Wintering Red Kites in Central Spain: Macrohabitat Selection and Population Density Estimate
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
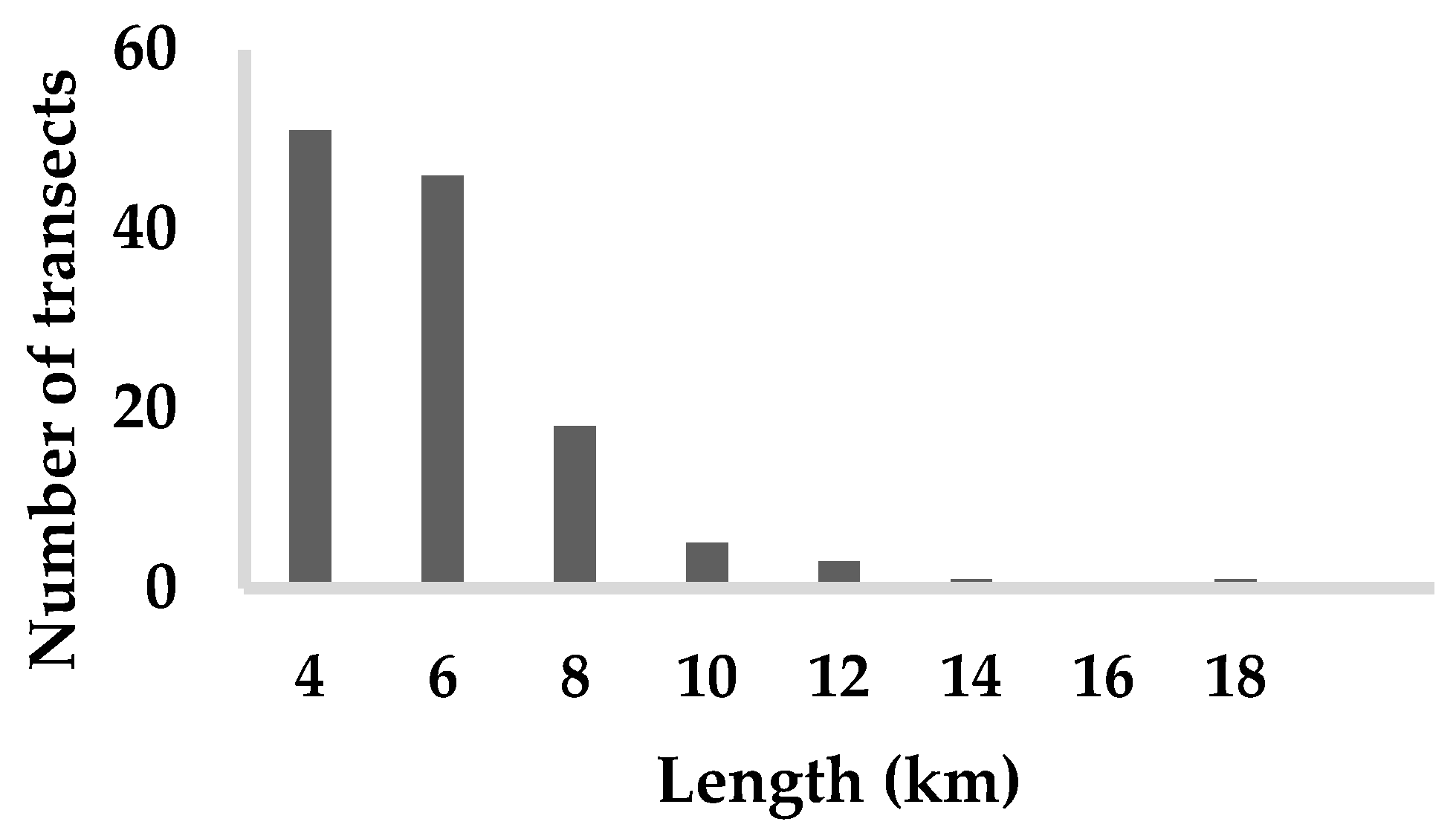
2.2. Red Kite Sampling
2.3. Habitat Characterization
2.4. Statistical Methods
3. Results
3.1. Presence of the Red Kite
3.2. Estimate Density of the Red Kite
3.3. Habitat Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cramp, S.; Simmons, K.E.L. Birds of the Western Palearctic, Vol. II; University Press: Oxford, UK, 1978. [Google Scholar]
- Del Hoyo, J.; Elliot, A.; Sargatal, J. Handbook of the Birds of the World. Vol.2 New World Vultures to Guineafowl; Lynx Edicions: Barcelona, Spain, 1994. [Google Scholar]
- Heneberg, P.; Dolinay, M.; Matušík, H.; Pfeiffer, T.; Nachtigall, W.; Bizos, J.; Šimčíková, D.; Literák, I. Conservation of the Red Kite Milvus milvus (Aves: Accipitriformes) is not affected by the establishment of a broad hybrid zone with the Black Kite Milvus migrans migrans in Central Europe. PLoS ONE 2016, 11, e0159202. [Google Scholar] [CrossRef]
- Westrip, J.R.S.; BirdLife International Milvus milvus (Mediterranean Assessment). The IUCN Red List of Threatened Species 2022: E.T22695072A210523277. Available online: https://doi.org/10.2305/IUCN.UK.2022-1.RLTS.T22695072A210523277.en (accessed on 23 September 2025).
- Lovegrove, R.; Elliott, G.; Smith, K. The Red Kite in Britain. RSPB Conserv. Rev. 1990, 4, 15–21. [Google Scholar]
- Mougeot, F.; Garcia, J.T.; Viñuela, J. Breeding biology, behaviour, diet and conservation of the Red Kite (Milvus milvus), with particular emphasis on Mediterranean populations. In Ecology and Conservation of European Forest-Dwelling Raptors; Zuberogoitia, I., Martínez, J.E., Eds.; Departamento de Agricultura de la Diputación Foral de Bizkaia: Biscay, Spain, 2011; pp. 190–204. [Google Scholar]
- Rodríguez, B.; Rodríguez, A.; Siverio, F.; Siverio, M. Causes of raptor admissions to a wildlife rehabilitation center in Tenerife (Canary Islands). J. Raptor Res. 2010, 44, 30–39. [Google Scholar] [CrossRef]
- Martínez-Abraín, A.; Quevedo, M.; Serrano, D. Translocation in relict shy-selected animal populations: Program success versus prevention of wildlife-human conflict. Biol. Conserv. 2022, 268, 109519. [Google Scholar] [CrossRef]
- Ortega, E.; Mañosa, S.; Margalida, A.; Sánchez, R.; Oria, J.; González, L.M. A demographic description of the recovery of the Vulnerable Spanish Imperial Eagle Aquila adalberti. Oryx 2009, 43, 113. [Google Scholar] [CrossRef]
- Torres-Esquivias, J.A. La Población española de Malvasía Cabeciblanca (Oxyura leucocephala) veinticinco años después del mínimo de 1977. Oxyura Rev. Sobre Las Zonas Húmedas 2003, 11, 5–43. [Google Scholar]
- BirdLife International Species Factsheet: Red Kite Milvus milvus. Available online: https://datazone.birdlife.org/species/factsheet/red-kite-milvus-milvus (accessed on 23 September 2025).
- Carter, I.; McQuaid, M.; Snell, N.; Steven, P. The Red Kite (Milvus milvus) reintroduction project: Modeling the impact of translocating kite young within England. J. Raptor Res. 2003, 33, 251–254. [Google Scholar]
- Smart, J.; Amar, A.; Sim, I.M.W.; Etheridge, B.; Cameron, D.; Christie, G.; Wilson, J.D. Illegal killing slows population recovery of a re-introduced raptor of high conservation concern—The Red Kite Milvus milvus. Biol. Conserv. 2010, 143, 1278–1286. [Google Scholar] [CrossRef]
- Stevens, M.; Murn, C.; Hennessey, R. Population change of Red Kites Milvus milvus in Central Southern England between 2011 and 2016 derived from line transect surveys and multiple covariate distance sampling. Acta Ornithol. 2019, 54, 243. [Google Scholar] [CrossRef]
- Wotton, S.R.; Carter, I.; Cross, A.V.; Etheridge, B.; Snell, N.; Duffy, K.; Thorpe, R.; Gregory, R.D. Breeding status of the Red Kite Milvus milvus in Britain in 2000. Bird Study 2002, 49, 278–286. [Google Scholar] [CrossRef]
- Migración y Ecología Espacial del Milano real en España; Urios, V., García-Macía, J., Eds.; Monografía; Sociedad Española de Ornitología: Madrid, Spain, 2022; ISBN 978-84-124888-1-4. [Google Scholar]
- García-Macía, J.; Pomares, A.; De la Puente, J.; Bermejo, A.; Martínez, J.; Álvarez, E.; Morollón, S.; Urios, V. Striking variability in the post-reproductive movements of Spanish Red Kites (Milvus milvus): Three strategies, sex differences, and changes over time. Animals 2022, 12, 2930. [Google Scholar] [CrossRef] [PubMed]
- García-Macía, J.; López-Poveda, G.; De La Puente, J.; Bermejo-Bermejo, A.; Galán, M.; Álvarez, E.; Morollón, S.; Urios, V. The variability of juvenile dispersal in an opportunistic raptor. Curr. Zool. 2023, 69, zoac039. [Google Scholar] [CrossRef]
- Literák, I.; Kyseláková, C.M.; Dostál, M.; Karlsson, C.; Škrábal, J.; Skyrpan, M.; Hrtan, E.; Haraszthy, L.; Raab, R. Evidence of genetic determination of annual movement strategies in medium-sized raptors. Sci. Rep. 2025, 15, 3159. [Google Scholar] [CrossRef]
- Heuck, C.; Brandl, R.; Albrecht, J.; Gottschalk, T.K. The potential distribution of the Red Kite in Germany. J. Ornithol. 2013, 154, 911–921. [Google Scholar] [CrossRef]
- García-Macía, J.; Vidal-Mateo, J.; de la Puente, J.; Bermejo, A.; Urios, V. Spatial ecology of the Red Kite (Milvus milvus) during the breeding period in Spain. Ornis Fenn. 2022, 99, 150–162. [Google Scholar] [CrossRef]
- Panter, C.T.; Literák, I.; Raab, R.; Tolhurst, B.A.; White, R.L. Age, landscape, and arrival date explain ranging behavior of wintering Red Kites in Southwest Europe. J. Wildl. Manag. 2022, 86, e22147. [Google Scholar] [CrossRef]
- Viñuela, J. Milano Real Milvus milvus. In Atlas de las aves en invierno en España 2007–2010; SEO/BirdLife: Madrid, Spain, 2012; pp. 163–165. [Google Scholar]
- García-Macía, J.; De La Puente, J.; Bermejo-Bermejo, A.; Raab, R.; Urios, V. High Variability and dual strategy in the wintering Red Kites (Milvus milvus). Diversity 2022, 14, 117. [Google Scholar] [CrossRef]
- Cody, M.L. Habitat Selection in Birds; Academic Press, Inc.: London, UK, 1985. [Google Scholar]
- Demerdzhiev, D.A.; Popgeorgiev, G.S.; Dobrev, D.D.; Arkumarev, V.S.; Terziev, N.G. Habitat requirements of the Lesser Spotted Eagle Clanga pomarina Brehm, 1831 (Aves: Accipitridae) at the southern periphery of the distribution range (Southeast Bulgaria). Acta Zool. Bulg. 2019, 14, 35–65. [Google Scholar]
- Mayor, S.J.; Schneider, D.C.; Schaefer, J.A.; Mahoney, S.P. Habitat selection at multiple scales. Écoscience 2009, 16, 238–247. [Google Scholar] [CrossRef]
- Simberlof, D. The contribution of population and community biology to conservation science. Annu. Rev. Ecol. Syst. 1988, 1, 473–511. [Google Scholar] [CrossRef]
- Woodford, J.E.; Eloranta, C.A.; Rinaldi, A. Nest density, productivity, and habitat selection of Red-Shouldered Hawks in a contiguous forest. J. Raptor Res. 2008, 42, 79–86. [Google Scholar] [CrossRef]
- Ferrer-Sánchez, Y.; Rodríguez-Estrella, R.; Martínez-Morales, M.Á. Improving conservation strategies of raptors through landscape ecology analysis: The case of the Endemic Cuban Black Hawk. Ecol. Evol. 2019, 9, 13808–13823. [Google Scholar] [CrossRef] [PubMed]
- Väli, Ü.; Treinys, R.; Lõhmus, A. Geographical variation in macrohabitat use and preferences of the Lesser Spotted Eagle Aquila pomarina. Ibis 2004, 146, 661–671. [Google Scholar] [CrossRef]
- Seoane, J.; Viñuela, J.; Dı́az-Delgado, R.; Bustamante, J. The effects of land use and climate on Red Kite distribution in the Iberian Peninsula. Biol. Conserv. 2003, 111, 401–414. [Google Scholar] [CrossRef][Green Version]
- Viñuela, J.; Marti, R.; Ruiz, A. El Milano Real En España; SEO/BirdLife: Madrid, Spain, 1999. [Google Scholar][Green Version]
- López de la Nieta, D.; Iniesta, P.; Gosálvez, R.U. Características geográficas de La Mancha Húmeda. In Avifauna acuática: Conservación en la Reserva de la Biosfera de la Mancha Húmeda; Editorial Cuarto Centenario: Toledo, Spain, 2021. [Google Scholar][Green Version]
- Ruiz Pulpón, Á.R. El acceso al agua como factor de identificación de problemas de desarrollo agrario sostenible en el territorio del Alto Guadiana. Estud. Geográficos 2008, 69, 665–686. [Google Scholar] [CrossRef][Green Version]
- Gosálvez, R.; Gil-Delgado, J.A.; Vives-Ferrándiz, C.; Sánchez, G.; Florín, M. Seguimiento de aves acuáticas amenazadas en lagunas de la Reserva de la Biosfera de La Mancha Húmeda (España Central). Polígonos Rev. Geogr. 2012, 22, 89. [Google Scholar] [CrossRef]
- Viñuela, J. Road transects as a large-scale census method for raptors: The case of the Red Kite Milvus milvus in Spain. Bird Study 1997, 44, 155–165. [Google Scholar] [CrossRef]
- Jeréz, O. La Reserva de La Biosfera de La Mancha Húmeda y La Cuenca Alta Del Guadiana. Guía Didáctica del medio Físico y de la Evolución de los Paisajes; Universidad de Castilla-La Mancha: Albacete, Spain, 2010. [Google Scholar]
- Jiménez, J.; del Moral, A.; Morillo, C.; Sánchez, M.J. Las aves del Parque Nacional de Las Tablas de Daimiel y otros humedales Manchegos; Lynx Edicions: Barcelona, Spain, 1992. [Google Scholar]
- Peinado, M.; Plaza, J. La Reserva de La Biosfera de La Mancha: Geografía, Territorio y Paisaje. In Reserva de la Biosfera de la Mancha Húmeda: Retos y oportunidades de futuro; García del Castillo, J., Rubio, M.A., López, A., Eds.; Ministerio de Transición Ecológica: Madrid, Spain, 2011; pp. 43–51. [Google Scholar]
- Agencia Estatal de Meteorología (AEMET) AEMET OpenData. Available online: https://www.aemet.es/es/datos_abiertos/AEMET_OpenData (accessed on 28 September 2025).
- Buckland, S.T.; Rexstad, E.A.; Marques, T.A.; Oedekoven, C.S. Distance Sampling: Methods and Applications; Methods in Statistical Ecology; Springer International Publishing: Cham, Germany, 2015; ISBN 978-3-319-19218-5. [Google Scholar]
- Sutherland, W.J. Ecological Census Techniques: A Handbook, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Pfeiffer, T.; Meyburg, B.-U. Flight altitudes and flight activities of adult Red Kites (Milvus milvus) in the breeding area as determined by GPS telemetry. J. Ornithol. 2022, 163, 867–879. [Google Scholar] [CrossRef]
- Britto, V.O.; Gil–Delgado, J.A.; Gosálvez, R.U.; López–Iborra, G.M.; Velasco, A. Foraging habitat selection by Gull–Billed Tern (Gelochelidon nilotica) in Central Spain (Castilla–La Mancha). Anim. Biodivers. Conserv. 2018, 41, 301–310. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Pearson Education Limited: Harlow, UK, 2014; ISBN 978-1-292-02404-2. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Thomas, L.; Buckland, S.T.; Rexstad, E.A.; Laake, J.L.; Strindberg, S.; Hedley, S.L.; Bishop, J.R.B.; Marques, T.A.; Burnham, K.P. Distance aoftware: Design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 2010, 47, 5–14. [Google Scholar] [CrossRef]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Introduction to Distance Sampling: Estimating Abundance of Biological Populations; Oxford University Press: Oxford, UK, 2001; ISBN 978-0-19-850649-2. [Google Scholar]
- Akaike, H. Information theory and an extension of the Maximum Likelihood Principle. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer Series in Statistics; Springer: New York, NY, USA, 1998; pp. 199–213. ISBN 978-1-4612-7248-9. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2010; ISBN 978-0-387-95364-9. [Google Scholar]
- Anderson, M.J.; Walsh, D.C.I.; Robert Clarke, K.; Gorley, R.N.; Guerra-Castro, E. Some solutions to the Multivariate Behrens–Fisher problem for dissimilarity-based analyses. Aust. N. Z. J. Stat. 2017, 59, 57–79. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Viñuela, J.; Deán, J.I.; de la Puente, J.; Bermejo, A. Milano Real Milvus milvus. In III Atlas de las aves en Época de Reproducción en España; Molina, B., Nebreda, A., Muñoz, R., Seoane, J., Real, R., Bustamante, J., del Moral, J.C., Eds.; SEO/BirdLife: Madrid, Spain, 2022. [Google Scholar]
- Nicolai, B.; Mammen, U.; Kolbe, M. Long-term changes in population and habitat selection of Red Kite Milvus milvus in the region with the highest population density. Vogelwelt 2017, 137, 194–197. [Google Scholar]
- Newton, I. Population Ecology of Raptors; T & AD Poyser Ltd.: Berkhamsted, UK, 1979. [Google Scholar]
- Newton, I.; Davis, P.E.; Moss, D. Distribution and breeding of Red Kites Milvus milvus in relation to afforestation and other land-use in Wales. J. Appl. Ecol. 1996, 33, 210–224. [Google Scholar] [CrossRef]


| Geographic Zone | Mean Temperature °C | Total Rainfall (mm) | Number of Municipalities | Area (km2) | Winter 1 + Winter 2 Transects | Breeding Transects | Total Transects |
|---|---|---|---|---|---|---|---|
| Landete * | 14.7 | 445.8 | 9 | 728.24 | 16 + 16 | 16 | 48 |
| Motilla del Palancar | 15.2 | 310.4 | 8 | 494.01 | 12 + 12 | 12 | 36 |
| Belmonte | 13.5 | 183.2 | 8 | 450.00 | 22 + 14 | 14 | 50 |
| Mota del Cuervo * | 13.5 | 183.2 | 3 | 443.40 | 20 + 11 | 14 | 45 |
| Pedro Muñoz * | 17.4 | 274.6 | 5 | 1307.04 | 14 + 8 | 14 | 36 |
| Quero * | 17.7 | 168 | 4 | 574.96 | 25 + 14 | 32 | 71 |
| Lillo * | 18.7 | 340 | 6 | 1025.10 | 16 + 12 | 11 | 39 |
| Total | 43 | 5023.35 | 125 + 87 | 113 | 325 |
| Model (Key Function/Adjustment Term + Pos-Stratification) | # Parameters a | ΔAIC | AIC | X2-p | p |
|---|---|---|---|---|---|
| Locality | |||||
| Hazard rate/simple polynomial | 2 | 0 | 248.98 | 0.002 | 0.16 |
| Half-normal/cosine | 2 | 9.598 | 258.58 | ||
| Uniform/cosine | 2 | 21.39 | 270.38 | ||
| Transect type | |||||
| Hazard rate/simple polynomial | 4 | 3.18 | 252.16 | ||
| Half-normal/cosine | 4 | 13.58 | 262.57 | ||
| Uniform/cosine | 4 | 48.19 | 297.18 | ||
| Hazard rate for roads | 108.33 | 108.09 | 0.16 | 0.18 | |
| Hazard rate for tracks | 144.25 | 144.08 | 0.02 | 0.14 | |
| Winter b | |||||
| Half-normal/cosine | 3 | 9.04 | 258.02 | ||
| Uniform/cosine | 4 | 60.55 | 309.53 | ||
| Half-normal for Winter2022 | 146.64 | 146.58 | 0.11 | 0.19 | |
| Half-normal for Winter2023 | 111.7 | 111.44 | 0.00002 | 0.22 | |
| Geographic Zone | Estimated Density | Lower Density Limit | Upper Density Limit | Lower Estimate < Population Esimate < Upper Estimate |
|---|---|---|---|---|
| Belmonte | 0.12 | 0.06 | 0.23 | 27 < 54 < 104 |
| Mota del C. | 0.35 | 0.22 | 0.57 | 90 < 155 < 253 |
| Pedro Muñoz | 0.35 | 0.19 | 0.63 | 249 < 458 < 824 |
| Quero | 0.62 | 0.43 | 0.91 | 247 < 357 < 523 |
| Longar | 0.40 | 0.24 | 0.66 | 195 < 410 < 677 |
| Total | 816 < 1434 < 2381 |
| Habitat Type | Average | SD | Ratio | avNon-Reserve | avMHBR | CuSum | p | |
|---|---|---|---|---|---|---|---|---|
| Ploughed with winter cereal | 0.163 | 0.121 | 1.366 | 38.290 | 26.887 | 0.229 | 0.001 | *** |
| Trellised vineyard | 0.110 | 0.071 | 1.546 | 2.820 | 23.113 | 0.383 | 0.001 | *** |
| Fallow land | 0.092 | 0.077 | 1.193 | 11.110 | 22.485 | 0.511 | 0.626 | |
| Holm oak woodland | 0.075 | 0.137 | 0.552 | 14.110 | 1.959 | 0.617 | 0.003 | ** |
| Goblet-pruned vineyard | 0.068 | 0.086 | 0.791 | 0.070 | 13.557 | 0.711 | 0.951 | |
| Scrubland | 0.056 | 0.069 | 0.839 | 11.540 | 0.835 | 0.792 | 0.001 | *** |
| Pinewood | 0.054 | 0.081 | 0.684 | 11.04 | 0.907 | 0.870 | 0.001 | *** |
| Almond crops | 0.032 | 0.044 | 0.712 | 4.96 | 3.082 | 0.914 | 0.059 | * |
| Olive crops | 0.026 | 0.035 | 0.760 | 1.750 | 4.505 | 0.951 | 0.589 | |
| Juniper | 0.014 | 0.047 | 0.300 | 2.790 | 0.000 | 0.971 | 0.001 | *** |
| Tamarisk | 0.009 | 0.024 | 0.367 | 0.000 | 1.742 | 0.983 | 0.986 | |
| Poplar | 0.005 | 0.012 | 0.402 | 0.960 | 0.000 | 0.990 | 0.001 | *** |
| Holm oak/Pinewood | 0.003 | 0.014 | 0.220 | 0.360 | 0.278 | 0.994 | 0.230 | |
| Reeds | 0.002 | 0.016 | 0.102 | 0.000 | 0.330 | 0.996 | 0.233 | |
| Broom shrubland | 0.001 | 0.005 | 0.207 | 0.000 | 0.206 | 0.998 | 0.629 | |
| Orchards | 0.001 | 0.003 | 0.297 | 0.180 | 0.000 | 0.999 | 0.001 | *** |
| Isolated buildings | 0.000 | 0.003 | 0.172 | 0.000 | 0.093 | 1.000 | 0.742 | |
| Other | 0.000 | 0.001 | 0.241 | 0.040 | 0.021 | 1.000 | 0.128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermúdez-Cavero, A.O.; Bernat-Ponce, E.; Gil-Delgado, J.A.; López-Peinado, A. Wintering Red Kites in Central Spain: Macrohabitat Selection and Population Density Estimate. Birds 2025, 6, 54. https://doi.org/10.3390/birds6040054
Bermúdez-Cavero AO, Bernat-Ponce E, Gil-Delgado JA, López-Peinado A. Wintering Red Kites in Central Spain: Macrohabitat Selection and Population Density Estimate. Birds. 2025; 6(4):54. https://doi.org/10.3390/birds6040054
Chicago/Turabian StyleBermúdez-Cavero, Alan Omar, Edgar Bernat-Ponce, José Antonio Gil-Delgado, and Andrés López-Peinado. 2025. "Wintering Red Kites in Central Spain: Macrohabitat Selection and Population Density Estimate" Birds 6, no. 4: 54. https://doi.org/10.3390/birds6040054
APA StyleBermúdez-Cavero, A. O., Bernat-Ponce, E., Gil-Delgado, J. A., & López-Peinado, A. (2025). Wintering Red Kites in Central Spain: Macrohabitat Selection and Population Density Estimate. Birds, 6(4), 54. https://doi.org/10.3390/birds6040054





