Reintroduction of Indian Grey Hornbills in Gir, India: Insights into Ranging, Habitat Use, Nesting and Behavioural Patterns
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
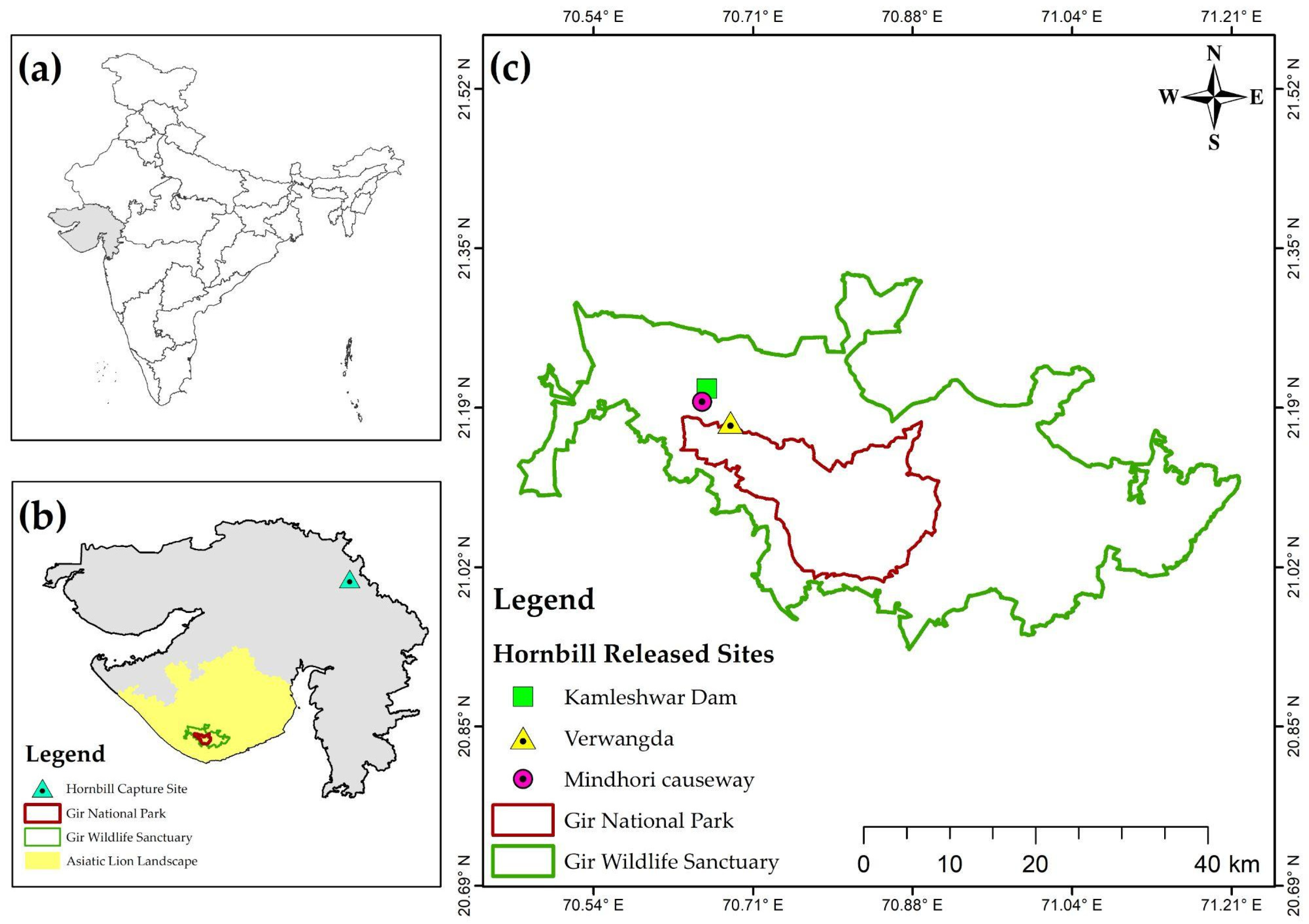
2.1. Study Area
2.2. Study Species
2.3. Capture and Release
2.4. Deployment of Transmitters and Colour Rings
2.5. Home Range Estimation
2.6. Movement Patterns
2.7. Habitat Selection
2.8. Breeding Behaviour
2.9. Statistical Analysis
3. Results
3.1. Home Ranges
3.2. Movement Patterns
3.3. Nest Site Selection
3.4. Behavioural Activities
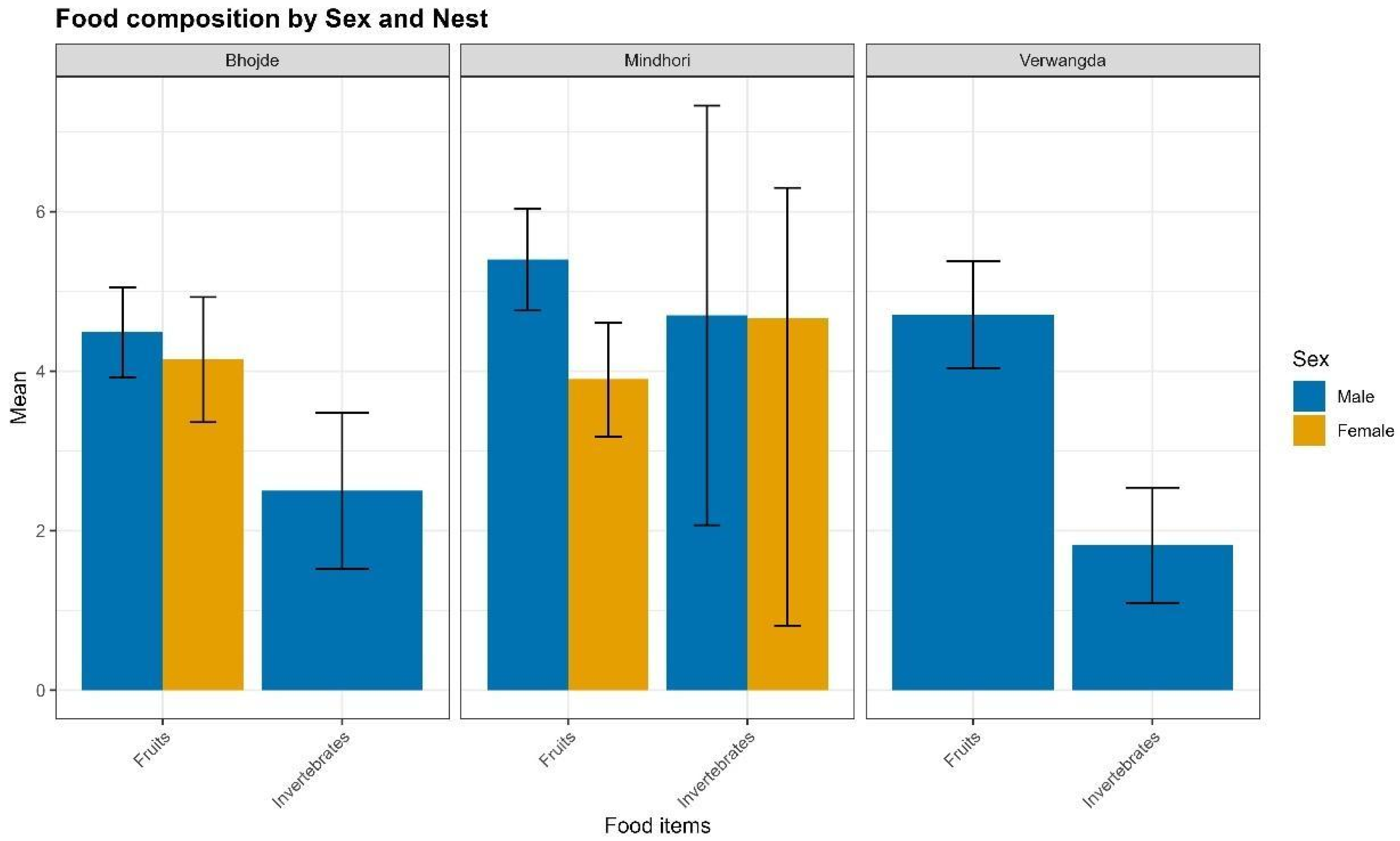
3.5. Feeding Behaviour
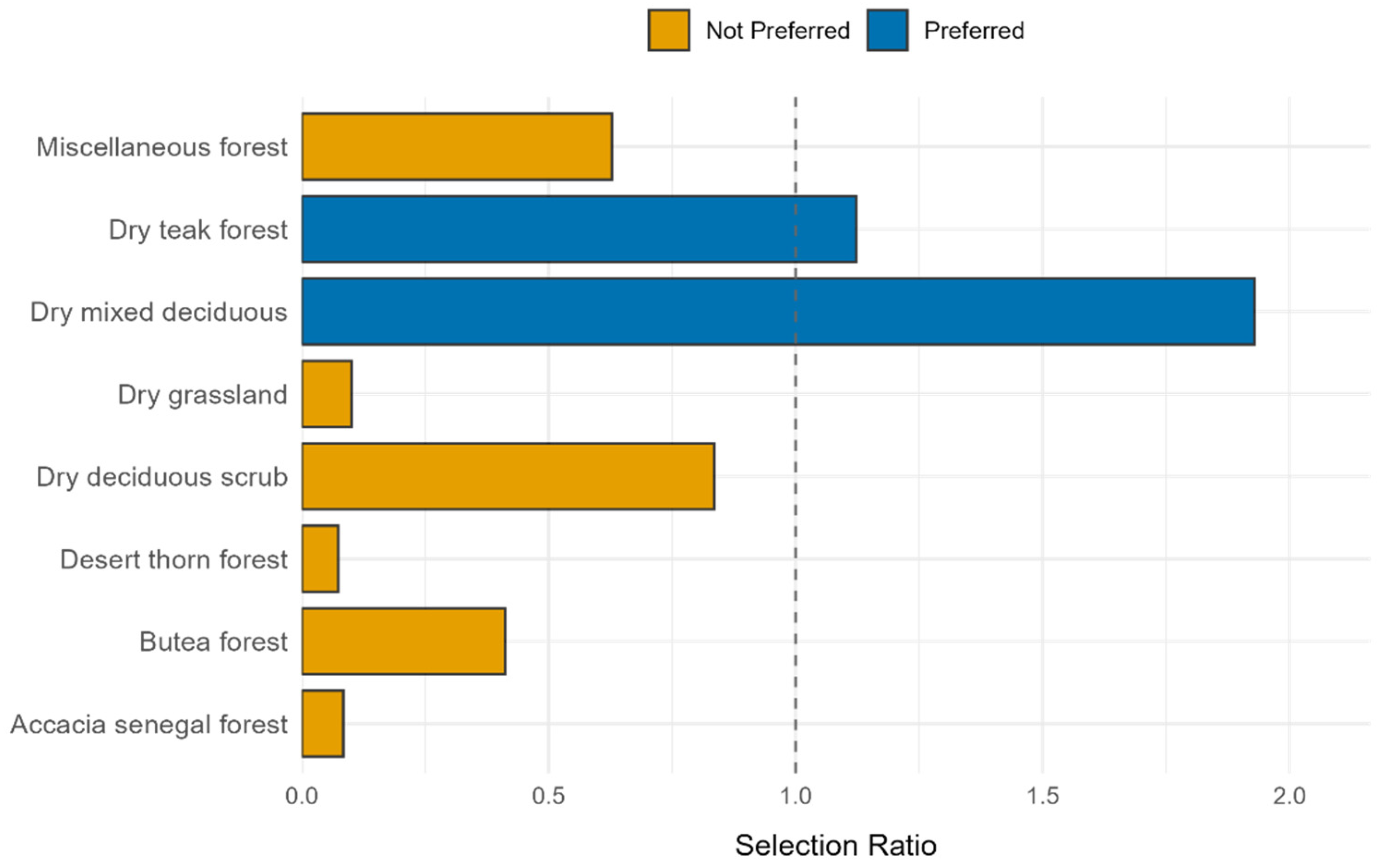
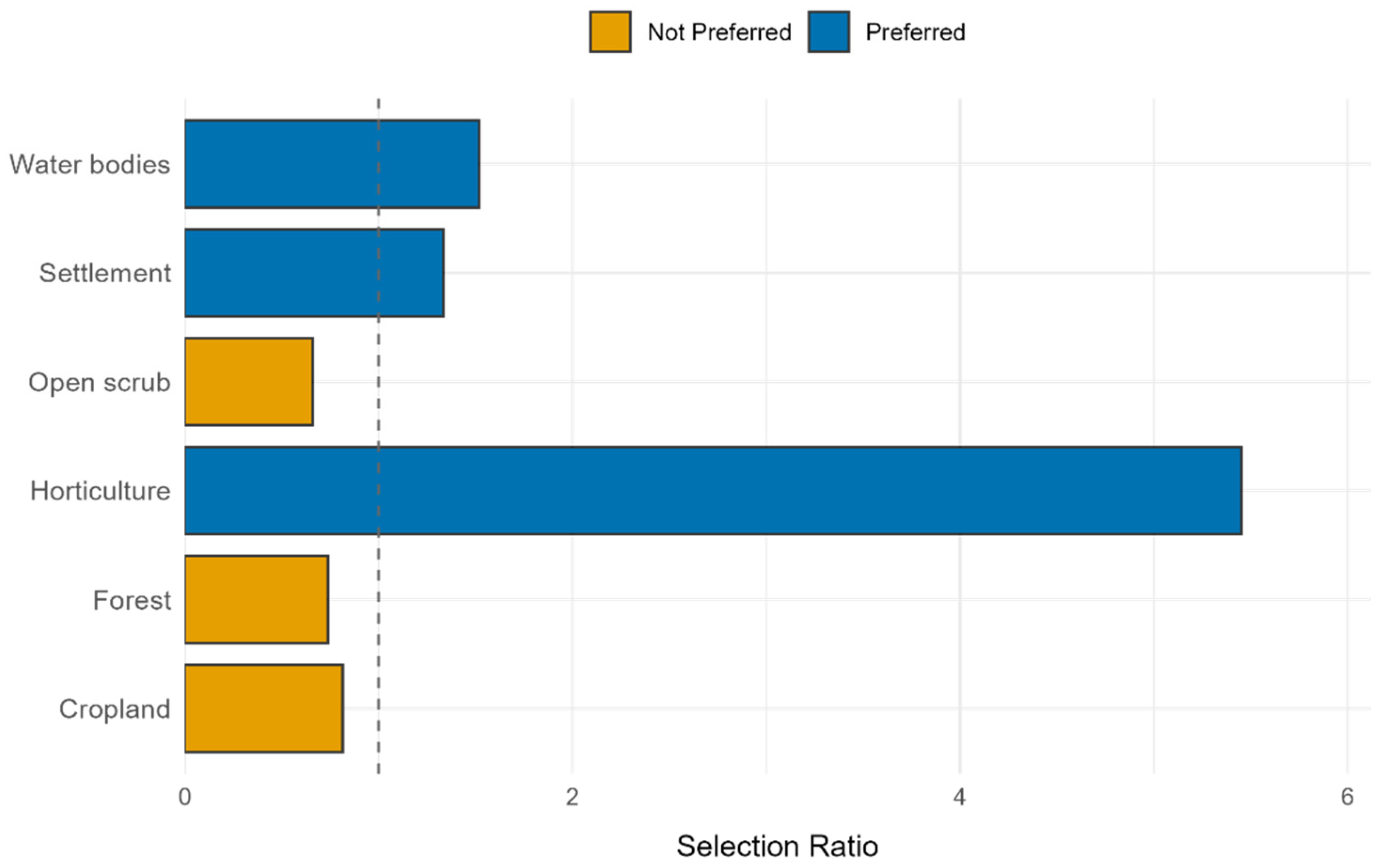
3.6. Habitat Selection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, M.J.; Gordon, I.J.; Pierson, J.C.; Neaves, L.E.; Wilson, B.A.; Brockett, B.; Ross, C.E.; Smith, K.J.; Rapley, S.; Andrewartha, T.A.; et al. Reintroduction Biology and the IUCN Red List: The Dominance of Species of Least Concern in the Peer-Reviewed Literature. Glob. Ecol. Conserv. 2022, 38, e02242. [Google Scholar] [CrossRef]
- Albert, C.; Luque, G.M.; Courchamp, F. The Twenty Most Charismatic Species. PLoS ONE 2018, 13, e0199149. [Google Scholar] [CrossRef] [PubMed]
- Merta, D.; Kobielski, J.; Theuerkauf, J.; Gula, R. Towards a Successful Reintroduction of Capercaillies—Activity, Movements and Diet of Young Released to the Lower Silesia Forest, Poland. Wildl. Biol. 2016, 22, 130–135. [Google Scholar] [CrossRef]
- Burnside, R.J.; Carter, I.; Dawes, A.; Waters, D.; Lock, L.; Goriup, P.; Székely, T. The UK Great Bustard Otis tarda Reintroduction Trial: A 5-Year Progress Report. Oryx 2012, 46, 112–121. [Google Scholar] [CrossRef]
- Vernon, C.J.; Dean, W.R.J. The Introduction of the Chukar Partridge Alectoris chukar to Southern Africa. Ostrich 2022, 93, 157–159. [Google Scholar] [CrossRef]
- Chiatante, G.; Meriggi, A. Habitat Selection and Density of Common Pheasant (Phasianus colchicus) in Northern Italy: Effects of Land Use Cover and Landscape Configuration. Eur. J. Wildl. Res. 2022, 68, 26. [Google Scholar] [CrossRef]
- Ashrafzadeh, M.R.; Khosravi, R.; Fernandes, C.; Aguayo, C.; Bagi, Z.; Lavadinović, V.M.; Szendrei, L.; Beuković, D.; Mihalik, B.; Kusza, S. Assessing the Origin, Genetic Structure and Demographic History of the Common Pheasant (Phasianus colchicus) in the Introduced European Range. Sci. Rep. 2021, 11, 21721. [Google Scholar] [CrossRef]
- Baratti, M.; Ammannati, M.; Magnelli, C.; Dessì-Fulgheri, F. Introgression of Chukar Genes into a Reintroduced Red-Legged Partridge (Alectoris rufa) Population in Central Italy. Anim. Genet. 2005, 36, 29–35. [Google Scholar] [CrossRef]
- Chaiyarat, R.; Kongprom, U.; Manathamkamon, D.; Wanpradab, S.; Sangarang, S. Captive Breeding and Reintroduction of the Oriental Pied Hornbill (Anthracoceros albirostris) in Khao Kheow Open Zoo, Thailand. Zoo Biol. 2012, 31, 683–693. [Google Scholar] [CrossRef]
- Kemp, L.V.; Kotze, A.; Jansen, R.; Dalton, D.L.; Grobler, P.; Little, R.M. Review of Trial Reintroductions of the Long-Lived, Cooperative Breeding Southern Ground-Hornbill. Bird Conserv. Int. 2020, 30, 533–558. [Google Scholar] [CrossRef]
- Seddon, P.J.; Soorae, P.S.; Launay, F. Taxonomic Bias in Reintroduction Projects. Anim. Conserv. 2005, 8, 51–58. [Google Scholar] [CrossRef]
- Destro, G.F.G.; De Marco, P.; Terribile, L.C. Threats for Bird Population Restoration: A Systematic Review. Perspect. Ecol. Conserv. 2018, 16, 68–73. [Google Scholar] [CrossRef]
- Kleiman, D.G. Reintroduction of Captive Mammals for Conservation: Guidelines for Reintroducing Endangered Species into the Wild. BioScience 1989, 39, 152–161. [Google Scholar] [CrossRef]
- IUCN. The IUCN Position Statement on Translocation of Living Organisms: Introductions, Re-Introductions and Re-Stocking; IUCN: Gland, Switzerland, 1987. [Google Scholar]
- Lyles, A.M.; May, R.M. Problems in Leaving the Ark. Nature 1987, 326, 245–246. [Google Scholar] [CrossRef]
- Magdalena Wolf, C.; Garland, T.; Griffith, B. Predictors of Avian and Mammalian Translocation Success: Reanalysis with Phylogenetically Independent Contrasts. Biol. Conserv. 1998, 86, 243–255. [Google Scholar] [CrossRef]
- Seddon, P.J.; Armstrong, D.P.; Maloney, R.F. Developing the Science of Reintroduction Biology. Conserv. Biol. 2007, 21, 303–312. [Google Scholar] [CrossRef]
- Armstrong, D.P.; Seddon, P.J. Directions in Reintroduction Biology. Trends Ecol. Evol. 2008, 23, 20–25. [Google Scholar] [CrossRef]
- Bernardo, C.S.S.; Lloyd, H.; Olmos, F.; Cancian, L.F.; Galetti, M. Using Post-Release Monitoring Data to Optimize Avian Reintroduction Programs: A 2-Year Case Study from the Brazilian Atlantic Rainforest. Anim. Conserv. 2011, 14, 676–686. [Google Scholar] [CrossRef]
- Kuwabara, R.; Ohsako, Y.; Funakoshi, M.; Deguchi, T. Which Combination of Release Techniques and Ages Minimizes Post-Release Dispersal during Oriental Stork Reintroduction? J. Field Ornithol. 2024, 95, 6. [Google Scholar] [CrossRef]
- Armstrong, D.P.; Castro, I.; Alley, J.C.; Feenstra, B.; Perrott, J.K. Mortality and Behaviour of Hihi, an Endangered New Zealand Honeyeater, in the Establishment Phase Following Translocation. Biol. Conserv. 1999, 89, 329–339. [Google Scholar] [CrossRef]
- Martinez, L.A.; Lombardi, J.V.; Parker, I.D.; East, F.; Campbell, T.A.; Lopez, R.R. Long Live the Cat: Ocelot Population Viability in a Planned Reintroduced Population in Texas, USA. Ecosphere 2024, 15, e70044. [Google Scholar] [CrossRef]
- Smetzer, J.R.; Greggor, A.L.; Paxton, K.L.; Masuda, B.; Paxton, E.H. Automated Telemetry Reveals Post-Reintroduction Exploratory Behavior and Movement Patterns of an Endangered Corvid, ‘Alalā (Corvus hawaiiensis) in Hawai’i, USA. Glob. Ecol. Conserv. 2021, 26, e01522. [Google Scholar] [CrossRef]
- Armstong, D.P.; McLean, I.G. New Zealand Translocations: Theory and Practice. Pac. Conserv. Biol. 1995, 2, 39–54. [Google Scholar] [CrossRef]
- Moseby, K.E.; Hill, B.M.; Lavery, T.H. Tailoring Release Protocols to Individual Species and Sites: One Size Does Not Fit All. PLoS ONE 2014, 9, e99753. [Google Scholar] [CrossRef] [PubMed]
- King, A.C.; Reed, J.M. Successful Population Establishment from Small Introductions Appears to Be Less Common than Believed. PeerJ 2016, 4, e2440. [Google Scholar] [CrossRef] [PubMed]
- Meretsky, V.; Snyder, N.F.R.; Beissinger, S.R.; Clendenen, D.A.; Wiley, J.W. Quantity versus Quality in California Condor Reintroduction: Reply to Beres and Starfield. Conserv. Biol. 2001, 15, 1449–1451. [Google Scholar] [CrossRef]
- BirdLife International IUCN. Red List of Threatened Species: Ocyceros birostris. IUCN Red List Threathened Species. 2020. Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-3.RLTS.T22682429A184951065.en (accessed on 12 March 2025).
- SoIB. State of India’s Birds, 2023: Range, Trends, and Conservation Status; The SoIB Partnership: New Delhi, India, 2023; Available online: https://stateofindiasbirds.in/ (accessed on 19 August 2025).
- Ali, S.; Ripley, S.D.; Society, B.N.H. Handbook of the Birds of India and Pakistan: Together with Those of Bangladesh, Nepal, Bhutan, and Sri Lanka, Compact ed.; Oxford University Press: Oxford, UK, 1983. [Google Scholar]
- Dharmakumarsinhji, R.S. Birds of Saurashtra; Times of India Press: Bombay, India, 1955. [Google Scholar]
- Dharmakumarsinhji, K.S. Wild Life Preservation in India. Annual Report for 1953 on the Western Region. J. Bombay Nat. Hist. Soc. 1954, 52, 865–873. [Google Scholar]
- Dharmakumarsinhji, K.S. The Changing Wildlife of Kathiawar. J. Bombay Nat. Hist. Soc. 1978, 75, 632–650. [Google Scholar]
- Gurubh, R. The Common Grey Hornbill in the Gir Forest. Newsl. Birdwatch. 1971, 11, 6–7. [Google Scholar]
- Khacher, L. The Birds of Gujarat—A Salim Ali Centenary Year Overview. J. Bombay Nat. Hist. Soc. 1996, 93, 331–373. [Google Scholar]
- Divyabhanusinh, C. The Story of Asia’s Lions; Marg Publication: Mumbai, India, 2005. [Google Scholar]
- Rahmani, A.R.; Islam, M.Z.; Kasambe, R.M. Important Bird and Biodiversity Areas in India: Priority Sites for Conservation (Revised and Updated); Bombay Natural History Society, Indian Bird Conservation Network, Royal Society for the Protection of Birds and BirdLife International (U.K.): Mumbai, India, 2016; pp. xii–1992. [Google Scholar]
- Ganpule, P. The Birds of Gujarat: Status and Distribution. Flamingo 2016, 8–12, 2–40. [Google Scholar]
- Jadeja, B.; Nakar, R. Phenological Studies of Some Tree Species from Girnar Reserve Forest, Gujarat India. Plant Arch. 2010, 10, 825–882. [Google Scholar]
- IUCN. Guidelines for Reintroductions and Other Conservation Translocations; IUCN: Gland, Switzerland, 2013; ISBN 978-2-8317-1609-1. [Google Scholar]
- Rashid, M.A. Wildlife and Its Conservation in Gujarat State. Cheetal 1979, 21, 36–42. [Google Scholar]
- Vasavada, D.T.; Rana, V.J.; Mohan, R. Management Plan for Gir Protected Areas; Wildlife Division, Gujarat Forest Department: Gandhinagar, India, 2022; Volume 1.
- Kitamura, S. Frugivory and Seed Dispersal by Hornbills (Bucerotidae) in Tropical Forests. Acta Oecol. 2011, 37, 531–541. [Google Scholar] [CrossRef]
- Naniwadekar, R.; Rathore, A.; Shukla, U.; Chaplod, S.; Datta, A. How Far Do Asian Forest Hornbills Disperse Seeds? Acta Oecol. 2019, 101, 103482. [Google Scholar] [CrossRef]
- Yu, X.; Li, X.; Huo, Z. Breeding Ecology and Success of a Reintroduced Population of the Endangered Crested Ibis Nipponia nippon. Bird Conserv. Int. 2015, 25, 207–219. [Google Scholar] [CrossRef]
- Rodgers, W.A.; Panwar, H.S. Planning a Wildlife Protected Area Network in India; Wildlife Institute of India: Dehradun, India, 1988. [Google Scholar]
- India Meteorological Department Met Centre Ahmedabad Current Weather and Climate Data. Available online: https://mausam.imd.gov.in/ahmedabad/ (accessed on 29 September 2025).
- Champion, H.G.; Seth, S.K. A Revised Survey of Forest Types of India; Government of India Press: New Delhi, India, 1968. [Google Scholar]
- Khan, J.A.; Chellam, R.; Rodgers, W.A.; Johnsingh, A.J.T. Ungulate Densities and Biomass in the Tropical Dry Deciduous Forests of Gir, Gujarat, India. J. Trop. Ecol. 1996, 12, 149–162. [Google Scholar] [CrossRef]
- Sridhar, S. Need for Designing and Fixing Artificial Nest Boxes to Conserve the Hornbills. Bird Behav. 2021, 61, 53. [Google Scholar]
- Kemp, A.C.; Woodcock, M. The Hornbills: Bucerotiformes; Bird Families of the World; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Patil, N.; Chaturvedi, N.; Hegde, V. Food of Common Grey Hornbill Tockus birostris (Scopoli). J. Bombay Nat. Hist. Soc. 1997, 94, 408–410. [Google Scholar]
- Kasambe, R. Indian Grey Hornbill: Study of the Ecology and Breeding Behaviour of Indian Grey Hornbill Ocyceros birostris in Central India; Lambert Academic Publishing: Saarbrücken, Germany, 2011. [Google Scholar]
- Kemp, A.C. A Review of the Hornbills: Biology and Radiation. Living Bird 1979, 17, 5–136. [Google Scholar]
- Balasubramanian, P.; Santhoshkumar, E.; Anbarasu, C. Vegetation Features and Restoration Initiatives in the Indian Grey Hornbill Habitats in Sathyamangalam Wildlife Sanctuary, Eastern Ghats, India. Raffles Bull Zool 2011, 24, 53–57. [Google Scholar]
- Poonswad, P.; Kemp, A.C.; Strange, M. Hornbills of the World: A Photographic Guide; Draco Publishing and Distribution Pte Limited: Singapore, 2013. [Google Scholar]
- Balasubramanian, P.; Vijayan, V.S.; Prasad, S.N.; Venkitachalam, R. Status and Distribution of the Hornbills in the Eastern Ghats. Project Report; Salim Ali Centre for Ornithology and Natural History: Coimbatore, India, 2005. [Google Scholar]
- Gadikar, A. Adaptations of the Indian Grey Hornbill Ocyceros birostris in an Urban Environment. Indian Birds 2017, 13, 167–168. [Google Scholar]
- Santhoshkumar, E.; Balasubramanian, P. Food Habits of Indian Grey Hornbill Ocyceros birostris in Sathyamangalam Forest Division, Eastern Ghats, India. J. Bombay Nat. Hist. Soc. (JBNHS) 2014, 111, 90–97. [Google Scholar] [CrossRef]
- Charde, P.; Kasambe, R.; Tarar, J. Breeding Behaviour of Indian Grey Hornbills in Central India. Raffles Bull. Zool. Suppl. 2011, 24, 59–64. [Google Scholar]
- Santhoshkumar, E.; Balasubramanian, P. Seed Dispersal by the Indian Grey Hornbill Ocyceros birostris in Eastern Ghats, India. Ecotropica 2011, 17, 71–77. [Google Scholar]
- Santhoshkumar, E.; Balasubramanian, P. Breeding Behaviour and Nest Tree Use by Indian Grey Hornbill Ocyceros birostris in the Eastern Ghats, India. Forktail 2010, 26, 82–85. [Google Scholar]
- Pittie, A. A Note on the Circumorbital Skin Colour of Indian Grey Hornbill Ocyceros birostris. J. Bombay Nat. Hist. Soc. 2003, 100, 141. [Google Scholar]
- Anggraini, K.; Kinnaird, M.; O’Brien, T. The Effects of Fruit Availability and Habitat Disturbance on an Assemblage of Sumatran Hornbills. Bird Conserv. Int. 2000, 10, 189–202. [Google Scholar] [CrossRef]
- Singh, H.S. Protected Areas and Natural Heritage Sites of Gujarat; Gujarat Biodiversity Board: Gandhinagar, India, 2014.
- Lee, S.C.R.; Hodgson, D.J.; Bearhop, S. What Has Biotelemetry Ever Done for Avian Translocations? Mov. Ecol. 2022, 10, 57. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B. An Assessment of the Published Results of Animal Relocations. Biol. Conserv. 2000, 96, 1–11. [Google Scholar] [CrossRef]
- Tavecchia, G.; Viedma, C.; Martínez-Abraín, A.; Bartolomé, M.-A.; Gómez, J.A.; Oro, D. Maximizing Re-Introduction Success: Assessing the Immediate Cost of Release in a Threatened Waterfowl. Biol. Conserv. 2009, 142, 3005–3012. [Google Scholar] [CrossRef]
- Donald, P.F. Adult Sex Ratios in Wild Bird Populations. Ibis 2007, 149, 671–692. [Google Scholar] [CrossRef]
- Sivakumar, K.; Jhala, Y.; Bhardwaj, G.; Mohan, A. Study on Ecology and Migration of Lesser Florican (Sypheotides indica) in Western India Using Satellite Techniques; Project Report, 80; Wildlife Institute of India: Uttarakhand, India, 2016. [Google Scholar]
- Ram, M.; Vasavada, D.; Tikadar, S.; Gadhavi, D.; Rather, T.A.; Jhala, L.; Zala, Y. Breeding and Non-Breeding Home Range, and Dispersal Patterns of The Critically Endangered Lesser Florican Sypheotides indicus (Miller, 1782). J. Bombay Nat. Hist. Soc. (JBNHS) 2022, 119, 3–10. [Google Scholar] [CrossRef]
- CLS Argos System. ARGOS Satellite Telemetry Data Portal. Available online: http://argos-system.cls.fr/argos-cwi2/login.html (accessed on 12 March 2023).
- Ornitela. GPS-GSM Wildlife Tracking Platform. Available online: https://cpanel.glosendas.net/ (accessed on 24 April 2023).
- Gurarie, E.; Andrews, R.D.; Laidre, K.L. A Novel Method for Identifying Behavioural Changes in Animal Movement Data. Ecol. Lett. 2009, 12, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Killick, R.; Eckley, I.A. Changepoint: An R Package for Changepoint Analysis. J. Stat. Softw. 2014, 58, 1–19. [Google Scholar] [CrossRef]
- Moehrenschlager, A.; Macdonald, D.W. Movement and Survival Parameters of Translocated and Resident Swift Foxes Vulpes velox. Anim. Conserv. 2003, 6, 199–206. [Google Scholar] [CrossRef]
- Datta, A. An Ecological Study of Sympatric Hornbills and Fruiting Patterns in a Tropical Forest in Arunachal Pradesh. Ph.D. Thesis, Saurashtra University, Rajkot, India, 2001. [Google Scholar]
- Manly, B.F.J.; McDonald, L.L.; Thomas, D.L.; McDonald, T.L.; Erickson, W.P. Resource Selection by Animals: Statistical Design and Analysis for Field Studies, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 46–82. ISBN 978-0-306-48151-2. [Google Scholar]
- Datta, A.; Rawat, G.S. Nest-Site Selection and Nesting Success of Three Hornbill Species in Arunachal Pradesh, North-East India: Great Hornbill Buceros bicornis, Wreathed Hornbill Aceros undulatus and Oriental Pied Hornbill Anthracoceros albirostris. Bird Conserv. Int. 2004, 14, S39–S52. [Google Scholar] [CrossRef]
- Calenge, C. The Package “Adehabitat” for the R Software: A Tool for the Analysis of Space and Habitat Use by Animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 4 December 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R Package Version 1.9. 0. 2023; R Foundation for Statistical Computing: Vienna, Austria, 2024. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 19 August 2025).
- Griffith, B.; Scott, J.M.; Carpenter, J.W.; Reed, C. Translocation as a Species Conservation Tool: Status and Strategy. Science 1989, 245, 477–480. [Google Scholar] [CrossRef]
- Brown, J.S.; Kotler, B.P. Hazardous Duty Pay and the Foraging Cost of Predation. Ecol. Lett. 2004, 7, 999–1014. [Google Scholar] [CrossRef]
- Schmitz, P.; Caspers, S.; Warren, P.; Witte, K. First Steps into the Wild-Exploration Behavior of European Bison after the First Reintroduction in Western Europe. PLoS ONE 2015, 10, e0143046. [Google Scholar] [CrossRef]
- Fryxell, J.M.; Hazell, M.; Börger, L.; Dalziel, B.D.; Haydon, D.T.; Morales, J.M.; McIntosh, T.; Rosatte, R.C. Multiple Movement Modes by Large Herbivores at Multiple Spatiotemporal Scales. Proc. Natl. Acad. Sci. USA 2008, 105, 19114–19119. [Google Scholar] [CrossRef]
- Mudappa, D.C.; Kannan, R. Nest-Site Characteristics and Nesting Success of the Malabar Gray Hornbill in the Southern Western Ghats, India. Wilson Bull. 1997, 109, 102–111. [Google Scholar]
- Taylor, G.; Canessa, S.; Clarke, R.H.; Ingwersen, D.; Armstrong, D.P.; Seddon, P.J.; Ewen, J.G. Is Reintroduction Biology an Effective Applied Science? Trends Ecol. Evol. 2017, 32, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Kenward, R.E. A Manual for Wildlife Radio Tagging; Academic Press: London, UK, 2000; ISBN 978-0-08-057420-2. [Google Scholar]
- Bridge, E.S.; Thorup, K.; Bowlin, M.S.; Chilson, P.B.; Diehl, R.H.; Fléron, R.W.; Hartl, P.; Kays, R.; Kelly, J.F.; Robinson, W.D.; et al. Technology on the Move: Recent and Forthcoming Innovations for Tracking Migratory Birds. BioScience 2011, 61, 689–698. [Google Scholar] [CrossRef]
- Hebblewhite, M.; Haydon, D.T. Distinguishing Technology from Biology: A Critical Review of the Use of GPS Telemetry Data in Ecology. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2303–2312. [Google Scholar] [CrossRef]
- Kays, R.; Crofoot, M.C.; Jetz, W.; Wikelski, M. Terrestrial Animal Tracking as an Eye on Life and Planet. Science 2015, 348, aaa2478. [Google Scholar] [CrossRef]

| Bird ID | Month | Core Area (50% KDE) Km2 | Overall Home Range (95% KDE) Km2 | Phase |
|---|---|---|---|---|
| IGHM2 | November-2021 | 5.99 | 70.12 | Initial |
| December-2021 | 6.10 | 59.93 | Initial | |
| January-2022 | 1.41 | 7.76 | Later | |
| February-2022 | 8.03 | 43.04 | Later | |
| March-2022 | 1.69 | 10.16 | Later | |
| April-2022 | 0.01 | 0.05 | Later | |
| May-2022 | 1.00 | 7.00 | Later | |
| June-2022 | 0.10 | 0.48 | Later | |
| IGHM3 | Feb–March 2021 | 0.11 | 1.11 | Initial |
| IGHM7 | January-2024 | 32.19 | 207.60 | Initial |
| February-2024 | 16.72 | 97.47 | Initial | |
| IGHM10 | January-2024 | 0.80 | 5.13 | Initial |
| February-2024 | 1.53 | 10.12 | Initial | |
| March-2024 | 0.59 | 3.70 | Later | |
| April-2024 | 1.57 | 8.60 | Later | |
| May-2024 | 0.51 | 2.75 | Later | |
| June-2024 | 0.62 | 3.96 | Later | |
| July-2024 | 0.30 | 1.75 | Later | |
| August-2024 | 0.24 | 1.02 | Later | |
| September-2024 | 0.08 | 0.40 | Later | |
| October-2024 | 0.09 | 0.43 | Later | |
| November-2024 | 0.05 | 0.23 | Later | |
| December-2024 | 0.09 | 0.41 | Later | |
| IGHM11 | January–February 2024 | 3.98 | 35.44 | Initial |
| Bird ID | Month | Monthly Distance (km) | Daily Distance (km) | Phase |
|---|---|---|---|---|
| IGHM2 | November 2021 | 64.83 | 2.16 | Initial |
| December 2021 | 60.18 | 1.94 | Initial | |
| January 2022 | 39.02 | 1.26 | Later | |
| February 2022 | 50.33 | 1.74 | Later | |
| March 2022 | 9.92 | 0.32 | Later | |
| April 2022 | 10.27 | 0.34 | Later | |
| May 2022 | 9.04 | 0.29 | Later | |
| June 2022 | 6.15 | 0.32 | Later | |
| IGHM3 | March 2022 | 151.78 | 4.34 | Initial |
| April 2022 | 71.40 | 4.46 | Initial | |
| IGHM7 | January 2024 | 158.41 | 5.11 | Initial |
| February 2024 | 317.05 | 10.93 | Initial | |
| IGHM10 | January 2024 | 89.76 | 2.9 | Initial |
| February 2024 | 79.08 | 2.73 | Initial | |
| March 2024 | 75.22 | 2.43 | Later | |
| April 2024 | 77 | 2.57 | Later | |
| May 2024 | 56.34 | 1.82 | Later | |
| June 2024 | 105.15 | 3.51 | Later | |
| July 2024 | 62.44 | 2.01 | Later | |
| August 2024 | 51.22 | 1.65 | Later | |
| September 2024 | 36.28 | 1.21 | Later | |
| October 2024 | 40.27 | 1.30 | Later | |
| November 2024 | 19.59 | 0.65 | Later | |
| December 2024 | 32.47 | 1.05 | Later | |
| IGHM11 | January 2024 | 115.61 | 3.73 | Initial |
| February 2024 | 37.62 | 4.7 | Initial |
| Activity | Mean-Male (± SD) | Mean-Female (± SD) | Statistic | p-Value | Adjusted p-Value | Test |
|---|---|---|---|---|---|---|
| Bill Cleaning | 0.46 ± 0.73 | 0.73 ± 1.86 | 176.00 | 0.06 | 0.40 | Mann–Whitney |
| Feeding | 1.11 ± 3.25 | 1.24 ± 1.94 | 41926.00 | 0.25 | 1.00 | Mann–Whitney |
| Nest Cleaning | 1.04 ± 0.72 | 1.45 ± 0.41 | 316.00 | 0.08 | 0.48 | Mann–Whitney |
| Perching | 0.76 ± 2.0 | 0.58 ± 1.90 | 33414.50 | 0.69 | 1.00 | Mann–Whitney |
| Preening | 1.80 ± 1.99 | 2.07 ± 2.32 | 37.00 | 0.48 | 1.00 | Mann–Whitney |
| Peeping inside Nest | 0.14 ± 0.66 | 0.16 ± 0.34 | −0.53 | 0.60 | 1.00 | t-test |
| Comparison | Z Statistic | p-Value | Adjusted p-Value |
|---|---|---|---|
| Bhojde-Mindhori | −8.5050 | 0.01 | 0.0001 |
| Bhojde-Verwangda | −2.8300 | 0.047 | 0.014 |
| Mindhori-Verwangda | 4.7990 | 0.01 | 0.0001 |
| Comparison | Statistic | Adjusted p-Value |
|---|---|---|
| Bhojde vs. Mindhori | 93.81 | 0.001 |
| Bhojde vs. Verwangda | 1.24 | 0.79 |
| Mindhori vs. Verwangda | 90.40 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ram, M.; Gadhavi, D.; Sahu, A.; Srivastava, N.; Rather, T.A.; Dagur, T.; Modi, V.; Jhala, L.; Zala, Y.; Jhala, D. Reintroduction of Indian Grey Hornbills in Gir, India: Insights into Ranging, Habitat Use, Nesting and Behavioural Patterns. Birds 2025, 6, 58. https://doi.org/10.3390/birds6040058
Ram M, Gadhavi D, Sahu A, Srivastava N, Rather TA, Dagur T, Modi V, Jhala L, Zala Y, Jhala D. Reintroduction of Indian Grey Hornbills in Gir, India: Insights into Ranging, Habitat Use, Nesting and Behavioural Patterns. Birds. 2025; 6(4):58. https://doi.org/10.3390/birds6040058
Chicago/Turabian StyleRam, Mohan, Devesh Gadhavi, Aradhana Sahu, Nityanand Srivastava, Tahir Ali Rather, Tanisha Dagur, Vidhi Modi, Lahar Jhala, Yashpal Zala, and Dushyantsinh Jhala. 2025. "Reintroduction of Indian Grey Hornbills in Gir, India: Insights into Ranging, Habitat Use, Nesting and Behavioural Patterns" Birds 6, no. 4: 58. https://doi.org/10.3390/birds6040058
APA StyleRam, M., Gadhavi, D., Sahu, A., Srivastava, N., Rather, T. A., Dagur, T., Modi, V., Jhala, L., Zala, Y., & Jhala, D. (2025). Reintroduction of Indian Grey Hornbills in Gir, India: Insights into Ranging, Habitat Use, Nesting and Behavioural Patterns. Birds, 6(4), 58. https://doi.org/10.3390/birds6040058






