | Polymers |
|---|---|
| Natural polymers | Cellulose [51] |
| Xanthan gum [52] | |
| Poly(hydroxyalkanoates) [53] | |
| Starch [54] | |
| Chitosan [55] | |
| Chitin [56] | |
| Pullulan [57] | |
| Alginate [58] | |
| Wheat gluten [59] | |
| Gelatin [60] | |
| Collagen [61] | |
| Dextrin [62] | |
| Fibrin [63] | |
| Zein [64] | |
| Poly(hydroxybutyrate) (PHB) [65] | |
| Synthetic polymers | Polyamide-6 [66] |
| Polycaprolactone (PCL) [67] | |
| Polylactic acid (PLA) [68] | |
| Poly(lactic-co-glycolic acid) (PLGA) [69] | |
| Polyvinyl alcohol (PVA) [70] | |
| Poly(glycolic acid) (PGA) [71] | |
| Poly(butylene succinate) [72] | |
| Poly(anhydride-ester) [73] | |
| Polyorthoesters [74] | |
| Polycarbonate [75] | |
| Polyanhydride [76] | |
| Polyfumarate [77] | |
| Polyphosphoester [78] | |
| Polyphosphazenes [79] | |
| Poly(urethane ester)urea [80] | |
| Poly(L-lactide-co-caprolactone) (PLCL) [81] | |
| Polyurethane (PU) [81] | |
| Poly(vinylidene fluoride) (PVDF) [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Rojas, G.G.; Gómez-Lazaro, B.; López-Saucedo, F.; Vera-Graziano, R.; Bucio, E.; Mendizábal, E. Electrospun Scaffolds for Tissue Engineering: A Review. Macromol 2023, 3, 524-553. https://doi.org/10.3390/macromol3030031
Flores-Rojas GG, Gómez-Lazaro B, López-Saucedo F, Vera-Graziano R, Bucio E, Mendizábal E. Electrospun Scaffolds for Tissue Engineering: A Review. Macromol. 2023; 3(3):524-553. https://doi.org/10.3390/macromol3030031
Chicago/Turabian StyleFlores-Rojas, Guadalupe Gabriel, Bélen Gómez-Lazaro, Felipe López-Saucedo, Ricardo Vera-Graziano, Emilio Bucio, and Eduardo Mendizábal. 2023. "Electrospun Scaffolds for Tissue Engineering: A Review" Macromol 3, no. 3: 524-553. https://doi.org/10.3390/macromol3030031
APA StyleFlores-Rojas, G. G., Gómez-Lazaro, B., López-Saucedo, F., Vera-Graziano, R., Bucio, E., & Mendizábal, E. (2023). Electrospun Scaffolds for Tissue Engineering: A Review. Macromol, 3(3), 524-553. https://doi.org/10.3390/macromol3030031