Dynamics of Sex and Age Correlation of Eurasian Woodcock (Scolopax rusticola L.) During Spring Migration in Hungary
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Hungarian Woodcock Bag Monitoring Programme
2.2. Statistical Analysis
- b6 = var2 first max. − var2min. or b6 = var2first min. − var2max.
- b3 = var2 sec. max. − var2min. or b3 = var2 sec. min. − var2 max.
- b4 = var1 first max. or var1 first min.
- b1 = var1 sec. max. or var1 sec. min.
- b0 = var2min.
- b5 = b2~0.05
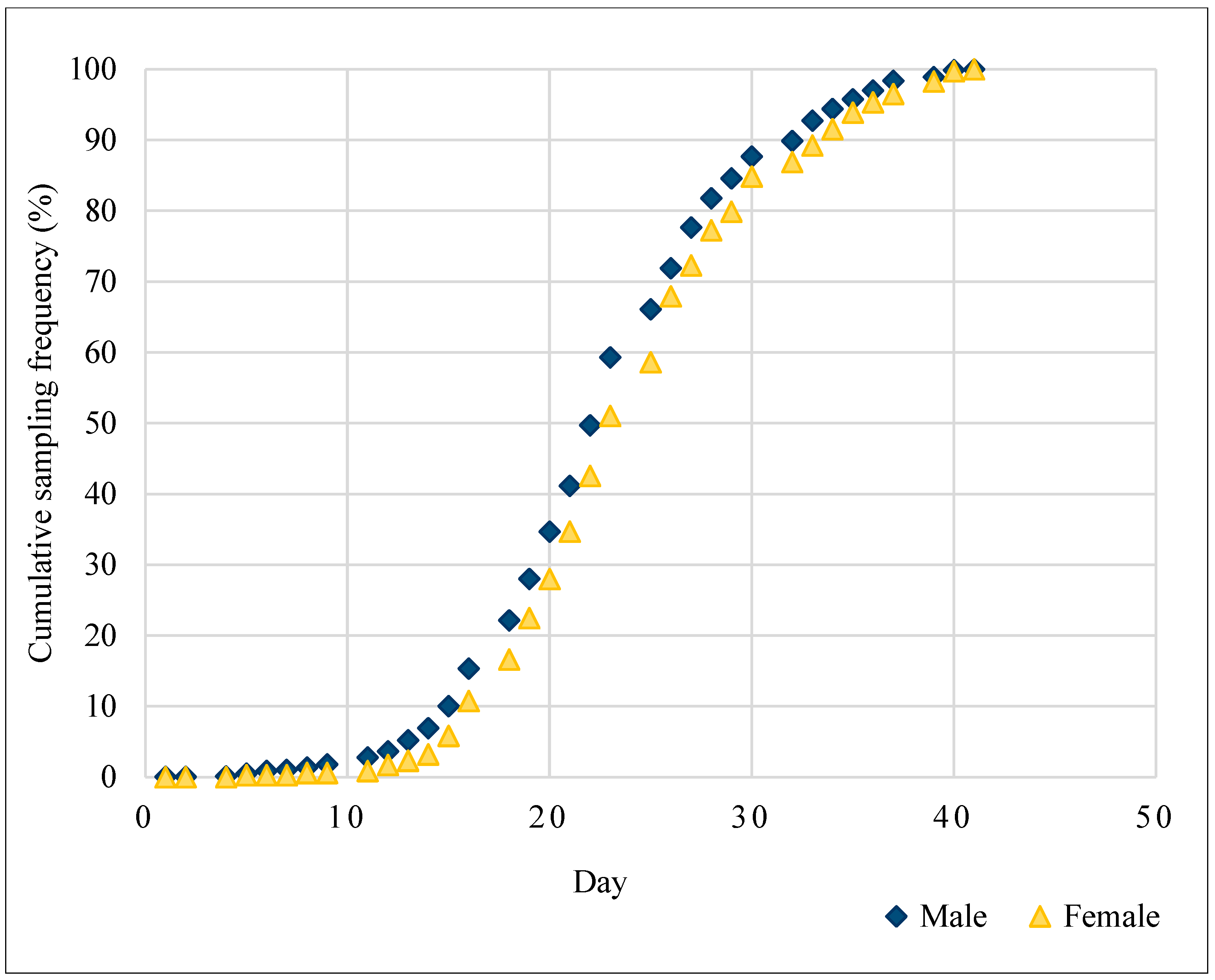
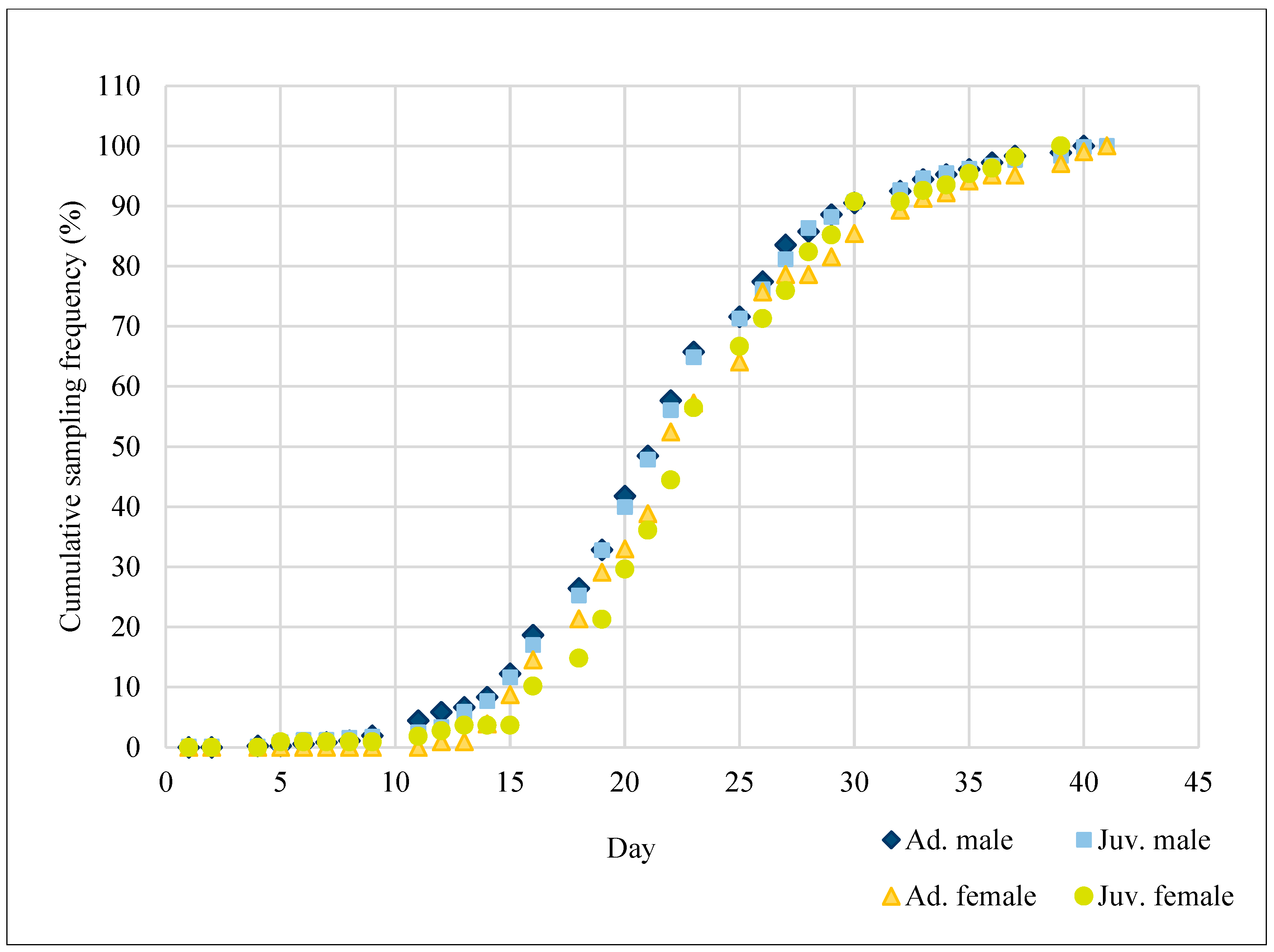
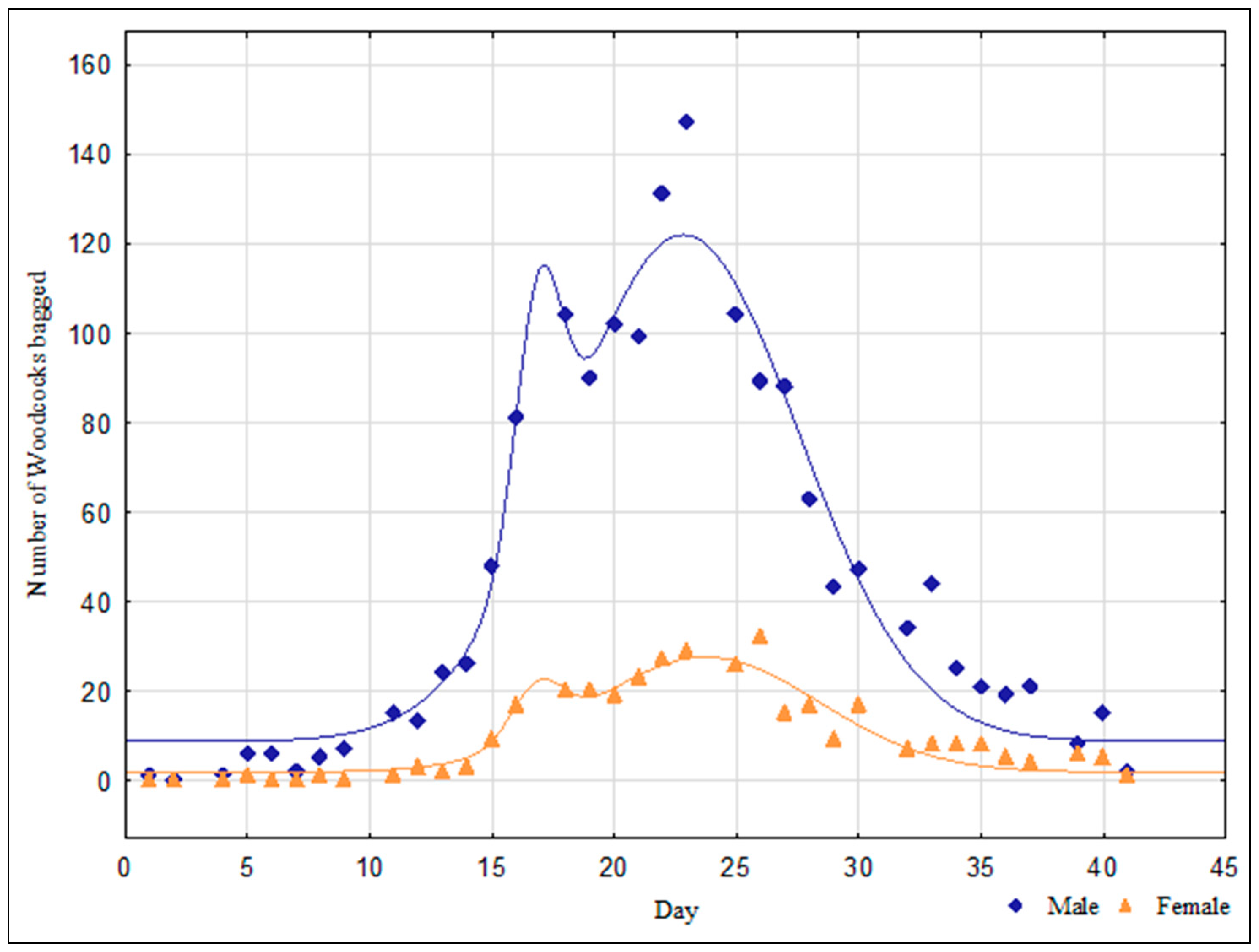
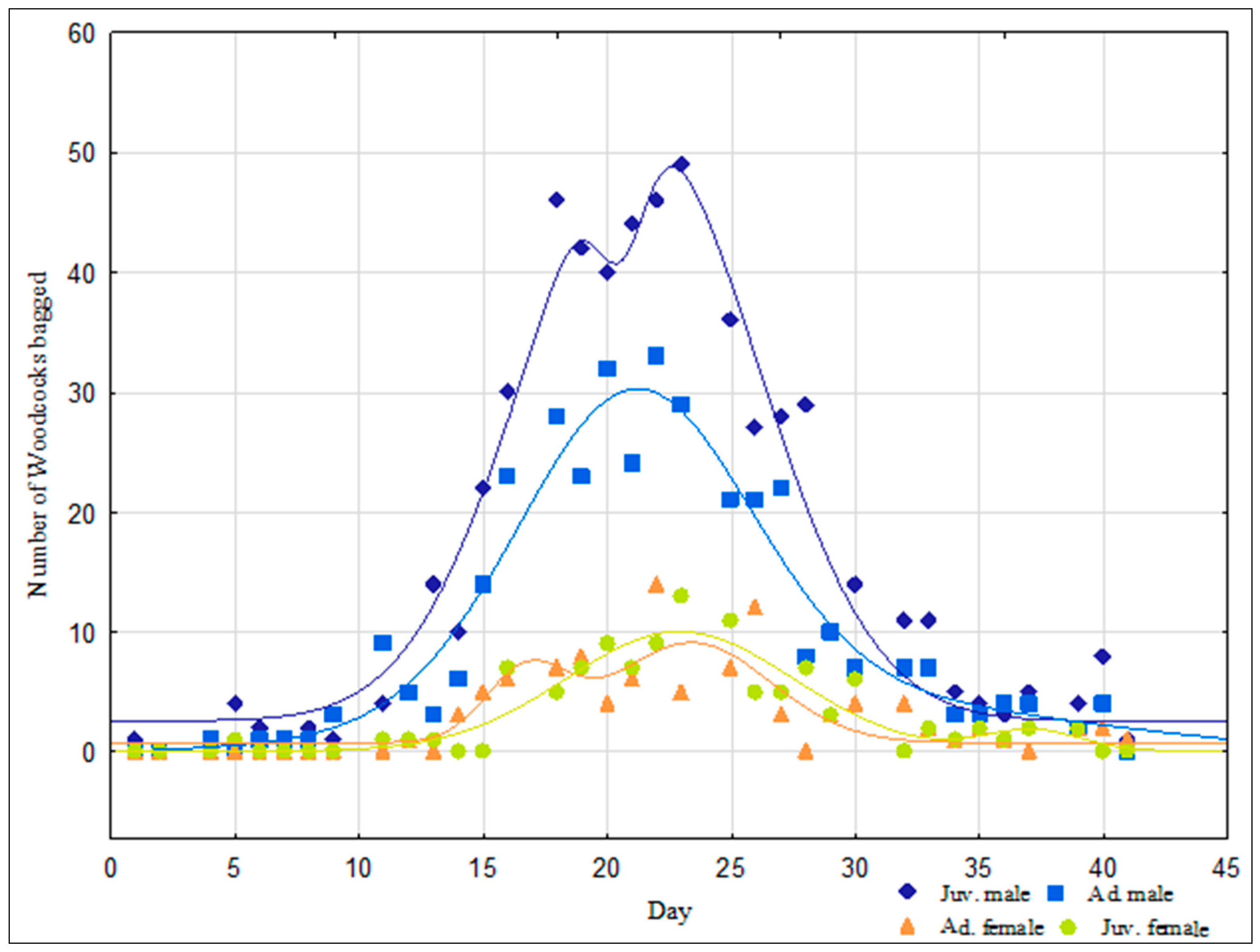
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dvořák, J.; Plhal, R.; Kamler, J.; Drimaj, J. Game Management; Department of Forest Protection and Wildlife Management, Faculty of Forestry and Wood Technology, Mendel University: Brno, Czech Republic, 2014. [Google Scholar]
- Ferrand, Y.; Gossmann, F. Elements for a woodcock (Scolopax rusticola) management plan. Game Wildl. Sci. 2001, 18, 115–139. [Google Scholar]
- Hirschfeld, A.; Heyd, A. Mortality of migratory birds caused by hunting in Europe: Bag statistics and proposals for the conservation of birds and animal welfare. Berichte Vogelschutz 2005, 42, 47–74. [Google Scholar]
- Christensen, T.K.; Fox, A.D.; Sunde, P.; Hounisen, P.J.; Andersen, L.W. Seasonal variation in the sex and age composition of the Woodcock bag in Denmark. Eur. J. Wildl. Res. 2017, 63, 52–61. [Google Scholar] [CrossRef]
- Keller, V.; Herrando, S.; Voríšek, P.; Franch, M.; Kipson, M.; Milanesi, P.; Martí, D.; Anton, M.; Klvanová, A.; Kalyakin, M.V.; et al. European Breeding Bird Atlas 2. Distribution, Abundance and Change; European Bird Census Council & Lynx Edicions: Barcelona, Spain, 2020. [Google Scholar]
- BirdLife International. Species factsheet: Eurasian Woodcock Scolopax rusticola. Available online: https://datazone.birdlife.org/species/factsheet/eurasian-woodcock-scolopax-rusticola (accessed on 6 June 2025).
- Hadarics, T.; Zalai, T. Magyarország Madarainak Névjegyzéke—Nomenclator Avium Hungariae—An Annotated List of the Birds of Hungary; MME, BirdLife International: Budapest, Hungary, 2008; p. 118. [Google Scholar]
- Bende, A.; László, R. Woodcock (Scolopax rusticola L.) nesting in Hungary from the second half of the 19th century to the present day. Ornis Hung. 2020, 28, 92–103. [Google Scholar] [CrossRef]
- Bende, A. Spring migration dynamics, age and sex ratio, and breeding biology of the Woodcock (Scolopax rusticola L.) in Hungary. Ph.D. Thesis, University of Sopron, Sopron, Hungary, 2021. [Google Scholar]
- Faragó, S. Erdei szalonka. In Magyar Madárvonulási Atlasz; Csörgő, T., Karcza, Z., Halmos, G., Magyar, G., Gyurácz, J., Szép, T., Bankovics, A., Schmidt, A., Schmidt, E., Eds.; Kossuth Kiadó Zrt.: Budapest, Hungary, 2009; pp. 304–305. [Google Scholar]
- Le Rest, K.; Hoodless, A.; Heward, C.; Cazenave, J.L.; Ferrand, Y. Effect of weather conditions on the spring migration of Eurasian Woodcock and consequences for breeding. Ibis 2019, 161, 559–572. [Google Scholar] [CrossRef]
- Faragó, S. A vadászható vízivadfajok magyarországi vonulása jelölt madarak megkerülése alapján. Hung. Waterfowl Publ. 2000, 6, 337–375. [Google Scholar]
- Cramp, S.; Simmons, K.E.L. Handbook of the Birds of Europe, the Middle East and North Africa; Oxford University Press: Oxford, UK, 1983; Volume 3, pp. 444–457. [Google Scholar]
- Glutz von Blotzheim, U.N. Handbuch der Vögel Mitteleuropas; Band 7. Charadriiformes; AULA: Wiesbaden, Germany, 1986; pp. 121–174. [Google Scholar]
- Hagemejer, W.; Blair, M. The EBCC Atlas of European Breeding Birds: Their Distribution and Abundance; Poyser: London, UK, 1997. [Google Scholar]
- Van Gils, J.; Wiersma, P.; Kirwan, G.M. Eurasian Woodcock (Scolopax rusticola), version 1.0. In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaka, NY, USA, 2020. [Google Scholar]
- Spina, F.; Baillie, S.R.; Bairlein, F.; Fiedler, W.; Thorup, K.; The Eurasian African Bird Migration Atlas. EURING/CMS. Available online: https://migrationatlas.org (accessed on 3 April 2025).
- Schally, G. Woodcock ringing in Hungary between 1913 and 2014. WI-WSSG Newsl. 2015, 41, 33–36. [Google Scholar]
- Schally, G.; Csányi, S.; Palatitz, P. Spring migration phenology of Eurasian Woodcocks tagged with GPS-Argos transmitters in Central Europe. Ornis Fenn. 2022, 99, 104–116. [Google Scholar] [CrossRef]
- Arizaga, J.; Crespo, A.; Telletxea, I.; Ibáñez, R.; Díez, F.; Tobar, J.; Minondo, M.; Ibarrola, Z.; Fuente, J.; Pérez, J. Solar/Argos PTTs contradict ring-recovery analyses: Woodcocks wintering in Spain are found to breed further east than previously stated. J. Ornithol. 2014, 156, 515–523. [Google Scholar] [CrossRef]
- Blokhin, Y.; Solokha, A.; Gorokhowsky, K.; Mezhnev, A.; Fokin, S. Hunting bags of Woodcock, snipes and other waders in Russia. WI-WSSG Newsl. 2015, 41, 13–18. [Google Scholar]
- Lutz, M.; Jensen, F.P. Draft European Union management Plan for Woodcock Scolopax rusticola 2006–2009; European Commission (DG ENV B2): Brussels, Belgium, 2005. [Google Scholar]
- Ferrand, Y.; Gossmann, F.; Bastat, C.; Guénézan, M. Monitoring of the wintering and breeding woodcock population in France. Rev. Catalana d’Ornitologia 2008, 24, 44–52. [Google Scholar]
- Ferrand, Y.; Gossmann, F. La bécasse des Bois; Effet de lisiére: Saint-Lucien, France, 2009. [Google Scholar]
- Sheldon, W.G. The Book of the American Woodcock; The University of Massachusetts Press: Amherst, MA, USA, 1967. [Google Scholar]
- Glasgow, L.L. Contributions to the Knowledge of the Ecology of the American Woodcock, Philohela Minor, on the Wintering Range in Louisiana. Ph.D. Thesis, Texas A&M, College Station, TX, USA, 1958. [Google Scholar]
- Clausager, I. Migration of Scandinavian Woodcock (Scolopax rusticola) with special reference to Denmark. Dan. Rev. Game Biol. 1974, 8, 38. [Google Scholar]
- Fokin, S.; Blokhin, Y. Roding activity, spring hunting and hunting bags of Woodcock (Scolopax rusticola) in Russia. In Fifth European Woodcock and Snipe Workshop—Proceedings of an International Symposium of the Wetlands International/IUCN Woodcock & Snipe Specialist Group; Kalchreuter, H., Ed.; Wetlands International Global Series No. 4; International Wader Studies 11: Wageningen, The Netherlands, 2000; pp. 19–24. [Google Scholar]
- Tavecchia, G.; Pradel, R.; Gossmann, F.; Bastat, C.; Ferrand, Y.; Lebreton, J.D. Temporal variation in annual survival probability of the Eurasian woodcock Scolopax rusticola wintering in France. Wildl. Biol. 2002, 8, 21–30. [Google Scholar] [CrossRef]
- Péron, G.; Ferrand, Y.; Gossmann, F.O.; Bastat, C.; Guénézan, M.; Gimenez, O. Escape migration decisions in Eurasian Woodcocks: Insights from survival analyses using large-scale recovery data. Behav. Ecol. Sociobiol. 2001, 65, 1949–1955. [Google Scholar] [CrossRef]
- Guzmán, J.L.; Caro, J.; Arroyo, B. Factors influencing mobility and survival of Eurasian Woodcock wintering in Spain. ACE 2017, 12, 21. [Google Scholar] [CrossRef]
- Stewart, R.L.M.; Francis, C.M.; Massey, C. Age-related differential timing of spring migration within sexes in passerines. Wilson Bull. 2002, 114, 264–271. [Google Scholar] [CrossRef]
- Lehikoinen, A.; Santaharju, J.; Anders, M. Sex-specific timing of autumn migration in birds: The role of sexual size dimorphism, migration distance and differences in breeding investment. Ornis Fenn. 2017, 94, 53–65. [Google Scholar] [CrossRef]
- Pedersen, L.; Jakobsen, N.M.; Strandberg, R.; Thorup, K.; Tøttrup, A.P. Sex-specific difference in migration schedule as a precursor of protandry in a long-distance migratory bird. Sci. Nat. 2019, 106, 1–10. [Google Scholar] [CrossRef]
- Wobker, J.; Heim, W.; Schmaljohann, H. Sex, age, moulting strategy, and migration distance explain the phenology of songbirds at a stopover along the East Asian flyway. Behav. Ecol. Sociobiol. 2021, 75, 1–14. [Google Scholar] [CrossRef]
- Logan, C.A.; Hyatt, L.E. Mate attraction by autumnal song in the Northern Mockingbird (Mimus polyglottos). Auk 1991, 108, 429–432. [Google Scholar]
- Forstmeier, W. Benefits of early arrival at breeding grounds vary between males. J. Anim. Ecol. 2002, 71, 1–9. [Google Scholar] [CrossRef]
- Mills, A.M. Protogyny in autumn migration: Do male birds ”Play Chicken”? Auk 2005, 122, 71–81. [Google Scholar] [CrossRef]
- Coppack, T.; Both, C. Predicting life-cycle adaptation of migratory birds to global climate change. Ardea 2002, 55, 369–378. [Google Scholar] [CrossRef]
- Remisiewicz, M.; Wennerberg, L. Differential migration strategies of the Wood Sandpiper (Tringa glareola)–genetic analyses reveal sex differences in morphology and spring migration phenology. Ornis Fenn. 2006, 83, 1–10. [Google Scholar]
- Bozó, L.; Heim, W. Sex-specific migration of Phylloscopus warblers at a stopover site in Far Eastern Russia. Ring. Migr. 2016, 31, 41–46. [Google Scholar] [CrossRef]
- Briedis, M.; Bauer, S.; Adamík, P.; Alves, J.A.; Costa, J.S.; Emmenegger, T.; Hahn, S. A full annual perspective on sex-biased migration timing in long-distance migratory birds. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182821. [Google Scholar] [CrossRef]
- Kokko, H.; Gunnarsson, T.G.; Morrell, L.J.; Gill, J.A. Why do female migratory birds arrive later than males? J. Anim. Ecol. 2006, 75, 1293–1303. [Google Scholar] [CrossRef]
- Morbey, Y.E.; Ydenberg, R.C. Protandrous arrival timing to breeding areas: A review. Ecol. Lett. 2001, 4, 663–673. [Google Scholar] [CrossRef]
- Jaatinen, K.; Lehikoinen, A.; Lank, D.B. Female-biased sex ratios and the proportion of cryptic male morphs of migrant juvenile ruffs (Philomachus pugnax) in Finland. Ornis Fenn. 2010, 87, 125–134. [Google Scholar] [CrossRef]
- Kokko, H. Competition for early arrival in migratory birds. J. Anim. Ecol. 1999, 68, 940–950. [Google Scholar] [CrossRef]
- McKinnon, E.A.; Fraser, K.C.; Stanley, C.Q.; Stutchbury, B.J. Tracking from the tropics reveals behaviour of juvenile songbirds on their first spring migration. PLoS ONE 2014, 9, e105605. [Google Scholar] [CrossRef] [PubMed]
- Brlík, V.; Pakanen, V.M.; Jaakkonen, T.; Arppe, H.; Jokinen, J.; Lakka, J.; Blomqvist, D.; Hahn, S.; Valkama, J.; Koivula, K. Survival fluctuation is linked to precipitation variation during staging in a migratory shorebird. Sci. Rep. 2022, 12, 19830. [Google Scholar] [CrossRef]
- Carneiro, C.; Gunnarsson, T.G.; Alves, J.A. Why are whimbrels not advancing their arrival dates into Iceland? Exploring seasonal and sex-specific variation in consistency of individual timing during the annual cycle. Front. Ecol. Evol. 2018, 7, 248. [Google Scholar] [CrossRef]
- Faragó, S.; László, R.; Sándor, G. Body size, age and sex ratio of the woodcock (Scolopax rusticola) in Hungary between 1990–1999. Hung. Waterfowl Publ. 2000, 6, 409–461. [Google Scholar]
- Faragó, S. Trend of Woodcock hunting bag in Hungary and its effect on the population. WI-WSSG Newsl. 2003, 29, 6–8. [Google Scholar]
- Faragó, S.; László, R. Long-term monitoring of the Hungarian Woodcock bag during 1995–2008. In Seventh European Woodcock and Snipe Workshop—Proceedings of an International Symposium of the IUCN/WI Woodcock & Snipe Specialist Group, Office National de la Chasse et de la Faune Sauvage; Ferrand, Y., Ed.; Office National de la Chasse et de la Faune Sauvage: Paris, France, 2013; pp. 41–44. [Google Scholar]
- Faragó, S.; László, R.; Bende, A. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2010-ben Magyarországon. Hung. Waterfowl Publ. 2012, 22, 285–296. [Google Scholar]
- Faragó, S.; László, R.; Bende, A. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2011-ben Magyarországon. Hung. Waterfowl Publ. 2012, 22, 297–310. [Google Scholar]
- Faragó, S.; László, R.; Bende, A. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2012-ben. Hung. Waterfowl Publ. 2014, 24, 283–295. [Google Scholar]
- Faragó, S.; László, R.; Bende, A. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2013-ban Magyarországon. Hung. Waterfowl Publ. 2015, 25, 289–302. [Google Scholar] [CrossRef]
- Faragó, S.; László, R.; Bende, A. Development of the woodcock (Scolopax rusticola) sex ratio in Hungary between 2010–2014. In V. Faculty Scientific Conference, Conference Publication; Bidló, A., Facskó, F., Eds.; University of Sopron Publishing: Sopron, Hungary, 2015; pp. 105–107. [Google Scholar]
- Faragó, S.; László, R.; Bende, A. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2014-ben Magyarországon. Hung. Waterfowl Publ. 2016, 27, 284–296. [Google Scholar]
- Directive 79/409/EEC of 2 April 1979 on the Conservation of Wild Birds. OJ L 103, 25.4.1979, pp. 1–18. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:31979L0409 (accessed on 12 June 2025).
- Faragó, S. History of the development of spring woodcock hunting in Hungary and the reasons for its maintenance. Hung. Waterfowl Publ. 2013, 23, 311–332. [Google Scholar]
- Blokhin, Y.Y.; Artemenkov, D.V. Impact of the Hunting Ban on the Population and Hunting Bag of the Eurasian Woodcock Scolopax rusticola (Scolopacidae, Aves) in European Russia. Povolzhskii Ekol. Zhurnal 2022, 4, 375–387. [Google Scholar] [CrossRef]
- Jackson, A.C. The moults and sequence of plumages of the British waders. Brit. Birds 1919, 12, 172–179. [Google Scholar]
- Dementiev, G.P.; Gladkov, N.A. Ptitsy Sovietskogo Soyuza; State Publishing House Soviet Science: Moscow, Russia, 1951; Volume III, pp. 320–326. [Google Scholar]
- Bende, A. Az erdei szalonka (Scolopax rusticola L.) tollváltása, a tollazat alapján történő kormeghatározás lehetőségei. Magy. Vízivad Közlemények 2023, 37, 45–58. [Google Scholar] [CrossRef]
- Bende, A.; Palinkas-Bodzsar, N.; Boa, L.; László, R. Sex determination of eurasian woodcock (Scolopax rusticola L.) by genetic and imaging diagnostic methods. Biodivers. Environ. 2023, 15, 29–35. [Google Scholar]
- Cochran, W.G. Sampling Techniques, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Csanády, V. Nonlinear regressions applied to practical examples. Dimens. Math. Publ. 2013, 1, 19–26. [Google Scholar]
- Csanády, V. Double Gaussian function application. Dimens. Math. Publ. 2019, 7, 37–47. [Google Scholar]
- Parris, R. Winplot for Windows 95/98/ME/2K/XP/Vista/7 (846K). In Peanut Software; Phillips Exeter Academy: Exeter, NH, USA, 2012. [Google Scholar]
- Dell Inc. Dell Statistica, (Version 13.0) [Computer Software]; Dell Inc.: Round Rock, TX, USA, 2016. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC. Available online: https://posit.co/download/rstudio/ (accessed on 4 March 2025).
- Cristol, D.A.; Baker, M.B.; Carbone, C. Differential migration revisited: Latitudinal segregation by age and sex class. Curr. Ornithol. 1999, 15, 33–88. [Google Scholar]
- Nebel, S. Differential migration of shorebirds in the east Asian–Australasian flyway. Emu 2007, 107, 14–18. [Google Scholar] [CrossRef]
- Barter, M. Sex-related differences in adult Curlew Sandpipers Caldiris ferruginea caught in Victoria. Stilt 1986, 8, 2–8. [Google Scholar]
- Barter, M. Are Curlew Sandpipers sexist—And if so, why? Stilt 1987, 11, 14–17. [Google Scholar]
- Barter, M. Bar-tailed Godwit Limosa lapponica in Australia. Part 1: Races, breeding areas and migration routes. Stilt 1989, 14, 43–48. [Google Scholar]
- Gosbell, K.; Minton, C. The biometrics and moult of Sanderling Calidris alba in Australia. Stilt 2001, 40, 7–22. [Google Scholar]
- McCloskey, J.T.; Thompson, J.E. Sex-related differences in migration chronology and winter habitat use of Common Snipe. Wilson Bull. 2000, 112, 143–148. [Google Scholar] [CrossRef]
- Cline, B.B.; Haig, S.M. Seasonal movement, residency, and migration patterns of Wilson’s Snipe (Gallinago delicata). Auk 2008, 128, 543–555. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H. Sex ratio variation in birds. Ibis 1986, 128, 317–329. [Google Scholar] [CrossRef]
- Merán, P. Some observations on Woodcock Migration in Austria and Western Hungary 1995. IWRB-WSRG Newsl. 1996, 22, 5–6. [Google Scholar]
- Merán, P. Some observations on Woodcock Migration in Austria and Western Hungary 1996. IWRB-WSRG Newsl. 1997, 23, 4–5. [Google Scholar]
- Merán, P. Some observations on Woodcock Migration in Austria and Western Hungary 1997. IWRB-WSRG Newsl. 1998, 24, 5–6. [Google Scholar]
- Merán, P. Some observations on Woodcock Migration in Austria and Western Hungary 1998. IWRB-WSRG Newsl. 1999, 25, 4–5. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2000-ben Magyarországon. Hung. Waterfowl Publ. 2002, 9, 323–340. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2001-ben Magyarországon. Hung. Waterfowl Publ. 2003, 11, 343–359. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2002-ben Magyarországon. Hung. Waterfowl Publ. 2005, 12, 247–261. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2003-ban Magyarországon. Hung. Waterfowl Publ. 2006, 13, 235–249. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2004-ben Magyarországon. Hung. Waterfowl Publ. 2007, 14, 211–225. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2005-ben Magyarországon. Hung. Waterfowl Publ. 2007, 15, 221–235. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2006-ban Magyarországon. Hung. Waterfowl Publ. 2008, 17, 215–229. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2007-ben Magyarországon. Hung. Waterfowl Publ. 2010, 18–19, 205–220. [Google Scholar]
- Faragó, S.; László, R. Az erdei szalonka (Scolopax rusticola) teríték monitoring eredményei 2008-ban Magyarországon. Hung. Waterfowl Publ. 2010, 18–19, 421–435. [Google Scholar]
- Sterbetz, I. Magyarországi adatok az erdei szalonka (Scolopax rusticola L., 1758) nászrepülésének fényviszonyairól. Állattani Közlemények 1982, 69, 123–126. [Google Scholar]
- Fadat, C. Modalités Zoogéographiques de la Migration et de L’hivernage en France de la Bécasse des Bois (Scolopax rusticola L.) et Gestion Cynégétique de ses Populations. 3 Tomes. Ph.D. Thesis, Université Montpellier, Montpellier, France, 1989. [Google Scholar]
- Boidot, J.-P.; Cau, J.-F.; Audrousseau, G. Evaluation of the 2014/15. WI-WSSG Newsl. 2015, 41, 49–50. [Google Scholar]
- Asbóth, R.; Hopp, F.; Molnár, L.; Novics, G.; Sasvári, Z.; Szabó, Z.; Szigethy, G. More data on woodcock. Nimród 1980, 104, 450. [Google Scholar]
- Faragó, S. Hunting Zoology; Agricultural Publishing House: Budapest, Hungary, 2007; pp. 206–214. [Google Scholar]
- Marcström, V. Removal of roding Woodcock. IWRB-WSRG Newsl. 1980, 6, 63–70. [Google Scholar]
- Ostermeyer, R.; Ferrand, Y. Approche du Comportement de la Bécasse des Bois Scolopax rusticola en Période de Reproduction en Forêt Domaniale de Compiègne (OiseFrance). Essai de Mise en Place D’une Méthode D’étude par Télémétrie; Office National de la Chasse Section Bécasse: Clermont l’Hérault, France, 1979. [Google Scholar]
- Pertunnen, E. The roding male count by hunting in Finland. IWRB—Woodcock Snipe Res. Group. Newsl. 1980, 6, 17–27. [Google Scholar]
- Shorten, M. The European Woodcock (Scolopax rusticola). A Search of the Literature since 1940. Rep. Game Conserv. Trust 1974, 21, 95. [Google Scholar]
- Hirons, G. The Significance of Roding by Woodcock Scolopax rusticola: An Alternative Explanation Based on Observations of Marked Birds. Ibis 1980, 122, 350–354. [Google Scholar] [CrossRef]
- Christensen, T.K.; Asferg, T. Woodcock hunting in Denmark–Status and recent changes. In Seventh European Woodcock and Snipe Workshop—Proceedings of an International Symposium of the IUCN/Wetlands International Woodcock & Snipe Specialist Group; Ferrand, Y., Ed.; ONCFS Publication: Paris, France, 2013; pp. 36–40. [Google Scholar]
- Cau, J.-F. Synthèse Nationale des lectures d’alies et études pondérales. Diagnose des echantillons sex-ratio age ratio repartition spatio-temporelle des oiseaux variations ponderales muses suspendues. Mordorée 2002, 224, 321–331. [Google Scholar]
- Cau, J.-F. Bilans concernant certains paramenters biomeriques releves cheé la becasse des bois sur le territorie Francais au niveau national, regional et departemental. Mordorée 2003, 228, 286–303. [Google Scholar]
- Cau, J.-F. Bilans concernant certains parameters biometriques et biologiques releves chez la becasse des bois sur le territoire Francais. Mordorée 2004, 232, 268–284. [Google Scholar]
- Cau, J.-F. Répartition des échantillons recus par département. Mordorée 2005, 236, 311–327. [Google Scholar]
- Cau, J.-F. La saison bécassiérre 2007/2008 par le C.N.B. Mordorée 2008, 247, 225–251. [Google Scholar]
- Boidot, J.-P. Répartition des échantillons recus par département saison 05/06. Mordorée 2006, 240, 319–331. [Google Scholar]
- Boidot, J.-P. Bilans CNB 2006/2007. La Mordorée 2007, 244, 337–347. [Google Scholar]
- Boidot, J.-P. La saison bécassiérre 2008/2009 par le C.N.B. Mordorée 2009, 252, 333–343. [Google Scholar]
- Boidot, J.-P. Bilans concernant certains paramèters biometriques et biologiques relevés chez la bécasse des bois lors de la saison 2009/2010 en France. Mordorée 2010, 256, 370–382. [Google Scholar]
- Boidot, J.-P. Bilans concernant certains paramèters biometriques et biologiques relevés chez la bécasse des bois lors de la saison 2010/2011 en France. Mordorée 2011, 259, 181–195. [Google Scholar]
- Boidot, J.-P. Bilans concernant certains paramèters biometriques et biologiques relevés chez la bécasse des bois lors de la saison 2011/2012 en France. Mordorée 2012, 263, 42–55. [Google Scholar]
- Aurousseau, G. Compte-rendu saison 2012/2013. Bilans concernant certains paramèters biometriques et biologiques relevés chez la bécasse des bois lors de la saison 2012/2013 en France. Mordorée 2013, 268, 3–13. [Google Scholar]
- Aurousseau, G. Saison Bécassière saison 2016–2017. Chass. Pays d’Azur 2017, 64, 12–14. [Google Scholar]
- Lepetit, J.-P. Bilans concernant certains paramèters biometriques et biologiques relevés chez la bécasse des bois lors de la saison 2013/2014 en France. Mordorée 2014, 271, 53–67. [Google Scholar]
- Bagnol, G. Tableau de Bord Saison 2017/2018. Fédération Départementale des Chasseurs du Gard 182 Route de Sauve—BP 57012—30910 NIMES Cedex 2. 2018; pp. 62–63.
- Mendall, H.L.; Aldous, C.M. The Ecology and Management of the American Woodcocok; USGS Maine Cooperative Fish and Wildlife Research Unit, University of Maine: Orono, ME, USA, 1943. [Google Scholar]
- Greeley, F. Sex and age studies in fall-shot woodcock (Philohela minor) from southern Wisconsin. J. Wildl. Manag. 1953, 17, 29–32. [Google Scholar] [CrossRef]
- Blankenship, L.H. Investigations of the American Woodcock in Michigan. Mich. Dep. Consum. Rep. 1957, 2123, 217. [Google Scholar]
- Prieto, N.; Tavecchia, G.; Telletxea, I.; Ibañez, R.; Ansorregi, F.; Galdos, A.; Urruzola, A.; Iriarte, I.; Arizaga, J. Survival probabilities of wintering Eurasian Woodcocks Scolopax rusticola in northern Spain reveal a direct link with hunting regimes. J. Ornithol. 2019, 160, 329–336. [Google Scholar] [CrossRef]
- Bende, A.; Faragó, S. Woodcock (Scolopax rusticola L.) management plan in Hungary. Hung. Waterfowl Publ. 2023, 37, 71–105. [Google Scholar]

| Year | Bagged Specimens | Known Sex (Specimens) | Known Age (Specimens) | Sex | Age | Sex and Age | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ♂ | ♀ | Ad. | Juv. | Ad. ♂ | Juv. ♂ | Ad. ♀ | Juv. ♀ | ||||
| 2010 | 2389 | 2385 | 2386 | 1988 | 397 | 1511 | 875 | 1278 | 709 | 230 | 166 |
| 2011 | 3402 | 3392 | 1913 | 2844 | 548 | 997 | 916 | 844 | 767 | 150 | 147 |
| 2012 | 1941 | 1925 | 1162 | 1671 | 354 | 476 | 686 | 370 | 573 | 105 | 112 |
| 2013 | 2913 | 2901 | 1738 | 2411 | 490 | 939 | 799 | 777 | 666 | 156 | 132 |
| 2014 | 2727 | 2705 | 1639 | 2250 | 455 | 766 | 873 | 646 | 727 | 120 | 143 |
| 2015 | 2787 | 2751 | 1661 | 2367 | 384 | 755 | 906 | 618 | 795 | 128 | 94 |
| 2016 | 2215 | 2192 | 1257 | 1784 | 408 | 605 | 652 | 469 | 539 | 127 | 104 |
| 2017 | 1683 | 1661 | 1325 | 1255 | 406 | 661 | 664 | 482 | 517 | 174 | 138 |
| 2018 | 2349 | 2267 | 2132 | 1876 | 391 | 1117 | 1015 | 891 | 811 | 182 | 174 |
| 2019 | 1761 | 1703 | 1694 | 1397 | 306 | 793 | 901 | 619 | 722 | 146 | 151 |
| Sum. | 24,167 | 23,882 | 16,907 | 19,843 | 4139 | 8620 | 8287 | 6994 | 6826 | 1518 | 1361 |
| Sex | Extreme Value Coordinates | Regression Coefficient (R) | |
|---|---|---|---|
| T Max. | N Max. | ||
| ♂ | 18 | 115 | 0.9768 |
| 23 | 121 | ||
| ♀ | 17 | 23 | 0.9539 |
| 24 | 28 | ||
| Sex × Age | |||
| Ad. ♂ | 21 | 30 | 0.9629 |
| Ad. ♀ | 17 | 8 | 0.8228 |
| 23 | 9 | ||
| Juv. ♂ | 19 | 43 | 0.9772 |
| 23 | 49 | ||
| Juv. ♀ | 23 | 10 | 0.9177 |
| Predictor | Estimate | 95% CI (Estimates) | Z-Value | p-Value |
|---|---|---|---|---|
| Constant | 0.8829 | [0.6974, 1.068] | 9.91 | p < 0.001 |
| Proportion ♀, ♂ | 0.0154 | [−0.3716, 0.4024] | 0.12 | p = 0.9045 |
| Proportion ad., juv. | 0.1007 | [−0.0215, 0.223] | 1.63 | p = 0.1033 |
| Proportion ♀, ♂ × ♀ Proportion ad., juv. | 0.0041 | [−0.0912, 0.0994] | 0.08 | p = 0.9332 |
| AR(1) (ar.L1) | 0.8854 | [0.8231, 0.9477] | 23.6 | p < 0.001 |
| MA(1) (ma.L1) | −0.4031 | [−0.5240, −0.2823] | −6.44 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bende, A.; Faragó, S.; László, R.; Csanády, V.; Fekete, I.; Pecsics, T.; Bozó, L. Dynamics of Sex and Age Correlation of Eurasian Woodcock (Scolopax rusticola L.) During Spring Migration in Hungary. Birds 2025, 6, 30. https://doi.org/10.3390/birds6020030
Bende A, Faragó S, László R, Csanády V, Fekete I, Pecsics T, Bozó L. Dynamics of Sex and Age Correlation of Eurasian Woodcock (Scolopax rusticola L.) During Spring Migration in Hungary. Birds. 2025; 6(2):30. https://doi.org/10.3390/birds6020030
Chicago/Turabian StyleBende, Attila, Sándor Faragó, Richárd László, Viktória Csanády, István Fekete, Tibor Pecsics, and László Bozó. 2025. "Dynamics of Sex and Age Correlation of Eurasian Woodcock (Scolopax rusticola L.) During Spring Migration in Hungary" Birds 6, no. 2: 30. https://doi.org/10.3390/birds6020030
APA StyleBende, A., Faragó, S., László, R., Csanády, V., Fekete, I., Pecsics, T., & Bozó, L. (2025). Dynamics of Sex and Age Correlation of Eurasian Woodcock (Scolopax rusticola L.) During Spring Migration in Hungary. Birds, 6(2), 30. https://doi.org/10.3390/birds6020030






