Avian Escape and Prevailing Light Levels
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FID | Flight Initiation Distance |
| Start_D | Starting Distance |
References
- Samia, D.S.; Nakagawa, S.; Nomura, F.; Rangel, T.F.; Blumstein, D.T. Increased tolerance to humans among disturbed wildlife. Nat. Commun. 2015, 6, 8877. [Google Scholar] [CrossRef]
- Lowry, H.; Lill, A.; Wong, B.M. Behavioural responses of wildlife to urban environments. Biol. Rev. 2013, 88, 537–549. [Google Scholar] [CrossRef]
- Hall, M.J.; Burns, A.L.; Martin, J.M.; Hochuli, D.F. Flight initiation distance changes across landscapes and habitats in a successful urban colonizer. Urban Ecosyst. 2020, 23, 785–791. [Google Scholar] [CrossRef]
- Ritzel, K.; Gallo, T. Behavior change in urban mammals: A systematic review. Front. Ecol. Evol. 2020, 8, 576665. [Google Scholar] [CrossRef]
- Rendall, A.R.; Plotz, R.D.; Yokochi, K.; Krauss, J.; Pengelly, A.; Di Stefano, S.A.; Swindell, S.; Ranawana, K.; Vidanapathirana, D.R.; Weston, M.A. Lifting the veil of darkness: Thermal technology facilitates collection of flight-initiation distances by night. Ecol. Evol. 2024, 14, e70450. [Google Scholar] [CrossRef]
- Falcón, J.; Torriglia, A.; Attia, D.; Viénot, F.; Gronfier, C.; Behar-Cohen, F.; Martinsons, C.; Hicks, D. Exposure to artificial light at night and the consequences for flora fauna and ecosystems. Front. Neurosci. 2020, 14, 602796. [Google Scholar] [CrossRef]
- Auman, H.J.; Bond, A.L.; Meathrel, C.E.; Richardson, A.M.M. Urbanization of the Silver Gull: Evidence of anthropogenic feeding regimes from stable isotope analyses. Waterbirds 2011, 34, 70–76. [Google Scholar] [CrossRef]
- Martin, G.R. Through birds’ eyes: Insights into avian sensory ecology. J. Ornithol. 2012, 153, 23–48. [Google Scholar] [CrossRef]
- Wolf, M.M.; Francis, C.D. Eye catching light: Anthropogenic light at night and its evolutionary influence on the avian eye. iScience 2025, 28, 112039. [Google Scholar] [CrossRef]
- Cantlay, J.C.; Portugal, S.J.; Martin, G.R. Visual fields, foraging and collision vulnerability in gulls (Laridae). Ibis 2025, 167, 386–396. [Google Scholar] [CrossRef]
- Victory, N.; Segovia, Y.; García, M. Foveal shape ultrastructure and photoreceptor composition in Yellow-legged Gull Larus michahellis (Naumann 1840). Zoomorphology 2021, 140, 151–167. [Google Scholar] [CrossRef]
- Fernández-Juricic, E.; Deisher, M.; Stark, A.C.; Randolet, J. Predator detection is limited in microhabitats with high light intensity: An experiment with Brown-headed Cowbirds. Ethology 2012, 118, 341–350. [Google Scholar] [CrossRef]
- Blumstein, D.T. Flight-initiation distance in birds is dependent on intruder starting distance. J. Wildl. Manag. 2003, 67, 852–857. [Google Scholar] [CrossRef]
- Lomas, S.C.; Whisson, D.A.; Maguire, G.S.; Tan, L.X.; Guay, P.J.; Weston, M.A. The influence of cover on nesting Red-capped Plovers: A trade-off between thermoregulation and predation risk? Victorian Nat. 2014, 131, 115–127. Available online: https://search.informit.org/doi/abs/10.3316/informit.651493023677570 (accessed on 4 July 2025).
- Morris, J.G.; Parsons, J.J. The various ways in which birds blink. Animals 2023, 13, 3656. [Google Scholar] [CrossRef]
- Blumstein, D.T.; Cooper, W. Escape from Predators; USA Cambridge University Press: Cambridge, UK, 2015; pp. 1–442. [Google Scholar]
- Møller, A.P. Flight distance of urban birds, predation and selection for urban life. Behav. Ecol. Sociobiol. 2008, 63, 63–75. [Google Scholar] [CrossRef]
- Mikula, P. Pedestrian density influences flight distances of urban birds. Ardea 2014, 102, 53–60. [Google Scholar] [CrossRef]
- Díaz, M.; Møller, A.P. Lockdown effects on fear revealed direct and indirect effects of human presence on perceived predation risk. Sci. Total Environ. 2023, 872, 162122. [Google Scholar] [CrossRef]
- Shuai, L.; Morelli, F.; Mikula, P.; Benedetti, Y.; Weston, M.A.; Ncube, E.; Tarakini, T.; Díaz, M.; Markó, G.; Jokimäki, J.; et al. A meta-analysis of the relationship between flock size and flight initiation distance in birds. Anim. Behav. 2024, 210, 1–9. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.; Singmann, H.; Dai, B.; Grothendieck, G.; Green, P.; Bolker, M.B. Package ‘lme4’. Convergence 2015, 6, 2. [Google Scholar]
- Hartig, F.; Lohse, L.; de Souza leite, M. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. 2024. Available online: https://cran.r-project.org/web/packages/DHARMa/index.html (accessed on 4 July 2025).
- Chaib, S.; Lind, O.; Kelber, A. Fast visual adaptation to dim light in a cavity-nesting bird. Proc. R. Soc. B 2023, 290, 1–8. [Google Scholar] [CrossRef]
- Monterroso, P.; Alves, P.C.; Ferreras, P. Catch me if you can: Diel activity patterns of mammalian prey and predators. Ethology 2013, 119, 1044–1056. [Google Scholar] [CrossRef]
- Yorzinski, J.L.; Platt, M.L. The difference between night and day: Antipredator behavior in birds. J. Ethol. 2012, 30, 211–218. [Google Scholar] [CrossRef]
- Gustin, M.; Giglio, G.; Pellegrino, S.C.; Frassanito, A.; Ferrarini, A. Nocturnal flights lead to collision risk with power lines and wind farms in Lesser Kestrels: A preliminary assessment through GPS tracking. Comput. Ecol. Softw. 2018, 8, 15–22. Available online: https://core.ac.uk/reader/201618090 (accessed on 4 July 2025).
- Ydenberg, R.C.; Dill, L.M. The economics of fleeing from predators. Adv. Study Behav. 1986, 16, 229–249. [Google Scholar] [CrossRef]
- MacLean, S.A.; Bonter, D.N. The sound of danger: Threat sensitivity to predator vocalizations, alarm calls, and novelty in gulls. PLoS ONE 2013, 8, e82384. [Google Scholar] [CrossRef]
- Dumont, F.; Pasquaretta, C.; Réale, D.; Bogliani, G.; von Hardenberg, A. Flight initiation distance and starting distance: Biological effect or mathematical artefact? Ethology 2012, 118, 1051–1062. [Google Scholar] [CrossRef]
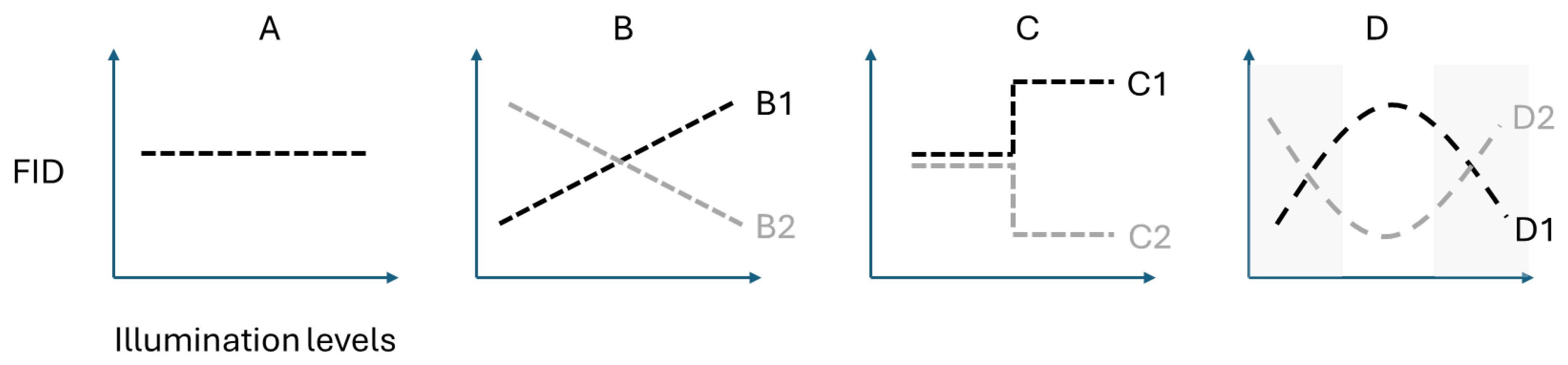
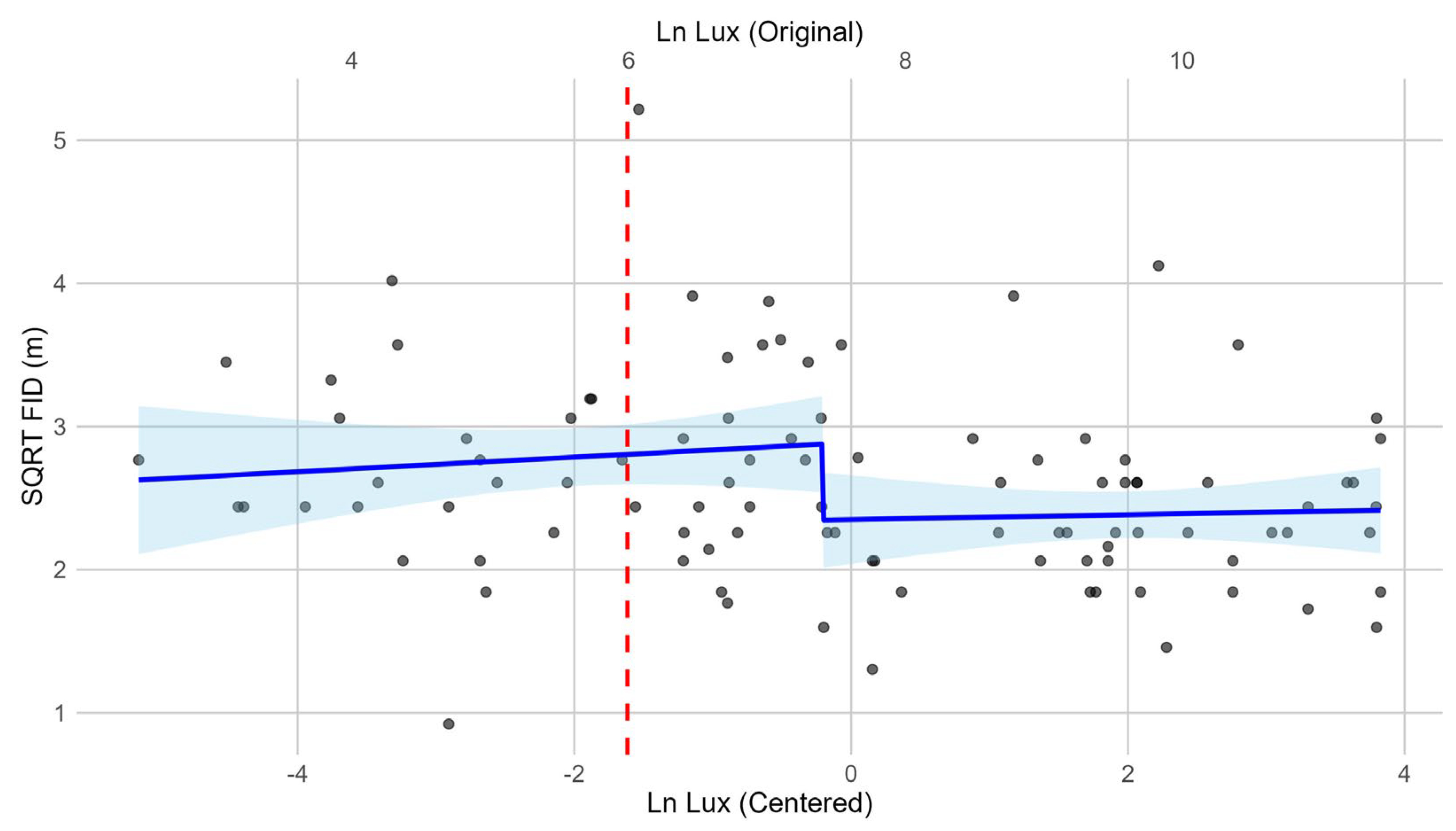
| Model | Intercept | Loge(Lux) | Loge(Start_D) | Loge(Lux)2 | AICc | ∆AICc | R2 (Adj.) |
|---|---|---|---|---|---|---|---|
| Step | 2.7235 ± 0.0547 p < 0.0001 | −0.3941 ± 0.0788 p < 0.0001 | 0.8638 ± 0.0889 p < 0.0001 | NA | 135.1364 | 0.0000 | 0.5259 |
| Linear | 2.5826 ± 0.0486 p < 0.0001 | −0.0860 ± 0.0209 p = 0.0001 | 0.8653 ± 0.0922 p < 0.0001 | NA | 141.9304 | 6.7940 | 0.4923 |
| Quadratic Forced (Inverted “U”; Concave Down) | 2.6052 ± 0.0682 p < 0.0001 | −0.0873 ± 0.0211 p = 0.0001 | 0.8641 ± 0.0926 p < 0.0001 | −0.0041 ± 0.0087 p = 0.6366 | 143.6965 | 8.5601 | 0.4881 |
| Unconstrained Quadratic (“U”; Concave Up) | 2.6052 ± 0.0682 p < 0.0001 | −0.0873 ± 0.0211 p = 0.0001 | 0.8641 ± 0.0926 p < 0.0001 | 0.0041 ± 0.0087 p = 0.6366 | 143.6965 | 8.5601 | 0.4881 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weston, M.A.; Yokochi, K. Avian Escape and Prevailing Light Levels. Birds 2025, 6, 47. https://doi.org/10.3390/birds6030047
Weston MA, Yokochi K. Avian Escape and Prevailing Light Levels. Birds. 2025; 6(3):47. https://doi.org/10.3390/birds6030047
Chicago/Turabian StyleWeston, Michael A., and Kaori Yokochi. 2025. "Avian Escape and Prevailing Light Levels" Birds 6, no. 3: 47. https://doi.org/10.3390/birds6030047
APA StyleWeston, M. A., & Yokochi, K. (2025). Avian Escape and Prevailing Light Levels. Birds, 6(3), 47. https://doi.org/10.3390/birds6030047







