Haemosporidian Infection Is Associated with the Oxidative Status in a Neotropical Bird
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
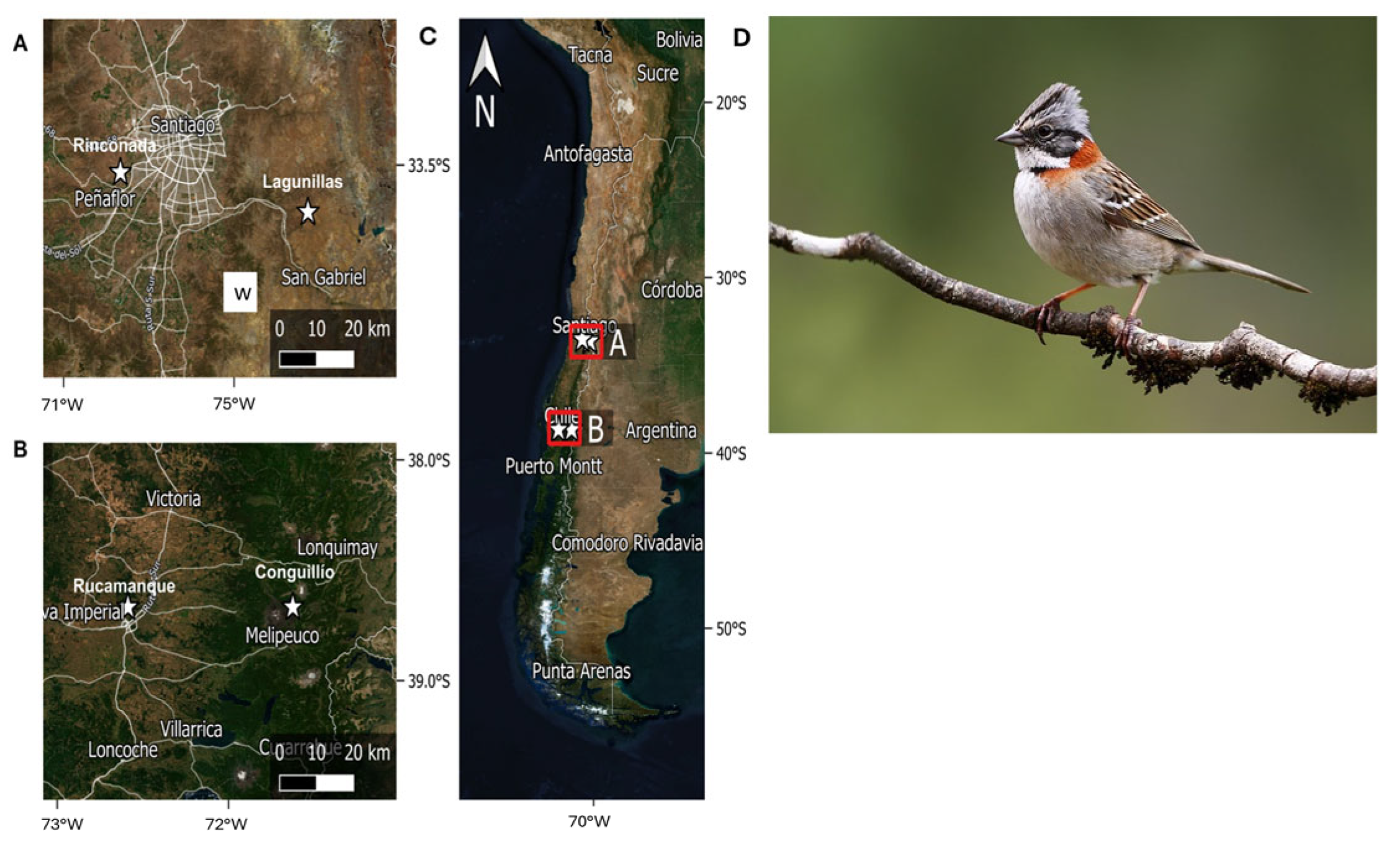
2.1. Study Species
2.2. Bird Sampling
2.3. Oxidative Status
2.4. DNA Extraction and Screening for Haemosporidian Parasites
2.5. Statistical Tests
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arneberg, P.; Skorping, A.; Grenfell, B.; Read, A.F. Host Densities as Determinants of Abundance in Parasite Communities. Proc. R. Soc. B Biol. Sci. 1998, 265, 1283–1289. [Google Scholar] [CrossRef]
- Frank, S.A. Evolution of Host-parasite Diversity. Evolution 1993, 47, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Costantini, D.; Møller, A.P. Does Immune Response Cause Oxidative Stress in Birds? A Meta-Analysis. Comp. Biochem. Physiol.—A Mol. Integr. Physiol. 2009, 153, 339–344. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Metcalfe, N.B.; Alonso-Alvarez, C. Oxidative Stress as a Life-History Constraint: The Role of Reactive Oxygen Species in Shaping Phenotypes from Conception to Death. Funct. Ecol. 2010, 24, 984–996. [Google Scholar] [CrossRef]
- Costantini, D. Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology: A Marriage Between Mechanistic and Evolutionary Approaches; Springer: Berlin/Heidelberg, Germany, 2014; p. 362. [Google Scholar]
- Costantini, D.; Verhulst, S. Does High Antioxidant Capacity Indicate Low Oxidative Stress? Funct. Ecol. 2009, 23, 506–509. [Google Scholar] [CrossRef]
- Van De Crommenacker, J.; Horrocks, N.P.C.; Versteegh, M.A.; Komdeur, J.; Tieleman, B.I.; Matson, K.D. Effects of Immune Supplementation and Immune Challenge on Oxidative Status and Physiology in a Model Bird: Implications for Ecologists. J. Exp. Biol. 2010, 213, 3527–3535. [Google Scholar] [CrossRef]
- Costantini, D.; Rowe, M.; Butler, M.W.; McGraw, K.J. From Molecules to Living Systems: Historical and Contemporary Issues in Oxidative Stress and Antioxidant Ecology. Funct. Ecol. 2010, 24, 950–959. [Google Scholar] [CrossRef]
- Dupré-Crochet, S.; Erard, M.; Nüβe, O. ROS Production in Phagocytes: Why, When, and Where? J. Leukoc. Biol. 2013, 94, 657–670. [Google Scholar] [CrossRef]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Involvement of Superoxide and Myeloperoxidase in Oxygen-Dependent Killing of Staphylococcus aureus by Neutrophils. Infect. Immun. 1996, 64, 3512–3517. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J.; Kettle, A.J.; Rosen, H.; Winterbourn, C.C.; Nauseef, W.M. Myeloperoxidase: A Front-Line Defender against Phagocytosed Microorganisms. J. Leukoc. Biol. 2013, 93, 185–198. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollier, H. Chemistry and Biochemistry of 4-Hydroxynonenal, Malonaldehyde and Related Aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [PubMed]
- Jentzsch, A.M.; Bachmann, H.; Fürst, P.; Biesalski, H.K. Improved Analysis of Malondialdehyde in Human Body Fluids. Free Radic. Biol. Med. 1996, 20, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.A.; McGraw, K.J.; Robinson, W.D. Serum Antioxidant Levels in Wild Birds Vary in Relation to Diet, Season, Life History Strategy, and Species. Oecologia 2009, 161, 673–683. [Google Scholar] [CrossRef]
- Jortzik, E.; Becker, K. Thioredoxin and Glutathione Systems in Plasmodium falciparum. Int. J. Med. Microbiol. 2012, 302, 187–194. [Google Scholar] [CrossRef]
- Cyktor, J.C.; Turner, J. Interleukin-10 and Immunity against Prokaryotic and Eukaryotic Intracellular Pathogens. Infect. Immun. 2011, 79, 2964–2973. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Sardar, A.H.; Mandal, A.; Saini, S.; Abhishek, K.; Kumar, A.; Purkait, B.; Singh, R.; Das, S.; Mukhopadhyay, R.; et al. Metabolic Reconfiguration of the Central Glucose Metabolism: A Crucial Strategy of Leishmania donovani for Its Survival during Oxidative Stress. FASEB J. 2015, 29, 2081–2098. [Google Scholar] [CrossRef]
- Dzik, J.M. Molecules Released by Helminth Parasites Involved in Host Colonization. Acta Biochim. Pol. 2006, 53, 33–64. [Google Scholar] [CrossRef]
- Shrestha, S.P.; Tomita, T.; Weiss, L.M.; Orlofsky, A. Proliferation of Toxoplasma Gondii in Inflammatory Macrophages in Vivo Is Associated with Diminished Oxygen Radical Production in the Host Cell. Int. J. Parasitol. 2006, 36, 433–441. [Google Scholar] [CrossRef]
- Hurford, A.; Day, T. Immune Evasion and the Evolution of Molecular Mimicry in Parasites. Evolution 2013, 67, 2889–2904. [Google Scholar] [CrossRef]
- Ludin, P.; Nilsson, D.; Mäser, P. Genome-Wide Identification of Molecular Mimicry Candidates in Parasites. PLoS ONE 2011, 6, e17546. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Atkinson, C.; Thomas, N.; Hunter, B. Parasitic Diseases of Wild Birds; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Pigeault, R.; Cozzarolo, C.; Choquet, R.; Strehler, M.; Jenkins, T.; Delhaye, J.; Bovet, L.; Wassef, J.; Glaizot, O.; Christe, P. Haemosporidian Infection and Co-Infection Affect Host Survival and Reproduction in Wild Populations of Great Tits. Int. J. Parasitol. 2018, 48, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Van de Crommenacker, J.; Richardson, D.S.; Koltz, A.M.; Hutchings, K.; Komdeur, J. Parasitic Infection and Oxidative Status Are Associated and Vary with Breeding Activity in the Seychelles warbler. Proc. R. Soc. B Biol. Sci. 2012, 279, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- De Angeli Dutra, D.; Silveira, P.; Ramos, J.A.; Sousa, J.P.; Braga, É.M.; Norte, A.C. Haemosporidian Infections Affect Antioxidant Defences in Great Tits Parus Major but Are Not Related to Exposure to Aerial Pollutants. Parasitol. Open 2017, 3, e4. [Google Scholar] [CrossRef][Green Version]
- Jiménez-Peñuela, J.; Ferraguti, M.; Martínez-De La Puente, J.; Soriguer, R.C.; Figuerola, J. Oxidative Status in Relation to Blood Parasite Infections in House sparrows Living along an Urbanization Gradient. Environ. Pollut. 2023, 316, 120712. [Google Scholar] [CrossRef] [PubMed]
- Schoenle, L.A.; Kernbach, M.; Haussmann, M.F.; Bonier, F.; Moore, I.T. An Experimental Test of the Physiological Consequences of Avian Malaria Infection. J. Anim. Ecol. 2017, 86, 1483–1496. [Google Scholar] [CrossRef]
- Delhaye, J.; Jenkins, T.; Christe, P. Plasmodium Infection and Oxidative Status in Breeding Great Tits, Parus major. Malar. J. 2016, 15, 531. [Google Scholar] [CrossRef] [PubMed]
- Poblete, Y.; Contreras, C.; Fernández, C.; Flores, C.R.; Vega, P.; Ávila, M.; Sabat, P. Geographic Variation in the Altitudinal Migration Patterns, Body Size, Oxidative Status and Exploratory Behavior in a Neotropical Bird. Ecol. Evol. 2023, 13, e9941. [Google Scholar] [CrossRef]
- Poblete, Y.; Gutiérrez, V.; Cid, V.; Newsome, S.D.; Sabat, P.; Vasquez, R.A. Intraspecific Variation in Exploratory Behavior and Elevational Affinity in a Widely Distributed Songbird. Oecologia 2018, 186, 931–938. [Google Scholar] [CrossRef]
- Sabat, P.; Cavieres, G.; Veloso, C.; Canals, M.; Bozinovic, F. Intraspecific Basal Metabolic Rate Varies with Trophic Level in Rufous-Collared Sparrows. Comp. Biochem. Physiol. Part A 2009, 154, 502–507. [Google Scholar] [CrossRef]
- Maldonado, K.; van Dongen, W.F.D.; Vásquez, R.; Sabat, P. Geographic Variation in the Association between Exploratory Behavior and Physiology in Rufous-Collared Sparrows. Physiol. Biochem. Zool. 2012, 85, 618–624. [Google Scholar] [CrossRef]
- Ruiz, G.; Novoa, F.F.; Sabat, P. Hematological Parameters and Stress Index in Rufous-Collared Sparrows Dwelling in Urban Environments. Condor 2002, 104, 162–166. [Google Scholar] [CrossRef]
- Poblete, Y.; Gutierrez, V.; González, P.L.; Wingfield, J.C.; Vásquez, R.A. Differences in Circulating Corticosterone Levels Associated with Elevation of Breeding Sites in Rufous—Collared Sparrows Zonotrichia capensis. J. Ornithol. 2020, 162, 487–496. [Google Scholar] [CrossRef]
- Lopez-Calleja, M.V. Dieta de Zonotrichia capensis (Emberizidae) Y Diuca diuca (Fringillidae): Efecto de La Variación Estacional de Los Recursos Tróficos y La Riqueza de Aves Granívoras En Chile Central. Rev. Chil. Hist. Nat. 1995, 68, 321–331. [Google Scholar]
- Clark, A.D.; Addis, E.A.; Vásquez, R.A.; Wingfield, J.C. Seasonal Modulation of the Adrenocortical Stress Responses in Chilean Populations of Zonotrichia capensis. J. Ornithol. 2019, 160, 61–70. [Google Scholar] [CrossRef]
- Chapman, F.M. The Post-Glacial History of Zonotrichia capensis. Bull. Am. Museum Nat. Hist. 1940, 78, 381–438. [Google Scholar]
- Doussang, D.; González-Acuña, D.; Torres-Fuentes, L.G.; Lougheed, S.C.; Clemente-Carvalho, R.B.; Greene, K.C.; Vianna, J.A. Spatial Distribution, Prevalence and Diversity of Haemosporidians in the Rufous-Collared Sparrow, Zonotrichia capensis. Parasites Vectors 2019, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Peig, J.; Green, A.J. New Perspectives for Estimating Body Condition from Mass/Length Data: The Scaled Mass Index as an Alternative Method. Oikos 2009, 118, 1883–1891. [Google Scholar] [CrossRef]
- Fridolfsson, A.K.; Ellegren, H. A Simple and Universal Method for Molecular Sexing of Non-Ratite Birds. J. Avian Biol. 1999, 30, 116–121. [Google Scholar] [CrossRef]
- Fernández, C.; Villaseñor, N.R.; Contreras, C.; Ávila, M.; Sabat, P.; Poblete, Y. Intra-Urban Variation in Body Condition, Body Size and Oxidative Status of Rufous-Collared Sparrow Relate to Urban Green Space Attributes in a Latin American Metropolis. Urban Ecosyst. 2023, 26, 575–586. [Google Scholar] [CrossRef]
- Sabat, P.; Narváez, C.; Peña-Villalobos, I.; Contreras, C.; Maldonado, K.; Sanchez-Hernandez, J.C.; Newsome, S.D.; Nespolo, R.; Bozinovic, F. Coping with Salt Water Habitats: Metabolic and Oxidative Responses to Salt Intake in the Rufous-Collared Sparrow. Front. Physiol. 2017, 8, 654. [Google Scholar] [CrossRef]
- Tapia-Monsalve, R.; Newsome, S.D.; Sanchez-Hernandez, J.C.; Bozinovic, F.; Nespolo, R.; Sabat, P. Terrestrial Birds in Coastal Environments: Metabolic Rate and Oxidative Status Varies with the Use of Marine Resources. Oecologia 2018, 188, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.S.; Sabat, P.; Castañeda, L.E.; Contreras, C.; Navarrete, L.; Peña-Villalobos, I.; Navedo, J.G. Oxidative Status and Metabolic Profile in a Long-Lived Bird Preparing for Extreme Endurance Migration. Sci. Rep. 2019, 9, 17616. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Otarola, N.; Maldonado, K.; Valdés-Ferranty, F.; Newsome, S.D.; Sabat, P. Seasonal Changes in Diet, Immune Function, and Oxidative Stress in Three Passerines Inhabiting a Mediterranean Climate. Oecologia 2023, 203, 395–405. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçağ, E. The Cupric Ion Reducing Antioxidant Capacity and Polyphenolic Content of Some Herbal Teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.P.N.; Magalhães, L.M.; Reis, S.; Lima, J.L.F.C.; Segundo, M.A. High-Throughput Total Cupric Ion Reducing Antioxidant Capacity of Biological Samples Determined Using Flow Injection Analysis and Microplate-Based Methods. Anal. Sci. 2011, 27, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Hellgren, O.; Waldenström, J.; Bensch, S. A New PCR Assay for Simultaneous Studies of Leucocytozoon, Plasmodium, and Haemoproteus from Avian Blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Burnham, K.; Anderson, D.R. A Practical Information-Theoretic Approach. In Model Selection and Multimodel Inference; Springer: New York, NY, USA, 1998; pp. 75–117. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, Inc.: Boston, MA, USA, 2024. [Google Scholar]
- Brooks, M.; Bolker, B.; Kristensen, K.; Maechler, M.; Magnusson, A.; McGillycuddy, M.; Skaug, H.; Nielsen, A.; Berg, C. Package ‘Glmmtmb’. R Packag Vers 2023, 1, 7. [Google Scholar]
- Sorci, G.; Faivre, B. Inflammation and Oxidative Stress in Vertebrate Host-Parasite Systems. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 71–83. [Google Scholar] [CrossRef]
- Jiménez-Peñuela, J.; Ferraguti, M.; Martínez-de la Puente, J.; Soriguer, R.; Figuerola, J. Urbanization and Blood Parasite Infections Affect the Body Condition of Wild Birds. Sci. Total Environ. 2019, 651, 3015–3022. [Google Scholar] [CrossRef]
- Razavi, S.M.; Nazifi, S.; Afsar, M.; Yazdanpanah, Z.; Rakhshandehroo, E. Evaluation of the Blood Oxidant-Antioxidant Interactions in Pigeons Naturally Infected with Haemoproteus columbae. Vet. Arh. 2016, 86, 395–405. [Google Scholar]
- Jiménez-Gallardo, L.; López-Arrabé, J.; Pérez-Tris, J.; Remacha, C. Young Male Blackcaps with Blood Parasite Coinfections Cope with Oxidative Stress Favouring Anthocyanin-Rich Food during Migratory Fattening. J. Avian Biol. 2024, e03214. [Google Scholar] [CrossRef]
- Pap, P.L.; Pătraş, L.; Osváth, G.; Buehler, D.M.; Versteegh, M.A.; Sesarman, A.; Banciu, M.; Vágási, C.I. Seasonal Patterns and Relationships among Coccidian Infestations, Measures of Oxidative Physiology, and Immune Function in Free-Living House Sparrows over an Annual Cycle. Physiol. Biochem. Zool. 2015, 88, 395–405. [Google Scholar] [CrossRef]
- Monaghan, P.; Metcalfe, N.B.; Torres, R. Oxidative Stress as a Mediator of Life History Trade-Offs: Mechanisms, Measurements and Interpretation. Ecol. Lett. 2009, 12, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Alvarez, C.; Bertrand, S.; Devevey, G.; Prost, J.; Faivre, B.; Sorci, G. Increased Susceptibility to Oxidative Stress as a Proximate Cost of Reproduction. Ecol. Lett. 2004, 7, 363–368. [Google Scholar] [CrossRef]
- Merrill, L.; Levengood, J.M.; England, J.C.; Osborn, J.M.; Hagy, H.M. Blood Parasite Infection Linked to Condition of Spring-Migrating Lesser Scaup (Aythya affinis). Can. J. Zool. 2018, 96, 1145–1152. [Google Scholar] [CrossRef]
- Marzal, A.; Reviriego, M.; Hermosell, I.G.; Balbontín, J.; Bensch, S.; Relinque, C.; Rodríguez, L.; Garcia-Longoria, L.; de Lope, F. Malaria Infection and Feather Growth Rate Predict Reproductive Success in House Martins. Oecologia 2013, 171, 853–861. [Google Scholar] [CrossRef] [PubMed]
- da Silva Rodrigues, R.; de Souza Penha, V.A.; Miwa, R.Y.; Branco, J.O.; Junior, O.M. Stress and Body Condition Predict Haemosporidian Parasitaemia in Birds from Cerrado, Southeastern Brazil. Ardea 2021, 109, 175–183. [Google Scholar] [CrossRef]
- Rivero de Aguilar, J.; Barroso, O.; Bonaccorso, E.; Cadena, H.; Hussing, L.; Jorquera, J.; Martinez, J.; Martínez-de la Puente, J.; Marzal, A.; León Miranda, F.; et al. Associations among MHC Genes, Latitude, and Avian Malaria Infections in the Rufous-Collared Sparrow (Zonotrichia capensis). Ecol. Evol. 2024, 14, e11634. [Google Scholar] [CrossRef]
- O’Connor, E.A.; Westerdahl, H.; Burri, R.; Edwards, S.V. Avian Mhc Evolution in the Era of Genomics: Phase 1.0. Cells 2019, 8, 1152. [Google Scholar] [CrossRef]
- Ellis, V.A.; Kunkel, M.R.; Ricklefs, R.E. The Ecology of Host Immune Responses to Chronic Avian Haemosporidian Infection. Oecologia 2014, 176, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Bosholn, M.; Anciães, M.; Gil, D.; Weckstein, J.D.; Dispoto, J.H.; Fecchio, A. Individual Variation in Feather Corticosterone Levels and Its Influence on Haemosporidian Infection in a Neotropical Bird. Ibis 2020, 162, 215–226. [Google Scholar] [CrossRef]
- Ilgūnas, M.; Bukauskaitė, D.; Palinauskas, V.; Iezhova, T.; Fragner, K.; Platonova, E.; Weissenböck, H.; Valkiūnas, G. Patterns of Plasmodium homocircumflexum Virulence in Experimentally Infected Passerine Birds. Malar. J. 2019, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Ilgūnas, M.; Bukauskaitė, D.; Fragner, K.; Weissenböck, H.; Atkinson, C.T.; Iezhova, T.A. Characterization of Plasmodium relictum, a Cosmopolitan Agent of Avian Malaria. Malar. J. 2018, 17, 184. [Google Scholar] [CrossRef]
- Cox, F.E.G. Concomitant Infections, Parasites and Immune Responses. Parasitology 2001, 122 (Suppl. S1), S23–S38. [Google Scholar] [CrossRef]
- Soares, L.; Latta, S.C.; Ricklefs, R.E. Dynamics of Avian Haemosporidian Assemblages through Millennial Time Scales Inferred From Insular Biotas of the West Indies. Proc. Natl. Acad. Sci. USA 2017, 114, 6635–6640. [Google Scholar] [CrossRef]
- De Angeli Dutra, D.; Fecchio, A.; Braga, É.M.; Poulin, R. Haemosporidian Taxonomic Composition, Network Centrality and Partner Fidelity Between Resident and Migratory Avian Hosts. Oecologia 2021, 197, 501–509. [Google Scholar] [CrossRef]
- Christe, P.; Glaizot, O.; Strepparava, N.; Devevey, G.; Fumagalli, L. Twofold Cost of Reproduction: An Increase in Parental Effort Leads to Higher Malarial Parasitaemia and to a Decrease in Resistance to Oxidative Stress. Proc. R. Soc. B Biol. Sci. 2012, 279, 1142–1149. [Google Scholar] [CrossRef]


| Effect on TBARS Levels | Estimate | SE | z Value | p- Value |
|---|---|---|---|---|
| Intercept | −8.51 | 0.14 | −59.47 | <0.00 ** |
| Infection status 1 | 0.25 | 0.11 | 2.33 | 0.02 * |
| Reproductive status 1 | −0.07 | 0.11 | −0.65 | 0.52 |
| Infection status 1: Reproductive status 1 | −0.20 | 0.15 | −1.35 | 0.18 |
| Random effects | σ | |||
| Location: Elevation | 0.05 | |||
| Effect on TAC Levels | Estimate | SE | z Value | p- Value |
| Intercept | −4.43 | 0.15 | −29.59 | <0.00 ** |
| Infection status 1 | −0.04 | 0.10 | −0.39 | 0.70 |
| Reproductive status 1 | −0.09 | 0.11 | −0.85 | 0.40 |
| Infection status 1: Reproductive status 1 | 0.19 | 0.13 | 1.43 | 0.15 |
| Random effects | σ | |||
| Location | 0.05 | |||
| Effect on TBARS/TAC Ratio | Estimate | SE | z Value | p- Value |
| Intercept | −4.06 | 0.10 | −39.6 | <0.00 *** |
| Infection status 1 | 0.48 | 0.12 | 3.9 | <0.00 *** |
| Reproductive status 1 | 0.00 | 0.13 | 0.01 | 0.98 |
| Infection status 1: Reproductive status 1 | −0.53 | 0.17 | −3.03 | <0.00 *** |
| Random effects | σ | |||
| Location: Elevation | 3.2 × 10−10 |
| Effect on TBARS | Estimate | SE | z Value | p- Value |
|---|---|---|---|---|
| Intercept | −9.17 | 0.17 | −53.18 | <0.00 *** |
| TAC levels | 47.21 | 12.54 | 3.76 | <0.00 *** |
| Infection status 1 | 0.53 | 0.18 | 2.95 | <0.00 *** |
| Sex 1 | 0.01 | 0.07 | 1.42 | 0.153 |
| BCI | −0.01 | 0.01 | −1.22 | 0.22 |
| TAC levels: Infection status 1 | −25.99 | 12.98 | −2.00 | 0.04 * |
| Random effects | σ | |||
| Location: Elevation | 0.01 |
| Effect on Body Condition | Estimate | SE | z Value | p- Value |
|---|---|---|---|---|
| Intercept | −1.48 | 1.02 | −1.44 | 0.18 |
| Infection status 1 | 1.07 | 0.81 | 1.33 | 0.19 |
| Sex 1 | 1.85 | 0.71 | 2.61 | 0.01 * |
| Breeding status 1 | 0.07 | 0.74 | 0.10 | 0.92 |
| Random effects | σ | |||
| Location: Elevation | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poblete, Y.; Contreras, C.; Ávila, M.; Carmona, M.P.; Fernández, C.; Flores, C.R.; Sabat, P. Haemosporidian Infection Is Associated with the Oxidative Status in a Neotropical Bird. Birds 2024, 5, 604-615. https://doi.org/10.3390/birds5030040
Poblete Y, Contreras C, Ávila M, Carmona MP, Fernández C, Flores CR, Sabat P. Haemosporidian Infection Is Associated with the Oxidative Status in a Neotropical Bird. Birds. 2024; 5(3):604-615. https://doi.org/10.3390/birds5030040
Chicago/Turabian StylePoblete, Yanina, Carolina Contreras, Miguel Ávila, María Paz Carmona, Carolina Fernández, Cristian R. Flores, and Pablo Sabat. 2024. "Haemosporidian Infection Is Associated with the Oxidative Status in a Neotropical Bird" Birds 5, no. 3: 604-615. https://doi.org/10.3390/birds5030040
APA StylePoblete, Y., Contreras, C., Ávila, M., Carmona, M. P., Fernández, C., Flores, C. R., & Sabat, P. (2024). Haemosporidian Infection Is Associated with the Oxidative Status in a Neotropical Bird. Birds, 5(3), 604-615. https://doi.org/10.3390/birds5030040





