The Correlation between Interleukin 33 and Psoriasis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. The Literature Searches and Study Selection
2.2. Data Extraction and Quality Assessment
2.3. Statistical Analyses
3. Results
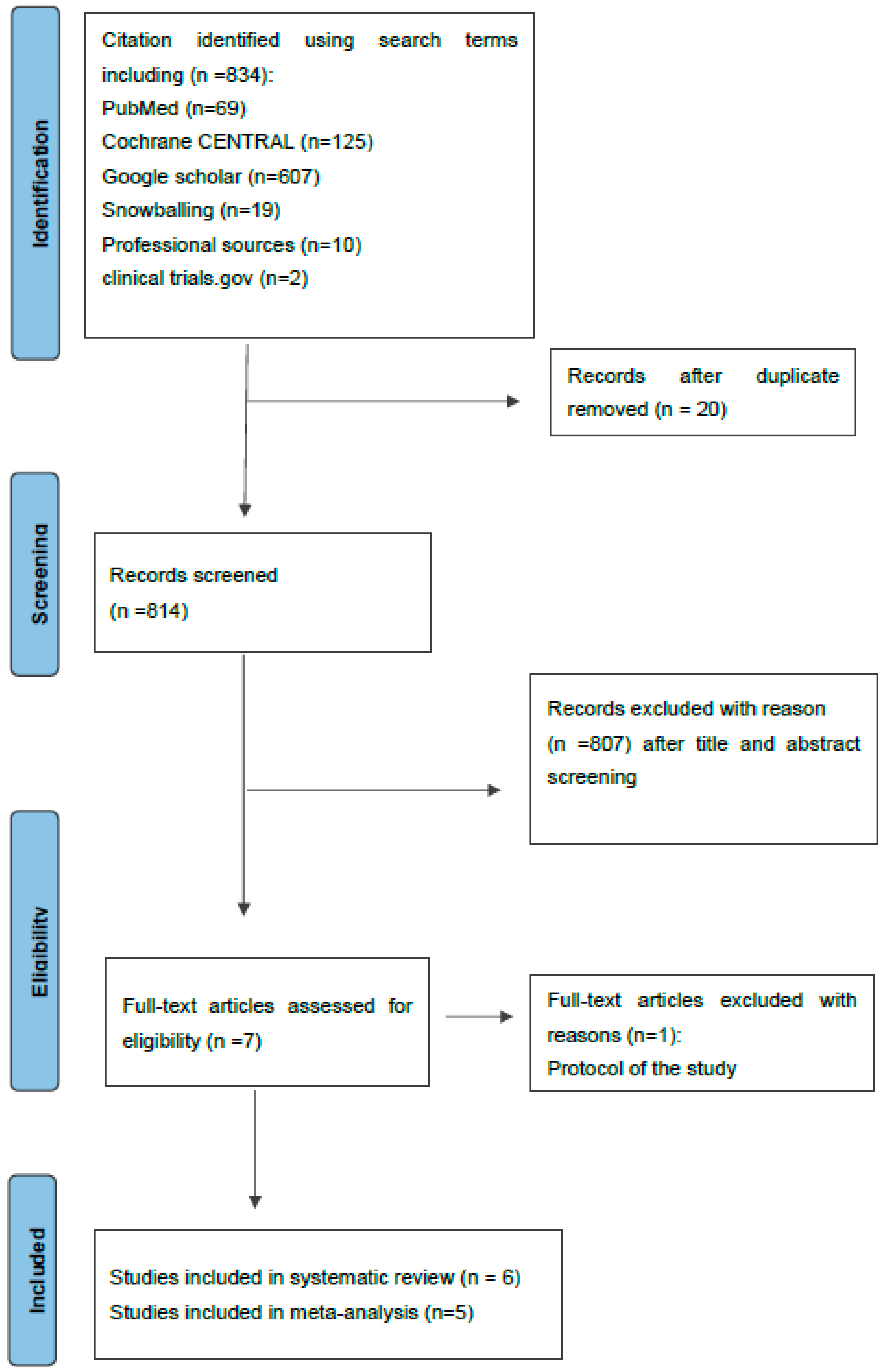
3.1. Study Characteristics and Search Results
3.2. Quality of the Individual Studies
3.3. Serum IL- 33 and Relation with Disease Severity or PASI Score
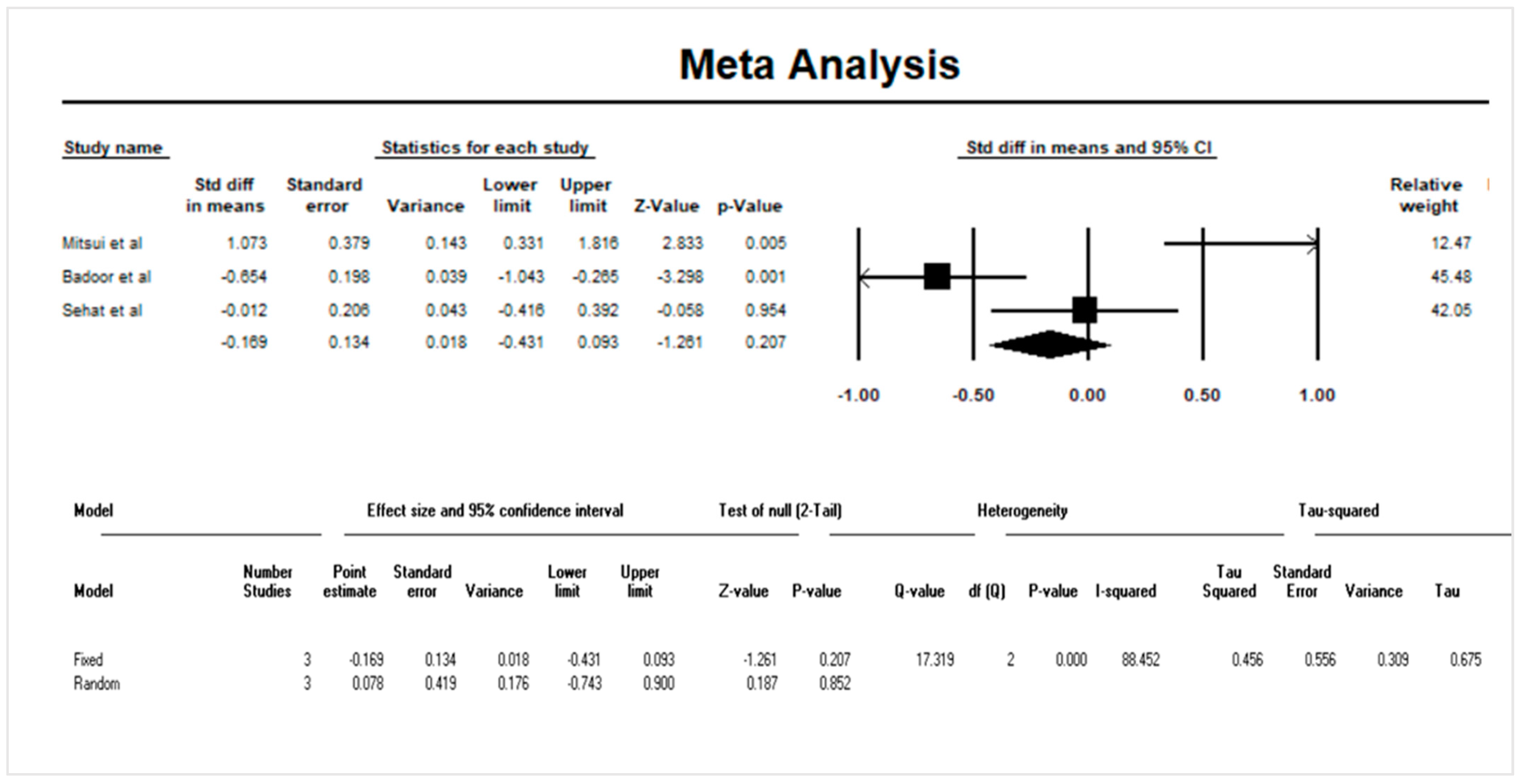
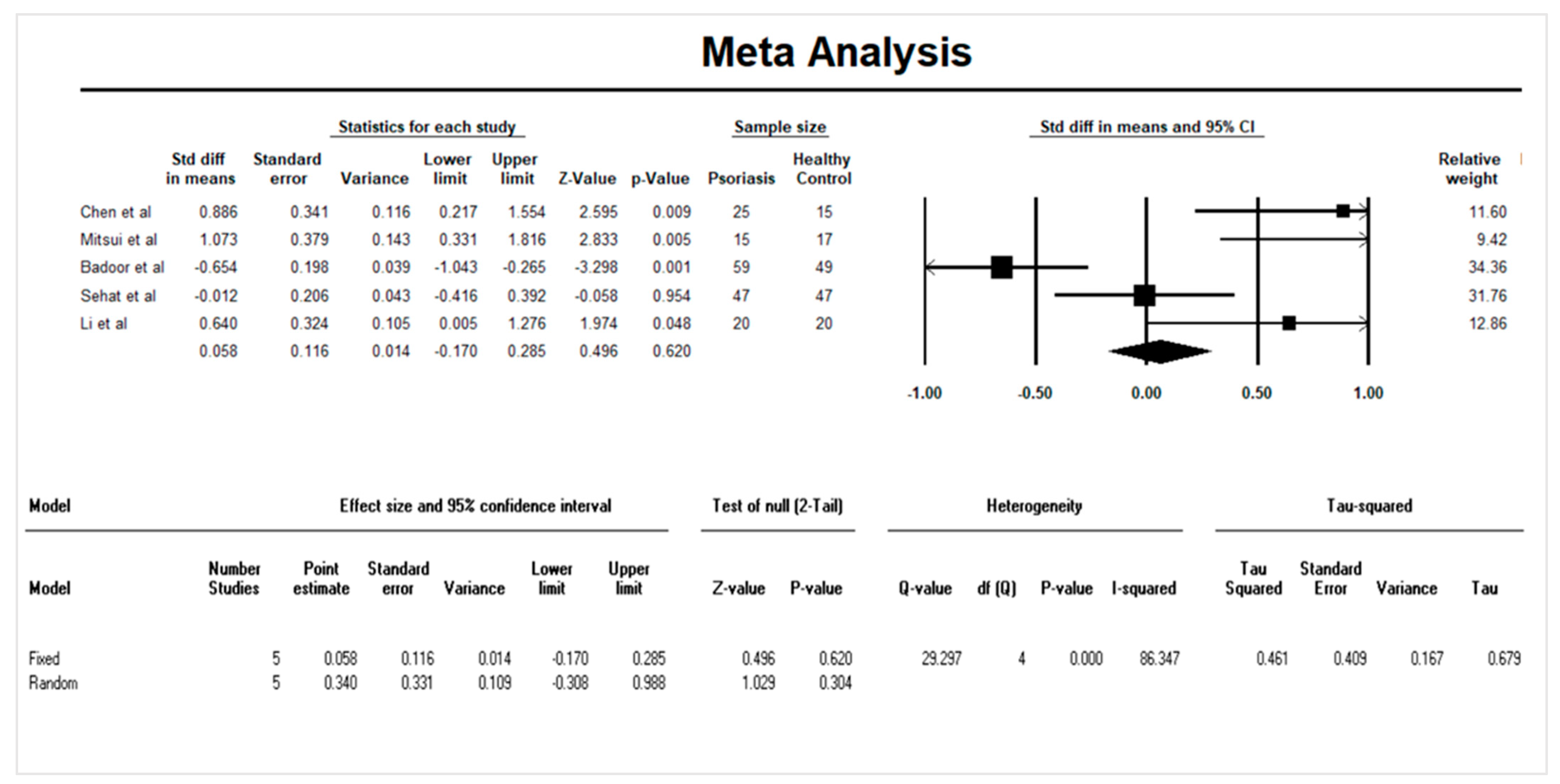
3.4. Quantitative Data Synthesis: Serum IL-33 and Psoriasis
3.5. Sensitivity Analysis
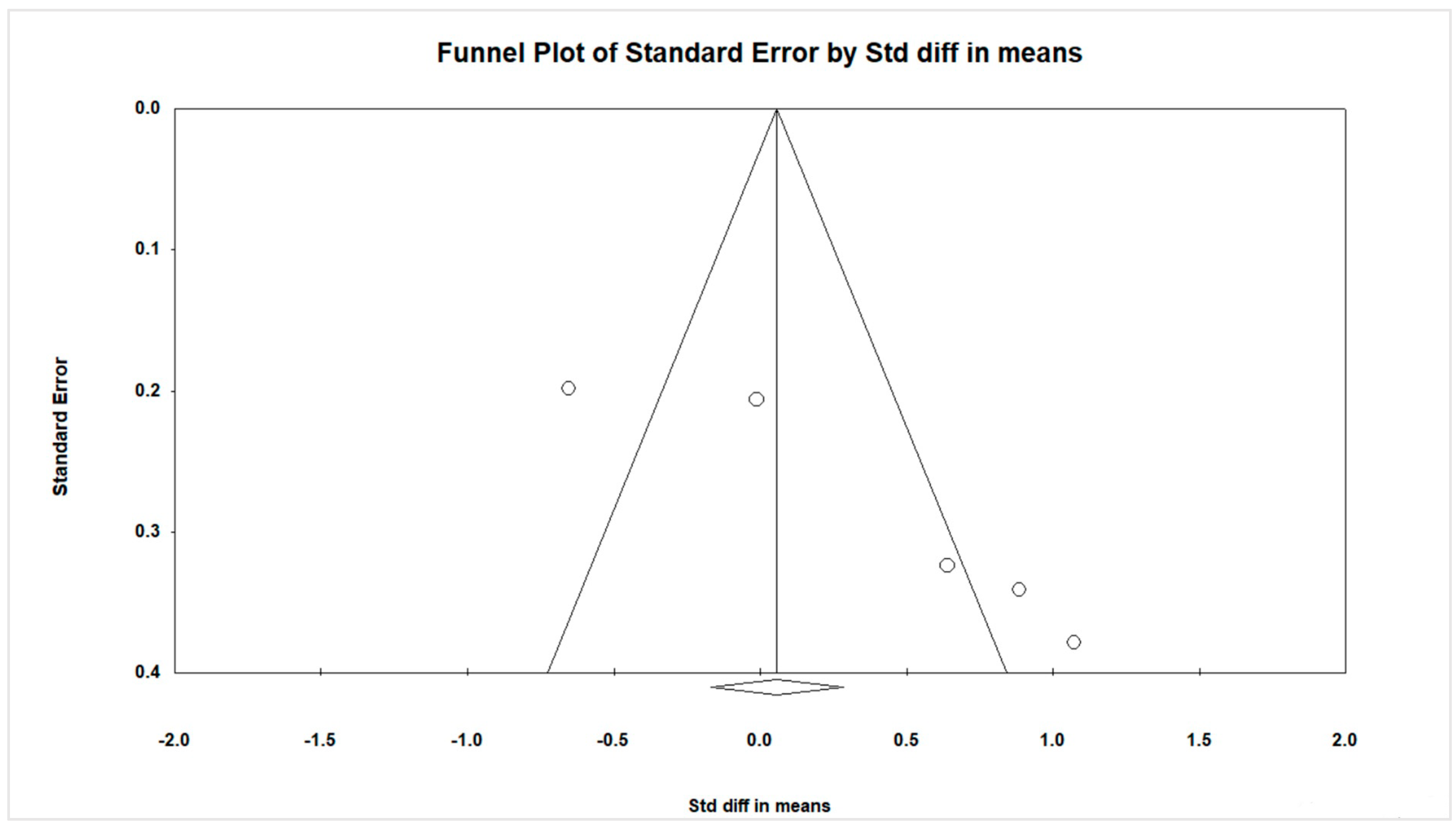
3.6. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Quality Assessment | Yes (n,%) | Quality | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | ||
| Borsky et al [23] | Yes | Yes | Not reported | Yes | Not reported | Yes | Yes | No | Yes | No | No | Not reported | Not reported | Not reported | 6.43% | Low |
| Chen et al [15] | Yes | No | Not reported | Yes | Not reported | Yes | Yes | No | Yes | No | Yes | Not reported | Not reported | Not reported | 6.43% | Low |
| Mitsui et al [7] | Yes | Yes | Not reported | Yes | Not reported | Yes | Yes | No | Yes | No | Yes | Not reported | Not reported | Not reported | 7.50% | Moderate |
| Badoor et al [8] | Yes | No | Not reported | Yes | Not reported | Yes | Yes | No | Yes | No | Yes | Not reported | Not reported | Yes | 7.50% | Moderate |
| Sehat et al [9] | Yes | No | Not reported | Yes | Not reported | Yes | Yes | Yes | Yes | No | Yes | Not reported | Not reported | Yes | 8.57% | Moderate |
| Li et al [11] | Yes | No | Not reported | Yes | Not reported | Yes | Yes | No | Yes | No | Yes | Not reported | Not reported | Not reported | 6.43% | Low |
| Q1. Was the research question or objective in this paper clearly stated? | ||||||||||||||||
| Q2. Was the study population clearly specified and defined? | ||||||||||||||||
| Q3. Was the participation rate of eligible persons at least 50%? Were all the subjects selected or recruited from the same or similar populations (including the same time period)? | ||||||||||||||||
| Q4. Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | ||||||||||||||||
| Q5. Was a sample size justification, power description, or variance and effect estimates provided? | ||||||||||||||||
| Q6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | ||||||||||||||||
| Q7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | ||||||||||||||||
| Q8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | ||||||||||||||||
| Q9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | ||||||||||||||||
| Q10. Was the exposure(s) assessed more than once over time? | ||||||||||||||||
| Q11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | ||||||||||||||||
| Q12. Were the outcome assessors blinded to the exposure status of participants? | ||||||||||||||||
| Q13. Was loss to follow-up after baseline 20% or less? | ||||||||||||||||
| Q14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | ||||||||||||||||
References
- WHO. Global Report on Psoriasis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Naldi, L. Epidemiology of psoriasis. Curr. Drug Targets Inflamm. Allergy 2004, 3, 121–128. [Google Scholar] [CrossRef]
- Danielsen, K.; Olsen, A.; Wilsgaard, T.; Furberg, A.S. Is the prevalence of psoriasis increasing? A 30-year follow-up of a population-based cohort. Br. J. Dermatol. 2013, 168, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Michalek, I.; Loring, B.; John, S. A systematic review of worldwide epidemiology of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Abuabara, K.; Azfar, R.S.; Shin, D.B.; Neimann, A.L.; Troxel, A.B.; Gelfand, J.M. Cause-specific mortality in patients with severe psoriasis: A population-based cohort study in the U.K. Br. J. Dermatol. 2010, 163, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, A.; Tada, Y.; Takahashi, T.; Shibata, S.; Kamata, M.; Miyagaki, T.; Fujita, H.; Sugaya, M.; Kadono, T.; Sato, S. Serum IL-33 levels are increased in patients with psoriasis. Clin. Exp. Dermatol. 2016, 41, 183–189. [Google Scholar] [CrossRef]
- Bodoor, K.; Al-Qarqaz, F.; al Heis, L.; Alfaqih, M.A.; Oweis, A.O.; Almomani, R.; Obeidat, M.A. IL-33/13 axis and IL-4/31 axis play distinct roles in inflammatory process and itch in psoriasis and atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2020, 13, 419. [Google Scholar] [CrossRef]
- Sehat, M.; Talaei, R.; Dadgostar, E.; Nikoueinejad, H.; Akbari, H. Evaluating serum levels of IL-33, IL-36, IL-37 and gene expression of IL-37 in patients with psoriasis vulgaris. Iran. J. Allergy Asthma Immunol. 2018, 17, 179–187. [Google Scholar]
- Pietrzak, A.T.; Zalewska, A.; Chodorowska, G.; Krasowska, D.; Michalak-Stoma, A.; Nockowski, P.; Osemlak, P.; Paszkowski, T.; Roliński, J.M. Cytokines and anticytokines in psoriasis. Clin. Chim. Acta 2008, 394, 7–21. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Rui, W.; Li, X.; Xuan, D.; Zheng, S.; Yu, Y.; Zhang, J.; Kong, N.; Zhu, X. New interleukins in psoriasis and psoriatic arthritis patients: The possible roles of interleukin-33 to interleukin-38 in disease activities and bone erosions. Dermatology 2017, 233, 37–46. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Hueber, A.J.; Alves-Filho, J.C.; Asquith, D.L.; Michels, C.; Millar, N.L.; Reilly, J.H.; Graham, G.J.; Liew, F.Y.; Miller, A.M.; McInnes, I.B. IL-33 induces skin inflammation with mast cell and neutrophil activation. Eur. J. Immunol. 2011, 41, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Zhang, B.; Kempuraj, D.; Tagen, M.; Vasiadi, M.; Angelidou, A.; Alysandratos, K.-D.; Kalogeromitros, D.; Asadi, S.; Stavrianeas, N. IL-33 augments substance P–induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc. Natl. Acad. Sci. USA 2010, 107, 4448–4453. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, Y.; Gong, Y.; Zhang, X.; Cui, L.; Chen, R.; Yu, Y.; Yu, Q.; Chen, Y.; Diao, H. Interleukin-33 alleviates psoriatic inflammation by suppressing the T helper type 17 immune response. Immunology 2020, 160, 382–392. [Google Scholar] [CrossRef]
- Meephansan, J.; Subpayasarn, U.; Ponnikorn, S.; Chakkavittumrong, P.; Juntongjin, P.; Komine, M.; Ohtsuki, M.; Poovorawan, Y. Methotrexate, but not narrowband ultraviolet B radiation, suppresses interleukin-33 mRNA levels in psoriatic plaques and protein levels in serum of patients with psoriasis. J. Dermatol. 2018, 45, 322–325. [Google Scholar] [CrossRef]
- Suttle, M.-M.; Enoksson, M.; Zoltowska, A.; Chatterjee, M.; Nilsson, G.; Harvima, I.T. Experimentally induced psoriatic lesions associate with rapid but transient decrease in interleukin-33 immunostaining in epidermis. Acta Derm. -Venereol. 2015, 95, 536–541. [Google Scholar] [CrossRef]
- Tamagawa-Mineoka, R.; Okuzawa, Y.; Masuda, K.; Katoh, N. Increased serum levels of interleukin 33 in patients with atopic dermatitis. J. Am. Acad. Dermatol. 2014, 70, 882–888. [Google Scholar] [CrossRef]
- Cannavò, S.P.; Bertino, L.; di Salvo, E.; Papaianni, V.; Ventura-Spagnolo, E.; Gangemi, S. Possible roles of IL-33 in the innate-adaptive immune crosstalk of psoriasis pathogenesis. Mediat. Inflamm. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Sapojnikova, N.; Kartvelishvili, T.; Asatiani, N.; Zinkevich, V.; Kalandadze, I.; Gugutsidze, D.; Shakarishvili, R.; Tsiskaridze, A. Correlation between MMP-9 and extracellular cytokine HMGB1 in prediction of human ischemic stroke outcome. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 1379–1384. [Google Scholar] [CrossRef]
- NIH. Study Quality Assessment Tools. 2013. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 4 October 2021).
- Borenstein, M. Comprehensive Meta-Analysis Software. 2021. Available online: https://www.meta-analysis.com/ (accessed on 4 October 2021).
- Borsky, P.; Fiala, Z.; Andrys, C.; Beranek, M.; Hamakova, K.; Malkova, A.; Svadlakova, T.; Krejsek, J.; Palicka, V.; Borska, L. Alarmins HMGB1, IL-33, S100A7, and S100A12 in psoriasis vulgaris. Mediat. Inflamm. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Ighani, A.; Partridge, A.C.; Shear, N.H.; Lynde, C.; Gulliver, W.P.; Sibbald, C.; Fleming, P. Comparison of management guidelines for moderate-to-severe plaque psoriasis: A review of phototherapy, systemic therapies, and biologic agents. J. Cutan. Med. Surg. 2019, 23, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Balato, A.; di Caprio, R.; Canta, L.; Mattii, M.; Lembo, S.; Raimondo, A.; Schiattarella, M.; Balato, N.; Ayala, F. IL-33 is regulated by TNF-α in normal and psoriatic skin. Arch. Dermatol. Res. 2014, 306, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Vageli, D.P.; Exarchou, A.; Zafiriou, E.; Doukas, P.G.; Doukas, S.; Roussaki-Schulze, A. Effect of TNF-α inhibitors on transcriptional levels of pro-inflammatory interleukin-33 and Toll-like receptors-2 and-9 in psoriatic plaques. Exp. Ther. Med. 2015, 10, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
| IL-33 Elevation | IL-33 Suppression | Constant IL-33 |
|---|---|---|
| Biliary atresia | HT of AIS | Heart failure |
| CKD | Poststroke depression | CKD |
| Rheumatoid arthritis | Long-term outcome after stroke | Stable angina pectoris, NSTEMI, STEMI |
| Rheumatoid arthritis | Intracerebral hemorrhage | NSTEMI |
| Psoriasis vulgaris | HF-REF | |
| Prostate cancer | Behçet’s disease | |
| Gastric cancer | Critically ill subjects | |
| Endometrial cancer | Osteoporosis | |
| Non–small cell lung cancer | Amyotrophic lateral sclerosis | |
| Breast cancer | Increased body weight | |
| Sepsis in infants |
| Study Name, Year of Publication and Location | Type of Study | Psoriatic Group | Control Group | Outcome Measure | |||
|---|---|---|---|---|---|---|---|
| IL-33 Level (pg/mL) | PASIx SCORE | Sample Size (n) | IL-33 Level (pg/mL) | Sample Size (n) | Methods Used for Measuring Serum IL-33 | ||
| Borsky 2020 [23] Czech Republic | Observational cohort study | 4.890 (median) IQR * (2.94–7.96) | Median 17.4 | Total: 63 Female: 47.62% Male: 52.38% | 3.11 (median) IQR * (2.16–5.20) | Total: 95 Female: 47.4% Male: 52.6% | ELISA + |
| Chen 2020 [15] China | Observational cohort study | 0.35 (mean) | Not reported | 25 | 0.15 (mean) | 15 | ELISA |
| Mitsui 2014 [7] Japan | Observational cohort study | Mean 586 pg/mL (234–3900) | Measured but not reported | Total: 15 Female: 6.7% Male: 93.3% | Mean 87.7 pg/mL (60–197) | Total: 17 Female: 41% Male: 59% | ELISA |
| Bodoor 2020 [8] Jordan | Observational cohort study | Mean 29.55 pg/mL | Measured but not reported | 59 | Mean 44.88 pg/mL | 49 | ELISA |
| Sehat 2018 [9] Iran | Observational cohort study | Mean 19.21 ± 9.43 pg/mL | Mild (<11): 38 (80.9%) Moderate (11–19): 4 (8.5%) Severe (>19): 5 (10.6%) | 47 | Mean 19.30 ± 6.58 | 47 | ELISA |
| Li 2017 [11] China | Observational cohort study | 95 (mean) | Median: 3 Range (0.3–7.2) | Total: 20 Female:30% Male: 70% | 0 (mean) | Total: 20 Female: 35% Male: 65% | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kc, K.; Hu, H.; Mahatara, T.; Koirala, S.; Shrestha, S.; Sharma, S.K.; Song, X.; Tian, Z. The Correlation between Interleukin 33 and Psoriasis: A Systematic Review and Meta-Analysis. Dermato 2023, 3, 13-24. https://doi.org/10.3390/dermato3010002
Kc K, Hu H, Mahatara T, Koirala S, Shrestha S, Sharma SK, Song X, Tian Z. The Correlation between Interleukin 33 and Psoriasis: A Systematic Review and Meta-Analysis. Dermato. 2023; 3(1):13-24. https://doi.org/10.3390/dermato3010002
Chicago/Turabian StyleKc, Keshav, Hua Hu, Tilak Mahatara, Sunil Koirala, Samjhana Shrestha, Shiv K. Sharma, Xiangfeng Song, and Zhongwei Tian. 2023. "The Correlation between Interleukin 33 and Psoriasis: A Systematic Review and Meta-Analysis" Dermato 3, no. 1: 13-24. https://doi.org/10.3390/dermato3010002
APA StyleKc, K., Hu, H., Mahatara, T., Koirala, S., Shrestha, S., Sharma, S. K., Song, X., & Tian, Z. (2023). The Correlation between Interleukin 33 and Psoriasis: A Systematic Review and Meta-Analysis. Dermato, 3(1), 13-24. https://doi.org/10.3390/dermato3010002