Abstract
Dendrimers are increasingly being studied in the context of encapsulation. Many potential applications of dendrimers are based on their properties. They are used in drug delivery systems, cosmetics, food and chemistry. This review is first devoted to different synthesis approaches for dendrimers and to their ability to encapsulate active molecules. Their applications in different fields, as well as their cytotoxicity, are then detailed. To conclude this review, the main works on the interaction of dendrimers with the stratum corneum (SC) are also presented.
1. Introduction
Dendrimers are a class of hyperbranched, nano-sized, radially symmetrical molecules with a well-defined, homogeneous and monodisperse tree-like structure [1,2,3]. These materials have many properties such as versatility, an ability to self-assemble and to involve electrostatic interactions, chemical stability in biological media, various cytotoxicity and increased solubility in aqueous media [4,5,6,7,8,9]. These specific properties have a great impact on their applications [4,9,10] and on their capacity to encapsulate active ingredients, especially in the therapeutic and cosmetic fields [4,7,9,10]. In recent years, we have developed various dendrimers containing biobased moieties to make these compounds more eco- or biocompatible. These have been used in catalysis, medical imaging, depollution and cosmetic fields [11,12,13,14,15,16].
In this review, we describe the major synthetic pathways of the most commonly used dendrimers and their applications in several fields, i.e., medical imaging, pharmaceuticals, catalysis, environment, food and cosmetics. Their cytotoxicity and interactions with membranes, especially with the stratum corneum (SC), are also described.
2. Dendrimers: Definition and Synthesis
The term dendrimer comes from “dendros”, its Greek name, meaning tree or branch, and “meros”, meaning a part of [17]. Chemically, dendrimers are nano-sized, radially symmetric molecules with a well-defined, homogeneous and monodisperse structure composed of tree-like arms or branches [1,2,3]. These hyper-branched molecules were first discovered by Vogtle et al. in 1978 [18] and were named cascade molecules. These branched macromolecules were later developed as starbust-dendritic or dendrimers by Tomalia et al. [19] in 1985, and at the same time by Newkome et al. [3]. The latter called the synthesized macromolecules “arborols”, which is Latin for “trees” or cascade molecule, but this term is not as well known as “dendrimers” [4].
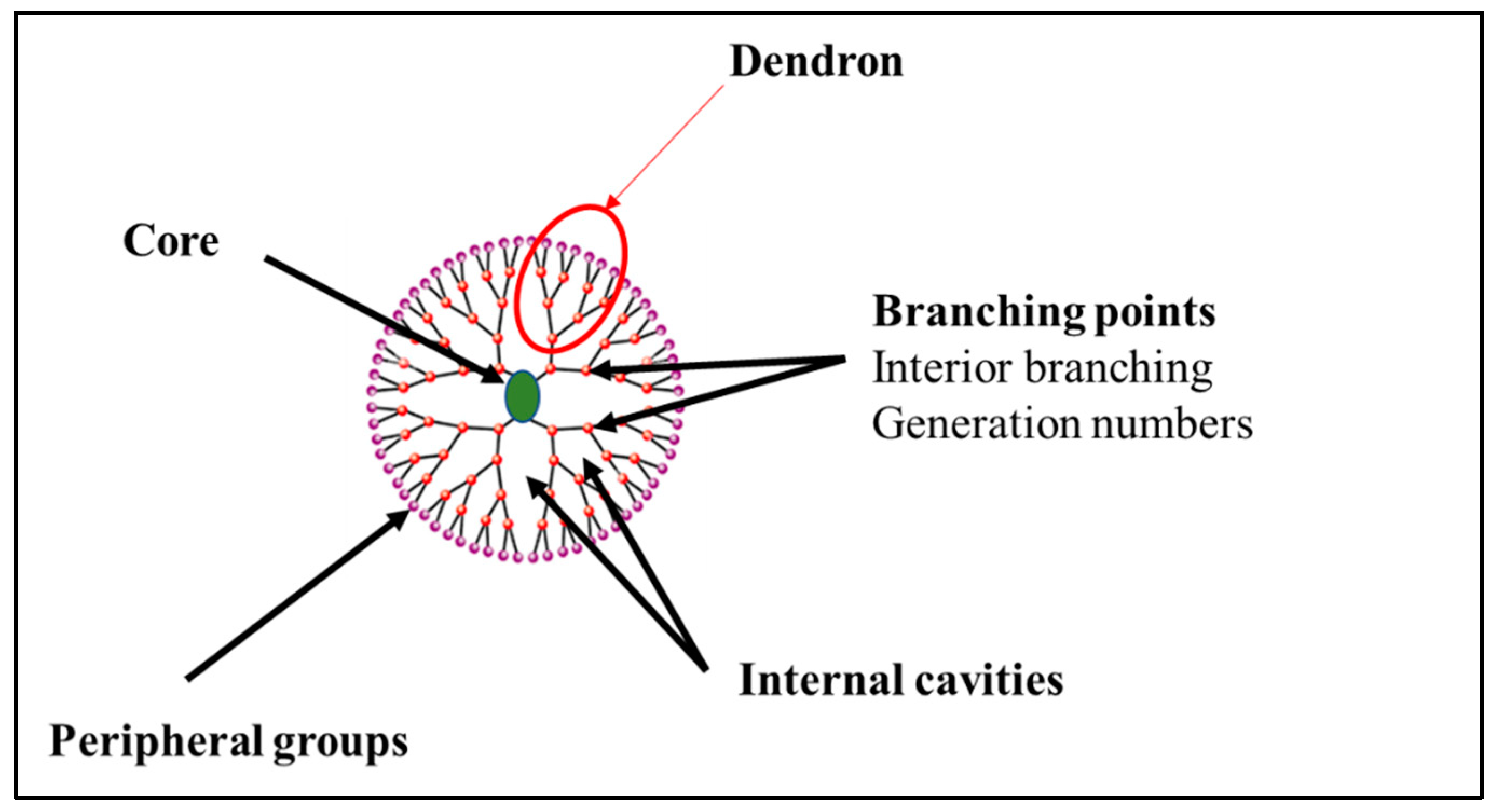
Dendrimers are composed of three parts. From the inside to the outside, the structure of a dendrimer consists of a core with a multitude of branches grafted to its internal cavities and terminal groups at the periphery (Figure 1). The identical parts connected to the core are called “dendrons” (Figure 1). Their number depends on the multivalence of the core used as a nucleus [7].
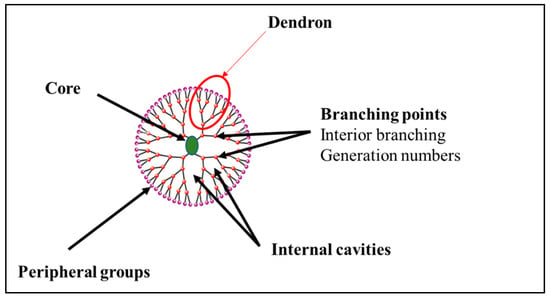
Figure 1.
General structure of a dendrimer.
Dendrimers are synthesized by a repetitive chemical reaction in which the formation of dendrimers takes place in a progressive manner, leading to a layer called generation [20]. They are mainly prepared using two types of approaches (Table 1), the divergent approach or the convergent one, or a combination of both [4].

Table 1.
Advantages and disadvantages of both convergent and divergent approaches.
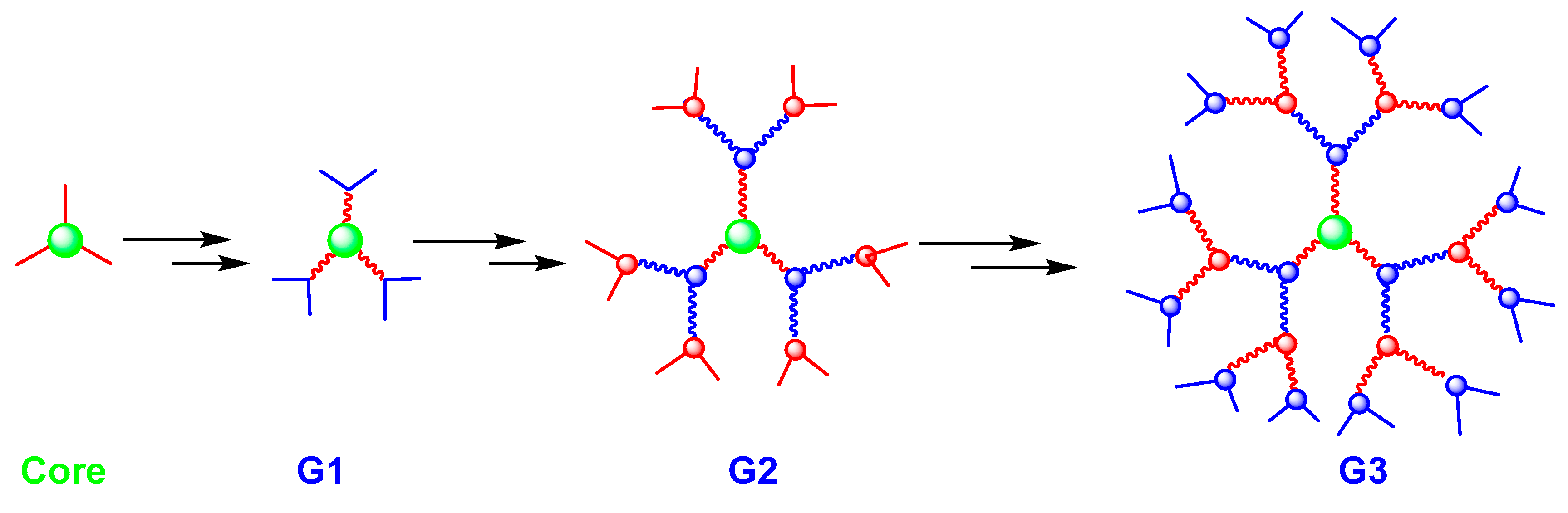
Following the divergent methods, the dendrimer is built from a core, which is a multifunctional molecule, toward the periphery (Figure 2). The core reacts with the monomer molecules containing one reactive group and two unreactive groups, resulting in the first-generation dendrimer. Then, the new periphery of the molecule is activated to react with the other monomers. The process is repeated to form other higher generations [4]. The divergent approach is effective to produce large quantities of dendrimers. However, problems can occur due to side reactions and incomplete reactions of peripheral groups, leading to structural defects. To solve these problems, a large excess of reagents is needed, inducing unfortunately major difficulties for the purification of the final product [19].
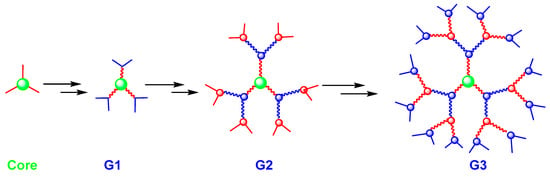
Figure 2.
Dendrimer synthesis using the divergent approach.
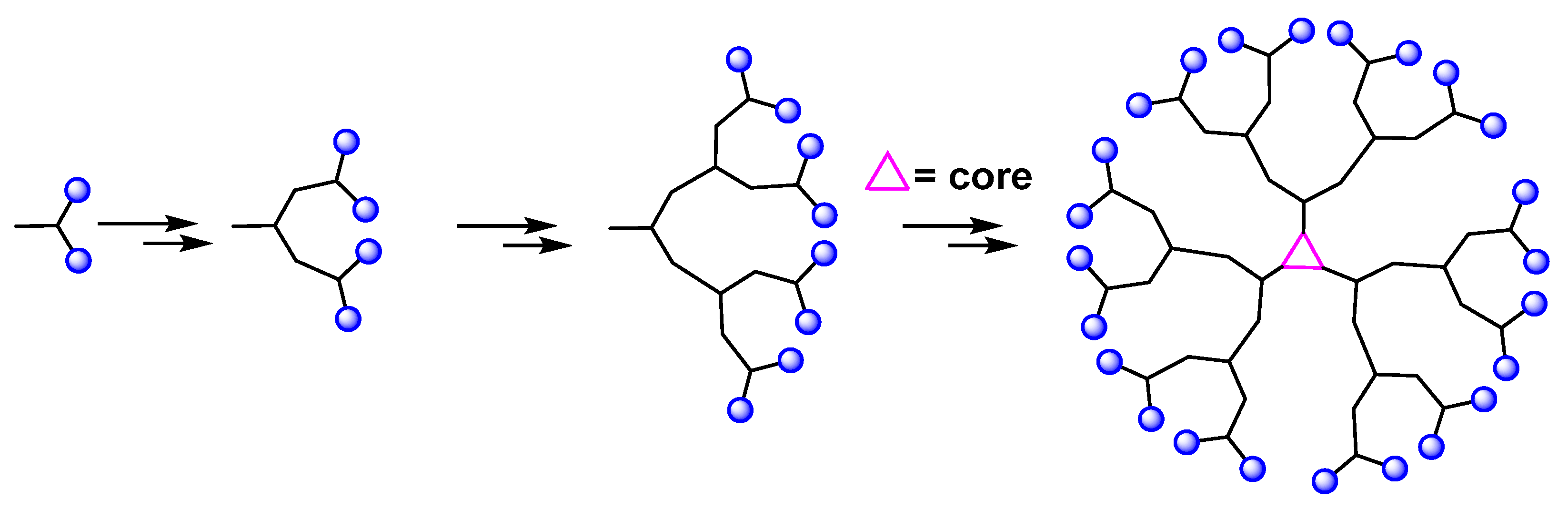
Convergent methods were developed with the aim of limiting the purification problems encountered in divergent methods. In this approach, the dendrimer is constructed in several steps, starting from the peripheral clusters, and progressing inwards to the core, unlike the divergent method (Figure 3). When the desired branching polymeric arms, i.e., dendrons. are formed, they are attached to a central multifunctional molecule [21]. The concept of using a convergent approach for the synthesis of dendritic macromolecules has several advantages, including the following: the small number of coupling reactions for each growth step allows for a better control of the synthesis; the appearance of defects in the final structure is minimized and large excesses of reagents are avoided, which simplifies the purification. However, this approach does not allow the formation of high generations because steric problems arise in the reactions between the dendrons and the central molecule [21,22]
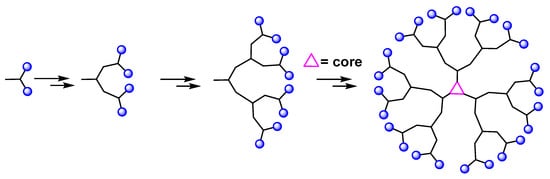
Figure 3.
Synthesis of dendrimer via the convergent approach.
The two main dendrimers that are commercially available are Poly(PropyleneImine) PPI [23,24] and Poly(AMidoAMine) PAMAM.
PAMAM was first synthesized by Tomalia et al. using a divergent approach [19]. The synthesis is performed in two steps, a Michael-type addition between ethylene diamine (EDA) or ammonia and methyl acrylate, followed by amidation of the resulting esters with an excess of ethylene diamine. Seven generations have thus been synthesized. Some properties of these dendrimers such as the symmetry and the ability to self-assemble were then studied [25].
The synthesis of other dendrimers of the PAMAM family comprising a cyclic core has also been performed using a divergent approach with the same reaction sequence from 4,7,10-tetraazacyclododecane, methyl acrylate and ethylene diamine [26]. Eleven generations (from G 0.5 to G 5.5) were synthesized and characterized. The surface of generations 4 and 5 of these dendrimers was then modified with 1-bromoacetyl-5-fluorouracil (5FU) to form the dendrimer-5FU; the latter was then hydrolyzed to control the release of 5FU. It was shown that this type of dendrimer can be used as a promising carrier for the controlled release of antitumor active ingredients [26].
The synthesis of PAMAM derivatives via a combination of convergent and divergent approaches was developed by Lee et al.; the azide functionalized PAMAM dendron is obtained via the divergent method using an azido-propylamine as a focal point. Then, using the convergent method, the dendrons formed are linked to cores possessing alkynes through a click reaction [27].
Poly(PropyleneImine) dendrimers, also called ‘POPAM’ [28], were developed by de Brabander-van den Berg et al. in 1993 using a divergent approach [23]; indeed, the repetitive double Michael addition of acrylonitrile with primary amines is followed by the hydrogenation of the nitriles via heterogeneous catalysis. 1,4-diaminobutane DAB and ethylene diamine EDA were used as the dendrimer core. This method of synthesis is widely described in the literature and is used very often [29,30].
Several surface modifications of PPI and PAMAM were also proposed. In most cases, the surface amine groups of PPIs and PAMAMs are replaced by more neutral entities in order to increase their solubility and/or to reduce their cytotoxicity, or by bioactive entities for specific applications [31].
In many cases, the terminal amines were functionalized by Poly (Ethylene Glycol) (PEG) [32,33,34,35,36], and the resulting dendrimers were widely used in the therapeutic field. The decoration of the dendrimers with sugars via glycosylation led to entities which play an important role in short-range molecular recognition, such as interactions with the extracellular matrix and neighboring cells or communication with the whole system, since most hormone proteins are glycosylated [10]. In 2015, Youngseon et al. developed dendrimers via the functionalization of PAMAM with bifunctional entities, fluorescein and curfolic acid linked together by DNA oligonucleotides to target cancer cells [37].
Over the past ten years, our research team developed new families of dendrimers derived from PAMAM and PPI by decorating the surface with hydroxyl groups, through the reaction of terminal amine groups with glycerol carbonate; many applications were developed (encapsulation of Gadolinium complexes for medical imaging, encapsulation of metallic nanoparticles for catalysis, encapsulation of emerging pollutants for depollution) [16,38,39,40].
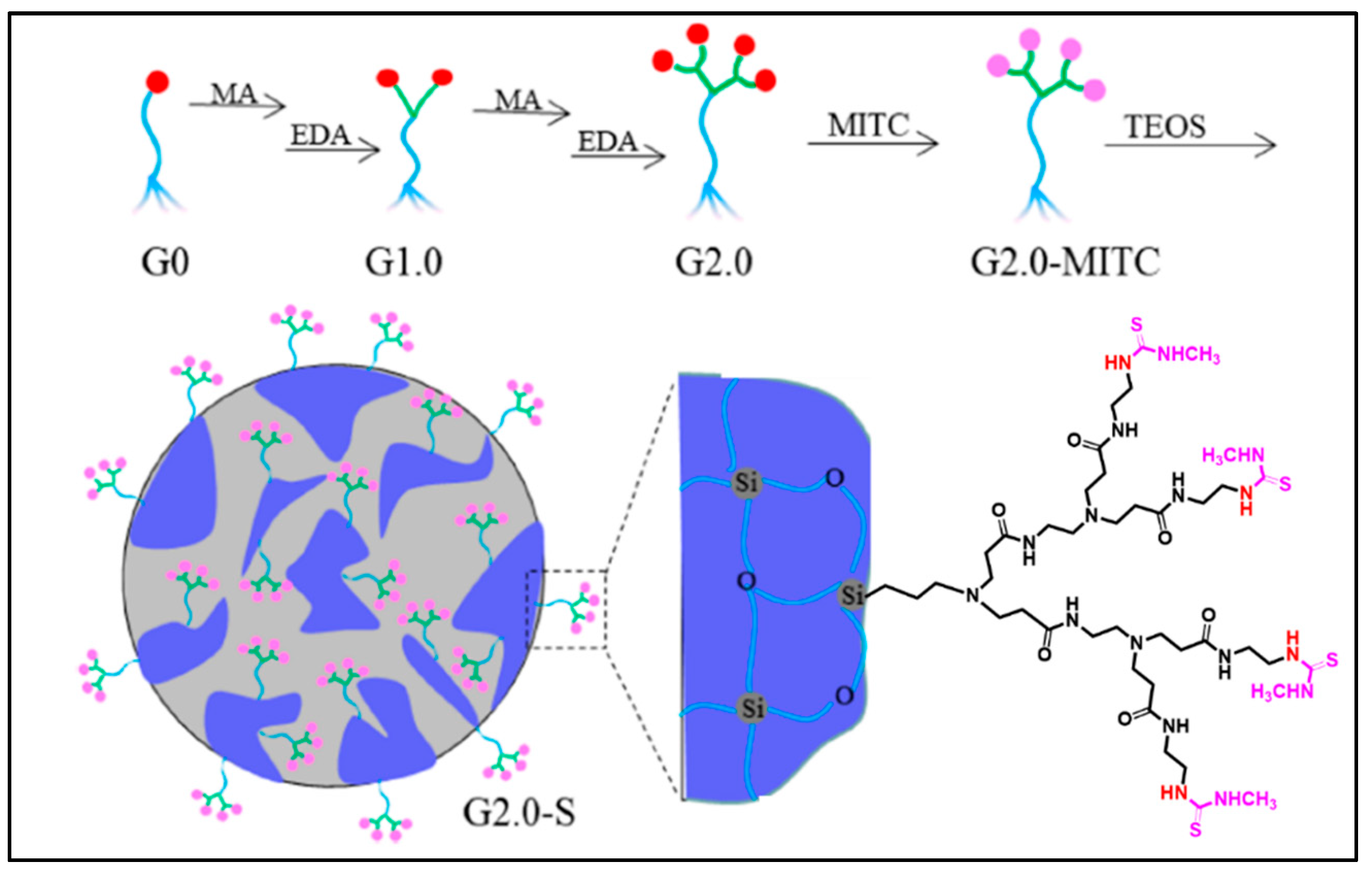
Recently, Luan et al. developed dendrimers also derived from PAMAM grafted onto mesoporous silica via functionalization with isothiocyanates in order to improve the absorption of Hg(II) and Cd(II) essentially for water treatment [41]. These PAMAM dendrimers/mesoporous silica composites decorated with methyl isothiocyanate groups were synthesized by the direct sol-gel reaction of functional PAMAM dendrimers containing alkoxysilyle groups (Figure 4).
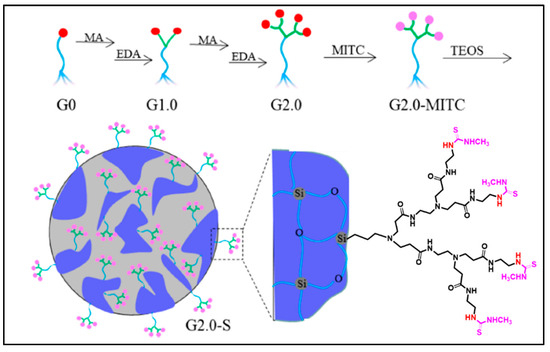
Figure 4.
Synthesis diagram of a PAMAM dendrimer grafted onto mesoporous silica decorated with methyl isothiocyanate, reproduced according to [41] with the permission of ACS.
New families of dendrimers derived from PAMAM, called tectodendrimers, were developed in recent years [42,43,44,45]. Tectodendrimers, also called megamers, are superstructured dendrimers whose cores are high-generation dendrimers, and the shells are low-generation dendrimers [43]. They have excellent properties similar to those of high-generation single dendrimers, but they also overcome some disadvantages such as limited encapsulation capacity and increased retention effect due to their small size [42]. Furthermore, the synthesis of tectodendrimers is much simpler than the synthesis of high-generation single dendrimers. Indeed, the number of repetitive reactions is limited; thus, the problem of steric hindrance encountered during the synthesis of a simple dendrimer of very high generation is avoided [43].
Furthermore, several families of dendrimers that are different than those derived from PAMAM or PPI were developed. Indeed, we developed two families of dendrimers from glycerol as a starting material using a divergent approach. The first family, called GlyceroClickDendrimer, is obtained via the click reaction between alkynes and azides, both derived from glycerol [46]. The second GlycerolADendrimer family is obtained via the reaction of glycerol with allyl bromide, followed by the oxidation of olefins [47].
In 2017, a new family of dendrimers was also developed using a divergent approach from carbosilanes functionalized with polyphenols, leading to a potential application as antioxidants in the cosmetic and pharmaceutical industries [48].
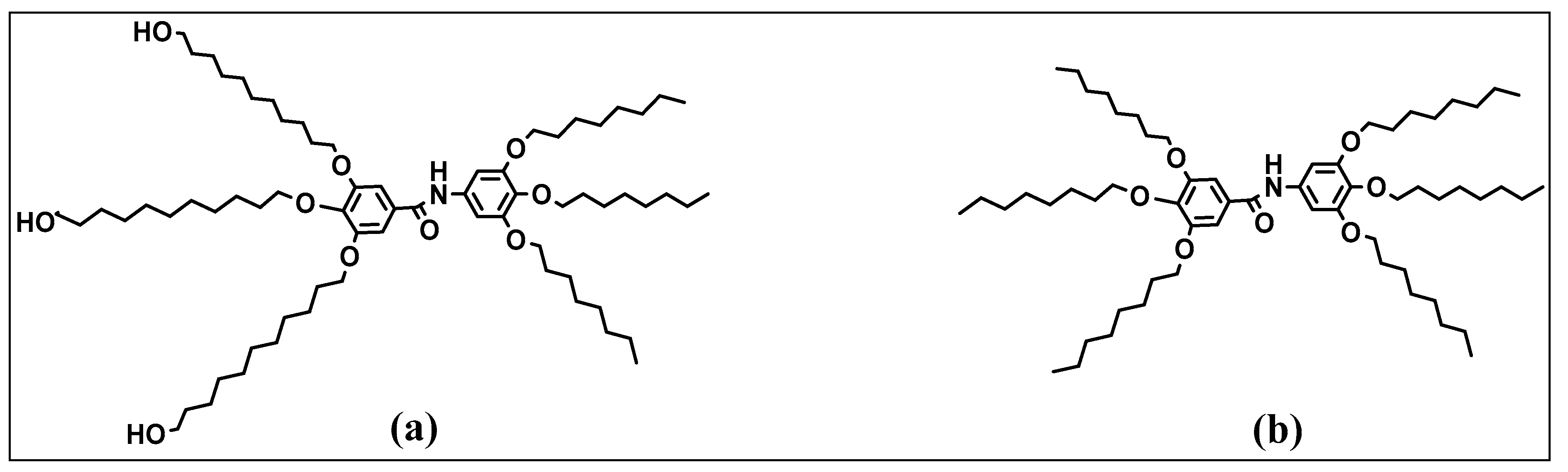
In the biomedical field, several approaches were also carried out with dendrimers. Nnadiekwe et al. synthesized biologically active benzamide-based Twin- and Janus-type dendrimers using a convergent approach from a primary precursor, methyl gallate (Figure 5); these dendrimers have peripheral groups with long octyloxy and hydroxyundecyloxy chains (identical functional groups for Twin and different for Janus) [49]. This functionalization makes it possible to increase the amphiphilic character favoring the self-assembly of the dendrimer, which, consequently, makes it possible to incorporate various supramolecular architectures such as columnar, globular and bilayer structures. Indeed, Janus-type dendrimers are among the most known dendrimers that exhibit self-assembly properties [50,51,52]. The self-assembly behavior of this kind of dendrimer arises from the selective interactions between the different faces. Janus dendrimers have a huge potential application other than in the biomedical field [8,50,53,54,55]. They can be used as catalysts because of their asymmetric surface properties [56,57,58]; the different functional groups on each face can facilitate specific catalytic reactions or enable spatially controlled reactions, leading to enhanced catalytic activity and selectivity. Janus dendrimers can be also employed in surface coatings and adhesives due to their amphiphilic nature [59,60,61]. By controlling the distribution of hydrophilic and hydrophobic functional groups, they can form self-assembled monolayers or coatings with tailored surface properties, such as wettability, adhesion and anti-fouling characteristics. Finally, Janus dendrimers can act as templates for nanoparticle synthesis [62,63,64]. By utilizing the different surface functionalities, they can selectively bind and stabilize nanoparticles, controlling their size, shape and surface properties. This enables the fabrication of well-defined nanoparticles for various applications, including catalysis, sensing and nanomedicine.
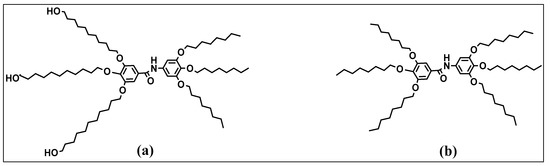
Figure 5.
Structures of 3,4,5-tris(11-hydroxyundecyloxy)-N-(3,4,5-tris(octyloxy)phenyl)benzamide (Janus) (a) and 3,4,5-tris(octyloxy)-N-(3,4,5-tris(octyloxy)phenyl)benzamide (Twin) (b) reproduced according to [49] with the permission of the Royal Society of Chemistry.
Various families of peptide dendrimers have also been developed in recent years [65,66,67]; these dendrimers are branched macromolecules consisting of a peptidyl core and/or peptide functional groups at the peripheries attached in a covalent manner [68]. They can be symmetrical or unsymmetrical and used as diagnostic reagents, anticancer and antiviral agents, vaccines and carriers of active principles and genes [65,69], thanks to their properties of self-assembly [67,70].
3. Applications of Dendrimers
Some applications of dendrimers were already mentioned above; they derive from their physico-chemical properties, such as structural versatility, self-assembly capability, stability and ability to engage in electrostatic interactions [4,5,6,70,71,72,73]. Thus, their capacity to encapsulate molecules of appropriate size in the internal cavities or the possibility of grafting molecules covalently to their periphery allow them to be used in many fields [74]. In general, dendrimers are in the nanoparticle range; several parameters can affect nano-encapsulation performance, including generation, type of end groups, surface charge, core structure, pH and environmental factors [75]. Symmetry also plays a very important role in determining the encapsulation properties [76] since this parameter directs the internal stacking modes, which differentiate key properties of dendrimers, such as densities, refractive indices and internal porosities. Thus, symmetrical dendrimers encapsulate more than unsymmetrical ones.
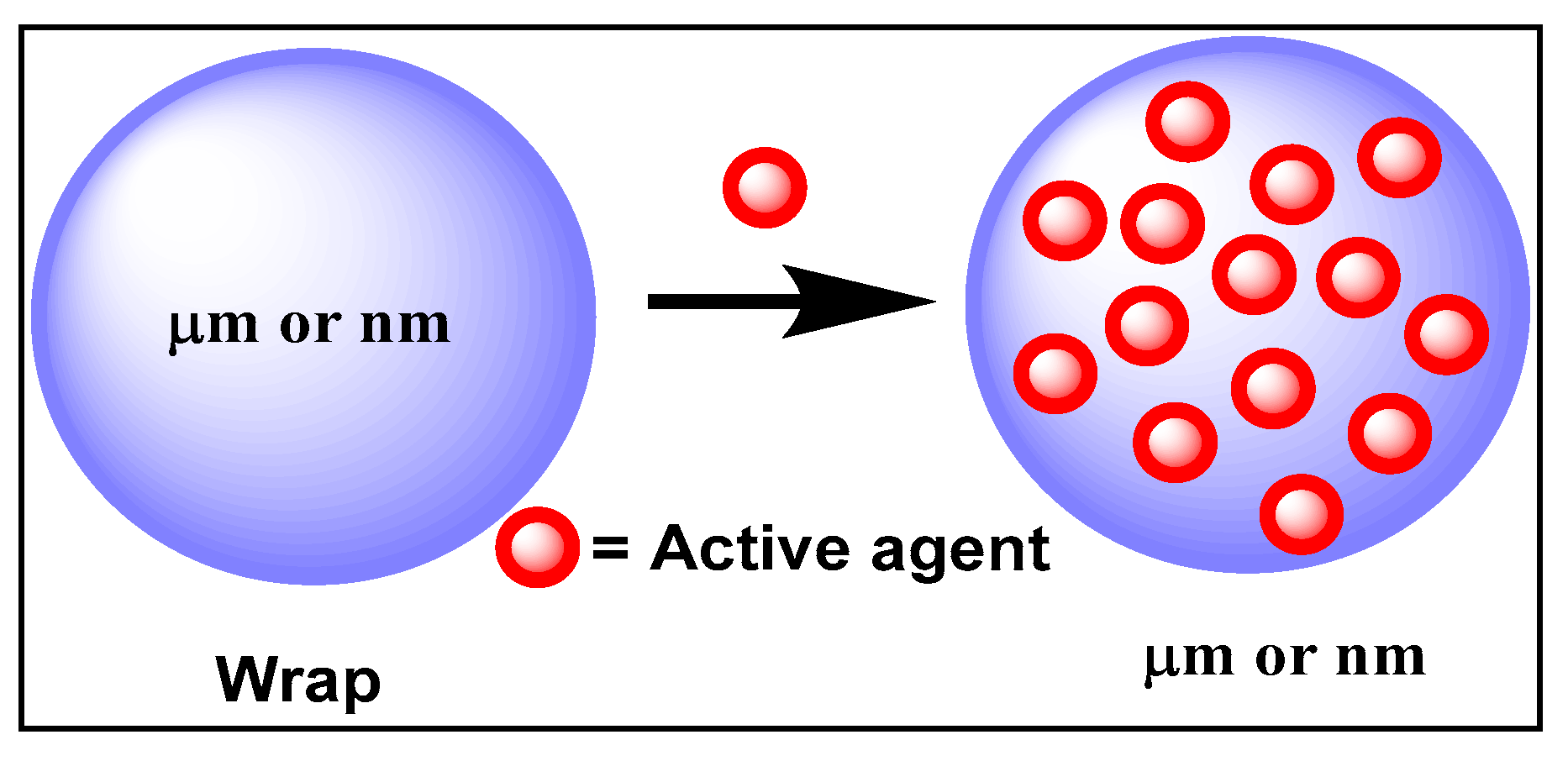
Encapsulation consists of trapping non-stable active agents in a protective sheath against undesirable reactions (e.g., oxidation or degradation due to light, air or pH). Two types of encapsulations can be used, including (i) nano-encapsulation, which consists of coating various substances in another material with a nanometric size, and (ii) micro-encapsulation, involving larger particles of the order of a micrometer (Figure 6) [77].
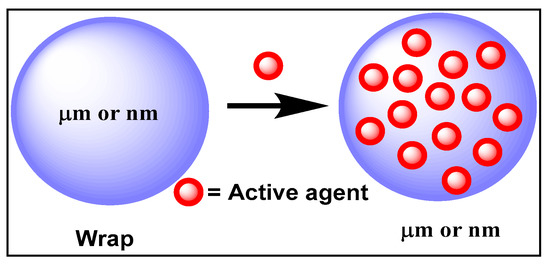
Figure 6.
Encapsulation principle.
For several years, encapsulation in dendrimers has been mainly used in the biomedical field (imaging, pharmaceuticals) but also in food and cosmetics. Dendrimers can also be used in the field of catalysis and the environment to purify water or soil by trapping organic and metallic pollutants. We thus detail below the different fields in which encapsulation in dendrimers is of interest.
3.1. Medical Imaging
Macromolecular contrast agents offer the possibility to measure local changes in cerebral blood volume associated with brain function or tumor grade [78]. Dendrimers, particularly dendrimers containing surface chelated ions [4], appear to be highly suitable for use as contrast agent carriers for magnetic resonance imaging (MRI).
In 1996, Wiener et al. studied the molecular dynamics of vanadyl chelate complexes covalently attached to the PAMAM surface with the aim of showing the effect of the vanadyl chelate ion on the relaxation time of PAMAM [79]. They showed that the relaxation time of the surface chelate looks like those of the inner carbons of PAMAM and increases with a higher molecular weight, which was not observed in the case of linear polymers. A comparison of the isotropic and anisotropic models showed that the anisotropic model better describes the motion-modulated line shapes than the isotropic model; thus, those dendritic complexes with ellipsoidal, flattened or elongated shapes or with rigid side chains are better MRI contrast agents. A study on the encapsulation of the contrast agent Gadolinium ion in high generation PAMAM dendrimers conjugated with the bifunctional chelate 2-(4-isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane-N,N8,N″,N‴-tetraacetate (p-SCN-Bz-DOTA) was performed by Henry Bryant et al. [80]. They showed that generation 5 dendrimers had an average of 127 chelates encapsulating 96 Gd+3 ions. The number of chelates and the encapsulation capacity increased with higher generations, too. They suggested that these results are relevant to the design of high generation dendrimer-based receptor agents. In the same spirit, the encapsulation of the gadolinium gadopentetate dimeglumine complex (GdBOPTA) in PPI dendrimers functionalized via glycerol carbonate [38] showed that this dendrimer is a very good encapsulating tool for contrast agents with a low cytotoxicity on lung fibroblasts. For imaging atherosclerotic plaques [81], the use of dendrimers represents an innovative and advantageous method compared to other nanoparticles, but the cost and complexity of synthesizing high generations and their toxicity toward cells (particularly in the case of cationic dendrimers) [81] are an obstacle.
Furthermore, it has been shown that dendrimers are also powerful tools for transporting fluorophores in biological systems. They can improve the optical properties and solubility of organic dyes in water, thus reducing the negative impact on the target object and increasing the intensity of fluorescence signals in vitro and in vivo. However, it is important to improve autofluorescence in bioimaging without using agents that could have effects on cell cultures or organisms [82]. Recently, a dendrimer-based signaling mechanism in which fluorescent probes are connected in an orderly fashion to each other was proposed to improve contrast in cell imaging, avoiding signal dispersion [83]. It was shown that the larger structure has a lower mobility and the accumulation of fluorescence from the connected probes has a high contrast.
3.2. Pharmaceutical
In the pharmaceutical field, the key factor in drug delivery is the release of the active molecule at the right place, at the right time and in the right concentration. However, there are many obstacles to this release, including the low solubility of the drug, its environmental or enzymatic degradation, its rapid rate of elimination from the body, its non-specific toxicity and its inability to cross biological barriers. Polymer or dendrimer-based carriers (surface coordination or internal encapsulation) can overcome these problems [84,85,86].
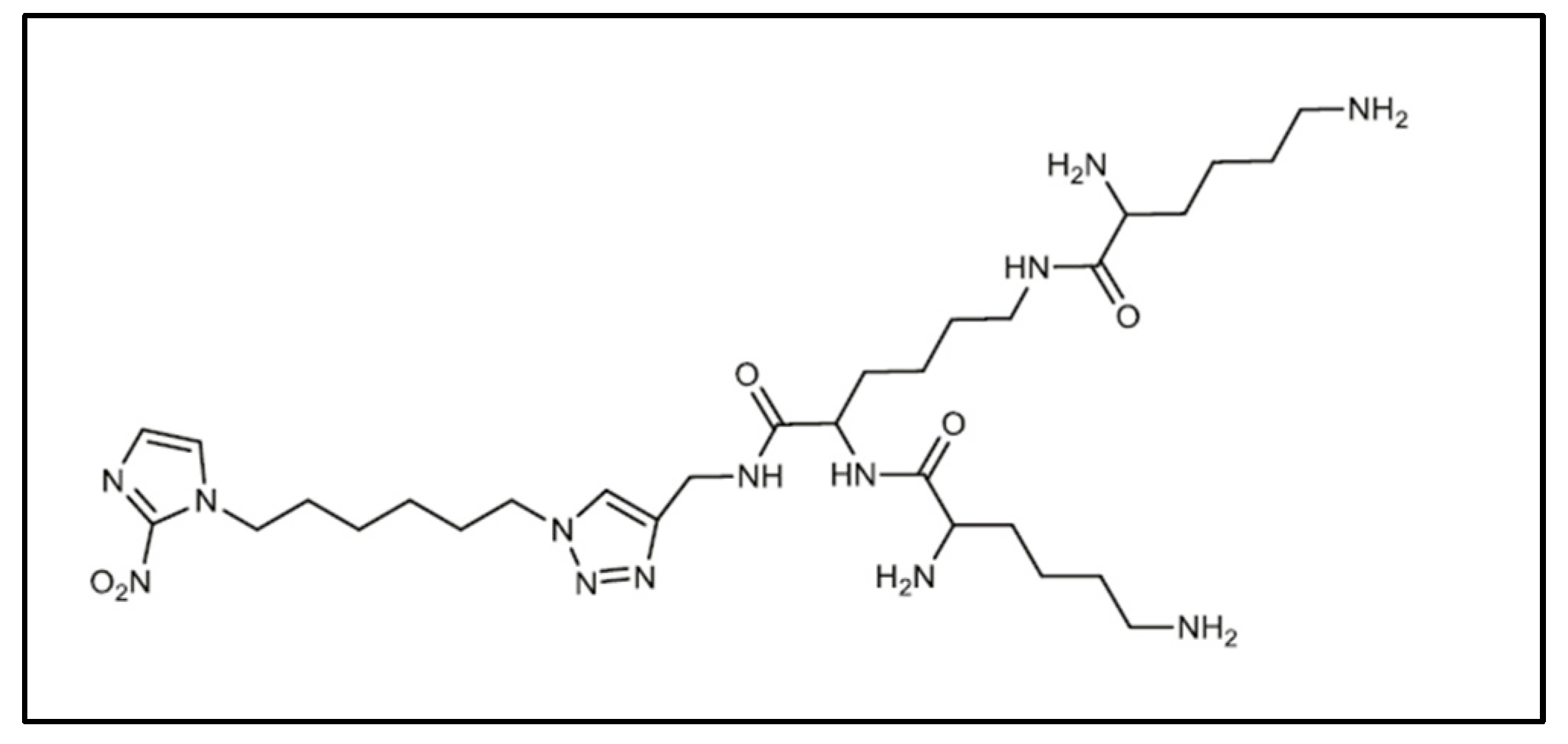
For example, in the field of cancer control, various tumor-specific strategies are used for targeting, imaging and therapy [10,87]. In 2013, Mollazade et al. compared the effect of free curcumin and curcumin encapsulated in the PAMAM dendrimer on the T47D breast cancer cell [88]. They showed that the PAMAM dendrimers had no cytotoxicity on the cells. However, the dendrimer enhanced the inhibitory effect of curcumin on telomerase activity and decreased the IC50 for breast cancer cell proliferation. Numerous studies on folic acid-functionalized PAMAM dendrimers have shown their value in the treatment of cancers (lung, breast, ovarian, brain and neck) [89] with the specific targeting of tumor cells for the delivery of encapsulated chemotherapy compounds. The ability of the PEG-functionalized PAMAM to encapsulate anticancer agents such as adriamycin and methotrexate increases with the generation of the dendrimer and the length of the PEG chain [90], but adriamycin and methotrexate are readily released in the presence of saline. A novel amphiphilic dendrimer (HAD) (Figure 7), developed by Click chemistry from a 2-nitroimidazole derivative with a polylysine dendron, encapsulates chlorin e6 and becomes a light-activated immunological adjuvant (LIA) for in situ cancer vaccination [91]. Through infrared activation, LIA induces tumor cell lysis and tumor antigen release. It effectively inhibits primary tumor growth and abscopal effect and induces a strong antigen-specific immune memory effect to prevent tumor metastasis and recurrence in vivo.
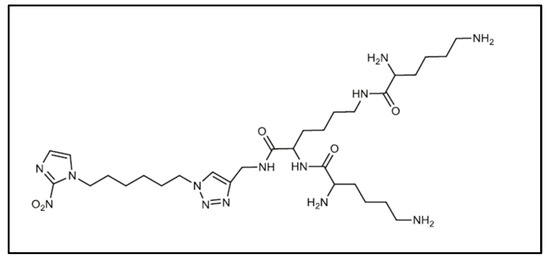
Figure 7.
First generation amphiphilic dendrimer (HAD) reproduced according to [91].
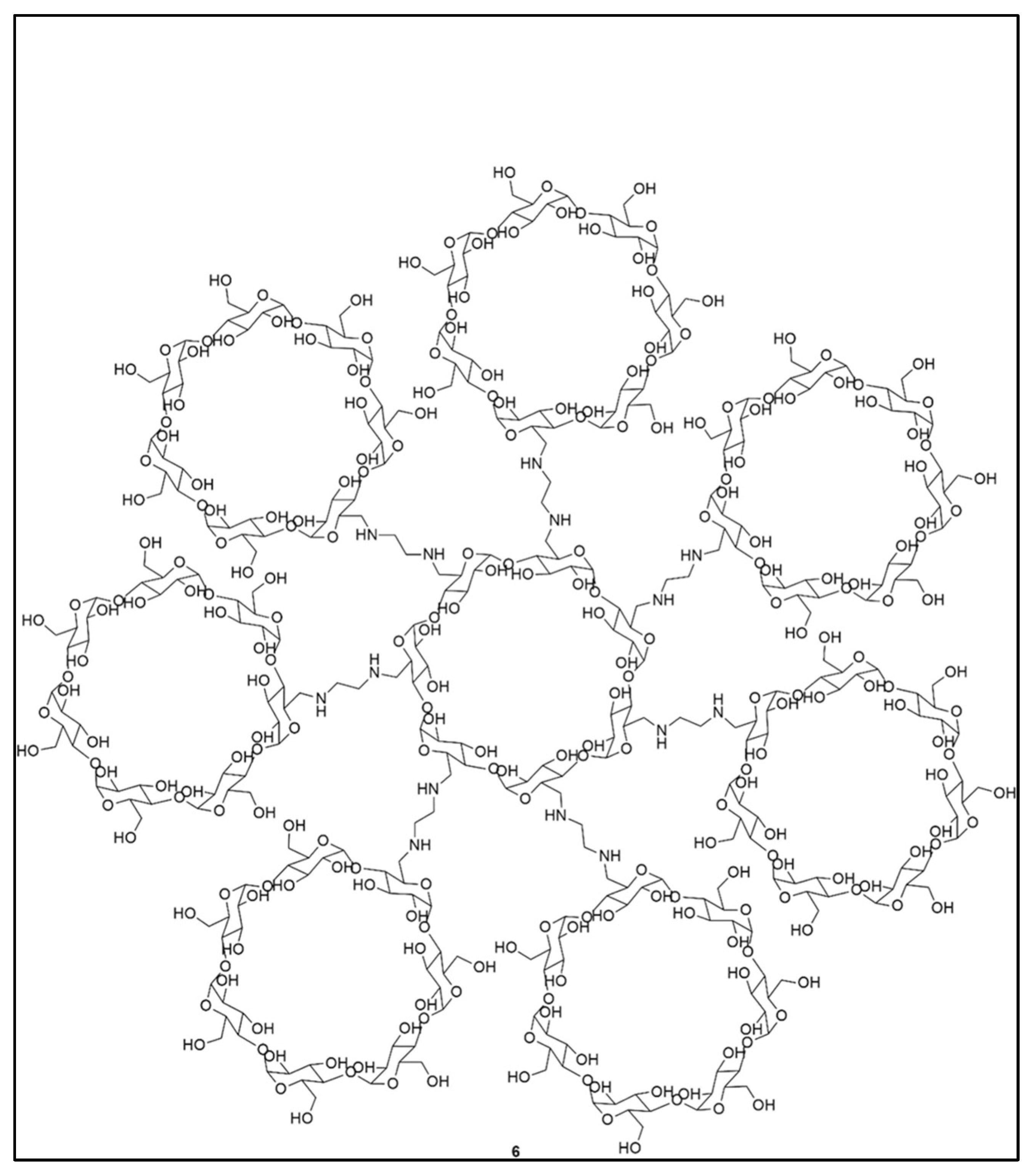
Dendrimers are also used to deliver active ingredients other than anticancer drugs, notably, anti-inflammatory drugs. An investigation of the ability of PAMAM-3 and PPI-4 to encapsulate phenylbutazone used as a model active ingredient showed that PAMAM-3 offered higher encapsulation, slower release and lower toxicity than PPI-4 [20]. PAMAMs of different generations are also good ophthalmic vehicles for the ocular administration of ilocarpine nitrate and tropicamide [92]. Other tri-block citric acid-poly(ethylene glycol)-citric acid (CPEGC) dendrimers, biocompatible compounds, have shown the ability to bind, solubilize and polarize hydrophobic molecules such as 5-amino salicylic acid (5-ASA), mefenamic acid and diclofenac [93]. Naproxen (anti-inflammatory) and naltrexone (with antagonistic properties used in the treatment of opiate addiction) were successfully encapsulated in cyclodextrin-based dendrimers by Namazi et al. (Figure 8) [94]. To finish this section on therapeutic applications, a PEG-functionalized polyether described by Mingjun Liu et al. [95] encapsulates cholesterol and amino acid derivatives, too.
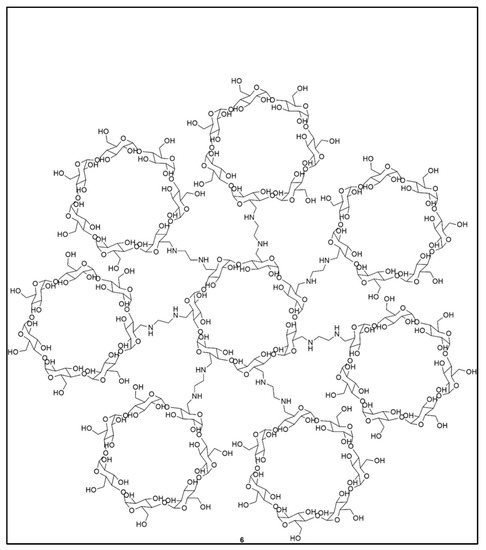
Figure 8.
First generation cyclodextrin dendrimers, reproduced according to [94] with the permission of Wiley.
3.3. Catalysis
Dendrimers offer a specific topology that allows fluid intradendritic coordination, controlling the size of nanoparticles (NPs) and preventing agglomeration [96]; in catalysis, they are templates for the preparation of a particle replica, but also stabilize nanoparticles and allow for improved catalyst solubility and catalytic selectivity [97]. Thus, these nano-objects have been used for a wide range of catalytic reactions, including hydrogenations, Heck coupling and Suzuki reactions in various solvents (water, organic solvents, biphasic organic/fluorinated solvents and supercritical CO2). In many cases, it is very simple to recycle the catalysts encapsulated in the dendrimers [97,98].
As examples, a new family of poly(propyleneimine) dendrimers functionalized with glycerol carbonate (GD-PPI) played the role of a recyclable basic catalyst for the ring opening of epoxides to carboxylic acid [39]. In addition, these glycerodendrimers were used for the preparation of stable platinum nanoparticles that showed good catalytic activity over several rings, for the reduction of α,β-unsaturated ketones to saturated ketones [40].
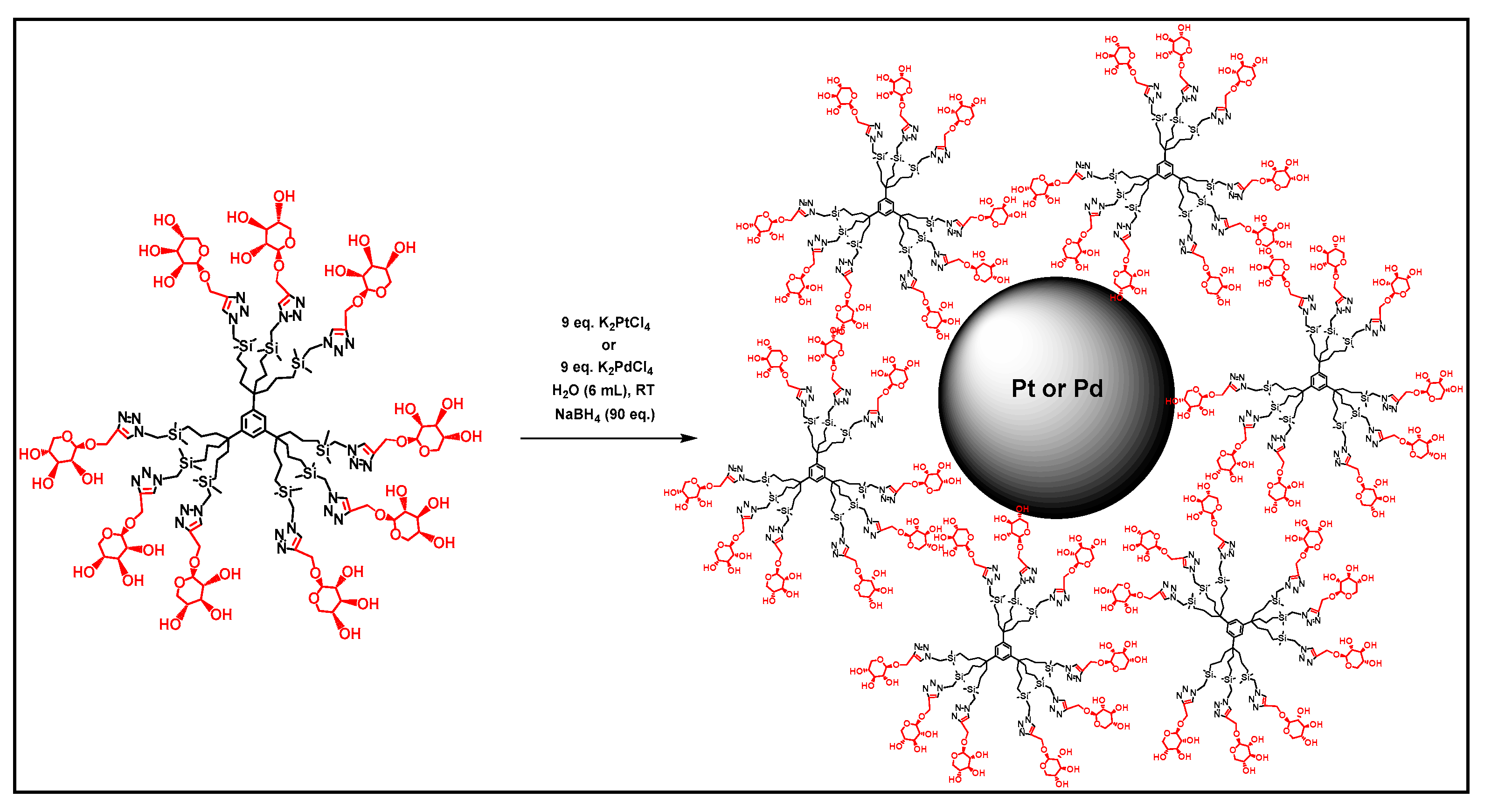
In 2008, Guangnan and his team worked on the encapsulation of Pd nanoparticles in dendrimers (PdDENs) derived from negatively charged PAMAM. These PdDENs are very stable in ionic liquids and can be used as highly active catalysts for the hydrogenation of styrene with an efficiency maintained after 12 recycles [99]. Other glycodendrimer-stabilized palladium nanoparticles (PdDENs) (Figure 9) showed good catalytic activity in water for the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) and in the C–C Miyaura–Suzuki coupling with substituted aryl bromide [96].
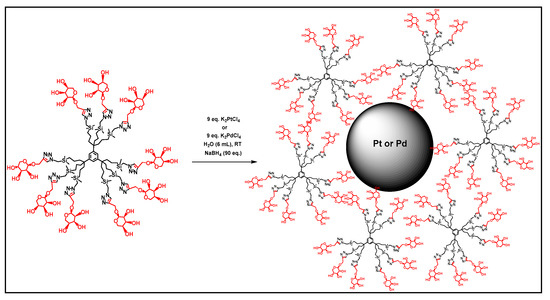
Figure 9.
Preparation of glycodendrimer-stabilized PdDENs, reproduced according to [96] with the permission of the author.
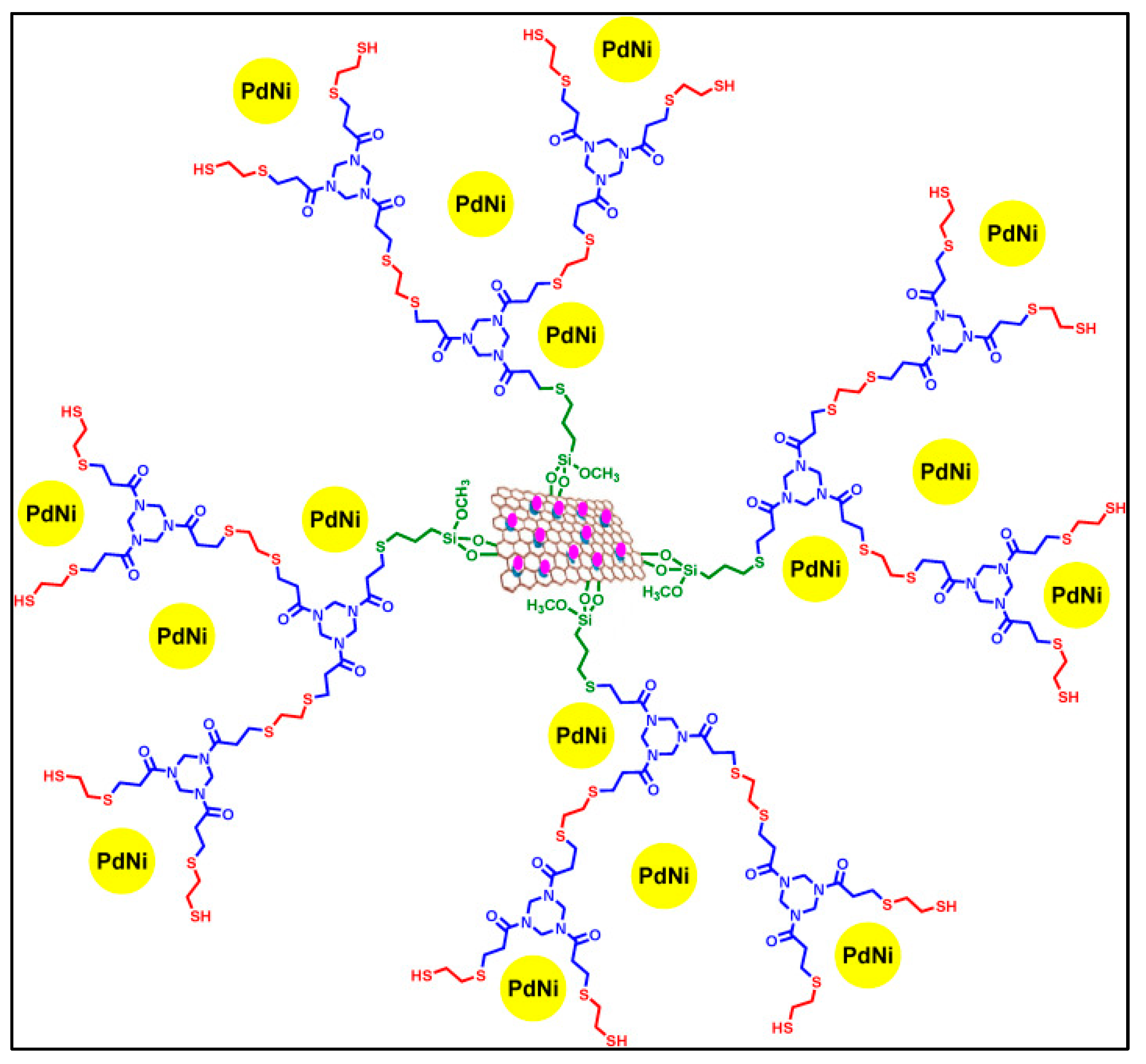
For several years, ferrocene-containing dendrimers have been used in the field of nanocatalysis [100] to stabilize Pd and Au nanoparticles that are very active in C–C cross-coupling, olefin hydrogenation and oxidation reactions. Ferrocene plays a crucial role in these dendritic supports with its redox activity and three-dimensionality stabilizing the nanoparticles. Other dendrimers grafted on the surface of a magnetic graphene oxide nanocomposite were developed by Niakan et al. (Figure 10) [101] and successfully used to immobilize bimetallic Pd/Ni nanoparticles active in the Suzuki–Miyaura reaction; the combination of Pd and Ni nanoparticles with the dendritic structure of the catalyst led to an excellent catalytic activity thanks to the presence of dendrimers with numerous cavities, thus allowing the fast absorption of the reagents and a synergy between the two metals.
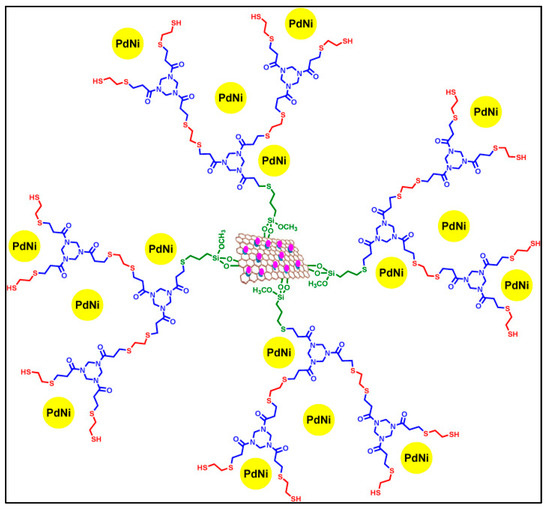
Figure 10.
Structure of nanoparticles stabilized by the dendrimer grafted on the surface of a second-generation magnetic graphene oxide nanocomposite GOF@Dm2@PdNi, reproduced according to [101] with the permission of Elsevier.
Finally, the cellulose/dendrimer combination, based on triacryloylhexahydrotriazine and the third generation ethane dithiol obtained by grafting via sulfur derivatives on the cellulose surface, was a heterogeneous catalyst [102] able to convert fructose into 5-hydroxymethylfurfural (96% conversion) and to be recycled at least five times without losing its stability and activity.
3.4. Environment
Water pollution caused by heavy metals represents a deleterious danger to public health and the ecological system. In the field of water purification, nanotechnology offers the possibility of effectively removing pollutants and germs [103]. The use of dendrimers as absorbents of organic and metallic pollutants has aroused considerable interest in recent years.
In 2005, Diallo et al. tested PAMAM-type dendrimers to recover Cu(II) ions from aqueous solutions [104]. They showed that Cu(II)-PAMAM bonds are much larger and more sensitive to the pH of the solution than Cu(II)-linear polymer bonds with amine groups, which makes it easy to recover the complex via filtration, and thus, to recycle the dendrimer. The same observation applies for soil pollution, where PAMAM dendrimers of different generations can be used as very good reusable extracting agents for the in situ removal of heavy metals from contaminated soils [105].
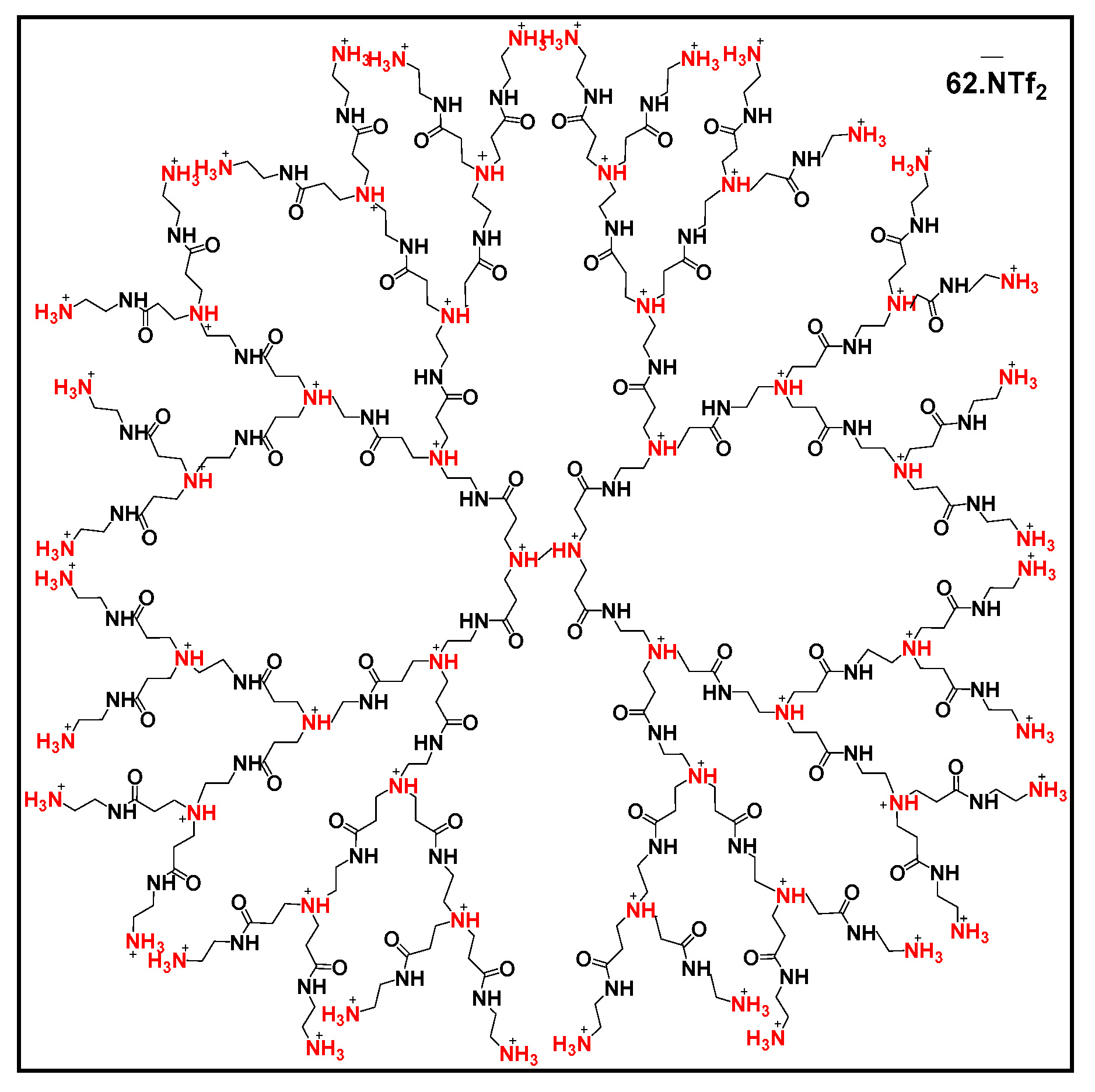
Recently, PAMAM-derived dendrimers grafted onto mesoporous silica via functionalization with sulfides were also developed for water treatment [41]. These dendrimers show better absorption properties for Hg(II) and Cd(II) compared to traditional absorbents. In addition, they show excellent selective absorption and regeneration properties (95.2% efficiency after five cycles). Other PAMAM-based materials such as carbon nanotubes/PAMAM (CNTs/PAMAM), graphene oxide/PAMAM (OG/PAMAM) and silica/PAMAM (SiO2/PAMAM) were developed to be used as sensors for the detection and quantification of metal ions (Cu2+, Hg2+, Zn2+, Pb2+ and Co2+) and organic pollutants (ketoprofen, tamoxifen and clofibric acid) [106]. In the same spirit, diclofenac salt, β-estradiol, atrazine and diuron were encapsulated by glycerodendrimers (GD-PAMAM and GD-PPI), and their release was studied as a function of pH [16]. Ibuprofen and naproxen could also be removed in water in the presence of PAMAM-halloysite (Hal-PAMAM) as an absorbent [107], with maximum absorption capacities (at pH 3–6) of 68.3 and 6.0 mg·g−1 for ibuprofen and naproxen, respectively. PAMAM G 2.5, this time grafted onto amidoximated membranes, was also used as an absorbent for antibiotics (lasalocid A, salinomycin and semduramicin) present in wastewater [108] with very good yields of 82 to 91%. Finally, ionic dendrimers are also used in the environmental field; indeed, the extraction of metal cations such as Cu2+, Ni2+, Pb2+, Cd2+ and Ag+ can be achieved with dendrimers derived from PAMAM or PPI (PAMAM G3 NH3+ Tf2N−, PPI G3 NH3+ Tf2N−, PPI G3NH2Me+ BF4− and PAMAM G3NH2Me+ BF4−) (Figure 11) [109]. Other imidazolium-functionalized ionic dendrimers (HPILs) stabilize uniformly sized silver nanoparticles stable in water [110] and can deliver biomolecules [111].
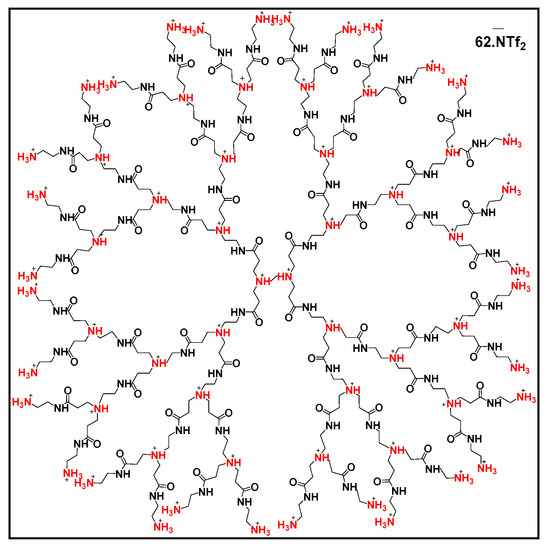
Figure 11.
Structure of PAMAM G3 NH3+ NTf2− dendrimer, reproduced according to [109] with the permission of the author.
3.5. Food
Many bioactive compounds used in the food industry are hydrophobic and insoluble or very slightly soluble in water. These include carotenoids, essential fatty acids, essential oils, some vitamins and phenolic compounds [112]. Their insolubility or low water solubility prevents them from being formulated at the intended dose, resulting in low bioavailability [112]. Therefore, delivery systems such as liposomes, nanotubes, proteins, biopolymers and dendrimers were developed to improve their solubility and bioavailability [113,114,115]. This also offers other advantages such as protection against evaporation (the case of essential oils), chemical reactions or migration into food, controlled delivery and preservation of the stability of bioactive compounds during processing and storage [115,116]. Dendrimers thus offer a new approach for the solubilization of these ingredients, providing specific delivery properties of bioactive compounds. Food applications of dendrimers are not as common as those of liposomes and micelles, but they may have a great future in this field [114].
The 2019 review by Yousefi et al. provides the main work on the synthesis of dendrimers and their ability to encapsulate bioactive ingredients such as resveratrol, curcumin, and vitamins C and B12 that can be used in food [112]. Four new families of glycerol-based dendrimers recently encapsulated cinnamon and lemongrass essential oils, too [12,15]. The encapsulation of β-carotene (BC) by the dendrimer derived from glucan octenyl succinate (OSA-SMDG) (corn sugar extract) was carried out to study the impact of the dendrimer on the storage stability and antioxidant activity of emulsions loaded with β-carotene (BC) as well as on its bioaccessibility [117]. Thus, the encapsulation capacity of β-carotene in both emulsions in the presence of 3% and 5% dendrimers is significant, and the antioxidant activity of both emulsions is higher than that of pure β-carotene. The extension to the encapsulation of coenzyme Q10 (CoQ10) also confirmed the interest of this type of dendrimer in the field of food encapsulation [118].
3.6. Cosmetics
In cosmetics, many biologically active substances are not stable and are sensitive to temperature, pH, light and oxidation. Therefore, it is important that they are protected from unwanted degradation to control the release of the active substance. The use of biodegradable polymers such as dendrimers for the encapsulation of cosmetic active ingredients can increase their efficacy and bioavailability, and also improve the stability and release of the active substances [119]. Dendrimers can be used in the formulation of various cosmetic products such as skin, hair, nail, lip and/or dental care and protection products [120]. Thus, for several years, the use of dendrimers in cosmetics has aroused the interest of researchers. A patent filed by L’Oréal [121] describes the use of polyester dendrimers functionalized by hydroxyl groups in cosmetic and dermatological compositions that can be applied to different supports (skin, nails, keratin fibers, semi-mucous membranes and/or mucous membranes). The cohesion between the drug and the support is strengthened and, moreover, the sensory properties are improved. We can also note the use of dendrimers in self-tanning compositions leading to an increased color intensity and good resistance to washing [122].
The use of PAMAM dendrimers in cosmetic and skincare applications such as creams, shampoos and conditioners, toners, make-up, and their use for the encapsulation of salicylic acid in anti-acne compositions, has also been reported. Salicylic acid is encapsulated in the cavities, but also tends to bind to amine groups on the surface of dendrimers, which stabilizes it prior to its application to the skin, where it is released. The use of this salicylic acid-PAMAM complex in various make-up compounds thus avoids the reaction with iron oxide pigments [123]. PAMAM-type dendrimers are also used in deodorant compositions and have additional odor absorption properties [124].
It has also been shown that various families of water-soluble PEG-derived dendrimers are suitable for the transport of ascorbic acid molecules due to their controlled multivalency [125]. The encapsulation of ascorbic acid can also be achieved in PAMAMs (G 2.5 to G4) in methanol [126] with better encapsulations for the 2.5 and 3.5 half generations. In 2010, Boisselier et al. worked on the encapsulation of vitamins C, B3 and B6 in dendrimers. The encapsulations are performed in water with two types of commercial dendrimers, PAMAM and PPI, with the different generations G2, G3 and G5 [127], and the encapsulation is proven by titration of the vitamins which are complexed to the primary and tertiary amines of the dendrimers; the highest number of encapsulated molecules is obviously observed for the higher generations.
Recently, we further studied the ability of glycerodendrimers GD-PAMAMs and GD-PPIs to encapsulate vitamin C and its interaction with the stratum corneum. We showed that GD-PAMAM-3 is the best candidate for cosmetic application due to its good vitamin C encapsulation capacity, its low cytotoxicity and its interaction with the stratum corneum [14].
Carbosilane dendrimers with polyphenol groups were also developed by De La Mata et al. as antioxidants in the cosmetic and pharmaceutical industries [48]. A copolymer with a carbosiloxane-based dendrimer was subsequently developed by Iimura et al. to improve formulation stability and to provide good water and oil resistance, gloss and a tactile feel and/or adhesive properties to hair and/or skin [128]. This dendrimer was recently patented for cosmetic application [129]. Many attempts were made to increase the solubility of quercetin using nano-encapsulation techniques. Indeed, Madaan et al. studied the solubility of quercetin in PAMAM solutions of various generations (G0 to G3) and concentrations; as a result, the solubility of quercetin could be increased, and the formation of quercitin/PAMAM complexes is confirmed by FTIR [130].
The PAMAM-3/caffeic acid compound (covalent bonds) recently reported in the literature exhibits dermo-cosmetic healing activity and a significant antioxidant capacity; used for wound healing and tissue regeneration, it can also be used in dermo-cosmetic compositions for skincare to reduce the appearance of wrinkles and expression lines [131]. In the same goal, ionic dendrimers derived from PAMAM or PPI and phenolic acids (ferulic acid, caffeic acid and phloretic acid) were also prepared [13], and the dendrimers derived from ferulic acid are the best candidates for use in cosmetics at concentrations not exceeding 100 mg/mL, due to their antioxidant activity and low cytotoxicity.
Finally, dendrimer/resveratrol formulations were also studied [132] to increase the solubility, stability and transdermal permeation of resveratrol for anti-aging products, shampoos, hair gels, sunscreens, sprays, lotions and anti-acne products [133].
4. Toxicity of Dendrimers
The study of toxicity, in general, is the measurement of the ability of a substance to cause adverse health effects on any life form [134]. It depends on its interaction with living matter, but also on the dose of the compound needed to produce an effect. This toxicity is expressed as a lethal dose capable of killing 50% of a population—the LD50 [134]. Three types of toxicity can be distinguished [111] as follows: (1) acute toxicity, for which the effects resulting from the massive administration of a substance at one time are immediate; (2) sub-acute toxicity, which is due to chronic absorption over several months (e.g., alcohol) and (3) long-term toxicity, where the effect appears after the accumulation of absorbed doses until the threshold dose is reached, as in the case for cumulative products (e.g., lead), irritant substances (e.g., passive smoking) or mutagens [134].
The cytotoxicity test is the most used to assess the toxicity of a substance or molecule. This test consists of assessing the potential of the substance/molecule to inhibit the growth of cells in an organism or to cause cell death. These in vitro tests can be performed using qualitative and quantitative methods [135]. The appropriate test method is chosen according to the nature of the sample to be evaluated, the type of cells and the culture conditions [135,136]. The cytotoxicity of a substance can be assessed experimentally using a variety of assay methods categorized as follows: (1) dye exclusion methods; (2) metabolic activity-based methods with Adenosine 5′-Triphosphate (ATP), sulforhodamine B and protease viability marker; (3) surviving clonogenic cells or (4) cell profiling via DNA synthesis [136]. The simplest methods are the dye exclusion methods such as the trypan blue dead cell indicator, and methods based on metabolic activity such as the MTT [137] and WST1 methods (Water Soluble Tetrazolium) [138]. For the latter, it is the reduction of the tetrazolium salt to formazan by mitochondrial NADH oxidoreductase enzymes in living cells that is observed. Table 2 provides some examples of the cytotoxic effects of various types of dendrimers evaluated using different methods on different cells.

Table 2.
Cytotoxic effects of various dendrimers on different cell types.
The cytotoxicity of dendrimers depends strongly on the number and nature of the surface functional groups. Cationic dendrimers often show high toxicity, while anionic and neutral dendrimers show little or no toxic effects [140,143]. The higher cytotoxicity of cationic dendrimers is often explained by the interaction of the positively charged dendrimer surface with negatively charged cell membranes. This interaction leads to the formation of nanopores in the cell membrane, with its damage and the subsequent leakage of the cell contents leading to cell death [144].
For PPI dendrimers, their toxicity increases with the number of surface groups. It is well demonstrated that fifth generation dendrimers (PPI-5) are more toxic than lower generation dendrimers [145]. We observed the same result with the PAMAM family, where higher generations show higher cytotoxicity [144], whereas carbosilane-poly(ethylene oxide) (CSi-PEO) dendrimers and other dendrimers with neutral or anionic end groups appear to be much less toxic [146]. It was also shown by comparing the two families of dendrimers, PPI and PAMAM, with the same number of functions at the periphery (32 NH2) that PAMAM G3 is less toxic than PPI G4 [20]. Therefore, it is recommended for some dendrimers to replace the surface groups with more neutral entities to reduce their cytotoxicity [32,143]. Thus, Stasko N.A et al. studied the cytotoxicity of PPI with surfaces modified with PEG or acetamide entities on cultured human endothelial cells (HUVEC). Cytotoxicity tests confirmed that the PPI functionalization effectively decreased the cytotoxicity of dendrimers [33]. The same observation was made when the surface of PPI was modified with maltotriose (tests performed on mouse blood cells) [147] or with glycerol carbonate (tests performed on lung fibroblasts) [38].
Regarding the PAMAM family, D’Emanuele and his team studied the influence of surface modification of PAMAMs on cytotoxicity toward human epithelial cells (Caco-2) [32]. Unmodified cationic PAMAMs are more toxic than anionic ones, and the toxicity of both increases with increasing generations. The same observations were made in cervical cancer cells [148]. The functionalization with glycerol carbonate leads to the same conclusion given above with PPI [16].
Finally, Frechet and coll. also showed that polyester dendrimers with peripheral hydroxyl groups were slightly toxic in vivo at very high concentrations in mice [149].
5. The Interaction of Dendrimers with the SC
The interactions of the dendrimers with the SC are important to (i) understand how these dendrimers act on the skin and (ii) optimize the design of the most efficient and least cytotoxic dendrimer.
5.1. The Skin
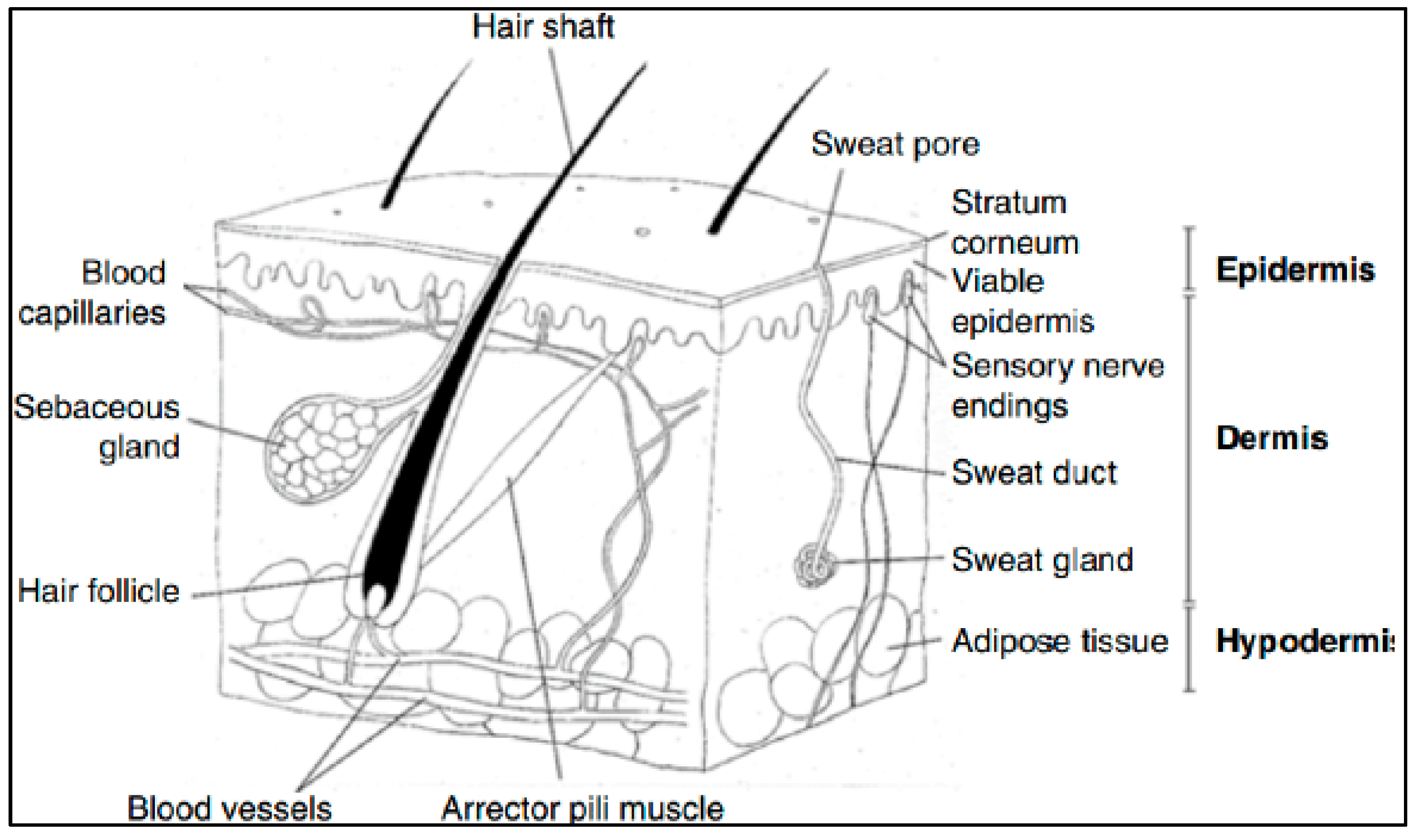
The skin is an organ made up of several layers and tissues, representing the largest organ in the human body, with approximately 2 m2 of surface area (for an adult) and 10% of the body weight [150]. Chemically, the skin consists of an average of 70% water, 27.5% protein, 2% fat and 0.5% minerals and trace elements [151]. Its main function is to protect the body from external physical, chemical and infectious aggressions, but also to play an active role in many biological and biochemical processes [152]. The skin is made up of the following three main layers (Figure 12): (i) the hypodermis or epidermal sub-layer, i.e., the deepest layer made up of fat cells, collagen fibers and blood vessels [152]; (ii) the dermis, i.e., the thickest, most elastic intermediate layer, made up of fibroblasts, collagen, elastin, connective tissue, lymphatic vessels, sensory receptors and hair roots [152] and (iii) the epidermis.
The epidermis is the outermost layer of skin, ranging in thickness from 0.06 mm in the eyelids to 0.8 mm in the heel [153]. It plays an important role in protecting the body from toxins, bacteria and fluid loss. It consists of five sublayers of keratinocytes [154] as follows: (1) the basal layer (stratum basale), also called the dermal–epidermal tight junction, which is the deepest layer where keratinocytes are produced; (2) the spinous layer (stratum spinosum), in which keratinocytes produce keratin (protein fibers) and become fusiform; (3) the granular layer (stratum granulosum), where keratinization begins and the cells produce hard granules, which develop into keratin and epidermal lipids; (4) the clear layer (stratum lucidium), in which the cells are highly compressed and flattened and (5) the stratum corneum, which is the outermost layer of the epidermis.
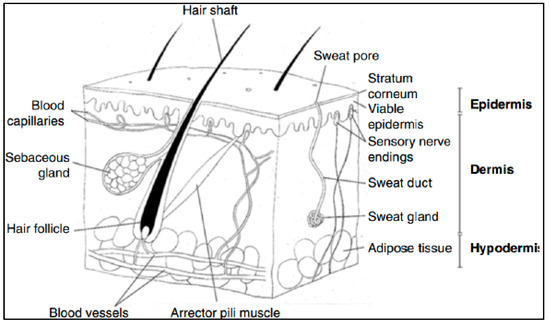
Figure 12.
A diagrammatic representation of the structure of human skin in cross section, reproduced from [155] with the permission of Springer Nature.
Figure 12.
A diagrammatic representation of the structure of human skin in cross section, reproduced from [155] with the permission of Springer Nature.
5.2. The Stratum Corneum
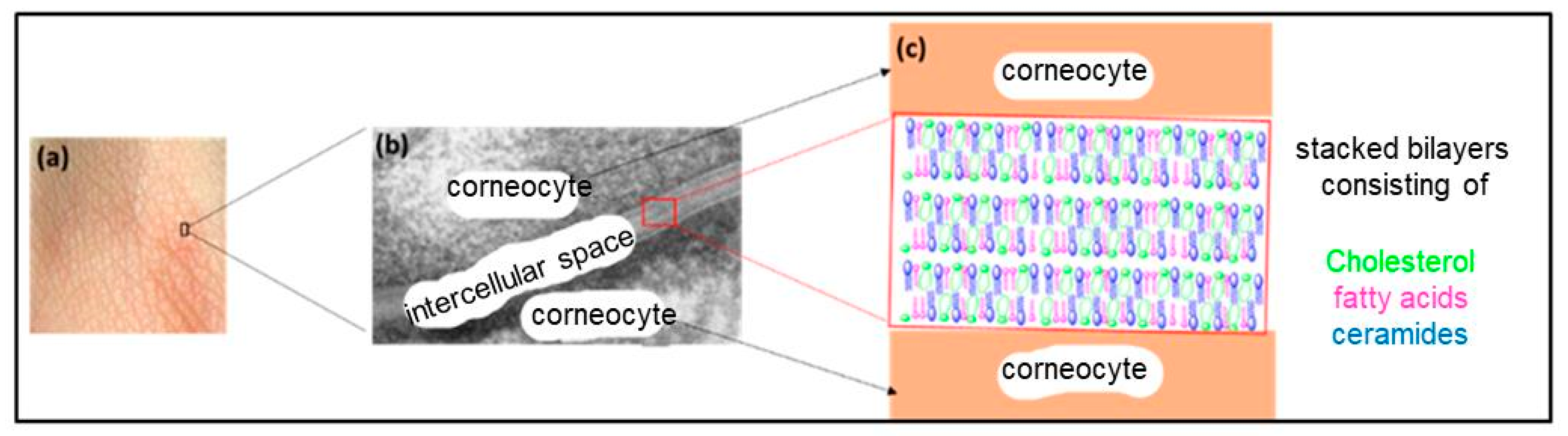
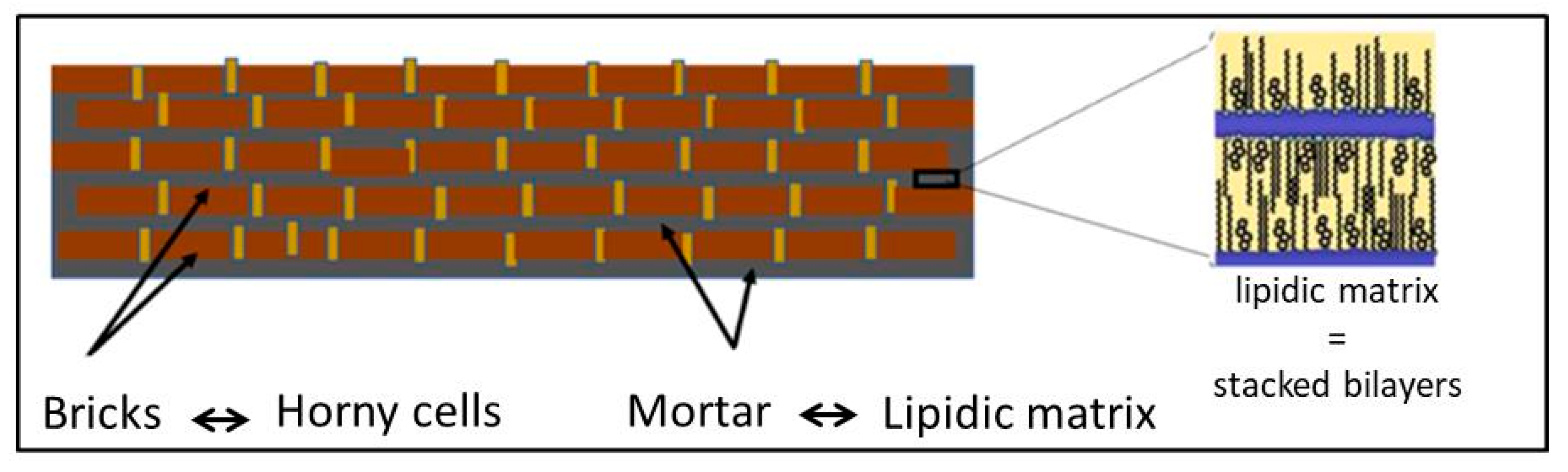
The SC is the outermost layer of the mammalian epidermis and is responsible for the barrier function of the skin [156,157]. It dictates the rate of absorption of exogenous molecules by the skin and prevents water loss from the body [156]. The SC is mainly composed of flattened, fully keratinized dead cells (corneocytes) embedded in an intercellular space (multilamellar lipid matrix) consisting of stacked bilayers (Figure 13) [158]. The “bricks and mortar” model is generally used to describe the structure of this layer, where the bricks represent the corneocytes and the mortar represents the lipid matrix (Figure 14) [159].
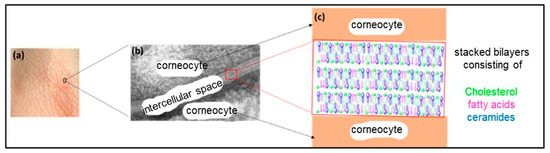
Figure 13.
(a) A surface image of the skin, (b) an electron micrograph of stacked lamellar membranes structured in an intercellular space, reproduced according to [160] with the permission of Elsevier, and (c) a structural model of the stratum corneum.
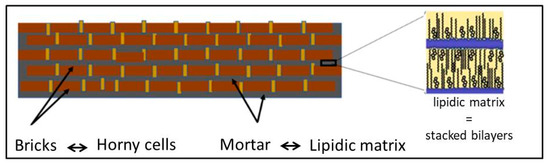
Figure 14.
Brick and mortar model of the stratum corneum, according to [159].
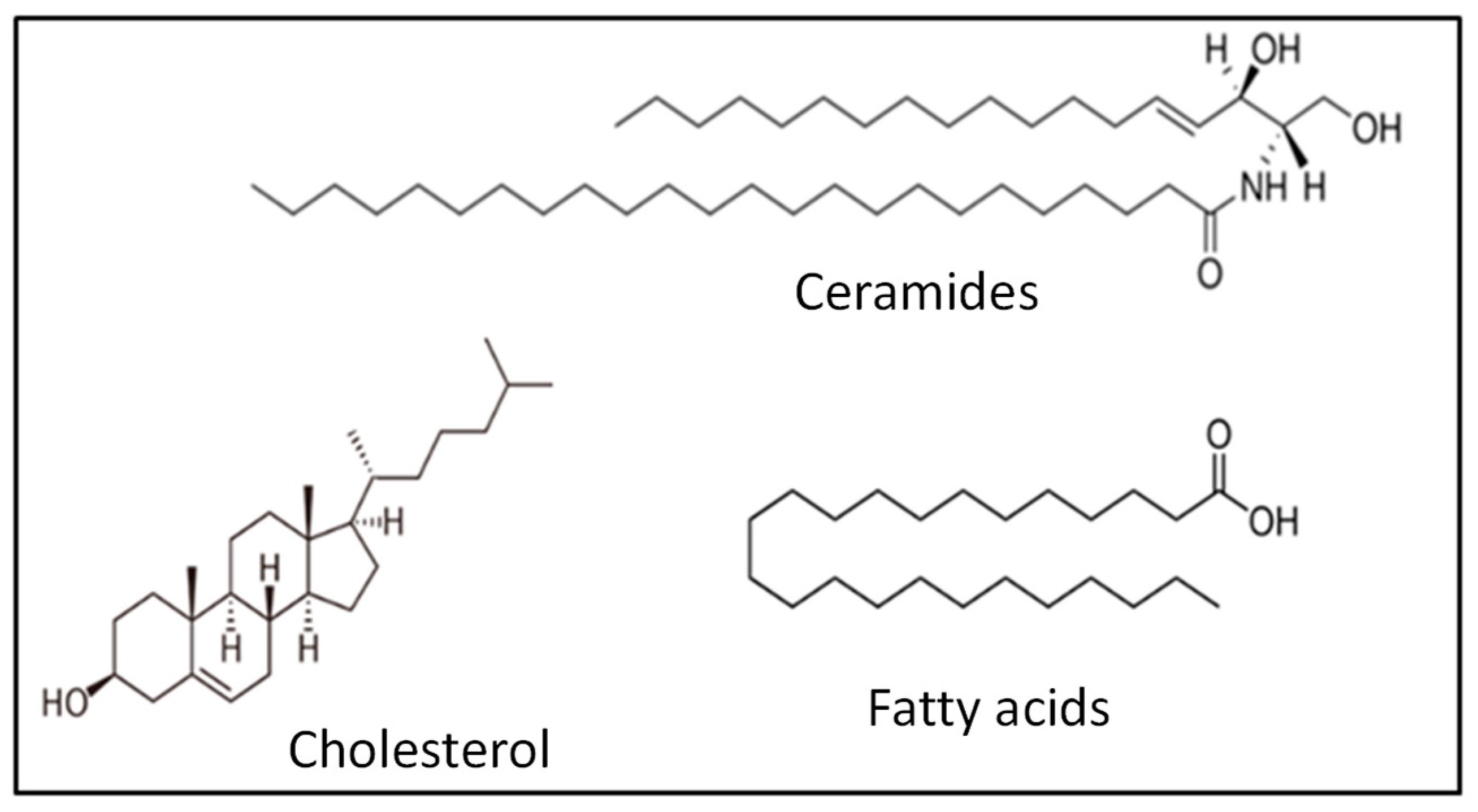
The phospholipid-free SC mainly consists of ceramides, cholesterol and long-chain free fatty acids in equimolar amounts (Figure 15) [161,162]. This lipid mixture is in the crystalline phase at a physiological temperature, which prevents the free diffusion of substances through the skin [163,164].
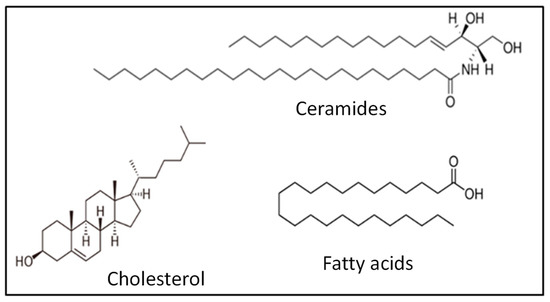
Figure 15.
Representative lipids of the main classes of lipids constituting the SC.
5.3. The Diffusion of Active Ingredients through the Skin
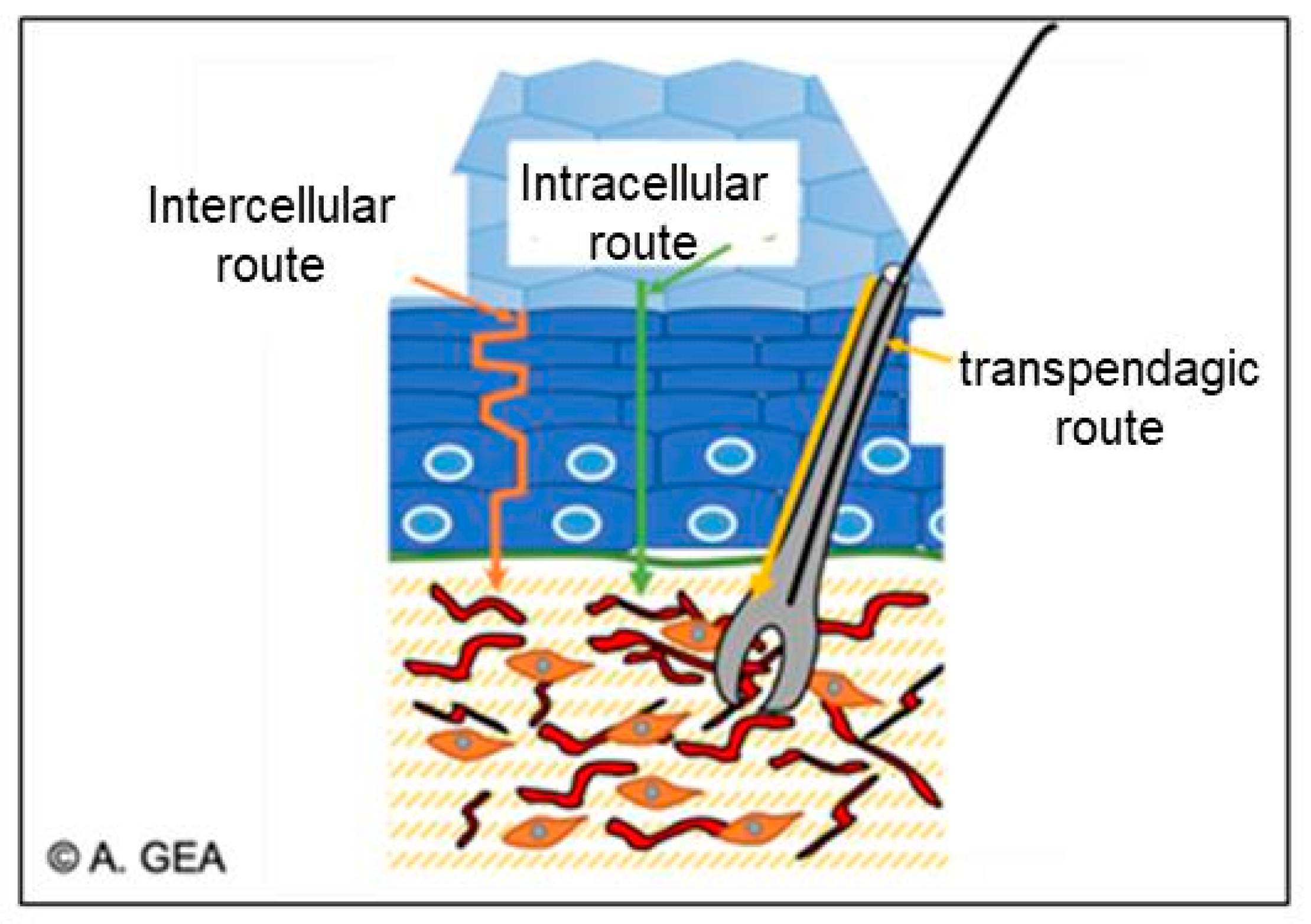
There are three main routes by which bioactive substances can enter the skin, as follows:
- -
- The intercellular route, which passes through the lipid matrix;
- -
- The intracellular pathway, which passes through the corneocytes;
- -
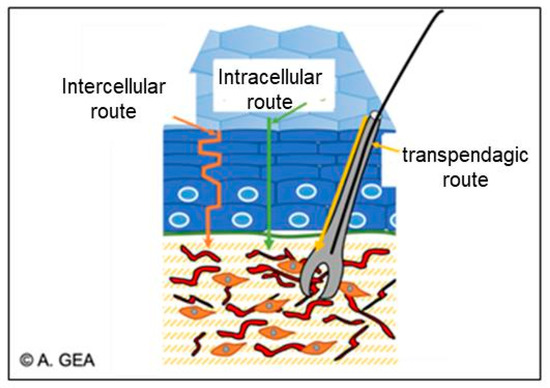 Figure 16. Different routes of diffusion through the skin, reproduced from [167] with the permission of the author.
Figure 16. Different routes of diffusion through the skin, reproduced from [167] with the permission of the author.
The lipid multilayer in the intercellular matrix is considered as the main transport barrier for active ingredients [168].
The delivery of active ingredients through the skin can be classified into two categories, including transdermal delivery, whereby the active substances diffuse through the different layers of the skin to reach the systemic circulation, or topical or local delivery, whereby the active substances are applied directly to the targeted pathological sites within the skin [169,170,171].
As the epidermis (or, more precisely, the stratum corneum (SC)) acts as a barrier, the delivery of drug molecules is limited. Only low molecular weight molecules (<500 Da) with optimal physicochemical properties (1 < log P < 3) can be passively transported through the SC [172]. Therefore, the use of biomaterials such as proteins, peptides, cyclodextrins and dendrimers has emerged as a new approach to increase the diffusion of active substances through the skin [170].
5.4. Biomimetic Skin Membranes
Biological membranes, in general, are very complex in terms of composition and organization. In order to understand the mechanisms at the molecular level and, in particular, the interactions between an external molecule and the membrane, it is necessary to simplify the study systems. For this reason, simplified membrane models are developed [173,174]. This represents a good compromise between simplicity and faithful representation of the native membrane.
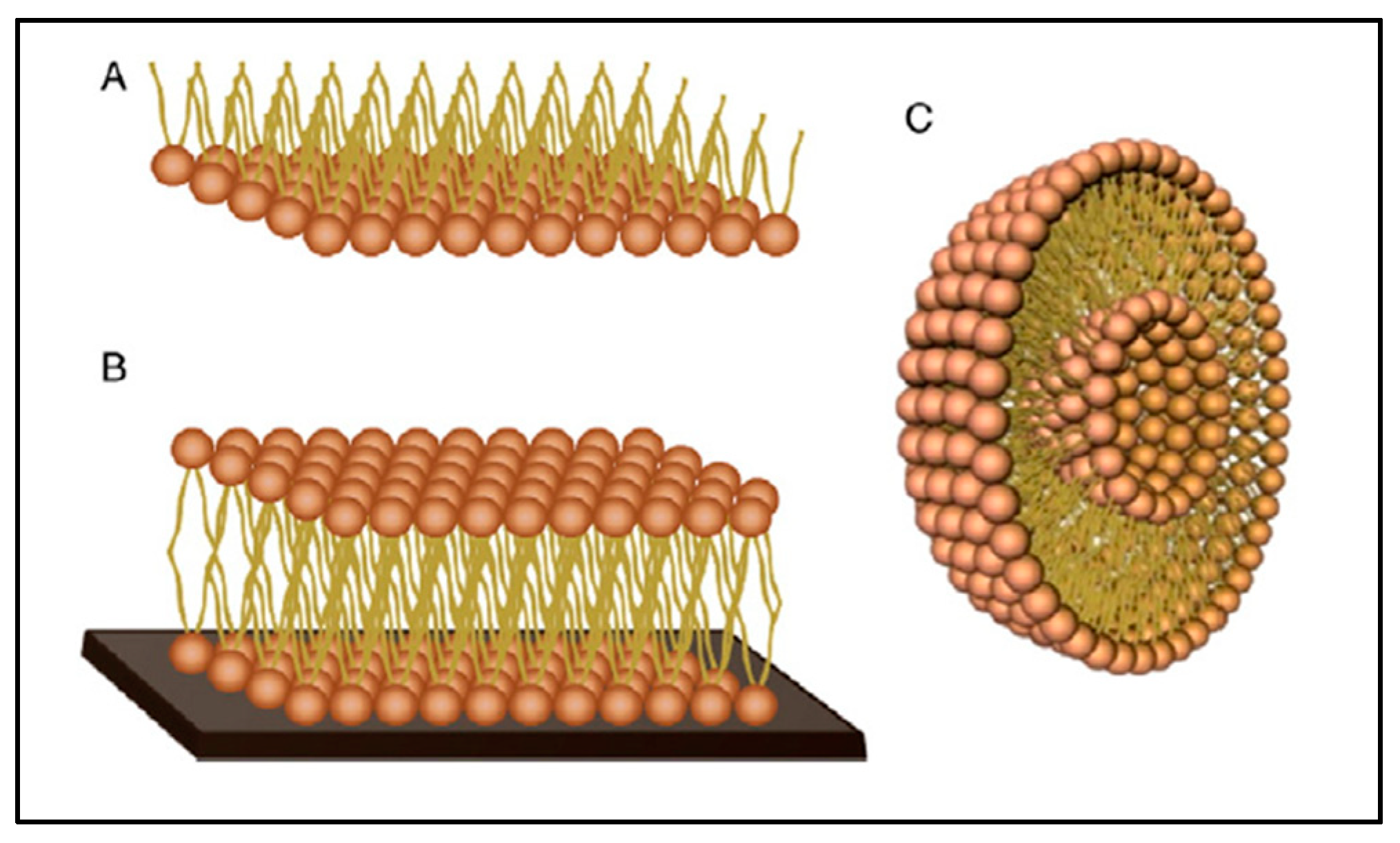
There are three main types of biomimetic membrane models; these include lipid monolayers, supported bilayers and liposomes (Figure 17) [173].
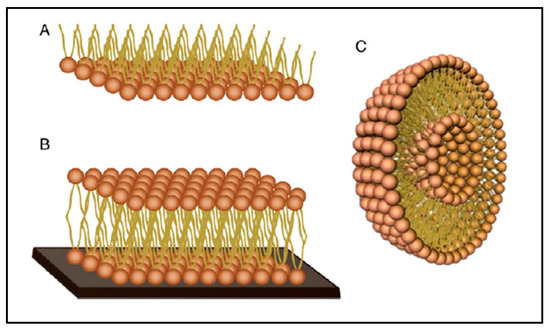
Figure 17.
Schematic representation of membrane models; (A) lipid monolayer, (B) supported lipid bilayer and (C) liposome, reproduced from [175] with the permission of the author.
Lipid monolayers, considered as half-layers, are the simplest models to mimic biological membranes. These models are often used to assess the ability of a substance to be inserted into the outer leaflet of the membrane and to characterize interactions in terms of kinetics and specificity [176]. Supported lipid bilayers are models consisting of a flat lipid bilayer supported on a solid surface. These systems can mimic some of the properties of the cell membrane such as lipid fluidity, domain formation and organization and protein support, but without the complexity of the real biological system [177]. Liposomes, also called lipid vesicles, are formed by one or more lipid bilayers containing aqueous compartments between the layers. They are classified into three groups according to their morphology and their size, which can vary from a few tens of nanometers to a few tens of microns; these three groups include small unilamellar vesicles (SUV), large unilamellar vesicles (LUV) and giant unilamellar vesicles (GUV) and multilamellar vesicles (MLV) made of several superposed bilayers [178]. These models are used to study the behavior of membrane phases (fluidity/stiffness) and membrane processes such as membrane fusion, molecular recognition, permeability and stability [179].
The choice of the lipid composition and the choice of the models depend, respectively, on the composition of the organism considered and on the property of the membrane to study [175].
In the case of the mammalian biological skin membrane, the SC is the layer responsible for the barrier function of the skin as discussed above [157]. The biomimetic membrane models of the SC consist of the major classes of preponderant skin lipids, ceramides, cholesterol and long chain free fatty acids [161,162]. Ceramides play an important role in structuring and maintaining the barrier function of the skin [156,180]. Cholesterol is one of the most present sterols in biological membranes and the only one in the SC [181]. Fatty acids are responsible for protecting the body from dehydration and play an important role through their anti-inflammatory effect [182].
Numerous studies using biomimetic lipid monolayers of the SC were typically performed using Langmuir balance or atomic force microscopy (AFM) [183,184,185]. The SC monolayer models exhibit an isotherm with no phase transition, characteristic of very rigid films [166]. By varying the ratios of the lipid constituents, the role of each lipid and its influence on the membrane properties can be determined [183].
Biomimetic liposome models of the skin are often prepared via hydration at a high temperature above 72 °C [161,186,187,188]. This is within the phase transition temperature range of the lipid composition from a lamellar gel-like phase to a cubic phase from 40–80 °C, respectively [186]. In a phase transition study of a biomimetic SC liposome, it was observed that the liposome had three transition points at 45 °C, 76 °C and 98 °C [189]. It was suggested that the transition observed near 45 °C is attributed to movements of the cholesterol side chains. The transition near 76 °C is attributed to the gel-to-crystalline phase change of the fatty acid and ceramide hydrocarbon chains, and the disruption of the association between the polar heads of the lipids. The transition at temperatures close to 100 °C is due to the vaporization of water molecules [189,190].
Studies on the SC liposome models have shown that the lipid composition and pH influence the liposome stability and structure [161,191,192]. For example, at a physiological pH, liposomes are not stable [192,193]. However, at 32 °C, the pH does not influence the membrane properties [187,193]. In addition, cholesterol fluidizes SC lipid membranes at temperatures below the main phase transition and condenses the membranes for higher temperatures [193]. The length of the ceramide chain influences the organization and properties of the bilayer. The longer the chain, the greater the electron density and the greater the bilayer thickness, which improves the barrier function [180]. In fact, an X-ray diffraction study showed that the SC model composed of short-chain ceramides is organized in three layers; two layers at the 50 Å ends are in the crystalline state, and an intermediate layer at 30 Å is in a liquid and easily permeable state [194]. The type of ceramide used also influences the permeability [188]. Indeed, when using dihydroceramides (dCer), the effect of acyl chain shortening is less pronounced than in the case of ceramides. For example, membranes composed of short-chain dCer are up to six times less permeable than membranes composed of corresponding short-chain ceramides. In addition, the same study showed, via infrared spectroscopy, that long dCer mixed less with fatty acids but formed ordered domains that were more thermally stable than the corresponding ceramide [188].
5.5. Dendrimer–Stratum Corneum Interaction
Understanding the interactions between dendrimers and biological membranes is essential for the use of dendrimers as delivery systems for active ingredients or genes [195]. In recent years, dendrimers have shown their potential to improve the cutaneous delivery of various molecules with a low skin irritation potential and a high active substance delivery capacity [171]. Several studies on the interaction of dendrimers with the skin membrane were performed, generally on human skin tissue [196,197,198] or animals [199,200]. However, to our knowledge, few studies of dendrimer interaction with biomimetic models of SC are described in the literature [201].
The nature, concentration, charge, size and hydrophobicity of the dendrimer influence both the penetration into the skin and the delivery of the active ingredient [202,203,204]. In the case of the study of the interaction of PAMAM dendrimers with porcine epidermal cells [204], it was shown that PAMAM G2 penetrates the layer better than PAMAM G4. Furthermore, the surface modification of PAMAM G2 by acetylation (cationic surface) or carboxylation (neutral surface) increases skin permeability. The functionalization of G2 dendrimers with oleic acid increases the 1-octanol/PBS partition coefficient, resulting in increased skin absorption and retention.
On the other hand, other studies on the same type of skin have shown that cationic dendrimers penetrate deeper layers of the skin, unlike anionic or neutral dendrimers [202,203], because their positive surface charge allows a greater interaction with the negatively charged skin surface.
In 2008, Venuganti et al. conducted a study on the effect of PAMAM dendrimers on porcine skin permeability of 5-fluorouracil (5FU) in the presence of phosphate buffer (PB), mineral oil (MO) or isopropyl myristate (IPM) [205]. They found that the simultaneous application of 5FU and dendrimer increased the flux of 5FU in the IPM and MO, whereas there was no change in the PB. The pretreatment of cells with dendrimer increased the permeability coefficient of 5FU in MO by 4-fold and by 2.5-fold in IPM, and this increased with the time of the pretreatment, whereas it decreased by half in the PB. Therefore, the pretreatment with dendrimers increased transepidermal water loss and decreased skin resistance (skin barrier modification), explaining the improvement in 5FU skin permeation.
The PAMAM dendrimer was also used as a carrier for 8-methoxypsoralen (8-MOP) to facilitate its penetration into the deep layer of the skin of a mouse [199]. The use of the dendrimer significantly increases the concentration of 8-MOP that penetrates the skin. The PAMAM G4 achieves a higher skin concentration of 8-MOP than the PAMAM G3 dendrimer. Another study also showed that PAMAM dendrimers can effectively improve the penetration of non-steroidal anti-inflammatory drugs such as ketoprofen and diflunisal through the skin [206].
In 2017, Volz et al. studied the interaction of fluorescein isothiocyanate (FITC)-labeled PAMAM-derived tectodendrimers with human skin tissue. They demonstrated via fluorescence microscopy (FLIM) that tectodendrimers penetrate the stratum corneum of human skin but do not cross the tight junction barrier or the stratum basale [207].
Furthermore, the effects of iontophoresis and sonophoresis on the permeation or deposition of peptide dendrimers through or within human skin could demonstrate that the skin permeation of dendrimers is enhanced by sonophoresis and iontophoresis, compared to conventional passive diffusion studies [196,198]. These dendrimers are stable for 6 h in the epidermis and dermis, and it is only after 24 h that certain small molecular weight dendrimers penetrate the epidermis [198].
Peptide dendrimers formed from three amino acids—glycine, arginine and lysine—have also been used for the cutaneous administration of ketoprofen covalently bound with these dendrimers [208]. This study, performed on mouse skin in vivo and in vitro, showed that these dendrimers improved the solubility of ketoprofen and significantly increased the skin permeability by sonophoresis after 6 h. The permeation of 5-fluorouracil through the human epidermis, assisted by these peptide dendrimers, is also improved [197], as well as the transdermal administration of the antioxidants silibinin and epigallocatechin-3-gallate (EGCG) [209].
6. Conclusions
Dendrimers are a class of macromolecules known for their architecture and their properties, particularly for the encapsulation and delivery of active substances in different fields. They are mainly synthesized by two approaches, divergent and convergent. The cytotoxicity of dendrimers depends on the nature of the charge and the number of generations.
Their interest for the cosmetic field is undeniable. They improve the permeability and diffusion of active ingredients in the skin. The nature, concentration, charge, size and hydrophobicity of the dendrimers influence both their penetration into the skin and the delivery of the active principle. The study of their interactions with the skin is essential for a better understanding of their activity and for the development of efficient and non-cytotoxic dendrimers. With the skin being a very complex system, the use of biomimetic models of the skin, and more particularly, of the SC, is a promising approach to obtain information at the molecular level.
Author Contributions
Conceptualization, K.B. and S.B.; methodology, K.B. and S.B.; validation, K.B., J.-P.M., C.C., M.D. and S.B.; investigation, K.B., M.D. and S.B.; resources, K.B., M.D. and S.B.; writing—original draft preparation, K.B. and S.B.; writing—review and editing, K.B. and S.B.; visualization, K.B. and S.B.; supervision, S.B.; project administration, M.D. and S.B.; funding acquisition, M.D. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-funded by the European Research Development Fund and Wallonia in the scope of the France-Wallonie-Vlaanderen Interreg 2014–2020 program via the project InTiCosm (project number 1.1.338 INTICOSM).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Donald, A.T.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Dendritic Macromolecules: Synthesis of Starburst Dendrimers. Macromolecules 1986, 19, 2466–2468. [Google Scholar] [CrossRef]
- Tomalia, D.A. The Dendritic State. Mater. Today 2005, 8, 34–46. [Google Scholar] [CrossRef]
- Newkome, G.R.; Yao, Z.-Q.; Baker, G.R.; Gupta, V.K. Cascade Molecules: A New Approach to Micelles. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Klajnert, B.; Bryszewska, M. Dendrimers: Properties and Applications. Acta Biochem. 2001, 48, 199–208. [Google Scholar] [CrossRef]
- Irfan, M.; Seiler, M. Encapsulation Using Hyperbranched Polymers: From Research and Technologies to Emerging Applications. Ind. Eng. Chem. Res. 2010, 49, 1169–1196. [Google Scholar] [CrossRef]
- Márquez-Miranda, V.; Araya-Durán, I.; Camarada, M.B.; Comer, J.; Valencia-Gallegos, J.A.; González-Nilo, F.D. Self-Assembly of Amphiphilic Dendrimers: The Role of Generation and Alkyl Chain Length in SiRNA Interaction. Sci. Rep. 2016, 6, 29436. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, Applications, and Properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Najafi, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Janus-Type Dendrimers: Synthesis, Properties, and Applications. J. Mol. Liq. 2022, 347, 118396. [Google Scholar] [CrossRef]
- Kokaz, S.F.; Deb, P.K.; Borah, P.; Bania, R.; Venugopala, K.N.; Nair, A.B.; Singh, V.; Al-Shar’i, N.A.; Hourani, W.; Gupta, G.; et al. Dendrimers: Properties and Applications in Biomedical Field. In Nanoengineering of Biomaterials; Wiley-VCH GmbH: New York, NY, USA, 2022; pp. 215–243. ISBN 9783527832095. [Google Scholar]
- Srinivasa-Gopalan, S.; Kevin, J.Y.; Kumar, C.S.S.R. Dendrimers in Cancer Treatment and Diagnosis. In Nanotechnologies for the Life Sciences; Wiley-VCH: New York, NY, USA, 2007; p. 423. ISBN 9783527313877. [Google Scholar]
- Mbakidi, J.P.; Barjhoux, I.; Aguibi, K.; Geffard, A.; Rioult, D.; Palos Ladeiro, M.; Bouquillon, S. Synthesis of New Betaine-Based Ionic Liquids by Using a “One-Pot” Amidation Process and Evaluation of Their Ecotoxicity through a New Method Involving a Hemocyte-Based Bioassay. ACS Sustain. Chem. Eng. 2021, 9, 15427–15441. [Google Scholar] [CrossRef]
- Maes, C.; Menot, B.; Hayouni, S.; Martinez, A.; Fauconnier, M.L.; Bouquillon, S. Preparation of New Glycerol-Based Dendrimers and Studies on Their Behavior toward Essential Oil Encapsulation. ACS Omega 2022, 7, 10277–10291. [Google Scholar] [CrossRef]
- Bacha, K.; Estager, J.; Brassart-Pasco, S.; Chemotti, C.; Fernandes, A.E.; Mbakidi, J.P.; Deleu, M.; Bouquillon, S. Synthesis and Activity of Ionic Antioxidant-Functionalized PAMAMs and PPIs Dendrimers. Polymers 2022, 14, 3513. [Google Scholar] [CrossRef] [PubMed]
- Bacha, K.; Chemotti, C.; Monboisse, J.C.; Robert, A.; Furlan, A.L.; Smeralda, W.; Damblon, C.; Estager, J.; Brassart-Pasco, S.; Mbakidi, J.P.; et al. Encapsulation of Vitamin C by Glycerol-Derived Dendrimers, Their Interaction with Biomimetic Models of Stratum Corneum and Their Cytotoxicity. Molecules 2022, 27, 8022. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Brostaux, Y.; Bouquillon, S.; Fauconnier, M.L. Use of New Glycerol-Based Dendrimers for Essential Oils Encapsulation: Optimization of Stirring Time and Rate Using a Plackett-Burman Design and a Surface. Foods 2021, 10, 207. [Google Scholar] [CrossRef]
- Menot, B.; Stopinski, J.; Martinez, A.; Oudart, J.B.; Maquart, F.X.; Bouquillon, S. Synthesis of Surface-Modified PAMAMs and PPIs for Encapsulation Purposes: Influence of the Decoration on Their Sizes and Toxicity. Tetrahedron 2015, 71, 3439–3446. [Google Scholar] [CrossRef]
- Les Dendrimeres: Des Nanomolecules Pour Le Transport de Medicaments. Available online: https://www.cinam.univ-mrs.fr/cinam/evenements/science-pour-tous/les-dendrimeres-des-nano-molecules-pour-le-transport-de-medicaments/ (accessed on 17 May 2023).
- Buhleier, E.; Wehner, W.; Vögtle, F. Cascade-and Nonskid-Chain-like Syntheses of Molecular Cavity Topologies. Synthesis 1978, 02, 155–158. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Shao, N.; Su, Y.; Hu, J.; Zhang, J.; Zhang, H.; Cheng, Y. Comparison of Generation 3 Polyamidoamine Dendrimer and Generation 4 Polypropylenimine Dendrimer on Drug Loading, Complex Structure, Release Behavior, and Cytotoxicity. Int. J. Nanomed. 2011, 6, 3361–3372. [Google Scholar] [CrossRef]
- Hawker, C.J.; Fréchet, J.M.J. Preparation of Polymers with Controlled Molecular Architecture. A New Convergent Approach to Dendritic Macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Hodge, P. Polymer Science Branches Out. Am. Nat. 1993, 362, 18–19. [Google Scholar] [CrossRef]
- Brabander-van den Berg, E.M.M.; Meijer, E.W. Poly(Propy1ene Imine) Dendrimers: Large-Scale Synthesis by Hetereogeneously Catalyzed Hydrogenation Angew. Angew. Chem. Int. 1993, 32, 1308–1311. [Google Scholar] [CrossRef]
- Worner, C.; Miilhaupt, R. Polynitrile-and Polyamine-Functional Poly(Trimethy1ene Imine) Dendrimers. Angew. Chem. Int. 1993, 32, 1306–1308. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms Macroscopic Matter. Angew. Chem. Int. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Zhuo, R.X.; Du, B.; Lu, Z.R. In Vitro Release of 5-Fluorouracil with Cyclic Core Dendritic Polymer. J. Control. Release 1999, 57, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, J.H.; Kim, B.K.; Kim, J.H.; Shin, W.S.; Jin, S.H. Convergent Synthesis of PAMAM Dendrimers Using Click Chemistry of Azide-Functionalized PAMAM Dendrons. Tetrahedron 2006, 62, 9193–9200. [Google Scholar] [CrossRef]
- Boas, U.; Christensen, J.B.; Heegaard, P.M.H. Dendrimers: Design, Synthesis and Chemical Properties. J. Mater. Chem. 2006, 16, 3785–3798. [Google Scholar] [CrossRef]
- Gupta, U.; Gupt, U.; Agashe, H.B.; Jain, N.K. Polypropylene Imine Dendrimer Mediated Solubility Enhancement: Effect of PH and Functional Groups of Hydrophobes. J. Pharm. Pharm. Sci. 2007, 10, 358–367. [Google Scholar]
- Gupta, U.; Dwivedi, S.K.D.; Bid, H.K.; Konwar, R.; Jain, N. Ligand Anchored Dendrimers Based Nanoconstructs for Effective Targeting to Cancer Cells. Int. J. Pharm. 2010, 393, 185–196. [Google Scholar] [CrossRef]
- Kaur, D.; Jain, K.; Mehra, N.K.; Kesharwani, P.; Jain, N.K. A Review on Comparative Study of PPI and PAMAM Dendrimers. J. Nanoparticle Res. 2016, 18, 146. [Google Scholar] [CrossRef]
- Jevprasesphant, R.; Penny, J.; Jalal, R.; Attwood, D.; Mckeown, N.B.; D’emanuele, A. The Influence of Surface Modification on the Cytotoxicity of PAMAM Dendrimers. Int. J. Pharm. 2003, 252, 263–266. [Google Scholar] [CrossRef]
- Stasko, N.A.; Johnson, C.B.; Schoenfisch, M.H.; Johnson, T.A.; Holmuhamedov, E.L. Cytotoxicity of Polypropylenimine Dendrimer Conjugates on Cultured Endothelial Cells. Biomacromolecules 2007, 8, 3853–3859. [Google Scholar] [CrossRef]
- Tack, F.; Bakker, A.; Maes, S.; Dekeyser, N.; Bruining, M.; Elissen-Roman, C.; Janicot, M.; Brewster, M.; Janssen, H.M.; de Waal, B.F.M.; et al. Modified Poly(Propylene Imine) Dendrimers as Effective Transfection Agents for Catalytic DNA Enzymes (DNAzymes). J. Drug Target. 2006, 14, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Toi, Y.; Harada, A.; Kono, K. Preparation of Poly (Ethylene Glycol)-Attached Dendrimers Encapsulating Photosensitizers for Application to Photodynamic Therapy. Bioconjugate Chem. 2007, 18, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, V.; Kumar, P.V.; Kumar Tekade, R. Novel PEGylated Nanoparticulate Architectures for Sustained Delivery of H2 Receptor Antagonist. Eur. J. Med. Chem. 2009, 44, 1155–1166. [Google Scholar] [CrossRef]
- Choi, Y.; Thomas, T.; Kotlyar, A.; Islam, M.T.; Baker, J.R. Synthesis and Functional Evaluation of DNA-Assembled Polyamidoamine Dendrimer Clusters for Cancer Cell-Specific Targeting. Chem. Biol. 2005, 12, 35–43. [Google Scholar] [CrossRef]
- Balieu, S.; Cadiou, C.; Martinez, A.; Nuzillard, J.M.; Oudart, J.B.; Maquart, F.X.; Chuburu, F.; Bouquillon, S. Encapsulation of Contrast Imaging Agents by Polypropyleneimine-Based Dendrimers. J. Biomed. Mater. Res.-Part A 2013, 101, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Balieu, S.; El Zein, A.; De Sousa, R.; Jérôme, F.; Tatibouët, A.; Gatard, S.; Pouilloux, Y.; Barrault, J.; Rollin, P.; Bouquillona, S. One-Step Surface Decoration of Poly(Propyleneimines) (PPIs) with the Glyceryl Moiety: New Way for Recycling Homogeneous Dendrimer-Based Catalysts. Adv. Synth. Catal. 2010, 352, 1826–1833. [Google Scholar] [CrossRef]
- Menot, B.; Salmon, L.; Bouquillon, S. Platinum Nanoparticles Stabilized by Glycerodendrimers: Synthesis and Application to the Hydrogenation of α,β-Unsaturated Ketones under Mild Conditions. Eur. J. Inorg. Chem. 2015, 2015, 4518–4523. [Google Scholar] [CrossRef]
- Luan, L.; Tang, B.; Liu, Y.; Xu, W.; Liu, Y.; Wang, A.; Niu, Y. Direct Synthesis of Sulfur-Decorating PAMAM Dendrimer/Mesoporous Silica for Enhanced Hg(II) and Cd(II) Adsorption. Langmuir 2022, 38, 698–710. [Google Scholar] [CrossRef]
- Mekuria, S.L.; Song, C.; Ouyang, Z.; Shen, M.; Janaszewska, A.; Klajnert-Maculewicz, B.; Shi, X. Synthesis and Shaping of Core-Shell Tecto Dendrimers for Biomedical Applications. Bioconjugate Chem. 2021, 32, 225–233. [Google Scholar] [CrossRef]
- Uppuluri, S.; Swanson, D.R.; Piehler, L.T.; Li, J.; Hagnauer, G.L.; Tomalia, D.A. Core-Shell Tecto(Dendrimers): I. Synthesis and Characterization of Saturated Shell Models. Adv. Mater 2000, 12, 796–800. [Google Scholar] [CrossRef]
- Song, C.; Ouyang, Z.; Guo, H.; Qu, J.; Gao, Y.; Xia, J.; Shen, M.; Shi, X. Core-Shell Tecto Dendrimers Enable Enhanced Tumor MR Imaging through an Amplified EPR Effect. Biomacromolecules 2021, 22, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, D.-Y.; Shen, M.; Shi, X. Design and Biomedical Applications of Core-Shell Tecto Dendrimers. Basic Clin. Med. 2022, 42, 15–25. [Google Scholar] [CrossRef]
- Bouquillon, S.; Hayouni, S.; Menot, B. Dendrimer-Type Compounds, Methods for Producing Same and Uses Thereof. PCT. Patent WO 2020/021032A1, 30 January 2020. [Google Scholar]
- Bouquillon, S.; Hayouni, S.; Menot, B. Dendrimer-Type Compounds, Methods for Producing Same and Uses Thereof. PCT. Patent WO 2020/021034A1, 30 January 2020. [Google Scholar]
- De La Mata, F.J.; Gómez Ramírez, R.; Ortega Ló-Pez, P.; Mencía Berlinches, G.; Maroto Díaz, M.; Natalia, S.D.O. Carbosilane Dendrimers Comprising Polyphenol Groups, And Uses Thereof. PCT. Patent WO 2017/220831Al, 28 December 2017. [Google Scholar]
- Nnadiekwe, C.C.; Nada, A.; Abdulazeez, I.; Imam, M.R.; Janjua, M.R.S.A.; Al-Saadi, A.A. UV-Absorbing Benzamide-Based Dendrimer Precursors: Synthesis, Theoretical Calculation, and Spectroscopic Characterization. New J. Chem. 2022, 46, 75–85. [Google Scholar] [CrossRef]
- Zhang, D.; Atochina-Vasserman, E.N.; Lu, J.; Maurya, D.S.; Xiao, Q.; Liu, M.; Adamson, J.; Ona, N.; Reagan, E.K.; Ni, H.; et al. The Unexpected Importance of the Primary Structure of the Hydrophobic Part of One-Component Ionizable Amphiphilic Janus Dendrimers in Targeted MRNA Delivery Activity. J. Am. Chem. Soc. 2022, 144, 4746–4753. [Google Scholar] [CrossRef]
- Zhang, D.; Xiao, Q.; Rahimzadeh, M.; Liu, M.; Rodriguez-Emmenegger, C.; Miyazaki, Y.; Shinoda, W.; Percec, V. Self-Assembly of Glycerol-Amphiphilic Janus Dendrimers Amplifies and Indicates Principles for the Selection of Stereochemistry by Biological Membranes. J. Am. Chem. Soc. 2023, 145, 4311–4323. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Rivera-Martinez, N.; Raab, C.J.; Bermudez, J.G.; Good, M.C.; Klein, M.L.; Percec, V. Co-Assembly of Liposomes, Dendrimersomes, and Polymersomes with Amphiphilic Janus Dendrimers Conjugated to Mono- and Tris-Nitrilotriacetic Acid (NTA, TrisNTA) Enhances Protein Recruitment. Giant 2022, 9, 100089–100101. [Google Scholar] [CrossRef]
- Duan, Y.; Zhao, X.; Sun, M.; Hao, H. Research Advances in the Synthesis, Application, Assembly, and Calculation of Janus Materials. Ind. Eng. Chem. Res. 2021, 60, 1071–1095. [Google Scholar] [CrossRef]
- Parida, P.; Nayak, B.P.; Mishra, S.C. Amphiphilic Janus like Particles for Biomedical Application. PharmaTutor Mag. 2014, 2, 92–97. [Google Scholar]
- Căta, A.; Ienașcu, I.M.C.; Ştefănuț, M.N.; Roșu, D.; Pop, O.R. Properties and Bioapplications of Amphiphilic Janus Dendrimers: A Review. Pharmaceutics 2023, 15, 589. [Google Scholar] [CrossRef]
- Liu, J.; Feng, Y.; Ma, B.; He, Y.M.; Fan, Q.H. Design and Synthesis of Janus-Type Chiral Dendritic Diphosphanes and Their Applications in Asymmetric Hydrogenation. Eur. J. Org. Chem. 2012, 2012, 6737–6744. [Google Scholar] [CrossRef]
- Liu, J.; Feng, Y.; He, Y.; Yang, N.; Fan, Q.H. Janus Dendritic Phosphines: Synthesis and Application in Suzuki Coupling Reactions. New J. Chem. 2012, 36, 380–385. [Google Scholar] [CrossRef]
- Feng, Y.; He, Y.M.; Zhao, L.W.; Huang, Y.Y.; Fan, Q.H. A Liquid-Phase Approach to Functionalized Janus Dendrimers: Novel Soluble Supports for Organic Synthesis. Org. Lett. 2007, 9, 2261–2264. [Google Scholar] [CrossRef] [PubMed]
- Casanellas, I.; Lagunas, A.; Vida, Y.; Pérez-Inestrosa, E.; Andrades, J.A.; Becerra, J.; Samitier, J. The Janus Role of Adhesion in Chondrogenesis. Int. J. Mol. Sci. 2020, 21, 5269. [Google Scholar] [CrossRef] [PubMed]
- Laine, R.M.; Roll, M.; Asuncion, M.; Sulaiman, S.; Popova, V.; Bartz, D.; Krug, D.J.; Mutin, P.H. Perfect and Nearly Perfect Silsesquioxane (SQs) Nanoconstruction Sites and Janus SQs. J. Sol.-Gel. Sci. Technol. 2008, 46, 335–347. [Google Scholar] [CrossRef]
- Han, Y.D.; Kim, H.S.; Park, Y.M.; Chun, H.J.; Kim, J.H.; Yoon, H.C. Retroreflective Janus Microparticle as a Nonspectroscopic Optical Immunosensing Probe. ACS App. Mater. Interfaces 2016, 8, 10767–10774. [Google Scholar] [CrossRef]
- Liu, Y.; Mu, S.; Liu, X.; Ling, Q.; Hang, C.; Ruiz, J.; Astruc, D.; Gu, H. Ferrocenyl Janus Mixed-Dendron Stars and Their Stabilization of Au and Ag Nanoparticles. Tetrahedron 2018, 74, 4777–4789. [Google Scholar] [CrossRef]
- Zhao, L.; Ling, Q.; Liu, X.; Hang, C.; Zhao, Q.; Liu, F.; Gu, H. Multifunctional Triazolylferrocenyl Janus Dendron: Nanoparticle Stabilizer, Smart Drug Carrier and Supramolecular Nanoreactor. Appl. Organomet. Chem. 2018, 32, e4000–e4011. [Google Scholar] [CrossRef]
- Plunkett, S.; El Khatib, M.; Şencan, İ.; Porter, J.E.; Kumar, A.T.N.; Collins, J.E.; Sakadžić, S.; Vinogradov, S.A. In Vivo Deep-Tissue Microscopy with UCNP/Janus-Dendrimers as Imaging Probes: Resolution at Depth and Feasibility of Ratiometric Sensing. Nanoscale 2020, 12, 2657–2672. [Google Scholar] [CrossRef]
- Sadler, K.; Tam, J.P. Peptide Dendrimers: Applications and Synthesis. Rev. Mol. Biotechnol. 2002, 90, 195–229. [Google Scholar] [CrossRef]
- Niederhafner, P.; Šebestík, J.; Ježek, J. Peptide Dendrimers. J. Pept. Sci. 2005, 11, 757–788. [Google Scholar] [CrossRef]
- Xie, F.; Li, R.; Shu, W.; Zhao, L.; Wan, J. Self-Assembly of Peptide Dendrimers and Their Bio-Applications in Theranostics. Mater. Today Bio 2022, 14, 100239–100247. [Google Scholar] [CrossRef] [PubMed]
- Sapra, R.; Verma, R.P.; Maurya, G.P.; Dhawan, S.; Babu, J.; Haridas, V. Designer Peptide and Protein Dendrimers: A Cross-Sectional Analysis. Chem. Rev. 2019, 119, 11391–11441. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhao, L.; Li, Y.; Xu, T. Design of Biocompatible Dendrimers for Cancer Diagnosis and Therapy: Current Status and Future Perspectives. Chem. Soc. Rev. 2011, 40, 2673–2703. [Google Scholar] [CrossRef] [PubMed]
- Sheveleva, N.N.; Tarasenko, I.I.; Vovk, M.A.; Mikhailova, M.E.; Neelov, I.M.; Markelov, D.A. NMR Studies of Two Lysine Based Dendrimers with Insertion of Similar Histidine-Arginine and Arginine-Histidine Spacers Having Different Properties for Application in Drug Delivery. Int. J. Mol. Sci. 2023, 24, 949. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Yang, H.; Liu, S.; Zhao, Z. Self-Assembly of Dendrimer-DNA Amphiphiles and Their Catalysis as G-Quadruplex/Hemin DNAzymes. Polymer 2023, 266, 125621–125627. [Google Scholar] [CrossRef]
- He, H.; He, J.; Zheng, K.; Ma, M.; Shi, Y.; Chen, S.; Wang, X. Fantastic Supramolecular Chiral Self-Assembly of POSS Based Dendrimers: From Helical Nano-Fibers to Nano-Toroids and Loofah-like Superstructures. Eur. Polym. J. 2023, 184, 111768–111773. [Google Scholar] [CrossRef]
- Chowdhury, S.; Toth, I.; Stephenson, R.J. Dendrimers in Vaccine Delivery: Recent Progress and Advances. Biomaterials 2022, 280, 121303–121316. [Google Scholar] [CrossRef]
- Lin, Q.; Jiang, G.; Tong, K. Dendrimers in Drug-Delivery Applications. Des. Monomers Polym 2010, 13, 301–324. [Google Scholar] [CrossRef]
- Yousefi, M.; Narmani, A.; Jafari, S.M. Dendrimers as Efficient Nanocarriers for the Protection and Delivery of Bioactive Phytochemicals. Adv. Colloid. Interface Sci. 2020, 278, 102125–102137. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Nixon, L.S.; Hedstrand, D.M. The Role of Branch Cell Symmetry and Other Critical Nanoscale Design Parameters in the Determination of Dendrimer Encapsulation Properties. Biomolecules 2020, 10, 642. [Google Scholar] [CrossRef]
- Suganya, V.; Anuradha, V. Microencapsulation and Nanoencapsulation: A Review. In. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef]
- Moseley, M.E.; Chew, W.M.; White, D.L.; Kucharczyk, J.; Litt, L.; Derugin, N.; Dupon, J.; Brasch, R.C.; Norman, D. Hypercarbia-Induced Changes in Cerebral Blood Volume in the Cat: A ’H MRI and Intravascular Contrast Agent Study. Magn. Reson. Med. 1992, 23, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wiener, E.C.; Auteri, F.P.; Chen, J.W.; Brechbiel, M.W.; Gansow, O.A.; Schneider, D.S.; Belford, R.L.; Clarkson, R.B.; Lauterbur, P.C. Molecular Dynamics of Ion-Chelate Complexes Attached to Dendrimers. ACS J. 1996, 118, 7774–7782. [Google Scholar] [CrossRef]
- Bryant, L.H.; Brechbiel, M.W.; Wu, C.; Bulte, J.W.M.; Herynek, V.; Frank, J.A. Synthesis and Relaxometry of High-Generation (G 5, 7, 9, and 10) PAMAM Dendrimer-DOTA-Gadolinium Chelates. J. Magn. Reson. Imag. 1999, 9, 348–352. [Google Scholar] [CrossRef]
- Anwaier, G.; Chen, C.; Cao, Y.; Qi, R. A Review of Molecular Imaging of Atherosclerosis and the Potential Application of Dendrimer in Imaging of Plaque. Int. J. Nanomed. 2017, 12, 7681–7693. [Google Scholar] [CrossRef] [PubMed]
- Trzepiński, P.; Klajnert-Maculewicz, B. Dendrimers for Fluorescence-Based Bioimaging. J. Chem. Technol. Biotechnol. 2017, 92, 1157–1166. [Google Scholar] [CrossRef]
- Deng, J.; Xu, J.; Ouyang, M.; Zou, Z.; Lei, Y.; Li, J.; Qing, Z.; Yang, R. Target-Triggered Hairpin-Free Chain-Branching Growth of DNA Dendrimers for Contrast-Enhanced Imaging in Living Cells by Avoiding Signal Dispersion. Chin. Chem. Lett. 2022, 33, 773–777. [Google Scholar] [CrossRef]
- Hudde, T.; Rayner, S.A.; Comer, R.M.; Weber, M.; Isaacs, J.D.; Waldmann, H.; Larkin, D.F.P.; George, A.J.T. Activated Polyamidoamine Dendrimers, a Non-Viral Vector for Gene Transfer to the Corneal Endothelium. Gene Ther. 1999, 6, 939–943. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Lajiness, M.S.; Vieth, M.; Erickson, J. Molecular Properties That Influence Oral Drug-like Behavior. Curr. Opin. Drug Discov. Devel. 2004, 7, 470–477. [Google Scholar]
- Singh, V.; Sahebkar, A.; Kesharwani, P. Poly (Propylene Imine) Dendrimer as an Emerging Polymeric Nanocarrier for Anticancer Drug and Gene Delivery. Eur. Polym. J. 2021, 158, 110683–110697. [Google Scholar] [CrossRef]
- Mollazade, M.; Nejati-Koshki, K.; Akbarzadeh, A.; Zarghami, N.; Nasiri, M.; Jahanban-Esfahlan, R.; Alibakhshi, A. PAMAM Dendrimers Augment Inhibitory Effects of Curcumin on Cancer Cell Proliferation: Possible Inhibition of Telomerase. Asian Pac. J. Cancer Prev. 2013, 14, 6925–6928. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Sheikh, A.; Hasan, N.; Sahebkar, A.; Riadi, Y.; Kesharwani, P. Folic Acid Conjugated Poly(Amidoamine) Dendrimer as a Smart Nanocarriers for Tracing, Imaging, and Treating Cancers over-Expressing Folate Receptors. Eur. Polym. J. 2022, 170, 111156–111170. [Google Scholar] [CrossRef]
- Kojima, C.; Kono, K.; Maruyama, K.; Takagishi, T. Synthesis of Polyamidoamine Dendrimers Having Poly(Ethylene Glycol) Grafts and Their Ability to Encapsulate Anticancer Drugs. Bioconjugate Chem. 2000, 11, 910–917. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, N.; Ma, C.; Zhang, Y.; Tan, H.; Qing, G.; Zhang, J.; Wang, Y.; Wang, J.; Chen, S.; et al. An Amphiphilic Dendrimer as a Light-Activable Immunological Adjuvant for in Situ Cancer Vaccination. Nat. Commun. 2021, 12, 4964–4979. [Google Scholar] [CrossRef]
- Vandamme, T.F.; Brobeck, L. Poly(Amidoamine) Dendrimers as Ophthalmic Vehicles for Ocular Delivery of Pilocarpine Nitrate and Tropicamide. J. Control. Release 2005, 102, 23–38. [Google Scholar] [CrossRef]
- Namazi, H.; Adeli, M. Dendrimers of Citric Acid and Poly (Ethylene Glycol) as the New Drug-Delivery Agents. Biomaterials 2005, 26, 1175–1183. [Google Scholar] [CrossRef]
- Namazi, H.; Heydari, A. Synthesis of β-Cyclodextrin-Based Dendrimer as a Novel Encapsulation Agent. Polym. Int. 2013, 63, 1447–1455. [Google Scholar] [CrossRef]
- Liu, M.; Kono, K.; J Fré Chet, J.M. Water-Soluble Dendrimer-Poly(Ethylene Glycol) Starlike Conjugates as Potential Drug Carriers. J. Polym. Sci. A Polym. Chem. 1999, 37, 3492–3503. [Google Scholar] [CrossRef]
- Gatard, S.; Salmon, L.; Deraedt, C.; Ruiz, J.; Astruc, D.; Bouquillon, S. Palladium Nanoparticles Stabilized by Glycodendrimers and Their Application in Catalysis. Eur. J. Inorg. Chem. 2014, 2014, 4369–4375. [Google Scholar] [CrossRef]
- Niu, Y.; Crooks, R.M. Dendrimer-Encapsulated Metal Nanoparticles and Their Applications to Catalysis. Comptes Rendus Chim. 2003, 6, 1049–1059. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Zolotukhina, A. Heterogeneous Dendrimer-Based Catalysts. Polymers 2022, 14, 981. [Google Scholar] [CrossRef]
- Ou, G.; Xu, L.; He, B.; Yuan, Y. Enhanced Stability of Charged Dendrimer-Encapsulated Pd Nanoparticles in Ionic Liquids. Chem. Commun. 2008, 35, 4210–4212. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ornelas, C.; Diallo, A.K.; Deraedt, C.; Wang, Y.; Lu, F.; Gu, H.; Astruc, D. Ferrocene-Based Dendritic Macromolecules as Efficient Supports in Nanocatalysis. Polymer 2022, 246, 124714–124722. [Google Scholar] [CrossRef]
- Niakan, M.; Masteri-Farahani, M. Pd–Ni Bimetallic Catalyst Supported on Dendrimer-Functionalized Magnetic Graphene Oxide for Efficient Catalytic Suzuki-Miyaura Coupling Reaction. Tetrahedron 2022, 108, 132655–132665. [Google Scholar] [CrossRef]
- Niakan, M.; Masteri-Farahani, M.; Karimi, S.; Shekaari, H. Sulfonic Acid Functionalized Dendrimer-Grafted Cellulose as a Solid Acid Catalyst for the High-Yield and Green Production of 5-Hydroxymethylfurfural. Sustain. Energy Fuels 2022, 6, 2514–2522. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Behari, J.; Sen, P. Application of Nanoparticles in Waste Water Treatment. World Appl. Sci. J. 2008, 3, 417–433. [Google Scholar]
- Diallo, M.S.; Christie, S.; Swaminathan, P.; Johnson, J.H.; Goddard, W.A. Dendrimer Enhanced Ultrafiltration. 1. Recovery of Cu(II) from Aqueous Solutions Using PAMAM Dendrimers with Ethylene Diamine Core and Terminal NH2 Groups. Environ. Sci. Technol. 2005, 39, 1366–1377. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, D. Removal of Copper from Contaminated Soil by Use of Poly(Amidoamine) Dendrimers. Environ. Sci. Technol. 2005, 39, 2369–2375. [Google Scholar] [CrossRef]
- Viltres, H.; López, Y.C.; Leyva, C.; Gupta, N.K.; Naranjo, A.G.; Acevedo–Peña, P.; Sanchez-Diaz, A.; Bae, J.; Kim, K.S. Polyamidoamine Dendrimer-Based Materials for Environmental Applications: A Review. J. Mol. Liq. 2021, 334, 116017–116040. [Google Scholar] [CrossRef]
- Kurczewska, J.; Cegłowski, M.; Schroeder, G. PAMAM-Halloysite Dunino Hybrid as an Effective Adsorbent of Ibuprofen and Naproxen from Aqueous Solutions. Appl. Clay Sci. 2020, 190, 105603–105612. [Google Scholar] [CrossRef]
- Cao, V.; Yunessnia lehi, A.; Bojaran, M.; Fattahi, M. Treatment of Lasalocid A, Salinomycin and Semduramicin as Ionophore Antibiotics in Pharmaceutical Wastewater by PAMAM-Coated Membranes. Environ. Technol. Innov. 2020, 20, 101103–101113. [Google Scholar] [CrossRef]
- Hayouni, S.; Robert, A.; Maes, C.; Conreux, A.; Marin, B.; Mohamadou, A.; Bouquillon, S. New Dendritic Ionic Liquids (DILs) for the Extraction of Metallic Species from Water. New J. Chem. 2018, 42, 18010–18020. [Google Scholar] [CrossRef]
- Tian, N.; Ni, X.; Shen, Z. Synthesis of Main-Chain Imidazolium-Based Hyperbranched Polymeric Ionic Liquids and Their Application in the Stabilization of Ag Nanoparticles. React. Funct. Polym. 2016, 101, 39–46. [Google Scholar] [CrossRef]
- Qin, T.; Li, X.; Chen, J.; Zeng, Y.; Yu, T.; Yang, G.; Li, Y. Dendritic Ionic Liquids Based on Imidazolium-Modified Poly(Aryl Ether) Dendrimers. Chem. Asian J. 2014, 9, 3641–3649. [Google Scholar] [CrossRef]
- Yousefi, M.; Orojzadeh, P.; Jafari, S.M. Nanoencapsulation of Food Ingredients by Dendrimers. In Biopolymer Nanostructures Food Encapsulation Purposes; Elsevier: Amsterdam, The Netherlands, 2019; Volume 20, pp. 607–625. ISBN 9780128156636. [Google Scholar]
- Assadpour, E.; Jafari, S.M. An Overview of Biopolymer Nanostructures for Encapsulation of Food Ingredients. In Biopolymer Nanostructures for Food Encapsulation Purposes; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 1–35. ISBN 9780128156636. [Google Scholar]
- Shatrohan Lal, R.K. Synthesis of Organic Nanoparticles and Their Applications in Drug Delivery and Food Nanotechnology: A Review. J. Nanomater. Mol. Nanotechnol. 2014, 03, 4–14. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2014, 7, 452–490. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An Overview of Encapsulation Technologies for Food Application. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, F.; Zhu, Y.; Miao, M. Development of Dendrimer-like Glucan-Stabilized Pickering Emulsions Incorporated with β-Carotene. Food Chem. 2022, 385, 132626–132633. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, F.; Chen, Y.; Hui, Q.; Miao, M. Dendrimer-like Glucan Nanoparticulate System Improves the Solubility and Cellular Antioxidant Activity of Coenzyme Q10. Food Chem. 2020, 333, 127510–127517. [Google Scholar] [CrossRef]
- Ammala, A. Biodegradable Polymers as Encapsulation Materials for Cosmetics and Personal Care Markets. Int. J. Cosmet. Sci. 2013, 35, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, P. Emerging Trends of Nanotechnology in Advanced Cosmetics. Colloids Surf. B Biointerfaces 2022, 214, 112440–112458. [Google Scholar] [CrossRef] [PubMed]
- Tournilhac, F.; Simon, P. Cosmetic or Dermatological Topical Compositions Comprisingdendritic Polyesters. U.S. Patent 6,287,552 B1, 11 September 2001. [Google Scholar]
- Allard, D.; Forestier, S. Self-Tanning Cosmetic Compositions. U.S. Patent 6, 399, 048 B1, 4 June 2002. [Google Scholar]
- Wolf, B.A.; Snyden, F. Cosmetic Compositions Having Keratolytic and Anti-Acne Activity. U.S Patent 5,449,519, 12 September 2001. [Google Scholar]
- Forestier, S.; Rollat-Corvol, I.; Rollat, C.I. Deodorant Composition and Use Thereof. U.S. Patent 6, 001 342, 14 December 1999. [Google Scholar]
- Astruc, D.; Ruiz, J.; Boisselier, E. Encapsulation de La Vitamine C Dans Des Dendrimères Solubles Dans l’eau. PCT. Patent WO 2009/112682A1, 17 September 2009. [Google Scholar]
- Wołowiec, S.; Laskowski, M.; Laskowska, B.; Magoń, A.; Mysliwiec, B.; Pyda, M. Dermatological Application of PAMAM-Vitamin Bioconjugates and Host-Guest Complexes-Vitamin C Case Study. In Stoichiometry and Research–The Importance of Quantity in Biomedicine; Innocenti, A., Ed.; IntechOpen: Rijeka, Croatia, 2012; Volume 8, pp. 195–210. [Google Scholar]
- Boisselier, E.; Liang, L.; Dalko-Csiba, M.; Ruiz, J.; Astruc, D. Interactions and Encapsulation of Vitamins C, B3, and B6 with Dendrimers in Water. Chem. A Eur. J. 2010, 16, 6056–6068. [Google Scholar] [CrossRef]
- Iimura, T.; Furukawa, H. Copolymer Having Carbosiloxane Dendrimer Structure, and Composition and Cosmetic Containing the Same. US. Patent 2012/0263662 A l, 18 October 2012. [Google Scholar]
- Souda, T.; Sugiura, T.; Kennoki, M.; Matsuba, M. Copolymère Ayant Une Structure de Dendrimère de Carbosiloxane, et Composition, Ingrédient Cosmétique, Agent de Formation de Revêtement et Produit Cosmétique Le Contenant. PCT. Patent WO 2020/203145 A1, 8 October 2020. [Google Scholar]
- Madaan, K.; Lather, V.; Pandita, D. Evaluation of Polyamidoamine Dendrimers as Potential Carriers for Quercetin, a Versatile Flavonoid. Drug Deliv. 2016, 23, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Guzman, L.; Castro, R.; Saffie, C.; Mandujano, P. Agent Nanocicatrisant Pour Soigner Des Blessures. PCT. Patent WO 2020/232561 Al, 26 November 2020. [Google Scholar]
- Chauhan, A.S. Dendrimer Nanotechnology for Enhanced Formulation and Controlled Delivery of Resveratrol. Ann. N. Y. Acad. Sci. 2015, 1348, 134–140. [Google Scholar] [CrossRef]
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594. [Google Scholar] [CrossRef]
- La Toxicité. Available online: https://www.futura-sciences.com/sante/definitions/medecine-toxicite-6517/ (accessed on 17 May 2023).
- La Cytotoxicité. Available online: https://www.eurofins.fr/pharma/accueil/services/prestations-analytiques/dispositifs-medicaux/evaluation-biologique/cytotoxicite (accessed on 17 May 2023).
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Biotechno. Pharm. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- Berridge, M.V.; Tan, A.S. Characterization of the Cellular Reduction of 3-(4,5-Dimethylthiazol-2- Yl)-2,5-Diphenyltetrazolium Bromide (MTT): Subcellular Localization, Substrate Dependence, and Involvement of Mitochondrial Electron Transport in MTT Reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef]
- Ishiyama, M.; Tominaga, H.; Shiga, M.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A Combined Assay of Cell Vability and in Vitro Cytotoxicity with a Highly Water-Soluble Tetrazolium Salt, Neutral Red and Crystal Violet. Biol. Pharm. Bull. 1996, 19, 1518–1520. [Google Scholar] [CrossRef]
- Lazniewska, J.; Milowska, K.; Zablocka, M.; Mignani, S.; Caminade, A.M.; Majoral, J.P.; Bryszewska, M.; Gabryelak, T. Mechanism of Cationic Phosphorus Dendrimer Toxicity against Murine Neural Cell Lines. Mol. Pharm. 2013, 10, 3484–3496. [Google Scholar] [CrossRef]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weenerc, J.W.; Meijerc, E.W.; Paulusd, W.; Duncana, R. Dendrimers: Relationship between Structure and Biocompatibility in Vitro, and Preliminary Studies on the Biodistribution of I-Labelled Polyamidoamine Dendrimers in Vivo. J. Control. Release 2000, 65, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Davoren, M.; Byrne, H.J. In Vitro Mammalian Cytotoxicological Study of PAMAM Dendrimers-Towards Quantitative Structure Activity Relationships. Toxicol. Vitro 2010, 24, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Ciolkowski, M.; Wróbel, D.; Petersen, J.F.; Ficker, M.; Christensen, J.B.; Bryszewska, M.; Klajnert, B. Modified PAMAM Dendrimer with 4-Carbomethoxypyrrolidone Surface Groups Reveals Negligible Toxicity against Three Rodent Cell-Lines. Nanomedicine 2013, 9, 461–464. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.C.; Bhalgat, M.K.; Zera, R.T. Preliminary Biological Evaluation of Polyamidoamine (PAMAM) StarburstTM Dendrimers. J. Biomed. Mater. Res. 1996, 30, 53–65. [Google Scholar] [CrossRef]
- Mishra, V.; Gupta, U.; Jain, N.K. Influence of Different Generations of Poly(Propylene Imine) Dendrimers on Human Erythrocytes. Pharmazie 2010, 65, 891–895. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Petersen, J.F.; Ficker, M.; Janaszewska, A.; Christensen, J.B.; Klajnert, B.; Bryszewska, M. Surface Modification of PAMAM Dendrimer Improves Its Biocompatibility. Nanomedicine 2012, 8, 815–817. [Google Scholar] [CrossRef]
- Ziemba, B.; Halets, I.; Shcharbin, D.; Appelhans, D.; Voit, B.; Pieszynski, I.; Bryszewska, M.; Klajnert, B. Influence of Fourth Generation Poly(Propyleneimine) Dendrimers on Blood Cells. J. Biomed. Mater. Res.-Part A 2012, 100, 2870–2880. [Google Scholar] [CrossRef]
- Albertazzi, L.; Serresi, M.; Albanese, A.; Beltram, F. Dendrimer Internalization and Intracellular Trafficking in Living Cells. Mol. Pharm. 2010, 7, 680–688. [Google Scholar] [CrossRef]
- Padilla De Jesús, O.L.; Ihre, H.R.; Gagne, L.; Fréchet, J.M.J.; Szoka, F.C. Polyester Dendritic Systems for Drug Delivery Applications: In Vitro and in Vivo Evaluation. Bioconjugate Chem. 2002, 13, 453–461. [Google Scholar] [CrossRef]
- Agache, P.; Lihoreau, T.; Mac-Mary, S.; Fanian, F.; Humbert, P. The Human Skin: An Overview. In Agache’s Measuring the Skin Non-Invasive Investigations, Physiology, Normal Constants; Humbert, P., Fanian, F., Maibach, H.I., Agache, P., Eds.; Springer Nature: Cham, Switzerland, 2016; Volume 1, pp. 1–4. [Google Scholar]
- Structures et Rôles de La Peau. Available online: https://sante.lefigaro.fr/mieux-etre/beaute/structures-roles-peau (accessed on 17 May 2023).
- Mélissopoulos, A.; Levacher, C.; Robert, L.; Ballotti, R. La Peau Structure et Physiologie, 2nd ed.; Mélissopoulos, A., Levacher, C., Eds.; Lavoisier: Paris, France, 2012; Volume 1, ISBN 9782743013691. [Google Scholar]
- Elias, P.M. Epidermal Lipids, Barrier Function, and Desquamation. J. Investig. Dermatol. 1983, 80, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Structure et Fonction de La Peau. Available online: https://www.eucerin.fr/a-propos-de-la-peau/comprendre-la-peau/structure-et-fonction-de-la-peau (accessed on 17 May 2023).
- Ng, K.W.; Lau, W.M. Skin Deep: The Basics of Human Skin Structure and Drug Penetration. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Drug Manipulation Strategies and Vehicle Effects; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. ISBN 9783662450130. [Google Scholar]
- Wertz, P.W.; van den Bergh, B. The Physical, Chemical and Functional Properties of Lipids in the Skin and Other Biological Barriers. Chem. Phys. Lipids 1998, 2, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. Lipids and the Epidermal Permeability Barrier. Arch. Dermatol. Res. 1981, 270, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Christophers, E.; Kligman, A.M. Visualization of the Cell Layers of the Stratum Corneum. J. Investig. Dermatol. 1964, 42, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Nemes, Z.; Steinert, P.M. Bricks and Mortar of the Epidermal Barrier. Exp. Mol. Med. 1999, 31, 5–19. [Google Scholar] [CrossRef]
- Madison, K.C. Barrier Function of the Skin: “‘La Raison d’Etre’” of the Epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W.; Abraham, W.; Landmann, L.; Downing, D.T. Preparation of Liposomes from Stratum Corneum Lipids. Soc. Investig. Dermatol. 1986, 87, 582–584. [Google Scholar] [CrossRef]
- Law, S.; Wertz, P.W.; Swartzendruber, D.C.; Squier, C.A. Regional Variation in Content, Composition and Organization of Porcine Epithelial Barrier Lipids Revealed by Thin-Layer Chromatography and Transmission Electron Microscopy. Arch. Oral. Biol. 1995, 40, 1085–1091. [Google Scholar] [CrossRef]
- Menon, G.K.; Cleary, G.W.; Lane, M.E. The Structure and Function of the Stratum Corneum. Int. J. Pharm. 2012, 435, 3–9. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Dubbelaar, F.E.R.; Gooris, G.S.; Ponec, M. The Lipid Organisation in the Skin Barrier. Acta Derm. Venereol. 2000, 208, 23–30. [Google Scholar] [CrossRef]
- Cannon, J.B. Lipids in Transdermal and Topical Drug Delivery. Am. Pharm. Rev. 2014, 17, 1–11. [Google Scholar]
- Banker, G.S.; Rhodes, C.T. Modern Pharmaceutics, 4th ed.; Banker, G.S., Rhodes, C.T., Eds.; Marcel Dekker: New York, NY, USA, 2002; Volume 1, ISBN 0824706749. [Google Scholar]
- Géa, A.; Banel, P. Physiologie et Huiles Essentielles, 1st ed.; Dunod: Malakoff, France, 2022; Volume 1. [Google Scholar]
- Elias, P.-M.; Friend, D.S. The Permeability Barrier in Mammalian Epidermis. J. Cell Biol. 1975, 65, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Penetration Enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, M.; Fang, L. Biomaterials as Novel Penetration Enhancers for Transdermal and Dermal Drug Delivery Systems. Drug Deliv. 2013, 20, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.; Krishna Venuganti, V.V. Dendritic Polymers for Dermal Drug Delivery. Ther. Deliv. 2017, 8, 1077–1096. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current Status and Future Potential of Transdermal Drug Delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Eeman, M.; Deleu, M. From Biological Membranes to Biomimetic Model Membranes. Biotechnol. Agron. Soc. Environ. 2010, 14, 691–708. Available online: https://hdl.handle.net/2268/81993 (accessed on 17 October 2022).
- Clifton, L.A.; Campbell, R.A.; Sebastiani, F.; Campos-Terán, J.; Gonzalez-Martinez, J.F.; Björklund, S.; Sotres, J.; Cárdenas, M. Design and Use of Model Membranes to Study Biomolecular Interactions Using Complementary Surface-Sensitive Techniques. Adv. Colloid Interface Sci. 2020, 277, 102118–102139. [Google Scholar] [CrossRef]
- Deleu, M.; Crowet, J.M.; Nasir, M.N.; Lins, L. Complementary Biophysical Tools to Investigate Lipid Specificity in the Interaction between Bioactive Molecules and the Plasma Membrane: A Review. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 3171–3190. [Google Scholar] [CrossRef]
- Brockman, H. Lipid Monolayers: Why Use Half a Membrane to Characterize Protein-Membrane Interactions? Curr. Opin. Struct. Biol. 1990, 9, 438–443. [Google Scholar] [CrossRef]
- Basit, H.; Lopez, S.G.; Keyes, T.E. Fluorescence Correlation and Lifetime Correlation Spectroscopy Applied to the Study of Supported Lipid Bilayer Models of the Cell Membrane. Methods 2014, 68, 286–299. [Google Scholar] [CrossRef]
- Hoebeke, M. The Importance of Liposomes as Models and Tools in the Understanding of Photosensitization Mechanisms. J. Photochem. Photobio. B Biol. 1995, 28, 189–196. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and Polymersomes: A Comparative Review towards Cell Mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Dwadasi, B.S.; Rai, B. Molecular Dynamics Simulation of Skin Lipids: Effect of Ceramide Chain Lengths on Bilayer Properties. J. Phys. Chem. B 2016, 120, 12536–12546. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.M.; Yardley, H.J. Lipid Compositions of Cells Isolated from Pig, Human, and Rat Epidermis. J. Lipid Res. 1975, 16, 434–440. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, M.; Song, L. A Review of Fatty Acids Influencing Skin Condition. J. Cosmet. Dermatol. 2020, 19, 3199–3204. [Google Scholar] [CrossRef]
- Eeman, M.; Francius, G.; Dufrêne, Y.F.; Nott, K.; Paquot, M.; Deleu, M. Effect of Cholesterol and Fatty Acids on the Molecular Interactions of Fengycin with Stratum Corneum Mimicking Lipid Monolayers. Langmuir 2009, 25, 3029–3039. [Google Scholar] [CrossRef] [PubMed]
- Eeman, M.; Deleu, M.; Paquot, M.; Thonart, P.; Dufrêne, Y.F. Nanoscale Properties of Mixed Fengycin/Ceramide Monolayers Explored Using Atomic Force Microscopy. Langmuir 2005, 21, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Neubert, R.H.H.; Opálka, L.; Kerth, A.; Brezesinski, G. Non-Ionic Surfactants as Innovative Skin Penetration Enhancers: Insight in the Mechanism of Interaction with Simple 2D Stratum Corneum Model System. Eur. J. Pharm. Sci. 2021, 157, 105620. [Google Scholar] [CrossRef]
- Blume, A.; Jansen, M.; Ghyczy, M.; Gareiss, J. Interaction of Phospholipid Liposomes with Lipid Model Mixtures for Stratum Corneum Lipids. Int. J. Pharm. 1993, 99, 219–228. [Google Scholar] [CrossRef]
- Mueller, J.; Trapp, M.; Neubert, R.H.H. The Effect of Hydrophilic Penetration/Diffusion Enhancer on Stratum Corneum Lipid Models: Part II*: DMSO. Chem. Phys. Lipids 2019, 225, 104816–104823. [Google Scholar] [CrossRef]
- Školová, B.; Jandovská, K.; Pullmannová, P.; Tesař, O.; Roh, J.; Hrabálek, A.; Vávrová, K. The Role of the Trans Double Bond in Skin Barrier Sphingolipids: Permeability and Infrared Spectroscopic Study of Model Ceramide and Dihydroceramide Membranes. Langmuir 2014, 30, 5527–5535. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W. Mode of Action of Penetration Enhancers in Human Skin. J. Control. Release 1987, 6, 85–97. [Google Scholar] [CrossRef]
- Kim, C.-K.; Hong, M.-S.; Kim, Y.-B.; Han, S.-K. Effect of Penetration Enhancers (Pyrrolidone Derivatives) on Multilamellar Liposomes of Stratum Corneum Lipid: A Study by UV Spectroscopy and Differential Scanning Calorimetry. Int. J. Pharm. 1993, 95, 43–50. [Google Scholar] [CrossRef]
- Hatziantoniou, S.; Nezis, I.P.; Margaritis, L.H.; Demetzos, C. Visualisation of Liposomes Prepared from Skin and Stratum Corneum Lipids by Transmission Electron Microscopy. Micron 2007, 38, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Downing, D.T.; Abraham, W.; Wegner, B.K.; Willman, K.W.; Marshall, J.L. Partition of Sodium Dodecyl Sulfate into Stratum Corneum Lipid Liposomes. Arch. Dermatol. Res. 1993, 285, 151–157. [Google Scholar] [CrossRef]
- Zbytovská, J.; Kiselev, M.A.; Funari, S.S.; Garamus, V.M.; Wartewig, S.; Palát, K.; Neubert, R. Influence of Cholesterol on the Structure of Stratum Corneum Lipid Model Membrane. Colloids Surf. A Physicochem. Eng. Asp. 2008, 328, 90–99. [Google Scholar] [CrossRef]
- Kiselev, M.A. Conformation of Ceramide 6 Molecules and Chain-Flip Transitions in the Lipid Matrix of the Outermost Layer of Mammalian Skin, the Stratum Corneum. Crystallogr. Rep. 2007, 52, 525–528. [Google Scholar] [CrossRef]
- Bandaru, R.; Sanket, A.S.; Rekha, S.; Kamble, O.; Dewangan, R.P.; Kesharwani, P.; Samal, S.K.; Dandela, R. Biological Interaction of Dendrimers. In Dendrimer-Based Nanotherapeutics; Elsevier: Amsterdam, The Netherlands, 2021; Volume 5, pp. 63–74. ISBN 9780128212509. [Google Scholar]
- Mutalik, S.; Nayak, U.Y.; Kalra, R.; Kumar, A.; Kulkarni, R.V.; Parekh, H.S. Sonophoresis-Mediated Permeation and Retention of Peptide Dendrimers across Human Epidermis. Skin Res. Technol. 2012, 18, 101–107. [Google Scholar] [CrossRef]
- Mutalik, S.; Shetty, P.K.; Kumar, A.; Kalra, R.; Parekh, H.S. Enhancement in Deposition and Permeation of 5-Fluorouracil through Human Epidermis Assisted by Peptide Dendrimers. Drug Deliv. 2014, 21, 44–54. [Google Scholar] [CrossRef]
- Mutalik, S.; Parekh, H.S.; Anissimov, Y.G.; Grice, J.E.; Roberts, M.S. Iontophoresis-Mediated Transdermal Permeation of Peptide Dendrimers across Human Epidermis. Skin Pharmacol. Physiol. 2013, 26, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Borowska, K.; Wołowiec, S.; Rubaj, A.; Głowniak, K.; Sieniawska, E.; Radej, S. Effect of Polyamidoamine Dendrimer G3 and G4 on Skin Permeation of 8-Methoxypsoralene—In Vivo Study. Int. J. Pharm. 2012, 426, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Manikkath, J.; Hegde, A.R.; Kalthur, G.; Parekh, H.S.; Mutalik, S. Influence of Peptide Dendrimers and Sonophoresis on the Transdermal Delivery of Ketoprofen. Int. J. Pharm. 2017, 521, 110–119. [Google Scholar] [CrossRef]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM Dendrimer-Cell Membrane Interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Venuganti, V.V.K.; Perumal, O.P. Poly(Amidoamine) Dendrimers as Skin Penetration Enhancers: Influence of Charge, Generation, and Concentration. J. Pharm. Sci. 2009, 98, 2345–2356. [Google Scholar] [CrossRef]
- Venuganti, V.V.; Sahdev, P.; Hildreth, M.; Guan, X.; Perumal, O. Structure-Skin Permeability Relationship of Dendrimers. Pharm. Res. 2011, 28, 2246–2260. [Google Scholar] [CrossRef]
- Yang, Y.; Sunoqrot, S.; Stowell, C.; Ji, J.; Lee, C.W.; Kim, J.W.; Khan, S.A.; Hong, S. Effect of Size, Surface Charge, and Hydrophobicity of Poly(Amidoamine) Dendrimers on Their Skin Penetration. Biomacromolecules 2012, 13, 2154–2162. [Google Scholar] [CrossRef]
- Venuganti, V.V.K.; Perumal, O.P. Effect of Poly(Amidoamine) (PAMAM) Dendrimer on Skin Permeation of 5-Fluorouracil. Int. J. Pharm. 2008, 361, 230–238. [Google Scholar] [CrossRef]
- Yiyun, C.; Na, M.; Tongwen, X.; Rongqiang, F.; Xueyuan, W.; Xiaomin, W.; Longping, W. Transdermal Delivery of Nonsteroidal Anti-Inflammatory Drugs Mediated by Polyamidoamine (PAMAM) Dendrimers. J. Pharm. Sci. 2007, 96, 595–602. [Google Scholar] [CrossRef]
- Volz, P.; Schilrreff, P.; Brodwolf, R.; Wolff, C.; Stellmacher, J.; Balke, J.; Morilla, M.J.; Zoschke, C.; Schäfer-Korting, M.; Alexiev, U. Pitfalls in Using Fluorescence Tagging of Nanomaterials: Tecto-Dendrimers in Skin Tissue as Investigated by Cluster-FLIM. Ann. N. Y. Acad. Sci. 2017, 1405, 202–214. [Google Scholar] [CrossRef]
- Hegde, A.R.; Rewatkar, P.V.; Manikkath, J.; Tupally, K.; Parekh, H.S.; Mutalik, S. Peptide Dendrimer-Conjugates of Ketoprofen: Synthesis and Ex Vivo and in Vivo Evaluations of Passive Diffusion, Sonophoresis and Iontophoresis for Skin Delivery. Eur. J. Pharm. Sci. 2017, 102, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.K.; Manikkath, J.; Tupally, K.; Kokil, G.; Hegde, A.R.; Raut, S.Y.; Parekh, H.S.; Mutalik, S. Skin Delivery of EGCG and Silibinin: Potential of Peptide Dendrimers for Enhanced Skin Permeation and Deposition. AAPS Pharm. Sci. Technol. 2017, 18, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).