Artificial Light at Night Increases Growth and Impairs Reproductive Success in Budgerigars (Melopsittacus undulatus) in a Duration Dose-Dependent Manner
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Housing
2.2. Experimental Design
2.3. Wb, Egg Production, and Hatchability
2.4. Droppings Collection
2.5. MLTS Assay
2.6. Statistical Analysis
3. Results
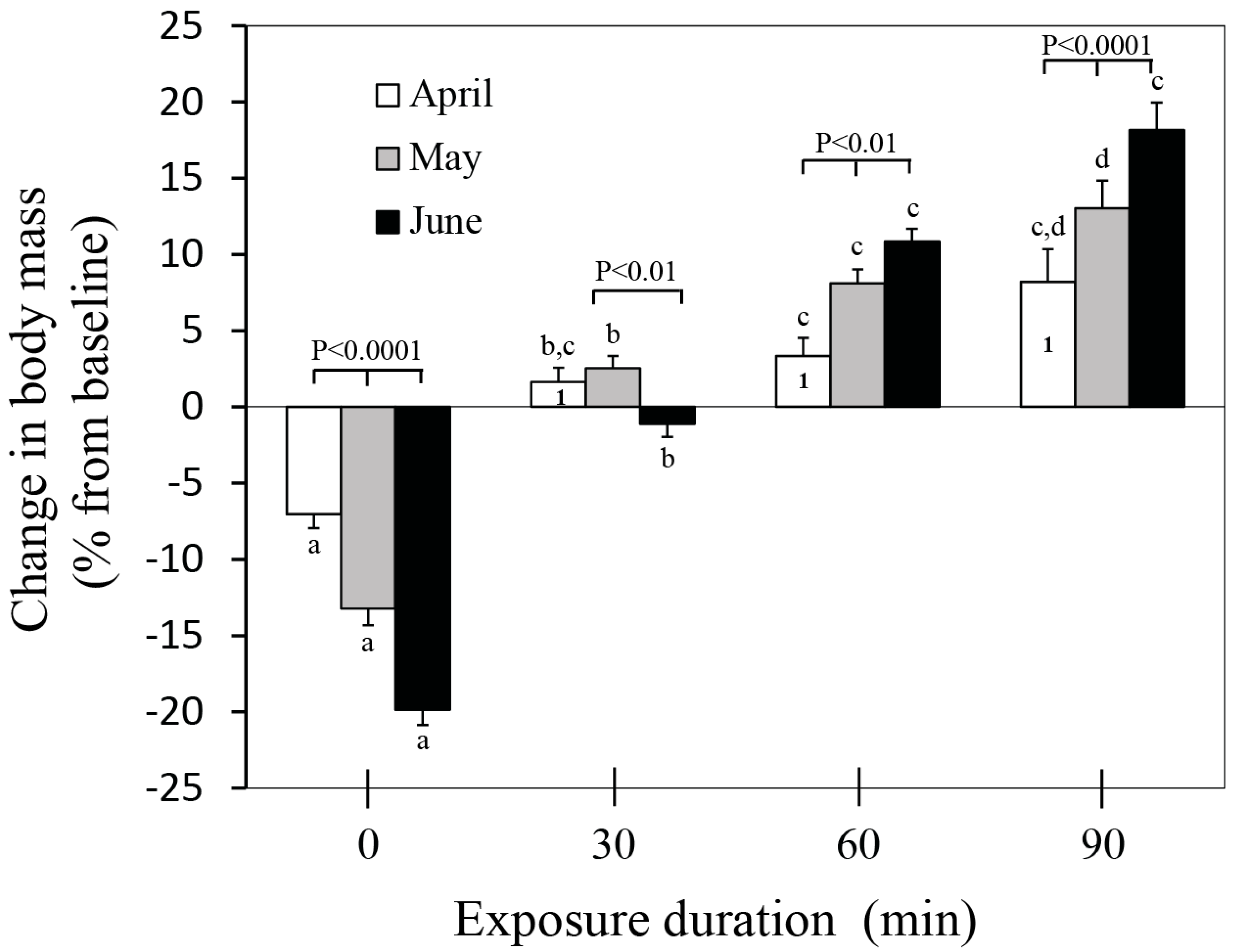
3.1. Body Mass Changes
3.2. Reproductive Success
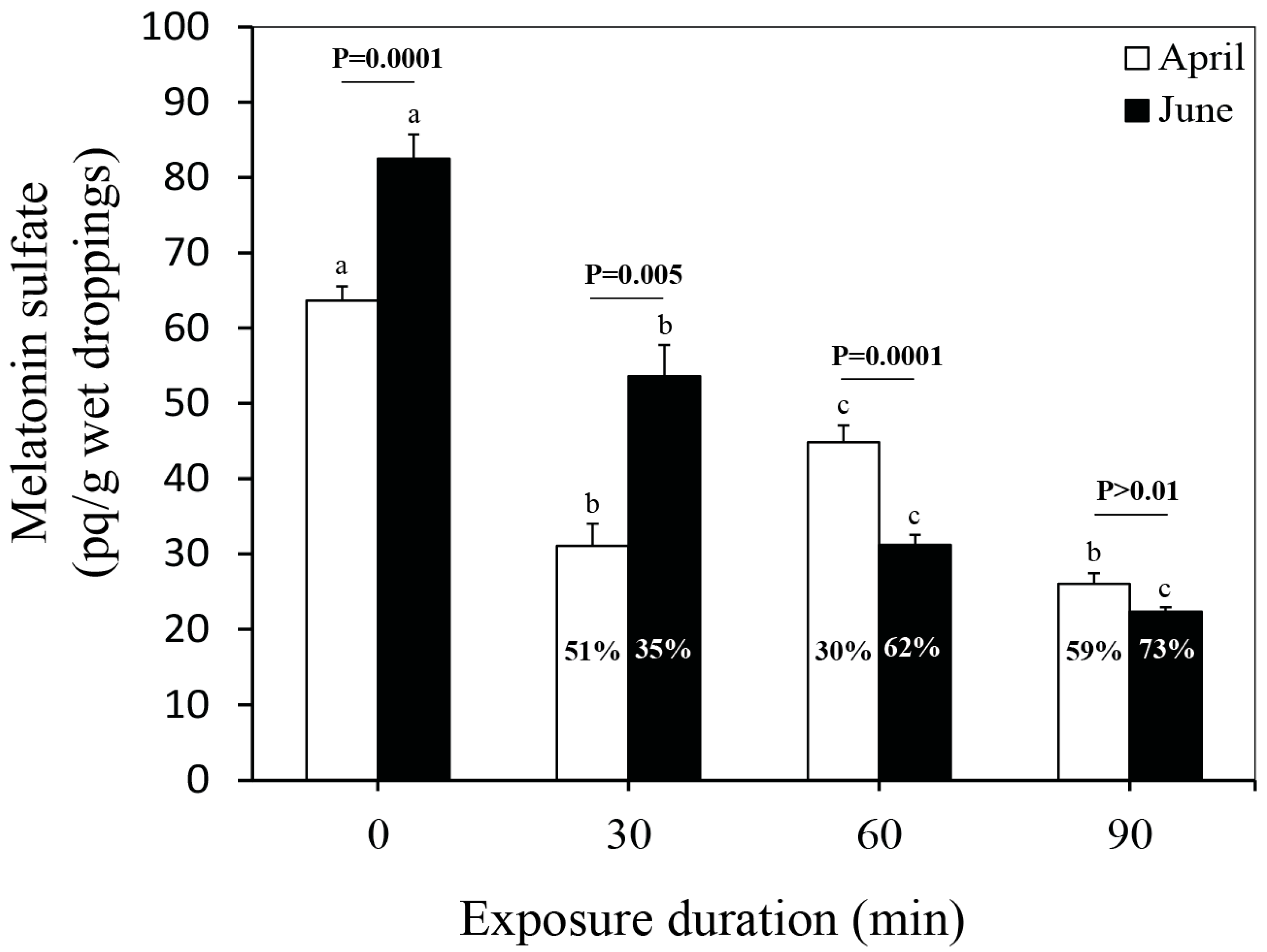
3.3. MLTS Levels
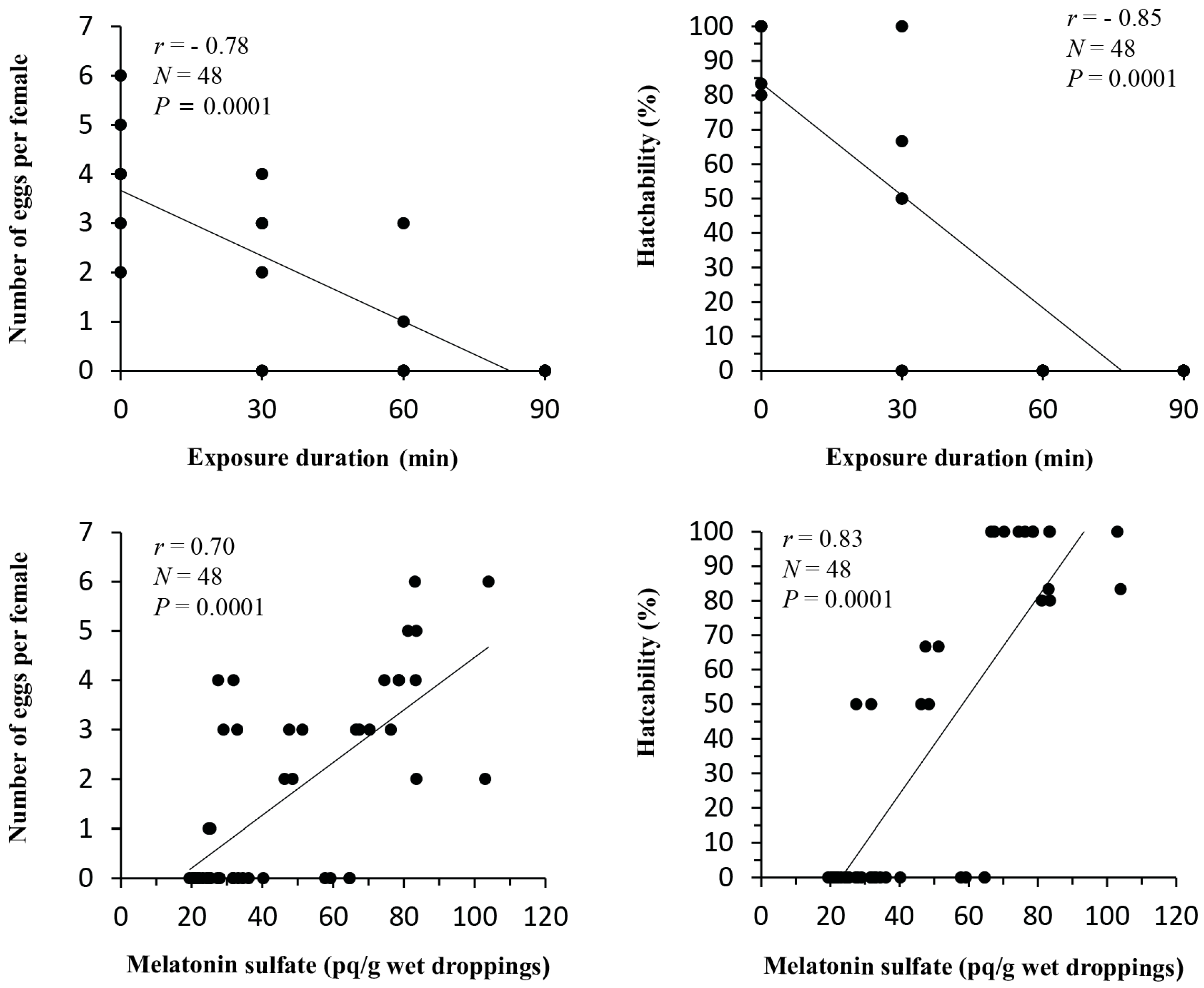
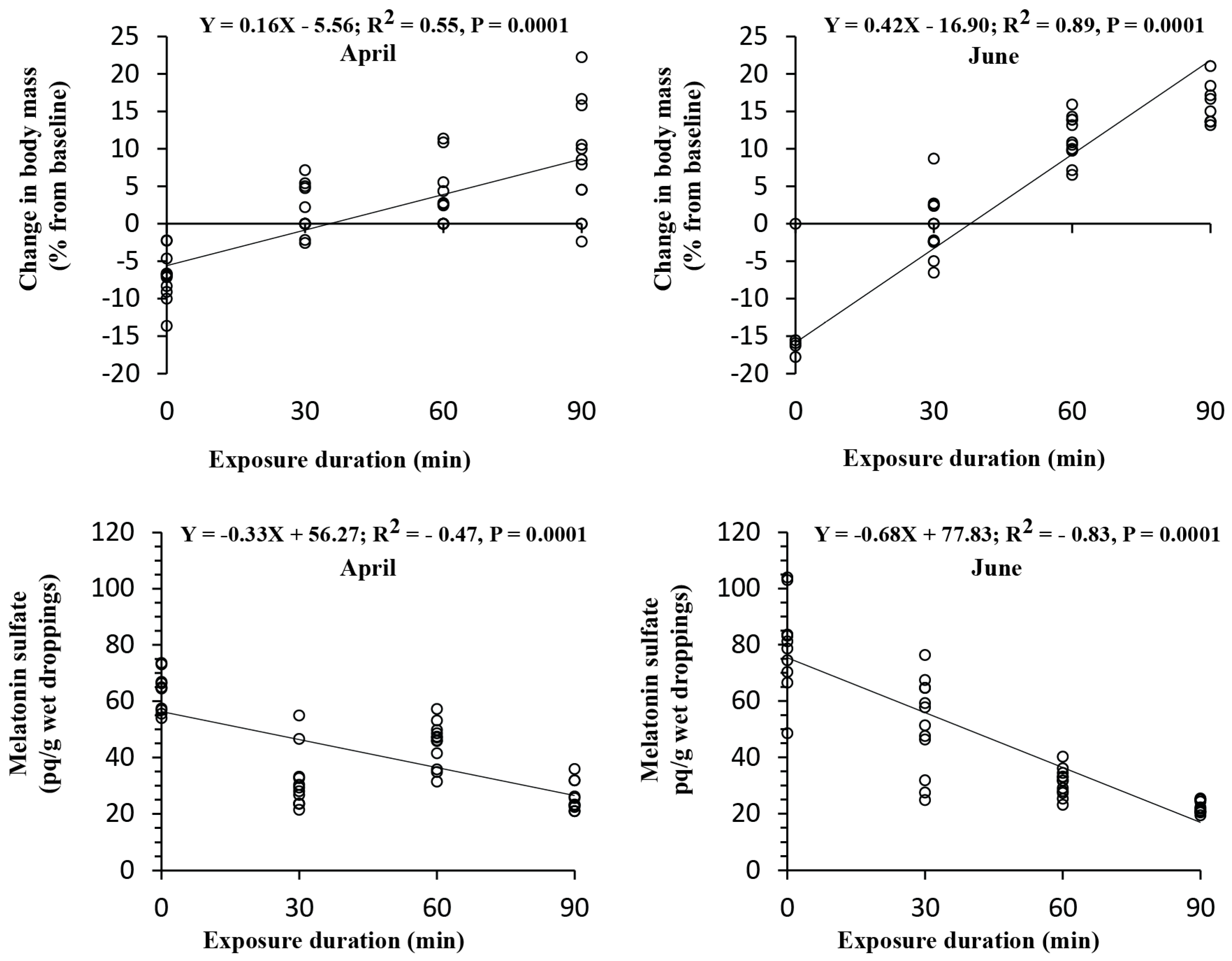
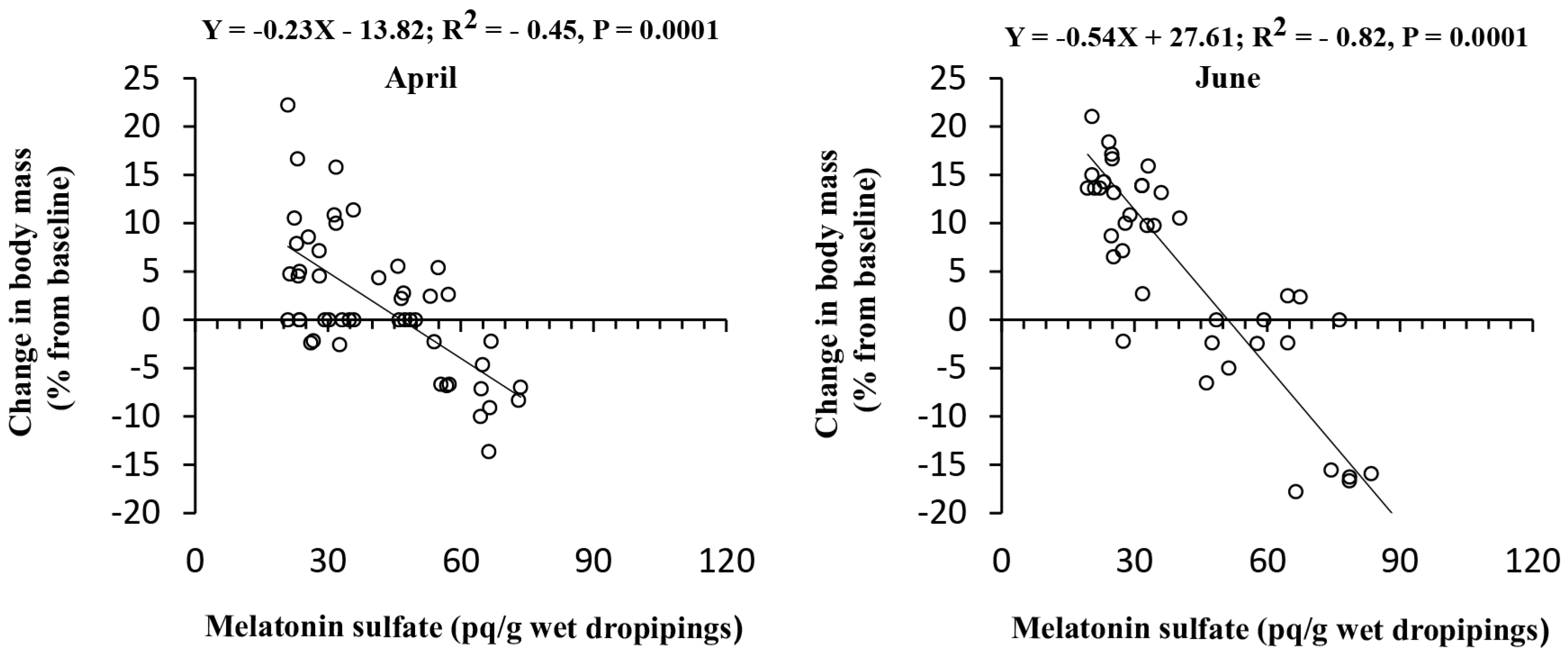
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fonken, L.K.; Workman, J.L.; Walton, J.C.; Weil, Z.M.; Morris, J.S.; Haim, A.; Nelson, R.J. Night at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664–18669. [Google Scholar] [CrossRef] [PubMed]
- Rich, C.; Longcore, T. Ecological Consequences of Artificial Night Lighting; Island Press: Washington, DC, USA, 2010. [Google Scholar]
- Zubidat, A.E.; Fares, B.; Fares, F.; Haim, A. Melatonin through DNA methylation constricts breast cancer growth accelerated by blue LED light at night in 4T1 tumor bearing mice. Gratis J. Cancer Biol. Ther. 2015, 1, 57–73. [Google Scholar] [CrossRef]
- Cassone, V.M. Avian circadian organization: A chorus of clocks. Front. Neuroendocrinol. 2014, 35, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, U.; Thakkar, N.; Das, P.; Pal Bhadra, M. Evolution of circadian rhythms: From bacteria to human. Sleep Med. 2017, 35, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Binkley, S. Circadian organization in mammals and birds. Photochem. Photobiol. 1982, 35, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.J. Photoperiodic regulation of seasonal breeding in birds. Ann. N. Y. Acad. Sci. 2005, 1040, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Raap, T.; Sun, J.; Pinxten, R.; Eens, M. Disruptive effects of light pollution on sleep in free-living birds: Season and/or light intensity-dependent? Behav. Process. 2017, 144, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.; Valcu, M.; Kempenaers, B. Light pollution alters the phenology of dawn and dusk singing in common European songbirds. Philos. Trans. Royal Soc. B 2015, 370, 20140126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, X.; Zhang, J.; Li, H. Differences in the reproductive hormone rhythm of tree sparrows (Passer montanus) from urban and rural sites in Beijing: The effect of anthropogenic light sources. Gen. Comp. Endocrinol. 2014, 206, 24–29. [Google Scholar] [CrossRef]
- Dominoni, D.M.; Goymann, W.; Helm, B.; Partecke, J. Urban-like night illumination reduces melatonin release in European blackbirds (Turdus merula): Implications of city life for biological time-keeping of songbirds. Front. Zool. 2013, 10, 60. [Google Scholar] [CrossRef]
- Oishi, T.; Yamao, M.; Kondo, C.; Haida, Y.; Masuda, A.; Tamotsu, S. Multiphotoreceptor and multioscillator system in avian circadian organization. Microsc. Res. Tech. 2001, 1, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Underwood, H.; Steele, C.T.; Zivkovic, B. Circadian organization and the role of the pineal in birds. Microsc. Res. Tech. 2001, 53, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Münch, M.; Kobialka, S.; Kräuchi, K.; Steiner, R.; Oelhafen, P.; Orgül, S.; Wirz-Justice, A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 2005, 90, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; Jeninga, L.; Ouyang, J.Q.; van Oers, K.; Spoelstra, K.; Visser, M.E. Dose-dependent responses of avian daily rhythms to artificial light at night. Physiol. Behav. 2016, 155, 172–179. [Google Scholar] [CrossRef]
- Dominoni, D.M.; De Jong, M.; Bellingham, M.; O’Shaughnessy, P.; van Oers, K.R.; Smith, B.; Visser, M.E.; Helm, B. Dose-response effects of light at night on the reproductive physiology of great tits (Parus major): Integrating morphological analyses with candidate gene expression. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Malek, I.; Haim, A.; Izhaki, I. Melatonin mends adverse temporal effects of bright light at night partially independent of its effect on stress responses in captive birds. Chronobiol. Int. 2020, 37, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.Q.; de Jong, M.; van Grunsven, R.H.A.; Matson, K.D.; Haussmann, M.F.; Meerlo, P.; Visser, M.E.; Spoelstra, K. Restless roosts: Light pollution affects behavior, sleep, and physiology in a free-living songbird. Glob. Chang. Biol. 2017, 23, 4987–4994. [Google Scholar] [CrossRef]
- Raap, T.; Casasole, G.; Costantini, D.; AbdElgawad, H.; Asard, H.; Pinxten, R.; Eens, M. Artificial light at night affects body mass but not oxidative status in free-living nestling songbirds: An experimental study. Sci. Rep. 2016, 6, 35626. [Google Scholar] [CrossRef] [PubMed]
- Crome, F.; Shields, J. Parrots and Pigeons of Australia (National Photographic Index of Australian Wildlife); Angus and Robertson: Sydney, Australia, 1992. [Google Scholar]
- Dominoni, D.M. The effects of light pollution on biological rhythms of birds: An integrated, mechanistic perspective. J. Ornithol. 2015, 156, S409–S418. [Google Scholar] [CrossRef]
- Warwic, C.; Jessop, M.; Arena, P.; Pilny, A.; Steedman, C. Guidelines for Inspection of Companion and Commercial Animal Establishments. Front. Vet. Sci. 2018, 5, 151–172. [Google Scholar] [CrossRef]
- Cissé, Y.M.; Nelson, R.J. Consequences of circadian dysregulation on metabolism. ChronoPhysiol. Ther. 2016, 6, 55–63. [Google Scholar]
- Moore, C.B.; Siopes, T.D. Effects of lighting conditions and melatonin supplementation on the cellular and humoral immune responses in Japanese quail Coturnix coturnix japonica. Gen. Comp. Endocrinol. 2000, 119, 95–104. [Google Scholar] [CrossRef]
- Tanaka, H.; Honda, Y.; Hirasawa, M.; Fujishima, T.; Abe, S. Budgerigar breeders’ hypersensitivity pneumonitis presenting as chronic bronchitis with purulent sputum. Intern. Med. 1995, 34, 676–678. [Google Scholar] [CrossRef][Green Version]
- der Strate, B.V.; Longdin, R.; Geerlings, M.; Bachmayer, N.; Cavallin, M.; Litwin, V.; Patel, M.; Passe-Coutrin, W.; Schoelch, C.; Companjen, A.; et al. Best practices in performing flow cytometry in a regulated environment: Feedback from experience within the European Bioanalysis Forum. Bioanalysis 2017, 9, 1253–1264. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Liang, W.; Wang, Y.; Zhang, S. Intensity dependent disruptive effects of light at night on activation of the HPG axis of tree sparrows (Passer montanus). Environ Pollut. 2019, 249, 904–909. [Google Scholar] [CrossRef]
- Goymann, W. Noninvasive monitoring of hormones in bird droppings: Physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Ann. N. Y. Acad. Sci. 2005, 1046, 35–53. [Google Scholar] [CrossRef]
- GFI Guidance for Industry—Bioanalytical Method Validation. 2018. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf (accessed on 1 July 2019).
- Zubidat, A.E.; Fares, B.; Fares, F.; Haim, A. Artificial light at night of different spectral compositions differentially affects tumor growth in mice: Interaction with melatonin and epigenetic pathways. Cancer Control 2018, 25, 1073274818812908. [Google Scholar] [CrossRef]
- Titulaer, M.; Spoelstra, K.; Lange, C.Y.; Visser, M.E. Activity patterns during food provisioning are affected by artificial light in free living great tits (Parus major). PLoS ONE 2012, 7, e37377. [Google Scholar] [CrossRef]
- Hinde, R.A.; Steel, E. The influence of daylength and male vocalizations on the estrogen-dependent behavior of female canaries and Budgerigars, with discussion of data from other species. Adv. Study Behav. 1987, 8, 39–73. [Google Scholar]
- Brockway, B.F. Roles of budgerigar vocalization in the integration of breeding behaviors. In Bird Vocalizations; Hinde, R., Ed.; Cambridge University Press: Cambridge, UK, 1969; pp. 131–158. [Google Scholar]
- Wang, G.; Harpole, C.E.; Paulose, J.; Cassone, V.M. The role of the pineal gland in the photoperiodic control of bird song frequency and repertoire in the house sparrow, Passer domesticus. Horm. Behav. 2014, 65, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Falchi, F.; Cinzano, P.; Elvidge, C.D.; Keith, D.M.; Haim, A. Limiting the impact of light pollution on human health, environment and stellar visibility. J. Environ. Manag. 2011, 92, 2714–2722. [Google Scholar] [CrossRef]
- Thapan, K.; Arendt, J.; Skene, D.J. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J. Physiol. 2001, 535, 261–267. [Google Scholar] [CrossRef]
- Alaasam, V.J.; Duncan, R.; Casagrande, S.; Davies, S.; Sidher, A.; Seymoure, B.; Shen, Y.; Zhang, Y.; Ouyang, J.Q. Light at night disrupts nocturnal rest and elevates glucocorticoids at cool color temperatures. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 465–472. [Google Scholar] [CrossRef]
- Russ, A.; Reitemeier, S.; Weissmann, A.; Gottschalk, J.; Einspanier, A.; Klenke, R. Seasonal and urban effects on the endocrinology of a wild passerine. Ecol. Evol. 2015, 5, 5698–5710. [Google Scholar] [CrossRef]
- Angelier, F.; Wingfield, J.C. Importance of the glucocorticoid stress response in a changing world: Theory, hypotheses and perspectives. Gen. Comp. Endocrinol. 2013, 190, 118–128. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Palanza, P.; Sacerdote, P.; Panerai, A.E.; Sgoifo, A.; Dantzer, R.; Parmigiani, S. Social factors and individual vulnerability to chronic stress exposure. Neurosci. Biobehav. Rev. 2005, 29, 67–81. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J.D.; Buler, J.J.; Schreckengost, T.; Smolinsky, J.A.; Boone, M.; Emiel van Loon, E.; Dawson, D.K.; Walters, E.L. Artificial light at night confounds broad-scale habitat use by migrating birds. Ecol. Lett. 2018, 21, 356–364. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itay, M.; Haim, A. Artificial Light at Night Increases Growth and Impairs Reproductive Success in Budgerigars (Melopsittacus undulatus) in a Duration Dose-Dependent Manner. Birds 2024, 5, 352-362. https://doi.org/10.3390/birds5030023
Itay M, Haim A. Artificial Light at Night Increases Growth and Impairs Reproductive Success in Budgerigars (Melopsittacus undulatus) in a Duration Dose-Dependent Manner. Birds. 2024; 5(3):352-362. https://doi.org/10.3390/birds5030023
Chicago/Turabian StyleItay, Malek, and Abraham Haim. 2024. "Artificial Light at Night Increases Growth and Impairs Reproductive Success in Budgerigars (Melopsittacus undulatus) in a Duration Dose-Dependent Manner" Birds 5, no. 3: 352-362. https://doi.org/10.3390/birds5030023
APA StyleItay, M., & Haim, A. (2024). Artificial Light at Night Increases Growth and Impairs Reproductive Success in Budgerigars (Melopsittacus undulatus) in a Duration Dose-Dependent Manner. Birds, 5(3), 352-362. https://doi.org/10.3390/birds5030023



