Simple Summary
When we compared breeding bird communities in cork oak patches located before and after a fire event, we did not observe any significant changes in the density of territorial pairs or in the diversity metrics. This counterintuitive response may be due to the characteristics of cork oak (Quercus suber), a sclerophilous tree that is very resilient to fires and able to recover foliage in the following spring season, thus allowing rapid bird recolonization.
Abstract
Forest fires are disturbance events that can impact biological assemblages at multiple scales. In this study, the structures of breeding bird communities in cork oak patches located in an agro-mosaic suburban landscape of central Italy (Rome) were compared at the local scale with a fine-grained mapping method before (2018) and after (2023) a fire event occurred in July 2022. The analyses did not reveal any significant changes in the density of territorial pairs or in the diversity metrics, both univariate (Shannon–Wiener index, evenness, Margalef normalized richness) and bivariate (Whittaker and k-dominance plots, abundance/biomass curves) of diversity. Even when the guilds of strictly forest-related species were compared, no differences emerged before and after the fire. This counterintuitive phenomenon may be due to the characteristics of the dominant tree, the cork oak (Quercus suber), a sclerophilous tree that is very resilient to fires and able to recover foliage in the following spring season, thus allowing rapid bird recolonization. However, other small-scale phenomena (e.g., the ‘crowding effect’ and local dispersal of territorial pairs from remnant wood patches not affected by fire) may explain this lack of change in breeding bird density and diversity. Further studies should be carried out at larger spatial and temporal scales and at different levels of fire frequency and intensity to confirm these responses at the guild/community level in suburban cork oak wood patches.
1. Introduction
Forest ecosystems can be impacted by anthropogenic fires [1]. Worldwide, several studies have demonstrated how fires impact bird communities in extremely different ways: bird assemblages occurring in burned areas undergo structural alterations depending on the fire regime (extent, duration, frequency, and intensity), as well as other environmental factors, especially vegetation cover and its resilience following the passage of fire [2,3,4,5,6,7,8]. Such disruption in the structure of bird communities can lead to an increase in ecotonal and generalist species, which are linked to edge and disturbed habitats, to the detriment of specialized species, which are linked to stable and mature environments, such as forest habitats [9,10,11,12]. Fires disrupt both foraging and nesting sites of many forest-specialized species, changing the patterns of niches and resources [9]. However, the effects and responses of birds are strongly scale-dependent and species-/context-specific [13,14,15,16].
Compared to temperate broad-leaved and coniferous woods (e.g., [17,18,19,20,21,22]), forest vegetation in the Mediterranean basin is different and often highly resistant and resilient to the effects of fire, as is the case for Mediterranean maquis forests and, more specifically, for cork oak (Quercus suber) forests. The bark of cork oak trees has a high insulating capacity and can reach a thickness of approximately 30 cm, thus protecting the cambium from the heat of fire [23,24]; see also [25] for central Italy. Therefore, cork oak forests recover quickly following a fire; however, this recovery depends on the disturbance regime [26].
In ‘Campagna Romana’, an agroforestry countryside area surrounding the city of Rome (central Italy; [27]), many cork oak patches occur. These remnants form interesting fragmented ‘archipelagoes’ of habitat islands (sensu [28]) of high ecological interest and conservation concern [29,30]. These landscape mosaics are often affected by anthropogenic disturbances linked to the suburban context and, among these disturbances, fires [31]. Since these are areas bordering urbanized sites, such fires (mainly small-size and accidental events linked to agricultural practices and influenced by socioeconomic conditions mainly linked to antisocial behaviors or linked to crop land management [32]) are quickly controlled, and the affected surfaces are always limited; however, at the local scale, they can still heavily alter forest cover and structure and related biological diversity [22,33]. Therefore, specific wildfire protection plans have been defined (e.g., for Rome, [34]).
In this study, which was carried out in a suburban agroforestry mosaic located in central Italy, we report data on the structure of breeding bird communities present before (2018) and after (2023) a fire event that occurred in the summer of 2022. The fire-affected patches were dominated by cork oaks that, beginning in the autumn following the event, presented recovery of leaf cover, despite the passage of the fire being particularly destructive at the local scale. Therefore, we hypothesized that species typically associated with the cork oak forest structure (forest-related guild) were not adversely affected by the fire.
2. Materials and Methods
2.1. Study Area
The study area, with a total extent of 38 hectares, consists of a residual corridor of the Roman countryside (‘Campagna Romana’; southwestern Rome suburban area), located between two nature reserves: “Valle dei Casali” and “Tenuta dei Massimi” (Latium Regional law n. 29/1997; size area: 38 hectares; 41°51′52.6″ N 12°24′53.6″ E; [35]; Figure 1). This is an agroforest mosaic with a landscape matrix composed of croplands and uncultivated fields dominated by Vulpio-Dasypiretum and Diplotaxio tenuifolii-Agropyretum repentis [36,37,38], including three forest patches (10.05% of the total area) dominated (>76% of tree frequency; n= 349; Supplementary Materials Table S1) by cork oaks (Quercus suber; [39]).
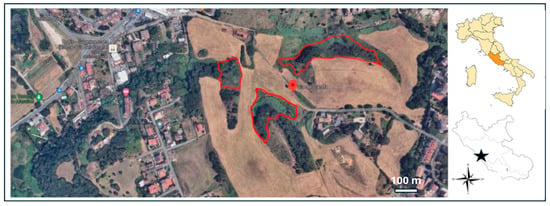
Figure 1.
Map of the study area and its location in Latium (star, on the right), central Italy, showing the suburban landscape mosaic. Cork oak (Quercus suber) patches are dark green. The polygons in red indicate the burnt areas where the fire event occurred (July 2022).
At the edges of the forest patches, there are characteristic ecotonal species belonging to the Mediterranean scrub vegetation (mock privet Phillyrea sp., Mediterranean buckthorn Rhamnus alaternus, laurel Laurus nobilis, and olive tree Olea europaea; Supplementary Materials Table S1). Furthermore, there are small ecotonal patches of hygrophilous vegetation with exotic species (eucalyptus Eucalyptus sp., stone pine Pinus pinea, Chinese privet Ligustrum lucidum) surrounding ponds and abandoned rural houses (details in [39]).
In 17 July 2022, a fire of great intensity (canopy crown fire [40]; duration: approximately five hours) affected 57.32% of the cork oak-dominated patches (the only wood patches occurring at the study site), resulting in a marked reduction in tree foliage cover [41]). The fire, probably started by bales of hay placed in the recently mown fields, left the cork oak tree structure intact, burning only the foliage and the 2nd- and 3rd-level branches. The fire had a heavy impact on the undergrowth (Mediterranean scrub, Rubus sp.), which disappeared. Since autumn 2022, and mainly in the following season (2023), signs of vegetation recovery have been observed both in the undergrowth and in the arboreal cork oaks (>7.5 cm).
2.2. Bird Surveys
A multivisit territory mapping method was used to study the breeding bird community, which involves a spatial recording of direct observations, vocalizations, song contacts and alarm calls of the territorial bird species locally present to define the number of breeding pairs [42,43].
Twenty-two surveys were carried out from 9 April to 30 May 2023 (research effort: appr. 40 h) in the first hours of the morning (08.00–11.00 a.m.). Every survey (n = 22) was carried out randomly inside the period along different paths. In any survey (almost 2 h/visit), we walked the whole area via different routes. The data obtained for the entire study area, without a subdivision into burnt and unburnt subareas, were compared with those published in the study carried out before the fire event (spring 2018; [39]), via the same paths. The data were separated into three sampling subperiods (II half of April, I half of May and II half of May) to test for possible phenological differences.
Each survey was carried out under clear or variable weather conditions in the absence of rain or strong winds, all of which could affect sampling [42,43]. Sampling was conducted by two of the authors (S.C., C.B.). Species-specific territories were obtained following the clustering procedure described in [42]. We considered a “territory” as a range area where a territorial species pair was considered to be breeding [42,43]. Species with crepuscular or nocturnal activity (e.g., Strigiformes and Caprimulgiformes) and individuals flying very high (> 25 m) were not considered. Compared with 2019, in 2023 we carried out a comparable research effort (in terms of the number of visits and time effort, i.e., approximately 40 h); one of the authors (C.B.) carried out the surveys both in 2019 and 2023 to avoid observer effects.
The data were analyzed at the community, guild, and species levels. At the species level, in our analyses, we considered only the territorial breeding species (therefore excluding nonterritorial/gregarious breeders, e.g., Passer sp., Sturnus vulgaris, and non-breeders as species in migratory transit). In this last case, we selected two ecological guilds: (i) forest species and (ii) open/edge species, including species linked to (i) strictly forest habitat types (w, in Table 1) and (ii) species linked to open habitats (croplands) and ecotones (wood/maquis/cropland fringe; e/o in Table 1).

Table 1.
The bird community in the study area (only territorially breeding species; non-territorial species or solely migratory non-breeding species were not included), before (2023) and after (2018) the 2022 fire event. Species and ecological guilds (w: forest species; e/o: open/edge species), densities (D, in breeding pairs/10 ha), and relative frequencies (FrD, as D/N, where D is species density and N is the total species density), and consuming biomass (Cb, in grams, and relative frequencies, FrCb) for each species are reported. Below: the values of the univariate diversity metrics at the community level: normalized richness (Margalef index; Dm), Shannon–Wiener diversity (H′) and evenness (J). Phenological status: s: sedentary; m: long-distance migrant.
For all species, we obtained (i) the ecological density (as D = breeding pairs/10 ha), i.e., the density calculated considering only the suitable habitat cover (e.g., cork oak patch size area for the forest birds and crop land/ecotone size areas for open/edge species), (ii) the consuming biomass (Cb; in g/10 ha), calculated as: Cb = Scb0.7 [44], where Scb, or the standing crop biomass, is the total body mass of all censused individuals (in g/10 ha). This value is directly proportional to the energy removed by individuals from the environment [44]. We used more consuming biomass than standing crop biomass because the former better explained the specific variations in metabolic rhythm, which were related mainly to individual size [44].
To assign species to different ecological guilds, we refer to [45,46,47,48,49]. To calculate the biomass values, the mean body mass values were obtained from [45,46,47,48,49]. When available, we used biomass data available for an area immediately surrounding (4 km away; Villa Pamphili urban park; [50]), and (iii) the relative frequencies were calculated both for density (frD, as D/N, where N is the total species density) and for biomass (frCb, expressed as the ratio of specific Cb/total Cb).
2.3. Data Preparation and Statistical Methods
To compare the taxonomic diversity of samples of different sizes and to verify the representativeness of our data, individual rarefaction curves were generated. These curves were also obtained by dividing the data into three subsampling periods (halves II of April, I and II of May) to verify possible phenological differences inside the study period at the level of species richness and diversity.
Univariate diversity metrics were then calculated: (i) Shannon–Wiener diversity index (H′; [51]), as H′ = −Σ frD [lnfrD]; (ii) evenness index (J; [52]), as J = H′/H′max, where H′max = lnS (with S = number of detected species); and (iii) normalized Margalef richness (Dm; [53]), as Dm = (S−1)/lnN.
With respect to bivariate diversity metrics, the following analyses were performed: (i) k-dominance plots [54,55], which allowed us to understand the maturity and stress level of the bird community (and of the forest species guild) on the basis of the uniformity of the frequency distributions of the different species; and (ii) ABC curves (abundance–biomass comparison [56,57]; an application for birds [58,59]), which make it possible to obtain information on the level of stress at the community level, rank the cumulative frequencies both for density (i.e., the normalized abundance) and biomass, and obtain the related curves. When abundance and biomass curves are compared, we may obtain information on the level of relative dominance of large- vs. small-body-mass species with structural and ecological implications (e.g., on the role of anthropogenic disturbances affecting the assemblages). ABC curves are based on a general assumption that, in human-disturbed habitats, small-sized species (i.e., those with low body masses and low trophic levels) tend to increase in abundance; consequently, the abundance curves approach an asymptote before the biomass curves do. Instead, in mature and undisturbed habitats, an opposite pattern may be observed, with the biomass curves accumulating before the abundance curves, indicating that a greater number of large-body-mass species of high trophic level occur in more complex and diverse assemblages [56,57]: under these conditions, the frequency of abundance is more evenly distributed than the frequency of biomass. Early cumulative abundance curves may indicate that the resources are used by a few dominant (i.e., more abundant) species with a broad spatial niche (i.e., generalists), whereas early cumulative biomass curves may indicate that species with a relatively high biomass largely occur in the assemblage (review in [60]).
To compare the relative frequencies of the ecological densities of each species between the two years, a χ2 test was carried out. To test for differences between the pairs of frequencies obtained in the two years before and after the fire, we used analysis of covariance (ANCOVA) [61,62]. To perform the statistical analyses, we used PAST software version 1.34 [63].
3. Results
In the 2023 spring period, 752 total records belonging to 13 different territorial breeding birds were obtained, both for forest and open/edge species. In 2018 (before the fire), 565 records were obtained: 62.5 territories were recorded (density: 16.45 pairs/10 ha), 37 of which were forest species (density: 9.74) and 25.5 of which were open/edge species (density: 6.71). In 2023 (after the fire), 61 territories were recorded (density: 16.05), of which 39 belonged to forest species (density: 10.26) and 22 to open/edge species (density: 5.79). The data at the species level reported in Table 1 were compared only for the territorial breeding species.
The individual rarefaction curves show a cumulation highlighting how the sample of collected data is representative both in the spring period and in the three sampling subperiods (II half of April, I half of May and II half of May; Figure 2).
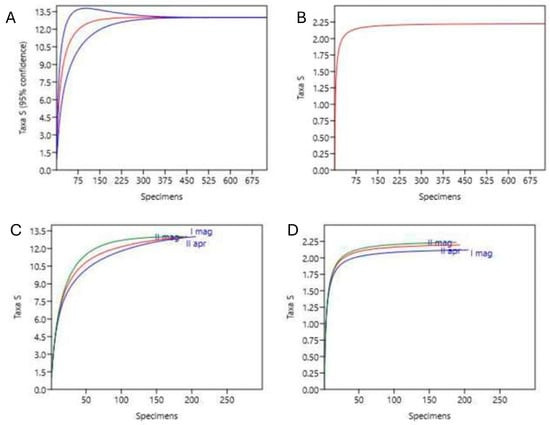
Figure 2.
Individual rarefaction curves for specific richness (left) and the Shannon diversity index H’ (right) for the entire sampling period (top) and for the three subperiods represented separately (bottom). For richness, the 95% confidence intervals are reported (in blue). (A,B) respectively, specific richness and Shannon index, entire period (in red line; in blue the confidence intervals); (C,D) respectively, specific richness and Shannon index, three subperiods [(C): I half of May: green; II May: red; II April: blue; (D): II May: green; II April: red; I May: blue].
The univariate diversity metrics (Margalef normalized richness, Shannon–Wiener and evenness indices), which were calculated for the 2023 data (after fire), revealed medium-high species diversity and a uniform abundance among species, comparable with those calculated in 2018 (before fire, Table 1). The same results were obtained by dividing the data at the guild level (forest and open/edge species; Supplementary Materials Tables S2 and S3).
Differences among species-specific frequencies between 2018 (before) and 2023 (after fire), both at the community and guild levels, were not significant (Table 2 and Supplementary Material Table S4).

Table 2.
Statistical comparisons between species frequencies (on density; FrD; as D/N, where D is species density and N is the total species density) calculated for the 2018 (before) and 2023 (after the 2022 fire event) data (with the χ2 test and p-value). Only data on territorial breeding species have been reported (non-territorial species or migratory passes have not been included).
The Whittaker and k-dominance plots, which compare the frequencies obtained both from the ecological densities and biomasses at the community level and at the forest guild level, show a comparable evenness before (2018) and after (2023) the fire. The analysis of covariance did not reveal a significant difference between the patterns in frequency both for abundance (Whittaker plots—bird community level: F = 7.757, p = 0.994; forest guild level: F = 0.0001, p = 0.997; k-dominance plots—bird community level: F = 0.245, p = 0.625; forest guild level: F = 0.992, p = 0.335; ANCOVA test; Figure 3) and biomass (Whittaker plots—bird community level: F = 0.001, p = 0.992; forest guild level: F = 0.0001, p = 0.981; k-dominance plots—bird community level: F = 0.084, p = 0.775; forest guild level: F = 0.007, p = 0.935; ANCOVA test; Figure 4).
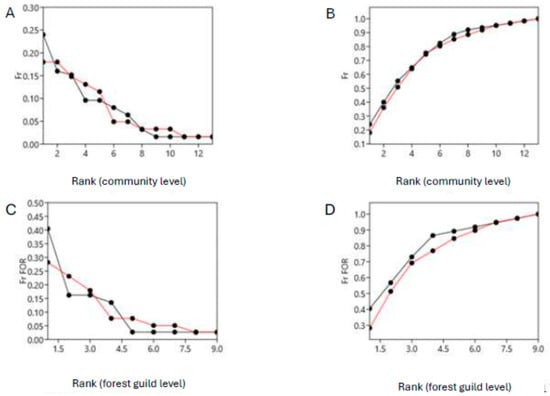
Figure 3.
Whittaker (left) and k-dominance plots (right) made by comparing frequencies derived from the ecological densities of the entire bird community (above) and forest guild (below) before (2018, lines in black) and after (2023, lines in red) the fire event (July 2022). (A,B): respectively, Whittaker plots and k-dominance plots for the entire bird community; (C,D): respectively, Whittaker plots and k-dominance plots for the forest guild.
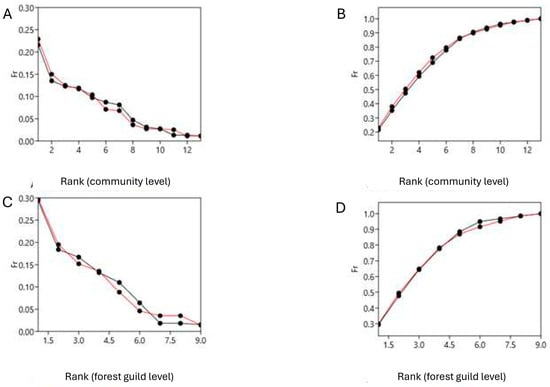
Figure 4.
Whittaker (left) and k-dominance plots (right) made by comparing frequencies derived from the biomass of the entire bird community (above) and only for the forest guilds (below) before (2018, lines in black) and after (2023, lines in red) the fire event (July 2022). (A,B): respectively, Whittaker plots and k-dominance plots for the entire bird community; (C,D): respectively, Whittaker plots and k-dominance plots for the forest guild.
The ABC curves also show a large overlap between the two periods before (2018) and after (2023), highlighting an absence of significant differences between the two patterns (ANCOVA test, 2018: F = 0.461, p = 0.504; 2023: F = 0.015, p = 0.902. Figure 5).

Figure 5.
Abundance/biomass comparisons (ABC) curves for the 2018 (A) and 2023 data (B). Black lines: abundance curves; red lines: biomass curves.
4. Discussion
At the local scale, the structure and composition of territorial species of breeding bird communities did not significantly change between the two periods before (2018) and after (2023) the 2022 fire event. Although an increase in open/edge birds was expected to be detrimental to forest species, the passage of the fire had no impact either at the community level or at the level of two selected guilds (forest and open/edge species). Our results are counterintuitive and different from those of many studies focusing on changes in abundance, richness and diversity where abrupt reductions in these metrics have been observed after fires (e.g., [64,65]).
Cork oaks are highly fire-resilient trees with recovery capacity already in the season following the fire, being the only European tree with stem and crown resprouting capability (through epicormic buds) after intense crown fires [66,67,68,69]. In our case study, the post-fire crown regeneration of the tree canopy, which began in the autumn following the fire, and the vegetative recovery of cork oak trees in the following spring (2023) have probably restored the habitat suitability for forest birds (see [70] for Algerian cork oak woods). Since this patch system occurs in a fragmented suburban agroforestry landscape, it is likely that other factors may have contributed to this lack of expected changes in our bird assemblages. For example, it is conceivable that, following the fire, an increase in density occurred in the residual areas not affected by the fire. This phenomenon, known as the ‘crowding effect’ [71], occurs in fragmented landscapes when specific disturbance-induced changes lead to an abrupt reduction in the size of suitable habitat for sensitive species (e.g., [72]). In fragmented agroforestry landscapes, it is also probable that in the presence of patches not affected by disturbances, rapid recolonization processes toward the burned areas occurred, thus re-establishing, in the short term, the structure and composition of the breeding bird communities (particularly in forest-related species).
According to Mendelsohn et al. [73], our data suggest that the observed responses of birds to fires may be attributed to (i) the availability of nearby unburned habitat patches (showing a role of refugia), (ii) the local suitability of post-fire resilient vegetation, and (iii) the species-specific dispersal capacity.
However, this is only an exploratory study, highlighting the post-fire resilience of breeding birds in suburban cork oak wood patches at a local scale and in a short time range. In this context, our considerations may not be valid in spatial and historical circumstances where fires act locally, with high frequency and intensity (e.g., as ‘pulse’ events; [74]). For example, Touhami et al. [75] highlighted how cork oak forests can severely degrade when fires and related disturbances (e.g., grazing) occur at a rate that does not allow for the recovery of forest systems (i.e., as ‘press’ disturbances [74]; Zeghdani et al. [70] showed long times to restore the pre-fire densities also for forest species).
Moreover, it is important to highlight some limitations of this study. First, since some species recorded are Eurasian or Eurasian/African (trans-Saharan) migrants, these species could be affected by large-scale (i.e., not local) events (climate conditions, availability of food sources, other disturbances) along their migratory routes [76]. Therefore, the observed lack of changes may not be directly attributable to local fire events.
Second, during the time gap between the pre-fire (2018) and post-fire (2023) periods, several changes unrelated to the fire could have occurred, affecting the bird communities. Without continuous monitoring throughout this period or at least more frequent surveys and given the lack of ‘control’ areas (i.e., comparable sites without fires), it is difficult to assign the observed changes (or lack of changes) directly to the fire event. However, although ‘Campagna Romana’ has undergone major transformations in recent decades [77,78], the local pattern of landscape mosaics has not changed in the last 2–5 years (pers. obs.). Our study area has been characterized by agro-mosaic stability over time (i.e., with few, recurrent and comparable disturbances occurring any year; the only other anthropogenic disturbance occurring locally is crop mowing in June). Therefore, we are confident that from 2018 to June 2022 (before fire), bird communities were very similar in structure, and we may consider our design as a before/after comparison.
Third, our local data at the species level could be affected by population trends at larger scales (i.e., national or regional ones). All the species here considered have shown a stable or increasing trend in the first decades of the 2000s (except the Eurasian Turtle Dove and Zitting Cisticola, which show stable/decreasing trends) and, particularly, the guild of forest species considered both individually and as a whole (see the Woodland Bird Index; for Europe: +7% from 2010 to 2022 [79]; for Italy: [80,81]; for Latium [82]; see also [83] for Northern Italy). Therefore, our conclusions may not be extendable to other species showing declining trends. However, since data in population size at national level population sizes were comparable in both study years, we think that our post-fire results indicate the impacts (or lack of impact) of fire and do not depend on other factors on a larger scale.
Finally, we carried out a study at the community and guild levels, assuming that all species associated with the forest complex were uniformly impacted by the fire. This represents an oversimplification, and further analyses are necessary at the single-species level. For example, some passerines (e.g., Curruca melanocephala and Hippolais polyglotta) show interesting responses in the first year after fires (e.g., [13,14]).
Therefore, further studies should confirm the fire responses at the species and community levels in suburban forest mosaics dominated by epicormic resprouting trees (such as, in our case, cork oaks), which are largely diffused worldwide (e.g., [84]), and at larger spatial and temporal scales for different disturbance regimes and use guilds obtained with different criteria (e.g., foraging- and phenological-based).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/birds5040042/s1, Supplementary Materials Table S1. Structure and floristic composition of the cork oak patches in the study area. The number of plants for each tree species and diameter class (in cm), and the total value (and relative frequency, fr) are reported. Data from [24]; Supplementary Materials Table S2. Ecological guild of forest species. Species recorded in 2018 (before) and 2023 (after 2022 fire event), density values (D), relative frequencies (FrD), consuming biomass (Cb) and relative frequencies (FrCb) have been reported. Below: the values of the univariate diversity metrics: normalized richness (Margalef index; Dm), Shannon–Wiener diversity (H’) and evenness (J); Supplementary Materials Table S3. Guild of open/edge species. Species recorded in 2018 (before) and 2023 (after the 2022 fire event), density values (D) and relative frequencies (FrD), consuming biomass (Cb), relative frequencies (FrCb) have been reported. Below: the values of the univariate diversity metrics: normalized richness (Margalef index; Dm), Shannon–Wiener diversity (H’) and evenness (J); Supplementary Materials Table S4. Guilds of forest and open/edge species. Relative frequencies (density) calculated for the 2018 and 2023 data (with the χ2 test and p-value).
Author Contributions
Conceptualization, data curation, formal analysis, C.B.; investigation and field sampling: S.C. and C.B.; methodology, C.B.; supervision, C.B. and M.S.; writing—original draft, C.B. and S.C.; writing—review and editing, C.B. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Marco Gustin (BirdLife International—LIPU), Massimo Brunelli and Fulvio Fraticelli (SROPU—Stazione Romana Osservazione e Protezione Uccelli, Rome, Italy), Giuseppe Dodaro (CIRF and Fondazione per lo Sviluppo Sostenibile) provided useful information about trends of common species at national and regional levels, when available. Three anonymous reviewers and Editor-in-Chief (Jukka Jokimäki) provided further useful comments and suggestions which largely improved the first and the second draft of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Trabaud, L.; Prodon, P. (Eds.) Fire in Mediterranean Ecosystems. Ecosystems Research Report, 5; Commission of the European Communities: Bruxelles, Belgium, 1993. [Google Scholar]
- Prodon, R.; Fons, R.; Peter, A.M. L’impact du feu sur la vegetation, les oiseaux et les micromammifères dans diverses formations mediterraneennes des Pyrenees Orientales: Premiers resultats. Rev. Ecol. 1984, 39, 128–158. [Google Scholar] [CrossRef]
- Jacquet, K.; Prodon, R. Measuring the postfire resilience of a bird–vegetation system: A 28-year study in a Mediterranean oak woodland. Oecologia 2009, 161, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Woinarski, J.C.Z. Effects of fire on the bird communities of tropical woodlands and open forests in northern Australia. Austral. J. Ecol. 1990, 15, 1–22. [Google Scholar] [CrossRef]
- Woinarski, J.C.Z.; Recher, H.F. Impact and response: A review of the effects of fire on the Australian avifauna. Pacific Conserv. Biol. 1997, 3, 183–205. [Google Scholar] [CrossRef]
- Gill, A.M.; York, A.; Woinarski, J.C.Z. Australia’s Biodiversity: Responses to Fire; Environment Australia: Camberra, ACT, Australia, 1999. [Google Scholar]
- Woinarski, J.C.Z. Fire and Australian birds: A review. In Australia’s Biodiversity: Responses to Fire: Plants, Birds and Invertebrates; Gill, A.M., Woinarksi, G.C.Z., York, A., Eds.; Environment Australia: Camberra, ACT, Australia, 1999; pp. 55–111. [Google Scholar]
- Imbeau, L.; Savard, J.P.L.; Gagnon, R. Comparing bird assemblages in successional black spruce stands originating from fire and logging. Canad. J. Zool. 2000, 77, 1850–1860. [Google Scholar] [CrossRef]
- Farina, A. Landscape structure and breeding bird distribution in a sub-Mediterranean agro-ecosystem. Lands. Ecol. 1997, 12, 365–378. [Google Scholar] [CrossRef]
- Battisti, C.; Ukmar, E.; Luiselli, L.; Bologna, M.A. Diversity/dominance diagrams show that fire disrupts the evenness in Mediterranean pinewood forest bird assemblages. Comm. Ecol. 2008, 9, 107–113. [Google Scholar] [CrossRef]
- Devictor, V.; Julliard, R.; Clavel, J.; Jiguet, F.; Lee, A.; Couvet, D. Functional biotic homogenization of bird communities in disturbed landscapes. Glob. Ecol. Biogeogr. 2008, 17, 252–261. [Google Scholar] [CrossRef]
- Ukmar, E.; Battisti, C.; Luiselli, L.; Bologna, M.A. The effect of fire on communities, guild and species of breeding birds in burnt and control pinewoods in central Italy. Biodiv. Conserv. 2007, 10, 1007–1021. [Google Scholar] [CrossRef]
- Pons, P.; Prodon, R. Short term temporal patterns in a Mediterranean shrubland bird community after wildfire. Acta Oecol. 1996, 17, 29–41. [Google Scholar]
- Izhaki, I.; Adar, M. The effects of post-fire management on bird community succession. Intern. J. Wildl. Fire 1997, 7, 335–342. [Google Scholar] [CrossRef]
- Schieck, J.; Song, S.J. Changes in bird communities throughout succession following fire and harvest in boreal forests of western North America: Literature review and meta-analyses. Canad. J. For. Res. 2006, 36, 1299–1318. [Google Scholar] [CrossRef]
- Lowe, J.; Pothier, D.; Rompré, G.; Savard, J.P.L. Long-term changes in bird community in the unmanaged post-fire eastern Québec boreal forest. J. Ornithol. 2012, 153, 1113–1125. [Google Scholar] [CrossRef]
- Trabaud, L.; Lepart, J. Diversity and stability in garrigue ecosystems after fire. Vegetatio 1980, 43, 49–57. [Google Scholar] [CrossRef]
- Lloret, F.; Verdu, M.; Noe, F.-H.; Alfonso, V.-B. Fire and resprouting in Mediterranean ecosystems: Insights from an external biogeographical region, the mexical shrubland. Am. J. Botany 1999, 86, 1655–1661. [Google Scholar] [CrossRef]
- Herrando, S.; Brotons, L. Forest bird diversity in Mediterranean areas affected by wildfires: A multi-scale approach. Ecography 2002, 25, 161–172. [Google Scholar] [CrossRef]
- Brotons, L.; Herrando, S.; Martin, J.L. Bird assemblages in forest fragments within Mediterranean mosaics created by wild fires. Landsc. Ecol. 2005, 19, 663–675. [Google Scholar] [CrossRef]
- Bellia, E. Diversità e struttura dell’avifauna in una successione post incendio di bosco meso-mediterraneo. Avocetta 2005, 29, 75–84. [Google Scholar]
- Sarà, M.; Bellia, E.; Milazzo, A. Fire disturbance disrupts co-occurrence patterns of terrestrial vertebrates in Mediterranean woodlands. J. Biogeogr. 2006, 33, 843–852. [Google Scholar] [CrossRef]
- Natividade, J.V. Subericultura; Lisboa, Ministério da Economia, Direcção Geral dos Serviços Florestais e Aquìcolas, Oficinas de Fotogravura de Marques Abreu: Porto, Portugal, 1950. [Google Scholar]
- Jackson, J.F.; Adams, D.C.; Jackson, U.B. Allometry of constitutive defense: A model and a comparative test with tree bark and fire regime. Am. Nat. 1999, 153, 614–632. [Google Scholar] [CrossRef]
- De Lillis, M.; Bianco, P.M.; Loreto, F. The influence of leaf water content and isoprenoids on flammability of some Mediterranean woody species. Intern. J. Wildl. Fire 2009, 18, 203–212. [Google Scholar] [CrossRef]
- Catry, F.X.; Moreira, F.; Pausas, J.G.; Fernandes, P.M.; Rego, F.; Cardillo, E.; Curt, T. Cork oak vulnerability to fire: The role of bark harvesting, tree characteristics and abiotic factors. PLoS ONE 2012, 7, e39810. [Google Scholar] [CrossRef] [PubMed]
- Grapow, L.C.; Fanelli, G. The vanishing landscape of the Campagna Romana. Lands. Urb. Plan. 1993, 24, 69–76. [Google Scholar] [CrossRef]
- Battisti, C.; Luiselli, L.; Frank, B.; Lorenzetti, E. Should fragment area reduction be considered a stress for forest bird assemblages? Evidence from diversity/dominance diagrams. Comm. Ecol. 2009, 10, 189–195. [Google Scholar] [CrossRef]
- Testi, A.; Lucattini, C. Contribution to the syntaxonomic knowledge of Quercus suber woodlands of Latium. Rendiconti Lincei 1994, 5, 247–259. [Google Scholar] [CrossRef]
- Spada, F.; Agrillo, E.; Casella, L.; Dowgiallo, G.; Schirone, B. Phytogeography of Quercus suber L. in Lazio (Central Italy): A causalistic approach. Ann. Bot. 2008, 8, 43–54. [Google Scholar]
- Gratani, L.; Amadori, M. Post-fire resprouting of shrubby species in Mediterranean maquis. Vegetatio 1991, 96, 137–143. [Google Scholar] [CrossRef]
- Ferrara, C.; Salvati, L.; Corona, P.; Romano, R.; Marchi, M. The background context matters: Local-scale socioeconomic conditions and the spatial distribution of wildfires in Italy. Sci. Tot. Environ. 2019, 654, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Barbero, M.; Bonin, G.; Loisel, R.; Quézel, P. Changes and disturbances of forest ecosystems caused by human activities in the western part of the Mediterranean basin. Vegetatio 1990, 87, 151–173. [Google Scholar] [CrossRef]
- Comune di Roma. Piano di Protezione Civile di Roma Capitale Piano Comunale di Protezione Civile (art. 12, comma 2, lett. e) del D.lgs. 1/2018) Piano di Emergenza Comunale (D.G.R. Lazio 363/2014). File 5 ‘Rischio Incendio Boschivo e di Interfaccia urbano-rurale’ (Forest Fire and Urban-Rural Interface Risk). Available online: https://www.comune.roma.it/web-resources/cms/documents/PPC2024_5_Rischio_Incendio_Boschivo.pdf (accessed on 6 September 2024).
- Battisti, C. Check-list of Vertebrates in the “Tenuta dei Massimi” nature reserve (Rome, central Italy) with some remarks on local conservation priorities. Nat. Hist. Sci. 2014, 1, 25–36. [Google Scholar] [CrossRef][Green Version]
- Fanelli, G.; Celesti-Grapow, L. La flora del bacino del fosso della Magliana (Roma). Ann. Di Bot. Roma 1994, 52 (Suppl. S11), 83–114. [Google Scholar]
- Fanelli, G. Analisi fitosociologica dell’area metropolitana di Roma. Braun-Blanquetia 2002, 27, 1–269. [Google Scholar]
- Fanelli, G.; Bianco, P.M. (Eds.) Memoria Illustrativa Della Carta Della Vegetazione Della Provincia di Roma. Provincia di Roma, Dip. VI. Governo del Territorio, Serv. 3; Sistema Informativo Geografico: Rome, Italy, 2007. [Google Scholar]
- Battisti, C.; Mandolini, R. La comunità ornitica nidificante in un settore residuale della campagna romana (corridoio tra le Riserve naturali ‘Valle dei Casali’ e ‘Tenuta dei Massimi’; Roma, Italia centrale). Alula 2018, 25, 1–9. [Google Scholar]
- Ottmar, R.D.; Sandberg, D.V.; Riccardi, C.L.; Prichard, S.J. An overview of the fuel characteristic classification system—Quantifying, classifying, and creating fuelbeds for resource planning. Canad. J. For. Res. 2007, 37, 2383–2393. [Google Scholar] [CrossRef]
- WWF Roma. 2022. Available online: https://www.wwfroma.it/aggiornamenti-dal-wwf-roma/1164-altro-incendio-a-roma-stavolta-alla-riserva-naturale-della-tenuta-dei-massimi (accessed on 2 July 2022).
- Bibby, C.J.; Burgess, N.D.; Hillis, D.; Mustoe, S. Bird Census Techniques; Academic Press: London, UK, 2000. [Google Scholar]
- Sutherland, W.J. (Ed.) Ecological Census Techniques: A Handbook; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Salt, G.W. An analysis of avifaunas in the Teton Mountains and Jackson Hole. Condor 1957, 59, 373–393. [Google Scholar] [CrossRef]
- Cramp, S.; Simmons, K.E.L. (Eds.) The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1977; Volume I. [Google Scholar]
- Cramp, S.; Simmons, K.E.L. (Eds.) The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1980; Volume II. [Google Scholar]
- Cramp, S.; Simmons, K.E.L. (Eds.) The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1983; Volume III. [Google Scholar]
- Cramp, S. (Ed.) The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1988; Volume V. [Google Scholar]
- Cramp, S.; Perrins, C.M. (Eds.) The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1993; Volume VII. [Google Scholar]
- Battisti, C.; Dodaro, G. Mapping bird assemblages in a Mediterranean urban park: Evidence for a shift in dominance towards medium-large body sized species after 26 years. Belg. J. Zool. 2016, 146, 81–89. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in ecology. Transl. Mem. R. Acad. Cienc. Artes. Barc. 1958, 32, 373–449. [Google Scholar]
- Lambshead, P.J.D.; Platt, H.M.; Shaw, K.M. The detection of differences among assemblages of marine benthic species based on an assessment of dominance and diversity. J. Nat. Hist. 1983, 17, 859–874. [Google Scholar] [CrossRef]
- Platt, H.M.; Shaw, K.M.; Lambshead, P.J.D. Nematode species abundance patterns and their use in the detection of environmental perturbatio. Hydrobiologia 1984, 118, 59–66. [Google Scholar] [CrossRef]
- Warwick, R.M. A new method for detecting pollution effects on marine macro-benthic communities. Mar. Biol. 1986, 92, 557–562. [Google Scholar] [CrossRef]
- Clarke, K.R. Comparisons of dominance curves. J. Experim. Mar. Biol. Ecol. 1990, 138, 143–157. [Google Scholar] [CrossRef]
- Battisti, C. Mapping breeding birds in a re-naturalized historical fortress: Composition, structure and considerations about abundance vs. biomass comparisons. Riv. Ital. Ornitol. 2023, 93, 3–10. [Google Scholar] [CrossRef]
- Battisti, C. Synanthropic-dominated biomass in an insular landbird assemblage. Comm. Ecol. 2018, 19, 203–210. [Google Scholar] [CrossRef]
- Battisti, C.; Poeta, G.; Fanelli, G. An Introduction to Disturbance Ecology: A Road Map for Wildlife and Conservation; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Krebs, C.J. Ecological Methodology; Harper Collins: New York, NY, USA, 1989. [Google Scholar]
- Dytham, C. Choosing and Using Statistics: A Biologist’s Guide; John Wiley and Sons: New York, NY, USA, 2011. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST–Palaeontological statistics, ver. 1.34. Palaeontol. Electr. 2005, 4, 1–66. [Google Scholar]
- Chalmandrier, L.; Midgley, G.F.; Barnard, P.; Sirami, C. Effects of time since fire on birds in a plant diversity hotspot. Acta Oecol. 2013, 49, 99–106. [Google Scholar] [CrossRef]
- Robinson, N.M.; Leonard, S.W.; Bennett, A.F.; Clarke, M.F. Refuges for birds in fire-prone landscapes: The influence of fire severity and fire history on the distribution of forest birds. For. Ecol. Manag. 2014, 318, 110–121. [Google Scholar] [CrossRef]
- Pausas, J. Resprouting of Quercus suber in NE Spain after fire. J. Veget. Sci. 1997, 8, 703–706. [Google Scholar] [CrossRef]
- Catry, F.X.; Moreira, F.; Duarte, I.; Acácio, V. Factors affecting post-fire crown regeneration in cork oak (Quercus suber L.) trees. Europ. J. For. Res. 2009, 128, 231–240. [Google Scholar] [CrossRef]
- Paula, S.; Arianoutsou, M.; Kazanis, D.; Tavsanoglu, C.; Lloret, F.; Buhk, C.; Ojeda, F.; Luna, B.; Moreno, M.; Rodrigo, A.; et al. Fire-related traits for plant species of the Mediterranean Basin. Ecology 2009, 90, 1420. [Google Scholar] [CrossRef]
- Silva, J.S.; Catry, F. Forest fires in cork oak (Quercus suber L.) stands in Portugal. Intern. J. Environ. Stud. 2006, 63, 235–257. [Google Scholar] [CrossRef]
- Zeghdani, N.; Prodon, R.; Benyacoub, S. Resilience of Bird Communities After Wildfires in Algerian Cork Oak Forests. Acta Ornithologica 2024, 58, 175–186. [Google Scholar] [CrossRef]
- Debinski, D.M.; Holt, R.D. A survey and overview of habitat fragmentation experiments. Conserv. Biol. 2000, 14, 342–355. [Google Scholar] [CrossRef]
- Vallejos, M.A.V.; Padial, A.A.; Vitule, J.R.S.; Monteiro-Filho, E.L.A. Effects of crowding due to habitat loss on species assemblage patterns. Conserv. Biol. 2020, 34, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, M.B.; Brehme, C.S.; Rochester, C.J.; Stokes, D.C.; Hathaway, S.A.; Fisher, R.N. Responses in bird communities to wildland fires in southern California. Fire Ecol. 2008, 4, 63–82. [Google Scholar] [CrossRef]
- Inamine, H.; Miller, A.; Roxburgh, S.; Buckling, A.; Shea, K. Pulse and press disturbances have different effects on transient community dynamics. Am. Nat. 2022, 200, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Touhami, I.; Chirino, E.; Aouinti, H.; El Khorchani, A.; Elaieb, M.T.; Khaldi, A.; Nasr, Z. Decline and dieback of cork oak (Quercus suber L.) forests in the Mediterranean basin: A case study of Kroumirie, Northwest Tunisia. J. For. Res. 2020, 31, 1461–1477. [Google Scholar] [CrossRef]
- Kirby, J.S.; Stattersfield, A.J.; Butchart, S.H.; Evans, M.I.; Grimmett, R.F.; Jones, V.R.; O’Sullivan, J.; Tucker, G.M.; Newton, I. Key conservation issues for migratory land-and waterbird species on the world’s major flyways. Bird Conserv. Internat. 2008, 18 (Suppl. S1), S49–S73. [Google Scholar] [CrossRef]
- Frondoni, R.; Mollo, B.; Capotorti, G. A landscape analysis of land cover change in the Municipality of Rome (Italy): Spatio-temporal characteristics and ecological implications of land cover transitions from 1954 to 2001. Landsc. Urban Plann. 2011, 100, 117–128. [Google Scholar] [CrossRef]
- Salvati, L. Agro-forest landscape and the ‘fringe’ city: A multivariate assessment of land-use changes in a sprawling region and implications for planning. Sci. Tot. Environm. 2014, 490, 715–723. [Google Scholar] [CrossRef]
- EBCC, 2023. Report on the Pan-European Common Bird Monitoring Scheme, December 2021. European Bird Census Council. Available online: https://pecbms.info/trends-and-indicators/species-trends/ (accessed on 10 September 2024).
- Fornasari, L.; Londi, G.; Buvoli, L.; Tellini Florenzano, G.; La Gioia, G.; Pedrini, P.; Brichetti, P.; de Carli, E. Distribuzione geografica e ambientale degli uccelli comuni nidificanti in Italia, 2000–2004 (dati del Progetto MITO2000). Avocetta 2010, 34, 5–224. [Google Scholar]
- Rete Rurale Nazionale & LIPU. Gli Andamenti di Popolazione Degli Uccelli Comuni in Italia 2000–2010. MiPAAF. Available online: https://www.reterurale.it/flex/cm/pages/ServeAttachment.php/L/IT/D/0%252F3%252Fb%252FD.81a4bb99845c9a5d989a/P/BLOB%3AID%3D3851/E/pdf (accessed on 10 September 2024).
- Brunelli, M.; Sarrocco, S.; Corbi, F.; Sorace, A.; Boano, A.; De Felici, S.; Guerrieri, G.; Meschini, A.; Roma, S. (Eds.) Nuovo Atlante Degli Uccelli Nidificanti nel LAZI; Edizioni ARP (Agenzia Regionale Parchi): Roma, Italy, 2011. [Google Scholar]
- Tirozzi, P.; Orioli, V.; Dondina, O.; Kataoka, L.; Bani, L. Species traits drive long-term population trends of common breeding birds in northern Italy. Animals 2021, 11, 3426. [Google Scholar] [CrossRef] [PubMed]
- Recher, H.F.; Davis, W.E., Jr. Response of birds to a wildfire in the Great Western Woodlands, Western Australia. Pac. Conserv. Biol. 2013, 19, 188–203. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).