Influence of Nesting Habitat and Nest Emplacement on the Breeding Success of the Black Francolin (Francolinus francolinus, Phasianidae): A Case Study from Pakistan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
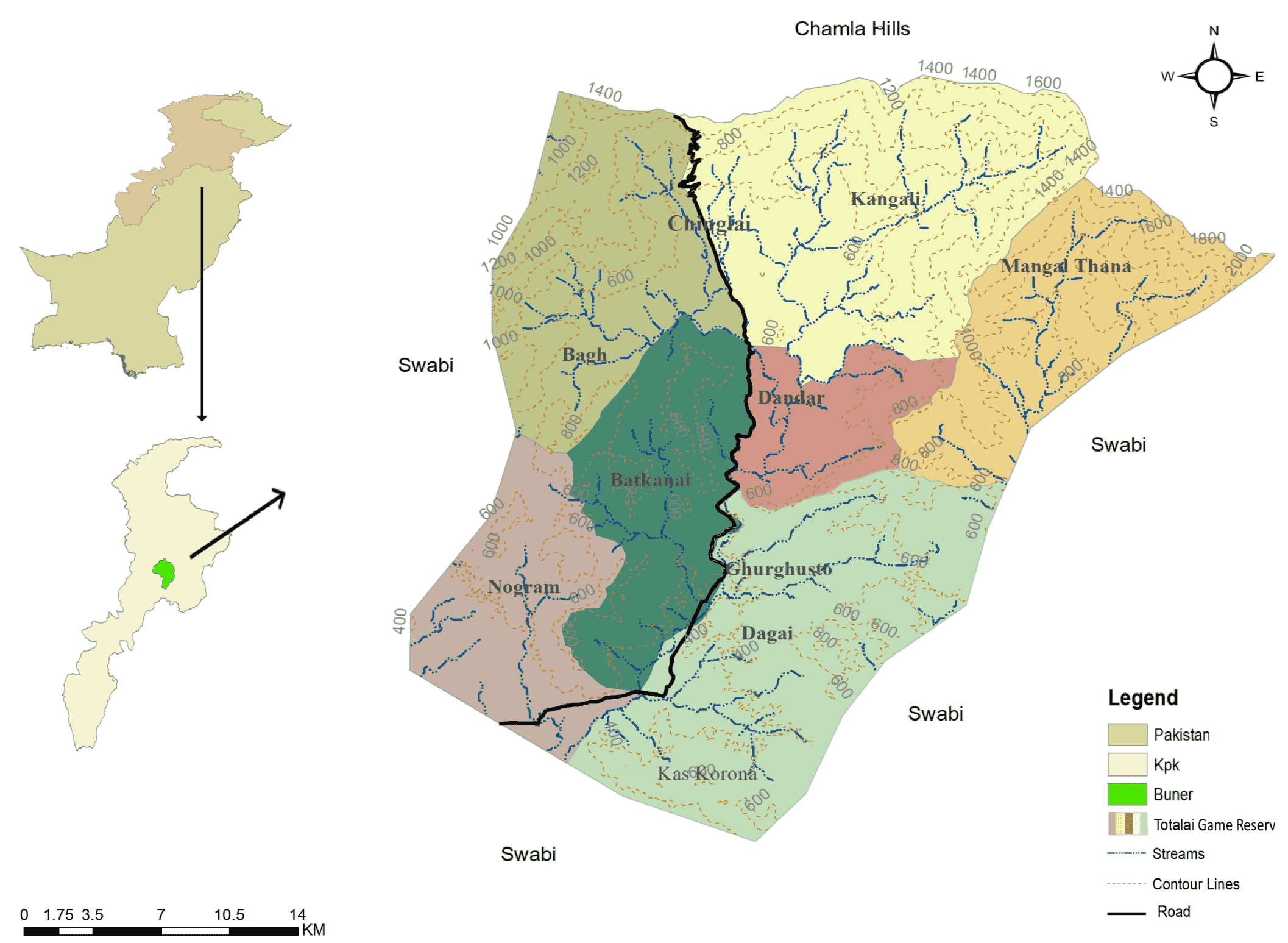
2.1. Study Area
2.2. Data Collection
2.2.1. Nesting Monitoring and Breeding Survey Methodology (BSM)
2.2.2. Nest Identification and Measurement Parameters
2.2.3. Measurement of Black Francolin Eggs
2.2.4. Assessment of Breeding Success of Black Francolin
2.3. Statistical Analysis of the Data
3. Results
3.1. Breeding Chronology
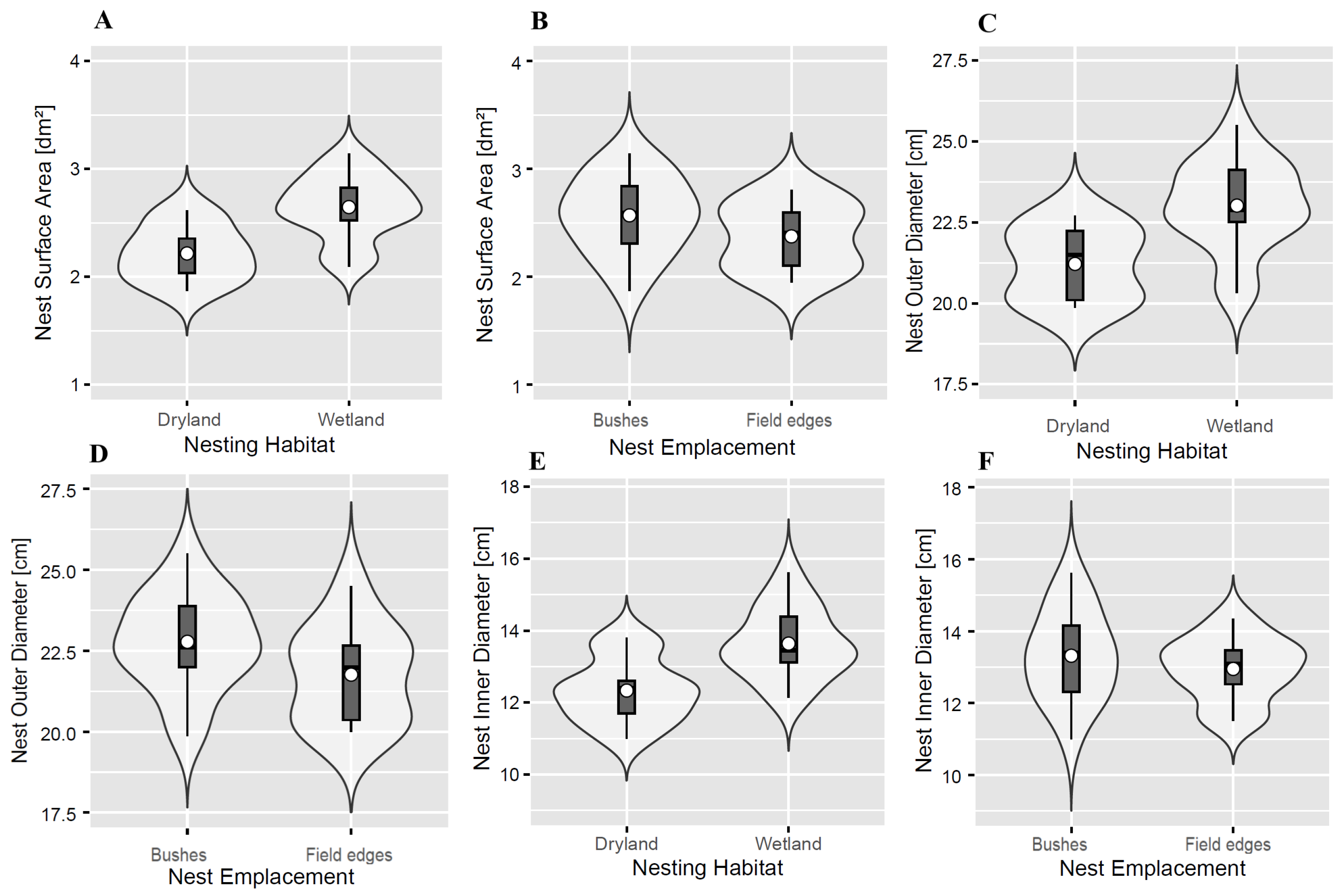
3.2. Placement and Measurements of Black Francolin Nests
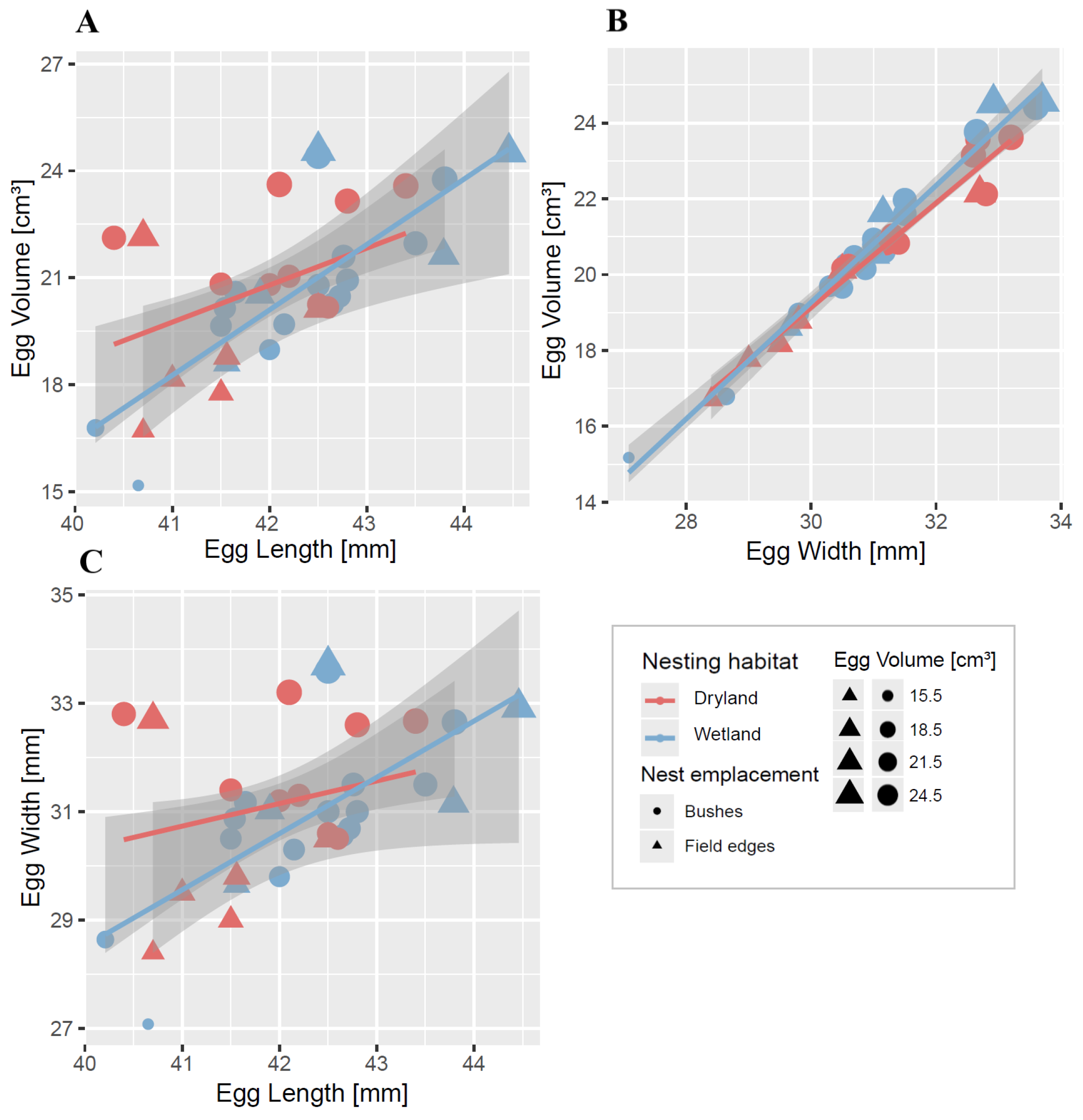
3.3. Characteristics of Black Francolin Eggs
3.4. Breeding Parameter Distributions Across Different Clutch Sizes
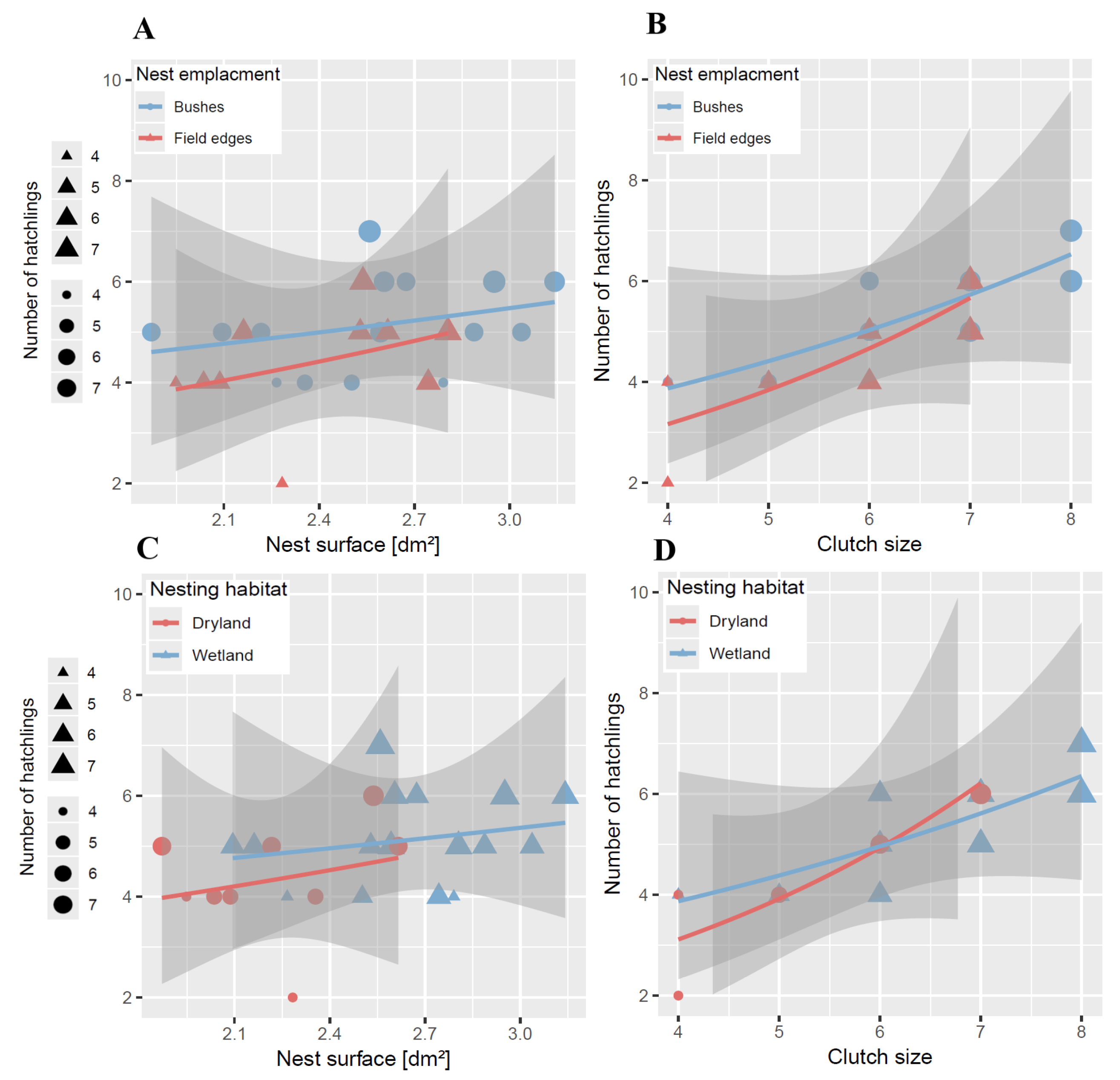
3.5. Impact of Nest Traits on Clutch Size
3.6. Variation in Hatchling Numbers
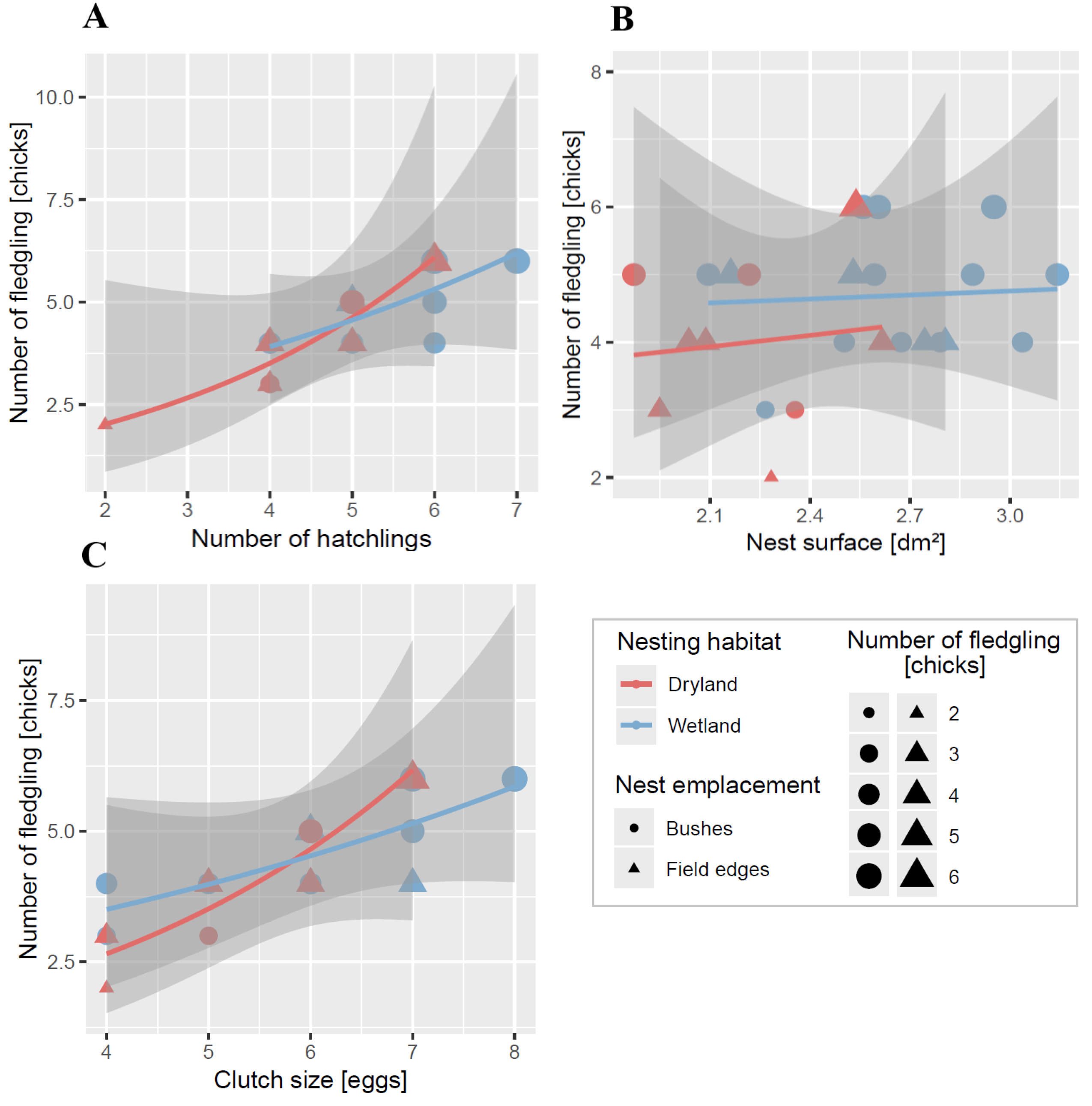
3.7. The Influence of Clutch Size, Hatching Success, and Nest Surface on the Number of Fledglings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forcina, G.; Guerrini, M.; Zeder, M.A.; Barbanera, F. The Black Francolin: Assessing the Origins of a Prized Courtly Bird in an Interdisciplinary Manner. In Animals and Courts: Europe, c. 1200–1800; Hengerer, M., Weber, N., Eds.; De Gruyter: Berlin, Germany, 2019; pp. 43–54. [Google Scholar]
- Kumar, S. Environmental Contaminants and Their Impact on Wildlife. In Toxicology and Human Health: Environmental Exposures and Biomarkers; Gupta, R.C., Abdelrasoul, G.N., Kucuk, O., Eds.; Springer: Cham, Switzerland, 2023; pp. 3–26. [Google Scholar]
- Fisher, Z.S.Y.; Cartwright, S.; Bealey, C.; Rayaleh, H.A.; McGowan, P.; Milner-Gulland, E.J. The Djibouti Francolin and Juniper Forest in Djibouti: The Need for Both Ecosystem and Species-Specific Conservation. Oryx 2009, 43, 542–551. [Google Scholar] [CrossRef]
- Boye, P. On the distribution and status of the Black Francolin, Francolinus francolinus, in Cyprus. Zool. Middle East 1990, 4, 17–22. [Google Scholar] [CrossRef]
- Forcina, G.; Guerrini, M.; Khaliq, I.; Khan, A.A.; Barbanera, F. Human-Modified Biogeographic Patterns and Conservation in Game Birds: The Dilemma of the Black Francolin (Francolinus francolinus, Phasianidae) in Pakistan. PLoS ONE 2018, 13, e0205059. [Google Scholar] [CrossRef]
- Khan, M. Studies on the Biology, Habitat, Distribution Pattern and Food of the Black Partridge (Francolinus francolinus) in Tehsil, Faisalabad [Pakistan]; UAF: Faisalabad, Pakistan, 1989; 118p. [Google Scholar]
- Mahmood, S.; Mahmood, T.; Rais, M.; Qureshi, I.Z.; Nadeem, M.S. A Comparative Study on the Populations and Habitats of the Grey Francolin (Francolinus pondicerianus) and the Black Francolin (Francolinus francolinus) in Lehri Nature Park, Punjab, Pakistan. Podoces 2010, 5, 42–53. [Google Scholar]
- Mandiwana-Neudani, T.G.; Little, R.M.; Crowe, T.M.; Bowie, R.C.K. Taxonomy, Phylogeny and Biogeography of ’True’ Francolins: Galliformes, Phasianidae, Phasianinae, Gallini; Francolinus, Ortygornis, Afrocolinus gen. nov., Peliperdix and Scleroptila spp. Ostrich 2019, 90, 191–221. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, D.K.; Lochan, R.; Dewan, S.; Negi, S. Relative Abundance, Habitat Preference, and Breeding Ecology of Asian Black Francolin, Francolinus francolinus asiae (Bonaparte, 1856) (Galliformes: Phasianidae) from North-Western Himalaya. J. Asia-Pac. Biodivers. 2020, 13, 162–168. [Google Scholar] [CrossRef]
- Shahriari, A. Distribution, population, and ecology of Black Francolin Francolinus francolinus bogdanovi on the Sistan Plain, in relation to plant coverage and drought. Podoces 2009, 4, 28–36. [Google Scholar]
- Negi, P.; Lakhera, P.C. Breeding habitat preference of Black Francolin (Francolinus francolinus asiae) in Chamoli district of Uttarakhand, Western Himalaya. Int. J. Adv. Res. 2015, 3, 540–544. [Google Scholar]
- Kakakhel, S.F.B.; Haq, N.U.; Haq, E.U. Captive Breeding and Reintroduction of Black Francolin, Grey Francolin and Chukar Partridge (2015–2020) in District Dir Lower, Khyber Pakhtunkhwa, Pakistan. Eur. J. Biol. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Negi, P.; Lakhera, P. Distribution Pattern and Habitat Preference of the Black Francolin (Francolinus francolinus asiae) in Uttarakhand, India. Zool. Ecol. 2019, 29, 86–92. [Google Scholar] [CrossRef]
- Siliguri, J. Black Francolin (Francolinus francolinus) in Bangladesh: Breeding Biology, Status, Threats and Conservation. Forktail 2014, 30, 142–143. [Google Scholar]
- Khan, M.F.; Awan, M.S.; Nayyer, A.Q.; Mehmood, K.; Khattak, M.N.K. A Comparative Study on the Population and Habitats of the Grey Francolin (Francolinus pondicerianus) and Black Francolin (Francolinus francolinus) in Mang Game Reserve, Haripur, Pakistan. J. Anim. Plant Sci. 2015, 25, 101–107. [Google Scholar]
- Basit, A.; Anwar, M.; Rakha, B.A.; Ansari, M.S.; Munawar, N.; Akhter, A. Population density of Black Francolin (Francolinus francolinus L.) in Kala Chitta range, Pakistan. JAPS J. Anim. Plant Sci. 2021, 31, 1537–1541. [Google Scholar]
- Fierro-Calderón, K.; Loaiza-Muñoz, M.; Sánchez-Martínez, M.A.; Ocampo, D.; David, S.; Greeney, H.F.; Londoño, G.A. Methods for collecting data about the breeding biology of Neotropical birds. J. Field Ornithol. 2021, 92, 315–341. [Google Scholar] [CrossRef]
- Crowe, T.M.; Harley, E.H.; Jakutowicz, M.B.; Komen, J.; Crowe, A.A. Phylogenetic, Taxonomic and Biogeographical Implications of Genetic, Morphological, and Behavioral Variation in Francolins (Phasianidae: Francolinus). Auk 1992, 109, 24–42. [Google Scholar] [CrossRef][Green Version]
- Hilaluddin; Shah, J.N.; Shawl, T.A. Nest site selection and breeding success by Cattle Egret and Little Egret in Amroha, Uttar Pradesh, India. Waterbirds 2003, 26, 444–448. [Google Scholar] [CrossRef]
- Kazantzidis, S.; Goutner, V.; Pyrovetsi, M.; Sinis, A. Comparative Nest Site Selection and Breeding Success in Two Sympatric Ardeids, Black-crowned Night-Heron (Nycticorax nycticorax) and Little Egret (Egretta garzetta) in the Axios Delta, Macedonia, Greece. Colon. Waterbirds 1997, 20, 505–517. [Google Scholar] [CrossRef]
- Ashoori, A.; Barati, A. Breeding success of Black-crowned Night Heron (Nycticorax nycticorax), Little Egret (Egretta garzetta) and Cattle Egret (Bubulcus ibis) (Aves: Ardeidae) in relation to nest height in the South Caspian Sea. Ital. J. Zool. 2013, 80, 149–154. [Google Scholar] [CrossRef]
- Zhao, J.M.; Yang, C.; Lou, Y.Q.; Shi, M.; Fang, Y.; Sun, Y.H. Nesting season, nest age, and disturbance, but not habitat characteristics, affect nest survival of Chinese grouse. Curr. Zool. 2020, 66, 29–37. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Deeming, D.C. Nest Design and Breeding Success: Replicability of Methodologies and Research Findings in Secondary Hole Nesting Passerines. Birds 2024, 5, 278–307. [Google Scholar] [CrossRef]
- Palmer, W.E. Pheasants, Partridges, and Grouse: A Guide to the Pheasants, Partridges, Quails, Grouse, Guineafowl, Buttonquails, and Sandgrouse of the World. Ornithology 2003, 120, 920. [Google Scholar] [CrossRef]
- Suárez, F.; Morales, M.B.; Mínguez, I.; Herranz, J. Seasonal variation in nest mass and dimensions in an open-cup ground-nesting shrub-steppe passerine: The Tawny Pipit Anthus campestris. Ardeola 2005, 52, 43–52. [Google Scholar]
- Persson, I.; Göransson, G. Nest attendance during egg laying in pheasants. Anim. Behav. 1999, 58, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Green, R.E. Breeding biology. In Bird Ecology and Conservation: A Handbook of Techniques; Sutherland, W.J., Newton, I., Green, R.E., Eds.; University Press: Oxford, UK, 2004; pp. 57–84. [Google Scholar]
- Dad, K.; Khan, S.; Akhtar, N.; Saeed, K. Exploring the wild avian fauna of Totalai Game Reserve district Buner, Khyber Pakhtunkhwa. J. Biodivers. Environ. Sci. 2014, 5, 150–157. [Google Scholar]
- Zippenfenig, P. Open-Meteo.com Weather API [Computer Software]. Zenodo 2023, 7970649. [Google Scholar] [CrossRef]
- Calladine, J.; Buner, F.; Aebischer, N.J. Temporal variations in the singing activity and the detection of Turtle Doves Streptopelia turtur: Implications for surveys. Bird Study 1999, 46, 74–80. [Google Scholar] [CrossRef]
- Little, R.; Crowe, T. The Breeding Biology of the Greywing Francolin Francolinus africanus and Its Implications for Hunting and Management. Afr. Zool. 1993, 28, 6–12. [Google Scholar]
- Fan, S.; Zhang, J.; Duan, Y.; Luo, X. First Description of the Breeding Biology of the Spectacled Fulvetta (Fulvetta ruficapilla sordidior) in Southwest China. Animals 2023, 13, 2157. [Google Scholar] [CrossRef]
- Metallaoui, S.; Dziri, H.; Bousseheba, A.; Heddam, S.; Chenchouni, H. Breeding ecology of the Cattle Egret (Bubulcus ibis) in Guerbes-Sanhadja wetlands of Algeria. Reg. Stud. Mar. Sci. 2020, 33, 100979. [Google Scholar] [CrossRef]
- Pietz, P.J.; Granfors, D.A. Identifying predators and fates of grassland passerine nests using miniature video cameras. J. Wildl. Manag. 2000, 64, 71–87. [Google Scholar] [CrossRef]
- Soler, J.J. Functional significance of nest size variation in the Rufous-bush Robin Cercotrichas galactotes. Ardea 1998, 86, 185–196. [Google Scholar]
- Campbell, B.; Lack, E. A Dictionary of Birds; A&C Black: London, UK, 2011; Volume 108. [Google Scholar]
- Narushin, V. Egg geometry calculation using the measurements of length and breadth. Poult. Sci. 2005, 84, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Panda, P. Shape and texture. In Text Book on Egg and Poultry Technology; Vikas Publishing House: New Delhi, India, 1996; Volume 426, p. 427. [Google Scholar]
- Parejo, D.; Sánchez-Guzmán, J.M.; Avilés, J.M. Breeding biology of the Cattle Egret Bubulcus ibis in southwest Spain. Bird Study 2001, 48, 367–372. [Google Scholar] [CrossRef]
- Jehle, G.; Yackel Adams, A.A.; Savidge, J.A.; Skagen, S.K. Nest Survival Estimation: A Review of Alternatives to the Mayfield Estimator. Condor 2004, 106, 472–484. [Google Scholar] [CrossRef]
- McCulloch, C.E.; Searle, S.R. Generalized Linear Mixed Models (GLMMs). In Wiley Series in Probability and Statistics; Shewhart, W.A., Wilks, S.S., Eds.; Wiley: New York, NY, USA, 2000; pp. 220–246. [Google Scholar]
- Akresh, M.E.; Ardia, D.R.; King, D.I. Effect of nest characteristics on thermal properties, clutch size, and reproductive performance for an open-cup nesting songbird. Avian Biol. Res. 2017, 10, 107–118. [Google Scholar] [CrossRef]
- Cardoni, D.A.; Isacch, J.P.; Baladrón, A. Causes and consequences of nest mass and structure variation in the Bay-capped Wren-spinetail Spartonoica maluroides. Acta Ornithol. 2017, 52, 51–58. [Google Scholar] [CrossRef]
- Kang, K.H.; Nam, K.B.; Kim, J.S.; Yoo, J.C. Nest characteristics and composition of the colonial nesting Azure-winged magpie Cyanopica cyanus in South Korea. PeerJ 2022, 10, e13637. [Google Scholar] [CrossRef]
- Soler, J.J.; De Neve, L.; Martínez, J.G.; Soler, M. Nest size affects clutch size and the start of incubation in magpies: An experimental study. Behav. Ecol. 2001, 12, 301–307. [Google Scholar] [CrossRef]
- De Neve, L.; Soler, J.J.; Soler, M.; Pérez-Contreras, T. Nest size predicts the effect of food supplementation to magpie nestlings on their immunocompetence: An experimental test of nest size indicating parental ability. Behav. Ecol. 2004, 15, 1031–1036. [Google Scholar] [CrossRef]
- Clauser, A.J.; McRae, S.B. King Rails (Rallus elegans) vary building effort and nest height in relation to water level. Waterbirds 2016, 39, 268–276. [Google Scholar] [CrossRef]
- Broughton, R.K.; Parry, W. A Long-tailed Tit Aegithalos caudatus Nest Constructed from Plastic Fibres Supports the Theory of Concealment by Light Reflectance. Ringing Migr. 2019, 34, 120–123. [Google Scholar] [CrossRef]
- Alambiaga, I.; Álvarez, E.; Diez-Méndez, D.; Verdejo, J.; Barba, E. “The Tale of the Three Little Tits”: Different Nest Building Solutions Under the Same Environmental Pressures. Avian Biol. Res. 2020, 13, 49–56. [Google Scholar] [CrossRef]
- Bump, G.; Bump, J.W. A Study and Review of the Black Francolin and the Gray Francolin; Number 81; US Department of the Interior, Bureau of Sport Fisheries and Wildlife: Washington, DC, USA, 1964. [Google Scholar]
- Khan, W.A. Studies on the Comparative Ecology of the South Persian Black Partridge, Francolinus francolinus henrici, and the Northern Grey Partridge, Francolinus pondicerianus interpositus, in Lal Suhanra National Park, Bahawalpur, Punjab, Pakistan. Ph.D. Thesis, Pir Mehr Ali Shah Arid Agriculture University, Rawalpindi, Pakistan, 2010. [Google Scholar]
- Ali, S.; Ripley, S.D. Handbook of the Birds of India and Pakistan; Oxford University Press: Oxford, UK; BNHS: Mumbai, India, 1983; Volume 32, p. 10. [Google Scholar]
- Pomeroy, D. Estimating Black Francolin Francolinus francolinus numbers in western Cyprus. Sandgrouse 2014, 36, 181–188. [Google Scholar]
- Baker, E.C.S. The Game Birds of India, Burma, and Ceylon. Pheasants and Bustard-Quail. Vol. III. Auk 1931, 48, 144–145. [Google Scholar] [CrossRef]
- Mentis, M.T.; Bigalke, R.C. Breeding, social behaviour and management of Greywing and Redwing Francolins. S. Afr. J. Wildl. Res. 1981, 11, 133–139. [Google Scholar]
- Robertson, P.A.; Dellelegn, Y.; Dejene, S.; Shimelis, A.; Alemayehu, M. Harwood’s Francolin Francolinus harwoodi: Recent observations on its status, distribution, habitat requirements, behaviour and threats. Bird Conserv. Int. 1997, 7, 275–282. [Google Scholar] [CrossRef]
- Hafner, H.; Fasola, M. The relationship between feeding habitat and colonially nesting Ardeidae. Manag. Mediterr. Wetl. Their Birds 1992, 20, 194–201. [Google Scholar]
- Weber, W.J. Notes on Cattle Egret Breeding. Auk 1975, 92, 111–117. [Google Scholar] [CrossRef]
- Berggren, H.; Nordahl, O.; Tibblin, P.; Larsson, P.; Forsman, A. Testing for local adaptation to spawning habitat in sympatric subpopulations of pike by reciprocal translocation of embryos. PLoS ONE 2016, 11, e0154488. [Google Scholar] [CrossRef]
- Riaz, M.; Khan, A.A.; Babar, M.; Akhter, N.; Muhammad, S.; Khaliq, I. High genetic diversity revealed by RAPD markers in the Black Francolin (Francolinus francolinus, Galliformes) of Pakistan. Pak. J. Zool. 2011, 43, 5. [Google Scholar]
- Walsberg, G.E. Nest-site Selection and the Radiative Environment of the Warbling Vireo. Condor 1981, 83, 86–88. [Google Scholar] [CrossRef]
- Khan, W.A.; Afsar Mian, A.M. Comparative Population Biology of Black (Francolinus francolinus) and Grey (F. pondicerianus) Francolins under Lal Suhanra National Park (Pakistan) Conditions. Pak. J. Zool. 2013, 45, 949–958. [Google Scholar]
- Van Niekerk, J. Social and Breeding Behaviour of the Crested Francolin in the Rustenburg District, South Africa. S. Afr. J. Wildl. Res.-24-Mon. Delayed Open Access 2001, 31, 35–42. [Google Scholar]
- Khalil, S.; Anwar, M.; Hussain, I. Breeding Biology of Grey Francolin (Francolinus pondicerianus) in Salt Range, Pakistan. Pak. J. Zool. 2016, 48, 115–123. [Google Scholar]
- Dhondt, A.A.; Kempenaers, B.; Adriaensen, F. Density-Dependent Clutch Size Caused by Habitat Heterogeneity. J. Anim. Ecol. 1992, 61, 643–648. [Google Scholar] [CrossRef]
- Julliard, R.; McCleery, R.H.; Clobert, J.; Perrins, C.M. Phenotypic Adjustment of Clutch Size Due to Nest Predation in the Great Tit. Ecology 1997, 78, 394–404. [Google Scholar] [CrossRef]
- Van Noordwijk, A.; Van Balen, J.; Scharloo, W. Heritability of Ecologically Important Traits in the Great Tit. Ardea 1980, 55, 193–203. [Google Scholar] [CrossRef]
- Postma, E.; van Noordwijk, A.J. Genetic Variation for Clutch Size in Natural Populations of Birds from a Reaction Norm Perspective. Ecology 2005, 86, 2344–2357. [Google Scholar] [CrossRef]
- Pettifor, R.A.; Perrins, C.M.; McCleery, R.H. Individual Optimization of Clutch Size in Great Tits. Nature 1988, 336, 160–162. [Google Scholar] [CrossRef]
- Hume, A.O.; Oates, E.W. The Nests and Eggs of Indian Birds; R. H. Porter: London, UK, 1889. [Google Scholar] [CrossRef]
- Hussain, I.; Khalil, S. Population Biology of the Grey Francolin (Francolinus pondicerianus) in an Agro-Ecosystem of the Pothwar Plateau, Pakistan. Avian Res. 2012, 3, 91–102. [Google Scholar] [CrossRef][Green Version]
- Lee, J.H.; Kim, S.Y.; Sung, H.C. Nest Site Selection, Nest Characteristics, and Breeding Ecology of the Eurasian Tree Sparrow, Passer montanus, Living in an Urban Area. Anim. Taxon. Ecol. 2024, 70, 46–60. [Google Scholar] [CrossRef]
- Klomp, H. The Determination of Clutch-Size in Birds: A Review. Ardea 1970, 55, 1–124. [Google Scholar] [CrossRef]
- Martyka, R.; Arct, A.; Kotowska, D.; Gustafsson, L. Age- and trait-dependent breeding responses to environmental variation in a short-lived songbird. Sci. Rep. 2023, 13, 14967. [Google Scholar] [CrossRef]
- Hamann, J.; Cooke, F. Age effects on clutch size and laying dates of individual female lesser Snow Geese Anser caerulescens. Ibis 1987, 129, 527–532. [Google Scholar] [CrossRef]
- Tobolka, M.; Dylewski, L.; Wozna, J.T.; Zolnierowicz, K.M. How weather conditions in non-breeding and breeding grounds affect the phenology and breeding abilities of White Storks. Sci. Total Environ. 2018, 636, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Hipfner, J.M.; Gaston, A.J.; de Forest, L.N. The role of female age in determining egg size and laying date of Thick-billed Murres. J. Avian Biol. 1997, 28, 271–278. [Google Scholar] [CrossRef]
- Mazgajski, T.D. Nest site choice in relation to the presence of old nests and cavity depth in the starling Sturnus vulgaris. Ethol. Ecol. Evol. 2003, 15, 273–281. [Google Scholar] [CrossRef]
- Stantial, M.L.; Cohen, J.B.; Darrah, A.J.; Farrell, S.; Maslo, B. Habitat-specific behavior, growth rate, and survival of Piping Plover chicks in New Jersey, USA. Ecosphere 2021, 12, e03782. [Google Scholar] [CrossRef]


| Egg Parameters | Statistics | Nesting Habitat | Nest Emplacement | Overall | ||
|---|---|---|---|---|---|---|
| Wetland (n = 20) | Dryland (n = 15) | Bushes (n = 24) | Field Edges (n = 11) | (n = 35) | ||
| Length [mm] | Mean ± SD | 42.4 ± 1.0 | 41.8 ± 0.9 | 42.2 ± 0.9 | 42.0 ± 1.2 | 42.0 ± 1.0 |
| IQR | 1.1 | 1.3 | 1.1 | 1.2 | 1.2 | |
| Range | [40–45] | [40–44] | [40–44] | [40–45] | [40–45] | |
| Width [mm] | Mean ± SD | 30.9 ± 1.6 | 31.1 ± 1.5 | 31.4 ± 1.2 | 30.8 ± 1.7 | 31.3 ± 1.6 |
| IQR | 1.0 | 2.5 | 1.2 | 2.4 | 1.8 | |
| Range | [27–34] | [28–33] | [27–34] | [28–34] | [27–34] | |
| Volume [cm3] | Mean ± SD | 20.7 ± 2.4 | 20.6 ± 2.1 | 20.9 ± 2.1 | 20.3 ± 2.7 | 20.6 ± 2.3 |
| IQR | 2.0 | 2.7 | 1.9 | 3.5 | 2.5 | |
| Range | [15–25] | [16–24] | [15–25] | [16–25] | [15–25] | |
| Shape index [%] | Mean ± SD | 73.1 ± 2.8 | 74.3 ± 3.6 | 73.6 ± 3.0 | 73.2 ± 3.6 | 73.6 ± 3.2 |
| IQR | 2.5 | 4.2 | 3.1 | 2.1 | 3.2 | |
| Range | [66–79] | [69–81] | [66–82] | [69–80] | [66–82] | |
| Variables | Estimate | Std. Error | df | t-Value | p-Value |
|---|---|---|---|---|---|
| Gaussian GLMM (Egg Volume) | |||||
| (Intercept) | −44.7 | 2.1 | 23 | −20.9 | 0.001 |
| Egg length | 0.6 | 0.1 | 23 | 13.4 | 0.001 |
| Egg width | 1.4 | 0.04 | 23 | 40.3 | 0.001 |
| Wetland habitat | 6.1 | 2.5 | 23 | 2.5 | 0.02 |
| Field edges | 8.1 | 3.5 | 23 | 2.3 | 0.03 |
| Wetland habitat × Field edges | −12.3 | 4.1 | 23 | −3.0 | 0.006 |
| Egg length × Field edges | −0.1 | 0.1 | 23 | −1.6 | 0.1 |
| Egg length × Wetland habitat | −0.1 | 0.1 | 23 | −1.6 | 0.1 |
| Egg width × Field edges | −0.1 | 0.1 | 23 | −2.2 | 0.04 |
| Egg width × Wetland habitat | −0.1 | 0.1 | 23 | −1.7 | 0.1 |
| Egg length × Wetland habitat × Field edges | 0.2 | 0.1 | 23 | 1.7 | 0.1 |
| Egg width × Wetland habitat × Field edges | 0.2 | 0.1 | 23 | 2.9 | 0.009 |
| Gaussian GLMM (Egg Length) | |||||
| (Intercept) | 45.6 | 9.2 | 26 | 5.0 | 0.001 |
| Egg width | −0.1 | 0.3 | 27 | −0.4 | 0.7 |
| Wetland habitat | −18.0 | 10.2 | 27 | −1.8 | 0.09 |
| Field edges | −3.9 | 11.8 | 26 | −0.3 | 0.7 |
| Wetland habitat × Field edges | 6.9 | 15.0 | 27 | 0.5 | 0.7 |
| Egg width × Wetland habitat | 0.6 | 0.3 | 27 | 1.8 | 0.08 |
| Egg width × Field edges | 0.1 | 0.4 | 27 | 0.3 | 0.8 |
| Egg width × Wetland habitat × Field edges | −0.2 | 0.5 | 27 | −0.4 | 0.7 |
| Gaussian GLMM (Egg Width) | |||||
| (Intercept) | 38.3 | 21.7 | 26.7 | 1.8 | 0.09 |
| Egg length | −0.2 | 0.5 | 27 | −0.3 | 0.8 |
| Wetland habitat | −56.6 | 26.1 | 27 | −2.2 | 0.04 |
| Field edges | −5.5 | 39.9 | 26.9 | −0.1 | 0.9 |
| Wetland habitat × Field edges | 27.5 | 47.6 | 27 | 0.6 | 0.6 |
| Egg length × Wetland habitat | 1.3 | 0.6 | 27 | 2.1 | 0.04 |
| Egg length × Field edges | 0.09 | 1.0 | 27 | 0.1 | 0.9 |
| Egg length × Wetland habitat × Field edges | −0.6 | 1.1 | 27 | −0.5 | 0.6 |
| Clutch Size | 4 | 5 | 6 | 7 | 8 | Total |
|---|---|---|---|---|---|---|
| Number of nests | ||||||
| Wetland habitat | 2 | 2 | 7 | 4 | 2 | 17 |
| Dryland habitat | 2 | 2 | 3 | 1 | - | 8 |
| field edges | 2 | 2 | 4 | 2 | - | 10 |
| Bushes | 2 | 2 | 6 | 3 | 2 | 15 |
| Overall | 4 | 4 | 10 | 5 | 2 | 25 |
| Sum of clutches | ||||||
| Wetland habitat | 8 | 5 | 42 | 28 | 16 | 99 |
| Dryland habitat | 8 | 15 | 18 | 7 | - | 43 |
| Field edges | 8 | 10 | 24 | 14 | - | 56 |
| Bushes | 8 | 10 | 36 | 21 | 16 | 91 |
| Overall | 16 | 20 | 60 | 35 | 16 | 147 |
| Number of hatchlings | ||||||
| Wetland habitat | 8 | 4 | 35 | 22 | 13 | 82 |
| Dryland habitat | 6 | 12 | 15 | 6 | - | 39 |
| Field edges | 6 | 8 | 19 | 11 | - | 44 |
| Bushes | 8 | 8 | 31 | 17 | 13 | 77 |
| Overall | 14 | 16 | 50 | 28 | 13 | 121 |
| Number of fledglings | ||||||
| Wetland habitat | 7 | 4 | 32 | 20 | 12 | 79 |
| Dryland habitat | 5 | 11 | 14 | 6 | - | 32 |
| Field edges | 5 | 8 | 18 | 10 | - | 41 |
| Bushes | 7 | 7 | 28 | 16 | 12 | 84 |
| Overall | 12 | 15 | 46 | 26 | 12 | 111 |
| Hatching success [%] | ||||||
| Wetland habitat | 100 | 80.0 | 83.3 | 78.6 | 81.3 | 82.9 |
| Dryland habitat | 75.0 | 80.0 | 83.3 | 85.7 | - | 82.0 |
| Field edges | 75.0 | 80.0 | 79.1 | 78.6 | - | 78.6 |
| Bushes | 100 | 80.0 | 86.1 | 80.9 | 81.3 | 84.6 |
| Overall | 87.5 | 80.0 | 83.3 | 80.0 | 81.3 | 82.3 |
| Fledging success [%] | ||||||
| Wetland habitat | 87.5 | 100 | 91.4 | 90.9 | 92.3 | 96.3 |
| Dryland habitat | 83.3 | 91.7 | 93.3 | 100 | - | 82.1 |
| Field edges | 83.3 | 100 | 94.7 | 90.9 | - | 61.4 |
| Bushes | 87.5 | 87.5 | 90.3 | 94.1 | 92.3 | 91.8 |
| Overall | 85.7 | 93.8 | 92 | 92.8 | 92.3 | 91.7 |
| Breeding success [%] | ||||||
| Wetland habitat | 87.5 | 80 | 76.2 | 71.4 | 75.0 | 80.0 |
| Dryland habitat | 62.5 | 73.3 | 77.8 | 85.7 | - | 74.4 |
| Field edges | 62.5 | 80 | 75.0 | 71.4 | - | 73.2 |
| Bushes | 87.5 | 70.0 | 77.8 | 76.2 | 75.0 | 92.3 |
| Overall | 75.0 | 75.0 | 76.7 | 74.4 | 75.0 | 75.5 |
| Variables | LR | p-Value | Sig. |
|---|---|---|---|
| GLM 1: Clutch size (AIC = 83.4, AIC = 6.1) | |||
| Nesting habitat | 4.2 | 0.06 | N.S |
| Nest placement | 0.1 | 0.8 | N.S |
| Nest surface area | 3.3 | 0.1 | N.S |
| Nesting habitat × Nest surface area | 0.1 | 0.8 | N.S |
| Nest placement × Nest surface area | 1.4 | 0.3 | N.S |
| GLM 2: Hatchling numbers (AIC = 49.3, AIC = 4.7) | |||
| Clutch size | 18.4 | 0.001 | ** |
| Nesting habitat | 0.2 | 0.5 | N.S |
| Nest placement | 0.8 | 0.1 | N.S |
| Nest surface area | 0.1 | 0.6 | N.S |
| Nesting habitat × Nest placement | 0.30 | 0.3 | N.S |
| Clutch size × Nest placement | 0.7 | 0.1 | N.S |
| Clutch size × Nesting habitat | 0.2 | 0.4 | N.S |
| Clutch size × Nesting habitat × Nest placement | 0.05 | 0.7 | N.S |
| GLM 3: Fledgling numbers (AIC = 39.0, AIC = 0.3) | |||
| Hatchlings | 18.7 | 0.001 | ** |
| Clutch size | 1.2 | 0.01 | * |
| Nesting habitat | 0.01 | 0.9 | N.S |
| Nest placement | 0.01 | 0.8 | N.S |
| Nest surface area | 0.8 | 0.05 | * |
| Clutch size × Nest placement | 0.1 | 0.5 | N.S |
| Clutch size × Nesting habitat | 2.0 | 0.002 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, A.; Shams, S.; Ayaz, S.; Ibáñez-Arancibia, E.; Siraj, U.; De los Rios-Escalante, P.R.; Badshah, F. Influence of Nesting Habitat and Nest Emplacement on the Breeding Success of the Black Francolin (Francolinus francolinus, Phasianidae): A Case Study from Pakistan. Birds 2025, 6, 16. https://doi.org/10.3390/birds6020016
Ullah A, Shams S, Ayaz S, Ibáñez-Arancibia E, Siraj U, De los Rios-Escalante PR, Badshah F. Influence of Nesting Habitat and Nest Emplacement on the Breeding Success of the Black Francolin (Francolinus francolinus, Phasianidae): A Case Study from Pakistan. Birds. 2025; 6(2):16. https://doi.org/10.3390/birds6020016
Chicago/Turabian StyleUllah, Asad, Sumaira Shams, Sultan Ayaz, Eliana Ibáñez-Arancibia, Unays Siraj, Patricio R. De los Rios-Escalante, and Farhad Badshah. 2025. "Influence of Nesting Habitat and Nest Emplacement on the Breeding Success of the Black Francolin (Francolinus francolinus, Phasianidae): A Case Study from Pakistan" Birds 6, no. 2: 16. https://doi.org/10.3390/birds6020016
APA StyleUllah, A., Shams, S., Ayaz, S., Ibáñez-Arancibia, E., Siraj, U., De los Rios-Escalante, P. R., & Badshah, F. (2025). Influence of Nesting Habitat and Nest Emplacement on the Breeding Success of the Black Francolin (Francolinus francolinus, Phasianidae): A Case Study from Pakistan. Birds, 6(2), 16. https://doi.org/10.3390/birds6020016






