Long-Term Changes in Bird Communities in the Urban Parks of Mar del Plata City, Argentina
Simple Summary
Abstract
1. Introduction
2. Study Area and Methods
2.1. Study Area
2.2. Sampling Methods
2.3. Park Classification
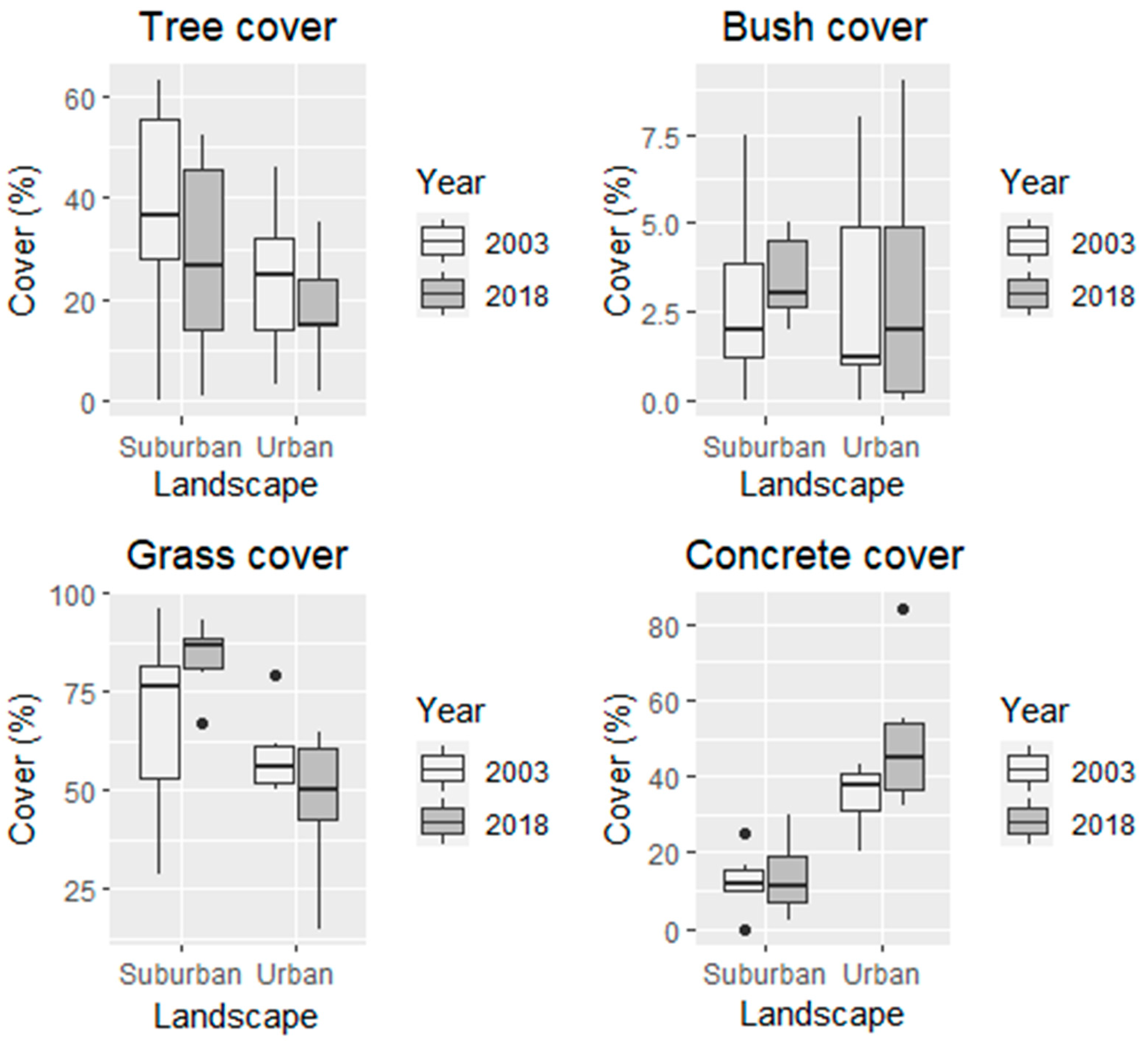
2.4. Habitat Structure in Parks
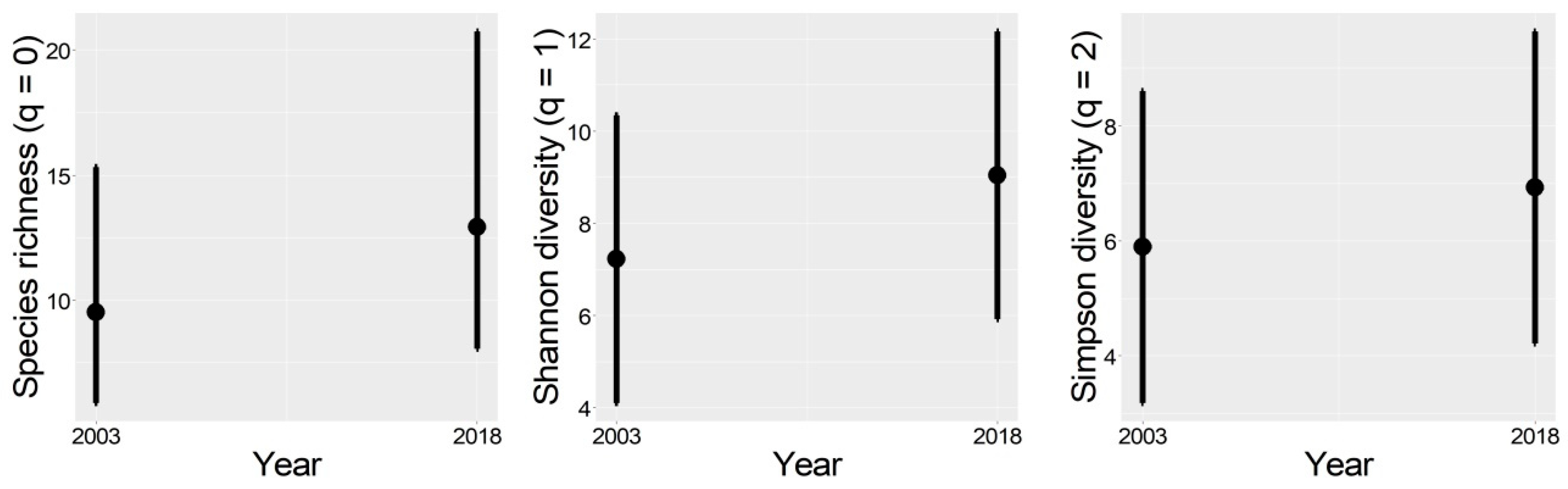
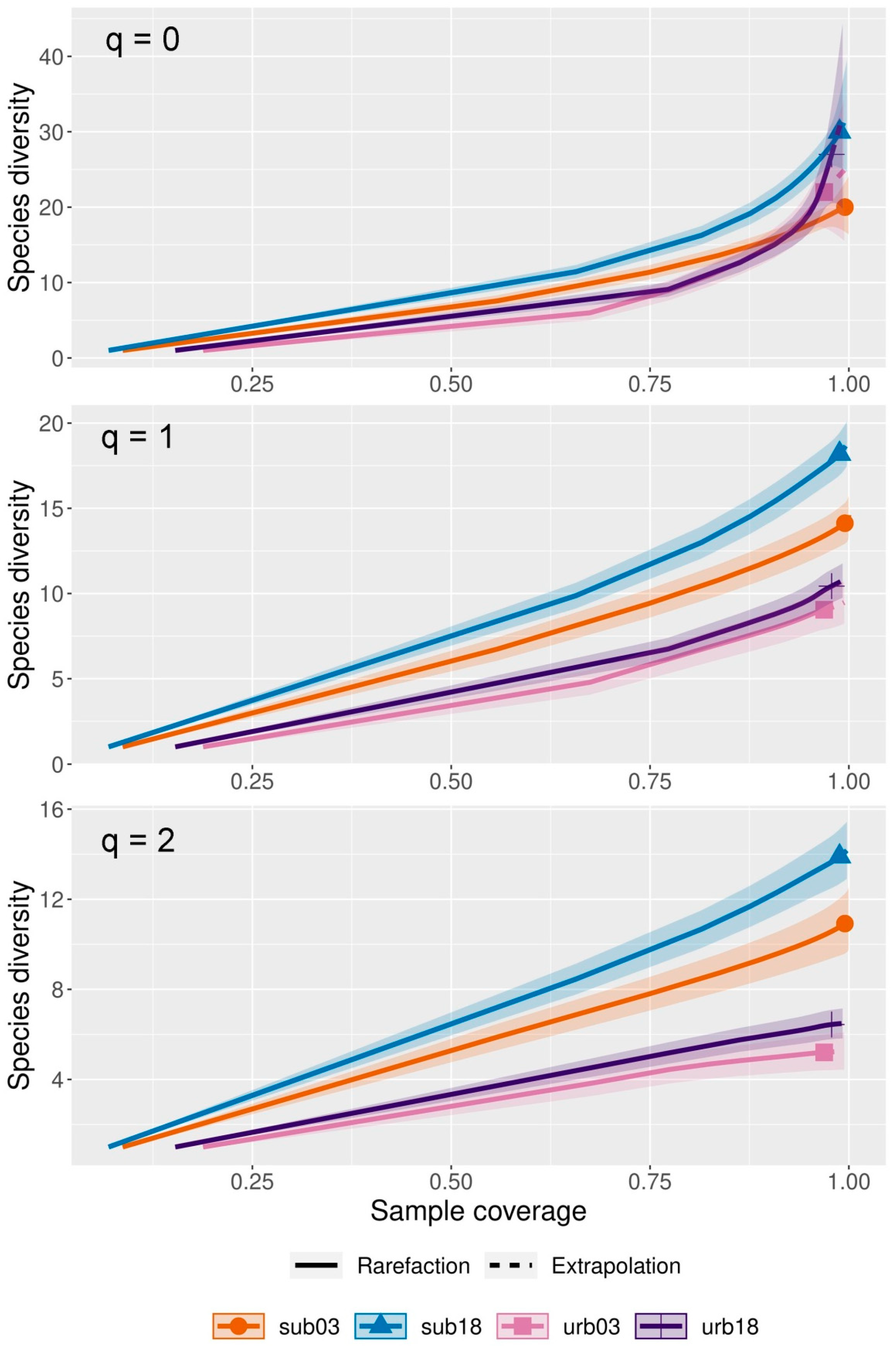
2.5. Species Richness and Diversity
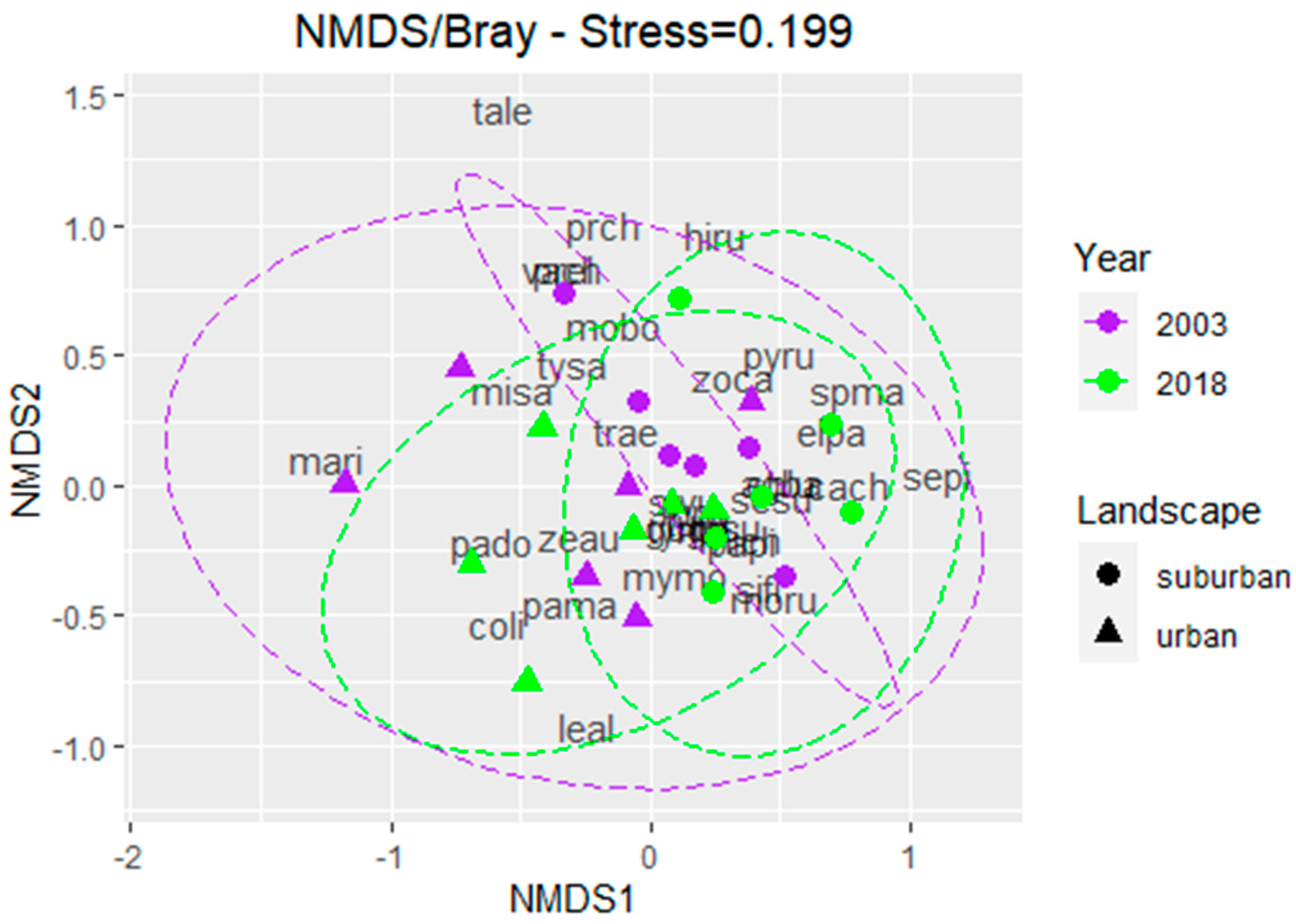
2.6. Species Composition
2.7. Data Analysis
2.7.1. Habitat Structure in Parks
2.7.2. Bird Richness and Diversity per Park
3. Results
3.1. Park Habitat Structure
3.2. Bird Richness and Diversity
3.3. Species Composition
4. Discussion
4.1. Bird Richness and Diversity Between Landscapes
4.2. Bird Richness and Diversity Between Years
4.3. Bird Species Composition Between Landscapes
4.4. Bird Species Composition Between Years
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berry, B.J.L. Urbanization. In The Earth as Transformed by Human Action; Cambridge University Press: Cambridge, UK, 1990; pp. 103–119. [Google Scholar]
- Meyer, W.B.; Turner, B.L. Human population growth and global land-use/cover change. Annu. Rev. Ecol. Syst. 1992, 23, 39–61. [Google Scholar] [CrossRef]
- Houghton, R.A. The worldwide extent of land-use change. BioScience 1994, 44, 305–313. [Google Scholar] [CrossRef]
- Marzluff, J.M.; Hamel, N. Land use issues. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 659–673. [Google Scholar]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Marzluff, J.M. Worldwide urbanization and its effects on birds. In Avian Ecology and Conservation in an Urbanizing World; Marzluff, J.M., Bowman, R., Donnelly, R., Eds.; Springer: Boston, MA, USA, 2001; pp. 19–47. [Google Scholar]
- Shanahan, D.F.; Strohbach, M.W.; Warren, P.S.; Fuller, R.A. The challenges of urban living. In Avian Urban Ecology; Oxford University Press: Oxford, UK, 2014; pp. 3–20. [Google Scholar]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Rahel, F.J. Homogenization of Freshwater Faunas. Annu. Rev. Ecol. Syst. 2002, 33, 291–315. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L. Toward a Mechanistic Understanding and Prediction of Biotic Homogenization. Am. Nat. 2003, 162, 442–460. [Google Scholar] [CrossRef]
- Johnston, R.F. Synanthropic birds of North America. In Avian Ecology and Conservation in an Urbanizing World; Marzluff, J.M., Bowman, R., Donnelly, R., Eds.; Springer: Boston, MA, USA, 2001; pp. 49–67. [Google Scholar]
- Beissinger, S.R.; Osborne, D.R. Effects of urbanization on avian community organization. Condor 1982, 84, 75–83. [Google Scholar] [CrossRef]
- Bezzel, E. Birdlife in intensively used rural and urban environments. Ornis Fenn. 1985, 62, 90–95. [Google Scholar]
- Emlen, J.T. An urban bird community in Tucson, Arizona: Derivation, structure, regulation. Condor 1974, 76, 184–197. [Google Scholar] [CrossRef]
- Leveau, L.M.; Leveau, C.M. Comunidades de aves en un gradiente urbano de la ciudad de Mar del Plata, Argentina. El Hornero 2004, 19, 13–21. [Google Scholar] [CrossRef]
- Chace, J.F.; Walsh, J.J. Urban effects on native avifauna: A review. Landsc. Urban Plan. 2006, 74, 46–69. [Google Scholar] [CrossRef]
- Jokimäki, J.; Suhonen, J.; Jokimäki-Kaisanlahti, M.L.; Carbó-Ramírez, P. Effects of urbanization on breeding birds in European towns: Impacts of species traits. Urban Ecosyst. 2016, 19, 1565–1577. [Google Scholar] [CrossRef]
- Clergeau, P.; Savard, J.P.L.; Mennechez, G.; Falardeau, G. Bird abundance and diversity along an urban-rural gradient: A comparative study between two cities on different continents. Condor 1998, 100, 413–425. [Google Scholar] [CrossRef]
- Conole, L.E.; Kirkpatrick, J.B. Functional and spatial differentiation of urban bird assemblages at the landscape scale. Landsc. Urban Plan. 2011, 100, 11–23. [Google Scholar] [CrossRef]
- Jokimäki, J.; Huhta, E. Artificial nest predation and abundance of birds along an urban gradient. Condor 2000, 102, 838–847. [Google Scholar] [CrossRef]
- Leveau, L.M. Bird traits in urban–rural gradients: How many functional groups are there? J. Ornithol. 2013, 154, 655–662. [Google Scholar] [CrossRef]
- Fernández-Juricic, E. Avifaunal Use of Wooded Streets in an Urban Landscape. Conserv. Biol. 2000, 14, 513–521. [Google Scholar] [CrossRef]
- Carbó-Ramírez, P.; Zuria, I. The value of small urban greenspaces for birds in a Mexican city. Landsc. Urban Plan. 2011, 100, 213–222. [Google Scholar] [CrossRef]
- Morneau, F.; Décarie, R.; Pelletier, R.; Lambert, D.; DesGranges, J.L.; Savard, J.P. Changes in breeding bird richness and abundance in Montreal parks over a period of 15 years. Landsc. Urban Plan. 1999, 44, 111–121. [Google Scholar] [CrossRef]
- Recher, H.F.; Serventy, D.L. Long term Changes in the Relative Abundances of Birds in Kings Park, Perth, Western Australia. Conserv. Biol. 1991, 5, 90–102. [Google Scholar] [CrossRef]
- Murgui, E. Population trends in breeding and wintering birds in urban parks: A 15-year study (1998–2013) in Valencia, Spain. Rev. Catalana D’Ornitologia 2014, 30, 30–40. [Google Scholar]
- Fidino, M.; Limbrick, K.; Bender, J.; Gallo, T.; Magle, S.B. Strolling through a Century: Replicating Historical Bird Surveys to Explore 100 Years of Change in an Urban Bird Community. Am. Nat. 2022, 199, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Skjelvik, C.E.; Dale, S. Bird population changes in urban green spaces explained by regional population trends. Urban Ecosyst. 2024, 27, 1339–1347. [Google Scholar] [CrossRef]
- Fidino, M.; Magle, S.B. Trends in Long-Term Urban Bird Research. In Ecology and Conservation of Birds in Urban Environments; Murgui, E., Hedblom, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 161–184. [Google Scholar]
- Stiles, F.G.; Rosselli, L.; De La Zerda, S. Changes over 26 years in the avifauna of the Bogotá region, Colombia: Has climate change become important? Front. Ecol. Evol. 2017, 5, 58. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; La Sorte, F.A.; Aronson, M.F.; Goddard, M.A.; MacGregor-Fors, I.; Nilon, C.H.; Warren, P.S. Global Patterns and Drivers of Urban Bird Diversity. In Ecology and Conservation of Birds in Urban Environments; Murgui, E., Hedblom, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 13–33. [Google Scholar]
- Rebele, F. Urban ecology and special features of urban ecosystems. Glob. Ecol. Biogeogr. Lett. 1994, 4, 173–187. [Google Scholar] [CrossRef]
- Lodge, D.M. Biological invasions: Lessons for ecology. Trends Ecol. Evol. 1993, 8, 133–137. [Google Scholar] [CrossRef]
- Pârâu, L.G.; Strubbe, D.; Mori, E.; Menchetti, M.; Ancillotto, L.; Kleunen, A.V.; White, R.L.; Luna, Á.; Hernández-Brito, D.; Louarn, M.L.; et al. Rose-ringed parakeet Psittacula krameri populations and numbers in Europe: A complete overview. Open Ornithol. J. 2016, 9, 1–13. [Google Scholar] [CrossRef]
- Craig, A.J.F.K. Common Starling (Sturnus vulgaris Linnaeus, 1758). In Invasive Birds: Global Trends and Impacts; Downs, C.T., Hart, L.A., Eds.; CABI: Wallingford, UK, 2020; pp. 9–24. [Google Scholar]
- Pinto, E.D.E. Range expansion of the European Starling Sturnus vulgaris in Argentina. Ardeola 2005, 52, 359–364. [Google Scholar]
- Pérez, J. Estornino pinto en la Capital Federal. Nuestras Aves 1988, 17, 359–364. [Google Scholar] [CrossRef]
- Ifran, N.R.; Fiorini, V.D. European starling (Sturnus vulgaris): Population density and interactions with native species in Buenos Aires urban parks. Ornitol. Neotrop. 2010, 21, 507–518. [Google Scholar]
- Feare, C. The Starling; Oxford University Press: Oxford, UK, 1984. [Google Scholar]
- Menguel, R.M. The Birds of Kentucky; Ornithological Monographs No. 3; 1965. [Google Scholar]
- Weitzel, N.H. Nest-site competition between the European Starling and native breeding birds in northwestern Nevada. Condor 1988, 90, 515–517. [Google Scholar] [CrossRef]
- Ingold, D.J. Nesting phenology and competition for nest sites among Red-headed and Red-bellied Woodpeckers and European Starlings. Auk 1989, 106, 209–217. [Google Scholar]
- Ibañez, L.M.; Girini, J.M.; Palacio, F.X.; Fiorini, V.D.; Montalti, D. Interacciones entre el estornino pinto (Sturnus vulgaris) y aves nativas de Argentina por el uso de cavidades. Rev. Mex. Biodivers. 2017, 88, 477–479. [Google Scholar] [CrossRef]
- Zárate, V.; Juncosa Polzella, A.S. Usurpación de Nido de Carpintero del Cardón (Melanerpes cactorum) por Parte de Estornino Pinto (Sturnus vulgaris). Nuestras Aves 2020, 65, 58–60. [Google Scholar] [CrossRef]
- Leveau, L.M. Long-term directional changes in urban bird communities of Mar del Plata City, Argentina. Front. Ecol. Evol. 2024, 12, 1457476. [Google Scholar] [CrossRef]
- Leveau, L.M.; Leveau, C.M. Does urbanization affect the seasonal dynamics of bird communities in urban parks? Urban Ecosyst. 2016, 19, 631–647. [Google Scholar] [CrossRef]
- Zhou, D.; Chu, L.M. How would size, age, human disturbance, and vegetation structure affect bird communities of urban parks in different seasons? J. Ornithol. 2012, 153, 1101–1112. [Google Scholar] [CrossRef]
- Morello, J.; Matteucci, S.D.; Rodriguez, A.F.; Silva, M.E.; Mesopotámica, P.; Llana, P. Ecorregiones y Complejos Ecosistémicos de Argentina. Orientación Gráfica Ed. B. Aires. 2012. [Google Scholar]
- Hutto, R.L.; Pletschet, S.M.; Hendricks, P. A fixed-radius point count method for nonbreeding and breeding season use. Auk 1986, 103, 593–602. [Google Scholar] [CrossRef]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A. Bird Census Techniques; Academic Press: London, UK, 1992. [Google Scholar]
- Bibby, C.J.; Jones, M.; Marsden, S. Bird Surveys; Expedition Advisory Centre: London, UK, 1998. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ferenc, M.; Sedláček, O.; Fuchs, R. How to improve urban greenspace for woodland birds: Site and local-scale determinants of bird species richness. Urban Ecosyst. 2014, 17, 625–640. [Google Scholar] [CrossRef]
- Jost, L.; González-Oreja, J. Midiendo la diversidad biológica: Más allá del índice de Shannon. Acta Zool. Lilloana 2012, 1–2, 3–14. [Google Scholar]
- Chao, A.; Chiu, C.H.; Jost, L. Unifying Species Diversity, Phylogenetic Diversity, Functional Diversity, and Related Similarity and Differentiation Measures Through Hill Numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- Li, D. hillR: Taxonomic, functional, and phylogenetic diversity and similarity through Hill Numbers. J. Open Source Softw. 2018, 3, 1041. [Google Scholar] [CrossRef]
- Chao, A.; Ma, K.H.; Hsieh, T.C. User’s guide for iNEXT online: Software for interpolation and Extrapolation of species diversity. Code 2016, 30043, 1–14. [Google Scholar]
- MacGregor-Fors, I.; Payton, M.E. Contrasting diversity values: Statistical inferences based on overlapping confidence intervals. PLoS ONE 2013, 8, e56794. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community ecology package. R Package Version 2013, 2, 321–326. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Zhou, D.; Chu, L.M. Do avian communities vary with season in highly urbanized Hong Kong? Wilson J. Ornithol. 2014, 126, 69–80. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, D.; Villeger, S.; De Bortoli, J.; Leprieur, F.; Logez, M.; Henriques-Silva, R.; Martinez-Santalla, S.; Martin-Devasa, R.; Gomez-Rodriguez, C.; et al. Betapart: Partitioning Beta Diversity into Turnover and Nestedness Components. R Package Version 1.5. 2; 2021. [Google Scholar]
- Baselga, A. Separating the two components of abundance-based dissimilarity: Balanced changes in abundance vs. abundance gradients. Methods Ecol. Evol. 2013, 4, 552–557. [Google Scholar] [CrossRef]
- Pinheiro, J. nlme: Linear and nonlinear mixed effects models. R Package Version 2011, 3, 1. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; Van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Zuur, A.F. (Ed.) Mixed Effects Models Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Lüdecke, D. sjPlot: Data visualization for statistics in social science. R Package Version 2021, 2, 1–106. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Garaffa, P.I.; Filloy, J.; Bellocq, M.I. Bird community responses along urban–rural gradients: Does the size of the urbanized area matter? Landsc. Urban Plan. 2009, 90, 33–41. [Google Scholar] [CrossRef]
- Leveau, L.M. Relaciones aves–habitat en el sector suburbano de Mar del Plata, Argentina. Ornitol. Neotrop. 2013, 24, 201–212. [Google Scholar]
- Fernández-Juricic, E.; Tellería, J.L. Effects of human disturbance on spatial and temporal feeding patterns of Blackbird Turdus merula in urban parks in Madrid, Spain. Bird Study 2000, 47, 13–21. [Google Scholar] [CrossRef]
- Fernández-juricic, E. Avian spatial segregation at edges and interiors of urban parks in Madrid, Spain. Biodivers. Conserv. 2001, 10, 1303–1316. [Google Scholar] [CrossRef]
- Patón, D.; Romero, F.; Cuenca, J.; Escudero, J.C. Tolerance to noise in 91 bird species from 27 urban gardens of Iberian Peninsula. Landsc. Urban Plan. 2012, 104, 1–8. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Morales-Pérez, L.; Quesada, J.; Schondube, J.E. Relationship between the presence of House Sparrows (Passer domesticus) and Neotropical bird community structure and diversity. Biol. Invasions 2010, 12, 87–96. [Google Scholar] [CrossRef]
- Blair, R.B. Creating a homogeneous avifauna. In Avian Ecology and Conservation in an Urbanizing World; Marzluff, J.M., Bowman, R., Donnelly, R., Eds.; Springer: Boston, MA, USA, 2001; pp. 459–486. [Google Scholar]
- Shaw, L.M.; Chamberlain, D.; Evans, M. The House Sparrow Passer domesticus in urban areas: Reviewing a possible link between post-decline distribution and human socioeconomic status. J. Ornithol. 2008, 149, 293–299. [Google Scholar] [CrossRef]
- Isacch, J.P.; Isacch, J. Estornino Pinto (Sturnus vulgaris) en la ciudad de Mar del Plata (Provincia de Buenos Aires, Argentina). Nuestras Aves 2004, 47, 33. [Google Scholar] [CrossRef]
- Jensen, R.F. Nuevos registros de estornino pinto (Sturnus vulgaris) para el sureste de la provincia de Entre Ríos, Argentina. Nuestras Aves 2008, 53, 22. [Google Scholar] [CrossRef]
- Munyenyembe, F.; Harris, J.; Hone, J.; Nix, H. Determinants of bird populations in an urban area. Aust. J. Ecol. 1989, 14, 549–557. [Google Scholar] [CrossRef]
- Kark, S.; Iwaniuk, A.; Schalimtzek, A.; Banker, E. Living in the city: Can anyone become an ‘urban exploiter’? J. Biogeogr. 2007, 34, 638–651. [Google Scholar] [CrossRef]
- Croci, S.; Butet, A.; Clergeau, P. Does urbanization filter birds on the basis of their biological traits. Condor 2008, 110, 223–240. [Google Scholar] [CrossRef]
- Narosky, T. Las aves de la Provincia de Buenos Aires: Distribución y Estatus; Asociación Ornitológica del Plata: Buenos Aires, Argentina, 1993. [Google Scholar]
- Krapovickas, S.; Di Giacomo, A.S. Conservation of pampas and campos grasslands in Argentina. Parks 1998, 8, 47–53. [Google Scholar]
- Dandapat, A.; Banerjee, D.; Chakraborty, D. The case of the Disappearing House Sparrow (Passer domesticus indicus). Vet. World 2010, 3, 97. [Google Scholar]
- Berigan, L.A.; Greig, E.I.; Bonter, D.N. Urban house sparrow (Passer domesticus) populations decline in North America. Wilson J. Ornithol. 2020, 132, 248–258. [Google Scholar] [CrossRef]
- Palacio, F.X.; Ibañez, L.M.; Maragliano, R.E.; Montalti, D. Urbanization as a driver of taxonomic, functional, and phylogenetic diversity losses in bird communities. Can. J. Zool. 2018, 96, 1114–1121. [Google Scholar] [CrossRef]
- Campbell, C.E.; Jones, D.N.; Awasthy, M.; Castley, J.G.; Chauvenet, A.L. Big changes in backyard birds: An analysis of long-term changes in bird communities in Australia’s most populous urban regions. Biol. Conserv. 2022, 272, 109671. [Google Scholar] [CrossRef]


| 2003 | 2018 | ||||
|---|---|---|---|---|---|
| Species | Abbreviation | Urban Parks | Suburban Parks | Urban Parks | Suburban Parks |
| Vanellus chilensis | vach | 0 | 0 | 1 | 1 |
| Columba livia | coli | 2 | 0 | 9 | 0 |
| Patagioenas picazuro | papi | 9 | 20 | 19 | 23 |
| Patagioenas maculosa | pama | 1 | 2 | 107 | 17 |
| Zenaida auriculata | zeau | 37 | 39 | 80 | 32 |
| Guira guira | gugu | 0 | 0 | 1 | 0 |
| Chlorostilbon lucidus | chlu | 1 | 0 | 0 | 2 |
| Leucochloris albicollis | leal | 0 | 0 | 1 | 1 |
| Milvago chimango | mich | 2 | 5 | 14 | 43 |
| Myiopsitta monachus | mymo | 0 | 3 | 18 | 41 |
| Furnarius rufus | furu | 10 | 21 | 15 | 19 |
| Elaenia parvirostris | elpa | 0 | 2 | 0 | 4 |
| Serpophaga subcristata | sesu | 2 | 0 | 1 | 3 |
| Pyrocephalus rubinus | pyru | 7 | 1 | 0 | 0 |
| Machetornis rixosus | mari | 1 | 3 | 2 | 0 |
| Pitangus sulphuratus | pisu | 6 | 17 | 9 | 12 |
| Tyrannus melancholicus | tyme | 1 | 6 | 3 | 3 |
| Tyrannus savana | tysa | 2 | 0 | 0 | 2 |
| Progne chalybea | prch | 0 | 0 | 1 | 4 |
| Progne elegans | prel | 0 | 0 | 1 | 1 |
| Tachycineta leucorrhoa | tale | 0 | 5 | 0 | 0 |
| Hirundo rustica | hiru | 0 | 0 | 2 | 15 |
| Troglodytes aedon | trae | 1 | 3 | 1 | 6 |
| Turdus rufiventris | turu | 5 | 8 | 12 | 13 |
| Mimus saturninus | misa | 14 | 9 | 7 | 4 |
| Sturnus vulgaris | stvu | 0 | 0 | 11 | 37 |
| Sicalis flaveola | sifl | 0 | 0 | 0 | 1 |
| Zonotrichia capensis | zoca | 4 | 11 | 1 | 8 |
| Setophaga pitiayumi | sepi | 0 | 0 | 0 | 5 |
| Agelaioides badius | agba | 1 | 7 | 3 | 4 |
| Molothrus bonariensis | mobo | 7 | 13 | 6 | 11 |
| Molothrus rufoaxillaris | moru | 0 | 0 | 2 | 2 |
| Chloris chloris | cach | 2 | 6 | 0 | 3 |
| Spinus magellanicus | spma | 5 | 0 | 2 | 6 |
| Passer domesticus | pado | 71 | 15 | 39 | 17 |
| Estimate | Std. Error | z/t Value | p | LRT | ||
|---|---|---|---|---|---|---|
| Species richness (q = 0) | Intercept | 2.260 | 0.094 | 24.139 | 0.000 | 9.646 *** |
| Year 2018 | 0.306 | 0.083 | 3.688 | 0.000 | ||
| Shannon diversity (q = 1) | Intercept | 7.226 | 0.918 | 7.867 | 0.000 | 8.708 ** |
| Year 2018 | 1.812 | 0.529 | 3.424 | 0.006 | ||
| Simpson diversity (q = 2) | Intercept | 5.900 | 0.830 | 7.105 | 0.000 | 4.630 * |
| Year 2018 | 1.026 | 0.451 | 2.276 | 0.044 |
| Species | Average Contribution | SD | Mean–SD Ratio | Average Abundance in Urban | Average Abundance in Suburban | Cumulative Contribution |
|---|---|---|---|---|---|---|
| Zenaida auriculata | 0.096 | 0.072 | 1.340 * | 9.750 | 5.917 | 0.138 |
| Passer domesticus | 0.088 | 0.103 | 0.849 | 9.167 | 2.667 | 0.265 |
| Patagioenas maculosa | 0.085 | 0.128 | 0.666 | 9.000 | 1.583 | 0.388 |
| Patagioenas picazuro | 0.042 | 0.039 | 1.064 * | 2.333 | 3.583 | 0.448 |
| Milvago chimango | 0.042 | 0.051 | 0.819 | 1.333 | 4.000 | 0.508 |
| Myiopsitta monachus | 0.041 | 0.054 | 0.763 | 1.500 | 3.667 | 0.568 |
| Sturnus vulgaris | 0.030 | 0.037 | 0.822 | 0.917 | 3.083 | 0.612 |
| Furnarius rufus | 0.029 | 0.025 | 1.140 * | 2.083 | 3.333 | 0.653 |
| Mimus saturninus | 0.024 | 0.035 | 0.707 | 1.750 | 1.083 | 0.688 |
| Molothrus bonariensis | 0.024 | 0.025 | 0.985 | 1.083 | 2.000 | 0.723 |
| Pitangus sulphuratus | 0.024 | 0.020 | 1.161 * | 1.250 | 2.417 | 0.757 |
| Turdus rufiventris | 0.022 | 0.021 | 1.048 * | 1.417 | 1.750 | 0.789 |
| Zonotrichia capensis | 0.019 | 0.018 | 1.048 * | 0.417 | 1.583 | 0.816 |
| Species | Average Contribution | SD | Mean–SD Ratio | Average Abundance in 2003 | Average Abundance in 2018 | Cumulative Contribution |
|---|---|---|---|---|---|---|
| Patagioenas maculosa | 0.101 | 0.139 | 0.730 | 0.250 | 10.333 | 0.144 |
| Zenaida auriculata | 0.092 | 0.071 | 1.304 * | 6.333 | 9.333 | 0.275 |
| Passer domesticus | 0.075 | 0.079 | 0.952 | 7.167 | 4.667 | 0.381 |
| Milvago chimango | 0.051 | 0.055 | 0.917 | 0.583 | 4.750 | 0.453 |
| Myiopsitta monachus | 0.050 | 0.061 | 0.832 | 0.250 | 4.917 | 0.524 |
| Sturnus vulgaris | 0.041 | 0.038 | 1.075 * | 0.000 | 4.000 | 0.582 |
| Patagioenas picazuro | 0.038 | 0.033 | 1.144 * | 2.417 | 3.500 | 0.637 |
| Furnarius rufus | 0.026 | 0.021 | 1.209 * | 2.583 | 2.833 | 0.673 |
| Molothrus bonariensis | 0.023 | 0.022 | 1.044 * | 1.667 | 1.417 | 0.706 |
| Mimus saturninus | 0.023 | 0.031 | 0.741 | 1.917 | 0.917 | 0.739 |
| Turdus rufiventris | 0.022 | 0.020 | 1.126 * | 1.083 | 2.083 | 0.771 |
| Pitangus sulphuratus | 0.021 | 0.017 | 1.228 * | 1.917 | 1.750 | 0.800 |
| Zonotrichia capensis | 0.015 | 0.015 | 0.959 | 1.250 | 0.750 | 0.821 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galiano, L.; Leveau, C.M.; Leveau, L.M. Long-Term Changes in Bird Communities in the Urban Parks of Mar del Plata City, Argentina. Birds 2024, 5, 814-831. https://doi.org/10.3390/birds5040054
Galiano L, Leveau CM, Leveau LM. Long-Term Changes in Bird Communities in the Urban Parks of Mar del Plata City, Argentina. Birds. 2024; 5(4):814-831. https://doi.org/10.3390/birds5040054
Chicago/Turabian StyleGaliano, Lourdes, Carlos M. Leveau, and Lucas M. Leveau. 2024. "Long-Term Changes in Bird Communities in the Urban Parks of Mar del Plata City, Argentina" Birds 5, no. 4: 814-831. https://doi.org/10.3390/birds5040054
APA StyleGaliano, L., Leveau, C. M., & Leveau, L. M. (2024). Long-Term Changes in Bird Communities in the Urban Parks of Mar del Plata City, Argentina. Birds, 5(4), 814-831. https://doi.org/10.3390/birds5040054






