Dendrimers: Synthesis, Encapsulation Applications and Specific Interaction with the Stratum Corneum—A Review
Abstract
1. Introduction
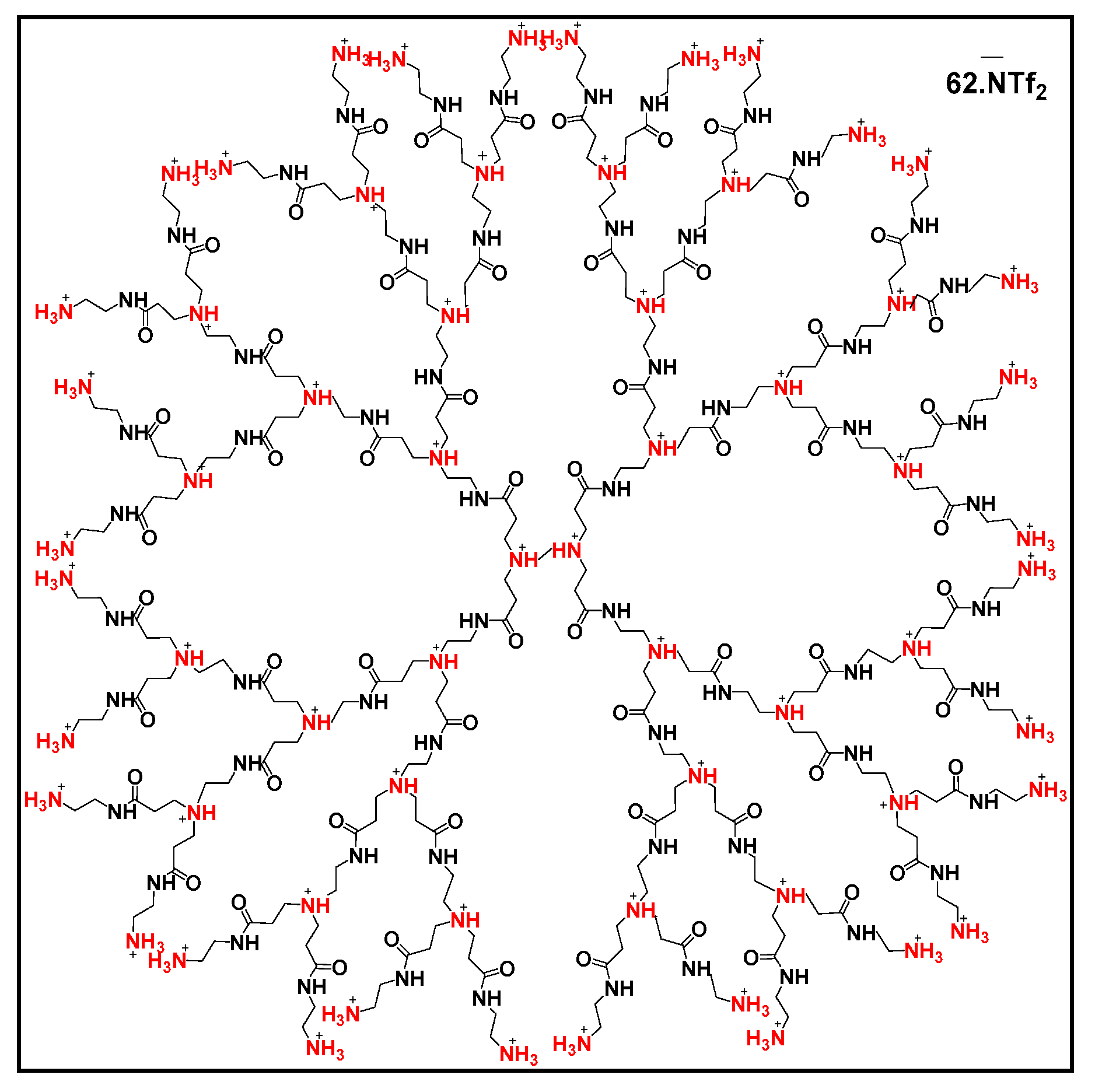
2. Dendrimers: Definition and Synthesis
3. Applications of Dendrimers
3.1. Medical Imaging
3.2. Pharmaceutical
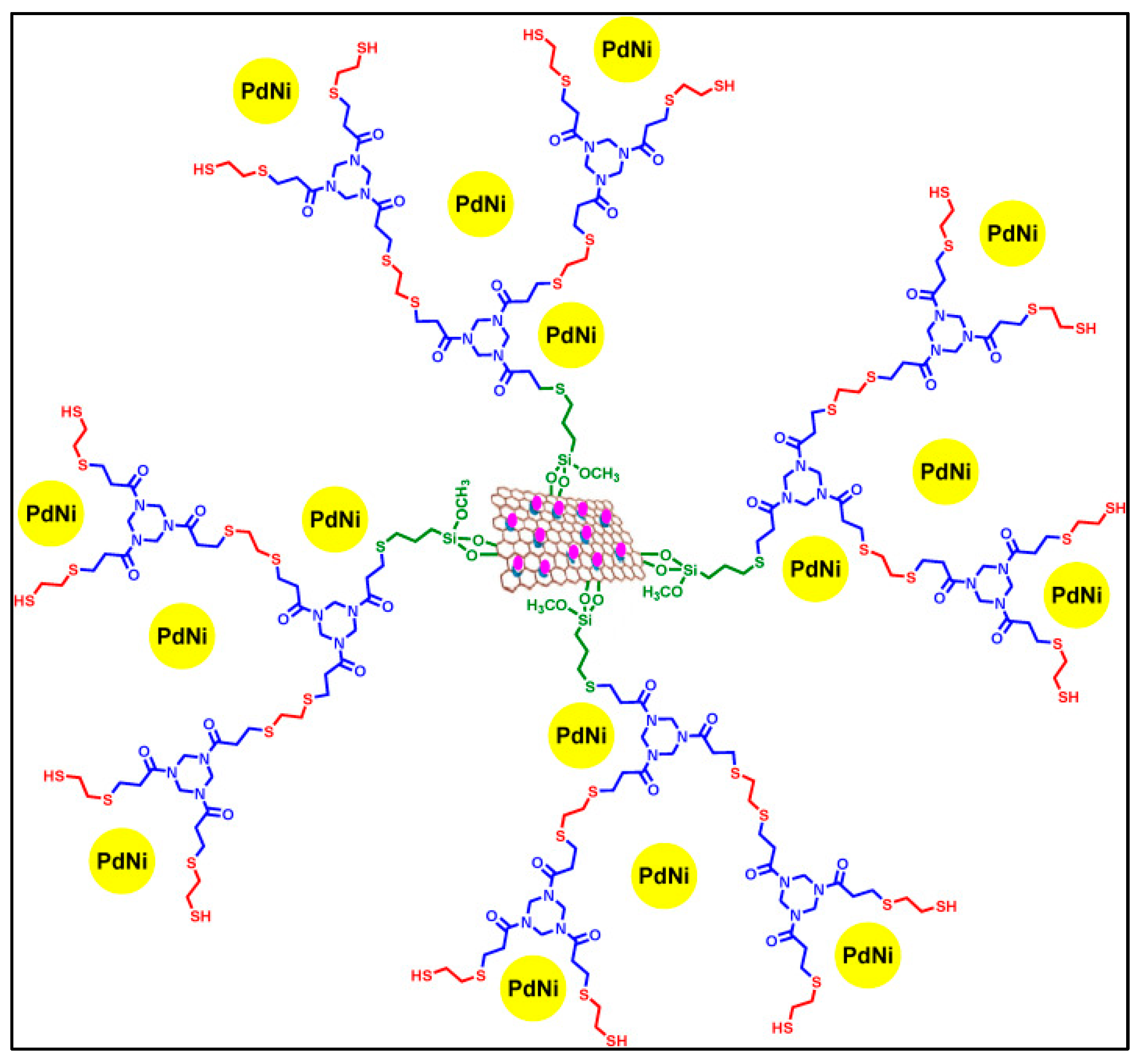
3.3. Catalysis
3.4. Environment
3.5. Food
3.6. Cosmetics
4. Toxicity of Dendrimers
5. The Interaction of Dendrimers with the SC
5.1. The Skin
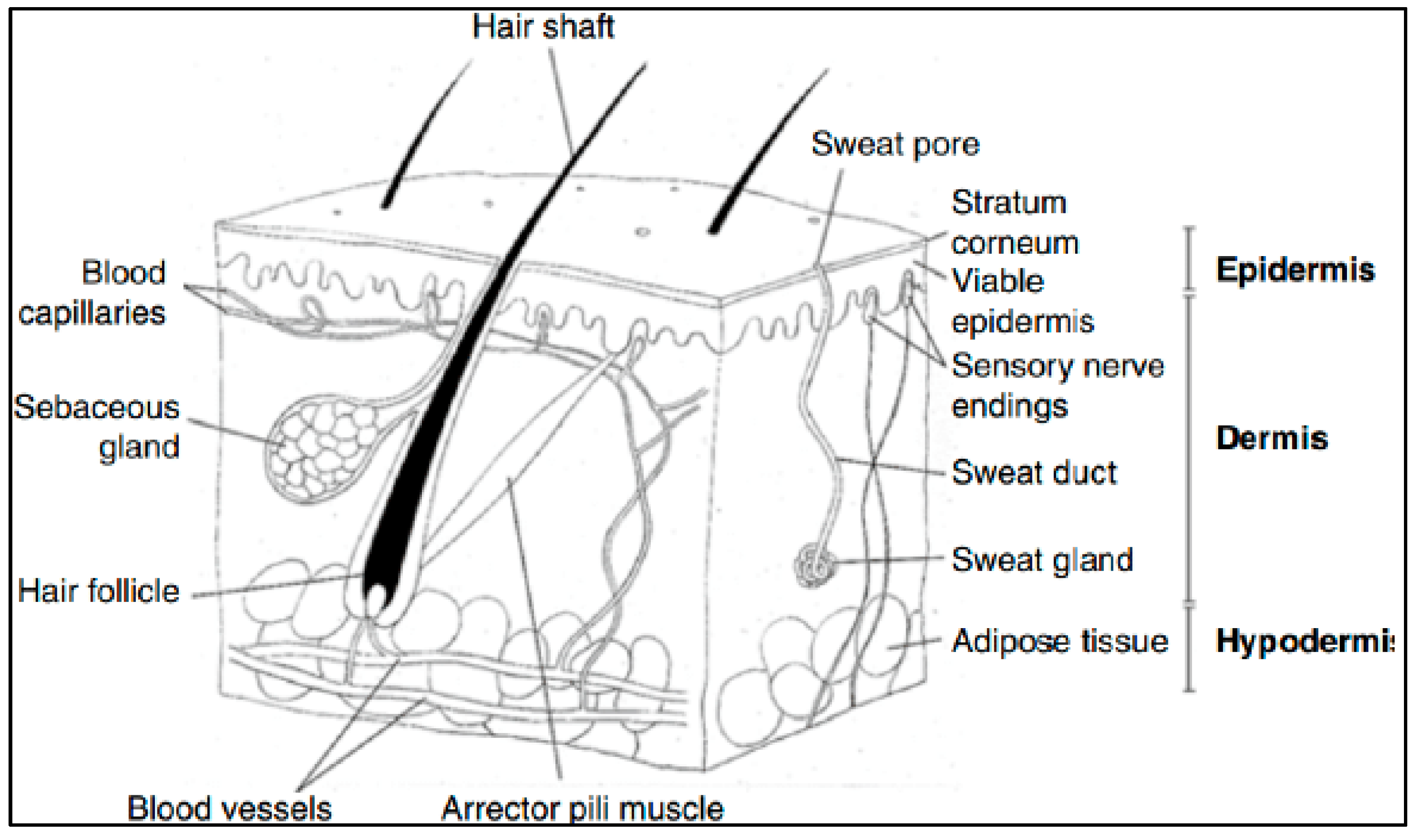
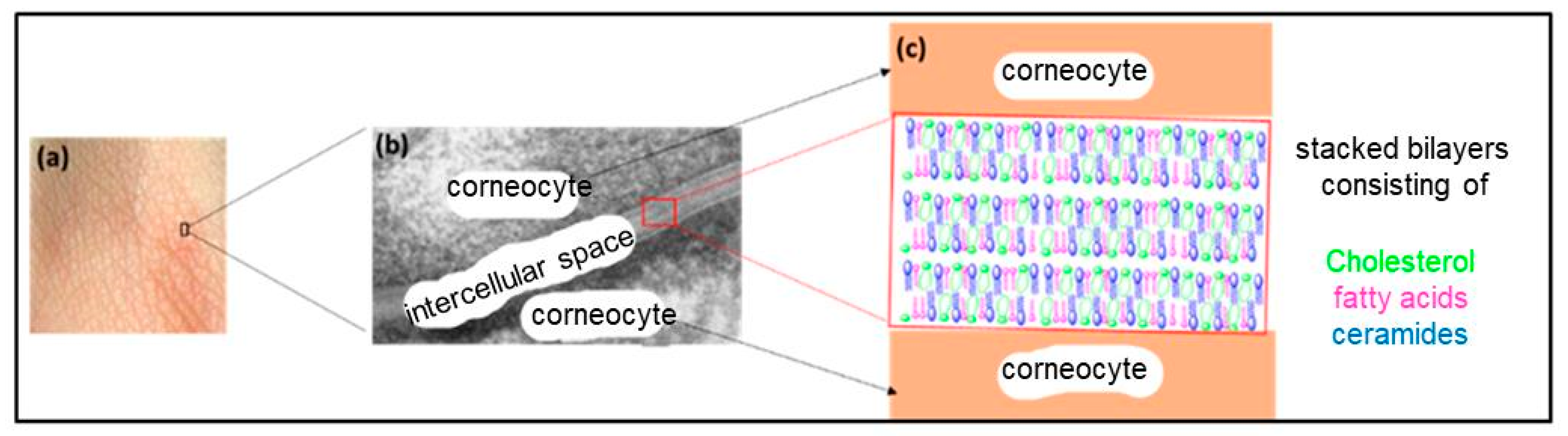
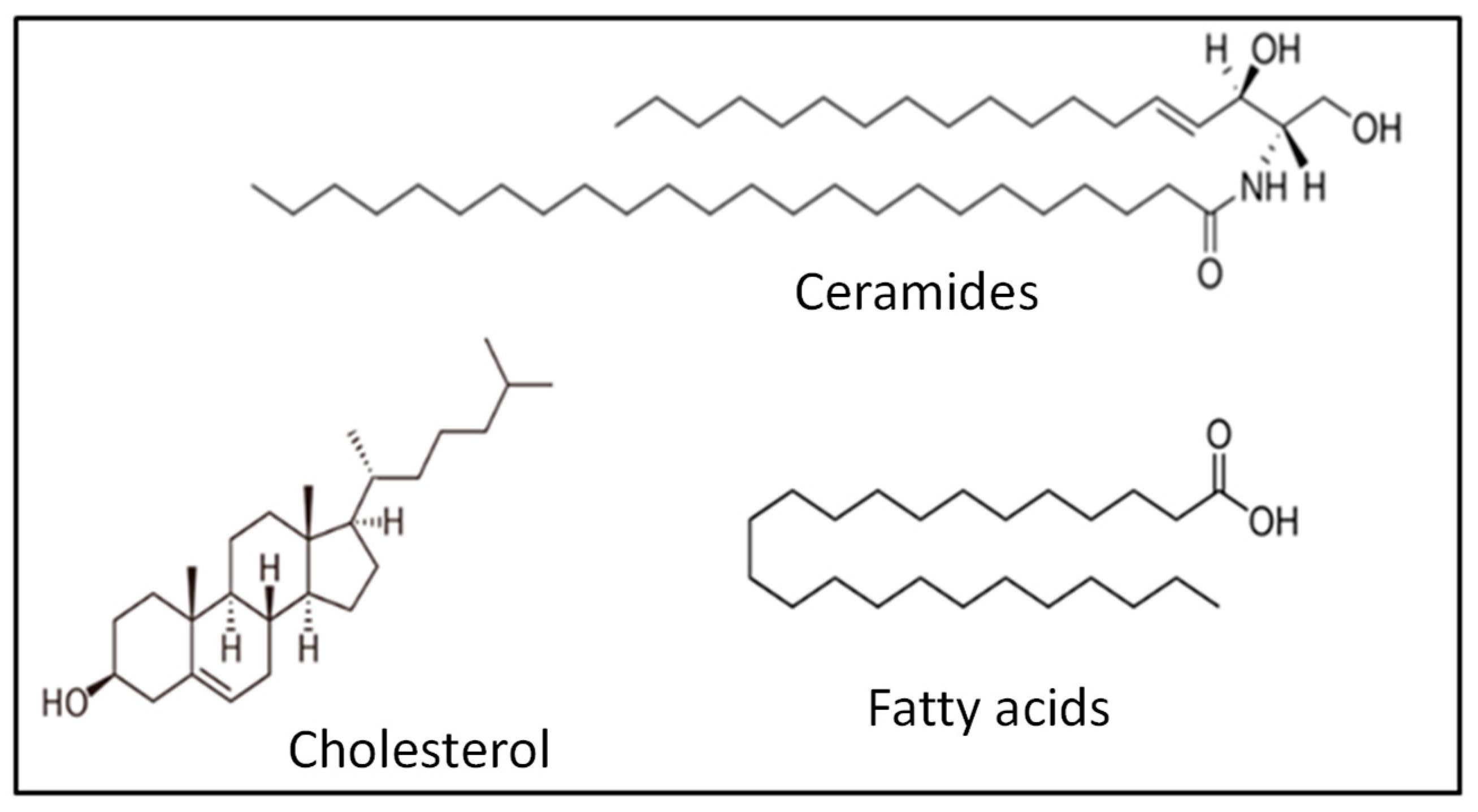
5.2. The Stratum Corneum
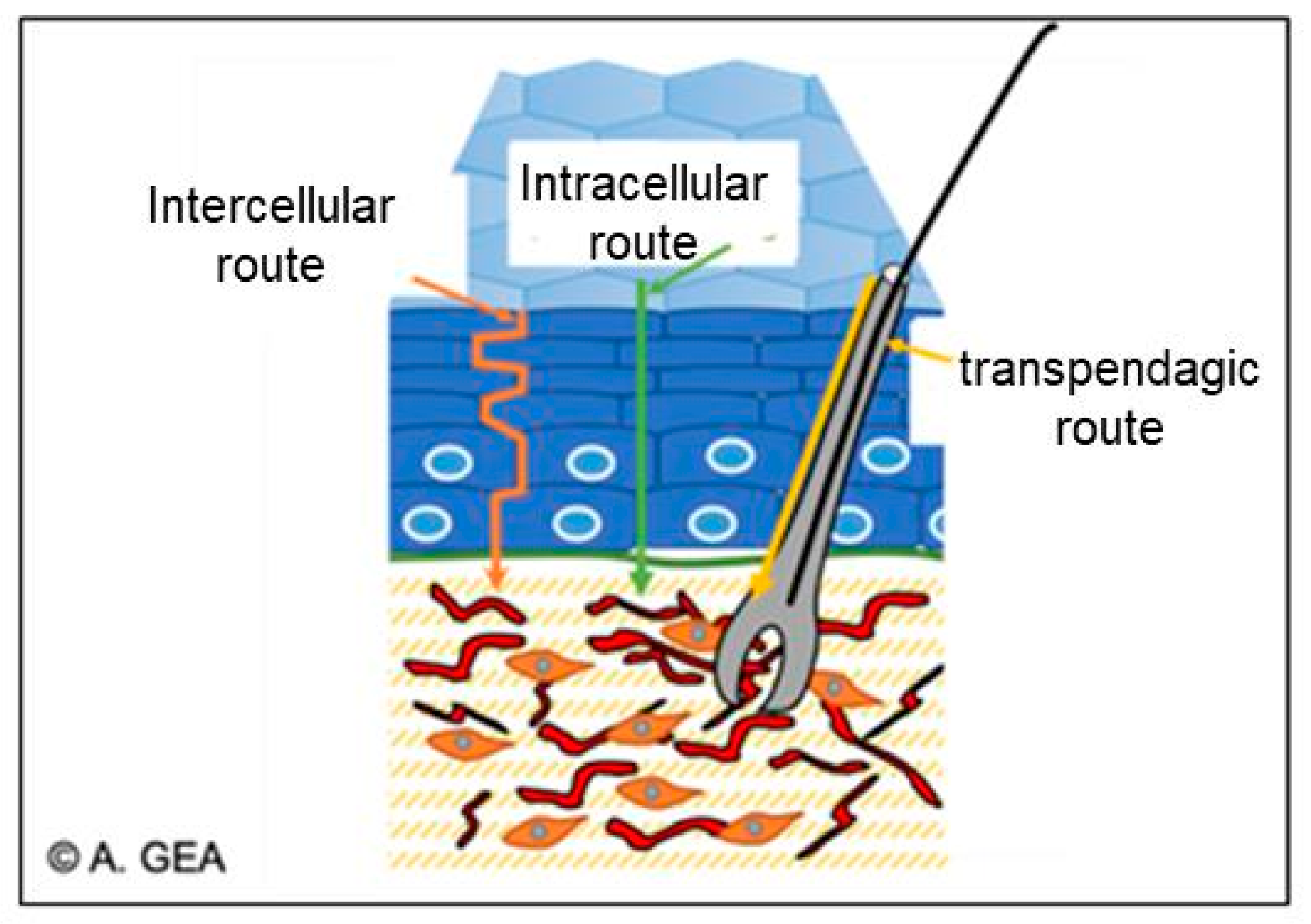
5.3. The Diffusion of Active Ingredients through the Skin
- -
- The intercellular route, which passes through the lipid matrix;
- -
- The intracellular pathway, which passes through the corneocytes;
- -
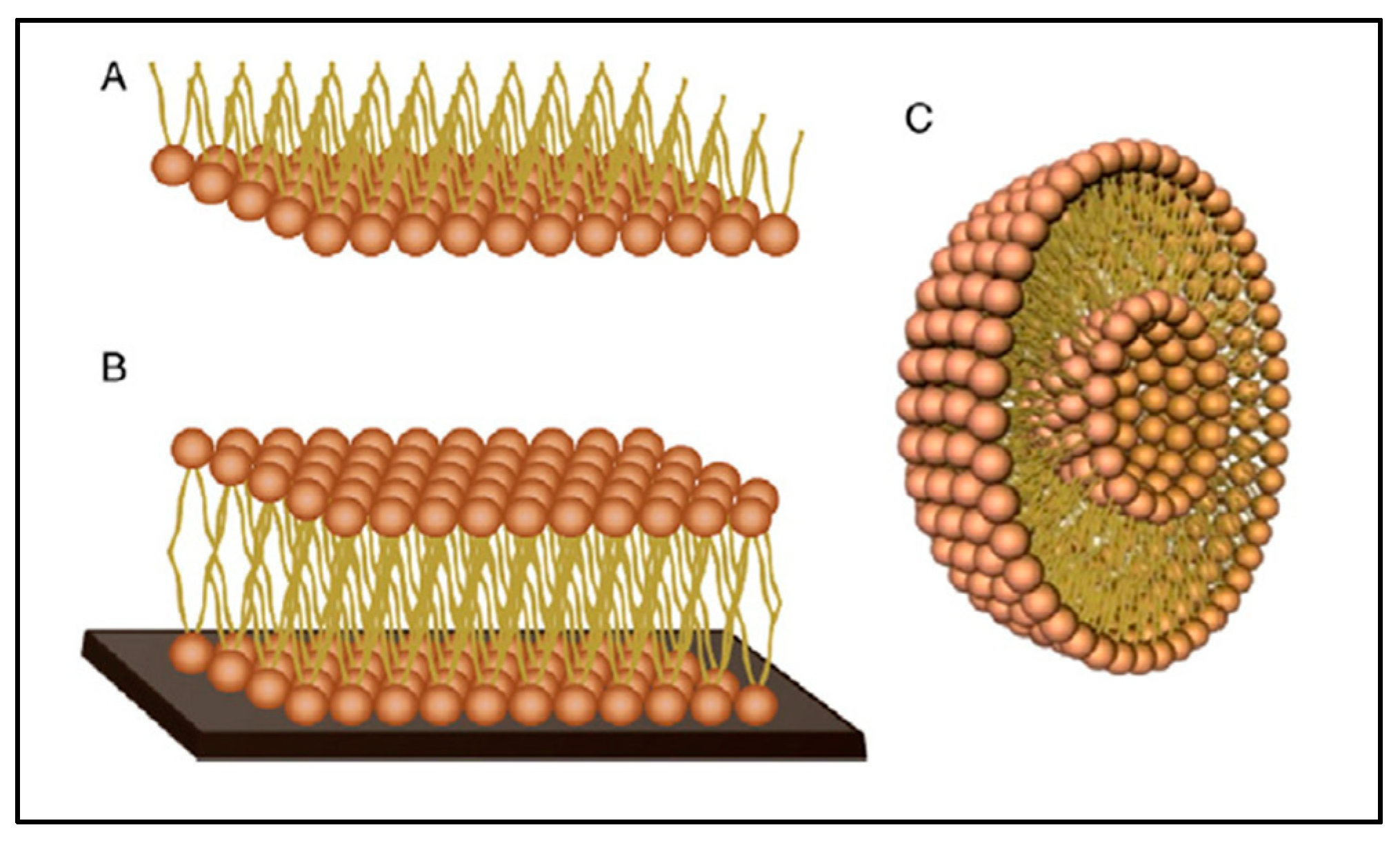
5.4. Biomimetic Skin Membranes
5.5. Dendrimer–Stratum Corneum Interaction
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Donald, A.T.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Dendritic Macromolecules: Synthesis of Starburst Dendrimers. Macromolecules 1986, 19, 2466–2468. [Google Scholar] [CrossRef]
- Tomalia, D.A. The Dendritic State. Mater. Today 2005, 8, 34–46. [Google Scholar] [CrossRef]
- Newkome, G.R.; Yao, Z.-Q.; Baker, G.R.; Gupta, V.K. Cascade Molecules: A New Approach to Micelles. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Klajnert, B.; Bryszewska, M. Dendrimers: Properties and Applications. Acta Biochem. 2001, 48, 199–208. [Google Scholar] [CrossRef]
- Irfan, M.; Seiler, M. Encapsulation Using Hyperbranched Polymers: From Research and Technologies to Emerging Applications. Ind. Eng. Chem. Res. 2010, 49, 1169–1196. [Google Scholar] [CrossRef]
- Márquez-Miranda, V.; Araya-Durán, I.; Camarada, M.B.; Comer, J.; Valencia-Gallegos, J.A.; González-Nilo, F.D. Self-Assembly of Amphiphilic Dendrimers: The Role of Generation and Alkyl Chain Length in SiRNA Interaction. Sci. Rep. 2016, 6, 29436. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, Applications, and Properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Najafi, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Janus-Type Dendrimers: Synthesis, Properties, and Applications. J. Mol. Liq. 2022, 347, 118396. [Google Scholar] [CrossRef]
- Kokaz, S.F.; Deb, P.K.; Borah, P.; Bania, R.; Venugopala, K.N.; Nair, A.B.; Singh, V.; Al-Shar’i, N.A.; Hourani, W.; Gupta, G.; et al. Dendrimers: Properties and Applications in Biomedical Field. In Nanoengineering of Biomaterials; Wiley-VCH GmbH: New York, NY, USA, 2022; pp. 215–243. ISBN 9783527832095. [Google Scholar]
- Srinivasa-Gopalan, S.; Kevin, J.Y.; Kumar, C.S.S.R. Dendrimers in Cancer Treatment and Diagnosis. In Nanotechnologies for the Life Sciences; Wiley-VCH: New York, NY, USA, 2007; p. 423. ISBN 9783527313877. [Google Scholar]
- Mbakidi, J.P.; Barjhoux, I.; Aguibi, K.; Geffard, A.; Rioult, D.; Palos Ladeiro, M.; Bouquillon, S. Synthesis of New Betaine-Based Ionic Liquids by Using a “One-Pot” Amidation Process and Evaluation of Their Ecotoxicity through a New Method Involving a Hemocyte-Based Bioassay. ACS Sustain. Chem. Eng. 2021, 9, 15427–15441. [Google Scholar] [CrossRef]
- Maes, C.; Menot, B.; Hayouni, S.; Martinez, A.; Fauconnier, M.L.; Bouquillon, S. Preparation of New Glycerol-Based Dendrimers and Studies on Their Behavior toward Essential Oil Encapsulation. ACS Omega 2022, 7, 10277–10291. [Google Scholar] [CrossRef]
- Bacha, K.; Estager, J.; Brassart-Pasco, S.; Chemotti, C.; Fernandes, A.E.; Mbakidi, J.P.; Deleu, M.; Bouquillon, S. Synthesis and Activity of Ionic Antioxidant-Functionalized PAMAMs and PPIs Dendrimers. Polymers 2022, 14, 3513. [Google Scholar] [CrossRef] [PubMed]
- Bacha, K.; Chemotti, C.; Monboisse, J.C.; Robert, A.; Furlan, A.L.; Smeralda, W.; Damblon, C.; Estager, J.; Brassart-Pasco, S.; Mbakidi, J.P.; et al. Encapsulation of Vitamin C by Glycerol-Derived Dendrimers, Their Interaction with Biomimetic Models of Stratum Corneum and Their Cytotoxicity. Molecules 2022, 27, 8022. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Brostaux, Y.; Bouquillon, S.; Fauconnier, M.L. Use of New Glycerol-Based Dendrimers for Essential Oils Encapsulation: Optimization of Stirring Time and Rate Using a Plackett-Burman Design and a Surface. Foods 2021, 10, 207. [Google Scholar] [CrossRef]
- Menot, B.; Stopinski, J.; Martinez, A.; Oudart, J.B.; Maquart, F.X.; Bouquillon, S. Synthesis of Surface-Modified PAMAMs and PPIs for Encapsulation Purposes: Influence of the Decoration on Their Sizes and Toxicity. Tetrahedron 2015, 71, 3439–3446. [Google Scholar] [CrossRef]
- Les Dendrimeres: Des Nanomolecules Pour Le Transport de Medicaments. Available online: https://www.cinam.univ-mrs.fr/cinam/evenements/science-pour-tous/les-dendrimeres-des-nano-molecules-pour-le-transport-de-medicaments/ (accessed on 17 May 2023).
- Buhleier, E.; Wehner, W.; Vögtle, F. Cascade-and Nonskid-Chain-like Syntheses of Molecular Cavity Topologies. Synthesis 1978, 02, 155–158. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Shao, N.; Su, Y.; Hu, J.; Zhang, J.; Zhang, H.; Cheng, Y. Comparison of Generation 3 Polyamidoamine Dendrimer and Generation 4 Polypropylenimine Dendrimer on Drug Loading, Complex Structure, Release Behavior, and Cytotoxicity. Int. J. Nanomed. 2011, 6, 3361–3372. [Google Scholar] [CrossRef]
- Hawker, C.J.; Fréchet, J.M.J. Preparation of Polymers with Controlled Molecular Architecture. A New Convergent Approach to Dendritic Macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Hodge, P. Polymer Science Branches Out. Am. Nat. 1993, 362, 18–19. [Google Scholar] [CrossRef]
- Brabander-van den Berg, E.M.M.; Meijer, E.W. Poly(Propy1ene Imine) Dendrimers: Large-Scale Synthesis by Hetereogeneously Catalyzed Hydrogenation Angew. Angew. Chem. Int. 1993, 32, 1308–1311. [Google Scholar] [CrossRef]
- Worner, C.; Miilhaupt, R. Polynitrile-and Polyamine-Functional Poly(Trimethy1ene Imine) Dendrimers. Angew. Chem. Int. 1993, 32, 1306–1308. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms Macroscopic Matter. Angew. Chem. Int. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Zhuo, R.X.; Du, B.; Lu, Z.R. In Vitro Release of 5-Fluorouracil with Cyclic Core Dendritic Polymer. J. Control. Release 1999, 57, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, J.H.; Kim, B.K.; Kim, J.H.; Shin, W.S.; Jin, S.H. Convergent Synthesis of PAMAM Dendrimers Using Click Chemistry of Azide-Functionalized PAMAM Dendrons. Tetrahedron 2006, 62, 9193–9200. [Google Scholar] [CrossRef]
- Boas, U.; Christensen, J.B.; Heegaard, P.M.H. Dendrimers: Design, Synthesis and Chemical Properties. J. Mater. Chem. 2006, 16, 3785–3798. [Google Scholar] [CrossRef]
- Gupta, U.; Gupt, U.; Agashe, H.B.; Jain, N.K. Polypropylene Imine Dendrimer Mediated Solubility Enhancement: Effect of PH and Functional Groups of Hydrophobes. J. Pharm. Pharm. Sci. 2007, 10, 358–367. [Google Scholar]
- Gupta, U.; Dwivedi, S.K.D.; Bid, H.K.; Konwar, R.; Jain, N. Ligand Anchored Dendrimers Based Nanoconstructs for Effective Targeting to Cancer Cells. Int. J. Pharm. 2010, 393, 185–196. [Google Scholar] [CrossRef]
- Kaur, D.; Jain, K.; Mehra, N.K.; Kesharwani, P.; Jain, N.K. A Review on Comparative Study of PPI and PAMAM Dendrimers. J. Nanoparticle Res. 2016, 18, 146. [Google Scholar] [CrossRef]
- Jevprasesphant, R.; Penny, J.; Jalal, R.; Attwood, D.; Mckeown, N.B.; D’emanuele, A. The Influence of Surface Modification on the Cytotoxicity of PAMAM Dendrimers. Int. J. Pharm. 2003, 252, 263–266. [Google Scholar] [CrossRef]
- Stasko, N.A.; Johnson, C.B.; Schoenfisch, M.H.; Johnson, T.A.; Holmuhamedov, E.L. Cytotoxicity of Polypropylenimine Dendrimer Conjugates on Cultured Endothelial Cells. Biomacromolecules 2007, 8, 3853–3859. [Google Scholar] [CrossRef]
- Tack, F.; Bakker, A.; Maes, S.; Dekeyser, N.; Bruining, M.; Elissen-Roman, C.; Janicot, M.; Brewster, M.; Janssen, H.M.; de Waal, B.F.M.; et al. Modified Poly(Propylene Imine) Dendrimers as Effective Transfection Agents for Catalytic DNA Enzymes (DNAzymes). J. Drug Target. 2006, 14, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Toi, Y.; Harada, A.; Kono, K. Preparation of Poly (Ethylene Glycol)-Attached Dendrimers Encapsulating Photosensitizers for Application to Photodynamic Therapy. Bioconjugate Chem. 2007, 18, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, V.; Kumar, P.V.; Kumar Tekade, R. Novel PEGylated Nanoparticulate Architectures for Sustained Delivery of H2 Receptor Antagonist. Eur. J. Med. Chem. 2009, 44, 1155–1166. [Google Scholar] [CrossRef]
- Choi, Y.; Thomas, T.; Kotlyar, A.; Islam, M.T.; Baker, J.R. Synthesis and Functional Evaluation of DNA-Assembled Polyamidoamine Dendrimer Clusters for Cancer Cell-Specific Targeting. Chem. Biol. 2005, 12, 35–43. [Google Scholar] [CrossRef]
- Balieu, S.; Cadiou, C.; Martinez, A.; Nuzillard, J.M.; Oudart, J.B.; Maquart, F.X.; Chuburu, F.; Bouquillon, S. Encapsulation of Contrast Imaging Agents by Polypropyleneimine-Based Dendrimers. J. Biomed. Mater. Res.-Part A 2013, 101, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Balieu, S.; El Zein, A.; De Sousa, R.; Jérôme, F.; Tatibouët, A.; Gatard, S.; Pouilloux, Y.; Barrault, J.; Rollin, P.; Bouquillona, S. One-Step Surface Decoration of Poly(Propyleneimines) (PPIs) with the Glyceryl Moiety: New Way for Recycling Homogeneous Dendrimer-Based Catalysts. Adv. Synth. Catal. 2010, 352, 1826–1833. [Google Scholar] [CrossRef]
- Menot, B.; Salmon, L.; Bouquillon, S. Platinum Nanoparticles Stabilized by Glycerodendrimers: Synthesis and Application to the Hydrogenation of α,β-Unsaturated Ketones under Mild Conditions. Eur. J. Inorg. Chem. 2015, 2015, 4518–4523. [Google Scholar] [CrossRef]
- Luan, L.; Tang, B.; Liu, Y.; Xu, W.; Liu, Y.; Wang, A.; Niu, Y. Direct Synthesis of Sulfur-Decorating PAMAM Dendrimer/Mesoporous Silica for Enhanced Hg(II) and Cd(II) Adsorption. Langmuir 2022, 38, 698–710. [Google Scholar] [CrossRef]
- Mekuria, S.L.; Song, C.; Ouyang, Z.; Shen, M.; Janaszewska, A.; Klajnert-Maculewicz, B.; Shi, X. Synthesis and Shaping of Core-Shell Tecto Dendrimers for Biomedical Applications. Bioconjugate Chem. 2021, 32, 225–233. [Google Scholar] [CrossRef]
- Uppuluri, S.; Swanson, D.R.; Piehler, L.T.; Li, J.; Hagnauer, G.L.; Tomalia, D.A. Core-Shell Tecto(Dendrimers): I. Synthesis and Characterization of Saturated Shell Models. Adv. Mater 2000, 12, 796–800. [Google Scholar] [CrossRef]
- Song, C.; Ouyang, Z.; Guo, H.; Qu, J.; Gao, Y.; Xia, J.; Shen, M.; Shi, X. Core-Shell Tecto Dendrimers Enable Enhanced Tumor MR Imaging through an Amplified EPR Effect. Biomacromolecules 2021, 22, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, D.-Y.; Shen, M.; Shi, X. Design and Biomedical Applications of Core-Shell Tecto Dendrimers. Basic Clin. Med. 2022, 42, 15–25. [Google Scholar] [CrossRef]
- Bouquillon, S.; Hayouni, S.; Menot, B. Dendrimer-Type Compounds, Methods for Producing Same and Uses Thereof. PCT. Patent WO 2020/021032A1, 30 January 2020. [Google Scholar]
- Bouquillon, S.; Hayouni, S.; Menot, B. Dendrimer-Type Compounds, Methods for Producing Same and Uses Thereof. PCT. Patent WO 2020/021034A1, 30 January 2020. [Google Scholar]
- De La Mata, F.J.; Gómez Ramírez, R.; Ortega Ló-Pez, P.; Mencía Berlinches, G.; Maroto Díaz, M.; Natalia, S.D.O. Carbosilane Dendrimers Comprising Polyphenol Groups, And Uses Thereof. PCT. Patent WO 2017/220831Al, 28 December 2017. [Google Scholar]
- Nnadiekwe, C.C.; Nada, A.; Abdulazeez, I.; Imam, M.R.; Janjua, M.R.S.A.; Al-Saadi, A.A. UV-Absorbing Benzamide-Based Dendrimer Precursors: Synthesis, Theoretical Calculation, and Spectroscopic Characterization. New J. Chem. 2022, 46, 75–85. [Google Scholar] [CrossRef]
- Zhang, D.; Atochina-Vasserman, E.N.; Lu, J.; Maurya, D.S.; Xiao, Q.; Liu, M.; Adamson, J.; Ona, N.; Reagan, E.K.; Ni, H.; et al. The Unexpected Importance of the Primary Structure of the Hydrophobic Part of One-Component Ionizable Amphiphilic Janus Dendrimers in Targeted MRNA Delivery Activity. J. Am. Chem. Soc. 2022, 144, 4746–4753. [Google Scholar] [CrossRef]
- Zhang, D.; Xiao, Q.; Rahimzadeh, M.; Liu, M.; Rodriguez-Emmenegger, C.; Miyazaki, Y.; Shinoda, W.; Percec, V. Self-Assembly of Glycerol-Amphiphilic Janus Dendrimers Amplifies and Indicates Principles for the Selection of Stereochemistry by Biological Membranes. J. Am. Chem. Soc. 2023, 145, 4311–4323. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Rivera-Martinez, N.; Raab, C.J.; Bermudez, J.G.; Good, M.C.; Klein, M.L.; Percec, V. Co-Assembly of Liposomes, Dendrimersomes, and Polymersomes with Amphiphilic Janus Dendrimers Conjugated to Mono- and Tris-Nitrilotriacetic Acid (NTA, TrisNTA) Enhances Protein Recruitment. Giant 2022, 9, 100089–100101. [Google Scholar] [CrossRef]
- Duan, Y.; Zhao, X.; Sun, M.; Hao, H. Research Advances in the Synthesis, Application, Assembly, and Calculation of Janus Materials. Ind. Eng. Chem. Res. 2021, 60, 1071–1095. [Google Scholar] [CrossRef]
- Parida, P.; Nayak, B.P.; Mishra, S.C. Amphiphilic Janus like Particles for Biomedical Application. PharmaTutor Mag. 2014, 2, 92–97. [Google Scholar]
- Căta, A.; Ienașcu, I.M.C.; Ştefănuț, M.N.; Roșu, D.; Pop, O.R. Properties and Bioapplications of Amphiphilic Janus Dendrimers: A Review. Pharmaceutics 2023, 15, 589. [Google Scholar] [CrossRef]
- Liu, J.; Feng, Y.; Ma, B.; He, Y.M.; Fan, Q.H. Design and Synthesis of Janus-Type Chiral Dendritic Diphosphanes and Their Applications in Asymmetric Hydrogenation. Eur. J. Org. Chem. 2012, 2012, 6737–6744. [Google Scholar] [CrossRef]
- Liu, J.; Feng, Y.; He, Y.; Yang, N.; Fan, Q.H. Janus Dendritic Phosphines: Synthesis and Application in Suzuki Coupling Reactions. New J. Chem. 2012, 36, 380–385. [Google Scholar] [CrossRef]
- Feng, Y.; He, Y.M.; Zhao, L.W.; Huang, Y.Y.; Fan, Q.H. A Liquid-Phase Approach to Functionalized Janus Dendrimers: Novel Soluble Supports for Organic Synthesis. Org. Lett. 2007, 9, 2261–2264. [Google Scholar] [CrossRef] [PubMed]
- Casanellas, I.; Lagunas, A.; Vida, Y.; Pérez-Inestrosa, E.; Andrades, J.A.; Becerra, J.; Samitier, J. The Janus Role of Adhesion in Chondrogenesis. Int. J. Mol. Sci. 2020, 21, 5269. [Google Scholar] [CrossRef] [PubMed]
- Laine, R.M.; Roll, M.; Asuncion, M.; Sulaiman, S.; Popova, V.; Bartz, D.; Krug, D.J.; Mutin, P.H. Perfect and Nearly Perfect Silsesquioxane (SQs) Nanoconstruction Sites and Janus SQs. J. Sol.-Gel. Sci. Technol. 2008, 46, 335–347. [Google Scholar] [CrossRef]
- Han, Y.D.; Kim, H.S.; Park, Y.M.; Chun, H.J.; Kim, J.H.; Yoon, H.C. Retroreflective Janus Microparticle as a Nonspectroscopic Optical Immunosensing Probe. ACS App. Mater. Interfaces 2016, 8, 10767–10774. [Google Scholar] [CrossRef]
- Liu, Y.; Mu, S.; Liu, X.; Ling, Q.; Hang, C.; Ruiz, J.; Astruc, D.; Gu, H. Ferrocenyl Janus Mixed-Dendron Stars and Their Stabilization of Au and Ag Nanoparticles. Tetrahedron 2018, 74, 4777–4789. [Google Scholar] [CrossRef]
- Zhao, L.; Ling, Q.; Liu, X.; Hang, C.; Zhao, Q.; Liu, F.; Gu, H. Multifunctional Triazolylferrocenyl Janus Dendron: Nanoparticle Stabilizer, Smart Drug Carrier and Supramolecular Nanoreactor. Appl. Organomet. Chem. 2018, 32, e4000–e4011. [Google Scholar] [CrossRef]
- Plunkett, S.; El Khatib, M.; Şencan, İ.; Porter, J.E.; Kumar, A.T.N.; Collins, J.E.; Sakadžić, S.; Vinogradov, S.A. In Vivo Deep-Tissue Microscopy with UCNP/Janus-Dendrimers as Imaging Probes: Resolution at Depth and Feasibility of Ratiometric Sensing. Nanoscale 2020, 12, 2657–2672. [Google Scholar] [CrossRef]
- Sadler, K.; Tam, J.P. Peptide Dendrimers: Applications and Synthesis. Rev. Mol. Biotechnol. 2002, 90, 195–229. [Google Scholar] [CrossRef]
- Niederhafner, P.; Šebestík, J.; Ježek, J. Peptide Dendrimers. J. Pept. Sci. 2005, 11, 757–788. [Google Scholar] [CrossRef]
- Xie, F.; Li, R.; Shu, W.; Zhao, L.; Wan, J. Self-Assembly of Peptide Dendrimers and Their Bio-Applications in Theranostics. Mater. Today Bio 2022, 14, 100239–100247. [Google Scholar] [CrossRef] [PubMed]
- Sapra, R.; Verma, R.P.; Maurya, G.P.; Dhawan, S.; Babu, J.; Haridas, V. Designer Peptide and Protein Dendrimers: A Cross-Sectional Analysis. Chem. Rev. 2019, 119, 11391–11441. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhao, L.; Li, Y.; Xu, T. Design of Biocompatible Dendrimers for Cancer Diagnosis and Therapy: Current Status and Future Perspectives. Chem. Soc. Rev. 2011, 40, 2673–2703. [Google Scholar] [CrossRef] [PubMed]
- Sheveleva, N.N.; Tarasenko, I.I.; Vovk, M.A.; Mikhailova, M.E.; Neelov, I.M.; Markelov, D.A. NMR Studies of Two Lysine Based Dendrimers with Insertion of Similar Histidine-Arginine and Arginine-Histidine Spacers Having Different Properties for Application in Drug Delivery. Int. J. Mol. Sci. 2023, 24, 949. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Yang, H.; Liu, S.; Zhao, Z. Self-Assembly of Dendrimer-DNA Amphiphiles and Their Catalysis as G-Quadruplex/Hemin DNAzymes. Polymer 2023, 266, 125621–125627. [Google Scholar] [CrossRef]
- He, H.; He, J.; Zheng, K.; Ma, M.; Shi, Y.; Chen, S.; Wang, X. Fantastic Supramolecular Chiral Self-Assembly of POSS Based Dendrimers: From Helical Nano-Fibers to Nano-Toroids and Loofah-like Superstructures. Eur. Polym. J. 2023, 184, 111768–111773. [Google Scholar] [CrossRef]
- Chowdhury, S.; Toth, I.; Stephenson, R.J. Dendrimers in Vaccine Delivery: Recent Progress and Advances. Biomaterials 2022, 280, 121303–121316. [Google Scholar] [CrossRef]
- Lin, Q.; Jiang, G.; Tong, K. Dendrimers in Drug-Delivery Applications. Des. Monomers Polym 2010, 13, 301–324. [Google Scholar] [CrossRef]
- Yousefi, M.; Narmani, A.; Jafari, S.M. Dendrimers as Efficient Nanocarriers for the Protection and Delivery of Bioactive Phytochemicals. Adv. Colloid. Interface Sci. 2020, 278, 102125–102137. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Nixon, L.S.; Hedstrand, D.M. The Role of Branch Cell Symmetry and Other Critical Nanoscale Design Parameters in the Determination of Dendrimer Encapsulation Properties. Biomolecules 2020, 10, 642. [Google Scholar] [CrossRef]
- Suganya, V.; Anuradha, V. Microencapsulation and Nanoencapsulation: A Review. In. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef]
- Moseley, M.E.; Chew, W.M.; White, D.L.; Kucharczyk, J.; Litt, L.; Derugin, N.; Dupon, J.; Brasch, R.C.; Norman, D. Hypercarbia-Induced Changes in Cerebral Blood Volume in the Cat: A ’H MRI and Intravascular Contrast Agent Study. Magn. Reson. Med. 1992, 23, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wiener, E.C.; Auteri, F.P.; Chen, J.W.; Brechbiel, M.W.; Gansow, O.A.; Schneider, D.S.; Belford, R.L.; Clarkson, R.B.; Lauterbur, P.C. Molecular Dynamics of Ion-Chelate Complexes Attached to Dendrimers. ACS J. 1996, 118, 7774–7782. [Google Scholar] [CrossRef]
- Bryant, L.H.; Brechbiel, M.W.; Wu, C.; Bulte, J.W.M.; Herynek, V.; Frank, J.A. Synthesis and Relaxometry of High-Generation (G 5, 7, 9, and 10) PAMAM Dendrimer-DOTA-Gadolinium Chelates. J. Magn. Reson. Imag. 1999, 9, 348–352. [Google Scholar] [CrossRef]
- Anwaier, G.; Chen, C.; Cao, Y.; Qi, R. A Review of Molecular Imaging of Atherosclerosis and the Potential Application of Dendrimer in Imaging of Plaque. Int. J. Nanomed. 2017, 12, 7681–7693. [Google Scholar] [CrossRef] [PubMed]
- Trzepiński, P.; Klajnert-Maculewicz, B. Dendrimers for Fluorescence-Based Bioimaging. J. Chem. Technol. Biotechnol. 2017, 92, 1157–1166. [Google Scholar] [CrossRef]
- Deng, J.; Xu, J.; Ouyang, M.; Zou, Z.; Lei, Y.; Li, J.; Qing, Z.; Yang, R. Target-Triggered Hairpin-Free Chain-Branching Growth of DNA Dendrimers for Contrast-Enhanced Imaging in Living Cells by Avoiding Signal Dispersion. Chin. Chem. Lett. 2022, 33, 773–777. [Google Scholar] [CrossRef]
- Hudde, T.; Rayner, S.A.; Comer, R.M.; Weber, M.; Isaacs, J.D.; Waldmann, H.; Larkin, D.F.P.; George, A.J.T. Activated Polyamidoamine Dendrimers, a Non-Viral Vector for Gene Transfer to the Corneal Endothelium. Gene Ther. 1999, 6, 939–943. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Lajiness, M.S.; Vieth, M.; Erickson, J. Molecular Properties That Influence Oral Drug-like Behavior. Curr. Opin. Drug Discov. Devel. 2004, 7, 470–477. [Google Scholar]
- Singh, V.; Sahebkar, A.; Kesharwani, P. Poly (Propylene Imine) Dendrimer as an Emerging Polymeric Nanocarrier for Anticancer Drug and Gene Delivery. Eur. Polym. J. 2021, 158, 110683–110697. [Google Scholar] [CrossRef]
- Mollazade, M.; Nejati-Koshki, K.; Akbarzadeh, A.; Zarghami, N.; Nasiri, M.; Jahanban-Esfahlan, R.; Alibakhshi, A. PAMAM Dendrimers Augment Inhibitory Effects of Curcumin on Cancer Cell Proliferation: Possible Inhibition of Telomerase. Asian Pac. J. Cancer Prev. 2013, 14, 6925–6928. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Sheikh, A.; Hasan, N.; Sahebkar, A.; Riadi, Y.; Kesharwani, P. Folic Acid Conjugated Poly(Amidoamine) Dendrimer as a Smart Nanocarriers for Tracing, Imaging, and Treating Cancers over-Expressing Folate Receptors. Eur. Polym. J. 2022, 170, 111156–111170. [Google Scholar] [CrossRef]
- Kojima, C.; Kono, K.; Maruyama, K.; Takagishi, T. Synthesis of Polyamidoamine Dendrimers Having Poly(Ethylene Glycol) Grafts and Their Ability to Encapsulate Anticancer Drugs. Bioconjugate Chem. 2000, 11, 910–917. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, N.; Ma, C.; Zhang, Y.; Tan, H.; Qing, G.; Zhang, J.; Wang, Y.; Wang, J.; Chen, S.; et al. An Amphiphilic Dendrimer as a Light-Activable Immunological Adjuvant for in Situ Cancer Vaccination. Nat. Commun. 2021, 12, 4964–4979. [Google Scholar] [CrossRef]
- Vandamme, T.F.; Brobeck, L. Poly(Amidoamine) Dendrimers as Ophthalmic Vehicles for Ocular Delivery of Pilocarpine Nitrate and Tropicamide. J. Control. Release 2005, 102, 23–38. [Google Scholar] [CrossRef]
- Namazi, H.; Adeli, M. Dendrimers of Citric Acid and Poly (Ethylene Glycol) as the New Drug-Delivery Agents. Biomaterials 2005, 26, 1175–1183. [Google Scholar] [CrossRef]
- Namazi, H.; Heydari, A. Synthesis of β-Cyclodextrin-Based Dendrimer as a Novel Encapsulation Agent. Polym. Int. 2013, 63, 1447–1455. [Google Scholar] [CrossRef]
- Liu, M.; Kono, K.; J Fré Chet, J.M. Water-Soluble Dendrimer-Poly(Ethylene Glycol) Starlike Conjugates as Potential Drug Carriers. J. Polym. Sci. A Polym. Chem. 1999, 37, 3492–3503. [Google Scholar] [CrossRef]
- Gatard, S.; Salmon, L.; Deraedt, C.; Ruiz, J.; Astruc, D.; Bouquillon, S. Palladium Nanoparticles Stabilized by Glycodendrimers and Their Application in Catalysis. Eur. J. Inorg. Chem. 2014, 2014, 4369–4375. [Google Scholar] [CrossRef]
- Niu, Y.; Crooks, R.M. Dendrimer-Encapsulated Metal Nanoparticles and Their Applications to Catalysis. Comptes Rendus Chim. 2003, 6, 1049–1059. [Google Scholar] [CrossRef]
- Karakhanov, E.; Maximov, A.; Zolotukhina, A. Heterogeneous Dendrimer-Based Catalysts. Polymers 2022, 14, 981. [Google Scholar] [CrossRef]
- Ou, G.; Xu, L.; He, B.; Yuan, Y. Enhanced Stability of Charged Dendrimer-Encapsulated Pd Nanoparticles in Ionic Liquids. Chem. Commun. 2008, 35, 4210–4212. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ornelas, C.; Diallo, A.K.; Deraedt, C.; Wang, Y.; Lu, F.; Gu, H.; Astruc, D. Ferrocene-Based Dendritic Macromolecules as Efficient Supports in Nanocatalysis. Polymer 2022, 246, 124714–124722. [Google Scholar] [CrossRef]
- Niakan, M.; Masteri-Farahani, M. Pd–Ni Bimetallic Catalyst Supported on Dendrimer-Functionalized Magnetic Graphene Oxide for Efficient Catalytic Suzuki-Miyaura Coupling Reaction. Tetrahedron 2022, 108, 132655–132665. [Google Scholar] [CrossRef]
- Niakan, M.; Masteri-Farahani, M.; Karimi, S.; Shekaari, H. Sulfonic Acid Functionalized Dendrimer-Grafted Cellulose as a Solid Acid Catalyst for the High-Yield and Green Production of 5-Hydroxymethylfurfural. Sustain. Energy Fuels 2022, 6, 2514–2522. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Behari, J.; Sen, P. Application of Nanoparticles in Waste Water Treatment. World Appl. Sci. J. 2008, 3, 417–433. [Google Scholar]
- Diallo, M.S.; Christie, S.; Swaminathan, P.; Johnson, J.H.; Goddard, W.A. Dendrimer Enhanced Ultrafiltration. 1. Recovery of Cu(II) from Aqueous Solutions Using PAMAM Dendrimers with Ethylene Diamine Core and Terminal NH2 Groups. Environ. Sci. Technol. 2005, 39, 1366–1377. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, D. Removal of Copper from Contaminated Soil by Use of Poly(Amidoamine) Dendrimers. Environ. Sci. Technol. 2005, 39, 2369–2375. [Google Scholar] [CrossRef]
- Viltres, H.; López, Y.C.; Leyva, C.; Gupta, N.K.; Naranjo, A.G.; Acevedo–Peña, P.; Sanchez-Diaz, A.; Bae, J.; Kim, K.S. Polyamidoamine Dendrimer-Based Materials for Environmental Applications: A Review. J. Mol. Liq. 2021, 334, 116017–116040. [Google Scholar] [CrossRef]
- Kurczewska, J.; Cegłowski, M.; Schroeder, G. PAMAM-Halloysite Dunino Hybrid as an Effective Adsorbent of Ibuprofen and Naproxen from Aqueous Solutions. Appl. Clay Sci. 2020, 190, 105603–105612. [Google Scholar] [CrossRef]
- Cao, V.; Yunessnia lehi, A.; Bojaran, M.; Fattahi, M. Treatment of Lasalocid A, Salinomycin and Semduramicin as Ionophore Antibiotics in Pharmaceutical Wastewater by PAMAM-Coated Membranes. Environ. Technol. Innov. 2020, 20, 101103–101113. [Google Scholar] [CrossRef]
- Hayouni, S.; Robert, A.; Maes, C.; Conreux, A.; Marin, B.; Mohamadou, A.; Bouquillon, S. New Dendritic Ionic Liquids (DILs) for the Extraction of Metallic Species from Water. New J. Chem. 2018, 42, 18010–18020. [Google Scholar] [CrossRef]
- Tian, N.; Ni, X.; Shen, Z. Synthesis of Main-Chain Imidazolium-Based Hyperbranched Polymeric Ionic Liquids and Their Application in the Stabilization of Ag Nanoparticles. React. Funct. Polym. 2016, 101, 39–46. [Google Scholar] [CrossRef]
- Qin, T.; Li, X.; Chen, J.; Zeng, Y.; Yu, T.; Yang, G.; Li, Y. Dendritic Ionic Liquids Based on Imidazolium-Modified Poly(Aryl Ether) Dendrimers. Chem. Asian J. 2014, 9, 3641–3649. [Google Scholar] [CrossRef]
- Yousefi, M.; Orojzadeh, P.; Jafari, S.M. Nanoencapsulation of Food Ingredients by Dendrimers. In Biopolymer Nanostructures Food Encapsulation Purposes; Elsevier: Amsterdam, The Netherlands, 2019; Volume 20, pp. 607–625. ISBN 9780128156636. [Google Scholar]
- Assadpour, E.; Jafari, S.M. An Overview of Biopolymer Nanostructures for Encapsulation of Food Ingredients. In Biopolymer Nanostructures for Food Encapsulation Purposes; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 1–35. ISBN 9780128156636. [Google Scholar]
- Shatrohan Lal, R.K. Synthesis of Organic Nanoparticles and Their Applications in Drug Delivery and Food Nanotechnology: A Review. J. Nanomater. Mol. Nanotechnol. 2014, 03, 4–14. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2014, 7, 452–490. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An Overview of Encapsulation Technologies for Food Application. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, F.; Zhu, Y.; Miao, M. Development of Dendrimer-like Glucan-Stabilized Pickering Emulsions Incorporated with β-Carotene. Food Chem. 2022, 385, 132626–132633. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, F.; Chen, Y.; Hui, Q.; Miao, M. Dendrimer-like Glucan Nanoparticulate System Improves the Solubility and Cellular Antioxidant Activity of Coenzyme Q10. Food Chem. 2020, 333, 127510–127517. [Google Scholar] [CrossRef]
- Ammala, A. Biodegradable Polymers as Encapsulation Materials for Cosmetics and Personal Care Markets. Int. J. Cosmet. Sci. 2013, 35, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, P. Emerging Trends of Nanotechnology in Advanced Cosmetics. Colloids Surf. B Biointerfaces 2022, 214, 112440–112458. [Google Scholar] [CrossRef] [PubMed]
- Tournilhac, F.; Simon, P. Cosmetic or Dermatological Topical Compositions Comprisingdendritic Polyesters. U.S. Patent 6,287,552 B1, 11 September 2001. [Google Scholar]
- Allard, D.; Forestier, S. Self-Tanning Cosmetic Compositions. U.S. Patent 6, 399, 048 B1, 4 June 2002. [Google Scholar]
- Wolf, B.A.; Snyden, F. Cosmetic Compositions Having Keratolytic and Anti-Acne Activity. U.S Patent 5,449,519, 12 September 2001. [Google Scholar]
- Forestier, S.; Rollat-Corvol, I.; Rollat, C.I. Deodorant Composition and Use Thereof. U.S. Patent 6, 001 342, 14 December 1999. [Google Scholar]
- Astruc, D.; Ruiz, J.; Boisselier, E. Encapsulation de La Vitamine C Dans Des Dendrimères Solubles Dans l’eau. PCT. Patent WO 2009/112682A1, 17 September 2009. [Google Scholar]
- Wołowiec, S.; Laskowski, M.; Laskowska, B.; Magoń, A.; Mysliwiec, B.; Pyda, M. Dermatological Application of PAMAM-Vitamin Bioconjugates and Host-Guest Complexes-Vitamin C Case Study. In Stoichiometry and Research–The Importance of Quantity in Biomedicine; Innocenti, A., Ed.; IntechOpen: Rijeka, Croatia, 2012; Volume 8, pp. 195–210. [Google Scholar]
- Boisselier, E.; Liang, L.; Dalko-Csiba, M.; Ruiz, J.; Astruc, D. Interactions and Encapsulation of Vitamins C, B3, and B6 with Dendrimers in Water. Chem. A Eur. J. 2010, 16, 6056–6068. [Google Scholar] [CrossRef]
- Iimura, T.; Furukawa, H. Copolymer Having Carbosiloxane Dendrimer Structure, and Composition and Cosmetic Containing the Same. US. Patent 2012/0263662 A l, 18 October 2012. [Google Scholar]
- Souda, T.; Sugiura, T.; Kennoki, M.; Matsuba, M. Copolymère Ayant Une Structure de Dendrimère de Carbosiloxane, et Composition, Ingrédient Cosmétique, Agent de Formation de Revêtement et Produit Cosmétique Le Contenant. PCT. Patent WO 2020/203145 A1, 8 October 2020. [Google Scholar]
- Madaan, K.; Lather, V.; Pandita, D. Evaluation of Polyamidoamine Dendrimers as Potential Carriers for Quercetin, a Versatile Flavonoid. Drug Deliv. 2016, 23, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Guzman, L.; Castro, R.; Saffie, C.; Mandujano, P. Agent Nanocicatrisant Pour Soigner Des Blessures. PCT. Patent WO 2020/232561 Al, 26 November 2020. [Google Scholar]
- Chauhan, A.S. Dendrimer Nanotechnology for Enhanced Formulation and Controlled Delivery of Resveratrol. Ann. N. Y. Acad. Sci. 2015, 1348, 134–140. [Google Scholar] [CrossRef]
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594. [Google Scholar] [CrossRef]
- La Toxicité. Available online: https://www.futura-sciences.com/sante/definitions/medecine-toxicite-6517/ (accessed on 17 May 2023).
- La Cytotoxicité. Available online: https://www.eurofins.fr/pharma/accueil/services/prestations-analytiques/dispositifs-medicaux/evaluation-biologique/cytotoxicite (accessed on 17 May 2023).
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Biotechno. Pharm. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- Berridge, M.V.; Tan, A.S. Characterization of the Cellular Reduction of 3-(4,5-Dimethylthiazol-2- Yl)-2,5-Diphenyltetrazolium Bromide (MTT): Subcellular Localization, Substrate Dependence, and Involvement of Mitochondrial Electron Transport in MTT Reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef]
- Ishiyama, M.; Tominaga, H.; Shiga, M.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A Combined Assay of Cell Vability and in Vitro Cytotoxicity with a Highly Water-Soluble Tetrazolium Salt, Neutral Red and Crystal Violet. Biol. Pharm. Bull. 1996, 19, 1518–1520. [Google Scholar] [CrossRef]
- Lazniewska, J.; Milowska, K.; Zablocka, M.; Mignani, S.; Caminade, A.M.; Majoral, J.P.; Bryszewska, M.; Gabryelak, T. Mechanism of Cationic Phosphorus Dendrimer Toxicity against Murine Neural Cell Lines. Mol. Pharm. 2013, 10, 3484–3496. [Google Scholar] [CrossRef]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weenerc, J.W.; Meijerc, E.W.; Paulusd, W.; Duncana, R. Dendrimers: Relationship between Structure and Biocompatibility in Vitro, and Preliminary Studies on the Biodistribution of I-Labelled Polyamidoamine Dendrimers in Vivo. J. Control. Release 2000, 65, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Davoren, M.; Byrne, H.J. In Vitro Mammalian Cytotoxicological Study of PAMAM Dendrimers-Towards Quantitative Structure Activity Relationships. Toxicol. Vitro 2010, 24, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Ciolkowski, M.; Wróbel, D.; Petersen, J.F.; Ficker, M.; Christensen, J.B.; Bryszewska, M.; Klajnert, B. Modified PAMAM Dendrimer with 4-Carbomethoxypyrrolidone Surface Groups Reveals Negligible Toxicity against Three Rodent Cell-Lines. Nanomedicine 2013, 9, 461–464. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.C.; Bhalgat, M.K.; Zera, R.T. Preliminary Biological Evaluation of Polyamidoamine (PAMAM) StarburstTM Dendrimers. J. Biomed. Mater. Res. 1996, 30, 53–65. [Google Scholar] [CrossRef]
- Mishra, V.; Gupta, U.; Jain, N.K. Influence of Different Generations of Poly(Propylene Imine) Dendrimers on Human Erythrocytes. Pharmazie 2010, 65, 891–895. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Petersen, J.F.; Ficker, M.; Janaszewska, A.; Christensen, J.B.; Klajnert, B.; Bryszewska, M. Surface Modification of PAMAM Dendrimer Improves Its Biocompatibility. Nanomedicine 2012, 8, 815–817. [Google Scholar] [CrossRef]
- Ziemba, B.; Halets, I.; Shcharbin, D.; Appelhans, D.; Voit, B.; Pieszynski, I.; Bryszewska, M.; Klajnert, B. Influence of Fourth Generation Poly(Propyleneimine) Dendrimers on Blood Cells. J. Biomed. Mater. Res.-Part A 2012, 100, 2870–2880. [Google Scholar] [CrossRef]
- Albertazzi, L.; Serresi, M.; Albanese, A.; Beltram, F. Dendrimer Internalization and Intracellular Trafficking in Living Cells. Mol. Pharm. 2010, 7, 680–688. [Google Scholar] [CrossRef]
- Padilla De Jesús, O.L.; Ihre, H.R.; Gagne, L.; Fréchet, J.M.J.; Szoka, F.C. Polyester Dendritic Systems for Drug Delivery Applications: In Vitro and in Vivo Evaluation. Bioconjugate Chem. 2002, 13, 453–461. [Google Scholar] [CrossRef]
- Agache, P.; Lihoreau, T.; Mac-Mary, S.; Fanian, F.; Humbert, P. The Human Skin: An Overview. In Agache’s Measuring the Skin Non-Invasive Investigations, Physiology, Normal Constants; Humbert, P., Fanian, F., Maibach, H.I., Agache, P., Eds.; Springer Nature: Cham, Switzerland, 2016; Volume 1, pp. 1–4. [Google Scholar]
- Structures et Rôles de La Peau. Available online: https://sante.lefigaro.fr/mieux-etre/beaute/structures-roles-peau (accessed on 17 May 2023).
- Mélissopoulos, A.; Levacher, C.; Robert, L.; Ballotti, R. La Peau Structure et Physiologie, 2nd ed.; Mélissopoulos, A., Levacher, C., Eds.; Lavoisier: Paris, France, 2012; Volume 1, ISBN 9782743013691. [Google Scholar]
- Elias, P.M. Epidermal Lipids, Barrier Function, and Desquamation. J. Investig. Dermatol. 1983, 80, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Structure et Fonction de La Peau. Available online: https://www.eucerin.fr/a-propos-de-la-peau/comprendre-la-peau/structure-et-fonction-de-la-peau (accessed on 17 May 2023).
- Ng, K.W.; Lau, W.M. Skin Deep: The Basics of Human Skin Structure and Drug Penetration. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Drug Manipulation Strategies and Vehicle Effects; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. ISBN 9783662450130. [Google Scholar]
- Wertz, P.W.; van den Bergh, B. The Physical, Chemical and Functional Properties of Lipids in the Skin and Other Biological Barriers. Chem. Phys. Lipids 1998, 2, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. Lipids and the Epidermal Permeability Barrier. Arch. Dermatol. Res. 1981, 270, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Christophers, E.; Kligman, A.M. Visualization of the Cell Layers of the Stratum Corneum. J. Investig. Dermatol. 1964, 42, 407–409. [Google Scholar] [CrossRef] [PubMed]
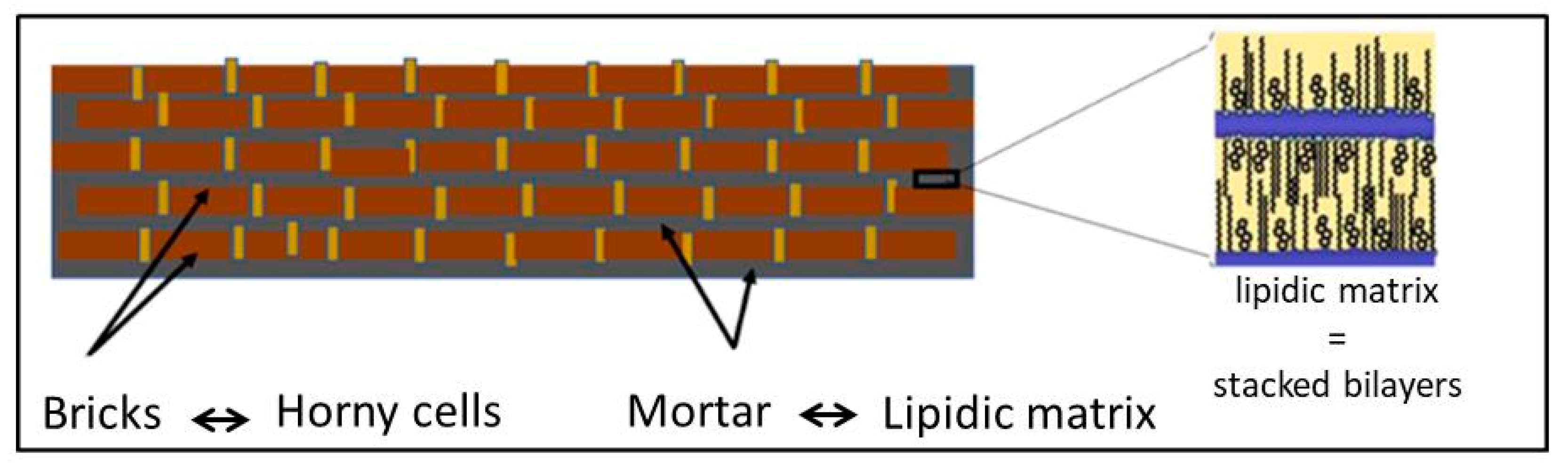
- Nemes, Z.; Steinert, P.M. Bricks and Mortar of the Epidermal Barrier. Exp. Mol. Med. 1999, 31, 5–19. [Google Scholar] [CrossRef]
- Madison, K.C. Barrier Function of the Skin: “‘La Raison d’Etre’” of the Epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Wertz, P.W.; Abraham, W.; Landmann, L.; Downing, D.T. Preparation of Liposomes from Stratum Corneum Lipids. Soc. Investig. Dermatol. 1986, 87, 582–584. [Google Scholar] [CrossRef]
- Law, S.; Wertz, P.W.; Swartzendruber, D.C.; Squier, C.A. Regional Variation in Content, Composition and Organization of Porcine Epithelial Barrier Lipids Revealed by Thin-Layer Chromatography and Transmission Electron Microscopy. Arch. Oral. Biol. 1995, 40, 1085–1091. [Google Scholar] [CrossRef]
- Menon, G.K.; Cleary, G.W.; Lane, M.E. The Structure and Function of the Stratum Corneum. Int. J. Pharm. 2012, 435, 3–9. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Dubbelaar, F.E.R.; Gooris, G.S.; Ponec, M. The Lipid Organisation in the Skin Barrier. Acta Derm. Venereol. 2000, 208, 23–30. [Google Scholar] [CrossRef]
- Cannon, J.B. Lipids in Transdermal and Topical Drug Delivery. Am. Pharm. Rev. 2014, 17, 1–11. [Google Scholar]
- Banker, G.S.; Rhodes, C.T. Modern Pharmaceutics, 4th ed.; Banker, G.S., Rhodes, C.T., Eds.; Marcel Dekker: New York, NY, USA, 2002; Volume 1, ISBN 0824706749. [Google Scholar]
- Géa, A.; Banel, P. Physiologie et Huiles Essentielles, 1st ed.; Dunod: Malakoff, France, 2022; Volume 1. [Google Scholar]
- Elias, P.-M.; Friend, D.S. The Permeability Barrier in Mammalian Epidermis. J. Cell Biol. 1975, 65, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Penetration Enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, M.; Fang, L. Biomaterials as Novel Penetration Enhancers for Transdermal and Dermal Drug Delivery Systems. Drug Deliv. 2013, 20, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.; Krishna Venuganti, V.V. Dendritic Polymers for Dermal Drug Delivery. Ther. Deliv. 2017, 8, 1077–1096. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current Status and Future Potential of Transdermal Drug Delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Eeman, M.; Deleu, M. From Biological Membranes to Biomimetic Model Membranes. Biotechnol. Agron. Soc. Environ. 2010, 14, 691–708. Available online: https://hdl.handle.net/2268/81993 (accessed on 17 October 2022).
- Clifton, L.A.; Campbell, R.A.; Sebastiani, F.; Campos-Terán, J.; Gonzalez-Martinez, J.F.; Björklund, S.; Sotres, J.; Cárdenas, M. Design and Use of Model Membranes to Study Biomolecular Interactions Using Complementary Surface-Sensitive Techniques. Adv. Colloid Interface Sci. 2020, 277, 102118–102139. [Google Scholar] [CrossRef]
- Deleu, M.; Crowet, J.M.; Nasir, M.N.; Lins, L. Complementary Biophysical Tools to Investigate Lipid Specificity in the Interaction between Bioactive Molecules and the Plasma Membrane: A Review. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 3171–3190. [Google Scholar] [CrossRef]
- Brockman, H. Lipid Monolayers: Why Use Half a Membrane to Characterize Protein-Membrane Interactions? Curr. Opin. Struct. Biol. 1990, 9, 438–443. [Google Scholar] [CrossRef]
- Basit, H.; Lopez, S.G.; Keyes, T.E. Fluorescence Correlation and Lifetime Correlation Spectroscopy Applied to the Study of Supported Lipid Bilayer Models of the Cell Membrane. Methods 2014, 68, 286–299. [Google Scholar] [CrossRef]
- Hoebeke, M. The Importance of Liposomes as Models and Tools in the Understanding of Photosensitization Mechanisms. J. Photochem. Photobio. B Biol. 1995, 28, 189–196. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and Polymersomes: A Comparative Review towards Cell Mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Dwadasi, B.S.; Rai, B. Molecular Dynamics Simulation of Skin Lipids: Effect of Ceramide Chain Lengths on Bilayer Properties. J. Phys. Chem. B 2016, 120, 12536–12546. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.M.; Yardley, H.J. Lipid Compositions of Cells Isolated from Pig, Human, and Rat Epidermis. J. Lipid Res. 1975, 16, 434–440. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, M.; Song, L. A Review of Fatty Acids Influencing Skin Condition. J. Cosmet. Dermatol. 2020, 19, 3199–3204. [Google Scholar] [CrossRef]
- Eeman, M.; Francius, G.; Dufrêne, Y.F.; Nott, K.; Paquot, M.; Deleu, M. Effect of Cholesterol and Fatty Acids on the Molecular Interactions of Fengycin with Stratum Corneum Mimicking Lipid Monolayers. Langmuir 2009, 25, 3029–3039. [Google Scholar] [CrossRef] [PubMed]
- Eeman, M.; Deleu, M.; Paquot, M.; Thonart, P.; Dufrêne, Y.F. Nanoscale Properties of Mixed Fengycin/Ceramide Monolayers Explored Using Atomic Force Microscopy. Langmuir 2005, 21, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Neubert, R.H.H.; Opálka, L.; Kerth, A.; Brezesinski, G. Non-Ionic Surfactants as Innovative Skin Penetration Enhancers: Insight in the Mechanism of Interaction with Simple 2D Stratum Corneum Model System. Eur. J. Pharm. Sci. 2021, 157, 105620. [Google Scholar] [CrossRef]
- Blume, A.; Jansen, M.; Ghyczy, M.; Gareiss, J. Interaction of Phospholipid Liposomes with Lipid Model Mixtures for Stratum Corneum Lipids. Int. J. Pharm. 1993, 99, 219–228. [Google Scholar] [CrossRef]
- Mueller, J.; Trapp, M.; Neubert, R.H.H. The Effect of Hydrophilic Penetration/Diffusion Enhancer on Stratum Corneum Lipid Models: Part II*: DMSO. Chem. Phys. Lipids 2019, 225, 104816–104823. [Google Scholar] [CrossRef]
- Školová, B.; Jandovská, K.; Pullmannová, P.; Tesař, O.; Roh, J.; Hrabálek, A.; Vávrová, K. The Role of the Trans Double Bond in Skin Barrier Sphingolipids: Permeability and Infrared Spectroscopic Study of Model Ceramide and Dihydroceramide Membranes. Langmuir 2014, 30, 5527–5535. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W. Mode of Action of Penetration Enhancers in Human Skin. J. Control. Release 1987, 6, 85–97. [Google Scholar] [CrossRef]
- Kim, C.-K.; Hong, M.-S.; Kim, Y.-B.; Han, S.-K. Effect of Penetration Enhancers (Pyrrolidone Derivatives) on Multilamellar Liposomes of Stratum Corneum Lipid: A Study by UV Spectroscopy and Differential Scanning Calorimetry. Int. J. Pharm. 1993, 95, 43–50. [Google Scholar] [CrossRef]
- Hatziantoniou, S.; Nezis, I.P.; Margaritis, L.H.; Demetzos, C. Visualisation of Liposomes Prepared from Skin and Stratum Corneum Lipids by Transmission Electron Microscopy. Micron 2007, 38, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Downing, D.T.; Abraham, W.; Wegner, B.K.; Willman, K.W.; Marshall, J.L. Partition of Sodium Dodecyl Sulfate into Stratum Corneum Lipid Liposomes. Arch. Dermatol. Res. 1993, 285, 151–157. [Google Scholar] [CrossRef]
- Zbytovská, J.; Kiselev, M.A.; Funari, S.S.; Garamus, V.M.; Wartewig, S.; Palát, K.; Neubert, R. Influence of Cholesterol on the Structure of Stratum Corneum Lipid Model Membrane. Colloids Surf. A Physicochem. Eng. Asp. 2008, 328, 90–99. [Google Scholar] [CrossRef]
- Kiselev, M.A. Conformation of Ceramide 6 Molecules and Chain-Flip Transitions in the Lipid Matrix of the Outermost Layer of Mammalian Skin, the Stratum Corneum. Crystallogr. Rep. 2007, 52, 525–528. [Google Scholar] [CrossRef]
- Bandaru, R.; Sanket, A.S.; Rekha, S.; Kamble, O.; Dewangan, R.P.; Kesharwani, P.; Samal, S.K.; Dandela, R. Biological Interaction of Dendrimers. In Dendrimer-Based Nanotherapeutics; Elsevier: Amsterdam, The Netherlands, 2021; Volume 5, pp. 63–74. ISBN 9780128212509. [Google Scholar]
- Mutalik, S.; Nayak, U.Y.; Kalra, R.; Kumar, A.; Kulkarni, R.V.; Parekh, H.S. Sonophoresis-Mediated Permeation and Retention of Peptide Dendrimers across Human Epidermis. Skin Res. Technol. 2012, 18, 101–107. [Google Scholar] [CrossRef]
- Mutalik, S.; Shetty, P.K.; Kumar, A.; Kalra, R.; Parekh, H.S. Enhancement in Deposition and Permeation of 5-Fluorouracil through Human Epidermis Assisted by Peptide Dendrimers. Drug Deliv. 2014, 21, 44–54. [Google Scholar] [CrossRef]
- Mutalik, S.; Parekh, H.S.; Anissimov, Y.G.; Grice, J.E.; Roberts, M.S. Iontophoresis-Mediated Transdermal Permeation of Peptide Dendrimers across Human Epidermis. Skin Pharmacol. Physiol. 2013, 26, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Borowska, K.; Wołowiec, S.; Rubaj, A.; Głowniak, K.; Sieniawska, E.; Radej, S. Effect of Polyamidoamine Dendrimer G3 and G4 on Skin Permeation of 8-Methoxypsoralene—In Vivo Study. Int. J. Pharm. 2012, 426, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Manikkath, J.; Hegde, A.R.; Kalthur, G.; Parekh, H.S.; Mutalik, S. Influence of Peptide Dendrimers and Sonophoresis on the Transdermal Delivery of Ketoprofen. Int. J. Pharm. 2017, 521, 110–119. [Google Scholar] [CrossRef]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM Dendrimer-Cell Membrane Interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Venuganti, V.V.K.; Perumal, O.P. Poly(Amidoamine) Dendrimers as Skin Penetration Enhancers: Influence of Charge, Generation, and Concentration. J. Pharm. Sci. 2009, 98, 2345–2356. [Google Scholar] [CrossRef]
- Venuganti, V.V.; Sahdev, P.; Hildreth, M.; Guan, X.; Perumal, O. Structure-Skin Permeability Relationship of Dendrimers. Pharm. Res. 2011, 28, 2246–2260. [Google Scholar] [CrossRef]
- Yang, Y.; Sunoqrot, S.; Stowell, C.; Ji, J.; Lee, C.W.; Kim, J.W.; Khan, S.A.; Hong, S. Effect of Size, Surface Charge, and Hydrophobicity of Poly(Amidoamine) Dendrimers on Their Skin Penetration. Biomacromolecules 2012, 13, 2154–2162. [Google Scholar] [CrossRef]
- Venuganti, V.V.K.; Perumal, O.P. Effect of Poly(Amidoamine) (PAMAM) Dendrimer on Skin Permeation of 5-Fluorouracil. Int. J. Pharm. 2008, 361, 230–238. [Google Scholar] [CrossRef]
- Yiyun, C.; Na, M.; Tongwen, X.; Rongqiang, F.; Xueyuan, W.; Xiaomin, W.; Longping, W. Transdermal Delivery of Nonsteroidal Anti-Inflammatory Drugs Mediated by Polyamidoamine (PAMAM) Dendrimers. J. Pharm. Sci. 2007, 96, 595–602. [Google Scholar] [CrossRef]
- Volz, P.; Schilrreff, P.; Brodwolf, R.; Wolff, C.; Stellmacher, J.; Balke, J.; Morilla, M.J.; Zoschke, C.; Schäfer-Korting, M.; Alexiev, U. Pitfalls in Using Fluorescence Tagging of Nanomaterials: Tecto-Dendrimers in Skin Tissue as Investigated by Cluster-FLIM. Ann. N. Y. Acad. Sci. 2017, 1405, 202–214. [Google Scholar] [CrossRef]
- Hegde, A.R.; Rewatkar, P.V.; Manikkath, J.; Tupally, K.; Parekh, H.S.; Mutalik, S. Peptide Dendrimer-Conjugates of Ketoprofen: Synthesis and Ex Vivo and in Vivo Evaluations of Passive Diffusion, Sonophoresis and Iontophoresis for Skin Delivery. Eur. J. Pharm. Sci. 2017, 102, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.K.; Manikkath, J.; Tupally, K.; Kokil, G.; Hegde, A.R.; Raut, S.Y.; Parekh, H.S.; Mutalik, S. Skin Delivery of EGCG and Silibinin: Potential of Peptide Dendrimers for Enhanced Skin Permeation and Deposition. AAPS Pharm. Sci. Technol. 2017, 18, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
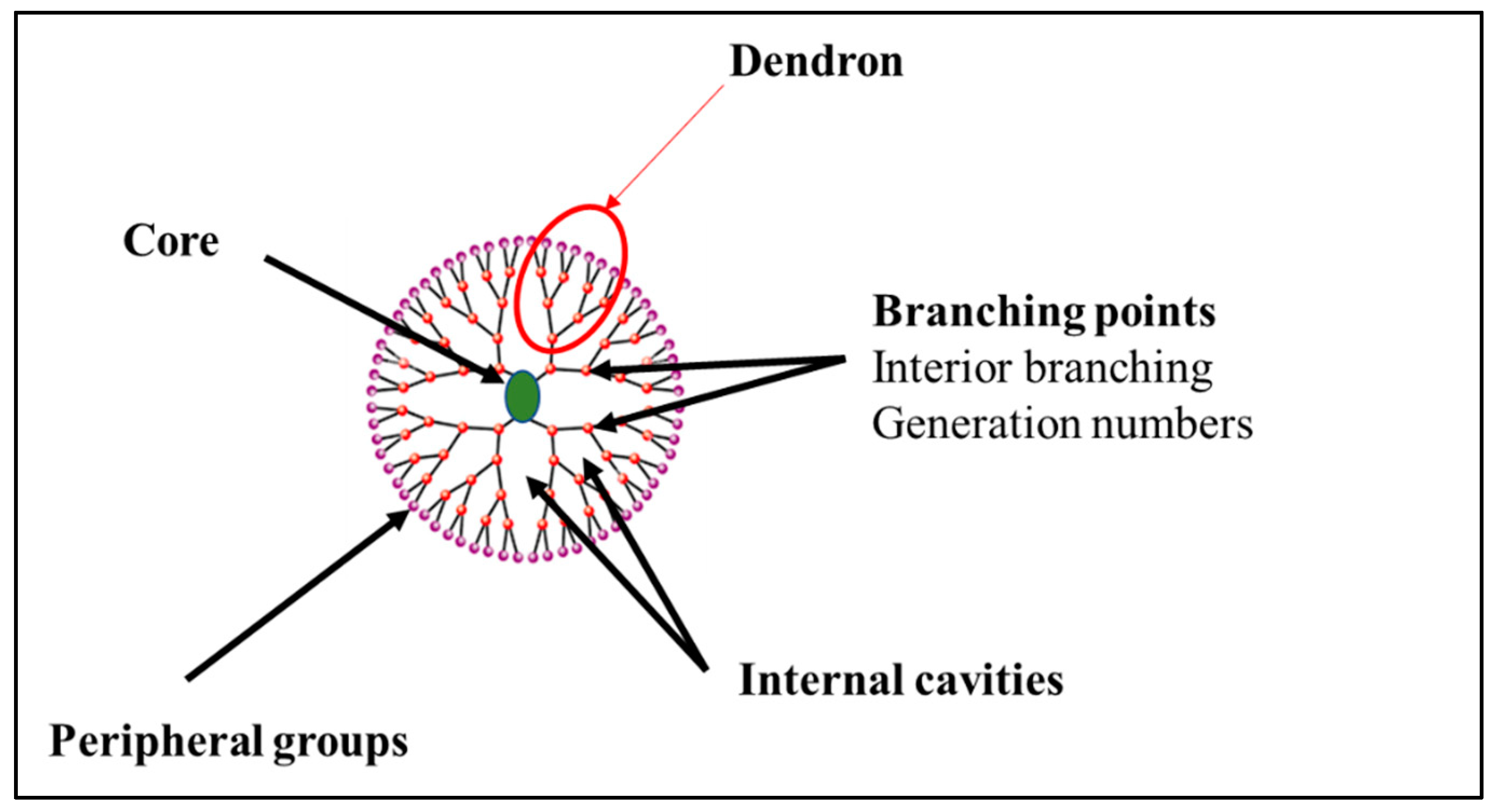
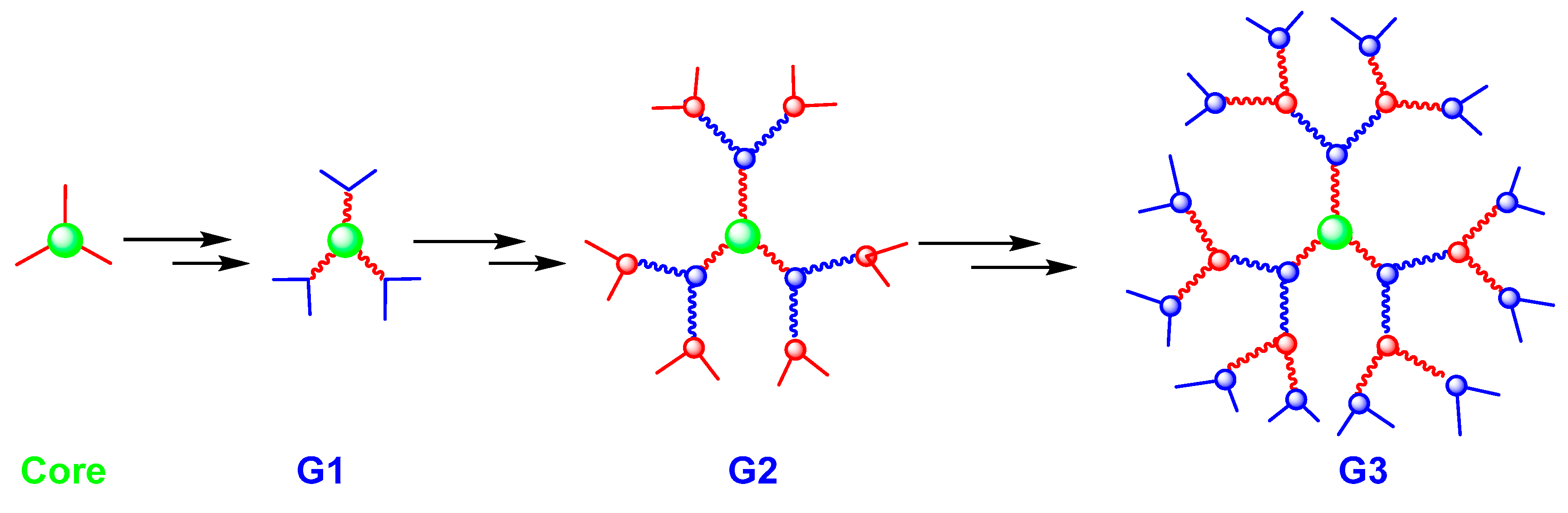
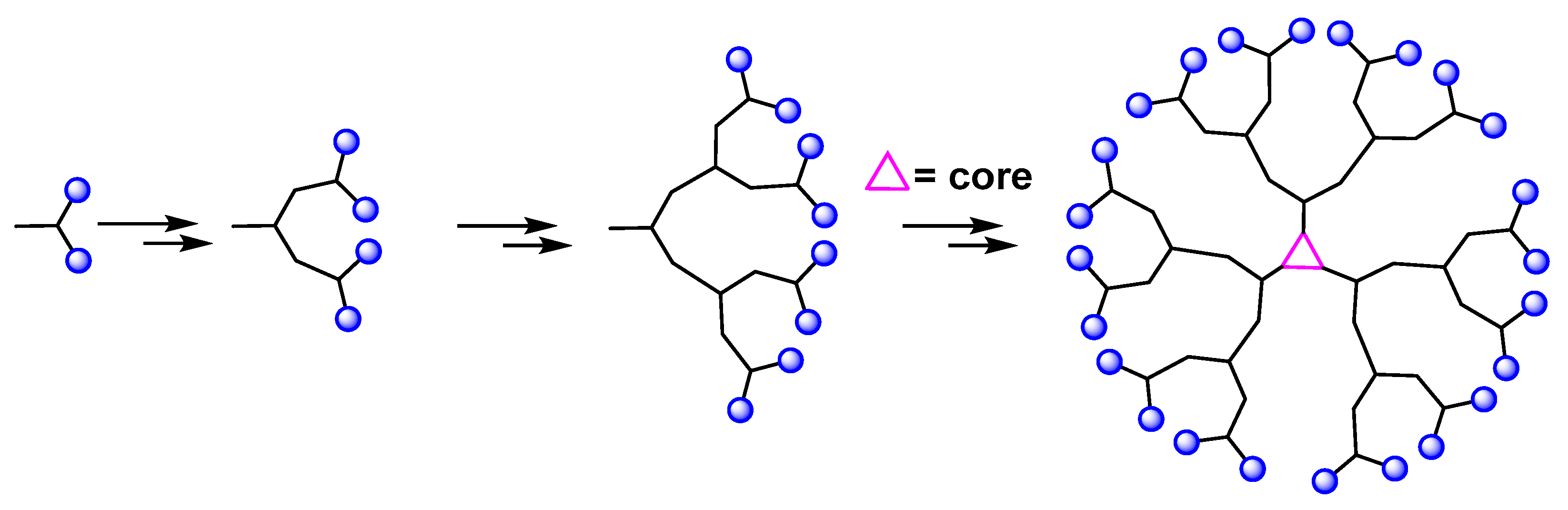
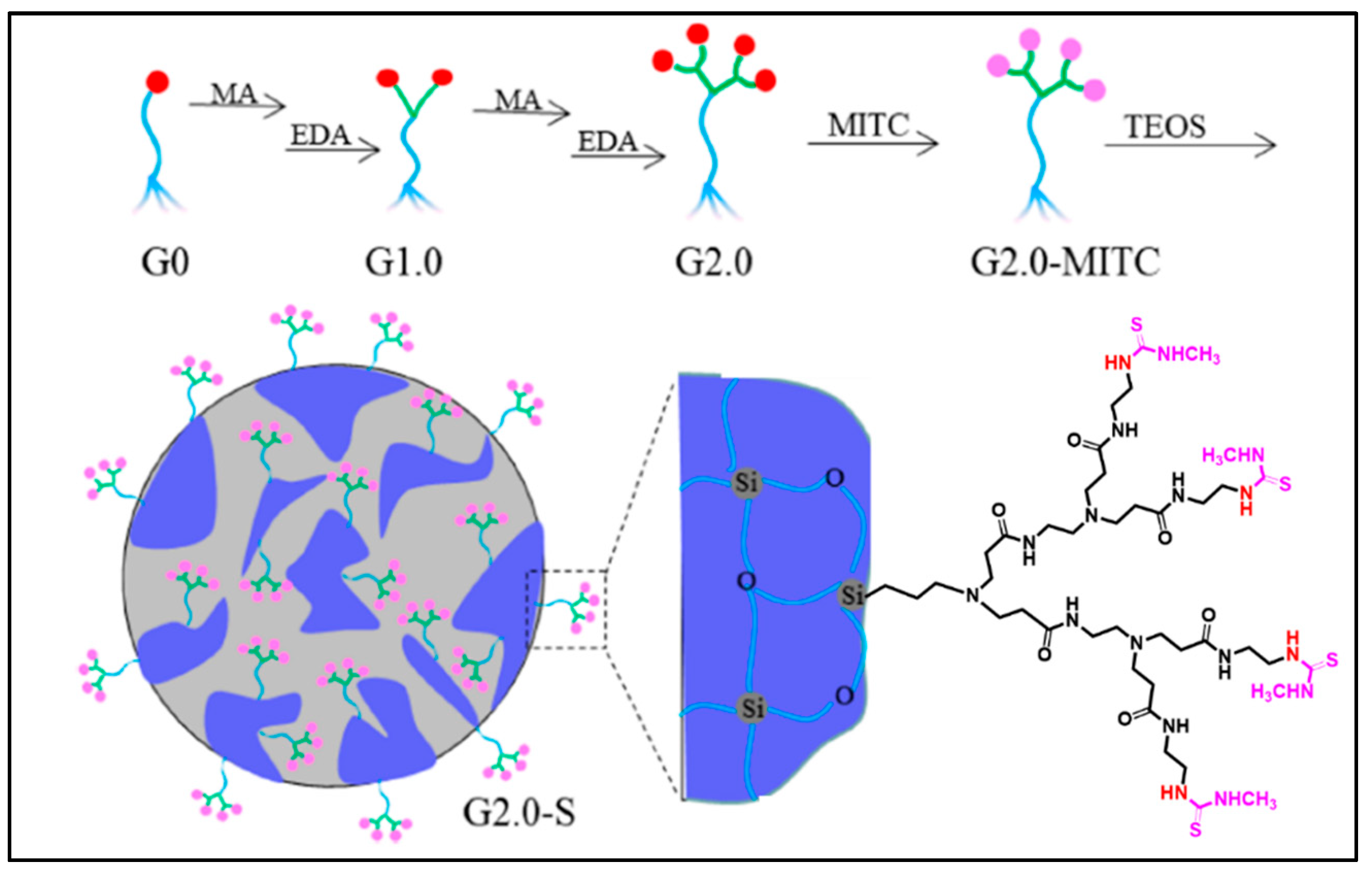

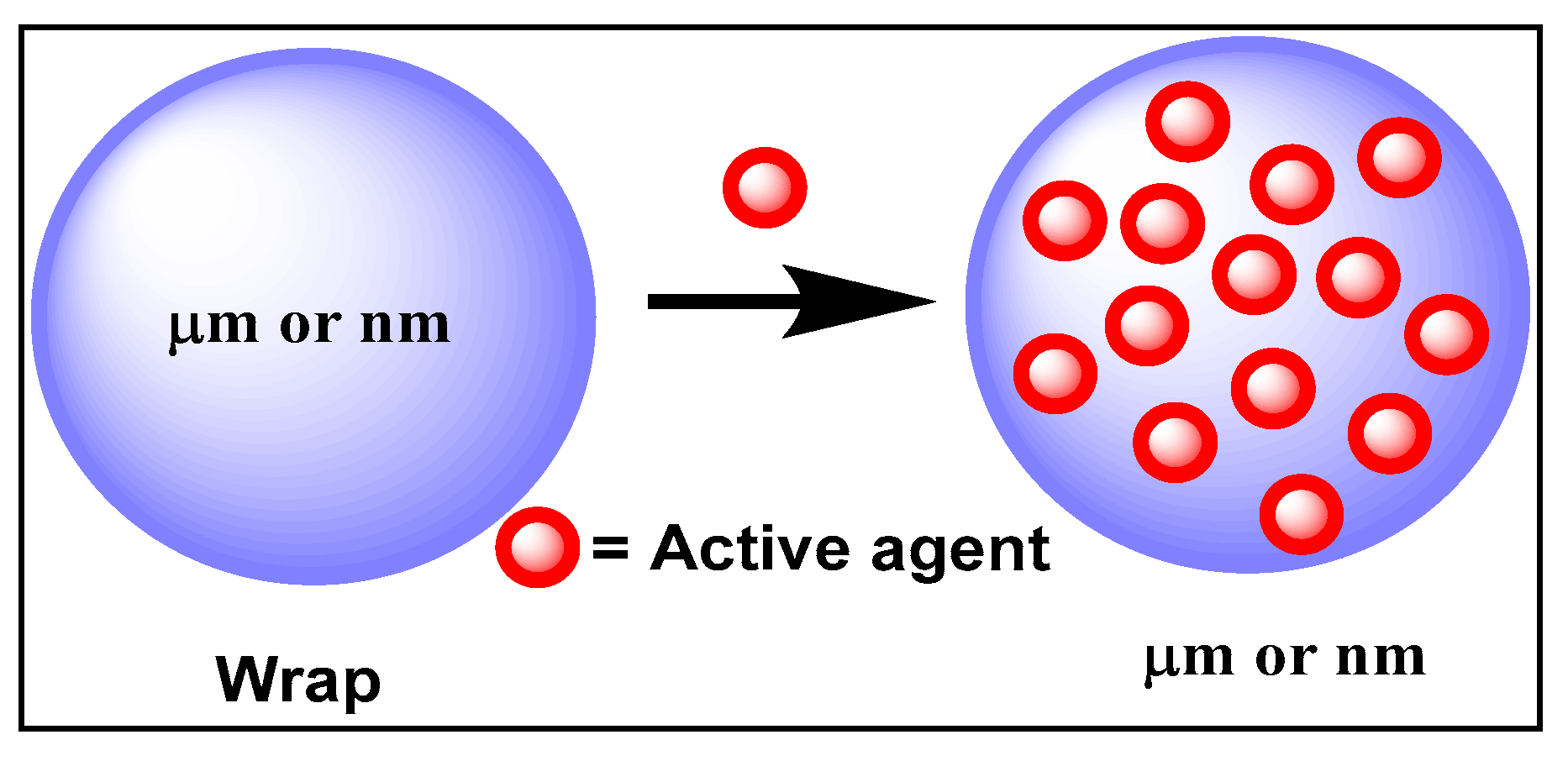
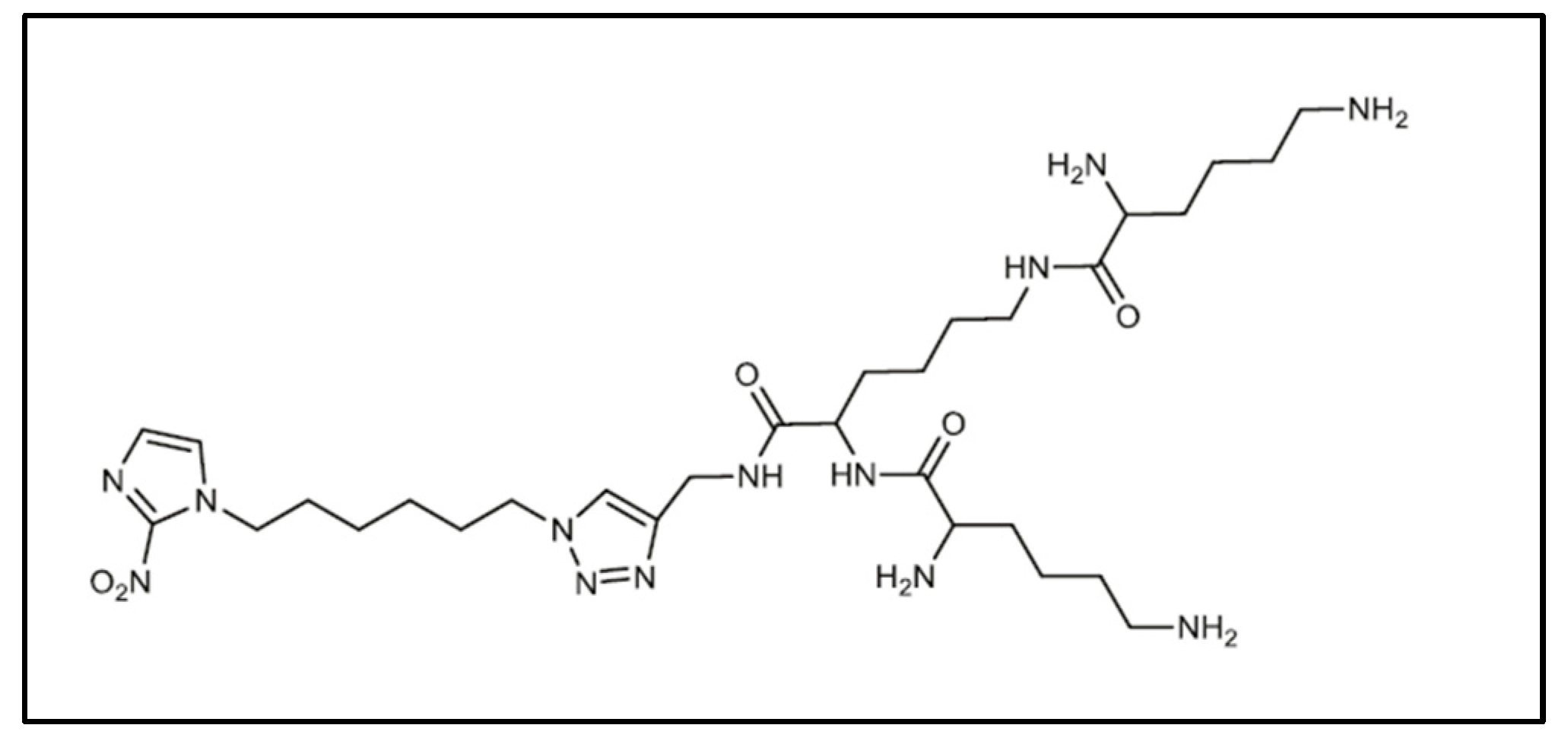
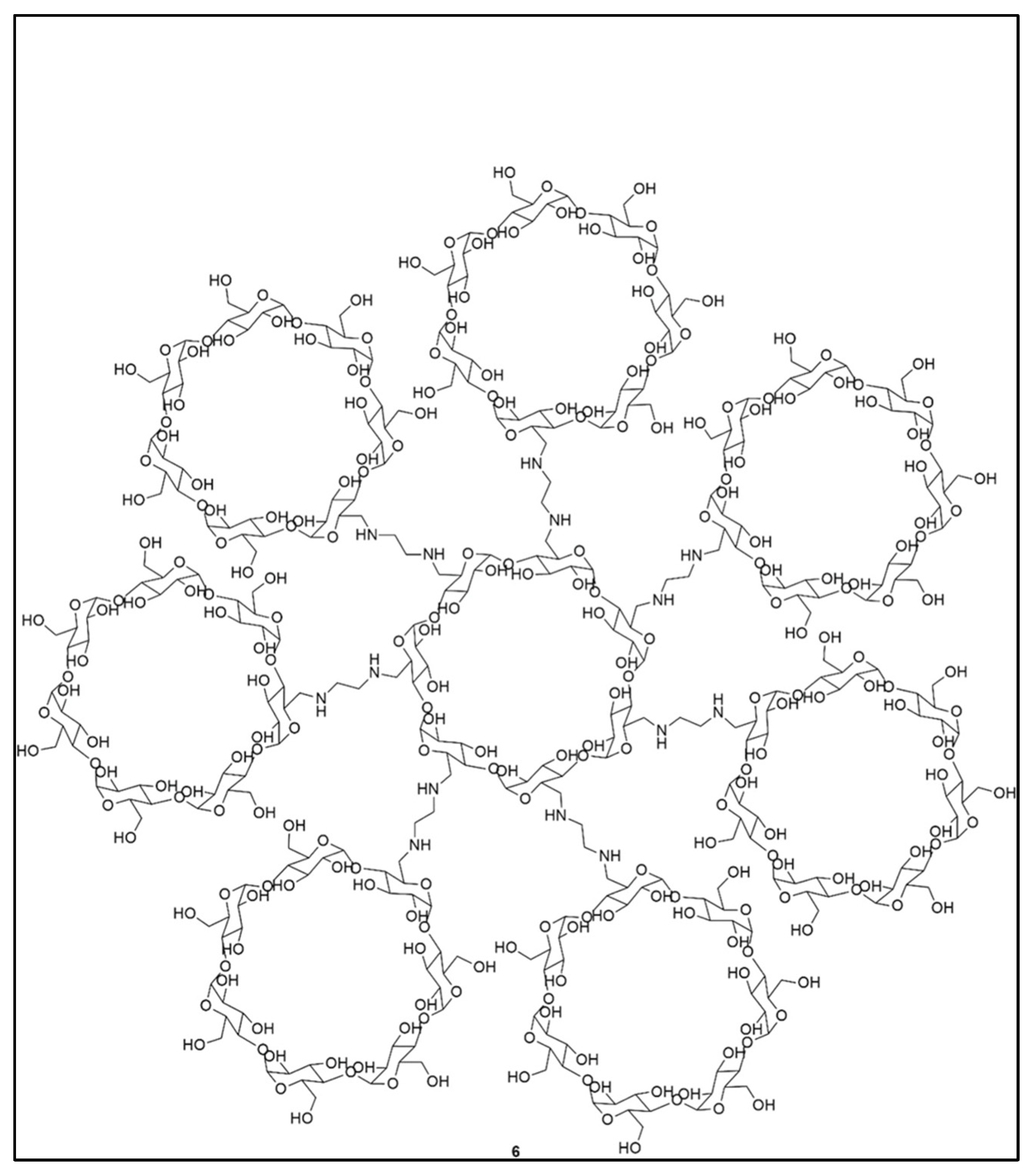

| Approaches | Principles | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Divergent | The dendrimer is built from a central multifunctional molecule toward the periphery. | Production of large quantities of dendrimers. | Secondary and incomplete reactions of peripheral groups. | [4] |
| Use of a large excess of reagents. | ||||
| Higher generation production. | Difficulties to purify the final product. | |||
| Convergent | Consists of building the dendrons separately and attaching them in a final step to a multifunctional core. | Better control of synthesis. | This approach does not allow the formation of high generations. Steric hindrance in the reactions between the dendrons and the central molecule. | [21,22] |
| Large excesses of reagents avoided. | ||||
| Limitation of purification problems. |
| Dendrimers | Cytotoxicity | Cells | Methods | Ref. |
|---|---|---|---|---|
| PAMAM-3 | IC50 (mM) = 0.14 ± 0 | Human epithelial cells (Caco-2) | MTT | [32] |
| PAMAM-3.5 | IC50 (mM) > 1 | |||
| 6-Lauroyl- PAMAM-3 | IC50 (mM) > 1 | |||
| CPD-2 | IC50 = 2.33 μM | Mouse embryonic hippocampal cells (mHippoE-18) | MTT | [139] |
| CPD-3 | IC50 = 1.31 μM | |||
| CPD-2 | IC50 = 1.84 μM | Mouse neuroblastoma cells (N2a) | ||
| CPD-3 | IC50 = 1.74 μM | |||
| PAMAM-2 | IC50 (mg/mL) > 2 | B16F10 melanocyte cells | MTT | [140] |
| DAB-2 | IC50 (mg/mL) = 0. 3 | |||
| PEA-2 | IC50 (mg/mL) > 0.1 | |||
| CSi–PEO-2 | IC50 (mg/mL) > 2 | |||
| PPI-4 | Cytotoxic at c (10 µg/mL) | Human lung fibroblasts MRC5 | WST1 | [38] |
| GD-PPI-4 | Cytotoxic at c > 100 µg/mL | |||
| PAMAM-3 | Cytotoxic at C (1000 µg/mL) | Human lung fibroblasts MRC5 | WST1 | [16] |
| GD-PAMAM-3 | ||||
| EC50 Values (µM) (Clonogenic test) | Human Keratinocytes (HaCaT) | Alamar Blue (AB) Neutral Red (NR) MTT Clonogenic | [141] | |
| PAMAM-4 | 2.63 [1.78–3.47] | |||
| PAMAM-5 | 0.57 [0.37–0.77] | |||
| PAMAM-6 | 0.19 [0.14–0.25] | |||
| EC50 Values(µM) (Clonogenic test) | Colon adenocarcinoma cells (SW480) | |||
| PAMAM-4 | 1.43 [1.27–1.59] | |||
| PAMAM-5 | 0.41 [0.39–0.42] | |||
| PAMAM-6 | 0.18 [0.15–0.2] | |||
| DAB-Ac40-FITC2 | Cytotoxic at 3 µM après 150 min Dead cells > 90% | Human umbilical vein endothelial cells (HUVEC) | Propidium Iodide (PI) and Lactate Dehydrogenase (LDH) | [33] |
| DAB-Ac59-FITC2 | No cytotoxic at 3 µM | |||
| DAB-Ac40-PEG-4-FITC2 | No cytotoxic at 3 µM | |||
| PAMAM–pyrrolidone dendrimère | No cytotoxic | Chinese hamster fibroblasts (B14) | MTT | [142] |
| Mouse embryonic hippocampal cells (mHippoE-18) | ||||
| Rat liver-derived cells (BRL-3A) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacha, K.; Chemotti, C.; Mbakidi, J.-P.; Deleu, M.; Bouquillon, S. Dendrimers: Synthesis, Encapsulation Applications and Specific Interaction with the Stratum Corneum—A Review. Macromol 2023, 3, 343-370. https://doi.org/10.3390/macromol3020022
Bacha K, Chemotti C, Mbakidi J-P, Deleu M, Bouquillon S. Dendrimers: Synthesis, Encapsulation Applications and Specific Interaction with the Stratum Corneum—A Review. Macromol. 2023; 3(2):343-370. https://doi.org/10.3390/macromol3020022
Chicago/Turabian StyleBacha, Katia, Catherine Chemotti, Jean-Pierre Mbakidi, Magali Deleu, and Sandrine Bouquillon. 2023. "Dendrimers: Synthesis, Encapsulation Applications and Specific Interaction with the Stratum Corneum—A Review" Macromol 3, no. 2: 343-370. https://doi.org/10.3390/macromol3020022
APA StyleBacha, K., Chemotti, C., Mbakidi, J.-P., Deleu, M., & Bouquillon, S. (2023). Dendrimers: Synthesis, Encapsulation Applications and Specific Interaction with the Stratum Corneum—A Review. Macromol, 3(2), 343-370. https://doi.org/10.3390/macromol3020022








